Abstract
The purpose of this study was to investigate the preparation of formulated water– in–soybean oil–in–water emulsions by repeated premix membrane emulsification method using a cellulose acetate membrane. The effect of selective membrane emulsification process parameters (concentration of the emulsifiers, number of passes of the emulsions through the membrane and storage temperature) on the properties and stability of the developed emulsions were also investigated. 1, 3, 6, 8-pyrenetetrasulfonic acid tetrasodium salt (PTSA) was used as a hydrophilic model ingredient for the encapsulation of bioactive substances. W/O emulsions with 7 wt% (weight percentage) PGPR displays homogeneous and very fine dispersions, with the median diameter at 0.640 μm. Meanwhile, emulsions prepared by membrane emulsification (fine W/O/W) showed the highest stability at Tween 80 concentrations of 0.5 wt.% (weight percentage). It concluded that at 7 wt.% (weight percentage) PGPR concentration and 0.5 wt.% (weight percentage) Tween 80 concentrations, the most uniform particles with minimum mean size of oil drops (9.926 μm) were obtained after four passes through the membrane. Thus, cellulose acetate membrane can be used for preparing a stable W/O/W emulsions by repeated premix ME due to low cost and relatively easy to handle.
Keywords: Water-in oil-in water, Repeated premix membrane emulsification, Cellulose acetate, And stability
Introduction
Emulsion is a mixture of at least two immiscible liquids (usually oil and water, but not always), with one of the liquids (the dispersed phase) being dispersed as small spherical droplets in the other (the continuous phase) (McClements et al. 2007). Emulsions can be classified as simple emulsions [(a) oil-in-water (O/W) emulsions, and (b) water-in-oil (W/O) emulsions] and multiple emulsions [(a) water-in-oil-in-water (W/O/W) emulsions and (b) oil-in-water-in-oil (O/W/O) emulsions].
A technique for producing emulsion known as ‘membrane emulsification’ (ME) has received growing attention in the late 1980s (Charcosset 2009) due to its simplicity, low energy consumption, lower amounts of surfactant is utilized and enabling the production of droplet-size with narrow distributions (Trentin et al. 2011). This is an innovative method to prepare emulsions with a homogeneous droplets size (Kim and Schroën 2008). Membrane emulsification (ME) can be carried out in two different ways: direct ME and premix ME (Trentin et al. 2011). In direct ME, membrane with uniform pore-size distribution is employed with low pressure to force the dispersed phase to permeate through a membrane into the continuous phase. In direct ME, the resulting droplet size is controlled predominantly by the choice of membrane (Trentin et al. 2011). In Ppremix ME, a coarse emulsion is forced through the membrane (Zhou et al. 2009). It is carried out in a two-step process: (i) the two liquids that cannot mix are mixed together first using a conventional stirrer mixer to form a preliminary emulsified coarse emulsion, which is then ii) passed through the membrane (Trentin et al. 2011).
Two-step emulsification methods are involved to prepare multiple emulsions. A simple emulsion (W/O or O/W) is produced, and then re-emulsified to form W/O/W and O/W/O emulsions, respectively (Morais et al. 2009). Typically, the primary emulsion is formed by exerting a high shear homogenization process to produce fine internal droplet. The second emulsification step is carried out in mild conditions in order to prevent rupture of the double emulsion droplets (Vladisavljevic and Williams 2005). If the secondary emulsification is performed by conventional methods; the harsh mechanical stresses may disrupt the primary emulsion droplets, result in reduction of the yield of multiple emulsions (Surh et al. 2007). In order to avoid this, Surh et al. (2007) used a low-shear mixer for the secondary emulsification step. Therefore; membrane emulsification may be a method of choice for the preparation of multiple emulsions owing to the low shear rates (Charcosset 2009). This method allows the production of emulsions with external droplets that exhibit a narrow size distribution and maintain a high encapsulation yield of the internal droplets (Vladisavljevic and Williams 2005). In a nutshell, formulation of double emulsions has great impact on the stability and droplet size of emulsions. In addition, the choice of the preparation method should be taken into account at the same time (Van der Graaf et al. 2005).
The aims of this study was to investigate the preparation of formulated water–in–soybean oil–in–water emulsions by repeated premix membrane emulsification method using a cellulose acetate membrane. 1, 3, 6, 8-pyrenetetrasulfonic acid tetrasodium salt (PTSA) was used as a hydrophilic model ingredient for the encapsulation of bioactive substances. PTSA was used as a hydrophilic model ingredient for the encapsulation because it is a highly water-soluble fluorescent dye model ingredient suitable to encapsulate active food supplements. Previous research reported that When a water-soluble dye PTSA as a model ingredient was loaded in the inner water phase, all W/O/W emulsions showed a high encapsulation efficiency of the dye (>90 %) in the inner water phase. The effect of selective membrane emulsification process parameters (concentration of the emulsifiers, number of passes of the emulsions through the membrane and storage temperature) on the properties and stability of the developed emulsions were also investigated.
Material & methods
Materials
Hydrophilic emulsifier, Tween 80 (Polyoxyethylene (20) sorbitan monooleate), sodium alginate and D (+) - glucose were obtained from Quality Reagent Chemical (Malaysia) Sdn Bhd. Lipophilic emulsifier, SY- Glyster CR 310 (polyglycerol polyricinoleate (PGPR)) was obtained from Sakamoto Yakuhin Kogyo Co. (Japan) and the soybean oil with a density at 25 °C of 0.92 g/mL was purchased from Sigma- Aldrich (St Louis, MO, USA). A fluorescent marker, 1,3,6,8- pyrenetetrasulfonic acid tetrasodium salt (PTSA, Mw 610.42), was purchased from Molecular Probes (Eugene, OR, USA).Disposable membrane filter cartridges, DISMIC 25CS080AN, which held cellulose acetate membrane with pore diameters of 0.8 μm, was purchased from Advantec Toyo (Tokyo Japan).The filtration area of each membrane was 4.0cm2. The disc diameter was 25 mm.A disposable SS-30ES syringe with a capacity of 30 ml was purchased from Terumo (Tokyo, Japan).
