Abstract
The aim of this study was to investigate the effect of cut type and pulsed light (PL) fluence on microbiological stability and quality of fresh-cut cantaloupes. Fresh-cut cantaloupes with various cut types (cuboid, triangular prism and sphere) were treated with PL technology at 6 J/cm2. Samples were exposed to PL treatment at fluences of 2.7, 7.8, 11.7 and 15.6 J/cm2 followed by storage at 4 ± 1 °C for 28 days. Microbiological quality, headspace composition, firmness, colour, pH, titratable acidity, total soluble solids, total phenolic content and ascorbic acid content of fresh-cut cantaloupes were determined. Spherical shape was found to be the most suitable shape for PL treatment of fresh-cut cantaloupes due to its significantly lowest (p ≤ 0.05) microbial counts before and after the PL treatment. No significant (p > 0.05) effect was observed for firmness, colour, total soluble solids and total phenolic content of fresh-cut cantaloupes throughout the storage study. Pulsed light treatment using 7.8 J/cm2 was the best for extending shelf life of fresh-cut cantaloupes with extension of 8 days longer at 4 ± 1 °C compared to the control while maintaining the ascorbic acid content. In conclusion, PL treatment is a potential technique for extending the shelf life of fresh-cut cantaloupes by inactivating microorganisms without compromising the nutritional value.
Keywords: Pulsed light, Fresh-cut cantaloupe, Cut type, Microbiological stability, Non-thermal
Introduction
Fresh-cut processing has gained an increase in popularity as it provides convenient and healthful fruits and vegetables while retaining freshness and nutrients of the non-processed products (Ramos-Villarroel et al. 2011). Fresh-cut fruit processing involves preparation processes such as peeling, slicing and/or cutting which increases the rate of respiration and biochemical reactions in fruit and resulted in negative effects on the quality and safety of the product (Soliva-Fortuny et al. 2001). Therefore, an appropriate technique for prolonging shelf life without detrimental effects on quality of fresh-cut products would be beneficial for consumer and producer.
Production of fresh-cut melons is of great interest due to its desirable sensorial attributes and difficulties for direct consumption because of large dimension and preparation required prior to eating (Amaro et al. 2012). Cantaloupe is popular mainly due to its sweet pulp and pleasant aroma. The fruits contain high amount of vitamin C and beta-carotene and provide potassium, iron and dietary fibre (Beaulieu and Lea 2007). However, cantaloupes are susceptible to food safety risk due to its neutral pH of flesh and netting on the rind that may harbour pathogens. As a result, fresh-cut cantaloupes are stored at <5 °C to control the microbial growth (FDA 2007). Besides, sanitation techniques can be applied to enhance safety of intact or cut cantaloupes.
Preservation technique for extending the shelf life of fresh-cut products is limited. Modified atmosphere packaging and refrigeration are the techniques used widely to extend shelf life of fresh-cut products but highly depends on the initial microbial loads and quality of the raw materials (Manzocco et al. 2011). Chemical treatments are also used by dipping fresh-cut products in chlorine-based chemicals, such as liquid chlorine, hypochlorite and chlorine dioxide. However, it was reported that the use of chlorine could form carcinogenic disinfection by-products which are harmful (Cao et al. 2010). Generally, microbial and enzymatic deterioration of fresh-cut products happens on the surface due to injury caused by cutting while the internal of the products is sterile (Manzocco et al. 2011). Due to these reasons, PL treatment, a surface decontamination technique without applying chemicals is suitable to increase safety of minimally processed products while maintaining its quality.
Pulsed light treatment is a technique used to inactivate microorganisms on surfaces by utilising intense pulses of broad spectrum in short duration. The light generated has broad spectrum wavelengths ranging from 100 to 1100 nm which consists of UV (100–400 nm), visible light (400–700 nm) and infrared (700–1100 nm) (Oms-Oliu et al. 2010). Lethal effect on microorganisms by PL treatment is contributed by UV-C part of the spectrum (200–280 nm) with mechanisms involving chemical modification and cleavage of DNA, protein denaturation and cellular materials alteration. According to Gómez-López et al. (2007), vegetables and fruits are appropriate for this technique.
Application of PL treatment on fresh-cut fruits is still limited. Pulsed light application at 8 J/cm2 was reported to maintain physical and nutritional qualities of fresh-cut mangoes (Charles et al. 2013). On the other hand, PL treatment at 12 J/cm2 reduced growth of Escherichia coli and Listeria innocua in fresh-cut avocado and watermelon but resulted in browning and softening (Ramos-Villarroel et al. 2011, 2012). As mentioned earlier, quality and safety of fresh-cut fruit can be negatively affected during fresh-cut processing (Soliva-Fortuny et al. 2001). Moreover, different serovars of Salmonella and Listeria were linked to foodborne outbreaks due to consumption of cantaloupe, occurred from 2008 to 2012 (CDC 2015). These issues suggested the need of applying a preservation technique such as PL to ensure the safety of fresh-cut cantaloupes for consumption while retaining its quality during storage. Therefore, the objective of this study was to investigate the effect of cut type and PL fluences on microbiological stability and quality changes of fresh-cut cantaloupes.
Materials and methods
Fruit preparation
Cantaloupes (Cucumis melo L. reticulatus cv. Glamour) at commercial maturity were harvested from a commercial farm and stored at 5 °C for 24–48 h. Fruits were selected to ensure uniformity in size and peel colouration. The cantaloupes and cutting utensils were washed with running potable water to remove foreign materials. The fruits were then halved using a sharp knife and the seeds were removed. Then, the fruits were cut into specific dimensions (cuboid, triangular prism and sphere). Approximately 150 g of fresh-cut cantaloupes was packed into polypropylene film (thickness of ~40 μm) and sealed before PL treatment.
Apparatus
Pulsed light treatment was carried out using the Steribeam SBS XeMatic-2 L-A system (SteriBeam Systems GmbH, Baden-Württemberg, Germany) composed of two lamps, one above and another below a quartz table that placed at the center to hold samples. The distance between the lamp and sample shelf is 10 cm. The emitted spectrum has wavelengths ranged from 180 nm to 1100 nm. The total fluence per pulse emitted is 0.3 J/cm2.
Pulsed light treatment
Effect of cut type
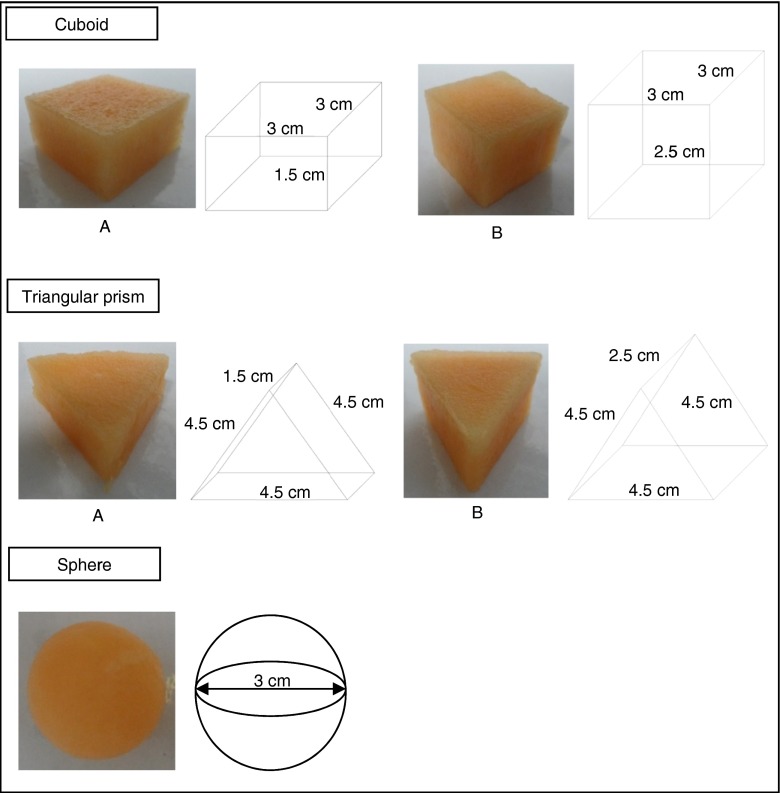
Cantaloupes were cut into shapes of cuboid, triangular prism and sphere as shown in Fig. 1. The surface area (A), volume (V) and A/V ratio were calculated based on the dimensions of each shape. The packages of fresh-cut cantaloupes were treated with PL at 6.0 J/cm2.
Fig. 1.
Cut type of fresh-cut cantaloupes
Effect of PL fluences
The packages of fresh-cut cantaloupes were exposed to PL. The number of pulses used were 9, 26, 39 and 52 with the respective fluences of 2.7, 7.8, 11.7 and 15.6 J/cm2.
