Abstract
The effects of heat treatment and extraction solvents (pure/aqueous acetone, ethanol, methanol) on antioxidant activity (AA) and components of clove (Syzygium aromaticum Linn) were studied. Clove was subjected to dry heat treatment (microwave and roasting) and the AA measured by free radical scavenging activity (FRSA), reducing power (RP), and phospho-molybdenum assay (TAA). Unheated samples served as controls. The antioxidant components estimated were total phenols, flavonoids and tannins. Using RP and FRSA, highest AA was observed in 80 % acetone extract of all samples (1.778–1448 and 84.5–86.0 %). TAA showed higher value in 80 % methanolic extract for all samples in the range 303.595–307.941 mmol ascorbic acid/g. Heated samples exhibited higher AA in all assays. Highest amount of phenols and flavonoids were extracted in 80 % acetone (4053-4064 mg/100 g) and 80 % methanol (11,271–11,370 mg/100 g) respectively. For tannins, maximum extraction was in 80 % acetone (control, 16441 mg/100 g), 80 % ethanol (microwave, 19,558 mg/100 g), and pure methanol (roasted, 15,823 mg/100 g). Total phenol and flavonoid contents were positively associated with AA determined using RP and FRSA. In conclusion, clove exhibited powerful AA in different extraction solvents which increased on dry heat treatments and correlated positively with antioxidant components. Hence, clove can be used as a natural antioxidant in food systems.
Keywords: Microwave heating, Roasting, Total phenols, Tannins, Free radical scavenging assay, Reducing power
Introduction
Spices and herbs used for culinary purposes to impart colour, flavor and aroma to dishes are also rich sources of powerful antioxidants. They exhibit antioxidant activity in whole or ground form, as extracts, in encapsulated form or as emulsions (Embuscado 2015). Natural antioxidants in processed foods are more favored by consumers than synthetic antioxidants even though they were permitted additives (Nanditha and Prabhasankar 2009). An exceptionally high polyphenolic content imparts antioxidant potential to spices indicating their possible role in disease prevention and encouraging their use as natural source of antioxidants. Syzygium aromaticum (S. aromaticum) (synonym: Eugenia cariophylata) commonly known as clove, is a median size tree (8–12 m) from the Myrtaceae family, which is native to the Maluku islands in East Indonesia. It was known as a valuable spice in trade for centuries and assisted in the economic development of this Asiatic region (Charles 2013). Cloves are the dried flower buds of Syzygium aromaticum. Clove oil is a natural preservative and flavouring substance used in food products without any harm (Azzouz and Bullerman 1982). Many bioactive compounds with antioxidant potential have been identified in clove. The primary components of essential oil are phenylpropanoids such as eugenol, eugenol acetate, carvacrol, thymol and cinnamaldehye. Clove also contain non-volatile bioactive substances such as sterols, flavonoids, galloyl tannin, phenolic acids and triterpenes (Brewer 2011; Charles 2013). Antioxidative activity has been reported in aroma extract of clove buds especially eugenol and eugenyl acetate (Lee and Shibamoto 2001).
Heat processing is a major way to convert foods to edible form and extend its shelf life. Besides, it breaks down the covalent bond form of phenolic compounds with insoluble polymers and liberates the natural antioxidants from plants (Duh et al. 2001; Lee et al. 2003; Nicoli et al. 1997; Nikousaleh and Prakash 2008). Each food possesses a complex mixture of compounds of varying polarity which have the potential synergistic action towards exhibiting antioxidant properties. Therefore different solvent systems with different nature have been used for the extraction of antioxidant components from various plant materials to fully understand their potential activity (Anwar et al. 2010; Sultana et al. 2009). Our earlier studies showed that among many spices studied, water extracts of dry heat treated clove exhibited higher antioxidant potential (Nikousaleh and Prakash 2008).
Spices are subjected to many processing treatments including cooking and dry heat treatments. Dry heat treatments enhance the flavour quality of spices, hence, it is a pre-requisite before they are ground and added to foods. The present study investigated the effect of dry heat treatments (roasting and microwaving), on the antioxidant activity of clove used in many ethnic cuisines. The heat treated samples were extracted in different media to evaluate the effects of different extraction solvents on the antioxidant activity and content of extractable components, namely, phenolics, flavonoids and tannins.
Materials and methods
Materials
The spice used for the study, whole clove (Syzygium aromaticum Linn.) was purchased from a supermarket in Mysore, India in a clean and packed form. Whole clove is the mature and dry bud from the tree, which is shelf stable and used in whole or powdered form for cooking. All the chemicals purchased were of analytical grade from different firms namely, Sd Fine Chemicals, and Qualigens Ltd., Mumbai, India. DPPH (1,1,-diphenyl-2-picrylhydrazyl) was procured from Sigma Chemical Co., U.S.A. Glass double distilled water was used for all analyses. All the components were estimated in triplicate and antioxidant activity were measured in 6 replicates for each sample.
Methods
A single batch of whole spice weighing 150 g was taken and divided into three parts for different treatments. Two sets were used for heat treatment and one set was left untreated which served as control. One set (50 g) was roasted on medium flame for 5 min with continuous stirring in a thick bottom pan. The end point was indicated by emission of a strong aroma typical of roasted clove. Another set was heated in microwave oven (Model number:BMO-700 T from BPL Sanyo Utilities and Appliances Ltd.) for a total of 2 min at high power (2450 MHz, 1200 watts) with intermittent stirring. The time of dry heat treatment was standardized after initial trials with each sample. The roasted spice was cooled and powdered immediately in a grinder to pass through a 40-mesh sieve and stored in airtight PET (polyethylene terephthalate) jars under refrigeration at 4 °C until further use. The unheated spice set was also powdered and stored in PET containers under similar conditions.