Research design
In this study, a simple membrane-filtration apparatus was designed to produce a fine W/O/W emulsion suitable for use in drug delivery systems using a disposable syringe and a membrane-filter unit. A coarse W/O/W emulsion prepared using a homogenizer was filtered using the apparatus to produce a fine emulsion. The fine emulsion is repeatedly passed through the same membrane a number of times to achieve additional droplet size reduction and enhance size uniformity. This method is called multi-stage batch premix ME. The fine emulsion is more stable than the coarse emulsion.Some process parameters such as effects of concentration of emulsifier, i.e. lipophilic emulsifier, polyglycerol polyricinoleate (PGPR) and hydrophilic emulsifier, Tween 80 (Polyoxyethylene (20) sorbitan monooleate), effect of transmembrane pressure, and effect of number of emulsification cycles on emulsification result were investigated. W/O/W multiple emulsions prepared was characterized by the encapsulation efficiency of a hydrophilic substance in the inner aqueous phase using a fluorescent molecule as a model marker. The effect of hydrophilic emulsifier concentration (Tween 80), number of passes through the membrane, mean droplet size, encapsulation efficiency and creaming stability were investigated.
Preparation of W/O/W emulsions
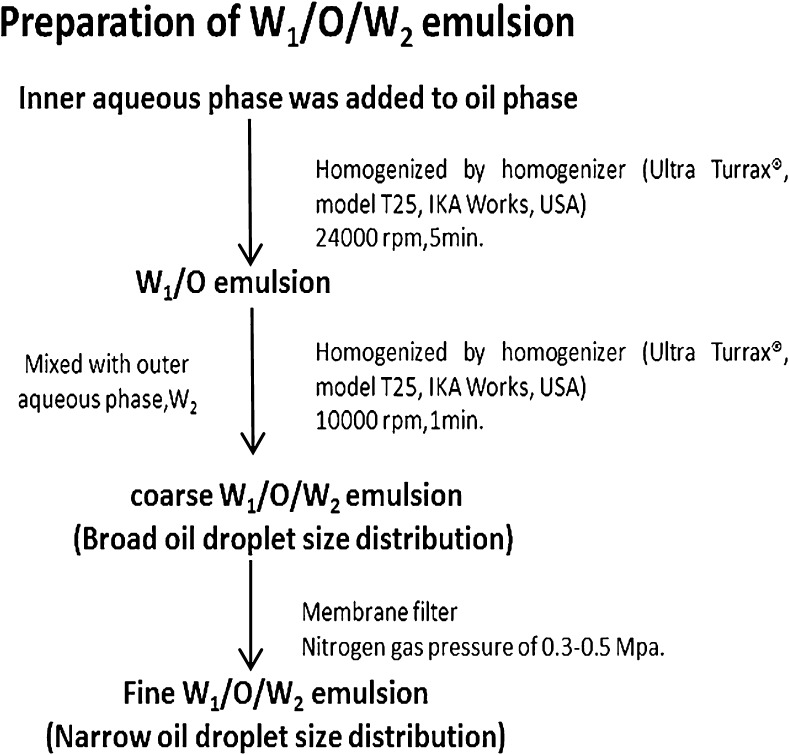
W/O/W emulsions were prepared by a two-step emulsification procedure. The inner aqueous phase (W1) was prepared by dissolving 5wt.% glucose in 30 ml of distilled water which contained 10−4mol/l PTSA (1, 3, 6, 8 - pyrenetetrasulfonic acid tetrasodium salt). The method used is modification of Mine et al. (1996) who produced successful formulations for the production of double emulsions (W1/O/W2) with membranes. (They use no additive for inner water phase (W1 covers 30vol. %), one of component in oil phase (O) using 0.5 % PGPR, 5 % glucose in outer water phase (W2)). The oil phase was prepared by dissolving 1–10 wt.% PGPR in soybean oil. PGPR was added to soybean oil and was heated to 60 to 70 °C., and were homogeneously mixed and dissolved. Then the inner aqueous phase was gradually added to the resultant mixture and were homogenized by means of a homogenizer (Ultra Turrax®, model T25, IKA Works, USA) at 24,000 rpm for 5 min. The mixture was gradually cooled to a temperature of from 40 to 20 °C to obtain a W1/O type emulsion. The Outer aqueous phase (W2) was prepared by dissolving 0.5–10 wt.% Tween 80, 1wt. % sodium alginate, and 5wt. % glucose in 30 ml of distilled water. The primary W1/O emulsion was then mixed with the outer aqueous phase W2 and was homogenized with the homogenizer at 10,000 rpm for 1 min to prepare the W1/O/W2 emulsions. Because the emulsion had a broad oil droplet size distribution, it was designated as the coarse W1/O/W2 emulsions. The coarse W1/O/W2 emulsion was passed through a membrane filter which held cellulose acetate membrane with pore diameters of 0.8 μm to reduce and monodisperse the diameter of the oil droplets.
A membrane cartridge or a membrane holder was connected to a disposable syringe. The coarse emulsion was put into the syringe and was forced to pass through the membrane by applying a pressure of 0.3–0.5 MPa with nitrogen gas. The filtrate was a W1/O/W2 emulsions having a narrow distribution of the oil droplet diameter. The median diameter of the oil droplet was also reduced. Therefore, the W1/O/W2 emulsions prepared by these procedures were designated as the fine W1/O/W2 emulsions. Figure 1 showed the steps for the preparation of W1/O/W2 emulsions.
Fig. 1.
Steps for the preparation of W1/O/W2 emulsions
Instrumentation and data analysis
Characterization of W/O emulsion
The properties of the W/O emulsion were characterized by measuring their particle size and sedimentation stability. In this study, the median diameter of the water droplets in W/O emulsion was measured immediately after preparation of W/O emulsion.
Characterization of fine W/O/W emulsions
The properties of the fine W/O/W emulsions were characterized by measuring their particle size, encapsulation efficiency of PTSA and creaming stability. The median diameter of the oil droplets in the fine W/O/W emulsions and the encapsulation efficiency of PTSA, were measured immediately after preparation of the fine W/O/W emulsion. The diameter of the oil droplets in the fine W/O/W emulsions and the encapsulation efficiency were also measured for the emulsion stored at 4 °C for 1 week or 8 days to assess the stability of the emulsion.
