Abstract
Apple pomace (AP), the residue that remains after the extraction of juice from apple accounts for ~25 % of total apple weight. Current study is aimed at identification of phytochemicals and utilization of Dehydrated apple pomace (DAP) in the preparation of bakery products with potential health benefits. DAP was prepared by drying the pomace obtained by crushing peeled apple fruits. DAP was incorporated into bakery products such as bun, muffin and cookies for value addition. Bioactivity such as free radical scavenging, cyto/DNA protectivity was evaluated in these products. DAP contained 17 g/100 g starch, 49.86 g/100 g fructose and 37 g/100 g dietary fibre. The phenolics and flavonoids content was 1.5 mg/g and 3.92 mg/g, respectively. Increase in DAP resulted in decreased volume and enhanced firmness of buns and muffins. DAP at 15 % in buns, 30 % in muffins and 20 % in cookies were found to be acceptable. DAP blended products exhibited better free radical scavenging as well as cyto/DNA protective properties suggesting the retention of bioactivity after baking. Addition of DAP potentially enhanced the bioactivity of the products evaluated.
Keywords: Apple pomace, Bakery products, Phenolics, Cytoprotective ability, Antioxidant activity, DNA protection
Introduction
Apple (Malus pumila) is a prominent fruit which is processed into products such as phenolic rich juice, cider and canned as fresh slices/cubes, baby foods, apple butter, jelly, vinegar etc. Further, apples lack phytic acid/phytates, an anti-nutritional substance that affects the absorption of iron, zinc and calcium and results in mineral deficiency (Masoodi et al. 2002). Apple pomace (AP) is the residue that remains after the extraction of juice from apple and it accounts to ~25 %. Use of AP is of paramount importance when tons of apples are crushed for juice every day. The fresh apple pomace being rich in sugars and dumping it in an open atmosphere invariably results in microbial growth rendering it unfit for any subsequent use in addition to causing environmental pollution. AP thus requires a suitable economic disposal. AP as a by-product of fruit industry contains significantly higher content (~37 %) of dietary fibre and hence it can be a potential source for health food preparations. Investigations have revealed that although apple fibres are superior due to the presence of bioactive components to that of wheat and oat bran, blending of apple fibre with wheat flour resulted in decreased baking efficiency (Chen et al. 1988a). Addition of 4 % of AP in cookie and muffin formulations, however gave an acceptable product quality (Chen et al. 1988b). Fibre concentrates from apple pomace and citrus peel were also used as potential sources for fibre enrichment. The dietary antioxidants from fruits or fruit wastes are effective against cyto/DNA oxidative damage caused by free radical reactive oxygen species during oxidative stress that causes many degenerative diseases such as cancer and cardio vascular diseases (Nanjarajurs et al. 2014). Earlier studies carried out indicated that a cake with 20 % AP provided the acceptable quality cakes with natural colorant and flavorant (Sudha et al. 2007). Masoodi et al. (2002) prepared cakes from AP - wheat flour blends at 5 %, 10 % and 15 % levels to enrich the cake with fibre content. In all these studies, products were presumed to have health potencies since fibre was added; validation of bioactivity of the product however was not carried out which was essential to understand the stability of functional ingredients under the conditions of processing. The current study was therefore attempted to determine the chemical composition and bioactivity of AP and its incorporation in different bakery products.
Materials and methods
Chemicals
Phenolic acid standards such as gallic, tannic, caffeic, coumaric, ferulic, gentisic, protocatechuic, syringic, vanillic and cinnamic acids and synthetic antioxidant namely butylated hydroxyanisole (BHA) were purchased from Sigma Chemical Co., St. Louis, MO, USA. Other chemicals and reagents such as 2, 2- diphenyl-1-picrylhydrazyl (DPPH),2 – Thiobarbituric acid (TBA), Folin Ciocalteau reagent, ferrous sulphate, ascorbic acid, agarose used in the experiments were of high quality and were obtained from Sisco Research Laboratories, Mumbai, India. HPLC grade solvents only were employed for HPLC analysis. Calf thymus DNA was from M/S Genei, Bangalore-58, India. The HPLC column (Shimpak C18) was obtained from Shimadzu Corp., Tokyo, Japan. Commercial wheat flour (WF) procured from local market having 11.2 % moisture, 10.1 % protein and 0.45 % ash was used in the study.
Preparation of AP
The apple fruits (Maharaja Variety) were purchased from the local market of Myrose, Karnataka, India. The fruits of medium size (~ 8 cm in diameter), oblong, with specific gravity of >1 were washed with water, air dried and peeled manually. Apple fruits were then passed through a pulper (of fine finish with 30 mesh sieve) for crushing. The crushed pulp was pressed with hydraulic press using a clean, wet and thick cloth to separate juice and pomace. The fractions of the fruit were peel (8.5 %), pulp (86.1 %), waste (5.3 %) and seed (0.1 %). The apple pomace (AP) thus obtained having a pH of 3.9 was dried in a hot air drier at 55 ± 2 °C for 8 h, powdered in apex mill and used for further estimations and preparation of products 6 kg of DAP was obtained from 100 kg of fresh apple (DAP).
Extraction
The dried powder (1 g) of DAP and 5 g each of bakery products prepared were extracted in 10 mL of hot water (~60 °C) and methanol, respectively after vortexing thoroughly at room temperature for 30 min. Supernatants were separated by centrifugation at 3000×g for 10 min at room temperature. Samples were designated as aqueous and methanol extracts of respective samples. Extracts were stored at 4 °C till the completion of the experiment.