Preparation of extract
The antioxidant activity of clove is associated both with essential oil and water soluble components. Hence, different solvents namely ethanol, methanol and acetone, and the combination of solvent with water like 80 % ethanol, 80 % methanol and 80 % acetone were used for extraction of the spice as suggested by Ismail et al. (2013). The selection of extraction media was based on many earlier studies wherein authors have repeated a better and higher extraction of antioxidant components in polar and non-polar solvent (Do et al. 2014; Wijekoon et al. 2011; Michiles et al. 2012; Alothman et al. 2009; Thippeswamy et al. 2013).
A 1.0 g portion of powdered spice sample was suspended in 100 ml of extraction solvent and shaken at a constant rotating speed of 150 rpm/min in a water bath shaker for 30 min. It was centrifuged for 10 min at 4000 × g and filtered through Whatman No. 1 filter paper to get a clear extract. Fresh extract was used for each experiment. Antioxidant activity of all extracts were measured by three standard techniques (reducing power, free radical scavenging activity by DPPH assay and total antioxidant activity by phosphomolybdenum complex assay). All extracts were made in duplicate and all analyses were conducted in triplicate. Hence, the results represent average and standard deviations of six determinations for all samples.
Determination of antioxidant activity
The reducing power (RP) was determined by the method of Oyaizu (1986). In brief the procedure was as follows - 1.0 ml of extract was mixed with 2.5 ml of phosphate buffer and 2.5 ml of potassium ferricyanide and incubated at 50 °C for 20 min. Thereafter, 2.5 ml of 10 % trichloro acetic acid was added to the mixture, followed by centrifugation. Finally, 2.5 ml of the supernatant solution was mixed with 2.5 ml distilled water and 0.5 ml FeCl3 solution and absorbance was measured at 700 nm. Increased absorbance of the reaction mixture indicated increasing reducing power. A typical blank contained the reagent and the solvent extract without sample in appropriate amount. Free radical scavenging activity (FRSA) was measured using the method of Shimada et al. (1992). A 1.0 ml solution of DPPH (0.1 mmol in methanol) was mixed with 3 ml of extract, incubated for 30 min and the absorbance measured at 517 nm. Solvent extract with reagent alone was used as blank. The DPPH concentration in the reaction medium was calculated as percent free radical scavenging activity (control OD- sample OD/control OD× 100).
The total antioxidant capacity (TAA) is based on the reduction of Mo (VI) to Mo (V) by the sample and the subsequent formation of a green phosphate/Mo (V) complex at acidic pH (Prieto et al. 1999). An aliquot of 0.1 ml of sample solution was combined with 1.0 ml of reagent solution and incubated at 95 °C for 90 min. After cooling the absorbance was measured at 695 nm against a blank. The antioxidant activity of extracts was expressed as equivalents of ascorbic acid.
Determination of antioxidant components
Antioxidant components namely, total phenols, tannins and flavonoids were measured following standard techniques in all solvent extracts of spice. Total phenols were measured by Folin-Ciocalteau method and concentration was calculated using tannic acid as standard. Results were expressed as mg tannic acid equivalents/100 g sample (Matthaus 2002). The total flavonoid content was determined using a standard curve with quercetin (0–100 mg/l) as the standard and expressed as mg of quercetin equivalents (QE)/100 g of extract following the method of Dowd (Meda et al. 2005). Tannins were measured colorimetrically based on the measurement of blue colour formed by reduction of phosphotungstomolybdic acid by tannin like compounds in alkaline solution, tannic acid was used as standard (AOAC 1970).
Statistical analysis
All values are expressed as mean and standard deviation derived from replicates. The analytical data were subjected to statistical analysis to determine the significant difference between the control and thermally treated samples using a student’s t-test. The level of probability was fixed at P < 0.05. The influence of antioxidant components on antioxidant activity was determined by correlation analysis (Rao 1996).
Results
The results of the study are compiled in Tables 1, 2, 3 and 4 and Fig. 1. A brief description is presented below.
Table 1.