Storage study
For storage study, sphere was selected as the shape for fresh-cut cantaloupes. Samples were randomly withdrawn for analysis at every 4 d interval for analysis up to 28 d of storage. Microbiological analysis, headspace composition determination, physical analysis (firmness and colour) and chemical analysis (pH, total soluble solids, titratable acidity, total phenolic content and ascorbic acid content) were conducted. Results were compared with a set of untreated samples.
Methods of analysis
Microbiological analysis
Microbiological analysis of cantaloupe pieces were performed by determining the total aerobic mesophilic microorganisms and yeast and mould populations based on the methods mentioned by Gómez et al. (2012). A piece of sample approximately 13–15 g was homogenised for 2 min with 0.1 % sterile peptone water in a ratio of 1:10 (sample:peptone water, w/v) using a BagMixer 400 stomacher lab blender (Interscience, Saint-Germain-en-Laye, France) under sterile conditions. Serial dilutions of the homogenates were plated onto plate count agar and incubated for 48 h at 37 ± 1 °C. Potato dextrose agar were used for yeast and mould count with incubation at 25 ± 1 °C for 5 days. The results were expressed in log cfu/g.
Headspace composition
The gas composition was measured using a 6600 headspace oxygen/carbon dioxide analyser (Illinois Instruments Inc., Illinois, US) by inserting the sampling needle into the packages. Percentages of O2 and CO2 were recorded when the readings were stabilised.
Physical analysis
Water activity
Water activity on the surface of cantaloupes was determined using AquaLab Series 3 TE water activity analyser (Decagon Devices, Inc., Washington, USA).
Firmness
Firmness of cantaloupes was measured using a TX-XT2i texture analyser (Stable Micro Systems Ltd., Surrey, UK). Settings used were set according to Munira et al. (2013) with some modifications. Cantaloupes were punctured by a 2 mm cylindrical probe with settings of pre-test speed at 1.50 mm/s, test speed at 1.00 mm/s, post-test speed at 10.00 mm/s, distance at 30 % strain and trigger force at 25 g. Firmness was expressed as the maximum load in Newtons (N).
Colour
Surface colour of cantaloupe pieces was determined using a Minolta Chroma Meter CR-300 (Konica Minolta, Inc., Tokyo, Japan). Values of L*(lightness), a*(green-red chromaticity) and b* (blue-yellow chromaticity) were recorded to calculate whiteness index (WI), chromaticity (C*) and Hue angle (h°) based on the following formulas.
Chemical analysis
Chemical properties
Cantaloupe pieces were blended using a Waring HGBTWTS3 blender (Waring Commercial, Connecticut, USA) and the pH of the juice was measured using a digital pH meter FE20 (Mettler-Toledo AG, Canton of Zurich, Switzerland) according to AOAC method 981.12 (AOAC 1990). Total soluble solids were measured using an Atago PR-101 digital refractometer (Atago Co., Ltd., Tokyo, Japan) and expressed in °Brix. Titratable acidity was determined using titration method according to AOAC method 942.15 (AOAC 1990). The results were expressed as percentage of citric acid.
Total phenolic content
Total phenolic content was determined according to Folin-Ciocalteu procedure (Fu et al. 2011). The cantaloupes were blended into slurry. The blended sample (1.00 g) was extracted using 9 mL of 50 % ethanol at room temperature for 30 min with a Harmony HTS-1003 hotplate stirrer (LMS Co., Ltd., Tokyo, Japan). The sample was then centrifuged at 4200 g for 30 min. A 0.50 mL of the diluted sample was mixed with 2.5 mL of 1:10 diluted Folin-Ciocalteu reagent. After 4 min, 2 mL of 75 g/L sodium carbonate solution were added. The mixture was incubated for 2 h at room temperature and the absorbance was measured using a Thermo Scientific G10S UV-Vis spectrophotometer (Thermo Fisher Scientific, Wisconsin, USA) at 760 nm. Gallic acid was used as a reference standard and the results were expressed in milligram gallic acid equivalents (mg GAE)/100 g wet weight of cantaloupes.
Ascorbic acid content
Ascorbic acid content was determined using 2,6-dichloroindophenol titration (967.21) (AOAC 1990). The results were expressed in mg/100 g.
Statistical analysis
All the treatments were performed in triplicate. For each treatment, sample analysis were repeated three times. The data was analysed using Minitab (v 16.0) based on analysis of variance and expressed as mean value ± standard deviation. The confidence level for statistical significance was set at a probability value of 0.05. Tukey’s test was used to determine significant difference of the data.
Results and discussion
Effect of cut type
Microbiological analysis
Table 1 shows the cut type of fresh-cut and its effect on microbial counts of fresh-cut cantaloupes treated with PL. In term of the cut type, sphere samples had a significantly lower (p ≤ 0.05) total plate count and yeast and mould count for both treated (1.43 log cfu/g) and untreated (2.82 and 2.88 log cfu/g, respectively) samples compared to cuboid and triangular prism. This was related to the A/V ratio of the sample. According to Danyen et al. (2011), higher A/V ratio would cause more wounds on the product which will result in higher rate of enzymatic hydrolysis of cell wall, leading to increase in microbial growth due to more electrolyte leakage. As a result, sphere with lowest A/V ratio had significantly lower microbial counts (p ≤ 0.05). Although A/V ratios of sphere and cuboid B were similar, the microbial counts of untreated sphere were significantly lower (p ≤ 0.05) compared to that of untreated cuboid B. This suggested that cutting method also played an important role on the quality of fresh-cut cantaloupes. Since more cutting steps were required to obtain fresh-cut cantaloupes with shapes of cuboid and triangular prism that sphere, this could lead to extra bruises to the fruits. Danyen et al. (2011) suggested that the tissue damage might enhance ethylene production which will increase activities of enzymes in hydrolysing cell walls of fruit tissues and thus results in more electrolyte leakage that favours microbial growth. This observation was similar to that observed by Argañosa et al. (2008) whereby papaya spheres (1.55 cm radius) had better physicochemical and microbiological properties than cube, parallelepiped and cylinder cuts. Based on Table 1, all treated samples had significantly lower (p ≤ 0.05) total plate count compared to untreated samples. The same trend was observed for yeast and mould count. This proved that PL is a potential decontamination technique in inactivating microorganisms. The percentages of microbial inactivation for sphere were higher (49.29 % and 50.35 % for total plate count and yeast and mould count, respectively) than that of cuboid A, cuboid B, triangular prism A and triangular prism B, which in the ranges of 25.41–40.78 % and 21.36–45.91 % for total plate count and yeast and mould count, respectively. This could be due to the reduced scattering of light around the edges of sphere samples. Kim et al. (2010) suggested that spherical or even conical shape was better than cubic or cylindrical cuts for irradiation due to non-uniformity of dose distribution around the product. Moreover, the significantly higher (p ≤ 0.05) initial microbial load of cuboid and triangular prism compared to sphere could lead to shielding effect among the microbial cells which protect them from light and thus affect the PL efficiency in inactivating the microorganisms (Gómez-López et al. 2007). In addition, PL-treated cantaloupe sphere had significantly lower (p ≤ 0.05) microbial count compared to cuboid and triangular prism (Table 1). As a consequence, sphere shape was selected as the appropriate shape for PL treatment in the subsequent study to investigate the effect of PL fluences on microbiological stability and quality changes of fresh-cut cantaloupes under refrigerated storage. The total plate count and yeast and mould count were not significant different for fresh-cut cantaloupes with shapes of cuboid (height of 1.50 and 2.50 cm) and triangular prism (length of 1.50 and 2.50 cm), respectively (Table 1). As PL penetration is restricted to the surface of product, the decontamination efficiency of PL became insignificant when two thick samples (1.5 and 2.5 cm) were compared.
Table 1.
