Abstract
The simultaneous optimization of a synergistic blend of oleoresin sage (SAG) and ascorbyl palmitate (AP) in sunflower oil (SO) was performed using central composite and rotatable design coupled with principal component analysis (PCA) and response surface methodology (RSM). The physicochemical parameters viz., peroxide value, anisidine value, free fatty acids, induction period, total polar matter, antioxidant capacity and conjugated diene value were considered as response variables. PCA reduced the original set of correlated responses to few uncorrelated principal components (PC). The PC1 (eigen value, 5.78; data variance explained, 82.53 %) was selected for optimization using RSM. The quadratic model adequately described the data (R2 = 0. 91, p < 0.05) and lack of fit was insignificant (p > 0.05). The contour plot of PC 1 score indicated the optimal synergistic combination of 1289.19 and 218.06 ppm for SAG and AP, respectively. This combination of SAG and AP resulted in shelf life of 320 days at 25 °C estimated using linear shelf life prediction model. In conclusion, the versatility of PCA–RSM approach has resulted in an easy interpretation in multiple response optimizations. This approach can be considered as a useful guide to develop new oil blends stabilized with food additives from natural sources.
Keywords: Salvia officinalis L, Ascorbyl palmitate, Sunflower oil, Multiple response optimization, Principal component analysis, Response surface methodology
Introduction
Over the years, considerable evidence has been gathered on the antioxidant effects of different compounds in food products. The natural antioxidants from plants have been solitary studied for their antioxidant potential in the food matrix. Among them, the spices and herbal extracts, tocopherols, ascorbic acid, citric acid and carotenoids are widely covered. Among spices, the sage (Salvia officinalis) has been used widely investigated for its food applications (Cuvelier et al. 1996; Frankel et al. 1996). It is commercially available in the market in different formulations such as oleoresin, seasoning and flavoring agent. In our previous investigation, the oleoresin sage (SAG) was tested for its in-vitro antioxidant activity by different assays (Upadhyay and Mishra 2014). Hamied et al. (2009) and Upadhyay and Mishra (2015a) reported an excellent preservative action of SAG to improve the oxidative stability of vegetable oils.
SAG is good source of primary antioxidants, which mainly possess radical scavenging and reducing capacity, whereas citric acid (CA) and ascorbyl palmitate (AP) are secondary antioxidants, which primarily act as chelating agent and oxygen scavengers, respectively (Kochhar and Rossell 1990). The synergistic effects of natural antioxidants in enhancing the oxidative stability of sunflower oil (SO) have been previously reported (Hras et al. 2000; Upadhyay and Mishra 2015b). These studies clearly highlighted the importance of effective concentration of antioxidants in defining the synergistic and antagonistic effects. In addition, there are reports which also indicated the antagonistic effect of natural antioxidants (Hopia et al. 1996; Hras et al. 2000). Therefore, the additional knowledge about the concentration dependent stabilization effect of natural antioxidants in a lipid based food matrix is required to progress in this area. It is very important to identify the optimal concentration of natural antioxidant mixtures which can significantly improve the oxidative stability of vegetable oils and oil based products.
There are different approaches for the blend optimization involving natural antioxidants by taking into consideration single response at a time, followed by optimization of other responses with the domain of desired limits (Jaswir and Man 1999; Jaswir et al. 2000). A relatively straightforward approach when there are only few response variables is to overlay the contour plots (Myers and Montgomery 2002). However, the overlaying of contour plots becomes difficult when there are more than three responses involved. Interestingly, there are useful approaches for simultaneous optimization of multiple response variables which are based on non-linear programming methods (Carlyle et al. 2000), desirability function (Harrington 1965), dual responses (Myers and Montgomery 2002) and square error loss (Ames et al. 1997). However, their application in the subject areas linked to the food research is rarely looked into.
In this work, a quite practical and effective strategy based on principal component analysis (PCA) and response surface methodology (RSM) was explored for tackling multiple response optimizations. This procedure was firstly presented by Bratchell (1989) and latter demonstrated by Ellekjaer et al. (1996) and Ribeiro et al. (2010). Although several statistical experimental design investigations involving natural antioxidants for the shelf life extension of vegetable oils can be found in literature (Kochhar and Rossell 1990; Jaswir and Man 1999; Hras et al. 2000; Jaswir et al. 2000), examples of optimization where simultaneous PCA and RSM approach to develop a synergistic blend of SAG and AP have not been undertaken. Through this work, we have attempted to evaluate the methodology by combining the PCA–RSM using central composite rotatable design (CCRD) to minimize the complexity analysis of several dependent variables. A wider concentration range of SAG and AP were tested to determine their oxidative stabilization effect in SO. The scheme was investigated to get the optimal concentration of SAG and AP to be added into refined, bleached and deodorized SO to improve its oxidative stability and prolong the shelf life.
Theoretical explanation of PCA–RSM approach
The routine physicochemical analyses of vegetable oils e.g., peroxide value, anisidine value and free fatty acids performed during the storage experiments often provide solutions which vary greatly in their interpretation. This is due to the difference in experimental conditions involved with different physicochemical parameters. This presents difficulty in determining the most influential parameter to be used as response variables in experimental designs pertaining shelf life extension of vegetable oils. Therefore, no experimental condition can be found under which design variables can be optimized. According to Ribeiro et al. (2010), if some or all of response variables are relatively correlated, then the original set of correlated responses can be reduced to one or very few uncorrelated PCA components. The scheme presented in this work minimized the complex analysis of multiple response variables using PCA coupled with optimization using RSM. The method is founded on two primary considerations, as follows:
Testing the correlations between the multiple response variables, i.e., the original responses in the experiment. If the responses exhibit reasonable mutual correlation (R > 0.6), the corresponding correlations are related to variations in the system (Montgomery and Runger 2003).