Measurement of particle-size distribution
The microscopic analysis was carried out in order to measure the particle size of water droplets in W/O emulsions and particle size oil droplets in W/O/W emulsions. This analysis was performed using an optical microscope (Olympus BX 51, Germany) equipped with an image analysis software (analySIS FIVE, Soft Imaging System, Germany.)
Measurement of the encapsulation efficiency
150 μl of the W/O/W emulsion and 1350 μl of purified water were poured into a sampling tube and mixed. The tube was then centrifuged at 17,000 g for 5 min. Six hundred μl of the clear part of the solution in the tube was taken up by a syringe equipped with a needle. The needle was replaced with a filter cartridge (Milex-VV, 0.1 μm pore diameter, Millipore), and then the solution was filtered to eliminate the oil droplets. 250 μl of the sample solution and 1750 μl of purified water were mixed in a cuvette. The PTSA concentration of the solution was determined with a fluorescence spectrometer (RF-1500, Shimadzu) at excitation and emission wavelength of 374 and 404 nm, respectively. The encapsulation efficiency of PTSA in the inner-water phase of a W/O/W emulsion was calculated by Eq. (1)
| 1 |
where m0 is the initial amount of PTSA loaded into the inner-water phase and was calculated from the composition of the W/O/W emulsion; msout is the amount of PTSA that leaked into the outer-phase solution and was calculated using the initial volume of the outer-water phase.
Measurement of sedimentation stability
Measurements were carried out on the W/O emulsions, where the water droplets tend to move downward because they are heavier than the surrounding oil phase. 10 ml of emulsion was transferred into a test tube (internal diameter) 15 mm, height) 125 mm), tightly sealed with a plastic cap, and then stored 7 days at two different temperature at 27 °C and 4 °C. After 7 days it was centrifuged at 6500 rpm for 30 min at room temperature. The height of the opaque emulsion phase was measured (ht) and compared to the initial emulsion height (h0) to determine sedimentation stability (S) (Rousseau and Hodge 2005):
| 2 |
Hence, the lower values of sedimentation stability indicate the lower extent of sedimentation (phase separation) which means the higher stability of the emulsions.
Measurement of creaming stability
Creaming stability measurement were carried out in the W/O/W emulsions, where the W/O droplets tend to move upward because they are lighter than the surrounding water phase. Ten grams of emulsion were transferred into a test tube (internal diameter = 15 mm, height = 125 mm), tightly sealed with a plastic cap, and then stored for 1 day and 7 days at room temperature. After storage, some emulsions separated into an optically opaque “cream” layer at the top and a transparent (or turbid) “serum” layer at the bottom. The serum layer was defined as the sum of any turbid and transparent layers. The total height of the emulsions (HE) and the height of the serum layer (Hs) were measured. The extent of creaming was characterized as % serum =100 (Hs/HE). The percent serum provided indirect information about the extent of droplet aggregation in an emulsion. All measurements were made on at three freshly prepared samples.
Process parameters that influenced premix membrane emulsification
Effects of the concentrations of the surfactants (emulsifier)
The effect of the lipophilic surfactant, SY-Glyster CR 310 (polyglycerol polyricinoleate (PGPR)) on the median diameters of the W/O emulsions was examined at the concentrations of 1, 3, 5, 7,9,10 and 12 % (w/v) in the oil phase solution. The PGPR concentration that was capable of forming W/O emulsions containing small water droplets with a narrow size distribution was selected for the subsequent preparation of W/O/W emulsions. The median diameter, encapsulation efficiency and the creaming stability of the W/O/W emulsion were also measured at 0.5, 2, 5, and 10 % (w/v) hydrophilic surfactant concentration, Tween 80 (Polyoxyethylene (20) sorbitan monooleate), in outer-phase solution.
Effect of number of passes (multistage premix)
Number of passes of the multiple emulsions, W/O/W, were increase form n = 1 to n = 5, through the same membrane without cleaning the membrane between the cycles. The emulsions samples obtained after each cycle were collected and analyzed. Effect of number of cycles on particle-size distribution of oil droplets, encapsulation efficiency and the creaming stability of W/O/W emulsions were studied.
Result & Discussion
Characterization of W/O emulsions
Effect of PGPR concentration on particle size distribution of W/O emulsions
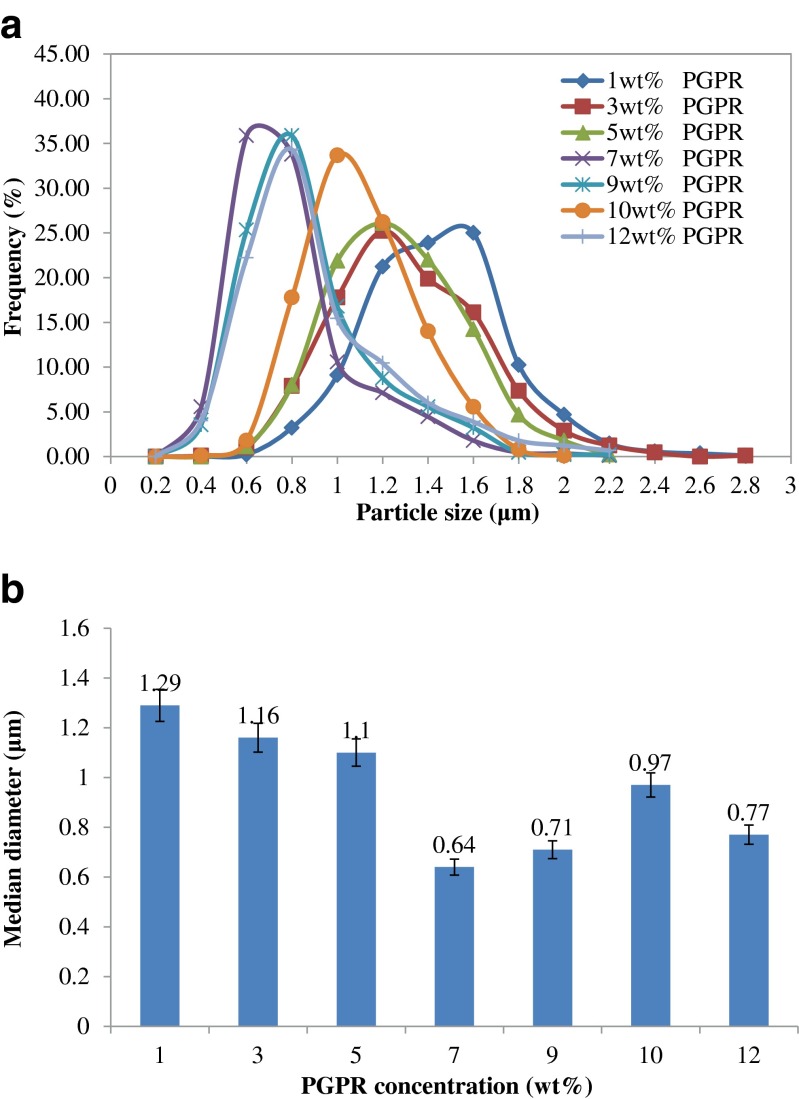
Based on the data obtained in the experimental measurements, the particle size distributions for the seven W/O emulsions were obtained through the graphic representation of the particle diameter according to their frequency. Figure 2 shows the resultant particle size distributions (a) of W/O emulsion prepared with different PGPR concentrations in the oil phase, (b) and median diameter. Hence it can be concluded that the distributions were mono modal at all PGPR concentrations. The W/O emulsion with 7 wt% PGPR and above showed fine dispersion compared to 5 wt% PGPR and below.