Cut type of fresh-cut cantaloupes and its effect on total plate count and yeast and mould count of fresh-cut cantaloupes treated with pulsed light
| Cut type | Cuboid | Triangular prism | Sphere | ||
|---|---|---|---|---|---|
| A | B | A | B | ||
| Cut Type | |||||
| Side (cm) | 3.00 | 3.00 | 4.50 | 4.50 | - |
| Height (cm) | 1.50 | 2.50 | 1.50 | 2.50 | - |
| Diameter (cm) | - | - | - | - | 3.00 |
| Surface area, A (cm2) | 36.00 | 48.00 | 37.79 | 51.29 | 28.27 |
| Volume, V (cm3) | 13.50 | 22.50 | 13.15 | 21.92 | 14.14 |
| A/V (cm−1) | 2.67 | 2.13 | 2.87 | 2.34 | 2.00 |
| Total Plate Count | |||||
| Untreated sample (log cfu/g) | 3.85 ± 0.28Aa | 4.05 ± 0.21Aa | 3.98 ± 0.08Aa | 3.66 ± 0.17Aa | 2.82 ± 0.25Ab |
| Treated sample (log cfu/g) | 2.28 ± 0.12Ba | 2.61 ± 0.38Ba | 2.72 ± 0.13Ba | 2.73 ± 0.08Ba | 1.43 ± 0.49Bb |
| Yeast and Mould Count | |||||
| Untreated sample (log cfu/g) | 3.42 ± 0.12Aab | 3.71 ± 0.13Aa | 3.70 ± 0.22Aa | 3.23 ± 0.31Aab | 2.88 ± 0.36Ab |
| Treated sample (log cfu/g) | 1.85 ± 0.60Ba | 2.17 ± 0.20Ba | 2.61 ± 0.25Ba | 2.54 ± 0.14Ba | 1.43 ± 0.49Bb |
*Different lowercase letters indicate significant differences within the same row (p ≤ 0.05)
*Different uppercase letters indicate significant differences within the same column (p ≤ 0.05)
Effect of fluences
Microbiological analysis
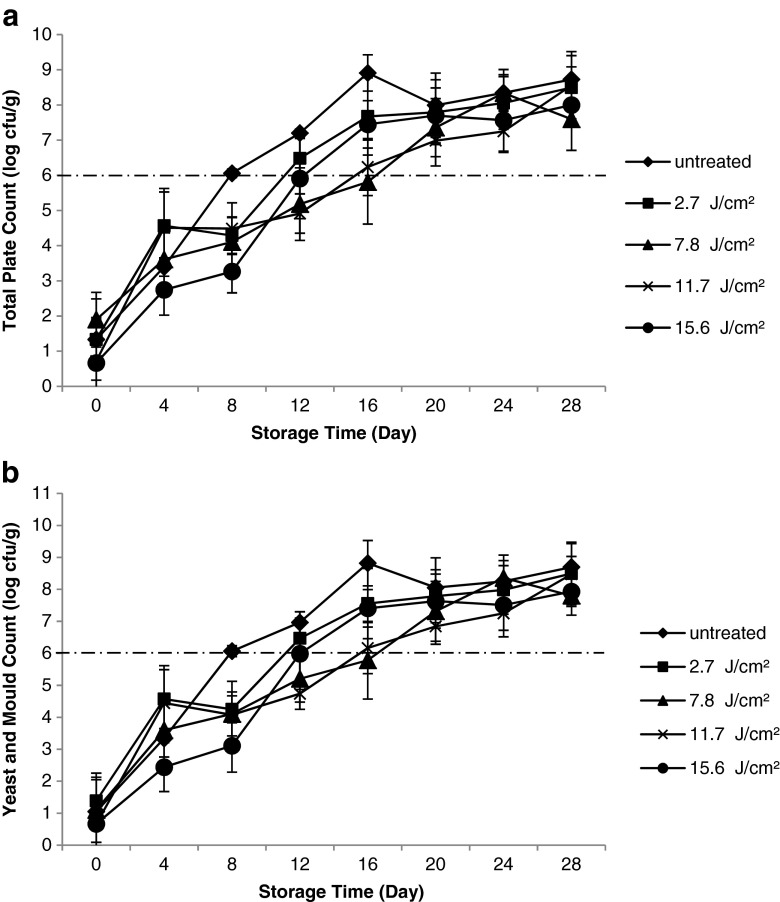
High pH and total sugar in cantaloupes promote microbial growth during storage (Munira et al. 2013). The findings of this study showed that PL treatment had significant lethal effect (p ≤ 0.05) against aerobic mesophilic microorganisms and yeast and moulds at higher fluences (7.8 and 11.7 J/cm2). Figure 2 showed that fresh-cut cantaloupes had highest microbiological stability at these fluences because the total plate count (5.81 and 6.24 log cfu/g for 7.8 and 11.7 J/cm2, respectively) and yeast and mould count (5.78 and 6.16 log cfu/g for 7.8 and 11.7 J/cm2, respectively) were lowest on Day 16. The microbial growth at these fluences increased after 16 days of storage.
Fig. 2.
Effect of fluences on microbial stability (a total plate count; b yeast and mould count) of fresh-cut cantaloupes treated with pulsed light
According to the Institute of Food Science and Technology, the microbial limit for minimally processed fruit and vegetable is 6 log cfu/g (IFST 1999), as indicated by the dotted lines in Fig. 2. Untreated fresh-cut cantaloupes reached this limit on day 8 when stored at 4 ± 1 °C. This finding indicated that PL treatment could inactivate microbial growth hence extended the shelf life of treated sample for 8 d longer relative to untreated fresh-cut cantaloupes. Water activity is an important factor for microbial growth. The water activity of untreated cantaloupe (0.95 ± 0.01) was found to be insignificant different compared to all the treated samples (0.95–0.96). This implies that the microbial inactivation on fresh-cut cantaloupes was mainly contributed by effect of PL on microorganisms. Treatment with PL causes sublethal damages to the microbe making the cells to be more sensitive in the subsequent stress such as low temperature storage (Lasagabaster and de Marañón 2014). However, no significant difference (p > 0.05) was observed for samples treated with 2.7 and 15.6 J/cm2 and the untreated sample throughout the storage. When 2.7 J/cm2 was applied, the light intensity was insufficient to inactivate the microorganisms. The light intensity decreases as absorption and scattering were reduced when the light travels through the samples (Abida et al. 2014). When PL fluence was increased to 15.6 J/cm2, no further microbial inactivation was observed even though the lowest microbial counts were noticed on day 8 compared to other fluences and untreated sample. Local surface roughness due to cutting technique during processing could protect the microorganisms and thus affects the lethal effect of PL (Ignat et al. 2014). Based on microscopic analysis shown by Chimbombi et al. (2013), bacterial internalisation in the fresh-cut cantaloupes was due to intercellular air spaces. Furthermore, growth of microbes throughout the storage could lead to shielding effect among the microbial cells which protect them from light (Gómez-López et al. 2007). Similarly, Ignat et al. (2014) reported that no further inactivation for total viable count and yeast when fresh-cut apple was treated by PL at higher fluence up to 15.75 J/cm2.
Headspace composition
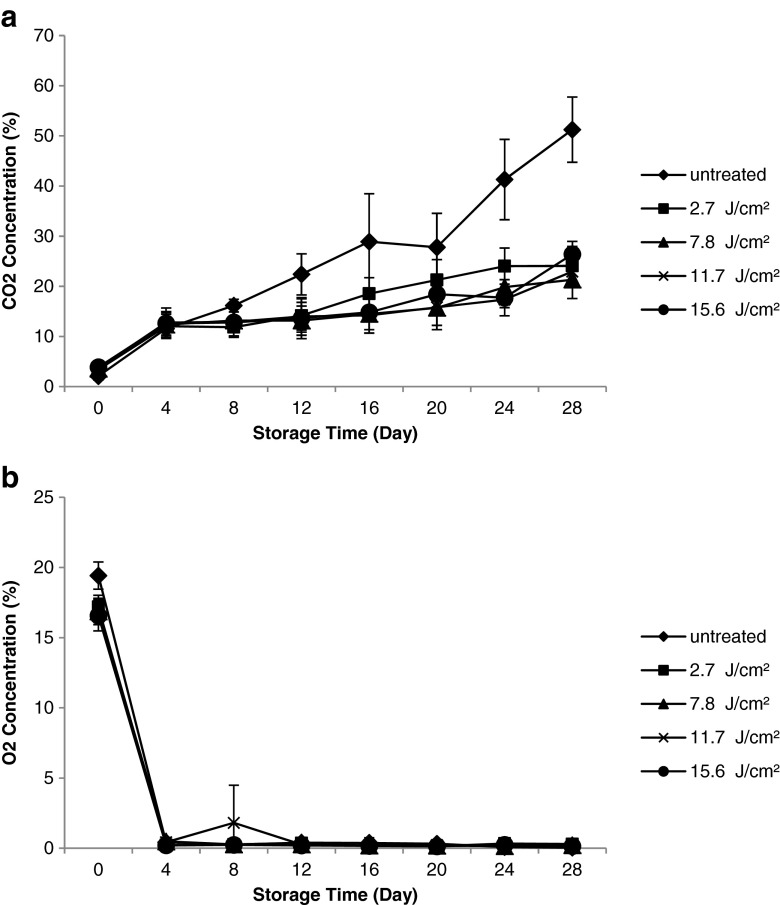
In Fig. 3, high CO2 production and sudden decrease in O2 on the fourth day of storage was due to high respiration rate of fresh-cut cantaloupes accounting for metabolism acceleration as a result of tissue wounding during cutting process (Amaro et al. 2012). The O2 consumption and CO2 production in fresh-cut cantaloupes during storage was attributed to tissue respiration and respiration of native flora (Ramos-Villarroel et al. 2014). PL had significant effect (p ≤ 0.05) on CO2 production throughout the storage. The higher rate of CO2 production in untreated fresh-cut cantaloupes during storage was due to greater proliferation of microbes compared to the treated ones. This difference was even significant (p ≤ 0.05) on day 16 until the end of storage. In contrary, fresh-cut avocado and watermelon exposed to PL treatment at 6 and 12 J/cm2 had significantly higher (p ≤ 0.05) CO2 production throughout the storage of 15 d compared to untreated samples (Ramos-Villarroel et al. 2011, 2012). Ramos-Villarroel et al. (2012) explained that PL treatment stimulates respiration of tissues leading to an increase in CO2 production during storage. However, the lower CO2 production observed for treated fresh-cut cantaloupes suggested that the effect of PL treatment on microbial inactivation was greater than stimulation of tissue respiration in this study. In this study, no significant effect (p > 0.05) in respiration was observed for PL-treated fresh-cut cantaloupes with increasing fluences. The increased CO2 and reduced O2 concentration in the sample packages may allow growth of anaerobes such as Clostridium botulinum and botulinal toxin production. However, it was found that Clostridium botulinum is a poor competitor in raw or minimally processed foods. In addition, botulinal toxin was reported to be absent in cantaloupe samples stored at 7 °C (Larson and Johnson 1999).