Applying PCA to reduce the redundant information when the original responses are correlated. Therefore, the response variables which are correlated can be grouped together into one main element which contained the important information as contained in the original set of responses (Ribeiro et al. 2010).
It is important to note that the PCA can only be applied when significant correlation between the principal components and original responses are observed. In this case, the original response variables can be replaced by the score values of a principal component which could explain the maximum variance in the data set. The score of first few principal components could act as new response variable for the optimization using RSM (Beebe et al. 1998).
Materials and methods
Materials
A fresh lot of refined, bleached and deodorized SO (without added antioxidant), SAG (Dalmatian variety/oil soluble formulation) and AP were procured from Synthite Industries Limited (Kerala, India). The SO used in the experiments had initial peroxide value ≤ 1.5 milliequivalents of O2/kg oil and fatty acid composition of 6.3, 3.6, 21.7 and 68.4 % of C16:0, C18:0, C18:1 and C18:2 fatty acids, respectively, as determined using gas chromatography (TSQ 8000, Triple Quadrupole GC-MS/MS, Thermo Fisher Scientific, USA). In our previous investigation, the major phenolic compounds in SAG viz., carnosic acid (CA) and carnosol (CAR) were quantified to be 11.20 and 5.33 %, respectively (Upadhyay and Mishra 2014). The chemical kit for the determination of antioxidant capacity of lipid-soluble compounds (Kits No. 360:003.24) in the oil samples was purchased from Analytik Jena, Germany. All the other chemicals and solvents (analytical grade) were purchased from Merck, India. Ultrapure water (conductivity < 3 μS/cm), purified using a Mili-Q-system (Milipore, Bedford, USA), was used for Rancimat test.
Sample preparation
The SO was blended with SAG and AP in accordance with the experimental plan as indicated in Table 1. SAG and AP were weighed accurately and added to the oil samples contained in ambered glass bottles (capped), followed by stirring for 20 min at 50 °C in a water bath to obtain different SO blends (SOB). Once the blend was dissolved and homogeneity achieved, the blends were removed from the water bath and transferred to an oven maintained at 60 ± 1 °C in the dark for an accelerated storage study. The oxidative stability of the blends was determined at scheduled time interval (5 days) by measuring different physicochemical parameters.
Table 1.
Central composite rotatable design (CCRD) for the two independent variables indicating the original response variable and principal component 1 (PC1) score as new response variable£
| Trial No. | Independent variables# (Coded values) | Original response variables¥ | New response | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| X 1 | X 2 | PVa† | AVb† | FFAc* | IPd* | TPMe† | CDVf† | ACLg† | PC1 score | |
| SOB4 | 1 (1309.62) | 1 (270.71) | 9.60 ± 0.23a | 5.43 ± 0.09abc | 0.07 ± 0.01a | 23.88 ± 0.22h | 9.69 ± 0.21a | 1.84 ± 0.04d | 2.39 ± 0.01ef | −1.125 |
| SOB5 | −1.414 (200) | 0 (200) | 17.16 ± 0.30f | 7.83 ± 0.21e | 0.09 ± 0.00a | 18.38 ± 0.14a | 10.49 ± 0.51g | 6.84 ± 0.12ef | 1.93 ± 0.04a | 2.184 |
| SOB9 | 0 (850) | 0 (200) | 11.08 ± 0.13b | 5.23 ± 0.15a | 0.07 ± 0.00a | 23.10 ± 0.34g | 9.68 ± 0.44a | 2.88 ± 0.11de | 2.32 ± 0.03de | −0.926 |
| SOB6 | 1.414 (1500) | 0 (200) | 11.14 ± 0.13b | 5.89 ± 0.06c | 0.08 ± 0.01a | 22.41 ± 0.11ef | 9.64 ± 0.18a | 2.66 ± 0.02b | 2.46 ± 0.01f | −0.732 |
| SOB1 | −1 (390.38) | −1 (129.29) | 14.46 ± 0.17d | 6.73 ± 0.06d | 0.09 ± 0.00a | 19.46 ± 0.22b | 10.34 ± 0.22f | 4.96 ± 0.03de | 2.07 ± 0.01b | 1.126 |
| SOB2 | 1 (1309.62) | −1 (129.29) | 15.55 ± 0.29e | 5.92 ± 0.13c | 0.08 ± 0.00a | 22.33 ± 0.34ef | 9.70 ± 0.19a | 2.11 ± 0.05a | 2.34 ± 0.01de | −0.364 |
| SOB12 | 0 (850) | 0 (200) | 11.41 ± 0.30bc | 5.64 ± 0.09abc | 0.08 ± 0.01a | 23.76 ± 0.13h | 9.75 ± 0.26bc | 3.22 ± 0.09de | 2.28 ± 0.02d | −0.634 |
| SOB8 | 0 (850) | 1.414 (300) | 11.26 ± 0.27b | 6.88 ± 0.09d | 0.09 ± 0.01a | 20.19 ± 0.17c | 9.84 ± 0.35c | 5.72 ± 0.05f | 2.17 ± 0.01c | 0.671 |
| SOB10 | 0 (850) | 0 (200) | 10.03 ± 0.28a | 5.82 ± 0.15bc | 0.07 ± 0.00a | 22.16 ± 0.23e | 9.64 ± 0.59a | 2.06 ± 0.10f | 2.19 ± 0.03c | −0.607 |
| SOB7 | 0 (850) | −1.414 (100) | 14.10 ± 0.29d | 6.88 ± 0.12d | 0.09 ± 0.00a | 20.43 ± 0.33c | 10.05 ± 0.19d | 4.71 ± 0.06c | 2.15 ± 0.01bc | 0.754 |
| SOB13 | 0 (850) | 0 (200) | 9.79 ± 0.16a | 5.83 ± 0.06bc | 0.08 ± 0.00a | 23.58 ± 0.41h | 9.73 ± 0.21b | 1.83 ± 0.03de | 2.28 ± 0.01d | −0.383 |
| SOB11 | 0 (850) | 0 (200) | 11.38 ± 0.18bc | 5.32 ± 0.07ab | 0.07 ± 0.01a | 22.56 ± 0.61b | 9.79 ± 0.24bc | 3.17 ± 0.03f | 2.16 ± 0.02c | −0.383 |
| SOB3 | −1 (390.38) | 1 (270.71) | 12.11 ± 0.28c | 7.14 ± 0.10d | 0.09 ± 0.01a | 21.76 ± 0.16d | 10.23 ± 0.32e | 4.33 ± 0.04d | 2.07 ± 0.02b | 0.818 |
£ All the CCRD trials were conducted in duplicates. The response variables were reported as mean ± standard error of four determinations, two for each set of CCRD trials
