Abstract
Humankind is presently engulfed by convenience quench, modern life style and urbanized diet system leading to progression in array of health disorders. The past decade confronted cardiometabolic disorder (21.8 %), lower respiratory and chronic obstructive lung disease (12.5 %) as the major causes of death world over. In anticipation, scientific communities' have demonstrated the role of healthy diets, especially those rich in fruits and vegetables, for management of such health related issues. These horticultural crops are considered as a good source of polyphenols such as dihydrochalcones, flavanols, flavonols, anthocyanins and phenolic acids. The present article reviews the efforts made to assess the potential of apple phenolic compounds present in fresh fruits, leaves, bark and pomace as dietary polyphenols. Considering the positive impact of such phytochemicals on human health, various nutraceuticals, dietary supplements and phenolic-rich food products are presently available on market shelves. On analytical front, improved instrumentation based on liquid chromatography (HPLC, UPLC, LC/MS/MS) have made the assessment of phenolics more rapid and reliable. Thus, owing to the emergent interest in natural compounds, it is pertinent to discuss the latest significant research findings on therapeutic aspects along with probable metabolic mechanisms of dietary polyphenols found in apples and their implications on human health.
Keywords: Apple, Phenolics, Antioxidant, Extraction, Bioactivity
Introduction
Convenience food and sedentary lifestyle may be responsible for the present state of human health, in which there is a high prevalence of metabolic disorders such as diabetes mellitus, cardiovascular disease, obesity and aging related syndromes. The World Health Organization (WHO) report revealed that the major causes responsible for human death world-over in past decade are ischaemic heart disease and stroke (21.8 %), lower respiratory infections and chronic obstructive lung disease (12.5 %), diarrhea (4.7 %), HIV/AIDS (3 %) and lung cancers (2.9 %) (WHO 2014). Overall deaths due to chronic disease like cancer have been reduced, however, the number of deaths caused by lung cancers (along with trachea and bronchus cancers) has increased in this decade (2.2 % deaths in 2000 to 2.9 % in 2012). The prime reason of wide occurrence of these diseases is, in part, associated with changing lifestyle and diet intake. Mediterranean diets or diets rich in fruits and vegetables are considered as good source of human health promoting constituents. Regular consumption of such diets are generally linked to management and prevention of various chronic diseases including cardiovascular diseases, cancer and diabetes (Boyer and Liu 2004; Hyson 2011).
Apple (Malus domestica Borkh.), a member of Rosaceae family, is an important and most widely grown temperate fruit crop in the world. As per FAO Statistical Year Book, the world apple production was approximately 76 million tonne during 2012 with China, USA, Turkey, Poland and India among the top five producers (FAO 2013). Besides fresh consumption, a significant amount of apple is also processed into juice, juice concentrate, cider and canned products. It is among the top of nutritionally rated and consumed fruits in the world (Boyer and Liu 2004). Apples are rich source of numerous phytonutrients, especially phenolic compounds and dietary carbohydrates. Apple phenolics are naturally occurring compounds that act as effective antioxidants by protecting the cell against damaging effects of free radicals and by inhibiting the oxidation of low density lipoproteins. Furthermore, apple consumption was reported to be related to positive effects on ageing and cognitive decline, weight management, bone health, asthma and pulmonary function, and gastrointestinal health (Hyson 2011). Much of these effects are associated to the presence of secondary metabolites such as flavonoids, isoflavonoids, carotenoids and phenolic acids, and dietary fibre. These molecules may be helpful in alleviating age-related cognitive decline resulted from oxidative stress. Studies revealed that supplementation of a vitamin-deficient, oxidative stress-promoting diet with apple juice concentrate helped aged mice against neurodegenerative effects (Tchantchou et al. 2005). Later, it was demonstrated that dietary intervention with apple juice concentrate was useful to augment remedial approach for age-related neurodegeneration as evident from alleviation of increased amyloid-β (Aβ) levels in normal adult mice, potentiated by apolipoprotein E deficiency (Chan and Shea 2009). Diet supplemented with fresh apples (annurca variety) showed a significant decrease in the anxiety level and improved ability to sustain long-term potentiating in aged rats (Viggiano et al. 2006). Reactive oxygen species (ROS) not only plays an important role in cardiometabolic disorders, but also responsible for development of gastric adenocarcinoma. Apple polyphenol extract too prevented ROS induced injury to gastric epithelial cells under in vitro (MKN 28 cells) and counteracted xanthine-xanthine oxidase induced lipid peroxidation under in vivo (Male Wistar rats) conditions (Graziani et al. 2005).
Major phenolics present in apple and its plant parts
The reviewed literature provides evidence suggesting that antioxidants-rich diet, to include apples, can exert beneficial effect on human health, which would be of benefit to the present health care system. Continuous efforts are being made to scientifically support the contribution of various apple phytochemicals in human health. The polyphenols present in various varieties of apples are almost identical although the concentrations differ (D’Archivio et al. 2007). The structural classes of polyphenols present in apples include flavonols (quercetin, kaempferol, and rutin), dihydrochalcones (phloretin and phloridzin), flavan-3-ols (epicatechin and procyanidins) and phenolic acids (caffeic acid and coumaric acid). In addition to apple fruits, apple leaves are also known to contain phenolic compounds such as 3-hydroxyphloridzin, phloridzin and quercetin-3-O-arabinoside and rutin (Walia et al. 2015). The major polyphenols present in fruits, plant parts and products are summarized in Table 1. Quantitatively, the prevailing polyphenols found in apples and its plant parts include hydroxycinnamic acids, flavan-3-ols, flavonols and dihydrochalcones (Fig. 1 & Table 2).
Table 1.
Profile of phenolics present in different parts of apple fruits
| Fruit type/part | Polyphenols (mg/kg dry weight) | ||||||
|---|---|---|---|---|---|---|---|
| Epicatechin | Procyanidin B2 | Chlorogenic acid | Phloridzin | Caffeic acid | Quercetin | Reference (s) | |
| Unripe fruit | 3281.0 | 2355.0 | 2063.0 | 838.0 | 725.0 | 21.3 | Yue et al. 2012 |
| Apple peel | 1080.0 | 1190.0 | 410.0 | 429.0 | ND | ND | Alonso-Salces et al. 2001 |
| Apple pulp | 220.0 | 340.0 | 540.0 | 59.0 | ND | ND | Alonso-Salces et al. 2001 |
| 15.5 | 219.4 | 62.8 | 28.1 | 83.7 | 61.4 | Bai et al. 2010 | |
| 596.0 | 243.0 | 686.0 | 1265.0 | ND | 46.47 | Lavelli and Corti 2011 | |
| Apple juice* | 46.6 | 38.7 | 37.6 | 4.1 | 4.8 | ND1 | Kahle et al. 2005 |
*Golden delicious variety, mg/L
Fig. 1.
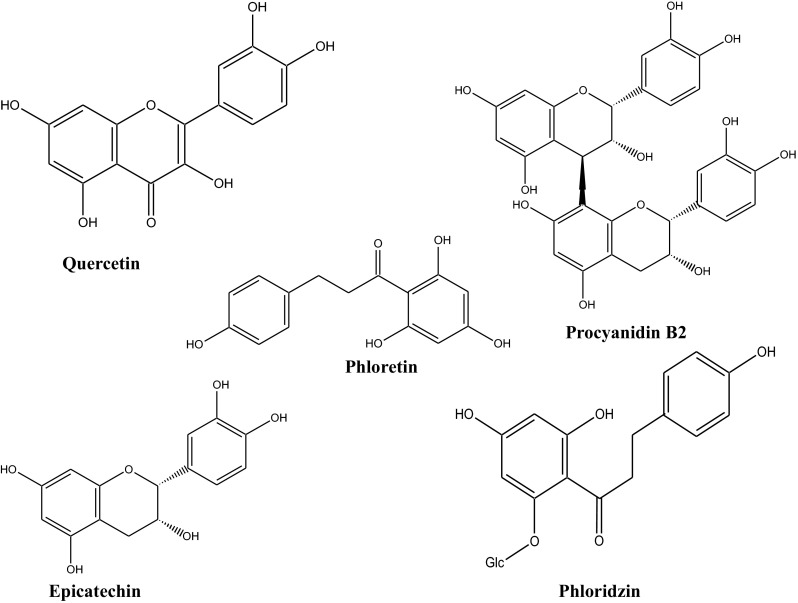
Major phenolics present in apple (Boyer and Liu 2004; Hyson 2011)
Table 2.