Fig. 2.
Particle size distributions a of W/O emulsion prepared with different PGPR concentrations in the oil phase, b and median diameter
Effect of PGPR concentration on stability and droplets size of W/O emulsions
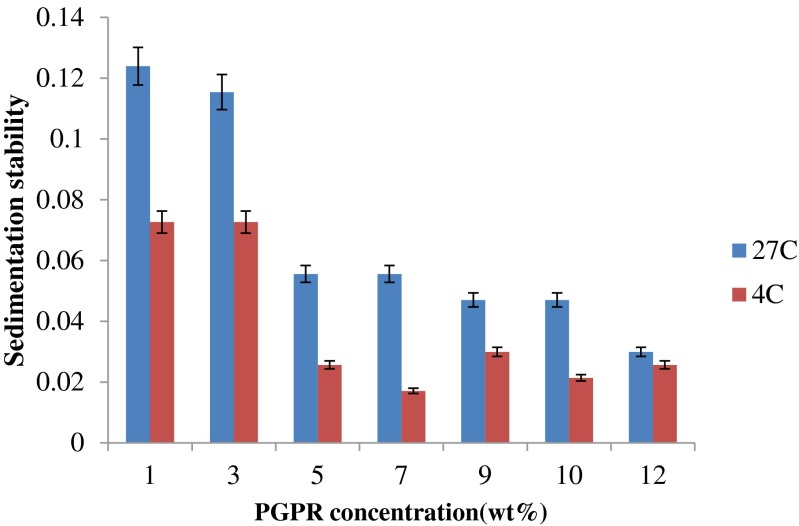
The stability of W/O emulsion increased with the increase of the emulsifier amount (PGPR). The effect of PGPR increase on the stability of W/O emulsions was reported previously in systems prepared with soybean oil and homogenized using a two stage valve homogenizer (Su et al. 2006). Figure 3 shows the sedimentation stability of W/O emulsion after 7 days storage at 4 °C and 27 °C.
Fig. 3.
Sedimentation stability of W/O emulsion after 7 days storage at 4 °C and 27 °C
In this study, the emulsion showed lowest stability at 1 wt% of PGPR concentration and highest stability at 7 wt% PGPR concentration. Increasing PGPR concentration from 3 wt% to 5 wt% caused a significant increase in stability of the emulsions (p < 0.05). This is because at low surfactant concentrations, the emulsion is not stable due to flocculation and coalescence of the water droplets. At higher surfactant concentration, lower initial size of water droplets were produced according to optical microscopy observations (Fig.4) and lower mean droplets diameters (Table 1).
Fig. 4.
Optical micrograph of W/O emulsions with 30 % aqueous phase, immediately after preparation. a 1 wt% PGPR; b 3 wt% PGPR; c 5 wt% PGPR; d 7 wt% PGPR; e 9 wt% PGPR; f 10 wt% PGPR; g 12 wt% PGPR
Table 1.
Mean droplets diameters for W/O emulsions with 30 % aqueous phase
| PGPR (%) | Mean diameter (μm) |
|---|---|
| 1 | 1.3340 ± 0.0733 a |
| 3 | 1.2100 ± 0.1264 a |
| 5 | 1.1553 ± 0.1323 a |
| 7 | 0.7180 ± 0.0842 b,c |
| 9 | 0.7817 ± 0.1323 b |
| 10 | 1.0067 ± 0.0671 b,d |
| 12 | 0.8230 ± 0.0980 b |
Mean values ±SD.,n = 3. Mean values with different letters are significantly different (p < 0.05)
As shown in Table 1, increasing PGPR concentration (between 1 to 5 wt%) had no significant effect on the mean droplets diameters (p > 0.05). Nevertheless, between 5 and 7wt% PGPR, the mean droplet diameters of the W/O emulsions were highly dependent on the PGPR concentrations. Increasing the PGPR concentration from 5 to 7 wt% caused a significant decrease in mean droplets diameters of water droplets (p < 0.05). From Table 1, at 5 wt% PGPR, the mean droplets diameter was 1.1553 ± 0.1323 μm whereas at 7 wt% PGPR, the mean droplets diameter was 0.7180 ± 0.0842 μm. In contrast, the mean droplets diameters prepared with 7 and 12 wt% PGPR showed no significant change (p > 0.05). These results showed that 7 wt% PGPR was capable to form W/O emulsions containing smallest water droplets and highest stability. The increase stability of W/O emulsions at 7 wt% PGPR was attributed to the lower mean droplets diameters. A lower droplets size decreases the coalescence process due to lower collision efficiency (Marquez et al. 2010). In addition, increase stability of the W/O emulsions may be due to the presence of sufficient PGPR in the oil phase to create a stable interfacial layer (Su et al. 2006). Moreover, increasing PGPR concentration increases the viscosity of the emulsion, which may lead to reduced rates of coalescence of water droplets (Marquez et al. 2010). Hence, 7 wt% PGPR concentration was selected for the preparation of water-in-soybean-oil-in-water emulsion (W/O/W) and the emulsions were stored at 4 °C due to it higher stability.