# X 1 and X 2 indicates the coded levels of sage (SAG) and ascorbyl palmitate (AP), respectively, added into the oil blends. Values in the parenthesis indicate the actual concentration (in ppm) of SAG and AP. ¥ Values within the same column denoted by different superscript letters are significantly different (p < 0.05)
aPeroxide value (meq O2/kg oil); bPara-anisidine value; cFree fatty acids (%); dInduction period (h); eTotal polar matter (%); fConjugated diene value (mmol/L); gAntioxidant capacity of lipid soluble compounds (μmol Trolox Equivalent/g); †Values indicative of day 5 of storage at 60 °C;* values indicative of day zero
Experiment design
A systematic optimization procedure based on RSM was carried out to obtain an optimized blend of SAG and AP added into SO. A CCRD with two independent factors, viz. SAG (200–1500 ppm) and AP (100–300 ppm) were coded at five levels (−1.414, −1, 0, 1, +1.414). The experimental design consisted of 13 points: 4 each at factorial (SOB1, 2, 3, 4) and axial points (SOB5, 6, 7, 8) and 5 (SOB9, 10, 11, 12, 13) at center points (Table 1). The replicates at center points were used to estimate pure error for the lack of fit test which indicates how well the selected model fits the data. With fewer than five or six replicates, the lack of fit test has very low power. The tested levels of SAG and AP were higher compared to literature reports (Jaswir and Man 1999; Hras et al. 2000) in order to relate the effect of concentration on the oxidative stability. All the experiments were performed in random order to minimize the introduction of bias into the measurements. Different physicochemical parameters, as described in the following section, were chosen as the response variables. PCA was performed on auto-scaled matrix of response variables to extract the principal components. As explained earlier, the principal component explaining the highest data variation was selected as new response variable for optimization. For the statistical analysis, the model coefficients were estimated by multiple regression analysis and validated by ANOVA. The optimal conditions were deduced by generating a contour plot using the fitted model equation followed by numerical optimization using desirability function methodology. To verify the adequacy of the developed model, triplicate trials was performed using optimized blend and compared with the predicted values.
Physicochemical analyses
The physicochemical parameters measured in this study were peroxide value (PV), para-anisidine value (AV), free fatty acids (FFA), total polar matter (TPM), induction period (IP), conjugated diene value (CDV) and antioxidant capacity of lipid soluble compounds (ACL). The PV (milliequivalents of O2/kg of oil), FFA (%) and TPM (%) were determined according to AOCS Official Methods (AOCS 2004), AOCS (1993) and IUPAC 2.507 method (IUPAC 1987), respectively. The AV and CDV were measured using a spectrophotometer (ELICO double beam SL 210 UV VIS spectrophotometer, India) according the IUPAC method described by Larmond (1987) and Saguy et al. (1996), respectively. The oxidative stability was determined by measuring the IP (h) using Rancimat 743 apparatus (Metrohm, Switzerland) operated under accelerated conditions (temperature: 100 °C, airflow rate: 20 L/h) (Farhoosh 2007). The ACL (μmol Trolox equivalent (TE)/g) of SOB samples were measured using photochemiluminescence (PCL) based assay using a Photochem® instrument (Analytik Jena, Germany) (Popov and Lewin 1999). The responses used for optimization by RSM viz., PV, AV, FFA, TPM and CDV were measured at day 5 of storage while IP and ACL values were determined prior to oxidation experiment at day zero.
Shelf life prediction
The shelf life of the fat and oil samples can be predicted within hours or days in tests conducted at elevated temperate. Since the rate of lipid oxidation is exponentially related to temperature, the shelf life of edible oils decreases logarithmically with increasing temperature (Farhoosh 2007). In order to estimate the shelf life of SOB samples, the Rancimat test was carried out at 4 different temperatures (100, 110, 120 and 130 °C) to determine the IP (h) at respective temperatures. The natural logarithm of IP (h) vs. absolute temperature (T, K) was plotted and the regression lines fitted to the data:
| 1 |
where a and b are the equation parameters as the slope and intercept, respectively. The Eq. (1) was used to predict the shelf life (days) of different SOB samples by extrapolating the IP to lower temperatures (25 °C). The radar charts were used to characterize and compare the oxidative stability of SO blends having different proportions of SAG and AP. A radar chart is a graphical method of displaying multivariate data in the form of a two-dimensional chart of three or more samples represented on axes starting from the same point. It consists of a sequence of equi-angular spokes, called radii, with each spoke representing one of the samples. In present case, the length of a spoke is proportional to the magnitude of IP of an oil sample. A line is drawn connecting the IP values for each oil sample at 4 different temperatures (100, 110, 120 and 130 °C).