Chemical analysis
The total soluble solids of fresh apple pomace was determined using a refractometer (ATAGO RX-5000) at 30 °C. The total soluble solids and titratable acidity were measured using fresh AP. AP was diluted with water (1:20) (v/v) and filtered through whatman No. 1 filter paper and titrable acidity of extract was measured by titration. Acidity was expressed as % citric acid. Starch in DAP was estimated according to Hassid and Neufeld (1964). Total fibre content was determined according to Asp et al. (1983). The ascorbic acid content in DAP was determined by HPLC (Watada 1982) using μBondapak C18 (10 μm) column (30 cm X 4.6 mm id), Waters Co., Ireland with isocratic mobile phase consisting of 0.08 % ortho phosphoric acid. L-ascorbic acid was used as standard. Total flavonoid present in DAP was measured using aluminium chloride colorimetric assay method and expressed as (+) - catechin equivalent (CAE) in mg per g of sample (Dewanto et al. 2002).
Estimation of sugars
Total sugars was determined according to Dubois et al. (1956). To the water extract of DAP (1 mL), 5 % of 0.6 mL phenol was added, followed by 3.6 mL of concentrated sulfuric acid along the sides of the tube. A yellowish orange color developed was read at 490 nm in spectrophotometer using distilled water as blank. The reducing sugars were determined by DNS method (Dubois et al. 1956). To the water extract of DAP (1 mL), DNS reagent (1 mL) was added, boiled for 10 min, distilled water (4 mL) was added to each tube, cooled and color developed was read at 540 nm. Distilled water served as blank. HPLC analysis was carried out for sugars using an amino column (25 cm X 4.6 mm id), Supelco, USA, with a refractive index detector operating at 190 nm. A solvent system consisting of acetonitrile/water (80:20 v/v) was used as mobile phase. The mobile phase was pumped with a LC – 10A pump at a flow rate of 1 mL/min. Standard sugars used were xylose, arabinose, fructose, glucose, galactose and sucrose.
Estimation of free phenolics
The free phenolics were extracted from DAP (1 g) in 5 mL of 70 % acetone in a Sonicator for 15 min at room temperature (28 °C ± 24 °C). The solution was filtered using Whatmann qualitative filter paper No. 1. The residue was then re-extracted with 3 mL of 70 % acetone, twice and evaporated to dryness under nitrogen atmosphere. The total phenolic content was determined spectrophotometrically using the Folin-phenol reagent. A sample aliquot of 100 μL was added to 900 μL of water, 1 mL of Folin-Ciocalteu reagent and 2 mL of 10 % sodium carbonate solution, mixed in a cyclomixer and incubated for 1 h at room temperature. The absorbance was measured at 765 nm with a Shimadzu UV-visible spectrophotometer. The standard curve was drawn using 10–100 μg of gallic acid. The total phenolic content was expressed as gallic acid equivalent (GAE) in mg per g of sample (Nethravathi et al. 2006). The concentrated sample was mixed in 1 mL of HPLC grade methanol and analyzed.
HPLC of phenolics
Phenolic acids in DAP extract was analyzed on a reversed phase Shimpak C18 column (4.6 × 150 mm), using a diode array detector (operating at 280 nm). The Shimpak C18 HPLC column was obtained from Shimadzu Co., Japan. A solvent system consisting of water/acetic acid/methanol (80:5:15) (v/v/v) was used as mobile phase at a flow rate of 1 mL/min. Phenolic acids such as gallic, tannic, caffeic, coumaric, ferulic, gentisic, protocatechuic, syringic, vanillic and cinnamic acids were used as standards to identify them in extracts. Quantitation of phenolic acids was achieved by the absorbance recorded in the chromatograms related to external standards at 280 nm (Khoddami et al. 2013).
Preparation of bakery products
Preparation of buns
Buns were prepared using straight dough method from WF-DAP blends (0, 10, 15 and 20 %) as per the method described by Sudha et al. 2012 with slight modification. Dough pieces (65 g) were rounded, proofed for 1 h and baked at 200 °C for 15 min. Buns were cooled and assessed for their weight and volume by rapeseed displacement method. Objective measurement of texture (crumb firmness) in triplicates was carried out in a texture analyser (TAHDi, Stable Micro Systems, Godalming, UK) by the standard AACC (2000) method (74–09). Six trained panelists carried out the sensory evaluation of buns on 9 - point hedonic scale by assigning different scores for surface characteristics, crumb color, texture and overall quality as described by Steel and Torrie (1980) at the significant level was established at P < 0.05. The acceptable level of apple pomace was chosen and the effect of additives namely gluten (2 %), glycerol monostearate (0.25 %), sodium stearoyl lactylate (0.25 %) and fungal α-amylase (1 mg) were added to improve the volume, texture and the sensory acceptability of buns.
Preparation of muffins
Muffins were prepared from WF-DAP blends (0, 10, 20, 30 and 40 %) according to Chetana et al. (2010). 65 g of batter was poured into the greased muffin tray and baked at 200 °C for 25 min. Volume of the muffins was measured using rapeseed displacement method. Weight (g) and specific volume (cc/g) of the muffins were measured. The textures of the muffins were measured objectively using Texture Analyzer (TAHDi, Stable Micro System, Godalming, Surrey, U.K.) as per the standard AACC (2000) methods. Six trained panelists carried out the sensory evaluation of muffins on 9 - point hedonic scale by assigning different scores for surface characteristics, crumb color, texture and overall quality as described by Steel and Torrie (1980) at the significant level was established at P < 0.05. The acceptable level was chosen and additives namely sodium stearoyl lactylate 0.5 % was added to improve the volume, texture and the sensory acceptability of muffins.
Preparation of cookies
The recipe and the formulation for baking of cookies were as per the method described by Sindhuja et al. (2005). Cookies were prepared from WF-DAP blends (0, 10, 20 and 30 %). Cookies after baking were cooled to room temperature (28 ± 2 °C) packed in polythene bags and kept in an airtight container. All the experiments were conducted in triplicates and at random the cookies were picked for subjective and objective quality evaluation. Cookies were subjectively evaluated for thickness, spread, spread ratio, texture and surface cracking pattern. The breaking strength was measured using the triple beam snap technique of (Gains 1991) using a texture analyser (TAHDi, Stable Micro Systems, Godalming, UK). Six trained panelists carried out the sensory evaluation of cookies on 9 - point hedonic scale by assigning different scores for surface cracking, crumb color, texture and overall quality as described by Steel and Torrie (1980) at the significant level was establish at P < 0.05. The acceptable level of apple pomace was chosen and additives namely glycerol monostearate (0.25 %), sodium stearoyl lactylate (0.25 %) were added to improve the spread, texture, islands and sensory acceptability of cookies.