Effect of dry heat treatments on antioxidant activity of clove extracted in different solvents using reducing power assay (absorbance at 700 nm)
| Extract | Treatment | Concentration (mg) | ||||
|---|---|---|---|---|---|---|
| 0.2 | 0.4 | 0.6 | 0.8 | P Value | ||
| Acetone | Control | 0.214 ± 0.026 | 0.379 ± 0.010 | 0.555 ± 0.004 | 0.742 ± 0.016 | - |
| Microwave | 0.256 ± 0.007 | 0.414 ± 0.022 | 0.578 ± 0.018 | 0.771 ± 0.010 | 0.000*** | |
| Roasted | 0.248 ± 0.01 | 0.396 ± 0.015 | 0.564 ± 0.005 | 0.751 ± 0.005 | 0.031* | |
| Acetone, 80 % | Control | 0.356 ± 0.016 | 0.658 ± 0.012 | 0.893 ± 0.007 | 1.178 ± 0.077 | - |
| Microwave | 0.544 ± 0.040 | 0.868 ± 0.029 | 1.156 ± 0.003 | 1.448 ± 0.025 | 0.000*** | |
| Roasted | 0.493 ± 0.035 | 0.820 ± 0.010 | 1.153 ± 0.015 | 1.442 ± 0.034 | 0.004** | |
| Ethanol | Control | 0.244 ± 0.004 | 0.425 ± 0.015 | 0.639 ± 0.013 | 0.844 ± 0.030 | - |
| Microwave | 0.265 ± 0.013 | 0.450 ± 0.002 | 0.642 ± 0.003 | 0.851 ± 0.006 | 0.043* | |
| Roasted | 0.267 ± 0.002 | 0.458 ± 0.010 | 0.658 ± 0.002 | 0.861 ± 0.0162 | 0.004** | |
| Ethanol, 80 % | Control | 0.315 ± 0.019 | 0.587 ± 0.069 | 0.862 ± 0.022 | 1.106 ± 0.014 | - |
| Microwave | 0.345 ± 0.045 | 0.598 ± 0.037 | 0.863 ± 0.001 | 1.118 ± 0.011 | 0.052 ns | |
| Roasted | 0.367 ± 0.004 | 0.610 ± 0.008 | 0.872 ± 0.003 | 1.122 ± 0.004 | 0.033* | |
| Methanol | Control | 0.365 ± 0.017 | 0.583 ± 0.005 | 0.780 ± 0.015 | 1.006 ± 0.031 | - |
| Microwave | 0.377 ± 0.009 | 0.585 ± 0.013 | 0.801 ± 0.019 | 1.018 ± 0.026 | 0.026* | |
| Roasted | 0.405 ± 0.020 | 0.649 ± 0.022 | 0.897 ± 0.051 | 1.074 ± 0.032 | 0.0099** | |
| Methanol, 80 % | Control | 0.432 ± 0.004 | 0.695 ± 0.009 | 0.924 ± 0.005 | 1.141 ± 0.043 | - |
| Microwave | 0.437 ± 0.004 | 0.706 ± 0.005 | 0.942 ± 0.020 | 1.152 ± 0.006 | 0.034* | |
| Roasted | 0.439 ± 0.007 | 0.714 ± 0.005 | 0.928 ± 0.005 | 1.172 ± 0.023 | 0.043* | |
Values indicate mean ± standard deviations. Significant difference between heat treated sample and control on application of Students T Test. *: P < 0.05; **: P < 0.01; ***: P < 0.001; ns: not significant
Table 2.
Effect of dry heat treatments on antioxidant activity of clove extracted in different solvents using phosphomolybdenum complex assay (mmol ascorbic acid/g sample)
| Extract | Control | Microwave | Roasted |
|---|---|---|---|
| Acetone | 196.160 ± 4.739 | 199.984 ± 4.064 | 260.784 ± 5.370 |
| P-value | - | 0.08 ns | 0.000*** |
| Acetone, 80 % | 234.200 ± 8.465 | 270.400 ± 8.973 | 269.300 ± 10.237 |
| P- value | - | 0.000*** | 0.000*** |
| Ethanol | 206.193 ± 2.419 | 228.252 ± 0.971 | 221.012 ± 5.622 |
| P- value | - | 0.000*** | 0.000*** |
| Ethanol, 80 % | 200.882 ± 4.109 | 226.075 ± 1.567 | 266.99 ± 2.531 |
| P- value | - | 0.000*** | 0.000*** |
| Methanol | 197.647 ± 0.196 | 197.941 ± 0.490 | 207.696 ± 0.309 |
| P- value | - | 0.000*** | 0.000*** |
| Methanol, 80 % | 303.595 ± 4.002 | 307.941 ± 0.653 | 305.115 ± 1.092 |
| P-value | - | 0.000*** | 0.024* |
Values indicate mean ± standard deviations. Significant difference between heat treated sample and control on application of Students T Test. *: P < 0.05; ***: P < 0.001; ns: not significant
Table 3.
Antioxidants components in dry heat treated clove extracted in different solvents (mg/100 g) of sample
| Antioxidant component | Extracts | Control | Microwave | Roasted |
|---|---|---|---|---|
| Total phenols | Acetone | 2402 ± 22 | 2483 ± 18* | 2486 ± 15* |
| Acetone, 80 % | 4053 ± 13 | 4060 ± 32ns | 4064 ± 10 ns | |
| Ethanol | 3362 ± 10 | 3365 ± 06 ns | 3410 ± 12** | |
| Ethanol, 80 % | 3817 ± 20 | 3855 ± 09 ns | 3919 ± 34** | |
| Methanol | 3599 ± 26 | 3608 ± 10 ns | 3622 ± 23 ns | |
| Methanol, 80 % | 3877 ± 19 | 3879 ± 27 ns | 3920 ± 08 ns | |
| Tannin | Acetone | 14696 ± 16 | 13814 ± 331* | 13706 ± 206* |
| Acetone, 80 % | 16441 ± 88 | 14382 ± 386** | 14950 ± 431* | |
| Ethanol | 13902 ± 103 | 16725 ± 278** | 14892 ± 189* | |
| Ethanol, 80 % | 14754 ± 348 | 19558 ± 281** | 15009 ± 17 ns | |
| Methanol | 15608 ± 334 | 14461 ± 250** | 15823 ± 193 ns | |
| Methanol, 80 % | 15647 ± 298 | 16598 ± 258* | 14127 ± 136** | |
| Flavonoid | Acetone | 1708 ± 36 | 1643 ± 11* | 1890 ± 75* |
| Acetone, 80 % | 7463 ± 12 | 7835 ± 101** | 7992 ± 14*** | |
| Ethanol | 2919 ± 68 | 2475 ± 22** | 2348 ± 26** | |
| Ethanol, 80 % | 9550 ± 87 | 8664 ± 36** | 8093 ± 0*** | |
| Methanol | 5331 ± 51 | 5310 ± 179 ns | 6364 ± 119** | |
| Methanol, 80 % | 11370 ± 78 | 11329 ± 41** | 11271 ± 36** |
Values indicate mean ± standard deviations. Significant difference between heat treated sample and control on application of Students T Test. *: P < 0.05; **: P < 0.01; ***: P < 0.001; ns: not significant
Table 4.