Statistical analysis
All the CCRD trials were conducted in duplicate sets. The response variables were reported as means of four determinations, two for each set of trials. Design expert (Version 7.0.0, Stat-Ease Int. Co., Minneapolis, USA) was used to construct the CCRD trials. The data obtained from the CCRD trials were statistically analyzed using ANOVA in order to test the model significance and suitability (p < 0.05). The multivariate analysis was carried out using OriginPro 9.1.0 statistical software (OriginLab Corporation, Northampton, USA). PCA was performed on the auto scaled data matrix, and the principal components were extracted so that the dimensionality of the original data matrix was reduced while retaining the maximum variability.
Results and discussion
The initial responses, considered in the statistical treatment and development of response surfaces, consisted of PV, AV, FFA, TPM, IP, CDV and ACL values obtained from the oxidation experiments of SOB samples. Since the multiple responses often make the statistical analysis complicated, the simultaneous optimization approach using PCA and RSM was applied to obtain an optimal synergistic combination of SAG and AP. The results obtained for different experimental trials during the oxidation experiments were summarized in the following sections.
Physicochemical parameters
Since the same lot of SO was used to formulate all the experimental combinations, the physicochemical parameters were found to have statistically similar values on day zero (data not shown). Hence, the physicochemical parameters of the SOB samples measured on day 5 of oxidation experiments were reported in Table 1. The experimental trials of SOB samples were found to have highly divergent range of PV, AV, FFA, TPM, IP, CDV and ACL which varied in accordance with the level of added SAG and AP. For instance the SOB4, which was found to be the most stable, had the highest value of ACL (2.39 μmol TE/g) and IP (23.88 h) and lowest values of PV (9.60 meq O2/ kg), AV (5.43), FFA (0.07 %), TPM (9.69 %) and CDV (1.84 mmol/L). In contrast the SOB5, the least stable sample, possessed the values (PV = 17.16 meq O2/kg, AV = 7.83, FFA = 0.09 %, IP = 18.38 h, TPM = 10.49 %, CDV = 6.84 mmol/L, ACL = 1.93 μmol TE/g) which were less desirable indicating relatively lesser oxidative stability. The preservative effect of SAG and AP was found to be concentration dependent from SOB5 (SAG: 200 ppm; AP: 200 ppm) upto SOB4 (SAG: 1309.62 ppm; AP: 271.71 ppm). This observation was consistent with the trend observed for PV, AV, FFA, TPM, CDV and IP, ACL which showed linear decrease and increase in their respective values. However, it is interesting to note that the oxidative stability of SOB6 (SAG: 1500 ppm; AP: 200 ppm) was lesser compared to SOB4 suggesting the pro-oxidant behavior. This observation was further supported by the findings of Rietjens et al. (2002) and Yen et al. (2002) which reported the pro-oxidant effects of phenolic compounds at higher concentration in the edible oils. Limited scientific investigations related to present study could not allow much comparison to be made with literature reports. However, the range of values obtained in this investigation for different physicochemical parameters could offer the benefit to other authors by allowing the comparisons to be made with other plant based extracts and lipid foods.
PCA
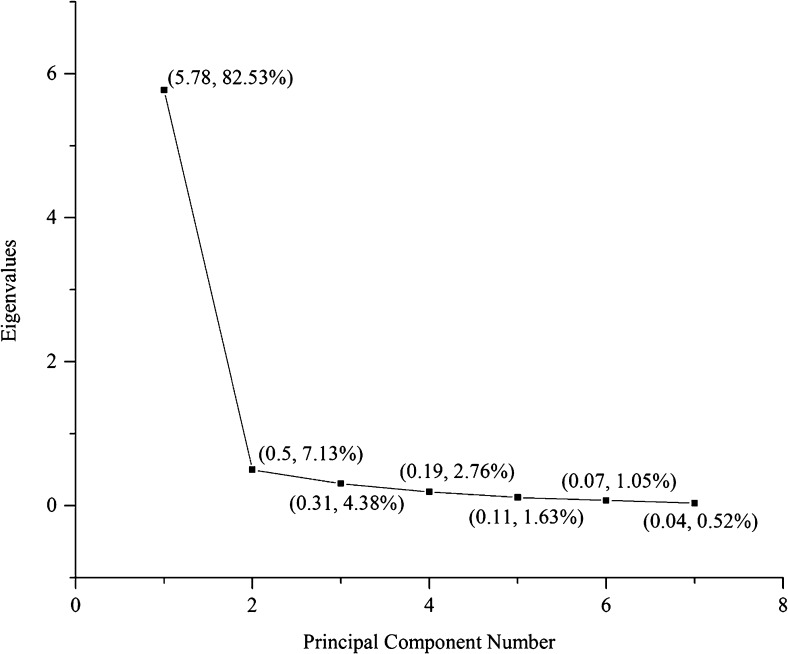
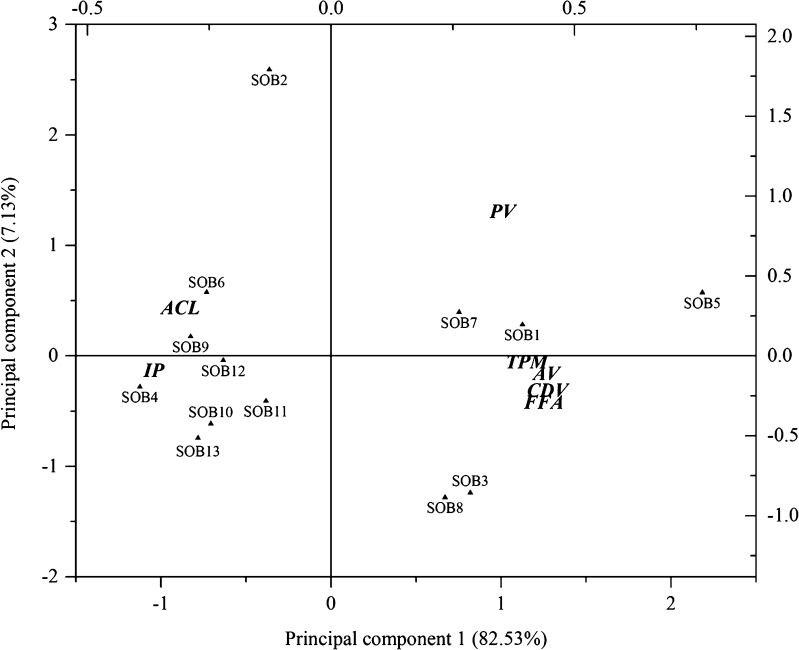