Antioxidant activity
Antioxidant activity of DAP blend extract was determined as free radical scavenging activity of the stable 2, 2-diphenyl-1-picrylhydrazyl (DPPH) free radical (Nethravathi et al. 2006; Srikanta et al. 2007). DAP extracts, both water and methanol extracts at various concentrations (5–30 μg GAE/mL) were added to 1 mL of 0.004 % methanol solution of DPPH. The mixture was shaken vigorously and left to stand for 20 min at room temperature in the dark. The absorbance of the resulting solution was measured spectrophotometrically at 517 nm. The capability to scavenge the DPPH radical was calculated using the following equation.
Cytoprotective ability
Effect on Red Blood Cells (RBC)
RBCs are fragile blood cells known to be affected during various oxidative stress conditions. This was employed as a model to evaluate the cytoprotective ability of apple pomace extract (Harish Nayaka et al. 2009). A 10 % suspension of erythrocytes in phosphate buffer solution (PBS), pH 7.4 was added to 150 μL of 50 mM ascorbic acid and 15 μL of 80 mM FeSO4 in presence and absence of 10–30 μg GAE of apple pomace extract. After incubating the sample at 37 °C for 30 min, the tubes were centrifuged and to 200 μL of the supernatant, 1 mL of 2-thiobarbituric acid (TBA) was added and boiled for 10 min at 95 °C. The tubes were cooled, vortexed, centrifuged and the supernatant was read at 535 nm. The percent protection was calculated as % of protection of RBCs
Effect on Buccal Cells (BC)
Buccal cells (1 × 104 cells/well) were exposed to UV radiation and a carcinogen – N-nitroso-N-methyl urea (MNU) (10 μg/mL for 1 X 104 cells/well), in presence and absence of 10 μg GAE of samples for 1 h at 37 °C. Twenty five microliters of cell suspension of both treated and untreated cells were mixed with 1 μL of dye mix containing 100 μg/mL each acridine orange and ethidium bromide and observed under the microscope at 40X. Viable cells stained green with acridine orange whereas damaged cells stained more orange due to ethidium bromide staining of nuclear components. Staining pattern between untreated and UV treated cells were compared and cytoprotective ability of DAP blend extracts was determined.
DNA protective ability
The DNA protective effect of DAP extract was determined electrophoretically (Submarine Electrophoresis System, Bangalore Genei, Bangalore, India) using calf thymus DNA (1 mg/mL) (Sathisha et al. 2009). The DNA was subjected to oxidation by Fenton’s reagent (100 μM ascorbic acid and 10 μM FeSO4) followed by gel electrophoresis. Relative difference in the migration between the native and the oxidized DNA was ensured on 1 % agarose gel electrophoresis after staining with ethidium bromide. Gels were documented and the intensity of the bands was determined. Protection of DNA was calculated based on the DNA band intensity comparing with that of the native in the presence and absence of extracts.
Statistical analysis
The data was subjected to statistical analysis by Duncan’s multiple range tests with different experimental groups. Completely randomized design with four replicates each as described by Steel and Torrie (1980) was performed at the significant level at P < 0.05. P value was calculated by the Mann-Whitney test. All the experiments were carried out in triplicates and all the results are expressed as mean ± Standard deviation (SD). Statistical programme SPSS for Windows; Version 10.0 was employed for the analysis.
Results and discussion
Chemical composition of apple pomace
The chemical properties of fresh AP & DAP provided in Table 1 indicated that AP had a pH of 3.9; total solids of 10 °Brix and titratable acidity of 0.234 %. The DAP had a starch content of 17 g/100 g, soluble dietary fibre of 6 g/100 g and insoluble dietary fibre 20 g/100 g. The ascorbic acid content was 2.86 g/100 g, where as the total sugars were ~31 g/100 g out of which the reducing sugars was 20 g/100 g. The spectrum of sugars in the 70 % ethanol extract was identified by HPLC as xylose, fructose, glucose and sucrose. The fructose (50 %) was the dominant sugar followed by glucose (12 %), sucrose (9 %) and xylose (<1 %). The total flavonoids present was 3.92 mg/g. These results indicate that DAP is a good source of dietary fiber and bioactive components.
Table 1.
Physico-chemical properties of fresh apple pomace (AP) and dried apple pomace (DAP)
| Parameters | Quantities |
|---|---|
| AP | |
| Moisture (%) | 84.64 ± 0.33 |
| pH | 3.9 ± 0.01 |
| Total Soluble Solids | 10 °B ± 0.3 |
| Titrable acidity | 0.234 ± 0.037 |
| DAP | |
| Starch (g/100 g on dw) | 17.0 ± 0.2 |
| Total sugar (g/100 g) | 30.89 ± 0.59 |
| Reducing sugar (g/100 g) | 20.40 ± 5.73 |
| Xylose (g/100 g) | 0.68 |
| Fructose (g/100 g) | 49.86 |
| Glucose (g/100 g) | 12.66 |
| Sucrose (g/100 g) | 8.94 |
| Insoluble fibre (g/100 g on dw) | 20.4 ± 0.3 |
| Soluble fibre (g/100 g on dw) | 6.4 ± 0.1 |
| Ascorbic acid (g/100 g on dw) | 2.86 ± 0.15 |
| Total flavonoids (mg/g on dw) | 3.92 ± 0.26 |
| Total phenolics (GAE) | 1.5 ± 0.14 |
| Phenolics constituents (mg/100 g) | |
| Gallic acid Catechin Chlorogenic acid Caffeic acid Epicatechin p-Coumaric acid Ferulic acid Protocatechuic acid Quercetin |
5.46 ± 0.13 9.98 ± 0.19 4.93 ± 0.10 60.28 ± 1.41 24.63 ± 0.87 0.78 ± 0.02 0.89 ± 0.03 1.45 ± 0.02 24.56 ± 0.78 |
Values are expressed as mean (n = 3) ± standard deviation; GAE- gallic acid equivalent
Phenolics in pomace
The total phenolics content of apple pomace was 1.5 ± 0.14 mg/g. The phenolics extracted from the apple pomace were subjected to HPLC for identifying the spectrum of phenolics present in the DAP. Among the free phenolics, caffeic acid (60.28 mg/100 g) dominated followed by epicatechin (24.63 mg/100 g) and quercitin (24.56 mg/100 g). Catechin (9.68 mg/100 g), gallic acid (5.46 mg/100 g), chlorogenic acid (4.93 mg/100 g) were in appreciable quantities. p-Coumaric acid and pro- catechuic acid were in minor quantities (Table 1).