Effect of storage temperature on stability and droplets size of W/O emulsions
Fig. 3 also shows the sedimentation stability of W/O emulsion after 7 days storage at 27 °C. Emulsions stability was improved as temperature decrease from 27 °C to 4 °C. At 1 wt%, 10 wt% and 12 wt% of PGPR, the stability improvement was insignificant (p > 0.05).
Emulsions stability was improved as temperature decrease from 27 °C to 4 °C. At 1 wt%, 10 wt% and 12 wt% of PGPR, the stability improvement was insignificant (p > 0.05). On the other hand, at 3 wt%, 5 wt%, 7 wt% and 9 wt% of PGPR, decreasing storage temperature from 27 °C to 4 °C significantly improved emulsion stability (p < 0.05). The decreased stability of increased temperature is due to the loss of viscosity and to the greater mobility of the system (Mikkonen et al. 2009). In a similar study, Mikkonen et al. (2009) prepared oil-in-water emulsions (O/W) and the emulsions were stored at 4 °C, room temperature (23 °C) and 45 °C for 14 days to examine the effect of temperature on the stability of those emulsions. Their results showed that increasing the storage temperature to 45 °C led to rapid breakdown of the emulsion, but a decreasing in storage temperature increased emulsion stability after 14 days. Meanwhile Su et al. (2006) prepared water-in-oil-in-water emulsions (W/O/W) and explained that the increase in Brownian motion on increasing temperature will increase the rate of flocculation leading to further instability in the multiple (W/O/W) emulsions.
Characterization of W/O/W emulsions
Effect of hydrophilic emulsifier concentration
Table 2 shows the creaming stability and encapsulation efficiency of coarse W/O/W emulsions after 7 days storage containing varying amounts of Tween 80 and a constant concentration of PGPR.
Table 2.
Main properties of coarse multiple emulsions after 7 days storage
| PGPR (wt%) | Tween 80 (wt %) | Creaming stability (%serum) | Encapsulation efficiency (%) |
|---|---|---|---|
| 7 | 0.5 | 34.61 | 95.60 |
| 7 | 2 | 42.86 | 87.83 |
| 7 | 5 | 37.18 | 93.84 |
| 7 | 10 | 52.14 | 77.72 |
At 7 wt% of PGPR concentration and 0.5 wt% of Tween 80 concentration, the emulsion showed the highest creaming stability and highest encapsulation efficiency. The encapsulation efficiency of the emulsions decreased as the hydrophilic surfactant (Tween 80) concentration increased. Shima et al. (2004a) have also shown the significant decrease of encapsulation efficiency of W/O/W emulsions as the hydrophilic surfactant (decaglycerol monolaurate) concentration increased. The reasons for the decrease in the encapsulation efficiency of the emulsion prepared at high surfactant concentration remains unclear. One of the major reasons may be Ostwald ripening (Shima et al. 2004b). Ng et al. (1996) suggested that the alleviators of Ostwald ripening are (a) an oil-insoluble solute in the inner-water phase and (b) an osmotic pressure excess more of the outer- water phase compared to the inner water phase. The first characteristic is included in this experimental design, i.e. the PTSA in the inner-water phase solution for the first approach (a). Therefore, the effect of Ostwald ripening would be partially counteracted by these features.
On the other hand, increasing Tween 80 concentration from 0.5 wt% to 10 wt% results in significant decrease (p < 0.05) in creaming stability. The most common initial manifestation of instability of oil-in-water emulsion is creaming which can lead to phase separation with a distinctive clear or semitransparent lower serum phase and cream (Klaypradit and Huang 2008). The same occurrence happens to W/O/W emulsion in this case. This may then be followed by droplets coalescence within the cream and oiling off at the top of the sample. The results of this study clearly indicated that lower Tween 80 concentration was capable to produce multiple emulsions with greater stability. Emulsifier concentration is one of the most important factors influencing the stability of emulsions. Several studies have shown that emulsifier concentration can strongly influence emulsion stability (Chen and Tao 2005). Table 2 shows that as the emulsifier concentration increased, emulsion stability decreased. This is due to the fact that at low emulsifier concentrations, the emulsion is not stable due to agglomeration of the oil droplets; at high surfactant concentrations, the emulsion destabilization occurs as a result of rapid coalescence (Chen and Tao 2005). These results are in agreement with Matsumoto et al. (1976) who suggested that high concentrations of water-soluble surfactants solubilise the oil-soluble surfactant and cause disruption of multiple droplets. On the contrary, Hou and Papadopoulos (1997) reported a negative synergistic effect of Tween 80 in stabilizing multiple droplets in their study of multiple emulsions containing Span 80 and Tween 80. Their report was in agreement with Opawale and Burgess (1998) who determined the interfacial elasticity of Span 83 and Tween 80 at the planar water/oil interface and reported that interfacial film strength decreased as Tween 80 concentration increased.
Effects of the hydrophilic emulsifier concentration on creaming stability and encapsulation efficiency in coarse and fine W/O/W emulsions
Figure 5 shows the encapsulation efficiency for the coarse and fine W/O/W emulsions on day 0 (a) and after 7 (b) days storage prepared using different amounts of hydrophilic surfactant (Tween 80) concentration. It was clearly observed that the encapsulation efficiency of the emulsions was decreased after membrane emulsification except for emulsions contain 0.5 wt% Tween 80 on day 0 and emulsions contain 10 wt% Tween 80 after 7 days storage.
Fig. 5.