The oxidation experimental data was examined by PCA to visualize the response patterns in the feature space of principal components (PC). Two PC’s (PC1 and PC2), explaining the cumulative 89.66 % of the data variance, were chosen on based on the Eigen values (>0.5) (Fig. 1). All the measured parameters (PV, AV, FFA, IP, TPM, CDV and ACL) and different SOB samples were distributed in the biplot plot according to their loading and score values (Fig. 2). As can be seen in Fig. 2, PV, AV, FFA, TPM and CDV were positively loaded on the PC1 indicating the higher value of these parameters during storage. On the other hand, IP and ACL were located on the negative side of PC1 indicating the decrease in their values with storage. The AV, FFA, TPM and CDV values were loaded far from the centre and close to each other suggesting a strong correlation between them which is also evident in Table 2. Among the oil blends, SOB1, 5 and 7 were situated on the upper positive quadrant along PC1, indicating that they had higher values for PV while SOB8 and 3 possessed higher values of AV, FFA, TPM and CDV. SOB5 was found to be least stable among the blends according to physicochemical parameters. The loadings of PC2 had a strong correlation with IP and ACL suggesting that the SOB 4, 6, 9, 10, 11, 12 and 13 located along PC2 have higher values for this parameter and therefore, possessed comparatively better oxidative stability. The SOB4 was found to be situated at extreme left quadrant along PC2 and far from PC1 indicating the highest oxidative stability. The results obtained from PCA revealed that the measured physicochemical parameters had provided adequate information to differentiate the relative oxidative stability of SOB samples as function of added antioxidants.
Fig. 1.
The scree plot showing percentage variances described by the PCA model. The values in parenthesis indicate the Eigen value and percentage variance explained by different principal components extracted
Fig. 2.
Biplot plot obtained by principal component analysis illustrating the separation of sunflower oil blends (SOB)
Table 2.
Correlation matrix between the original response variables†
| PVa | AVb | FFAc | IPd | ACLe | TPMf | CDVg | |
|---|---|---|---|---|---|---|---|
| PV | 1.00 | ||||||
| AV | 0.67 | 1.00 | |||||
| FFA | 0.64 | 0.93 | 1.00 | ||||
| IP | −0.74 | −0.87 | −0.84 | 1.00 | |||
| ACL | −0.55 | −0.77 | −0.77 | 0.78 | 1.00 | ||
| TPM | 0.71 | 0.86 | 0.87 | −0.82 | −0.86 | 1.00 | |
| CDV | 0.63 | 0.86 | 0.91 | −0.89 | −0.80 | 0.83 | 1.00 |
† Significantly correlated at p < 0.05
a Peroxide value; b Para-anisidine value; c Free fatty acids; d Induction period; e Antioxidant capacity of lipid soluble compounds; f Total polar matter; g Conjugated diene value
Optimization using PCA–RSM
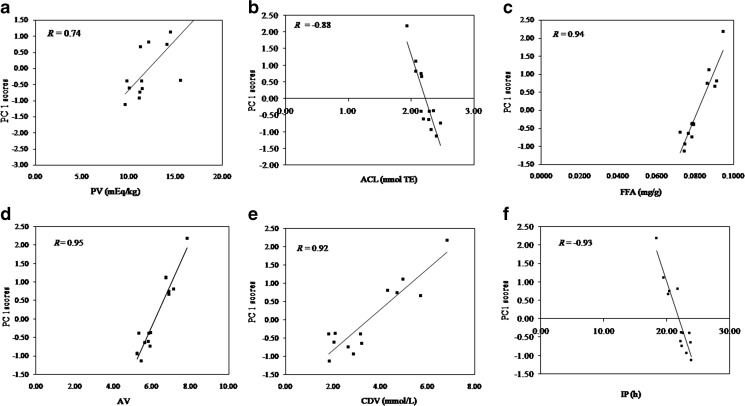
Since the PCA method was used to group the correlated variables, it is expected that the different responses with similar variations, as a function of changes in the experimental condition of the system, would be correlated. A direct correlation could be observed among multiple responses measured in this study (Table 2). The strategy for multiple response analysis takes advantage of minimizing the complex analysis of several dependent variables using PCA coupled with the experimental design. According to Ribeiro et al. (2010), once the correlation among the multiple responses is observed, the PCA can be used. The multiple responses can be supplanted by the scores of principal component (PC) explaining the highest percentage variance in the data. However, it is very important to verify the variance explained by the selected PC and the correlation between the scores from this component and each original response variable used in the PCA. If the explained variance and the correlations are satisfactory, the multiple response analysis will be substantive and reliable. In the present work, the PC 1 explained 82.53 % of the data variance and showed satisfactory correlation with all the measured original response variables (Fig. 3). Therefore, the statistical calculation was performed using PC 1 scores as a new response variable for the optimization using RSM.