The total phenolic contents determined according to Folin – Cioacalteu method were higher than the sums of the individual phenolics identified by HPLC. This difference can be explained by the fact that Folin – Cioacalteu method is not an absolute measurement of the amount of phenolics because some other substances such as organic acids, residual sugars, amino acids, proteins and other hydrophilic compounds interfere with this assay. In addition, phenolic compounds in fruits are found in many forms of simple phenols, viz., phenolic acids, flavnoids, tannins and coumarins, all of which can be detected through Folin-Cioacalteu based assay and also various phenolic compounds display different responses in Folin–Cioacalteu assay (Khoddami et al. 2013).
Quality characteristics of apple pomace - incorporated baked products
Buns
The results presented in Table 2 show that with an increase in DAP in the blend, the volume of the buns decreased from 235 to 100 cm3, thereby reducing the specific volume from 3.95 to 1.74 cm3/g. This is also reflected in the increase in firmness values from 375 g to 870 g. The crust and crumb colour scores decreased with increase in DAP. The fruit flavour increased with increase in the DAP content. The sensory scores for grain and texture were however lower for DAP incorporated samples when compared to control. Based on the data incorporation of 15 % DAP was found to be acceptable. To improve the quality characteristics, additives namely glycerol monostearate – GMS (0.25 %), sodium stearoyl lactylate – SSL (0.25 %), gluten (2 %) and α- amylase (1 mg) were added. Addition of additives increased the volume and specific volume while the firmness values reduced. The sensory scores of the buns with additives also increased, thereby increasing the overall quality scores. Kamel and Ponte (1993) reported that emulsifiers might have bound to the hydrophobic surface of the protein, resulting in a stronger protein network, thereby increasing the volume and better texture. Serna-Saldivar et al. (1988) reported that the volume depressing effect which was observed by the addition of soya bean and sesame meals was overcome by using the dough conditioner (SSL). Addition of the dough conditioner improved the bread volume, increased height and decreased bread density. These dough conditioners interact hydrophilically with glutenin, hydrophobically with gliadins and starch and hydrophobically/hydrophilically with soya bean protein. Thus the dough network becomes more extensible and has more gas retention capacity. Also amylases break the starch into dextrins which are acted upon by the yeast. It could be concluded that addition of GMS, SSL, gluten and α-amylase in combination helped in improving the quality characteristics of buns with 15 % DAP blend.
Table 2.
Quality characteristics of buns, muffins and cookies as affected by DAP
| Sample | Volume (cm3) | Specific volume (cm3/g) | Curst colour (9) | Crumb Colour (9) | Grain (9) | Texture | Overall Quality (9) | |
|---|---|---|---|---|---|---|---|---|
| O (g force) | S (9) | |||||||
| BUNS | ||||||||
| CON | 235 ± 4.3a | 3.95 ± 0.34a | 8.0a | 8.5a | 8.0a | 335 ± 3.6d | 8.0a | 8.0a |
| 10%DAP | 190 ± 2.3b | 3.25 ± 0.43b | 7.5b | 7.5b | 7.5a | 463 ± 2.9c | 7.5b | 7.5a |
| 15%DAP | 135 ± 4.1d | 2.33 ± 0.27c | 7.0c | 6.5c | 6.5b | 675 ± 4.2b | 6.5c | 6.5b |
| 20%DAP | 100 ± 3.0e | 1.74 ± 0.29d | 5.5d | 5.0d | 5.5c | 870 ± 7.9a | 5.5d | 5.5c |
| 15%DAP ± Add | 175 ± 3.3c | 3.01 ± 0.35b | 7.5b | 7.0b | 7.0ab | 451 ± 2.8c | 7.0b | 7.0b |
| SEM (+) | 2.56 | 0.13 | 0.14 | 0.11 | 0.13 | 3.16 | 0.16 | 0.12 |
| MUFFINS | ||||||||
| CON | 100 ± 3.1a | 2.22 ± 0.31a | 8.0a | 8.5a | 8.0a | 995 ± 3.8a | 8.0a | 8.0a |
| 10%DAP | 90 ± 2.2b | 1.98 ± 0.23b | 7.5b | 7.5b | 7.5a | 881 ± 3.0b | 7.5b | 7.5a |
| 20%DAP | 87 ± 3.0c | 1.92 ± 0.08b | 7.1c | 6.5c | 7.0b | 833 ± 4.4c | 7.0c | 7.0b |
| 30%DAP | 75 ± 3.3d | 1.66 ± 0.26c | 6.5d | 6.1d | 6.5c | 757 ± 4.1e | 6.5d | 6.5c |
| 40%DAP | 70 ± 2.7d | 1.53 ± 0.18c | 6.0d | 5.5e | 5.5d | 707 ± 5.1f | 5.5e | 5.5d |
| 30%DAP + Add | 90 ± 3.1b | 1.99 ± 0.15b | 7.0c | 6.6c | 7.0b | 795 ± 5.8d | 7.0c | 7.0b |
| SEM (+) | 1.24 | 0.11 | 0.14 | 0.11 | 0.13 | 5.12 | 0.16 | 0.12 |
| COOKIES | Spread (mm) | Spread ratio | Surface cracking (9) | Crumb colour (9) | Texture | Overall quality (9) | ||
| O (g force) | S (9) | |||||||
| CON | 86.2 ± 4.0a | 6.46 ± 0.14a | 8.0a | 8.0a | 3640 ± 3.6d | 8.0a | 8.0a | |
| 10%DAP | 83.4 ± 2.1b | 6.24 ± 0.20b | 7.5b | 7.5ab | 3833 ± 2.9c | 7.4b | 7.7a | |
| 20%DAP | 80.3 ± 1.9c | 6.01 ± 0.17c | 7.0c | 6.5c | 4103 ± 4.2b | 6.5c | 6.5b | |
| 30%DAP | 75.4 ± 3.1d | 5.76 ± 0.19d | 6.0d | 6.1d | 5248 ± 4.9a | 5.5d | 5.5c | |
| 20%DAP + Add | 83.2 ± 3.8b | 6.24 ± 0.15b | 7.5b | 7.0b | 3860 ± 2.8c | 7.5b | 7.0ab | |