Fig. 3.
Effect of fluences on headspace composition (a CO2 concentration; b O2 concentration) of fresh-cut cantaloupes treated with pulsed light
Physical analysis
Firmness
As presented in Table 2, firmness of fresh-cut cantaloupes was retained in the range of 1.49–2.08 N throughout the storage and was not significantly (p > 0.05) affected by PL treatment at the fluences used in this work. The insignificant alteration (p > 0.05) in texture of samples stored at 4 °C denoted that little or no senescence or cell degradation had occurred. Texture was claimed to be affected due to energy absorption in the UV range by membrane components (phospholipids and glycolipids) and cell wall components (proteins and lignin) and oxidative stress caused by released of reactive oxygen species by UV-C (Ramos-Villarroel et al. 2014). In contrary, the firmness retention for PL-treated fresh-cut cantaloupes in this research could be due to the diameter (~3 cm) of fresh-cut cantaloupe pieces used in this study which restricts PL penetration to the inner part of product. The firmness of PL-treated fresh-cut cantaloupes (1.65–2.08 N) was slightly higher compared to that of untreated (1.49–1.88 N) during storage, however no significant different (p > 0.05) was observed. Charles et al. (2013) suggested that UV-C treatment could result in higher levels of polyamines. Similar to calcium, polyamines form cation cross-links with pectic acid limiting the accessibility of the cell wall to degradative enzymes. In addition, photothermal effect of PL could result in development of a thin dried film on the surface of fruit that helped to protect against physiological degradation (Charles et al. 2013). Charles et al. (2013) also discovered maintenance of firmness in fresh-cut mango cubes treated with PL fluence of 8 J/cm2 during 7 days of storage.
Table 2.
Effect of fluences on firmness (expressed in N) of fresh-cut cantaloupes treated with pulsed light
| Storage time (day) | Untreated | Firmness at different fluences (N) | |||
|---|---|---|---|---|---|
| 2.7 J/cm2 | 7.8 J/cm2 | 11.7 J/cm2 | 15.6 J/cm2 | ||
| 0 | 1.88 ± 0.19Aa | 1.88 ± 0.10Aa | 1.98 ± 0.23Aa | 1.93 ± 0.19Aa | 2.08 ± 0.22Aa |
| 4 | 1.61 ± 0.41Aa | 1.93 ± 0.26Aa | 1.95 ± 0.14Aa | 1.91 ± 0.32Aa | 2.08 ± 0.48Aa |
| 8 | 1.61 ± 0.24Aa | 1.84 ± 0.14Aa | 1.90 ± 0.46Aa | 1.98 ± 0.29Aa | 1.97 ± 0.26Aa |
| 12 | 1.69 ± 0.33Aa | 1.81 ± 0.15Aa | 1.82 ± 0.26Aa | 1.98 ± 0.24Aa | 1.99 ± 0.50Aa |
| 16 | 1.58 ± 0.57Aa | 1.65 ± 0.05Aa | 1.98 ± 0.58Aa | 1.77 ± 0.43Aa | 1.88 ± 0.25Aa |
| 20 | 1.49 ± 0.41Aa | 1.83 ± 0.26Aa | 1.85 ± 0.10Aa | 1.99 ± 0.35Aa | 1.85 ± 0.32Aa |
| 24 | 1.73 ± 0.50Aa | 1.72 ± 0.11Aa | 2.05 ± 0.40Aa | 1.91 ± 0.31Aa | 1.78 ± 0.09Aa |
| 28 | 1.78 ± 0.38Aa | 1.88 ± 0.33Aa | 2.07 ± 0.30Aa | 1.80 ± 0.12Aa | 1.93 ± 0.43Aa |
*Different lowercase letters indicate significant differences within the same row (p ≤ 0.05)
*Different uppercase letters indicate significant differences within the same column (p ≤ 0.05)
Colour
The colour of fresh-cut cantaloupes was maintained well among different treatments without significant difference (p > 0.05) throughout the storage at 4 ± 1 °C. In Table 3, the colour parameters remained in the range of 64.56–68.01, 48.92–54.72, 30.24–34.98 and 75.16–77.23 for lightness, whiteness index, chromaticity and hue angle respectively for both untreated and treated fresh-cut cantaloupes. This indicated that PL did not cause negative effect on the colour of fresh-cut cantaloupes under refrigerated storage compared to the control and fresh cantaloupes. Good maintenance of quality in term of colour and firmness during storage could be contributed by the fruit initial quality, pre-cooling before processing, sharpness of utensils used for cutting process and application of strict temperature control. As with firmness, the surface treatment of PL could have minimal negative effect on thick (~3 cm) samples used in this research. In addition, it was reported that cantaloupes had low polyphenoloxidase (PPO) activity and the absence of oxidisable phenolic compounds indicated that enzymatic browning is unlikely to be the deterioration factor for fresh-cut cantaloupes (Lamikanra et al. 2000). Browning occurs when decompartmentation process permits the contact of phenolic compounds with PPO, an oxidation enzyme leading to formation of quinones that subsequently convert to melanins and brown pigment. Lamikanra et al. (2000) also reported that there was no observable colour changes in fresh-cut cantaloupes stored at low temperatures regardless of treatments. Maintenance of colour in fresh-cut cantaloupes in this study correlated well with total phenolic content, which seems to be the limiting factor of discolouration process. Thermal damage caused by PL seemed insignificant to cause non-enzymatic browning in this study as no discolouration of fresh-cut cantaloupes was detected.
Table 3.