Major structural classes of phenolics present in apple
| Polyphenol Class | |
| Flavonoids | |
| Anthocyanins | |
| Cyanidin 3-O-arabinoside Cyanidin 3-O-galactoside Cyanidin 3-O-xyloside | |
| Dihydrochalcones | |
| 3- Hydroxyphloretin 2′-O-glucoside Phloridzin Phloretin Phloretin-2-xyloglucoside | |
| Flavanols | |
| Catechin Epicatechin Procyanidin B1 Procyanidin B2 Procyanidin C1 Procyanidin-tetramer Procyanidin-pentamer | |
| Flavonols | |
| Quercetin-3-arabinopyranoside Quercetin-3-arabinofuranoside Quercetin 3-O-glactoside Quercetin 3-O-glucoside Quercetin 3-O-rhamnoside Quercetin 3-O-rutinoside Quercetin 3-O-xyloside Quercetin (aglycone) | |
| Phenolic acids | Hydroxy benzoic acid |
| Syringic acid | |
| Hydroxy cinnamic acid | |
| Caffeic acid 4-p-Coumaroylquinic acid 4-Caffeoylquinic acid Ferulic acid p-Coumaric acid |
Purification and analysis of apple polyphenols
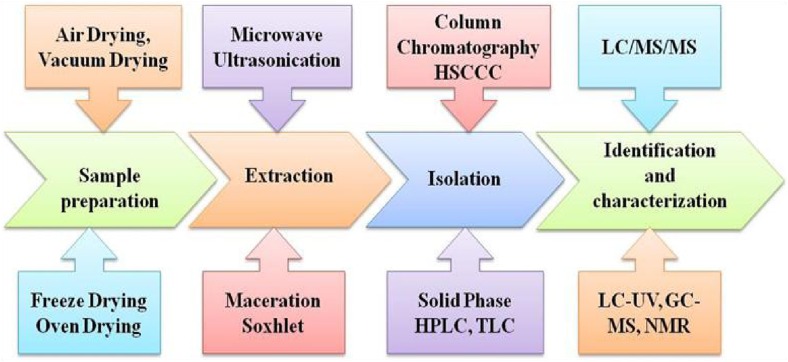
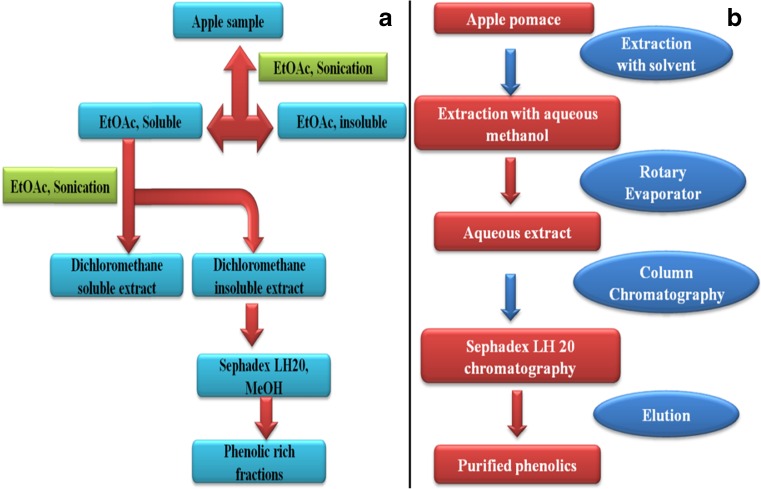
There are various validated analytical methods for the assessment and analysis of phenolics from apple and its plant parts (Table 3). The common techniques used for the determination of phenolics (Table 4) includes liquid chromatography (LC) coupled with mass spectroscopy (MS), electrospray ionization (ESI), LC/MS/MS, high performance liquid chromatography (HPLC/nuclear magnetic resonance (NMR)/mass spectroscopy (MS), LC-Ultraviolet (UV), and gas chromatography (GC)/MS (Sanchez-Rabaneda et al. 2004). In addition, sephadex-chromatography (Sephadex LH 20), high-speed countercurrent chromatography (HSCCC), (Cao et al. 2009) supercritical carbon dioxide chromatographic techniques were also employed for the separation of phenolics (Shibusawa et al. 2000). Spectrophotometric method such as Folin–Ciocalteu assay also used for quantification of total polyphenol contents (Rana et al. 2014). However, these assays have some limitation of proteins interference. The determination of phenolics by HPLC was more accurate in comparison to UV/Vis spectrophotometer. It is most reliable, fast, precise and sensitive, and useful technique for separation and identification of phenolics (Rana et al. 2014). In HPLC, the widely used detectors are diode array detector (DAD) and UV-VIS detector that makes detection of phenolics more accurate. Recently, we have reported a simple, fast and reproducible reversed-phase high-performance liquid chromatography (RP-HPLC) method using DAD for separation of eight phenolics simultaneously from industrial apple pomace (Rana et al. 2014). The separation of all the phenolics was achieved within 25 min using C12 stationary phase. Selection of stationary phase for the separation of phenolics is also important, however, most investigators reported use of reverse phase columns. Preparative high speed counter current chromatography was used for the separation and purification of phloretin from apple tree bark (Xu et al. 2010). The separation and identification of quercetin and phloretin glycosides from apple peels was achieved by HPLC/NMR/MS system (Lommen et al. 2000). Also phenolics can be analyzed by their derivatization with methyl esters employing GC and GC-MS techniques. The graphical representation of extraction, purification and characterization of phenolics from apple and apple parts is shown in Fig. 2. Owing to high moisture contents, apple tissues were dried either by lyophilization or oven drying before extraction to avoid degradation of indigenous polyphenolic constituents. However, drying at high temperature (above 60 °C) is detrimental to the most of phenolic composition, thus should be avoided. Different solvents (acetone, methanol, ethanol and ethyl acetate) of varying polarity were used for the efficient extraction of phenolics. Occasionally, solvent fractionation technique (Fig. 3a) using mixture of solvents was also employed for purification of phenolics. In order to break the strong association of phenolics with macronutrients such as proteins, effective method such as accelerated solvent, ultrasonication and microwave are advocated. Microwave extraction technique (microwave power 650.4, extraction time 53.7 s, solvent concentration 62.1 %) was efficiently used for extraction of phenolic compounds from apple pomace (Bai et al. 2010). Liquid–liquid and solid-phase extraction and chromatographic separation are also suggested by few investigators, out of which, solid-phase extraction was found more suitable. In case of dried apple pomace, extraction with aqueous acetone was done at room temperature. The obtained extract was concentrated using rotary evaporator and loaded onto a Sephadex LH-20 column, before separation by aqueous methanol (Fig. 3b). Majority of the phenolics were obtained in 80 % methanol (Foo and Lu 1999).
Table 3.
Characterization of major phenolics using validated protocols
| Apple Plant parts/products | Analytical methods | Major Phenolics | Calibration curve | Calibration range | R2 | LOD | LOQ | Reference(s) |
|---|---|---|---|---|---|---|---|---|
| Leaf | UPLC/MS/MS | Phloretin | y = 21199× −38.94 | 0.195–25 (μg mL−1) | 0.999 | 0.09 (μg mL−1) | 0.27(μg mL−1) | Walia et al. 2015 |
| Rutin | y = 75721× −13.04 | 0.195–25 (μg mL−1) | 0.999 | 0.19 (μg mL−1) | 0.57 (μg mL−1) | |||
| 3Hydroxyphloridzin | y = 62793× −35.17 | 0.58–75 (μg mL−1) | 0.999 | 0.29 (μg mL−1) | 0.87(μg mL−1) | |||
| Pomace | RP-HPLC-DAD | Phloridzin | Y = 0.0149× −0.0725 | 50–200 (μg mL−1) | 0.9962 | 0.14 (μg mL−1) | 0.48(μg mL−1) | Rana et al. 2014 |
| Phloretin | Y = 0.0328× −0.2057 | 50–200 (μg mL−1) | 0.9931 | 0.14(μg mL−1) | 0.48(μg mL−1) | |||
| Quercetin | Y = 0.0113× −0.066 | 50–200(μg mL−1) | 0.9957 | 0.58(μg mL−1) | 1.95(μg mL−1) | |||
| Leaves and peel | HPLC-DAD | Phloridzin | – | 0.5–90 (μg mL−1) | 0.9994 | 0.1 (μg mL−1) | 0.2(μg mL−1) | Kindt et al. 2007 |
| Quercetin | – | 0.5–90 (μg mL−1) | 0.9994 | 0.1 (μg mL−1) | 0.2(μg mL−1) | |||
| Phloretin | – | 0.5–90 (μg mL−1) | 0.9995 | 0.1(μg mL−1) | 0.2(μg mL−1) | |||
| Juice cider | UHPLC-MS/MS | Phloridzin | Y = −4619.6 + 198410X | 0.33–100 ng | 0.997 | <0.03 ng | 0.03 ng | Verdu et al. 2013 |
| Rutin | Y = −10,222 + 570740X | 0.070–5 ng | 0.9991 | 0.003 ng | 0.007 ng | |||
| Quercitrin | Y = −218,580 + 174400X | 2.0–150 ng | 0.9972 | <0.05 ng | 0.05 ng | |||
| Fruits | UHPLC-DAD/ESI-am-MS | Phloridzin | – | 0.5–3925 (ng mL-1) | 0.9992 | 20 (ng g −1) | 40(ng g-1) | De Paepe et al. 2013 |
| Quercetin | – | 0.5–3906 (ng mL-1) | 0.9994 | 40 (ng g-1) | 80 (ng g-1) | |||
| Phloretin | – | 0.5–3695 (ng mL-1) | 0.9991 | 40 (ng g-1) | 80 (ng g-1) |
Table 4.