| SEM(±) | 2.09 | 0.11 | 0.12 | 0.11 | 2.9 | 0.16 | 0.14 | |
Values in the parenthesis indicate the maximum score; Values for a particular column followed by different letters differ significantly (p < 0.05); SEM - Standard error of mean at 25 degrees of freedom, Values are means ± standard deviation (n = 4); CON- wheat flour (100 %); Add-Additives-gluten-2 %, Glycerol monostearate-0.25 %, Sodium stearoyl lactylate-0.25 % and fungal α-amylase-1 mg for buns; Sodium stearoyl lactylate 0.5 % for muffins; Glycerol monostearate-0.25 % and Sodium stearoyl lactylate-0.25 % for cookies; O-Objective; S-Subjective
Muffins
Muffins were prepared by replacing wheat flour at 10, 20, 30 and 40 % levels of DAP. As the incorporation of DAP increased from 0 to 40 %, the volume and specific volume of the muffin decreased from 100 to 70 cm3 and 2.22 cm3/g to 1.53 cm3/g (Table 2). The texture of the muffins measured objectively using texture analyzer showed that the muffins became softer with increase in levels of DAP. Sensory evaluation of the muffins showed that the scores for crust and crumb colour decreased significantly in 30 and 40 % levels of DAP. The shape of the muffins was symmetrical and normal up to 20 % level and with further increase to 40 % the muffins became slightly flat. Up to 20 %, the crumb colour was appealing. There was a gradual reduction in the scores for crumb grain, texture and taste with increase in DAP. Though the overall quality scores of acceptability were lowered, the muffins prepared from 20 % DAP were highly acceptable. The scores for crumb color decreased significantly as it changed from creamish yellow to brown color and with increasing levels of DAP and the muffins had pleasant fruit flavour. Though the overall quality scores reduced, the cakes prepared from 20 % DAP was highly acceptable. To improve the volume and texture, SSL (0.5 %) was added as SSL is more hydrophilic in nature with an HLB value of 10–12 which help in incorporation of air (Kamel and Ponte 1993).
Cookies
The results presented in Table 2 indicated that with an increase in the level of DAP from 0 to 30 %, the spread of the cookies decreased from 86.6 to 75.4 mm and the spread ratio decreased from 6.46 to 5.76. A decrease in the spread ratio of cookies was reported when wheat flour was supplemented with non-wheat flours (Sindhuja et al. 2005). This may be due to increase in dough viscosity by composite flour and it forms aggregates by competing with limited free water available in cookie dough. The breaking strength, which is the force required to break the cookies, increased from 3640 to 5248 g indicating an increase in the hardness with the addition of increasing levels of DAP. Effect of DAP on sensory characteristics of cookies showed that the surface cracking pattern which is an important quality attribute for cookies, was uniform up to 10 % DAP. However, at 20 % DAP level, the cookies possessed medium size islands and became big size at 30 % DAP incorporation. The texture of cookies containing more than 10 % DAP was very hard. At 20 % level of DAP, the cookies were slightly sweeter and had pleasant fruit flavour. 30 % DAP incorporated cookies were predominantly sweeter and has prominent fruit flavour. The above data indicates that the overall quality score of cookies decreased with increase in DAP. To improve the quality of cookies with 20 % DAP, additives namely glycerol monostearate (0.25 %) in combination with sodium stearoyllactylate (0.25 %) was used. The spread and spread ratio increased, whereas the breaking strength values decreased. Emulsifiers enhance the incorporation of air by creating great numbers of air bubbles thereby disperse the shortening in sufficiently small particles. Kamel and Ponte (1993) reported that these emulsifiers help in providing many nucleating sites for the water vapour to expand during baking which in turn resulting improve the texture of the biscuits. It could be concluded that acceptable quality cookies can be prepared using 20 % DAP.
Total phenolics
The total phenolic content in the water and methanol extracts of apple pomace (DAP) and the products prepared out of apple pomace was determined by Folin – Ciocalteau method. Results indicated higher amount of total phenolics in the methanol extract (DAPME) than water extract (DAPWE). Nearly 2.3 folds increase in total phenolics was observed in DAPME – 3.42 ± 0.42 mg/g when compared to 1.5 ± 0.14 mg/g in DAPWE. Similarly products prepared with DAP blending also showed increased phenolic content both in the methanol and water extract suggesting that DAP addition to bun, cookies and muffins may be responsible for the increase in phenolics as shown in Table 3.
Table 3.