Effect of fluences on colour parameters of fresh-cut cantaloupes treated with pulsed light
| Storage time (day) | Untreated | Fluences (J/cm2) | |||
|---|---|---|---|---|---|
| 2.7 | 7.8 | 11.7 | 15.6 | ||
| Lightness | |||||
| 0 | 66.82 ± 4.16Aa | 64.56 ± 4.75Aa | 65.68 ± 2.44Aa | 65.12 ± 3.01Aa | 65.34 ± 3.30Aa |
| 4 | 67.03 ± 3.45Aa | 65.47 ± 2.61Aa | 67.92 ± 2.58Aa | 66.13 ± 3.74Aa | 66.89 ± 3.40Aa |
| 8 | 64.68 ± 2.63Aa | 65.91 ± 2.52Aa | 65.89 ± 2.30Aa | 65.64 ± 2.02Aa | 67.68 ± 2.83Aa |
| 12 | 65.34 ± 2.15Aa | 64.89 ± 2.67Aa | 68.01 ± 2.13Aa | 66.38 ± 4.27Aa | 66.01 ± 3.22Aa |
| 16 | 65.18 ± 2.08Aa | 67.55 ± 3.26Aa | 67.57 ± 2.07Aa | 65.56 ± 3.05Aa | 66.24 ± 3.74Aa |
| 20 | 65.80 ± 2.31Aa | 65.18 ± 1.78Aa | 66.17 ± 1.93Aa | 66.54 ± 2.82Aa | 66.21 ± 2.19Aa |
| 24 | 62.46 ± 1.59Aa | 67.08 ± 2.12Aa | 66.19 ± 0.94Aa | 65.69 ± 3.57Aa | 65.72 ± 3.03Aa |
| 28 | 65.28 ± 2.13Aa | 67.02 ± 3.13Aa | 66.62 ± 2.59Aa | 64.97 ± 1.74Aa | 66.41 ± 4.36Aa |
| Whiteness Index | |||||
| 0 | 51.62 ± 3.88Aa | 52.63 ± 4.85Aa | 53.14 ± 2.57Aa | 53.73 ± 2.11Aa | 52.16 ± 2.16Aa |
| 4 | 51.85 ± 3.42Aa | 51.92 ± 2.60Aa | 54.20 ± 3.72Aa | 53.00 ± 3.18Aa | 53.65 ± 3.23Aa |
| 8 | 50.86 ± 2.03Aa | 52.01 ± 3.00Aa | 52.83 ± 2.05Aa | 52.67 ± 2.25Aa | 54.72 ± 2.78Aa |
| 12 | 51.89 ± 2.82Aa | 51.87 ± 3.61Aa | 54.09 ± 1.25Aa | 52.70 ± 3.64Aa | 52.94 ± 2.59Aa |
| 16 | 50.72 ± 1.84Aa | 53.62 ± 4.18Aa | 53.78 ± 2.20Aa | 53.29 ± 2.88Aa | 53.56 ± 3.26Aa |
| 20 | 51.54 ± 1.60Aa | 51.55 ± 2.38Aa | 52.81 ± 2.31Aa | 53.28 ± 2.79Aa | 52.45 ± 2.03Aa |
| 24 | 48.92 ± 0.80Aa | 53.42 ± 2.06Aa | 51.89 ± 1.02Aa | 52.33 ± 2.45Aa | 52.80 ± 2.95Aa |
| 28 | 51.22 ± 2.14Aa | 52.84 ± 3.69Aa | 51.73 ± 2.68Aa | 51.45 ± 1.38Aa | 51.97 ± 4.77Aa |
| Chromaticity | |||||
| 0 | 34.87 ± 1.41Aa | 31.37 ± 2.09Aa | 31.84 ± 1.20Aa | 30.24 ± 1.20Aa | 32.86 ± 1.44Aa |
| 4 | 34.98 ± 1.43Aa | 33.41 ± 1.22Aa | 32.61 ± 2.81Aa | 32.50 ± 0.92ABa | 32.37 ± 1.30Aa |
| 8 | 34.07 ± 0.89Aa | 33.68 ± 2.46Aa | 32.52 ± 0.63Aa | 32.46 ± 1.23ABa | 31.63 ± 1.37Aa |
| 12 | 33.28 ± 2.23Aa | 32.89 ± 2.69Aa | 32.87 ± 0.77Aa | 33.12 ± 1.00ABa | 32.48 ± 0.55Aa |
| 16 | 34.71 ± 2.46Aa | 33.11 ± 2.68Aa | 32.89 ± 1.10Aa | 31.51 ± 1.53ABa | 31.76 ± 1.11Aa |
| 20 | 34.27 ± 0.19Aa | 33.68 ± 1.64Aa | 32.89 ± 1.49Aa | 32.55 ± 1.10ABa | 33.39 ± 0.66Aa |
| 24 | 34.60 ± 0.65Aa | 32.86 ± 2.00Aa | 34.20 ± 0.61Aa | 32.99 ± 0.93ABa | 32.40 ± 1.29Aa |
| 28 | 34.22 ± 1.35Aa | 33.66 ± 2.10Aa | 34.82 ± 1.45Aa | 33.55 ± 0.21Ba | 34.22 ± 2.87Aa |
| Hue Angle | |||||
| 0 | 76.74 ± 2.20Aa | 78.25 ± 3.34Aa | 77.12 ± 2.01Aa | 77.18 ± 1.63Aa | 76.51 ± 1.29Aa |
| 4 | 76.90 ± 1.74Aa | 76.37 ± 2.07Aa | 77.23 ± 2.18Aa | 75.99 ± 2.65Aa | 76.38 ± 2.10Aa |
| 8 | 76.49 ± 1.74Aa | 76.62 ± 2.53Aa | 76.20 ± 1.84Aa | 75.54 ± 1.95Aa | 76.38 ± 2.18Aa |
| 12 | 75.69 ± 0.28Aa | 76.70 ± 2.92Aa | 77.23 ± 1.42Aa | 75.94 ± 1.85Aa | 76.06 ± 1.52Aa |
| 16 | 76.57 ± 1.19Aa | 76.79 ± 3.58Aa | 75.88 ± 2.19Aa | 75.84 ± 2.34Aa | 76.09 ± 2.09Aa |
| 20 | 76.31 ± 0.73Aa | 76.29 ± 2.14Aa | 76.52 ± 2.32Aa | 76.41 ± 2.64Aa | 75.47 ± 0.46Aa |
| 24 | 76.21 ± 0.53Aa | 76.35 ± 1.68Aa | 75.81 ± 1.39Aa | 76.33 ± 2.34Aa | 76.33 ± 1.84Aa |
| 28 | 76.30 ± 0.57Aa | 75.63 ± 3.09Aa | 75.49 ± 1.85Aa | 75.16 ± 1.19Aa | 75.33 ± 2.09Aa |
*Different lowercase letters indicate significant differences within the same row (p ≤ 0.05)
*Different uppercase letters indicate significant differences within the same column (p ≤ 0.05)
Chemical analysis
pH, titratable acidity and total soluble solids
Initial ranges of pH and titratable acidity of fresh-cut cantaloupes were 6.63–6.96 and 0.07–0.10 % citric acid, respectively. Based on Table 4, decrease in pH for untreated fresh-cut cantaloupes was significantly sooner (p ≤ 0.05) than that treated with PL as observed on day 8. In terms of storage effect, the decrease in pH continued for 2.7 and 15.6 J/cm2 without significant difference (p > 0.05) compared to the untreated fresh-cut cantaloupes in the subsequent storage whereas 7.8 and 11.7 J/cm2 showed important maintenance of pH with values of 6.56 and 6.65, respectively until 24 days of storage. Although significantly lower in (p ≤ 0.05) titratable acidity was only observed in the end of the storage for fresh-cut cantaloupes treated with 7.8, 11.7 and 15.6 J/cm2, the titratable acidity of fresh-cut cantaloupes generally corresponded to the pH reported in this study. The decrease in pH and increase in acidity during storage of fresh-cut cantaloupes seemed to correlate well with microbial growth and production of CO2. Untreated fresh-cut cantaloupes that had high microbial growth followed by high CO2 production demonstrated a faster rate of decrease in pH and increase in acidity during the storage period. On day 8, the pH for untreated fresh-cut cantaloupes (5.83) started to decrease while the pH for treated fresh-cut cantaloupes (6.82–6.88) remained significantly higher (p ≤ 0.05) than that of untreated samples. Among the treated fresh-cut cantaloupes, although insignificant pH and titratable acidity (p > 0.05) was observed between the fluences, the pH of fresh-cut cantaloupes treated with 7.8 and 11.7 J/cm2 generally remained high while the acidity remained low up to 24 days of storage due to its lower microbial growth compared to that treated with 2.7 and 15.6 J/cm2. In general, the pH tend to increase starting from day 4 and then began to decrease on day 12 for all the treated fresh-cut cantaloupes. The increase in pH could be due to acids metabolism during respiration (Danyen et al. 2011). During respiration, complex substrate molecules including organic acids in plant cells are broken down into simpler molecules such as CO2 and H2O (Saltveit 2002). Moreover, utilisation of organic acids increases as the microbial loads increase. On the other hand, the decrease in pH was due to the increase in microbial growth during further storage period. Salama et al. (1995) indicated that cantaloupes may be contaminated by Lactococcus lactis ssp. Lactis from field before harvest. As mentioned earlier, increase in O2 uptake and CO2 production tend to give rise to anaerobic conditions which induces the growth of Lactococcus lactis that produces energy principally through anaerobic glycolysis. All the strains ferment glucose and fructose and some strains ferment sucrose in the fruit causing production of lactic acid (Lamikanra et al. 2000). Hence, the production of lactic acid could explain the decrease in pH and increase in acidity of fresh-cut cantaloupes observed in this study. In addition, high levels of CO2 can acidify cytoplasm and dissolve in the water of the tissues resulting a decrease in the pH of the medium (Pintó et al. 2001).
Table 4.