Correlation between antioxidants components and antioxidant activity of clove in different media (R value)
| Treatment | Antioxidant assay | Total phenols | Flavonoid | Tannin |
|---|---|---|---|---|
| Control | Reducing power | 0.705 | 0.690 | 0.041 |
| FRSA | 0.604 | 0.791 | 0.554 | |
| TAAa | 0.161 | 0.359 | −0.318 | |
| Microwave | Reducing power | 0.787 | 0.676 | 0.036 |
| FRSA | 0.686 | 0.824 | 0.356 | |
| TAAa | 0.354 | 0.468 | −0.073 | |
| Roasted | Reducing power | 0.816 | 0.741 | 0.174 |
| FRSA | 0.503 | 0.774 | 0.409 | |
| TAAa | −0.068 | 0.213 | −0.801 |
a= total antioxidant activity by phosphomolybdenum complex method
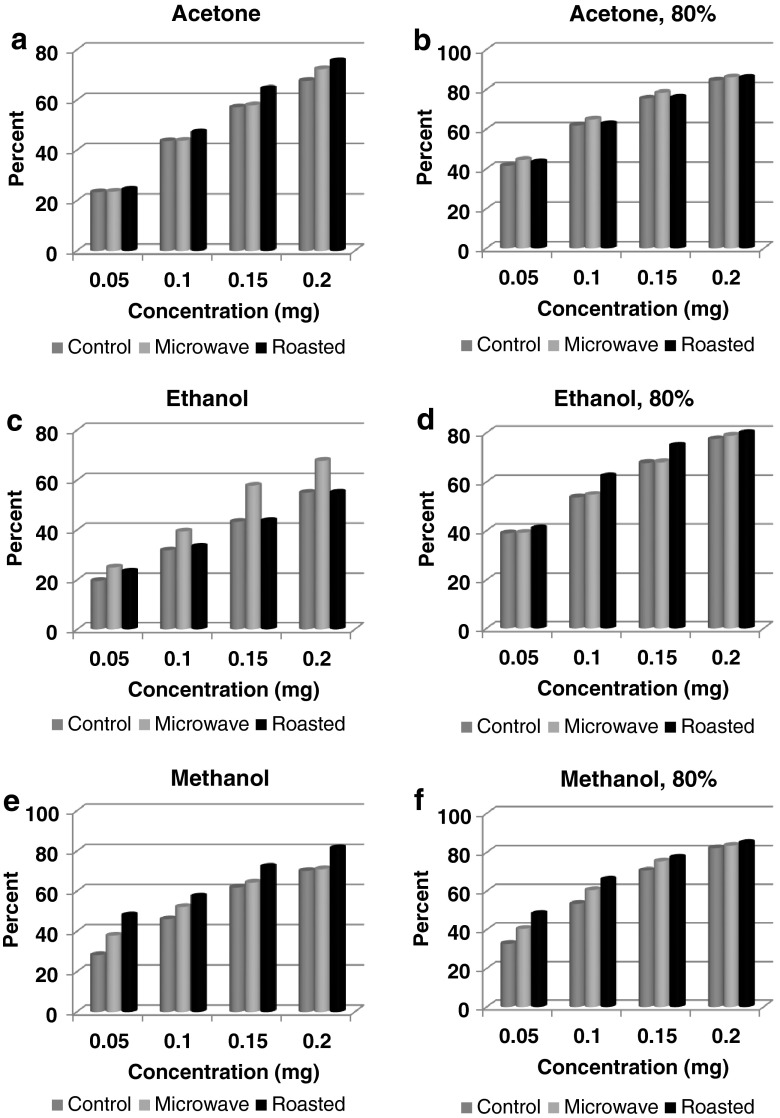
Fig. 1.
Effect of heating on free radical scavenging activity of clove in different solvent extracts. a Acetone. b Acetone, 80 %. c Ethanol. d Ethanol, 80 %. e Methanol. f Methanol, 80 %
Effect of dry heat treatments on antioxidant activity of clove
The observations of effect of dry heat treatments on antioxidant activity of clove extracted in different solvents using reducing power (RP) assay are given in Table1. The concentration of the spice used for RP assay were in the range of 0.2–0.8 mg. Results indicated that antioxidant activity in all samples was dose dependent and higher activity was seen at the highest concentration of the sample (0.8 mg). The dry heat treated extracts in all solvents showed slightly higher values which were marginally significant in relation to sample without heat treatment. The absorbance at 700 nm in different solvents for roasted and microwaved sample (at 0.8 mg concentration) was in the range of 0.751–1.442 and 0.771–1.448 respectively and followed the ascending order for acetone, ethanol, methanol, 80 % ethanol, 80 % methanol and 80 % acetone respectively. The absorbance of control sample (not heated) extracted with different solvents was in the range of 0.742 to 1.178 at 700 nm (acetone and 80 % acetone, respectively) and followed a similar order, though with significantly lower values than heat treated samples.