Extraction and analysis of apple phenolics
| Sample type | Extraction system(s) | Extraction solvent (s) | Major Phenolics | Analytical technique (s) | References |
|---|---|---|---|---|---|
| Apple leaves | Phloridzin | X-5 resin | Huai-De et al. 2010 | ||
| Homogenization, Centrifugation | Methanol, Acetone | Flavanols, Hydroxycinnamic acids, Dihydrochalcones, Flavonols | HPLC | Kindt et al. 2007 | |
| Apple tree bark | Phloretin | HSCCC | Xu et al. 2010 | ||
| Apple pulp and peel | Pressurized liquid extraction | Methanol | Catechins, Phloretin Glycosides, Quercetin glycosides, Hydroxycinnamic acids | HPLC-DAD | Alonso-Salces et al. 2001 |
| Solvent extraction | Phloretin, Quercetin glycosides | HPLC-NMR-MS | Lommen et al. 2000 | ||
| Ultrasonic extraction | Hexane, Methanol, water | Rutin, Cyanidin-3- galactoside, Phloridzin, Catechin, Quercetin glycoside | HPLC | Mehrabani et al. 2011 | |
| Ultrasonication | Chloroform, acetate, Acetone, Methanol, water | Quercetin glycosides | HPLC/MS/MS | Rupasinghe et al. 2011 | |
| Solvent extraction | Methanol, ethanol and acetone | Catechin, Chlorogenic acid, Epicatechin, Procyanidins, Phloridzin, Quercetin, Cyanidin | HPLC | Karaman et al. 2013 | |
| Homogenization, centrifugation | Methanol | Flavanols, Hydroxycinnamic acids, Dihydrochalcones, Flavonols | HPLC | Kindt et al. 2007 | |
| Apple fruit | Ultrasonic extraction | Hexane Methanol, water | Quercetin-3-galactoside, Cyanidin-3-galactoside, Epicatechin, Chlorogenic acid | HPLC | Mehrabani et al. 2012 |
| Phenolic compounds mixture | UHPLC-DAD/ESI-am-MS | De Paepe et al. 2013 | |||
| Pressurized hot water extraction | Phenolic acid, Flavanol | HPLC-DAD-ECD-CAD | Plaza et al. 2014 | ||
| Solvent fraction | Methanol | Phloridzin, Catechin, Quercetin, Rutin, Coumaroylquinic acid, Procyanidin | UHPLC-MS | Ceymann et al. 2011 | |
| Ultrasonication | Methanol, Ethylacetate | Protocatechuic acid, Chlorogenic acid, Caffeic acid, Rutin, Gallic acid | HPLC/DAD | Chen et al. 2011 | |
| Apple products | Solid phase extraction, liquid liquid extraction | Water, methanol, ethyl acetate | Phenolic mixture | HPLC | Picinelli et al. 1997 |
| Apple Juice | Solid phase extraction | Catechins, Hydoxybenzioic acids, Hydroxycinnamic acids, Hydroxyhydrocinnamic acids, Hydroxyphenylacetic acids | GC-MS | Loots et al. 2006 | |
| Homogenization | Flavan-3-ols, Phenolic acids, Flavonols, Dihydrochalcones | UHPLC-UV and UHPLC-MS/MS | Verdu et al. 2013 | ||
| Apple pomace | Solvent Extraction | Acetone, Methanol, Ethanol, Ethyl acetate | Phloridzin, Phloretin, Quercetin | RP-HPLC-DAD | Rana et al. 2014 |
| Microwave | Ethanol | Chlorogenic acid, Caffeic acid, Syrigin, Procyanidin B2, (−)- Epicatechin, Cinnamic acid, | RP-HPLC | Bai et al. 2010 | |
| Solvent Extraction | Ethanol | Chlorogenic acid, Quercetin, and its glycosides | HSCCC, HPLC-MS, Sephadex- LH20, | Cao et al. 2009 | |
| Sonication | Ethylacetate, dichloromethane | Mixture of phenolics | LC/MS/MS | Sanchez-Rabaneda et al. 2004 | |
| Solvent extraction | Acetone | Procyanidins | Sephadex LH-20, 13C-NMR | Foo and Lu 1999 |
Fig. 2.
General scheme of extraction, purification and characterization of phenolics
Fig. 3.
a) Fractionation of phenolics (Sanchez-Rabaneda et al. 2004) and b) Isolation of phenolics from apple pomace (Foo and Lu 1999)
Biological properties of apple phenolics
Antioxidant potential
Antioxidants are bioactive molecules, which act as scavengers of free radicals to significantly decrease the adverse effects of ROS and reactive nitrogen species. Free radicals are highly reactive species that can cause chain reactions and may cause oxidative damage of DNA, proteins and cell membrane, leading to development of chronic diseases such as cancer, autoimmune disease and heart disease. Apples are the major dietary source of phenolic constituents or antioxidants and the metabolic conversion of these phenolics generates degradation products that has protective effect on cells. Earlier investigations demonstrated that apple juice and their gut fermented products act as effective antioxidant against human colon carcinoma cell line Caco-2 (Bellion et al. 2008). It was recognized that the antioxidant activity was higher in peel as compared to the flesh, which was likely due to the high phenolics content in peel (Vieira et al. 2011) and also found to inhibit the HepG2 human liver cancer cell proliferation (Wolfe et al. 2003). Phenolic rich apple peel extracts were found to be effective inhibitors of polyunsaturated fatty acid oxidation as demonstrated by a stronger inhibitory effect (40–62 %) on fish oil oxidation at concentration of 200 μg/ml (Sekhon-Loodu et al. 2013). Lipid stabilizing capability of the peel extracts of different apple cultivars was also evaluated using aqueous emulsion system of methyl linolenate (Huber and Rupasinghe 2007). In another study, authors demonstrated good antioxidant activity that ranged from 6.07 to 21.040 mmol of Fe2+eq./kg in the harvested apple fruits of various cultivars (Donno et al. 2012). Dihydrochalcone phloretin possess dihydroxyacetophenone pharmacophore, a reported potent antioxidant. The published data showed lower IC50 value (3.1 μM) of phloretin to scavenge 50 % of the peroxynitrite radical, whereas much higher concentration (24µM) to inhibit 50 % of the lipid peroxidation (Rezk et al. 2002). Epicatechin was found to reduce lipid peroxidation and also inhibit human blood platelet aggregation. Evidence suggested dose dependent (20–200 µg ml-1) inhibitory effect on blood platelets stimulated with arachidonic acid (300 μM), ADP (3 μM) and epinephrine (6 μM) (Neiva et al. 1999). Furthermore, epicatechin showed protective effect against neuronal cell death provoked by oxidative stress (Abd El Mohsen et al. 2002). In our earlier study, results showed good free radical scavenging activities (IC50 value of 1.72 mg ml−1 by ABTS and 5.65 mg ml−1 by DPPH assay) in ethylacetate fractionated 70 % aqueous methanolic phenolics extract of apple pomace (Rana et al. 2014).
Antiproliferative property
Apple phenolics demonstrated strong antiproliferative activity. Investigations revealed that apple peel extract (12.4 ± 0.4 mg/mL) inhibit the growth of HepG2 liver cancer cells (Wolfe et al. 2003). Antiproliferative and pro-apoptotic activities of phenolic enriched peel extracts of different cultivars of apple such as Annurca, Red Delicious and Golden Delicious on HL-60 cell line was also studied. All three extracts were able to decrease cell viability from 50 to 80 %, however, annurca apples peel extract was more efficient to do so (Mari et al. 2010). The apple pomace which is generally considered as waste also exhibit good antiproliferative potential and showed IC50 value of 26.40 mg ml-1, 22.47 mg ml-1 and 21.26 mg ml-1 against HeLa, HT-29, MCF7 cancer cell lines, respectively (Savatovic et al. 2008). In a different study, quercetin and quercetin-3-O-D-glucopyranoside reported to have strong antiproliferative activity against HepG2 and MCF-7 cells (He and Liu 2008). The recorded EC50 value of quercetin and its glycoside was nearly 41 and 49 μM against HepG2 cells and 138 and 24 μM against MCF-7 cells, respectively. Recently, phloretin reported to inhibit the proliferation of SMMC-7721 hepatoma cells (Wang et al. 2012). The possible cause of apoptosis demonstrated to be inhibition of DNA synthesis by reduction in mitochondrial membrane potential and calcium homeostasis interference. Phloretin also showed significant anticarcinogenic properties by inhibiting the growth of colon cancer cells (HT29 and LoVo). This dihydrochalcone was found to decrease the viability of human colon cancer cell line sHT29 and LoVo at concentration of 260 and170 μmol/L after 24-h incubation (Przybylska et al. 2007). The antiproliferative effect of phloretin on H-Ras-transformed MCF10A human breast epithelial (H-Ras MCF10A) cells was also studied. It was found that dihydrochalcone phloretin decreases the H-Ras MCF10A cell proliferation in a dose dependent manner. Phloretin treated MCF10A cells results in apoptosis of cells due to the activation of JNK, p38, and Caspase-3 as these elements are involved in stimulation of apoptosis in various human cancer cells (Kim et al. 2009). In vivo studies revealed the anti-proliferative activities of procyanidins on human metastatic colon carcinoma-derived SW620 cells. An intraperitoneal injection of azoxymethane was administered to wistar rats for 2 weeks to induce colon carcinogenesis. Results indicated that apple procyanidins inhibit the progression of colon cancer by reducing the number of hyperproliferative crypts and ACFs on surface of the colon of rats (Gosse et al. 2005). In vitro antiproliferative activity of apple extract and compounds was summarized in Table 5. The reviewed literature is suggestive of the potential of apple polyphenols in management or prevention of chronic diseases.
