Abstract
Psidium guajava L. var. ‘Lalit’ is a perishable fruit with delicate skin which is prone to damage. The objective of this study was to determine the effect of edible coating made up of hydroxypropyl methyl cellulose and palm oil on ripening of guava. Coating solution was applied over fruits and coated fruits were stored at 24 ± 1 °C and 65 ± 5%RH. Changes in fruit colour, texture softening, respiration rate, weight loss, ascorbic acid content, soluble solids, titrable acidity, chlorophyll content, total reducing sugars, total phenolic content were studied during post-harvest ripening. Fruits coated with 1 % of hydroxypropyl methyl cellulose and 0.3 % of palm oil showed significant delay in weight loss, fruit firmness as well as colour change (p < 0.05). Coating delayed the enzyme activities of peroxidase and polyphenol oxidase of the fruit. Results suggest that overall quality of coated fruit was maintained by edible coating formulation extending the shelf life of fruit up to 12 days with appreciable retention of all quality parameters tested.
Keywords: Lalit guava, Edible composite coating, Shelf life extension, Textural softening, Peroxidase activity
Introduction
Guava (Psidium guajava L.) is a rich source of ascorbic acid, minerals and a very popular fruit of India. It is a tropical, climacteric fruit which is harvested while still green and transported to market. According to the Department of Agricultural Research and Education, ICAR, Government of India, in 1999–2000, guava variety ‘Lalit’ has been released by the CISH, Lucknow, for commercial cultivation. Its fruits are medium sized with attractive saffron-yellow colour and red blush. Its flesh is firm and pink with good blend of sugar and acid. It gives 24 % higher yield than popular variety ‘Allahabad Safeda’ (http://www.cishlko.org/achievements). Guava softens readily and, therefore, has a very short shelf life, which in turn makes transportation and storage difficult (Jain et al. 2003). Storage below 10 °C may cause severe chilling injury symptoms in the form of skin surface pitting and flesh browning, susceptibility to chilling injury limit the potential for its commercialization (Wang et al. 2009). High perishability of guava make them ideal study model to study the changes in ripening process.
Researchers have made continuous attempts to delay the softening process of detached guava by making use of different technologies; a few of them have been proven to be effective in some parameters such as texture or colour but not in others like flavour and physiological aspects. Increasing public concern regarding human health issues and environmental protection has led to increased interest in development of natural biodegradable edible coatings for maintaining postharvest quality of fruit. The use of edible coatings can increase the shelf life by creating an internally modified atmosphere that modulates fruit metabolism and moisture loss.
Cellulose is the most abundant polysaccharide on planet and its derivatives have different permeabilities to water vapour and gases and are good film formers. Hydroxypropyl methylcellulose (HPMC) is approved as direct food additive for the purpose of film former, stabilizer, thickener, and suspending agent (Baldwin 1994). Poor moisture barrier properties of HPMC can be enhanced by incorporation of hydrophobic compounds, such as fatty acids into the cellulose ether matrix to develop a composite film (Morillon et al. 2002). Palm oil (PO) is one of the saturated vegetable fat which is commercially used due to its lower cost and high oxidative stability (Che Man et al. 1999). Combination of HPMC and PO in an edible composite coating can be effective in controlling ripening process of climacteric guava fruits.
Here, we report changes in chemical composition and the activities of enzymes during ripening of guava cv. Lalit. Also the changes occurring after coating fruits with edible coating formulation of HPMC were studied.
Materials and methods
Materials
Fully mature fruit of Lalit guava variety were harvested from an orchard located at Pune, India; during November 2014. Food grade HPMC was procured from Dow Chemicals, USA. Glycerol was purchased from Merck, India. Tween 80, sodium hypochlorite and gallic acid were purchased from Hi-Media, India. Edible grade PO was purchased from local store. Acetone was purchased from SD Fine Chemicals Limited, India. All chemicals used in analysis were of analytical grade. Ethylene gas standard of 91.2 % purity was purchased from Alchemie Gases Pvt. Ltd., India.