Bioactivities of blended and unblended bakery products by DAP
| Samples | Total Phenolics Wet wt | Total Phenolics Dry Wt | IC50 μg/mL (FRS) Aqueous extract | IC50 μg/mL (FRS) Methanol extract | Expected % activity by AP | % FRS in Aqueous Extract | RBC protection (IC50 μg/mL GAE phenolics) | Fold difference in the Total activity |
|---|---|---|---|---|---|---|---|---|
| DAPWE | 1.5 ± 0.14b | - | 38.5 ± 3.04a | - | - | - | - | |
| DAPME | 3.42 ± 0.42a | - | - | 19.7 ± 1.54a | - | - | - | - |
| Bun - DAP | 0.27 ± 0.04f | 0.38 ± 0.05c | 6.4 ± 0.40d | 16.5 ± 1.28c | - | 13 | 2.25 ± 0.21c | 1.0 |
| Bun + DAP | 0.50 ± 0.07d | 0.59 ± 0.08b | 6.9 ± 0.42d | 17.5 ± 1.32b | 7 | 25 (>1.7 fold) | 3.79 ± 0.45b | 1.1 |
| Muffin- DAP | 0.14 ± 0.02g | 0.27 ± 0.04d | 8.0 ± 0.72c | 9.7 ± 1.1d | - | 6 | 1.77 ± 0.11d | 1.0 |
| Muffin + DAP | 0.32 ± 0.05e | 0.33 ± 0.05c | 5.9 ± 0.78e | 9.2 ± 1.07d | 4 | 14 (>2.0 fold) | 2.19 ± 0.22c | 3.96 |
| Cookies - DAP | 0.21 ± 0.03f | 0.21 ± 0.08d | 9.4 ± 1.04c | 19.3 ± 1.48a | - | 4 | 1.28 ± 0.14d | 1.0 |
| Cookies + DAP | 0.69 ± 0.09c | 0.761 ± 0.09a | 20.2 ± 1.41b | 18.1 ± 1.41b | 2.4 | 6 (>1.1 fold) | 4.98 ± 0.94a | 1.0 |
Values are means ± standard deviation (n = 3); values sharing same superscript (a-e) in column are not statistically significant at p < 0.05. Expected % activity of DAP is calculated by values obtained for added DAP in the product
Fold increase in the activity is expressed as difference between DAP blended product when compared to the respective unblended product. Data thus clearly reveals fold increase in total activity in blended product
DPPH radical scavenging activity
DPPH radical scavenging activity of water and methanol extracts was evaluated by DPPH radical scavenging assay. Table 3 shows percent DPPH radical scavenging activity of phenolic extracts. 80 % inhibition with an IC50 of 1.2 ± 0.270 μg/mL was observed for gallic acid. The phenolic extracts showed free radical scavenging activity with an IC50 ranging from 5.9–20 μg/mL in water extracts; while 9–19 μg/mL in methanol extracts. 2 - fold better activities were observed in Apple pomace methanol extract (DAPME) (IC50 – 19.7 ± 1.54 μg/mL) than that of water extracts- DAPWE (IC50- 38.5 ± 3.04 μg/mL). However water extracts of DAP blended products exhibited better activity than methanol extracts. Blended muffin showed better radical scavenging activity than the unblended product. No significant difference however was observed in bun. Cookies showed reduced radical scavenging activity in the blended samples suggesting the loss of free radical scavenging potential during processing of cookies. Addition of DAP showed different effects on DPPH activity from unblended bakery products to blended bakery products, where in aqueous extract, blended muffins showed ~1.35 folds better antioxidant activity. But, cookies exhibited ~2.14 folds lesser activity and there were no significant difference observed with respect to buns. Whereas, in methanol extract there was no significant difference between unblended to blended samples of all the three bakery products. Table 3 thus may suggest some inadvertent interaction of DAP with constituents of cookies during its preparation.
Cytoprotective ability
RBC protection
Red Blood Cells (RBCs) were used as a model to study the cytoprotective ability of extracts of DAP blended products. Free radicals generated by Fenton’s reagent by its thermal decomposition attacked the erythrocytes to induce a chain oxidation of lipid and protein, disturbing the membrane organization and eventually leading to haemolysis. 250 μL of 100 mM and 10 mM concentration of ascorbic acid and ferrous sulphate to 1:9 (v/v) dilutions could induce 100 % haemolysis in human erythrocytes. A protective effect was evident as we could see the inhibition of haemolysis as measured at A535 nm. Results indicated that DAPWE showed a potent RBC protective ability with an IC50 of 0.97 ± 0.10 μg/mL. Although no significant changes were observed at IC50 concentration, total activity was found to be either equal or better in the blended sample, due to increased phenolic contents as shown in Table 3. Activity was much higher in the blended muffin > bun > cookie suggesting that conditions of baking is important for preserving bioactivity of the blended sample (Fig. 1).
Fig. 1.
RBC protectivity of PBS extract of DAP control and DAP incorporated products
Protectivity against buccal mammalian cells was studied. As indicated in Fig. 2 oxidants induced cellular damage as revealed by disruption of cellular components. Treatment of cells with DAP blended products offered better protection than unblended products suggesting that DAP blending is contributing to cytoprotective ability of the product.
Fig. 2.
Buccal cell Protective ability of aqueous extracts of DAP control and DAP incorporated products
DNA protection
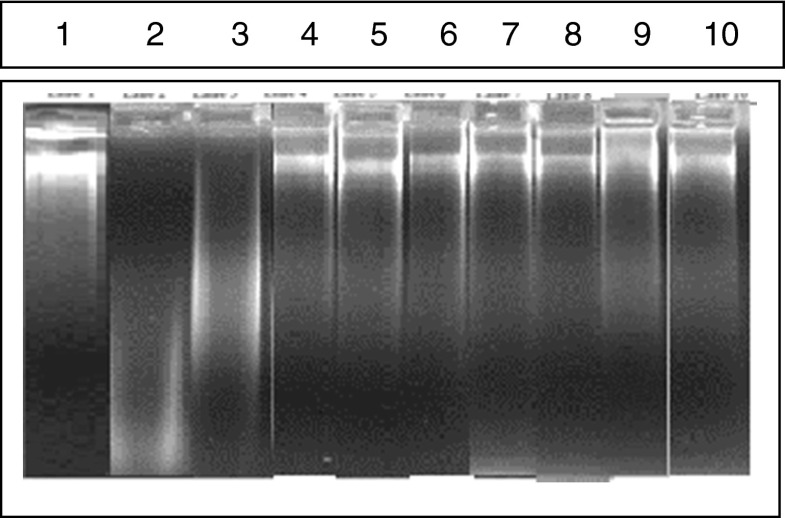
The efficiency of extracts of DAP/DAP blended product in preventing oxidative damage of DNA was also evaluated. The hydroxyl radical generated by Fenton’s reagent caused DNA damage which resulted in faster migration. This fragmentation was recovered by the treatment of different extracts in addition to BHA, the known standard antioxidant. The intensity of bands was measured by densitometry and percentage of protection of DNA was calculated. IC50 of the extracts is as follows – 10.0, 3.2, 5.4 and 3.1 μg/mL for water extracts of DAP, unblended bun, cake and cookie respectively. No significant difference was observed in the blended sample also (Fig. 3).