Table 5.
In vitro antiproliferative activity of apple extract and compounds
| Extract/Compound | Assay used | Cell lines used | Results | References |
|---|---|---|---|---|
| Apple peel | MTS-based Cell Titer 96 nonradioactive cell proliferation assay | HepG2 Human Liver cancer cell line | IC 50 value 12.4 ± 0.4 mg/mL | Wolfe et al. 2003 |
| Apple extracts | MTS-based cell titer 96 non-radioactive cell proliferation assay | HepG2 Human liver cancer, Caco-2 human colon cancer cells | EC50 value of apple extract against HepG2 and Caco-2 was recorded 56.6 ± 0.29 and 42.5 ± 2.64 mg/mL. | Liu and Sun 2003 |
| Granny Smith apple pomace | MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) colorimetric assay | Cervix epitheloid carcinoma (HeLa), colon adenocarcinoma (HT-29) and breast adenocarcinoma (MCF7) cell lines | The IC50 value of extract against HeLa, HT-29 and MCF7 was 26.40, 22.47 and 21.26 mg/mL | Savatovic et al. 2008 |
| Quercetin Quercetin-3-O- D-glucopyranoside | – | HepG2 human liver cancer cells and MCF-7 human breast cancer cells | The EC 50 value of quercetin and Quercetin-3-O- -D-glucopyranoside against HepG2 was 40.9 ± 1.1 and 49.2 ± 4.9 μM and against MCF-7 cells was137.5 ± 2.6 and 23.9 ± 3.9 μM, respectively. | He and Liu 2008 |
| Apple extracts and quercetin 3-β-D-glucoside (Q3G) | Methylene blue assay | MCF-7 human breast cancer cell | The EC50 value of apple extracts and Q3G in inhibiting MCF-7 cell proliferation was 70.7 ± 5.7 mg/ml and 46.4 ± 1.3 μM. | Yang and Liu 2009 |
Anticancer activity
A recent study by Zheng, et al. suggested that apple polyphenols inhibit in vitro proliferation and induce apoptosis in a metastatic oral adenoid cystic carcinoma cell line by down regulating the expression of vascular endothelial growth factor receptor (VEGFR-2) and activation of Caspase-3 expression at 150 and 250 µg ml-1 concentration (Zheng et al. 2013). It was revealed that apple phenolics and and their gut fermented products decrease the survival of human colon cell lines (LT97 and HT29) in time and dose dependent manner (Veeriah et al. 2007). Kern and co-workers revealed that apple phenolics influence protein kinase C activity in HT29 cells after 24 hr of incubation and also caused apoptosis in human colon carcinoma cells (Kern et al. 2007). The flavonoid procyanidin reported to possess pleiotropic anticancer effects on different cancer cells, such as cutaneous carcinoma, oral carcinoma, breast carcinoma, bronchogenic carcinoma, liver carcinoma, pancreatic carcinoma and gastric carcinoma (Ye et al. 1999). Procyanidins were found to play significant role in tumor cell apoptosis. Miura and co-workers found that the procyanindins induced apoptosis by activating Caspase 3 & 9 in mitochondrial pathway (Miura et al. 2008). In another study, procyanidin was found to inhibit growth of human breast cancer cells (MDA MB-231, 436, 468, SKBR-3, and MCF-7) by down regulating the multiple proteins involved in cell cycle regulation (Ramljak et al. 2005). It was reported that procyanidins induces necrosis and apoptosis of prostate cancer cell ‘PC-3’ for 6 and 24 hr with different concentration (100-300 µg ml-1) of procyanidins (Shang et al. 2009). Studies also revealed the apoptotic and cardioprotective properties of phloretin on H2O2-induced primary culture (Malekova et al. 2007). Phloretin demonstrated strong anti-tumor effects by inhibiting the growth of several cancer cell lines (Kobori et al. 1999). It’s ability to induce apoptosis relies on inhibition of protein kinase C activity as reported in HL60 human leukemia cells. This dihydrochalcone also inhibits HT-29 human colon cancer cells growth by inducing apoptosis in a dose-dependent manner, owing to activation of the caspase pathways (Park et al. 2007). Administration of phloretin and its glycoside through intraperitoneal injection revealed inhibition of tumor cell growth in rat mammary adenocarcinoma and fischer bladder carcinoma cell lines (Nelson and Falk 1993). The above discussed results able to demonstrate the potential of procyanidins and phloretin in development of new anticancer drugs.
Anti-inflammatory
Apple phenolics act as strong anti-inflammatory agents by protecting intestinal inflammation instigated by chronic inflammatory bowel diseases (IBD). Severe release of chemokines and several proinflammatory cytokines such as IL-12, IFN-c, TNF-a, IL-4, IL-5, and IL-13 by different cells and inflammatory circumstances of the colon and small intestine (ulcerative colitis and Crohn’s disease) are characteristic of IBD. In vitro studies showed the inhibitory potential of polyphenolic apple juice extracts on inflammatory gene expression in immunorelevant human cell lines (DLD-1, T84, MonoMac6, Jurkat). Results of this study demonstrated that procynanidin and phloretin rich apple juice extract (100–200 μg ml-1) inhibited the inflammatory enzymes i.e. cyclooxygenase-2, CYP3A4 and also inhibit the expression of NF-kB regulated proinflammatory genes such as TNF-a, IL-1b, CXCL9 and CXCL10 in LPS/IFN-c stimulated MonoMac6 cells (Jung et al. 2009). Phenolic compounds also inhibit the transcription factors such as STAT1, IRF1 responsible for the occurrence of inflammation. Prostaglandins and nitric oxide, the main mediators responsible for commencement of inflammation, are produced by nitric oxide synthase (iNOS) and cyclooxygenase (COX-2). It was demonstrated that phenolic compounds especially flavonoids inhibit both enzymes, as well as other intermediaries involved in inflammation process (Gonzalez-Gallego et al. 2007). Kaempferol and quercetin found to inhibit the activation of STAT-1 and NF-κB transcription factors of nitric oxide synthase (iNOS) in macrophages that were exposed to an inflammatory lipopolysaccharide, thus controlling nitric oxide production. Quercetin was found to inhibit the LPS-induced activation of NF-κB by 80 %, whereas kaempferol showed the moderate inhibitiory effect (57–72 %) (Hamalainen et al. 2007). Quercetin was also reported to inhibit IFNγ-induced activation of STAT-1 in mouse BV-2 microglia (Chen et al. 2005). In case of differentiated white adipocytes (3 T3-L1) epicatechin prevented (TNF α)-induced activation of cell signals, insulin resistance (NF-κB, protein kinases (MAPKs), AP-1 and peroxisome proliferator activated receptor γ (PPARγ). Results showed that epicatechin at dose dependent manner (0.5–10 μΜ) causes decrease in TNFα-mediated JNK, ERK1/2, and p-38 phosphorylation (Vazquez-Prieto et al. 2012). Phloretin acts as antiinflammatory agent by reducing the level of proinflammatory cytokines in RAW264.7 cells treated with 10μM concentration (Chang et al. 2012). It was also demonstrated to exhibit similar properties in intestinal inflammatory diseases. It reduces E. coli O157:H7 biofilm formation as it does not harm commensal E. coli K-12 biofilms (Lee et al. 2011). Phloretin specifically inhibits phosphoinositide hydrolysis induced by Prostaglandins (PGs) PGD and PGF, through interfering with PGF specific receptors in cultured rat astrocytes (Kitanaka et al. 1993).