Effect of fluences on pH, titratable acidity and total soluble solids of fresh-cut cantaloupes treated with pulsed light
| Storage time (day) | Untreated | Fluences (J/cm2) | |||
|---|---|---|---|---|---|
| 2.7 | 7.8 | 11.7 | 15.6 | ||
| pH | |||||
| 0 | 6.96 ± 0.51Aa | 6.63 ± 0.13Aa | 6.63 ± 0.12ABa | 6.65 ± 0.12ABa | 6.66 ± 0.13ABCa |
| 4 | 6.91 ± 0.36Aa | 6.83 ± 0.12Aa | 6.83 ± 0.16ABa | 6.75 ± 0.09Aa | 6.79 ± 0.10ABa |
| 8 | 5.83 ± 0.70Aa | 6.87 ± 0.16Ab | 6.88 ± 0.12Ab | 6.85 ± 0.12Ab | 6.82 ± 0.12Ab |
| 12 | 6.02 ± 0.84Aa | 6.39 ± 0.44Aa | 6.58 ± 0.29ABa | 6.80 ± 0.12Aa | 6.07 ± 0.46Ca |
| 16 | 5.41 ± 0.85Aa | 6.15 ± 0.54ABab | 6.67 ± 0.16ABb | 6.77 ± 0.08Ab | 6.47 ± 0.04ABCab |
| 20 | 5.35 ± 0.70Aa | 5.83 ± 0.49ABCab | 6.64 ± 0.14ABb | 6.67 ± 0.06Ab | 6.30 ± 0.15ABCab |
| 24 | 5.31 ± 0.81Aa | 5.25 ± 0.61BCa | 6.56 ± 0.26ABa | 6.65 ± 0.45ABa | 6.17 ± 0.21BCa |
| 28 | 5.20 ± 0.70Aab | C4.86 ± 0.38Cb | 6.13 ± 0.52Ba | 6.02 ± 0.38Bab | 6.11 ± 0.27Ca |
| Titratable acidity (% citric acid) | |||||
| 0 | 0.07 ± 0.04Aa | 0.10 ± 0.01Aa | 0.10 ± 0.01Aa | 0.10 ± 0.02Aa | 0.10 ± 0.02ABCa |
| 4 | 0.08 ± 0.04Aa | 0.09 ± 0.02Aa | 0.09 ± 0.01Aa | 0.10 ± 0.02Aa | 0.09 ± 0.01ABa |
| 8 | 0.15 ± 0.05Aa | 0.09 ± 0.00Aa | 0.09 ± 0.01Aa | 0.09 ± 0.01Aa | 0.09 ± 0.01Aa |
| 12 | 0.14 ± 0.06Aa | 0.13 ± 0.06Aa | 0.12 ± 0.04Aa | 0.09 ± 0.00Aa | 0.11 ± 0.03ABCa |
| 16 | 0.22 ± 0.12Aa | 0.15 ± 0.07Aa | 0.11 ± 0.03Aa | 0.10 ± 0.02Aa | 0.12 ± 0.03ABCa |
| 20 | 0.25 ± 0.10Aa | 0.17 ± 0.05Aa | 0.11 ± 0.02Aa | 0.11 ± 0.02Aa | 0.14 ± 0.02ABCa |
| 24 | 0.24 ± 0.11Aa | 0.23 ± 0.08ABa | 0.12 ± 0.03Aa | 0.11 ± 0.01Aa | 0.14 ± 0.01BCa |
| 28 | 0.26 ± 0.09Aab | 0.35 ± 0.10Bab | 0.17 ± 0.04Ab | 0.16 ± 0.02Bb | 0.15 ± 0.02Cb |
| Total soluble solids (°Brix) | |||||
| 0 | 11.03 ± 1.33Aa | 10.13 ± 1.16Aa | 10.37 ± 1.42Aa | 10.38 ± 0.82Aa | 10.09 ± 0.99Aa |
| 4 | 11.20 ± 0.95Aa | 10.64 ± 0.83Aa | 10.54 ± 0.95Aa | 10.67 ± 0.61Aa | 10.73 ± 0.70Aa |
| 8 | 11.14 ± 0.70Aa | 10.58 ± 1.03Aa | 10.87 ± 0.78Aa | 10.91 ± 0.77Aa | 10.66 ± 0.91Aa |
| 12 | 10.82 ± 0.55Aa | 10.57 ± 0.87Aa | 10.57 ± 0.77Aa | 10.93 ± 0.91Aa | 10.62 ± 0.97Aa |
| 16 | 10.80 ± 0.95Aa | 10.59 ± 0.86Aa | 10.70 ± 0.95Aa | 10.80 ± 1.06Aa | 10.41 ± 1.09Aa |
| 20 | 10.91 ± 1.04Aa | 10.61 ± 0.69Aa | 10.59 ± 1.17Aa | 10.67 ± 1.18Aa | 10.48 ± 0.58Aa |
| 24 | 10.21 ± 0.32Aa | 10.27 ± 0.94Aa | 10.36 ± 1.05Aa | 10.47 ± 0.85Aa | 10.10 ± 1.20Aa |
| 28 | 10.04 ± 0.32Aa | 10.22 ± 1.14Aa | 10.73 ± 0.84Aa | 10.53 ± 1.05Aa | 10.41 ± 0.63Aa |
*Different lowercase letters indicate significant differences within the same row (p ≤ 0.05)
*Different uppercase letters indicate significant differences within the same column (p ≤ 0.05)
Initial total soluble solids of fresh-cut cantaloupes were 10.08–11.03 °Brix (Table 4). The total soluble solids of fresh-cut cantaloupes remained stable throughout the storage and between treatments. Total soluble solids had been reported to be correlated to occurrence of metabolic activity (Lamikanra et al. 2000). The low storage temperature (4 ± 1 °C) used in this study resulted in low respiration rates of fresh-cut cantaloupes and thus insignificant changes (p > 0.05) in the total soluble solids as sugars are the first substrates for respiration. Furthermore, the low O2 and high CO2 concentration detected in the packages may inhibit ethylene production since O2 is required in the biosynthesis of 1-amino-cyclopropane-1-carboxylic acid which is further converted to ethylene by enzyme (Ramos-Villarroel et al. 2011). As a consequence, the decrease in ethylene production will slow down the ripening process. Stability of total soluble solids was also reported for fresh-cut cantaloupe melon stored under refrigeration temperature (Lamikanra et al. 2000).
Total phenolic content
The initial total phenolic content of fresh-cut cantaloupes was 21.13–22.68 mg GAE/100 g. The total phenolic content was maintained in fresh-cut cantaloupes without marked difference (p > 0.05) among the treatments during the storage as presented in Table 5. Since PL is a surface treatment, the phenols could be protected as they are located in vacuoles (Charles et al. 2013). The changes of total phenolic content in a sample are related to enzyme activity of polyphenoloxidase (PPO) and phenylalanine ammonia lyase (PAL). The interaction of phenolic compounds with PPO leads to formation of quinones that subsequently converts to melanins (Charles et al. 2013). Lamikanra and Watson (2001) mentioned that low PPO activity was observed in cantaloupes. Thus, low PPO activity in cantaloupes indicated insignificant changes (p > 0.05) of total phenolic content in this study. Retention of total phenolic content was also observed in fresh-cut cantaloupes stored at low temperature by Lamikanra and Watson (2001). Although there was no important effect (p > 0.05) shown by PL treatment, the total phenolic content was generally higher for fresh-cut cantaloupes treated with PL (21.13–26.05 mg GAE/100 g) during storage compared to the control (19.96–22.68 mg GAE/100 g) as presented in Table 5. Dunn et al. (1989) reported that PL reduced PPO activity which could explain the retention of slightly higher total phenolic content in PL-treated fresh-cut cantaloupes during storage. In addition, abiotic stress caused by PL may induce the production of polyphenolic compounds due to the increase in PAL activity in response to cell acclimation against stress in plants. Phenylalanine ammonia lyase serves as the primary enzyme of phenylpropanoid pathway involving the conversion of 1-phenylalanine into trans-cinnamic acid by deamination in plants (Murugesan et al. 2012). Modification of the package headspace atmosphere of fresh-cut cantaloupes during storage could also induce stress which increases the PAL activity (Oms-Oliu et al. 2008). Therefore, the total phenolic content was conserved in fresh-cut cantaloupes throughout the storage in this research. Charles et al. (2013) also discovered conservation of phenol content in fresh-cut mangoes for both control and that treated with PL fluence of 8 J/cm2 during 7 d of storage under 6 °C.
Table 5.