Fig. 3.
The correlations between original response variables and the PC 1 score. Correlation between (a) Peroxide value (PV) and PC 1 scores (b) Antioxidant capacity of lipid soluble compounds (ACL) and PC 1 scores (c) free fatty acids (FFA) and PC 1 scores (d) Para-anisidine value (AV) and PC 1 scores (e) Conjugated diene value (CDV) and PC 1 scores and (f) Induction period (IP) and PC 1 scores
The PC 1 scores obtained from the experimental data of the CCRD trials were reported in Table 1. The regression analysis of developed model using ANOVA and the estimated regression coefficients for the independent variables were summarized in the Table 3. A second order polynomial model (Eq. (2)) satisfactorily described the experimental data which showed that PC 1 scores were significantly influenced by SAG and AP. The overall model adequately fitted to the experimental data (p < 0.05, R2 = 0.91, adjusted R2 = 0.84) and lack of fit found to be insignificant (p > 0.05). These results suggested that the fitted response model can be applied to determine the optimal points of a combination of SAG and AP.
| 2 |
where, Y represents the PC1 score while X1 and X2 represents the concentration of SAG and AP, respectively.
Table 3.
ANOVA of the quadratic model terms (Eq. (2)) applied to the values of principal component 1 scores as new response variable
| Variation source | SSa | dfb | MSc | F value |
p-value Prob > F |
|---|---|---|---|---|---|
| Regressiond | 10.51 | 5 | 2.10 | 13.49 | 0.0018 |
| X 1 | 7.14 | 1 | 7.14 | 45.82 | 0.0003 |
| X 2 | 0.18 | 1 | 0.18 | 1.13 | 0.3233 |
| X 1 X 2 | 0.05 | 1 | 0.05 | 0.33 | 0.5838 |
| X 1 2 | 1.80 | 1 | 1.80 | 11.57 | 0.0114 |
| X 2 2 | 1.75 | 1 | 1.75 | 11.26 | 0.0122 |
| Residual | 1.09 | 7 | 0.16 | ||
| Lack of Fit | 0.90 | 3 | 0.30 | 6.50 | 0.0512 |
| Pure Error | 0.19 | 4 | 0.05 | ||
| Total SS | 11.60 | 12 | |||
| R 2 | 0.91 |
a SS Sum of squares, b df Degree of freedom, c MS Mean squares, d X 1, SAG and X 2, AP
The constant term in Eq. (2) is negative which indicates that the desired value of PC 1 score should be in the negative scale. Therefore, the lower value of PC1 score is desired to obtain the oil blends with higher oxidative stability. The oxidative stability of oil blends is directly proportional to the IP and ACL values which are located on the negative side of PC 1 and possess the negative loading values (Fig. 2). Therefore, the greater the PC 1 score on negative scale, the higher will be the oxidative stability of oil blends. From the developed model, it can be observed that the linear effects of SAG and AP were negative (p < 0.05). A linear increase in the concentration of SAG and AP would leads to an overall decrease in PC 1 score. This appears to have protective effect in stabilizing the oil blends. This behavior is highly desirable and suggesting an important role of linear increase in concentration on the oxidative stability. On the other hand, the quadratic effects of SAG and AP were positive (p < 0.05) which indicated the pro-oxidant effects at higher concentrations. At higher concentration, a substantial reduction in oxidative stability of SO was observed as indicated from the PV, AV, FFA, TPM, IP, CDV and ACL values. The observed pattern is indicative of concentration dependent synergistic and antagonistic effects of natural antioxidants which has been previously reported (Rietjens et al. 2002; Yen et al. 2002; Upadhyay and Mishra 2015b), thus justifying the finding of the present investigation. Interestingly, the interactive effect of SAG and AP has negative sign (p < 0.05) which indicates their synergistic effect in improving the oxidative stability of oil blends. According to Hras et al. (2000), the primary and secondary antioxidant, acts synergistically to achieve better antioxidant effects. Murakami et al. (2003) had also observed the synergistic antioxidant effects of α-tocopherols and ascorbic acid with polyphenolic compounds. The fundamental mechanism involved in the redox reaction between the α-tocopherols and ascorbic acid where ascorbic acid helped in the regeneration of oxidized α-tocopherols to its reduced form. In that respect are other studies which suggested the in-vivo and in-vitro synergistic antioxidative effects of mixed natural antioxidants (Ock-Sook et al. 1991; Jaswir and Man 1999). Therefore, it can be proposed that the synergistic effects observed in this study could have a similar mechanism of action where AP could help in regeneration of oxidized antioxidant compounds in SAG. The results obtained in this study were encouraging and supporting the findings of our previous work (Upadhyay and Mishra 2015c). It can be implied that the synergistic interaction between SAG and AP could significantly improve the oxidative stability of SO compared to their individual effects.
Numerical optimization was performed with an aim to minimize the PC 1 score value. This numerical optimization evaluates a point that maximizes the desirability function. The two dimensional representation of RSM is illustrated in a contour plot (Fig. 4) which shows the effect of different levels of SAG and AP on PC 1 scores. Using the methodology of desirability function, an optimized synergistic blend of SAG and AP was obtained which consisted of 1289.19 ppm of SAG and 218.06 ppm of AP. This combination has a desirability of 0.982 yielding a predicted PC 1 score of −1.065. This predicted PC 1 value was found to be statistically close (p > 0.05) to PC 1 value (−1.125) for SOB4, with standard error of prediction of 0.44. The optimized combination was analyzed (n = 3) at day 5 of storage experiment at 60 °C for PV, AV, FFA, TPM and CDV giving 9.74 meq O2/ kg, 5.51, 0.07, 9.71 and 1.82 mmol/L, respectively. Likewise, on day zero of storage experiment, the ACL and IP values at 100, 110, 120 and 130 °C were measured to be 2.30 μmol TE/g and 23.21, 11.01, 5.37 and 2.26 h, respectively, which were close to SOB4 (p > 0.05). This indicates that the optimized blend exhibited almost comparable oxidative stabilization effects as possessed by SOB4, the most stable blend identified in this investigation.