Antidiabetic properties
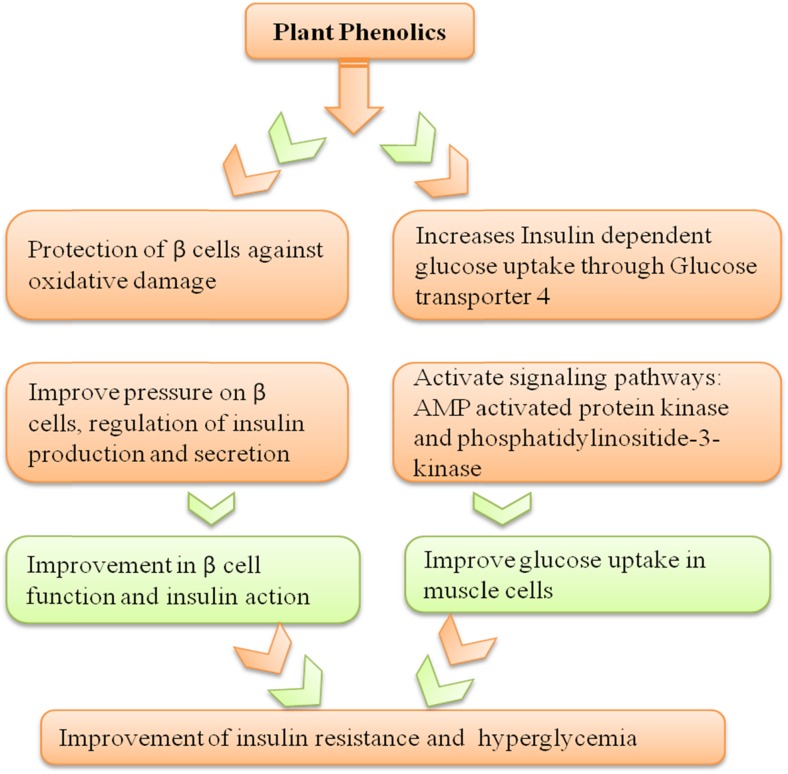
Diabetes is one of the leading chronic diseases affecting human population world-over. Defects in insulin secretion and action, or some time both resulted in hyperglycemia, which is the characteristic feature of this metabolic disease. Long-term damage causes complications in various tissues of human body especially eyes, nerves system, kidneys, blood vessels and heart (American Diabetes Association, Diagnosis and classification of Diabetes mellitus2010). Diabetes is categorized into ‘type 1’ and ‘type 2’ Diabetes mellitus (DM). The former (5–10 % of total incidence) results from damaged insulin-producing β-cells, thus causing insulin deficiency; whereas latter is results from insulin resistance to produce hyperglycemia. In an recent study with streptozotocin-induced rat model for ‘type I diabetes’, oral administration of phloridzin showed substantial decrease in blood glucose level and improvement in dyslipidemia, thus exhibiting its ability to act as antihyperglycemic and antihyperlipidemic agent (Najafian et al. 2012). An in silico study was conducted by our group to understand the mechanisms by which phloridzin could be critical at altering disease incidence (Randhawa et al. 2013). The authors were able to identify the key targets of type 2 DM by network analysis. MAPK1, EP300, and SMAD2 were reported as the central proteins and phloridzin found to have potential involvement in making critical interactions with MAPK1. An in vivo study using mouse model of type 2 diabetes, phloridzin treated mice shown improvement of hyperglycemia condition (Zhao et al. 2004). Furthermore, apple phenolics also found to inhibit sodium/glucose co-transporters in the intestine (SGLT1) and kidney (SGLT2), and subsequently inhibit renal glucose reabsorption. The role of apple polyphenols on management of diabetes is shown in Fig. 4. In addition, apple dihydrochalcone phloridzin causes reduction of β-cell mass that leads to the development of insulin resistance, and improvement of hyperglycemia without change in insulin levels, therefore may aid in controlling insulin sensitivity (Rossetti et al. 1987). Diabetic cardiomyopathy is a complicated disease associated with type 2 DM. In a recent article, Cai et al. have illustrated the role of phloridzin in prevention of diabetic cardiomyopathy in db/db mice. Researchers have found that phloridzin considerably decreased body weight gain and the levels of fasting blood glucose, total cholesterol, triglycerides and advanced glycation end products. It was found to regulate the expression of concerned protein, responsible for cardiac damage (Cai et al. 2013). In an in vivo study employing spontaneously diabetic torii (SDT) rats, administration of phlorizin (100 mg kg-1) inhibited intestinal glucose uptake and resulted in improved glycemic control. Administration of phlorizin had also improved liver function by increasing the mRNA levels of glucokinase enzymes that are linked with the glycogen cascade (Ohta et al. 2012). Quercetin was also reported to prevent diabetic vascular complications in insulin deficiency as well as resistance. It had protective consequences against β cell damage because of its multiple effects. Studies on the effect of quercetin on the streptozotocin induced diabetes in male Albino rats revealed significant decrease in the blood glucose levels of treated group as compared to control. Administration of streptozotocin caused degeneration of islet Beta cells but treatment with quercetin inverted most of the pancreatic morphological changes (Rifaai et al. 2012).
Fig. 4.
Role of phenolics in diabetes (Bahadoran et al. 2013)
Cardio-protective effects
Heart diseases and stroke are the leading cause of human deaths world-over in last decade (FAO 2013). These chronic diseases occurred on behest of free radicals generated in human body during various catabolic processes. However, antioxidant rich diets may help in scavenging these harmful radicals by their ability to stabilize the unstable molecules. Apple bioactive constituents reported to be related to a decreased risk of cardiovascular diseases owing to their cholesterol lowering effect (Serra et al. 2012). Atherosclerosis inception is the consequence of oxidative modification of LDL by free radicals species (Steinberg et al. 1989). Apple polyphenols inhibit LDL oxidation and lower blood LDL, thus possibly preventing cardiovascular disorders (Chu and Liu 2005). Animal study showed that supplementation of 20 % lyophilized apple to obese Zucker rats lowered plasma and LDL cholesterol (Aprikian et al. 2002) and also reduced the accumulation of triglyceride in heart and liver. It was also found that consumption of apples suppressed the level of glucosuria and proteinuria in obese Zucker rats. Chai et al. conducted a 1-year clinical trial in postmenopausal women to evaluate the effect of apple consumption in reducing cardiovascular disease. Authors revealed that daily intake of apple in diet by postmenopausal women lowered serum levels of total cholesterol and low-density lipoprotein (Chai et al. 2012). Male Wistar rats fed with cholesterol-enriched diet demonstrated that an apple rich diet significantly decreased LDL cholesterol and serum levels of triglycerides. The cholesterol and oxidized LDL-lowering effects mainly attributed to the epicatechin and procyanidin B1, and β-carotene present in apples (Serra et al. 2012). Researchers demonstrated that these phenolics posses good correlation with reduction ability of total cholesterol and oxidized LDL (Serra et al. 2012). Apple flavonoid phloretin may have advantageous effects in the commencement of cardiovascular diseases as it reduces activation of human blood platelets and also limit the TNF-α induced expression of endothelial adhesion molecules in human umbilical vein endothelial cells in a dose dependent manner (1–100 µmol L-1) (Stangl et al. 2005). Apple procyanidins played important role in decreasing cholesterol accumulation and also provide protection against oxidative stress. These procyanidins would considerably bind to plasma HDLs and thus reverse transport and metabolism of cholesterol (Tenore et al. 2013). Increased consumption of quercetin found to be related to decreased mortality from ischemic heart disease. A study to assess the relationship between quercetin intake and risk of chronic diseases revealed that the individuals (10,054 men and women) with higher intake of quercetin showed lower mortality from ischemic heart disease (Knekt et al. 2002).
Future perspective
Fruits and vegetables based diet are associated with good human health and sufficient experimental evidence indicative of their positive role in management, if not, prevention of chronic diseases like cardiovascular, inflammation and diabetes. The strong evidence from in vitro and in vivo studies revealed the potential use of apple phenolics as either purified compound or extracts (apple leaves, bark, skin or processed products) as effective food supplements or nutraceuticals for the management of chronic diseases. However, interaction of various fractions/extracts with food ingredient, major & micronutrients and individual or synergistic effect of polyphenols at molecular level needs comprehensive studies before put into task. Also, the bioavailability of these phytochemicals after ingestion needs comprehensive R&D efforts before advocating their consumption as supplement or nutraceuticals.
Acknowledgments
The authors wish to thank the Director, CSIR-IHBT Palampur for continuous encouragement and for providing necessary facilities. Authors also acknowledge Council of Scientific and Industrial Research, New Delhi (AGROPATHY Network Project) and Department of Biotechnology, Ministry of Science and Technology, GOI, for financial support.
Compliance with ethical standards
Conflict of interest
The authors declare that there is no conflict of interest.
Footnotes
Research highlights
• Potential of phenolic present in different parts of apple fruit as dietary polyphenols.