Effect of fluences on total phenolic content (expressed in mg GAE/100 g) and ascorbic acid content (expressed in mg/100 g) of fresh-cut cantaloupes treated with pulsed light
| Storage time (day) | Untreated | Fluences (J/cm2) | |||
|---|---|---|---|---|---|
| 2.7 J/cm2 | 7.8 J/cm2 | 11.7 J/cm2 | 15.6 J/cm2 | ||
| Total phenolic content (mg GAE/100 g) | |||||
| 0 | 22.68 ± 2.37Aa | 21.13 ± 3.58Aa | 22.03 ± 5.04Aa | 21.93 ± 5.39Aa | 22.27 ± 4.33Aa |
| 4 | 22.59 ± 0.43Aa | 22.09 ± 3.62Aa | 22.38 ± 3.58Aa | 22.49 ± 2.64Aa | 23.36 ± 2.93Aa |
| 8 | 21.37 ± 1.64Aa | 22.93 ± 3.13Aa | 23.82 ± 2.95Aa | 23.66 ± 2.86Aa | 23.27 ± 2.84Aa |
| 12 | 22.05 ± 0.85Aa | 22.46 ± 2.57Aa | 23.08 ± 2.62Aa | 23.42 ± 2.83Aa | 22.37 ± 3.70Aa |
| 16 | 21.80 ± 0.59Aa | 22.78 ± 2.83Aa | 24.01 ± 3.69Aa | 23.82 ± 3.33Aa | 24.06 ± 4.25Aa |
| 20 | 21.51 ± 1.09Aa | 22.36 ± 5.09Aa | 24.10 ± 4.75Aa | 23.76 ± 4.06Aa | 24.32 ± 4.25Aa |
| 24 | 21.01 ± 1.04Aa | 24.03 ± 3.74Aa | 26.05 ± 0.72Aa | 24.10 ± 4.85Aa | 24.29 ± 2.41Aa |
| 28 | 19.96 ± 0.97Aa | 24.33 ± 3.45Aa | 23.05 ± 5.63Aa | 22.62 ± 4.27Aa | 22.87 ± 5.51Aa |
| Ascorbic acid Content (mg/100 g) | |||||
| 0 | 21.29 ± 1.33Aa | 24.42 ± 3.69Aa | 24.82 ± 3.14Aa | 25.28 ± 2.96Aa | 24.23 ± 1.37Aa |
| 4 | 21.87 ± 2.87Aa | 23.78 ± 1.06Aa | 23.44 ± 2.04Aa | 24.35 ± 1.14Aa | 22.86 ± 1.02ABa |
| 8 | 22.71 ± 1.79Aa | 22.86 ± 1.21Aa | 23.06 ± 2.06Aa | 22.47 ± 0.26ABa | 22.61 ± 1.85ABa |
| 12 | 22.20 ± 2.31Aa | 21.73 ± 1.11Aa | 21.43 ± 0.62Aa | 22.82 ± 1.20ABa | 21.29 ± 2.00ABa |
| 16 | 21.62 ± 0.45Aa | 20.85 ± 1.43Aa | 21.28 ± 0.98Aa | 21.46 ± 0.99ABa | 22.09 ± 1.36ABa |
| 20 | 21.23 ± 2.19Aa | 20.40 ± 3.25Aa | 21.95 ± 1.88Aa | 21.70 ± 2.21ABa | 20.73 ± 2.20ABa |
| 24 | 20.48 ± 0.96Aa | 19.40 ± 0.74Aa | 21.51 ± 2.02Aa | 20.93 ± 1.36ABa | 22.16 ± 0.59ABa |
| 28 | 19.01 ± 0.58Aa | 17.04 ± 5.68Aa | 19.64 ± 1.63Aa | 19.82 ± 0.99Ba | 18.41 ± 2.35Ba |
*Different lowercase letters indicate significant differences within the same row (p ≤ 0.05)
*Different uppercase letters indicate significant differences within the same column (p ≤ 0.05)
Ascorbic acid content
From Table 5, the ascorbic acid content in PL-treated fresh-cut cantaloupes was higher (24.23–25.28 mg/100 g) than the untreated samples (21.29 mg/100 g) on day 0, although the difference was not significant (p > 0.05). The slightly greater ascorbic acid content in PL-treated fresh-cut cantaloupes could be due to the abiotic stress exerted by the PL irradiation. Oliveira et al. (2013) explained stresses, either biotic or abiotic, leading to oxidative stress in plants whereby the oxidative signalling involves in synthesis and accumulation of secondary metabolites. The ascorbic acid content in fresh-cut cantaloupes did not show significant difference (p > 0.05) among the untreated and treated samples at the different fluences throughout the storage study. Low temperature storage could help to retain vitamin C content. However, the ascorbic acid content generally decreased gradually during storage with significant effect (p ≤ 0.05) shown by fresh-cut cantaloupes treated with 11.7 and 15.6 J/cm2. Accumulation of CO2 in the package could have negative effect on the ascorbic acid content of fresh-cut fruit due to stimulation of oxidation by ascorbate peroxidase and inhibition of monodehydro- and/or dehydroascorbate-reductase (Agar et al. 1997). Ascorbic acid content retention is also lower in non-acidic fruits like cantaloupes compared to acidic fruits (Lamikanra and Watson 2001). Temperature increase caused by PL is dependent upon the number of pulses and fluences (Ramos-Villarroel et al. 2012). At lower fluences of 2.7 and 7.8 J/cm2, the temperature increase was mild, hence the ascorbic acid, a heat sensitive compound was maintained throughout the storage. At higher fluences of 11.7 and 15.6 J/cm2, ascorbic acid content reduced significantly (p ≤ 0.05) at the end of storage as compared to the initial ascorbic acid content for the respective treatment. This could partly be due to the thermal damage employed by high PL fluences. Oms-Oliu et al. (2010) also reported that PL application of 28 J/cm2 significantly reduced the vitamin C content in fresh-cut mushrooms.
Conclusions
Samples with spherical shape was the most suitable for PL treatment of fresh-cut cantaloupes based on microbial inactivation. Pulsed light had positive effect on microbiological stability of fresh-cut cantaloupes stored at 4 ± 1 °C when the fluence was increased to 7.8 J/cm2, but no additional inactivation was observed with further increased in the PL fluence. Changes in concentration of CO2, pH and titratable acidity seemed to be correlated to microbial growth in fresh-cut cantaloupes. No effect of PL fluences was observed for firmness, colour, total soluble solids and total phenolic content of fresh-cut cantaloupes during storage at 4 ± 1 °C. Ascorbic acid content in fresh-cut cantaloupes was conserved throughout the storage study at low fluences (2.7 and 7.8 J/cm2) PL treatment. Application of PL treatment using 7.8 J/cm2 was the best condition for extending the shelf life of fresh-cut cantaloupes at 4 ± 1 °C compared to the control. On day 16, the total plate count and yeast and mould count of sample remained lower than the safe microbial limit with the retention of ascorbic acid content up to 8 days of extended storage. Pulsed light treatment at moderate fluence could maintain microbiological stability of fresh-cut cantaloupes under storage at 4 ± 1 °C without compromising the physical appearance and nutritional value of cantaloupes.
Acknowledgments
The authors are grateful to Universiti Putra Malaysia for supporting this study through Project No. 05-02-12-2047RU.