Fig. 3.
Electrophoresis analysis of DNA protection by extracts of DAP – and DAP + Products. Lane-3: : BHA treated DNA; Lane-4: : Apple pomace treated DNA; Lane-5: : Bun - DAP treated DNA; Lane-6: : Bun + DAP treated DNA; Lane-7: : Muffin - DAP treated DNA; Lane-8: : Muffin + DAP treated DNA; Lane-9: : Cookie - DAP treated DNA; Lane-10: : Cookie + DAP treated DNA
AP is the main by-product of cider industry and has physico-chemical composition suitable to use it as a source for functional foods via value addition, particularly with various biological activities. Earlier studies although have revealed the presence of carbohydrates, dietary fibre, phenolics and flavonoids, precise composition of sugar and phenolic acids present was not reported. Further, the usage of AP for health products preparations were based on the potential health benefits that can be offered by dietary fibres and phenolic antioxidants. In the current study we report for the first time the other biological activities also such as cytoprotectivity, DNA protectivity against the oxidative damage in the products that were prepared with DAP, in addition to proven antioxidant activity.
Bakery products such as bun, cookies and muffins that were prepared with DAP were evaluated for their acceptability, product texture and bioactivity. Precise measurement of activity before and after product preparation is highly warranted since the baking/processing conditions are known to affect the activity. Current study as expressed in the Table 3 clearly revealed, that the bioactivities were retained to different extent in various baked products and antioxidant/cyto/DNA protective properties were retained. Providing bioactivity in the end product is very essential, since the end users are either younger population of the globe, who like to take more of bakery products than the conventional foods; or the older population, who cannot take conventional foods for various reasons. The selected group of baked products is well known for wider acceptability for all age groups. Further, addition of DAP also improved the flavour to the product and this therefore enhanced the sensory scores also.
As reported in Table 1 presence of enriched amounts of dietary fibres (26.4 ± 0.3 %), phenolic acids (1.5 ± 0.14 %) and flavonoids (3.92 ± 0.26 %) in AP justifies the biological activity that is demonstrated. As we have reported in our earlier investigations, enhanced amount of caffeic acid potentially contributes to H+K+-ATPase inhibitory activity (Siddaraju and Dharmesh 2007a, b), which has an impact in reducing the gastric ulcers. Epicatechin (24.63 mg/100 g), quercetin (24.56 mg/100 g), catechin (9.68 mg/100 g) and gallic acid (5.46 mg/100 g) present in significant amounts may contribute to cyto/DNA protectivity by virtue of antioxidant activity. Free oxidative radicals are known to attack cells and DNA leading to cellular and DNA damage (Harish Nayaka et al. 2010) during various chronic disease conditions. Consumption of antioxidant components containing AP-based products may therefore envisage protection against several health hazardous conditions that can be encountered during one’s life time due to unavoidable factors such as exposure to UV, pollution, infective agents, etc. Since basal activity was also exhibited in the products that were prepared without DAP, it may mean that the ground substance that were used for the preparation of the above mentioned bakery products may also contribute towards the activity. Addition of DAP however, potentially may enhance their bioactive potential (Table 3; Fig. 3).
Conclusion
Current study provided evidence for the use of AP in functional food preparation, particularly in bakery products that can contribute effectively to health beneficial properties against several life style disorders such as diabetes, cancer, ulcer, atherosclerosis, etc. Contribution to health beneficial properties from flavonoids and other constituents present in addition to phenolic acids and dietary fibres cannot be ruled out.
Acknowledgment
We are grateful to the Director of the Institute, for constant encouragement throughout the course of study. One of the authors Ms. Hasitha P acknowledges University Grant Commission, New Delhi for financial assistance as JRF and SRF.