• Assessment of different analytical methods for identification of phenolics
• Extraction and analysis of phenolics
• Deciphering the biological properties of apple phenolics
References
- Abd El Mohsen MM, Kuhnle G, Rechner AR, Schroeter H, Rose S, Jenner P, Rice-Evans CA. Uptake and metabolism of epicatechin and its access to the brain after oral ingestion. Free Radic Biol Med. 2002;33:1693–1702. doi: 10.1016/S0891-5849(02)01137-1. [DOI] [PubMed] [Google Scholar]
- Alonso-Salces RM, Korta E, Barranco A, Berrueta LA, Gallo B, Vicente F. Pressurized liquid extraction for the determination of polyphenols in apple. J Chromatogr A. 2001;933:37–43. doi: 10.1016/S0021-9673(01)01212-2. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association, Diagnosis and classification of diabetes mellitus (2010) Diab Care 33:S62-S69. [DOI] [PMC free article] [PubMed]
- Aprikian O, Busserolles J, Manach C, Mazur A, Morand C, Davicco MJ, Besson C, Rayssiguier Y, Remesy C, Demigne C. Lyophilized apple counteracts the development of hypercholesterolemia, oxidative stress, and renal dysfunction in obese zucker rats. J Nutr. 2002;132:1969–1976. doi: 10.1093/jn/132.7.1969. [DOI] [PubMed] [Google Scholar]
- Bahadoran Z, Mirmiran P, Azizi F. Dietary polyphenols as potential nutraceuticals in management of diabetes: a review. J Diabetes Metab Disord. 2013;12:43. doi: 10.1186/2251-6581-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai XL, Yue TL, Yuan YH, Zhang HW. Optimization of microwave-assisted extraction of polyphenols from apple pomace using response surface methodology and HPLC analysis. J Sep Sci. 2010;33:3751–3758. doi: 10.1002/jssc.201000430. [DOI] [PubMed] [Google Scholar]
- Bellion P, Hofmann T, Pool-Zobel BL, Will F, Dietrich H, Knaup B, Richling E, Baum M, Eisenbrand G, Janzowski C. Antioxidant effectiveness of phenolic apple juice extracts and their gut fermentation products in the human colon carcinoma cell line caco-2. J Agric Food Chem. 2008;56:6310–6317. doi: 10.1021/jf8005068. [DOI] [PubMed] [Google Scholar]
- Bhushan S, Kalia K, Sharma M, Singh B, Ahuja PS. Processing of apple pomace for bioactive molecules. Crit Rev Biotechnol. 2008;28:285–296. doi: 10.1080/07388550802368895. [DOI] [PubMed] [Google Scholar]
- Boyer J, Liu RH. Apple phytochemicals and their health benefits. Nutr J. 2004;3:5. doi: 10.1186/1475-2891-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q, Li B, Yu F, Lu W, Zhang Z, Yin M, Gao H (2013) Investigation of the protective effects of phlorizin on diabetic cardiomyopathy in db/db mice by quantitative proteomics. J Diab Res Article ID 263845. [DOI] [PMC free article] [PubMed]
- Cao X, Wang C, Pei H, Sun B. Separation and identification of polyphenols in apple pomace by high-speed counter-current chromatography and high-performance liquid chromatography coupled with mass spectrometry. J Chromatogr A. 2009;1216:4268–4274. doi: 10.1016/j.chroma.2009.01.046. [DOI] [PubMed] [Google Scholar]
- Ceymann M, Arrigoni E, Scharer H, Baumgartner D, Nising AB, Hurrell RF. Rapid high performance screening method using UHPLC-MS to quantify 12 polyphenol compounds in fresh apples. Anal Methods. 2011;3:1774–1778. doi: 10.1039/c1ay05152k. [DOI] [Google Scholar]
- Chai SC, Hooshmand S, Saadat RL, Payton ME, Brummel-Smith K, Arjmandi BH. Daily apple versus dried plum: impact on cardiovascular disease risk factors in postmenopausal women. J Acad Nutr Diet. 2012;112:1158–1168. doi: 10.1016/j.jand.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Chan A, Shea TB. Dietary supplementation with apple juice decreases endogenous amyloid-β levels in murine brain. J Alzheimers Dis. 2009;16:167–171. doi: 10.3233/JAD-2009-0959. [DOI] [PubMed] [Google Scholar]
- Chang WT, Huang WC, Liou CJ (2012) Evaluation of the anti-inflammatory effects of phloretin and phlorizin in lipopolysaccharide-stimulated mouse macrophages. Food Chem 134:972–979 [DOI] [PubMed]
- Chen JC, Ho FM, Chao PDL, Chen CP, Jeng KCG, Hsu HB, Lee ST, Wu WT, Lin WW. Inhibition of iNOS gene expression by quercetin is mediated by the inhibition of IκB kinase, nuclear factor-κ B and STAT1, and depends on heme oxygenase-1 induction in mouse BV-2 microglia. Eur J Pharmacol. 2005;521:9–20. doi: 10.1016/j.ejphar.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Chen NN, Zhao SC, Deng LG, Guo CY, Mao JS, Zheng H, Yang GS, Lu X, Aboul-Enein HY. Determination of five polyphenols by HPLC/DAD and discrimination of apple varieties. Chromatographia. 2011;73:595–598. doi: 10.1007/s10337-011-1929-2. [DOI] [Google Scholar]
- Chu YF, Liu RH (2005) Cardioprotective potentials of apple phytochemicals in LDL oxidation and LDL receptor expression. Cornell Institute of Food Science Symposium May 22–24.
- D’Archivio M, Files C, Di Benedetto R, Gargiulo R, Giovannini C, Masella R. Polyphenols, dietary sources and bioavailability. Annali Dell’Istituto Superiore di Sanita. 2007;43:348–361. [PubMed] [Google Scholar]
- De Paepe D, Servaes K, Noten B, Diels L, De Loose M, Van Droogenbroeck B, Voorspoels S. An Improved mass spectrometric method for identification and quantification of phenolic compounds in apple fruits. Food Chem. 2013;136:368–375. doi: 10.1016/j.foodchem.2012.08.062. [DOI] [PubMed] [Google Scholar]
- Donno D, Beccaro GL, Mellano MG, Torello Marinoni D, Cerutti AK, Canterino S, and. Bounous G (2012) Application of sensory, nutraceutical and genetic techniques to create a quality profile of ancient apple cultivars. J Food Qual 35:169–181.
- FAO (2013) Statistical Year Book, Food and agriculture organization of the united nations, Rome
- Foo LY, Lu Y. Isolation and identification of procyanidins in apple pomace. Food Chem. 1999;64:511–518. doi: 10.1016/S0308-8146(98)00150-2. [DOI] [Google Scholar]
- Gonzalez-Gallego J, Sanchez-Campos S, Tunon MJ. Anti-inflammatory properties of dietary flavonoids. Nutr Hosp. 2007;22:287–293. [PubMed] [Google Scholar]
- Gosse F, Guyot S, Roussi S, Lobstein A, Fischer B, Seiler N, Raul F. Chemopreventive properties of apple procyanidins on human colon cancer-derived metastatic SW620 cells and in a rat model of colon carcinogenesis. Carcinogenesis. 2005;26:1291–1295. doi: 10.1093/carcin/bgi074. [DOI] [PubMed] [Google Scholar]
- Graziani G, D'Argenio G, Tuccillo C, Loguercio C, Ritieni A, Morisco F, Del Vecchio Blanco C, Fogliano V, Romano M (2005) Apple polyphenol extracts prevent damage to human gastric epithelial cells in vitro and to rat gastric mucosa in vivo. Gut 54:193–200 [DOI] [PMC free article] [PubMed]
- Hamalainen M, Nieminen R, Vuorela P, Heinonen M, Moilanen E (2007) Anti-Inflammatory Effects of Flavonoids: Genistein, Kaempferol, Quercetin, and Daidzein Inhibit STAT-1 and NF-κB Activations, Whereas Flavone, Isorhamnetin, Naringenin, and Pelargonidin Inhibit only NF-κB Activation along with Their Inhibitory Effect on iNOS Expression and NO Production in Activated Macrophages. Mediat Inflamm Volume 2007, Article ID 45673. doi:10.1155/2007/45673 [DOI] [PMC free article] [PubMed]
- He X, Liu RH. Phytochemicals of apple peels: isolation, structure, elucidation, and their antiproliferative and antioxidant activities. J Agric Food Chem. 2008;56:9905–9910. doi: 10.1021/jf8015255. [DOI] [PubMed] [Google Scholar]
- Huai-De X, Lin-Bin W, Li-Jia Z (2010) Purification and antioxidant activity of polyphenols from apple tree leaves. Food Sci 31:72–78
- Huber GM, Rupasinghe HP (2007) Phenolic profiles and antioxidant properties of apple skin extracts. J Food Sci 74:693–700 [DOI] [PubMed]