Encapsulation efficiency for the coarse and fine W/O/W emulsions prepared using different amounts of hydrophilic surfactant (Tween 80) concentration. a Emulsions at day 0. b Emulsions after 7 days storage
These results suggested that the emulsification method affected the relationship between the amounts of surfactants and the encapsulation efficiency of the W/O/W emulsion. Shima et al. (2004a) have also shown the slightly decreased in encapsulation efficiency of W/O/W emulsions after membrane emulsification. In the study, they prepared coarse and fine W/O/W emulsions using different hydrophilic surfactants. The hydrophilic surfactants were pentaglycerol monolaurate, decaglycerol monolaurate, pentaglycerol monomyristate, decaglycerol monomyristate, and lysolecithin. Their results showed that at low concentration of decaglycerol monolaurate and decaglycerol monomyristate (lower than 4 % (w/v)), the encapsulation efficiency decrease due to the membrane-emulsification. This would be ascribed mainly to the difference in the polyglycerol residue of these surfactants. These results suggested that the encapsulation efficiency of the W/O/W emulsion prepared using the high HLB surfactants at a low concentration was more affected by the membrane-filtration than that of the emulsion prepared using the relatively low HLB surfactants. In this study, Tween 80 as the high-HLB surfactant was used and the encapsulation efficiency of the emulsions decrease after membrane emulsification.
The storage stability of emulsions prepared by homogenizer (coarse W/O/W) was compared with those of emulsions prepared by membrane emulsification (fine W/O/W). The creaming stability of W/O/W emulsions prepared by homogenizer and membrane emulsification are shown in Fig. 6 for comparison.
Fig. 6.
Creaming stability for the coarse and fine W/O/W emulsions stored at 4 °C for 7 days. Emulsions were prepared using different amounts of hydrophilic surfactant (Tween 80) concentration
Emulsions prepared by membrane emulsification are found to be more stable than the emulsions prepared by homogenizer. The percentage serum of the emulsions was decreased after passing through membrane (fine W/O/W) which indicates higher emulsions stability. The decrease stability of emulsions prepared by homogenizer indicates significant coalescence happen in homogenized emulsions. Emulsions prepared by membrane emulsification (fine W/O/W) showed highest stability at Tween 80 concentrations of 0.5 wt%. These results indicate that membrane emulsification with 0.5 wt% Tween 80 produce emulsions with greater stability. Hence, 0.5 wt% Tween 80 concentration was selected for the preparation of W/O/W emulsions by membrane emulsification in the subsequent experiments.
Effects of the hydrophilic emulsifier concentration on droplet sizes of fine W/O/W emulsions
The particle size distribution of W/O/W emulsions prepared with a range of Tween 80 concentrations (between 0.5 and 10 wt%) is shown in Fig. 7.
Fig. 7.
Cumulative particle size distributions of W/O/W emulsions prepared with different Tween 80 concentrations in the external aqueous phase, W2.The number of passes through the membrane is 1
The emulsions were prepared by membrane emulsification by passing one time through the homogenizer. It was observed that Tween 80 concentration (between 0.5 and 10wt%) had no significant effect on the droplet size distributions (p > 0.05).
Effect of number of passes through the membrane homogenizer on droplets sizes of fine W/O/W emulsions
The W/O/W emulsions produced by multi-stage premix ME were examined for their droplets size distributions (Fig. 8).
Fig. 8.
Influence of the number of passes through the membrane homogenizer on droplet size distribution of W/O/W emulsions
The droplets of a pre-emulsion are disrupted into smaller droplets during their permeation through the membrane in premix ME (Surh et al. 2008). At all stages process (n = 1 to n = 5), the droplets size distributions of W/O/W emulsions were mono-modal. At n = 1, about 92.56 % from the particles have a diameter of between 5.000 and 30.000 μm. Meanwhile at n = 2, 91.89 % from the particles have a diameter of between 5.000 and 35.000 μm. After the second pass, the particle size distributions of prepared emulsions were narrower compared to the emulsions prepared after first pass and second passes through the membrane. At n = 3, 91.11 % from the particles have a diameter of between 5.000 and 25.000 μm. In addition, the most uniform particles were prepared after fourth passes, since the majority of droplets fell within a fairly narrow particle size range around 10.000 μm. After five passes through the membrane, the majority of droplets also fell within particle size range around 10.000 μm but the particle size distribution of prepared emulsions was broader than emulsions prepared after fourth passes.
The influence of number of homogenization cycles on mean particle size also plays an important role. The mean particle size tended to decrease with increasing the number of passes. The mean particles size are 15.950 μm, 18.010 μm, 12.000 μm, 9.926 μm and 10.010 μm for n = 1, 2, 3, 4, and 5 respectively. Clearly, at four homogenization cycles, the lowest mean size of oil drops was obtained (9.926 μm). Hence, the most uniform particles with minimum mean size of oil drops were prepared after four passes through the membrane. These results are in agreement with Vladisavljevic et al. (2004) who showed that the optimum conditions with regard to particle size uniformity were three homogenization cycles at a pressure difference of 100kPa, under which the mean size of outer W/O particles was 9.000 μm and the span of particle size distribution was as low as 0.280. Their results showed that the higher the transmembrane pressure, the smaller number of cycles was necessary to reach a narrow particle size distribution. However, much better results with regard to particle size uniformity were obtained using several passes (usually 2–4) at smaller pressures, compare to a single pass at higher pressures.
The micrographs of emulsion particles after homogenization taken by optical microscope under the same magnification (10X) are shown in Fig. 9.
Fig. 9.
Optical microscopy images of W/O/W emulsions prepared by membrane emulsification using different numbers of passes through the homogenizer
As shown in Fig. 9, the particles of emulsion after 1 pass through the membrane were relatively large. After fourth passes, the most uniform particles were prepared since the majority of droplets fell within a fairly narrow particle size range around 10.000 μm. After five passes through the membrane, the majority of droplets also fell within particle size range around 10.000 μm but the particle size distribution of prepared emulsions was broader than emulsions prepared after fourth passes.