References
- Abida J, Rayees B, Masoodi FA. Pulsed light technology: a novel method for food preservation. Int Food Res J. 2014;21:839–848. [Google Scholar]
- Agar IT, Streif J, Bangerth F. Effect of high CO2 and controlled atmosphere (CA) on the ascorbic and dehydroascorbic acid content of some berry fruits. Postharvest Biol Tec. 1997;11:47–55. doi: 10.1016/S0925-5214(97)01414-2. [DOI] [Google Scholar]
- Amaro AL, Beaulieu JC, Grimm CC, Stein RE, Almeida DPF. Effect of oxygen on aroma volatiles and quality of fresh-cut cantaloupe and honeydew melons. Food Chem. 2012;130:49–57. doi: 10.1016/j.foodchem.2011.06.052. [DOI] [Google Scholar]
- AOAC . Official methods of analysis of the association of office analytical chemists. 15th. Washington, D.C: Association of Official Analytical Chemists; 1990. [Google Scholar]
- Argañosa ACSJ, Raposo MFJ, Teixeira PCM, Morais AMMB. Effect of cut-type on quality of minimally processed papaya. J Sci Food Agric. 2008;88:2050–2060. doi: 10.1002/jsfa.3309. [DOI] [Google Scholar]
- Beaulieu JC, Lea JM. Quality changes in cantaloupe during growth, maturation, and in stored fresh-cut cubes prepared from fruit harvested at various maturities. J Am Soc Hortic Sci. 2007;132:720–728. [Google Scholar]
- Cao S, Hu Z, Pang B, Wang H, Xie H, Wu F. Effect of ultrasound treatment on fruit decay and quality maintenance in strawberry after harvest. Food Control. 2010;21:529–532. doi: 10.1016/j.foodcont.2009.08.002. [DOI] [Google Scholar]
- Centers of Disease Control and Prevention (CDC) (2015) List of selected multistate foodborne outbreak investigations. Centers of Disease Control and Prevention. http://www.cdc.gov/foodsafety/outbreaks/multistate-outbreaks/outbreaks-list.html. Accessed 29 April 2015
- Charles F, Vidal V, Olive F, Filgueiras H, Sallanon H. Pulsed light treatment as new method to maintain physical and nutritional quality of fresh-cut mangoes. Innov Food Sci Emerg. 2013;18:190–195. doi: 10.1016/j.ifset.2013.02.004. [DOI] [Google Scholar]
- Chimbombi E, Moreira RG, Castell-Perez EM, Puerta-Gomez AF. Assessing accumulation (growth and internal mobility) of salmonella typhimurium LT2 in fresh-cut cantaloupe (cucumis melo L.) for optimization of decontamination strategies. Food Control. 2013;32:574–581. doi: 10.1016/j.foodcont.2013.01.042. [DOI] [Google Scholar]
- Danyen MS, Boodia N, Ruggoo A. Effect of cutting shapes and thicknesses on the quality of minimally processed pineapple (annas comosus), cv. ‘queen Victoria’. Afr J Food Agric Nutr Dev. 2011;11:5525–5538. [Google Scholar]
- Dunn JE, Clark RW, Asmus JF, Pearlman JS, Boyer K, Painchaud F (1989) US Patent Number:4871559
- Food and Drug Administration (FDA) (2007) Guide to minimise microbial food safety hazards for fresh-cut fruits and vegetables. Food and Drug Administration. http://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/ucm064458.htm. Accessed 14 November 2014
- Fu L, Xu BT, Xu XR, Gan RY, Zhang Y, Xia EQ, Li HB. Antioxidant capacities and total phenolic contents of 62 fruits. Food Chem. 2011;129:345–350. doi: 10.1016/j.foodchem.2011.04.079. [DOI] [PubMed] [Google Scholar]
- Gómez PL, García-Loredo A, Nieto A, Salvatori DM, Guerrero S, Alzamora SM. Effect of pulsed light combined with an antibrowning pretreatment on quality of fresh cut apple. Innov Food Sci Emerg. 2012;16:102–112. doi: 10.1016/j.ifset.2012.05.011. [DOI] [Google Scholar]
- Gómez-López VM, Ragaert P, Debevere J, Devlieghere F. Pulsed light for food decontamination: a review. Trends Food Sci Technol. 2007;18:464–473. doi: 10.1016/j.tifs.2007.03.010. [DOI] [Google Scholar]
- Institute of Food Science and Technology (IFST) (1999) Development and use of microbiological criteria for foods. Institute of Food Science and Technology. http://pdfs.findtheneedle.co.uk/16898.pdf. Accessed 12 June 2014
- Ignat A, Manzocco L, Maifreni M, Bartolomeoli I, Nicoli MC. Surface decontamination of fresh-cut apple by pulsed light: effects on structure, color and sensory properties. Postharvest Biol Tec. 2014;91:122–127. doi: 10.1016/j.postharvbio.2014.01.005. [DOI] [Google Scholar]
- Kim J, Moreira R, Castell-Perez E. Simulation of pathogen inactivation in whole and fresh-cut cantaloupe (cucumis melo) using electron beam treatment. J Food Eng. 2010;97:425–433. doi: 10.1016/j.jfoodeng.2009.10.038. [DOI] [Google Scholar]
- Lamikanra O, Watson MA. Effects of ascorbic acid on peroxidase and polyphenoloxidase activities in fresh-cut cantaloupe melon. J Food Sci. 2001;66:1283–1286. doi: 10.1111/j.1365-2621.2001.tb15202.x. [DOI] [Google Scholar]
- Lamikanra O, Chen JC, Banks D, Hunter PA. Biochemical and microbial changes during the storage of minimally processed cantaloupe. J Agric Food Chem. 2000;48:5955–5961. doi: 10.1021/jf0000732. [DOI] [PubMed] [Google Scholar]
- Larson AE, Johnson EA. Evaluation of botulinal toxin production in packaged fresh-cut cantaloupe and honeydew melons. J Food Prot. 1999;62:948–952. doi: 10.4315/0362-028x-62.8.948. [DOI] [PubMed] [Google Scholar]
- Lasagabaster A, de Marañón IM. Survival and growth of listeria innocua treated by pulsed light technology: impact of post-treatment temperature and illumination conditions. Food Microbiol. 2014;41:76–81. doi: 10.1016/j.fm.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Manzocco L, Da Pieve S, Maifreni M. Impact of UV-C light on safety and quality of fresh-cut melon. Innov Food Sci Emerg. 2011;12:13–17. doi: 10.1016/j.ifset.2010.11.006. [DOI] [Google Scholar]
- Munira ZA, Rosnah S, Zaulia O, Russly AR. Effect of postharvest storage of whole fruit on physico-chemical and microbial changes of fresh-cut cantaloupe (cucumis melo L. reticulatus cv. Glamour) Int Food Res J. 2013;20:501–508. [Google Scholar]
- Murugesan R, Orsat V, Lefsrud M. Effect of pulsed ultraviolet light on the total phenol content of elderberry (sambucus nigra) fruit. Food Nutr Sci. 2012;3:774–783. doi: 10.4236/fns.2012.36104. [DOI] [Google Scholar]
- Oliveira AB, Moura CFH, Gomes-Filho E, Marco CA, Urban L, Miranda MRA. The impact of organic farming on quality of tomatoes is associated to increased oxidative stress during fruit development. PLoS One. 2013;8 doi: 10.1371/journal.pone.0056354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oms-Oliu G, Soliva-Fortuny R, Martín-Belloso O. Using polysaccharide-based edible coatings to enhance quality and antioxidant properties of fresh-cut melon. LWT Food Sci Technol. 2008;41:1862–1870. doi: 10.1016/j.lwt.2008.01.007. [DOI] [Google Scholar]
- Oms-Oliu G, Martín-Belloso O, Soliva-Fortuny R. Pulsed light treatments for food preservation. A review. Food Bioprocess Tech. 2010;3:13–23. doi: 10.1007/s11947-008-0147-x. [DOI] [Google Scholar]
- Pintó E, Lentheric I, Vendrell M, Larrigaudière C. Role of fermentative and antioxidant metabolisms in the induction of core browning in controlled-atmosphere stored pears. J Sci Food Agric. 2001;81:364–370. doi: 10.1002/1097-0010(200102)81:3<364::AID-JSFA828>3.0.CO;2-N. [DOI] [Google Scholar]
- Ramos-Villarroel AY, Martín-Belloso O, Soliva-Fortuny R. Bacterial inactivation and quality changes in fresh-cut avocado treated with intense light pulses. Eur Food Res Technol. 2011;233:395–402. doi: 10.1007/s00217-011-1533-6. [DOI] [PubMed] [Google Scholar]
- Ramos-Villarroel AY, Aron-Maftei N, Martín-Belloso O, Soliva-Fortuny R. Influence of spectral distribution on bacterial inactivation and quality changes of fresh-cut watermelon treated with intense light pulses. Postharvest Biol Tec. 2012;69:32–39. doi: 10.1016/j.postharvbio.2012.03.002. [DOI] [Google Scholar]
- Ramos-Villarroel A, Aron-Maftei N, Martín-Belloso O, Soliva-Fortuny R. Bacterial inactivation and quality changes of fresh-cut avocados as affected by intense light pulses of specific spectra. Int J Food Sci Technol. 2014;49:128–136. doi: 10.1111/ijfs.12284. [DOI] [Google Scholar]
- Salama MS, Musafija-Jeknic T, Sandine WE, Giovannoni SJ. An ecology study of lactic acid bacteria: isolation of new strains of Lactococcus lactis subspecies cremoris. J Dairy Sci. 1995;78:1004–1017. doi: 10.3168/jds.S0022-0302(95)76716-9. [DOI] [Google Scholar]
- Saltveit ME (2002) Respiratory metabolism. United States Department of Agriculture. http://www.ba.ars.usda.gov/hb66/respiratoryMetab.pdf. Accessed 10 December 2014
- Soliva-Fortuny RC, Grigelmo-Miguel N, Odriozola-Serrano I, Gorinstein S, Martín-Belloso O. Browning evaluation of ready-to-eat apples as affected by modified atmosphere packaging. J Agric Food Chem. 2001;49:3685–3690. doi: 10.1021/jf010190c. [DOI] [PubMed] [Google Scholar]