- Hyson DA. A comprehensive review of apples and apple components and their relationship to human health. Adv Nutr. 2011;2:408–420. doi: 10.3945/an.111.000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung M, Triebel S, Anke T, Richling E, Erkel G. Influence of apple polyphenols on inflammatory gene expression. Mol Nutr Food Res. 2009;53:1263–1280. doi: 10.1002/mnfr.200800575. [DOI] [PubMed] [Google Scholar]
- Kahle K, Kraus M, Richling E. Polyphenol profiles of apple juices. Mol Nutr Food Res. 2005;49:797–806. doi: 10.1002/mnfr.200500064. [DOI] [PubMed] [Google Scholar]
- Karaman S, Tutem E, Baskan KS, Apak R (2013) Comparison of antioxidant capacity and phenolic composition of peel and flesh of some apple varieties. J Sci Food Agric 93:867–875 [DOI] [PubMed]
- Kern M, Pahlke G, Balavenkatraman KK, Bohmer FD, Marko D. Apple polyphenols affect protein kinase C activity and the onset of apoptosis in human colon carcinoma cells. J Agric Food Chem. 2007;55:4999–5006. doi: 10.1021/jf063158x. [DOI] [PubMed] [Google Scholar]
- Kim MS, Kwon JY, Kang NJ, Lee KW, Lee HJ. Phloretin induces apoptosis in H-ras MCF10A human breast tumor cells through the activation of p53 via JNK and p38 mitogen-activated protein kinase signaling. Ann N Y Acad Sci. 2009;1171:479–483. doi: 10.1111/j.1749-6632.2009.04692.x. [DOI] [PubMed] [Google Scholar]
- Kindt M, Orsini MC, Costantini B. Improved high-performance liquid chromatography–diode array detection method for the determination of phenolic compounds in leaves and peels from different apple varieties. J Chromatogr Sci. 2007;45:507–514. doi: 10.1093/chromsci/45.8.507. [DOI] [PubMed] [Google Scholar]
- Kitanaka JI, Ishibashi T, Baba A (1993) Phloretin as an antagonist of prostaglandin f, receptor in cultured rat astrocytes. J Neuro Chem 60:704–708 [DOI] [PubMed]
- Knekt P, Kumpulainen J, Jarvinen R, Rissanen H, Heliovaara M, Reunanen A, Hakulinen T, Aromaa A. Flavonoid intake and risk of chronic diseases. Am J Clin Nutr. 2002;76:560–568. doi: 10.1093/ajcn/76.3.560. [DOI] [PubMed] [Google Scholar]
- Kobori M, Iwashita K, Shinmoto H, Tsushida T (1999) Phloretin induced apoptosis inB16 melanoma 4A5 and and HL60 human leukemia. Biosci Biotech Boich 63:719–725 [DOI] [PubMed]
- Lamperi L, Chiuminatto U, Cincinelli A, Galvan P, Giordani E, Lepri L, Del Bubba M. Polyphenol levels and free radical scavenging activities of four apple cultivars from integrated and organic farming in different Italian areas. J Agric Food Chem. 2008;56:6536–6546. doi: 10.1021/jf801378m. [DOI] [PubMed] [Google Scholar]
- Lavelli V, Corti S. Phloridzin and other phytochemicals in apple pomace: stability evaluation upon dehydration and storage of dried product. Food Chem. 2011;129:1578–1583. doi: 10.1016/j.foodchem.2011.06.011. [DOI] [Google Scholar]
- Lee JH, Regmi S, Kim JA, Cho MH, Yun H, Lee CS, Lee J. Apple flavonoid phloretin inhibits Escherichia coli o157:h7 biofilm formation and ameliorates colon inflammation in rats. Infect Immun. 2011;79:4819–4827. doi: 10.1128/IAI.05580-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RH, Sun J. Antiproliferative activity of apples is not due to phenolic-induced hydrogen peroxide formation. J Agric Food Chem. 2003;51:1718–1723. doi: 10.1021/jf026162r. [DOI] [PubMed] [Google Scholar]
- Lommen A, Godejohann M, Venema DP, PCH Hollman, Spraul M. Application of directly coupled HPLC − NMR − MS to the identification and confirmation of quercetin glycosides and phloretin glycosides in apple peel. Anal Chem. 2000;72:1793–1797. doi: 10.1021/ac9912303. [DOI] [PubMed] [Google Scholar]
- Loots DT, Westhuizen FHV, Jerling J. Polyphenol composition and antioxidant activity of kei-apple (dovyalis caffra) juice. J Agric Food Chem. 2006;54:1271–1276. doi: 10.1021/jf052697j. [DOI] [PubMed] [Google Scholar]
- Malekova L, Tomaskova J, Novakova M, Stefanik P, Kopacek J, Lakatos B, Pastorekova S, Krizanova O, Breier A, Ondrias K. Inhibitory effect of DIDS, NPPB, and phloretin on intracellular chloride channels. Pflugers Arch - Eur J Physiol. 2007;455:349–357. doi: 10.1007/s00424-007-0300-9. [DOI] [PubMed] [Google Scholar]
- Mari A, Tedesco I, Nappo A, Russo GL, Malorni A, Carbone V. Phenolic compound characterization and antiproliferative activity of “annurca” apple, a southern Italian cultivar. Food Chem. 2010;123:157–164. doi: 10.1016/j.foodchem.2010.04.023. [DOI] [Google Scholar]
- Mehrabani LV, Dadpour MR, Delazar A, Movafeghi A, Hassanpouraghdam MB (2011) Quantification of phenolic compounds in peel and pulp of ‘Zonouz’ apple cultivar from Iran. Rom Biotech Lett 16:6390–6395
- Mehrabani LV, Hassanpouraghdam MB, Dadpour MR (2012) HPLC assisteddetermination of phenolic compounds in two apple cultivars from Iran. J Food Agri Environ 10:233–235
- Miura T, Chiba M, Kasai K, Nozaka H, Nakamura T, Shoji T, Kanda T, Ohtake Y, Sato T. Apple procyanidins induce tumor cell apoptosis through mitochondrial pathway activation of caspase-3. Carcinogenesis. 2008;29:585–593. doi: 10.1093/carcin/bgm198. [DOI] [PubMed] [Google Scholar]
- Najafian M, Jahromi MZ, Nowroznejhad MJ, Khajeaian P, Kargar MM, Sadeghi M, Arasteh A. Phloridzin reduces blood glucose levels and improves lipids metabolism in streptozotocin-induced diabetic rats. Mol Biol Rep. 2012;39:5299–5306. doi: 10.1007/s11033-011-1328-7. [DOI] [PubMed] [Google Scholar]
- Neiva TJ, Morais L, Polack M, Simoes CM, D’Amico EA. Effects of catechins on human blood platelet aggregation and lipid peroxidation. Phytother Res. 1999;13:597–600. doi: 10.1002/(SICI)1099-1573(199911)13:7<597::AID-PTR512>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Nelson JA, Falk RE. The efficacy of phloridzin and phloretin on tumor cell growth. Anticancer Res. 1993;13:2287–2292. [PubMed] [Google Scholar]
- Neveu V, Perez-Jiménez J, Vos F, Crespy V, Chaffaut L, Mennen L, Knox C, Eisner R., Cruz J, Wishart D, Scalbert (2010). Phenol explorer: an online comprehensive database on polyphenol contents in food. Database (Oxford). doi:10.1093/database/bap024 [DOI] [PMC free article] [PubMed]
- Ohta T, Morinaga H, Yamamoto T, Yamada T. Effect of phlorizin on metabolic abnormalities in spontaneously diabetic Torii (SDT) rats. Open J Animal Sci. 2012;2:113–118. doi: 10.4236/ojas.2012.22016. [DOI] [Google Scholar]
- Park SY, Kim EJ, Shin HK, Kwon DY, Kim MS, Surh YJ, Park JHY. Induction of apoptosis in HT-29 colon cancer cells by phloretin. J Med Food. 2007;10:581–586. doi: 10.1089/jmf.2007.116. [DOI] [PubMed] [Google Scholar]
- Picinelli A, Suarez B, Mangas JJ. Analysis of polyphenols in apple products. Z Lebensm Unters Forsch A. 1997;204:48–51. doi: 10.1007/s002170050035. [DOI] [Google Scholar]
- Plaza M, Kariuki J, Turner C. Quantification of individual phenolic compounds' contribution to antioxidant capacity in apple: a novel analytical tool based on liquid chromatography with diode array, electrochemical, and charged aerosol detection. J Agric Food Chem. 2014;62:409–418. doi: 10.1021/jf404263k. [DOI] [PubMed] [Google Scholar]
- Przybylska K, Bennett RN, Kromer K, Gee JM (2007) Assessment of the antiproliferative activity of carrot and apple extracts. Polish J Food Nut Sci 57:307–314
- Ramljak D, Romanczyk LJ, Metheny-Barlow LJ, Thompson N, Knezevic V, Galperin M, Ramesh A, Dickson RB. Pentameric procyanidin from theobroma cacao selectively inhibits growth of human breast cancer cells. Mol Cancer Ther. 2005;4:537–546. doi: 10.1158/1535-7163.MCT-04-0286. [DOI] [PubMed] [Google Scholar]
- Rana S, Rana A, Gulati A, Bhushan S. RP-HPLC-DAD determination of phenolics in industrial apple pomace. Food Anal Methods. 2014;7:1424–1432. doi: 10.1007/s12161-013-9765-7. [DOI] [Google Scholar]