Effect of number of passes through the membrane homogenizer on encapsulation efficiency of fine W/O/W emulsions
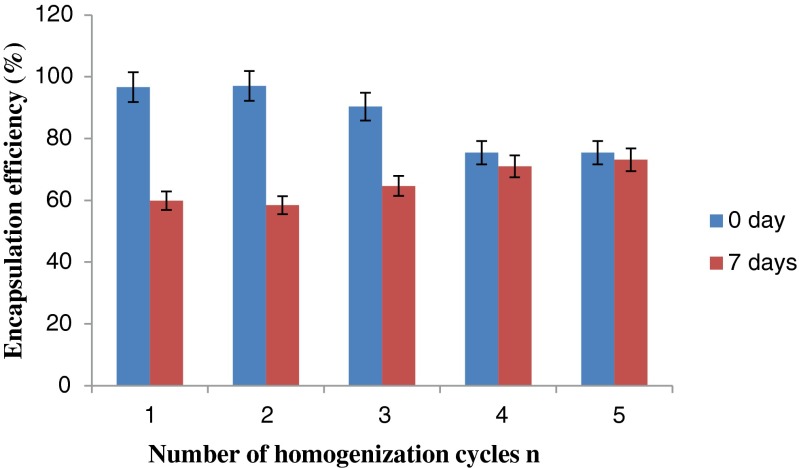
Fig. 10 shows the effects of the number of emulsification cycles on the encapsulation efficiency of PTSA in the W/O/W emulsions on day 0 and after 7 days storage. There was no significant dependence of the encapsulation efficiency on the number of homogenization cycles on day 0 and after 7 days storage (except, the encapsulation efficiency of the emulsions on day 0 significantly decreased as the number of homogenization cycles increased from 2 to 3 (p < 0.05)).
Fig. 10.
Encapsulation efficiency for the fine W/O/W emulsions as a function of number of emulsification cycles
Seven-day storage at 4 °C caused a decrease in the encapsulation efficiency of the emulsions. However, no significant decrease of encapsulation efficiency was observed in all W/O/W emulsions after 7 days storage except those prepared at 2 passes of homogenization cycles which showed 39.79 % decrement (p < 0.05). On the other hand, the emulsions prepared after four and five passes through the membrane demonstrate slight decrease (5.87 % and 3.05 % respectively) in the encapsulation efficiency after 7 days storage.
After 7 days storage, the encapsulation efficiency greater than 70 % was achieved for the emulsions prepared after four and five passes. This value was comparatively higher than those of the emulsions prepared at 3 passes of homogenization cycles and below. After 7 days storage, less than 65 % of encapsulation efficiency was obtained for the emulsions prepared at 3 passes of homogenization cycles and below. Shima et al. (2004b) obtained the encapsulation efficiency of PTSA greater than 90 % in the homogenization of a W/O/W coarse emulsion through disposable cellulose acetate filter with a mean pore size of 0.700–3.000 μm. The encapsulation efficiency was less than 65 % at 3 passes of homogenization cycles and below, because the mean size of oil drops were larger than the emulsions prepared at 4 and 5 passes through the membrane, as shown in Fig. 10.
Conclusion
In this study, a formulated and stable W/O/W multiple emulsions using membrane emulsification has been developed. The effects of the concentration of the emulsifier, i.e. lipophilic emulsifier, polyglycerol polyricinoleate (PGPR) and hydrophilic emulsifier, Tween 80 (Polyoxyethylene (20) sorbitan monooleate), number of emulsification cycles and storage temperature on emulsification result were investigated. From the preliminary study, the stability of W/O emulsions greatly depends upon the particle size distributions of the emulsions. The particle size distributions showed mono-modal at all PGPR concentrations (1-12wt%). W/O emulsions with 7 wt% PGPR shows homogeneous and very fine dispersions, with the median diameter at 0.640 μm. The emulsion showed lowest stability at 1 wt% of PGPR concentration and highest stability at 7 wt% PGPR concentration. At low surfactants concentrations the emulsion is not stable due to flocculation and coalescence of the water droplets.
The properties and stability of the W/O/W emulsions were examined, i.e. droplets size, particle size distribution, encapsulation efficiency, and creaming stability. It was found that the encapsulation efficiency of the emulsions decreased as the hydrophilic surfactant (Tween 80) concentration increased. Increasing Tween 80 concentration from 0.5 wt% to 10 wt% results in significant decrease (p < 0.05) in creaming stability. The most uniform particles with minimum mean size of oil drops were prepared after four passes through the membrane. Hence this study reveals the potential of cellulose acetate membrane to be used for low cost preparation of formulated water–in–oil–in–water (W/O/W) emulsions by repeated premix membrane emulsification.
Acknowledgments
We would like to thanks the Department of Bioprocess Engineering, Faculty of Chemical Engineering, Cardiovascular Engineering Centre, IJN-UTM, Faculty of Bioscience and Medical Engineering and Grant from Research Management Centre UTM for support of this study.
Footnotes
Research Highlight
1. Preparation of formulated water– in–soybean oil–in– water emulsions by repeated premix membrane emulsification method using a cellulose acetate membrane.
2. The effect of selective membrane emulsification process parameters on the properties and stability of the developed emulsions were also investigated.
3. 1,3,6,8-pyrenetetrasulfonic acid tetrasodium salt (PTSA) was used as a hydrophilic model ingredient for the encapsulation of bioactive substances.
4. Cellulose acetate membrane did not provide excellent performance in preparing a stable W/O/W emulsions by repeated premix ME. However this membrane is low cost, with acceptable performance and relatively easy to handle.
Contributor Information
Ida Idayu Muhamad, Phone: +6075535577, Email: idayu@cheme.utm.my.
Suguna Selvakumaran, Phone: 607-5535577, Email: Shannen89@yahoo.com.