For FRSA of solvent extracts of clove, the concentrations of sample used were in the range of 0.05 to 0.2 mg. Similar to RP assay, antioxidant activity was dose dependent and the highest antioxidant activity was seen at 0.2 mg (Fig. 1a-f). The results are discussed taking the data of the highest concentration. Depending upon the extracting media, higher antioxidant activity was shown by 80 % acetone extract and least was seen in ethanol extract in the range of 55.0 % - 85.9 % for roasted sample, 67.7 % - 86.1 % for microwave heated and 54.9 % - 84.5 % for control sample. Heated samples exhibited higher antioxidant activity in comparison to control with few exceptions (ethanolic extract of roasted clove and methanolic extract of microwaved clove were similar to control at higher concentrations). In acetone extracted sample, FRSA was 67.6, 72.3 and 75.5 % for control, microwave and roasted sample respectively. The difference was not significant in microwave sample and marginally significant in roasted sample (P ≤ 0.05). The sample extract in 80 % acetone exhibited activity equivalent to 84.5 % for control, 85.9 % for roasted and 86.1 % for microwaved sample. In ethanol extracts the values were lower at 54.9, 67.7 and 55.0 % for similar samples respectively. The rest of the extracting media showed FRSA in the range of 70.1 % - 84.4 % and all heated samples had significantly higher antioxidant activity than control. The level of significance varied from P ≤ 0.05 to P ≤ 0.01.
When the TAA was measured in clove sample using phosphomolybdenum assay, the control sample had least activity in acetone followed by methanol, 80 % ethanol, ethanol, 80 % acetone and 80 % methanol (Table 2). The heated samples however followed a different order with least value in methanol, and showed higher activity than control which was highly significant for all except acetone extract of microwave clove sample. The spread of values for all samples were between 196.16 and 307.941 mmol ascorbic acid/g, with varied differences among different solvent extracts.
Antioxidants components in dry heat treated clove extracted in different solvents
Since the antioxidant activity is dependent on the concentration of extracted components, an attempt was made to estimate the components in all extraction media. Total phenols, tannin and flavonoid content of unheated and dry heat treated samples, extracted in different solvents are presented in Table 3 and discussed below. The extraction efficiency of solvents was different for each of the components and a large variation could be observed in the amount of antioxidant components extracted. The results are discussed for each of the component for ease of comprehension.
The amount of total phenols extracted in different extracts varied from 2402 to 4064 mg/100 g of samples. However, between the phenolic content of control and microwave treated sample, differences were not significant with the exception of acetone, which was marginally higher for heat treated samples. In roasted sample, phenolic content was significantly higher in ethanolic media, and rest were in similar range. These results indicate that heat treatment did not make a major difference in content of extractable phenols. Among solvents, 80 % acetone could extract highest content of total phenols for all samples (range, 4053–4064 mg/100 g), this was followed by 80 % ethanol and 80 % methanol extracts in the range of 3817 and 3920 mg/100 g. Pure ethanol and methanol also extracted considerable amount of phenols ranging from 3362 to 3622 mg/100 g, while it was least for acetone.
The extraction of tannins was highest in 80 % acetone for control sample (16,441 mg/100 g), in 80 % ethanol for microwaved sample (19,558 mg/100 g) and in methanol for roasted sample (15,823 mg/100 g). The acetone and 80 % acetone media extracted higher tannins from control compared to heat treated sample (range, 13,706–14,950 mg/100 g). In ethanol and 80 % ethanol extracts, the microwave treated sample had significantly higher tannin content than control. Roasted sample also showed a higher extraction of tannins in comparison to control and it was marginally significant for ethanol, and not significant for 80 %ethanol.
The pattern of variations observed for total phenols and tannins content was also seen for flavonoids with values ranging from 1643 to 11,370 mg/100 g sample. Highest extraction was observed in 80 % methanol with values in the range of 11,271 to 11,370 mg/100 g. Pure acetone and ethanol could extract least amount of flavonoid (1643–1890 and 2348–2919 mg/100 g respectively). The microwave sample extracted in 80 % acetone and roasted sample extracted in pure and aqueous acetone, and pure methanol had significantly higher content of flavonoid than control, whereas all others were lower. Ethanol, 80 % ethanol and 80 % methanol extracted significantly lesser contents of tannins from heat treated samples.