Abbrevations
- DPPH
2, 2- diphenyl-1-picrylhydrazyl
- BHA
Butylated hydroxyl anisole
- MNU
N-methyl – N-Nitrosourea
- FRS
Free Radical Scavenging
- DAP
Dehydrated apple pomace
- PBS
Phosphate buffer saline
- DNA
Deoxy ribo Nucleir acid
Footnotes
Research highlights
1. This study was taken up in order to utilize the biowaste from fruit processing industry.
2. The apple pomace was chemically characterized in order to understand its probable bioactivity.
3. To study the acceptability levels by incorporating DAP in bakery products.
4. To develop a value added product and to look for the activity spectrum in it after baking.
5. The developed baked product proved as a potential health food.
References
- AACC, Approved methods of the AACC, (10th Ed.,) (2000) American Association of Cereal Chemists, St. Paul, MN (Methods 08–01, 30–25, 44-15A, 46–10, 54–10, 54–21)
- Asp NG, Jahnson CG, Hallmer H, Silijestrom M. Rapid enzymatic assay of insoluble and soluble dietary fiber. J Agri Food Chem. 1983;31(3):476–482. doi: 10.1021/jf00117a003. [DOI] [PubMed] [Google Scholar]
- Chen H, Rubenthaler GL, Schanu EG. Effect of apple fiber and cellulose on the physical properties of wheat flour. J Food Sci. 1988;53(1):304–305. doi: 10.1111/j.1365-2621.1988.tb10242.x. [DOI] [Google Scholar]
- Chen H, Rubenthaler GL, Leung HK, Barnowki JD. Chemical, physical and baking properties of apple fiber compared with wheat and oat bran. Cereal Chem. 1988;65(3):244–247. [Google Scholar]
- Chetana SML, Khyrunnisa B, Ramasarma PR. Nutritional characteristics of linseed/flaxseed (Linum usitatissimum) and its application in muffin making. J Texture Stud. 2010;41:563–578. doi: 10.1111/j.1745-4603.2010.00242.x. [DOI] [Google Scholar]
- Dewanto V, Wu X, Adom KK, Liu RH. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agric Food Chem. 2002;50:3010–3014. doi: 10.1021/jf0115589. [DOI] [PubMed] [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method of determination of sugars and related substances. Anal Chem. 1956;28(3):350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- Gains CS. Instrumental measurement of hardness of cookies and crackers. Cereal Foods World. 1991;36:989, 991–994, 996. [Google Scholar]
- Harish Nayaka MA, Sathisha UV, Dharmesh SM. Cytoprotective and antioxidant activity of free, conjugated and insoluble-bound phenolic acids from swallow root (Decalepis hamiltonii) Food Chem. 2010;119(4):1307–1312. doi: 10.1016/j.foodchem.2009.08.044. [DOI] [Google Scholar]
- Harish Nayaka MA, Sathisha UV, Manohara MP, Chandrashekara KB, Dharmesh SM. Cytoprotective and antioxidant activity studies of jaggery sugar. Food Chem. 2009;115(1):113–118. doi: 10.1016/j.foodchem.2008.11.067. [DOI] [Google Scholar]
- Hassid WZ, Neufeld EF (1964) Quantitative determination of starch in plant tissue. In: Methods in Carbohydrate Chemistry, Whistler RL(ed.) Vol 4. Academic Press, New York, pp 33–36
- Khoddami A, Wilkes MA, Roberts TH. Techniques for analysis of plant phenolic compounds. Molecules. 2013;18:2328–2375. doi: 10.3390/molecules18022328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamel B, Ponte J. Emulsifiers in baking. In: Kamel B, Stauffer C, editors. Advances in baking technology. London: Blakie; 1993. pp. 179–222. [Google Scholar]
- Masoodi FA, Bhavana S, Chauhan GS. Use of apple pomace as a source of dietary fiber in cakes. Plant Food Hum Nutr. 2002;57(2):121–128. doi: 10.1023/A:1015264032164. [DOI] [PubMed] [Google Scholar]
- Nanjarajurs SM, Dharmesh SM, Shivaleela B, Mallikarjuna SE, Rajarathnam S. Health and wellness product from mangosteen (Garcinia mangostana l.) rind: bioactive potentials. Int J Biotechnol Wellness Ind. 2014;3:111–120. doi: 10.6000/1927-3037.2014.03.04.1. [DOI] [Google Scholar]
- Nethravathi GP, Sathisha UV, Dharmesh SM, Shashirekha MN, Rajarathnam S. Anti-oxidant activity of indigenous edible mushrooms. J Agri Food Chem. 2006;54(26):9764–9772. doi: 10.1021/jf0615707. [DOI] [PubMed] [Google Scholar]
- Sathisha UV, Smitha L, Dharmesh SM. Antiproliferative, antioxidant and cyto/DNA protective properties in Andrographis serpyllifolia: role of andrographolide and phenolic acids. J Complementary Iintegr Med. 2009;6:1553–3840. [Google Scholar]
- Serna-Saldivar SO, Lopez-Ahumada G, Ortega-Ramirez R, Abril Dominguez R. Effect of sodium stearoyl-2- lactylate on the rheological and baking properties of wheat bread fortified with defatted soybean and sesame meal. J Food Sci. 1988;53(211–214):230. [Google Scholar]
- Siddaraju MN, Dharmesh SM. Inhibition of gastric H+,K+-ATPase and Helicobacter pylori growth by phenolic antioxidants of Curcuma amada. J Agri Food Chem. 2007;55(18):7377–7386. doi: 10.1021/jf070719r. [DOI] [PubMed] [Google Scholar]
- Siddaraju MN, Dharmesh SM (2007b) Inhibition of gastric H+,K+-ATPase and Helicobacterpylori growth by phenolic antioxidants of Zingiber officinale. Mol Nut Food Res 51(3):324–332 [DOI] [PubMed]
- Sindhuja A, Sudha ML, Rahim A. Effect of incorporation of Amaranth flour on the quality of cookies. Eur Food Res Technol. 2005;221(5):597–601. doi: 10.1007/s00217-005-0039-5. [DOI] [Google Scholar]
- Srikanta BM, Siddaraju MN, Dharmesh SM. A novel phenol-bound pectic polysaccharide from Decalepis hamiltonii with multi-step ulcer preventive activity. World J Gastroenterol. 2007;13(39):5196–5207. doi: 10.3748/wjg.v13.i39.5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel RGD, Torrie JH. Principles and procedures of statistics. 2nd. New York: McGraw-Hill; 1980. [Google Scholar]
- Sudha ML, Rajeswari G, Venkateswara Rao G. Chemical composition, rheological, quality characteristics and storage stability of buns enriched with coriander and curry leaves. J Food Sci Technol. 2012;51(12):3785–3793. doi: 10.1007/s13197-013-0930-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudha ML, Baskaran V, Leelavathi K. Apple pomace as a source of dietary fiber and polyphenols and its effect of the rheological characteristics of cake making. Food Chem. 2007;104(2):686–692. doi: 10.1016/j.foodchem.2006.12.016. [DOI] [Google Scholar]
- Watada AE. A high-performance liquid chromatography method for determining ascorbic acid content of fresh fruits and vegetables. Hortic Sci. 1982;17(3):334–335. [Google Scholar]