- Randhawa V, Sharma P, Bhushan S, Bagler G. Identification of key nodes of type 2 diabetes mellitus protein interactome and study of their interactions with phloridzin. OMICS. 2013;17:302–317. doi: 10.1089/omi.2012.0115. [DOI] [PubMed] [Google Scholar]
- Rezk BM, Haenen GRMM, Vijgh WJFV, Bast A. The antioxidant activity of phloretin: the disclosure of a new antioxidant pharmacophore in flavonoids. Biochem. Biophys Res Commun. 2002;2954:9–13. doi: 10.1016/S0006-291X(02)00618-6. [DOI] [PubMed] [Google Scholar]
- Rifaai RH, El-Tahawy NF, Saber EA, Ahmed R (2012) Effect of quercetin on the endocrine pancreas of the experimentally induced diabetes in male albino rats: a histological and immunohistochemical study. J Diabetes Metab 3:182
- Rossetti L, Smith D, Shulman GI, Papachristou D, DeFronzo RA. Correction of hyperglycemia with phloridzin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest. 1987;79:1510–1515. doi: 10.1172/JCI112981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupasinghe HPV, Kathirvel P, Huber GM. Ultrasonication-assisted solvent extraction of quercetin glycosides from ‘idared’ apple peels. Molecules. 2011;16:9783–9791. doi: 10.3390/molecules16129783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Rabaneda F, Jauregui O, Lamuela-Raventos R, Viladomat F, Bastida J, Codina C. Qualitative analysis of phenolic compounds in apple pomace using liquid chromatography coupled to mass spectrometry in tandem mode. Rapid Commun Mass Spectrom. 2004;18:553–563. doi: 10.1002/rcm.1370. [DOI] [PubMed] [Google Scholar]
- Savatovic SM, Cetkovic GS, Dilas SM, Tumbas VT, Canadanovic-Brunet JM, Cetojevic-Simin DD, Mandic AI. Antioxidant and antiproliferative activity of granny smith apple pomace. Apteff. 2008;39:201–212. doi: 10.2298/APT0839201S. [DOI] [Google Scholar]
- Sekhon-Loodu S, Warnakulasuriya SN, Rupasinghe HPV, Shahidi F. Antioxidant ability of fractionated apple peel phenolics to inhibit fish oil oxidation. Food Chem. 2013;140:189–196. doi: 10.1016/j.foodchem.2013.02.040. [DOI] [PubMed] [Google Scholar]
- Serra AT, Rocha J, Sepodes B, Matias AA, Feliciano RP, Carvalho A, Bronze MR, Duarte CM, Figueira ME. Evaluation of cardiovascular protective effect of different apple varieties –correlation of response with composition. Food Chem. 2012;135:2378–2386. doi: 10.1016/j.foodchem.2012.07.067. [DOI] [PubMed] [Google Scholar]
- Shang XJ, Yao G, Ge JP, Sun Y, Teng WH, Huang YF. Procyanidin induces apoptosis and necrosis of prostate cancer cell line PC-3 in a mitochondrion-dependent manner. J Androl. 2009;30:122–126. doi: 10.2164/jandrol.108.005629. [DOI] [PubMed] [Google Scholar]
- Shibusawa Y, Yanagida A, Ito A, Ichihashi K, Shindo H, Ito Y. High-speed counter-current chromatography of apple procyanidins. J Chromatogr A. 2000;886:65–73. doi: 10.1016/S0021-9673(00)00448-9. [DOI] [PubMed] [Google Scholar]
- Stangl V, Lorenz M, Ludwig A, Grimbo N, Guether C, Sanad W, Ziemer S, Martus P, Baumann G, Stangl K (2005) The flavonoid phloretin suppresses stimulated expression of endothelial adhesion molecules and reduces activation of human platelets. J Nutr 135:172–178 [DOI] [PubMed]
- Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989;320:915–924. doi: 10.1056/NEJM198901053200122. [DOI] [PubMed] [Google Scholar]
- Tchantchou F, Chan A, Kifle L, Ortiz D, Shea TB. Apple juice concentrate prevents oxidative damage and impaired maze performance in aged mice. J Alzheimers Dis. 2005;8:283–287. doi: 10.3233/jad-2005-8306. [DOI] [PubMed] [Google Scholar]
- Tenore GC, Campiglia P, Ritieni A, Novellino E. In vitro bioaccessibility, bioavailability and plasma protein interaction of polyphenols from annurca apple (M. pumila miller cv annurca) Food Chem. 2013;141:3519–3524. doi: 10.1016/j.foodchem.2013.06.051. [DOI] [PubMed] [Google Scholar]
- Vazquez-Prieto MA, Bettaieb A, Haj FG, Fraga CG, Oteiza PI. (−)-Epicatechin prevents TNFα-induced activation of signaling cascades involved in inflammation and insulin sensitivity in 3 T3-L1 adipocytes. Arch Biochem Biophys. 2012;527:113–118. doi: 10.1016/j.abb.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeriah S, Hofmann T, Glei M, Dietrich H, Will F, Schreier P, et al. Apple polyphenols and products formed in the gut differently inhibit survival of human cell lines derived from colon adenoma (LT97) and carcinoma (HT29) J Agric Food Chem. 2007;55:2892–2900. doi: 10.1021/jf063386r. [DOI] [PubMed] [Google Scholar]
- Verdu CF, Gatto VJ, Freuze I, Richomme P, Laurens F, Guilet D. Comparison of two methods, UHPLC-UV and UHPLC-MS/MS, for the quantification of polyphenols in cider apple juices. Molecules. 2013;18:10213–10227. doi: 10.3390/molecules180910213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira FGK, Borges GDSC, Copetti C, Pietro PFD, Nunes EDC, Fett R. Phenolic compounds and antioxidant activity of the apple flesh and peel of eleven cultivars grown in Brazil. Sci Hort. 2011;128:261–266. doi: 10.1016/j.scienta.2011.01.032. [DOI] [Google Scholar]
- Viggiano A, Viggianoa A, Monda M, Turco I, Incarnato L, Vinno V, Viggianoa E, Baccari ME, Luca BD. Annurca apple-rich diet restores long-term potentiation and induces behavioral modifications in aged rats. Exp Neurol. 2006;199:354–361. doi: 10.1016/j.expneurol.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Walia M, Kumar S, Agnihotria VK. UPLC-PDA quantification of chemical constituents of two different varieties (golden and royal) of apple leaves and their antioxidant activity. J Sci Food Agric. 2015 doi: 10.1002/jsfa.7239. [DOI] [PubMed] [Google Scholar]
- Wang H, Wang D, Pu Y, Pan D, Guan W, Ma Y. Phloretin induced apoptosis of human hepatoma cells SMMC-7721 and its correlative biological mechanisms. African J Pharmacy Pharmacol. 2012;6:648–659. [Google Scholar]
- WHO (2014)Fact Sheet, The top 10 causes of death.
- Wolfe K, Wu Xianzhong, Liu RH. Antioxidant activity of apple peel. J Agric Food Chem. 2003;51:609–614. doi: 10.1021/jf020782a. [DOI] [PubMed] [Google Scholar]
- Xu K, Lu H, Qu B, Shan H, Song J. High-speed counter-current chromatography preparative separation and purification of phloretin from apple tree bark. Sep Purif Technol. 2010;72:406–409. doi: 10.1016/j.seppur.2010.02.020. [DOI] [Google Scholar]
- Yang J, Liu RH. Synergistic effect of apple extracts and quercetin 3-β-D-glucoside combination on antiproliferative activity in MCF-7 human breast cancer cells in vitro. J Agric Food Chem. 2009;57:8581–8586. doi: 10.1021/jf8039796. [DOI] [PubMed] [Google Scholar]
- Ye X, Krohn RL, Liu W, Joshi SS, Kuszynski CA, McGinn TR, Bagchi M, Preuss HG, Stohs SJ, Bagchi D. The cytotoxic effects of a novel IH636 grape seed proanthocyanidin extract on cultured human cancer cells. Mol Cell Biochem. 1999;196:99–108. doi: 10.1023/A:1006926414683. [DOI] [PubMed] [Google Scholar]
- Yue T, Shao D, Yuan Y, Wang Z, Qiang C (2012) Ultrasound-assisted extraction, HPLC analysis, and antioxidant activity of polyphenols from unripe apple. J Sep Sci 35:2138–2145 [DOI] [PubMed]
- Zhao H, Yakar S, Gavrilova O, Sun H, Zhang Y, Kim H, Setser J, Jou W, LeRoith D. Phloridzin improves hyperglycemia but not hepatic insulin resistance in a transgenic mouse model of type 2 diabetes. Diabetes. 2004;53:2901–2909. doi: 10.2337/diabetes.53.11.2901. [DOI] [PubMed] [Google Scholar]
- Zheng CQ, Qiao B, Wang M, Tao Q. Mechanisms of apple polyphenols-induced proliferation inhibiting and apoptosis in a metastatic oral adenoid cystic carcinoma cell line. Kaohsiung J Med Sci. 2013;29:239–245. doi: 10.1016/j.kjms.2012.09.001. [DOI] [PubMed] [Google Scholar]