Fig. 4.
The contour plot showing the effect of SAG and AP on PC 1 scores
Shelf life estimation
In the present study, the relationship between ln (IP) and T values for all the trial runs of SOB samples showed a linear dependency with good regression coefficients (R2 > 0.95). The oxidative stability measure of SOB samples was also illustrated in the form of a radar chart, by plotting their respective IP values obtained at 4 different temperatures (100, 110, 120 and 130 °C) (Fig. 5). As can be seen from the radar chart, SOB4 showed highest values of IP at all tested temperatures with highest predicted shelf life of 347 days at 25 °C. The results clearly highlighted the influence of concentration of added antioxidants on the shelf life of oil blends. The estimated shelf lives of SOB samples were consistent with the findings of our previous investigation where a similar experimental combination of ROS and AP were tested to develop mathematical models for shelf life prediction (Upadhyay and Mishra 2015b). The results obtained from shelf life estimation were found to be consistent with PCA–RSM optimization results. The predicted shelf life using optimized synergistic blend (SAG, 1289.19 ppm; AP, 218.06 ppm) was estimated to be 320 days which was close to the shelf life predicted for SOB4 (347 days) (SAG, 1309.62 ppm; AP, 270.71 ppm).
Fig. 5.
Radar chart illustrating the induction period (IP) values of sunflower oil blends (SOB) at different temperatures (100 °C, IP100; 110 °C, IP110; 120 °C, IP120 and 130 °C, IP130). The values in parenthesis indicate the estimated shelf life at 25 °C
Conclusion
The purpose of PCA for data compression prior to plotting the response surfaces was important to find the optimal level of synergistic blends of SAG and AP. With this approach, the multiple responses could be simultaneously analyzed without the requirement to use complex methodologies. The high correlation among the original response variables makes the role of the first component of PCA as the new response variable. The response surface analyses have shown the importance of synergistic effects of SAG and AP in slowing down the oxidation of SO. An optimal combination of 1289.19 ppm of SAG and 218.06 ppm of AP was obtained using PCA–RSM approach with a shelf life estimate of 320 days at 25 °C. In addition, synergistic effects of natural antioxidants from different sources in SO could be performed observing the approach investigated in this study. The versatility of PCA–RSM approach could permit an easy and efficient interpretation of experimental results in multiple response optimization studies. This scheme can help in preserving the precious data analyses time without loss of information while keeping a maximum diversity of original experimental data sets.
Acknowledgments
The authors gratefully acknowledge Synthite Industries Limited, India for providing the raw materials (oleoresin SAG and refined SO) and chemical standard (AP) to conduct this study. The financial support by Department of Biotechnology, Government of India (No. BT/FNS/01/05/2008 dated 25/03/2008) for this research is highly acknowledged. The authors have no conflict of interest to declare.
Footnotes
Research Highlights
• Sunflower oil blended with sage and ascorbyl palmitate at different levels.
• CCRD was used as experimental design to obtain an optimized synergistic blend.
• A newer methodology based on simultaneous optimization using PCA-RSM approach used.
• Optimized blend contain 1289.19 and 218.06 ppm of sage and ascorbyl palmitate.
• Versatility of PCA-RSM simplified the interpretation of multiresponse optimization.
References
- Ames AE, Mattucci N, Macdonald S, Szonyi G, Hawkins DM. Quality loss functions for optimization across multiple response surfaces. J Qual Technol. 1997;29:339–346. [Google Scholar]
- AOCS . Official methods and recommended practices of the American oil chemists’ society. Champaign: AOCS Press; 1993. [Google Scholar]
- AOCS (2004) Official methods and recommended practices of the American oil chemists’ society, Champaign
- Beebe KR, Pell RJ, Seasholtz MB. Chemometrics: a practical guide. New York: Wiley; 1998. p. 348. [Google Scholar]
- Bratchell N. Multivariate response surface modeling by principal components analysis. J Chemometr. 1989;3:579–588. doi: 10.1002/cem.1180030406. [DOI] [Google Scholar]
- Carlyle WM, Montgomery DC, Runger GC. Optimization problems and methods in quality control and improvement. J Qual Technol. 2000;32:1–17. [Google Scholar]
- Cuvelier ME, Richard H, Berset C. Antioxidative activity & phenolic composition of pilot plant & commercial extracts of sage & rosemary. J Am Oil Chem Soc. 1996;73:645–652. doi: 10.1007/BF02518121. [DOI] [Google Scholar]
- Ellekjaer MR, Ilseng MA, Naes T. A case study of the use of experimental design and multivariate analysis in product improvement. Food Qual Prefer. 1996;7:29–36. doi: 10.1016/0950-3293(95)00018-6. [DOI] [Google Scholar]
- Farhoosh R. The effect of operational parameters of the Rancimat method on the determination of the oxidative stability measures and shelf life prediction of soybean oil. J Am Oil Chem Soc. 2007;84:205–209. doi: 10.1007/s11746-006-1030-4. [DOI] [Google Scholar]