Discussion
The overall observation of RP shows that among all extracts of clove, 80 % acetone showed the highest activity. This was followed by 80 % methanol and 80 % ethanol. Pure solvents had lower reducing power values. This could be related to the nature of antioxidants present in clove, as stated earlier. Clove has both fat soluble and water soluble bioactive constituents, hence aqueous solvents could solubilize a higher quantity of antioxidant components and exhibit higher antioxidant activity (Brewer 2011; Charles 2013). Clove is also recognized for a very high content of antioxidant components among spices (Srinivasan 2014). Wojdyło et al. (2007) in their study on antioxidant activity and phenolic compounds of spices reported the presence of 8.96 mg of phenols as gallic acid equivalents/100 g of dry weight of clove. Ho et al. (2008) reported the presence of 527 mg of gallic acid equivalents/g extract solid for total phenolic and 163.3 CE/g extract solid for flavonoid content of clove. Aqueous extracts of clove have been shown to have very high phenolic content and strong antioxidant properties by many workers (Duodonne et al. 2009; Kim et al. 2011; Gülçin 2011).
The heat treated samples in all cases exhibited significantly higher reducing power activity indicating that using this assay, a positive effect of heat treatment on clove could be seen. With FRSA, similar results were observed, where highest increase was seen in methanolic media. TAA also showed an increase in antioxidant activity of heat treated samples. There are many studies in literature supporting similar observation, which indicate that thermal treatments improve the antioxidant potential of spices (Raj and Arulmozhi 2013; Khatun et al. 2006). Raj and Arulmozhi (2013) studied efficacy of heat treatment on the phenolic content, flavonoid content and total antioxidant capacity of clove, cinnamon, and black pepper and stated that after heat treatment antioxidant activities in all spices were retained. Khatun et al. (2006) studied effect of thermal treatment on clove and demonstrated an increase in total phenol content as well as antioxidant activity in heated sample. Polyphenols occur in foods as conjugates with proteins and polysaccharides. Non-covalent and covalent associations of polyphenols with food macromolecules are two of the most important factors affecting quality of polyphenol rich foods, in processed foods, non-covalent polyphenol macromolecule interactions are largely due to weak associations and result from a combinations of hydrogen bonds and hydrophobic interactions. They are typically released or become bioaccessible when the food is subjected to heat treatment and/or enzymic digestion. Heat treatment denatures proteins and hydrolyzes or gelatinizes starch breaking or weakening the complex facilitating a higher antioxidant activity. In addition, formation of some non-nutrient antioxidants such as Maillard’s reaction products compensate for the loss of natural antioxidants which may occur through the heat processing (Le Bourvellec and Renard 2012; Parada and Aguilera 2007).
It has been reported that the nature of extraction solvents has an effect on yield and antioxidant activity of extracted plant materials. Polyphenol contents and their antioxidant activity are said to be strongly affected by polarity of extraction solvents. Due to this reason different solvents are used for the extraction of antioxidant components from different plant materials (Anwar et al. 2010; Ghasemzadeh et al. 2011; Siddhuraju and Becker 2003; Sultana et al. 2007; Sultana et al. 2009; Turkmen et al. 2006; Wijekoon et al. 2011). Methanol and ethanol have been reported as effective solvents for extraction of phenolic compounds from various plant matrices (Anwar et al. 2010; Do et al. 2014; Siddhuraju and Becker 2003; Sultana et al. 2009; Sultana and Anwar 2008).
These observations support our results which showed that the heat treated samples of clove showed higher activity in comparison with the control sample. Since clove is one of the spices used for culinary purpose, the heat treatments have a positive effect on the antioxidant components and activities. It can be said that in different extraction media, different levels of antioxidant components were solubilized. Solvent mixed with 20 % water showed higher quantity of antioxidant component in comparison to pure solvents. The variations observed in antioxidant activities of samples extracted in different solvent could be attributed to the solubility of their antioxidant components in respective solvent.
Correlation between antioxidants components and antioxidant activity of clove in different media
Since the analysis of extraction solvents revealed the presence of significant quantities of antioxidant components, it was interesting to see whether there was a correlation between the antioxidant activity and components and the correlation coefficients (R values) are presented in Table 4. The RP assay showed positive correlation with phenols and flavonoids for all samples with R values in the range of 0.676–816. A positive correlation was also seen with FRSA for all samples (range of R value, 0.503–0.824). Tannins, though positive, exhibited a very weak association in RP assay (0.036–0.174) and medium association with FRSA (R value, 0.356–0.554). The correlation between antioxidant components and TAA showed low R values for control (0.161–0.359) and microwave heated sample (0.354–0.468). For tannins, all association with TAA was negative and for total phenols, roasted sample also showed negative R value. The results indicate better association between antioxidant activity of clove with phenols and flavonoids when assayed with RP and FRSA.
Several studies indicate that phenolic contents in plants have antioxidant properties and high amounts of phenolic compounds reveal high antioxidant capabilities in foods (Chen et al. 2001; Kumar et al. 2010). Gülçin et al. (2012) in their study on antioxidant activity of clove oil found it to be an effective antioxidant in different in vitro assays in comparison to standard antioxidant compounds such as BHA, BHT, tocopherol and trolox.
To summarize, results of the study clearly indicate that clove is a powerful antioxidant demonstrating various levels of antioxidant activity in different solvents. These activities can be attributed to their antioxidant components which showed good correlation with the activities. Heat treatments were shown to have a positive effect in liberating some of these components, thus resulting in increased antioxidant activities in the heated samples. Hence clove has the potential to be used as an antioxidant in foods in heated form and processing has a positive effect on antioxidant properties of clove.