References
- Charcosset C. Review. Preparation of Emulsions and Particles by Membrane Emulsification for the Food Processing Industry. J Food Eng. 2009;92:241–249. doi: 10.1016/j.jfoodeng.2008.11.017. [DOI] [Google Scholar]
- Chen G, Tao D. An experimental study of stability of oil–water emulsion. Fuel Process Technol. 2005;86:499–508. doi: 10.1016/j.fuproc.2004.03.010. [DOI] [Google Scholar]
- Hou W, Papadopoulos KD. W1/O/W 2 and O1/W/O 2 globules stabilized with span 80 and tween 80. Colloids Surf. 1997;125:181–187. doi: 10.1016/S0927-7757(96)03861-7. [DOI] [Google Scholar]
- Kim CT, Schroën CGPH. Nano- and microtechnology for emulsification. Food Sci Technol. 2008;22(4):19–22. [Google Scholar]
- Klaypradit W, Huang YW. Fish oil encapsulation with chitosan using ultrasonic atomizer. LWT. 2008;41:1133–1139. doi: 10.1016/j.lwt.2007.06.014. [DOI] [Google Scholar]
- Marquez AL, Medrano A, Panizzolo LA, Wagner JR. Effect of calcium salts and surfactant concentration on the stability of water-in-oil (w/o) emulsions prepared with polyglycerol polyricinoleate. J Colloid Interface Sci. 2010;341:101–108. doi: 10.1016/j.jcis.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Matsumoto S, Kita Y, Yonezawa D. An attempt at preparing water-in-oil-in-water multiple-phase emulsions. J Colloid Interface Sci. 1976;57:353–361. doi: 10.1016/0021-9797(76)90210-1. [DOI] [Google Scholar]
- McClements DJ, Decker EA, Weiss J. Emulsion-based delivery systems for lipophilic bioactive components. J Food Sci. 2007;72:109–124. doi: 10.1111/j.1750-3841.2007.00507.x. [DOI] [PubMed] [Google Scholar]
- Mikkonen KS, Tenkanen M, Cooke P, Xu C, Rita H, Willfor S, Holmbom B, Hicks KB, Yadav MP. Mannans as stabilizers of oil-in-water beverage emulsions. LWT Food Sci Technol. 2009;42:849–855. doi: 10.1016/j.lwt.2008.11.010. [DOI] [Google Scholar]
- Mine Y, Shimizu M, Nakashima T (1996) Preparation and stabilization of simple and multiple emulsions using a microporous glass membrane. Colloids Surf B 6:261–268
- Morais JM, Rocha-Filho PA, Burgess DJ. Influence of phase inversion on the formation and stability of one-step multiple emulsions. Am Chem Soc. 2009;25:7954–7961. doi: 10.1021/la9007125. [DOI] [PubMed] [Google Scholar]
- Ng JD, Lorber B, Witz J, Théobald-Dietrich A, Kern D, Giegé R (1996) The crystallization of biological macromolecules from precipitates: evidence for Ostwald ripening. J Cryst Growth 168:50–62
- Opawale F, Burgess DJ. Influence of interfacial rheological properties of mixed emulsifier films on the stability of water-in-oil-in-water emulsions. J Pharm Pharmacol. 1998;50:965–973. doi: 10.1111/j.2042-7158.1998.tb06910.x. [DOI] [PubMed] [Google Scholar]
- Rousseau D, Hodge SM. Stabilization of water-in-oil emulsions with continuous phase crystals. Colloids Surf A Physicochem Eng Asp. 2005;260:229–237. doi: 10.1016/j.colsurfa.2005.02.035. [DOI] [Google Scholar]
- Shima M, Kobayashi Y, Kimura Y, Adachi S, Matsuno R. Effect of the hydrophilic surfactants on the preparation and encapsulation efficiency in course and fine W/O/W type emulsions. Colloids Surf A Physicochem Eng Asp. 2004;238:83–90. doi: 10.1016/j.colsurfa.2004.02.018. [DOI] [Google Scholar]
- Shima M, Kobayashi Y, Fujii T, Tanaka M, Kimura Y, Adachi S, Matsuno R. Preparation of fine W/O/W emulsion through membrane filtration of coarse W/O/W emulsion and disappearance of the inclusion of outer phase solution. Food Hydrocoll. 2004;18:61–70. doi: 10.1016/S0268-005X(03)00042-0. [DOI] [Google Scholar]
- Su J, Flanagan J, Hemar Y, Singh H. Synergistic effects of polyglycerol ester of polyricinoleic acid and sodium caseinate on the stabilisation of water–oil–water emulsions. Food Hydrocoll. 2006;20:261–268. doi: 10.1016/j.foodhyd.2004.03.010. [DOI] [Google Scholar]
- Surh J, Jeong YG, Vladisavljevic GT. On the preparation of lecithin-stabilized oil-in-water emulsions by multi-stage premix membrane emulsification. J Food Eng. 2008;89:164–170. doi: 10.1016/j.jfoodeng.2008.04.023. [DOI] [Google Scholar]
- Surh J, Vladisavljevic GT, Mun S, McClements DJ. Preparation and characterization of water/oil and water/oil/water emulsions containing biopolymer-gelled water droplets. J Agric Food Chem. 2007;55:175–184. doi: 10.1021/jf061637q. [DOI] [PubMed] [Google Scholar]
- Trentin A, Lamo SD, Guell C, Lopez F, Ferrando M. Protein-stabilized emulsions containing beta-carotene produced by premix membrane emulsification. J Food Eng. 2011;106:267–274. doi: 10.1016/j.jfoodeng.2011.03.013. [DOI] [Google Scholar]
- Van der Graaf S, Chroen CGPH, Boom RM. Preparation of double emulsions by membrane emulsificationsa review. J Membr Sci. 2005;251:7–15. doi: 10.1016/j.memsci.2004.12.013. [DOI] [Google Scholar]
- Vladisavljevic GT, Shimizu M, Nakashima T. Preparation of monodisperse multiple emulsions at high production rates by multi-stage premix membrane emulsification. J Membr Sci. 2004;244:97–106. doi: 10.1016/j.memsci.2004.07.008. [DOI] [Google Scholar]
- Vladisavljevic GT, Williams RA. Recent developments in manufacturing emulsions and particulate products using membranes. Adv Colloid Interf Sci. 2005;113:1–20. doi: 10.1016/j.cis.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Zhou QZ, Ma GH, Su ZG. Effect of membrane parameters on the size and uniformity in preparing agarose beads by premix membrane emulsification. J Membr Sci. 2009;326:694–700. doi: 10.1016/j.memsci.2008.11.012. [DOI] [Google Scholar]