- Frankel EN, Huang SW, Aeschbach R, Prior E. Antioxidant activity of rosemary extract and its constituents, carnosoic acid, carnosol, and rosmarinic acid, in bulk oil and oil-in-water emulsion. J Agric Food Chem. 1996;44:131–135. doi: 10.1021/jf950374p. [DOI] [Google Scholar]
- Hamied AA, Nassar AG, Badry NE. Investigations on antioxidant & antibacterial activities of some natural extracts. World J Dairy Food Sci. 2009;4:1–7. [Google Scholar]
- Harrington J. The desirability function. Ind Qual Control. 1965;21:494–498. [Google Scholar]
- Hopia AI, Huang SW, Schwarz K, German JB, Frankel EN. Effect of different lipid systems on antioxidant activity of rosemary constituents carnosol and carnosic acid with and without α-tocopherol. J Agric Food Chem. 1996;44:2030–2036. doi: 10.1021/jf950777p. [DOI] [Google Scholar]
- Hras AR, Hadolin M, Knez Z, Bauman D. Comparison of antioxidative and synergistic effects of rosemary extract with α-tocopherol, Ascorbyl palmitate and citric acid in sunflower oil. Food Chem. 2000;71:229–233. doi: 10.1016/S0308-8146(00)00161-8. [DOI] [Google Scholar]
- IUPAC . Standard methods for the analysis oils and fats and derivatives. 7. Oxford: Pergamon; 1987. [Google Scholar]
- Jaswir I, Man YBC. Use optimization of natural antioxidants in refined, bleached, and deodorized palm olein during repeated deep-fat frying using response surface methodology. J Am Oil Chem Soc. 1999;76:341–348. doi: 10.1007/s11746-999-0241-x. [DOI] [Google Scholar]
- Jaswir I, Man YBC, Kitts DD. Optimization of physicochemical changes of palm olein with phytochemical antioxidants during deep-fat frying. J Am Oil Chem Soc. 2000;77:1161–1168. doi: 10.1007/s11746-000-0182-6. [DOI] [Google Scholar]
- Kochhar SP, Rossell JB. Detection, estimation and evaluation of antioxidants in food systems. In: Hudson BJF, editor. Food antioxidants. New York: Elsevier; 1990. pp. 19–64. [Google Scholar]
- Larmond E. Laboratory methods for sensory evaluation of food, publication 1637/E. Canada Department of Agricultural Research Branch: Ottawa; 1987. [Google Scholar]
- Montgomery DC, Runger GC. Applied statistics and probability for engineers. New York: Wiley; 2003. [Google Scholar]
- Murakami M, Yamaguchi T, Takamura H, Matoba T. Effects of ascorbic acid and α-tocopherol on antioxidant activity of polyphenolic compounds. Food Chem Toxicol. 2003;68:1622–1625. [Google Scholar]
- Myers RH, Montgomery DC. Response surface methodology. 2. New York: Wiley; 2002. p. 798. [Google Scholar]
- Ock-Sook YI, Han D, Shin HK. Synergistic antioxidative effects of tocopherol and ascorbic acid in fish oil/lecithin/water system. J Am Oil Chem Soc. 1991;68:881–883. doi: 10.1007/BF02660606. [DOI] [Google Scholar]
- Popov I, Lewin G. Photochemiluminescent detection of antiradical activity. VI. Antioxidant characteristics of human blood plasma, low density lipoprotein, serum albumin and amino acids during in-vitro oxidation. Luminescence. 1999;14:169–174. doi: 10.1002/(SICI)1522-7243(199905/06)14:3<169::AID-BIO539>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Ribeiro JS, Teófilo RF, Augusto F, Ferreira MMC. Simultaneous optimization of the microextraction of coffee volatiles using response surface methodology and principal component analysis. Chemom Intell Lab Syst. 2010;102:45–52. doi: 10.1016/j.chemolab.2010.03.005. [DOI] [Google Scholar]
- Rietjens IMCM, Boersma MG, Haan L, Spenkelink B, Awad HM, Cnubben NHP, Zanden JJ, Woude H, Alink GM, Koeman JH. The pro-oxidant chemistry of the natural antioxidants vitamin C, vitamin E, carotenoids and flavonoids. Environ Toxicol Pharmacol. 2002;11:321–333. doi: 10.1016/S1382-6689(02)00003-0. [DOI] [PubMed] [Google Scholar]
- Saguy IS, Shani A, Weinberg P, Garti N. Utilization of jojoba oil for deep-fat frying of foods. Food Sci Technol. 1996;29:573–577. [Google Scholar]
- Upadhyay R, Mishra HN. Antioxidant activity measurement of oleoresin from rosemary and sage. Ind Crop Prod. 2014;61:453–459. doi: 10.1016/j.indcrop.2014.07.043. [DOI] [Google Scholar]
- Upadhyay R, Mishra HN (2015a) Multivariate analysis for kinetic modeling of oxidative stability and shelf life estimation of sunflower oil blended with sage (Salvia officinalis) extract under Rancimat conditions. Food Bioprocess Technol 8: 801–810
- Upadhyay R, Mishra HN. Predictive modeling for shelf life estimation of sunflower oil blended with oleoresin rosemary (Rosmarinus officinalis L.) and ascorbyl palmitate at low and high temperatures. Food Sci Technol. 2015;60:42–49. [Google Scholar]
- Upadhyay R, Mishra HN. A multivariate approach to optimise the synergistic blend of oleoresin rosemary (Rosmarinus officinalis L.) and ascorbyl palmitate added into sunflower oil. Int J Food Sci Technol. 2015;50:974–981. doi: 10.1111/ijfs.12738. [DOI] [Google Scholar]
- Yen GC, Duh PD, Tsai HL. Antioxidant and pro-oxidant properties of ascorbic acid and gallic acid. Food Chem. 2002;79:307–313. doi: 10.1016/S0308-8146(02)00145-0. [DOI] [Google Scholar]