References
- Alothman M, Bhat R, Karim AA. Antioxidant capacity and phenolic content of selected tropical fruits from Malaysia extracted with different solvents. Food Chem. 2009;115:785–788. doi: 10.1016/j.foodchem.2008.12.005. [DOI] [Google Scholar]
- Anwar F, Qayyum HMA, Hussian AI, Iqbal S. Antioxidant activity of 100 % and 80 % methanol extracts from barley seeds (Hordeum vulgare L.): stabilization of sunflower oil. Grasas Aceites. 2010;61:237–243. doi: 10.3989/gya.087409. [DOI] [Google Scholar]
- AOAC (1970). Official Methods of Analysis. 11thEdn, Association of Official Analytical Chemists, Washington DC, USA, P: 154
- Azzouz M A, Bullerman L B. Comparative antimycotic effects of selected herbs, spices, plant components and commercial antifungal agents. J Food Prot. 1982;45:1298–1301. doi: 10.4315/0362-028X-45.14.1298. [DOI] [PubMed] [Google Scholar]
- Brewer MS. Natural antioxidants: sources, compounds, mechanisms of action and potential applications. Compre Rev Food Sci Food Safety. 2011;10:221–248. doi: 10.1111/j.1541-4337.2011.00156.x. [DOI] [Google Scholar]
- Charles DJ 2013. Clove. In the book, Antioxidant properties of spices, herbs, and other sources. Springer, New York. 245–254
- Chen H, Zuo Y, Deng Y. Separation and determination of flavonoids and other phenolic compounds in cranberry juice by high performance liquid chromatography. J Chromatogr A. 2001;913:387–395. doi: 10.1016/S0021-9673(00)01030-X. [DOI] [PubMed] [Google Scholar]
- Do QD, Angkawijaya AE, Tran-Nguyen PL, Huynh LH, Soetaredjo FE, Ismadji S, Ju YH. Effect of extraction solvent on total phenol content, total flavonoids content and antioxidant activity of limnophila aromatica. J Food Drug Anal. 2014;22:296–302. doi: 10.1016/j.jfda.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duh PD, Yen GC, Yen WJ, Chang LW. Antioxidant effects of water extracts from barley (Hordeum vulgare L.) prepared under different roasting temperatures. J Agric Food Chem. 2001;49:1455–1463. doi: 10.1021/jf000882l. [DOI] [PubMed] [Google Scholar]
- Duodonne S, Vitrae X, Coutiere P, Woillez M, Merillon JM. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD and ORAC assays. J Agric Food Chem. 2009;57:1768–1774. doi: 10.1021/jf803011r. [DOI] [PubMed] [Google Scholar]
- Embuscado ME. Spices and herbs: natural sources of antioxidants – a mini review. J Funct Foods Corrected Proof Available Online Elsevier Publishers. 2015 [Google Scholar]
- Ghasemzadeh A, Jaafar HZE, Rahmat A. Effects of solvent type on phenolics and flavonoids content and antioxidant activities in two varieties of young ginger (zingiber officinale roscoe) extracts. J Med Plants Res. 2011;5(7):1147–1154. [Google Scholar]
- Gülçin İ. Antioxidant activity of eugenol: a structure activity relationship study. J Med Food. 2011;14:975–985. doi: 10.1089/jmf.2010.0197. [DOI] [PubMed] [Google Scholar]
- Gülçin İ, Elmastas M, Aboul-Enein HY. Antioxidant activity of clove oil – a powerful antioxidant source. Arab J Chem. 2012;5:489–499. doi: 10.1016/j.arabjc.2010.09.016. [DOI] [Google Scholar]
- Ho SC, Tsai TH, Tsai PJ, Lin CC. Protective capacities of certain spices against peroxynitrite-mediated biomolecular damage. Food Chem Toxicol. 2008;46:920–928. doi: 10.1016/j.fct.2007.10.028. [DOI] [PubMed] [Google Scholar]
- Ismail A, Azlan A, Khoo HE, Prasad N, Kong KW (2013) Antioxidant assays principles, methods and analysis. Universiti Putra Malaysia Serdang Malaysia 3
- Khatun M, Eguchi S, Yamaguchi T, Takamura H, Matoba T. Effect of thermal treatment on radical scavenging activity of some spices. Food Sci Technol Res. 2006;12:178–185. doi: 10.3136/fstr.12.178. [DOI] [Google Scholar]
- Kim IS, Yang MR, Lee OH, Kang SN. Antioxidant activity of hot water extracts from various spices. Int J Mol Sci. 2011;12:4120–4131. doi: 10.3390/ijms12064120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Rani A, Dixit AK, Pratap D, Bhatnagar D. A comparative assessment of total phenolic content, ferric reducing-anti-oxidative power, free radical-scavenging activity, vitamin C and isoflavones content in soybean with varying seed coat colour. Food Res Int. 2010;43:323–328. doi: 10.1016/j.foodres.2009.10.019. [DOI] [Google Scholar]
- Le Bourvellec C, Renard CMCG. Interactions between polyphenols and macromolecules: quantification methods and mechanisms. Crit Rev Food Sci Nutr. 2012;52:213–248. doi: 10.1080/10408398.2010.499808. [DOI] [PubMed] [Google Scholar]
- Lee KG, Shibamoto T. Antioxidant property of aroma extract isolated from clove buds [Syzygium aromaticum (L.) Merr. Et Perry] Food Chem. 2001;74:443–448. doi: 10.1016/S0308-8146(01)00161-3. [DOI] [Google Scholar]
- Lee SC, Kim JH, Jeong SM, Kim DR, Ha JU, Nam KC, Ahn DU. Effect of far-infrared radiation on the antioxidant activity of rice hulls. J Agric Food Chem. 2003;51:4400–4403. doi: 10.1021/jf0300285. [DOI] [PubMed] [Google Scholar]
- Matthaus B. Antioxidant activity of extracts obtained from residues of different oilseeds. J Agric Food Chem. 2002;50:3444–3452. doi: 10.1021/jf011440s. [DOI] [PubMed] [Google Scholar]
- Meda A, Lamien CE, Romito M, Millogo J, Nacoulma OG. Determination of the total phenolic, flavonoid and proline contents in burkinafasan honey as well as their radical scavenging activity. Food Chem. 2005;91:571–577. doi: 10.1016/j.foodchem.2004.10.006. [DOI] [Google Scholar]
- Michiles JA, Kevers C, Pincemail J, Defraigne JO, Dommes J. Extraction efficiency can greatly influence antioxidant capacity assays in plant food materials. Food Chem. 2012;130:986–993. doi: 10.1016/j.foodchem.2011.07.117. [DOI] [Google Scholar]
- Nanditha B, Prabhasankar P. Antioxidants in bakery products: a review. Crit Rev Food Sci Nutr. 2009;49:1–27. doi: 10.1080/10408390701764104. [DOI] [PubMed] [Google Scholar]
- Nicoli MC, Anese M, Manzocco L, Lerici CR. Antioxidant properties of coffee brews in relation to the roasting degree. LWT Food Sci Technol. 1997;30(3):292–297. doi: 10.1006/fstl.1996.0181. [DOI] [Google Scholar]
- Nikousaleh A, Prakash J. Effect of dry heat treatment of six spices on antioxidant activity of their spice extracts. Foods. 2008;2(2):139–144. [Google Scholar]
- Oyaizu M. Studies on product of browning reaction produced from glucose amine. Japan J Nut. 1986;44:307–315. doi: 10.5264/eiyogakuzashi.44.307. [DOI] [Google Scholar]
- Parada J, Aguilera JM. Food microstructure affects the bioavailability of selected nutrients. J Food Sci. 2007;72:R21–R23. doi: 10.1111/j.1750-3841.2007.00274.x. [DOI] [PubMed] [Google Scholar]
- Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem. 1999;269:337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- Raj N, Arulmozhi K. Efficacy of heat treatment on the antioxidant activity of selected spices. Int J Curr Microbiol App Sci. 2013;11:13–18. [Google Scholar]
- Rao KV (1996). Biostatistics. A Manual of statistical methods for use in health, nutrition and anthropometry. Jaypee Brothers Bangalore, India. P 64, 82, 183
- Shimada K, Fujikawa K, Yahara K, Nakamura T. Antioxidative properties of xanthine on autoxidation of soybean oil in cyclodextrin emulsion. J Agric Food Chem. 1992;40:945–948. doi: 10.1021/jf00018a005. [DOI] [Google Scholar]
- Siddhuraju P, Becker K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agro climatic origins of drumstick tree (moringa oleifera Lam.) leaves. J Agric Food Chem. 2003;51:2144–2155. doi: 10.1021/jf020444+. [DOI] [PubMed] [Google Scholar]
- Srinivasan K. Antioxidant potential of spices and their active constituents. Crit Rev Food Sci Nutr. 2014;54:352–372. doi: 10.1080/10408398.2011.585525. [DOI] [PubMed] [Google Scholar]
- Sultana B, Anwar F. Flavonols (kaempferol, quercetin, myricetin) contents of selected fruits, vegetables and medicinal plants. Food Chem. 2008;108:879–884. doi: 10.1016/j.foodchem.2007.11.053. [DOI] [PubMed] [Google Scholar]
- Sultana B, Anwar F, Przybylski R. Antioxidant activity of phenolic components present in barks of barks of Azadirachta indica, Terminalia arjuna, acacia nilotica, and Eugenia jambolana Lam. Trees. Food Chem. 2007;104:1106–1114. doi: 10.1016/j.foodchem.2007.01.019. [DOI] [Google Scholar]
- Sultana B, Anwar F, Ashraf M. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules. 2009;14:2167–2180. doi: 10.3390/molecules14062167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thippeswamy NB, Naidu KA, Achur RN. Antioxidant and antibacterial properties of phenolic extracts from carum carvi L. J Pharm Res. 2013;7:352–357. [Google Scholar]
- Turkmen N, Sari F, Velioglu YS. Effect of extraction solvents on concentration and antioxidant activity of black and black mate polyphenols determined by ferrous tartrate and folin-ciocalteu methods. Food Chem. 2006;99:838–841. doi: 10.1016/j.foodchem.2005.08.034. [DOI] [Google Scholar]
- Wijekoon MMJO, Bhat R, Karim AA. Effect of extraction solvents on the phenolic compounds and antioxidant activities of bunga katon (etlingera elatior jack.) inflorescence. J Food Compos Anal. 2011;24:615–619. doi: 10.1016/j.jfca.2010.09.018. [DOI] [Google Scholar]
- Wojdyło A, Oszmiański J, Czemerys R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007;105:940–949. doi: 10.1016/j.foodchem.2007.04.038. [DOI] [Google Scholar]