Methods
Plant material selection and pre-treatment
Fruits of similar size (59.0 ± 1.5 mm) were selected and sorted on the basis of shape (round fruits), colour (L* value- 45.0 ± 2.0, a* value- 10.0 ± 1.0) and absence of visual defects. They were dipped in 0.1 % sodium hypochlorite solution for 15 min, washed with deionized water and dipped in deionized water for another 10 min. Further they were surface dried and were randomly divided into two lots of 40 fruits each for coating (El Ghaouth et al. 1991).
Edible coating formulation and application
Each component concentration was optimized in preliminary lab study and taken for the experiment. 1 % of HPMC was dissolved in distilled water and heated at 90 °C for 30 min followed by 30 min of hydration at 20 °C. Other components were added in the order of tween 80, PO and glycerol at concentration of 0.03, 0.3 and 0.3 % respectively in HPMC solution. The mixture was stirred on magnetic stirrer till complete mixing of components and then homogenized at 10,000 rpm for 3 min using a shear homogenizer (Tudor Machine). Prepared stable emulsion was kept in vacuum oven at 24 °C to remove air bubbles and then stored at 10 °C and used within 48 h. Fruits were coated following the procedure of Ozden and Bayindirli (2002). After coating, samples were surface dried, weighed and kept in controlled atmospheric conditions at 24 ± 1 °C and 65 ± 5%RH throughout storage period.
Physical analysis
Physiological weight loss (PWL)
Three fruits from coated and control lot were randomly selected and weighed at the start of the experiment every day. The differential weight loss was calculated for each day and converted into percentage by dividing the change with the initial weight recorded while storing them (Waskar et al. 2009).
Analysis of colour, texture and gas composition
The peel colour was measured using Hunter Lab Colorimeter (Hunter Associates Laboratory, Inc., USA) with CIELab scale (L*, a*, and b*). Peel and flesh firmness was evaluated by puncture test using Texture Analyzer (TA.TX2i Stable Micro Systems) using 2 mm stainless steel cylinder probe (P/2 Probe). Fruits were enclosed in an air tight glass container and gases were concentrated for 1 h. CO2 and O2 gases accumulated in headspace were estimated using gas analyzer (CheckMate 9900, PBI Dansensor) (Gontard et al. 1996). Ethylene released by fruits was measured using Gas chromatography (Agilent Technologies 7820 A) with FID detector using a 3.2 mm × 44 cm stainless steel column packed with Poropak Q 80/100. Inlet temperature, oven temperature and detector temperature was kept at 200, 40 and 250 °C respectively. Hydrogen, air and nitrogen flow was kept as 50, 300 and 25 ml/min.
Chemical analysis
Total soluble solids (TSS) and titrable acidity (TA)
Ten grams fruits devoid of seeds were crushed in a blender by adding 90 ml distilled water and then filtered through cheese cloth. An aliquot of 20 ml was titrated against 0.1 N NaOH, the titrable acidity was expressed as percentage malic acid (Antoniali et al. 2007).
Total chlorophyll content and lycopene content
Guava peels were extracted for chlorophyll in a mortar pestle to a fine pulp with the addition of 20 ml of 80 % acetone. Absorbance was taken at 645 and 663 nm (Salunkhe et al. 1991). Lycopene from pulp was extracted in hexane and absorbance of the extract was measured at 503 nm following the method of Kong and Ismail (2011).
Total phenolic content
One gram of sample (fresh pulp) was extracted in 10 ml, 80 % methanol and sonicated in ice for 1 min using a probe sonicator. Samples were then centrifuged at 3000 rpm at 4 °C for 15 min. Procedure was repeated twice and supernatant was pooled together to a final volume of 25 ml. Total phenolic content was estimated (Wolfe et al. 2003) using Gallic acid as a standard.
Total reducing sugars, total fructose and ascorbic acid content
Total reducing sugars were extracted using hot alcoholic extraction and estimated using well known DNSA method (Miller 1972). Total fructose content of extract was estimated using resorcinol method (Ashwell 1957). Vitamin C was extracted from fresh pulp in oxalic acid and estimated using colorimetric assay involving a reduction reaction with ammonium molybdate (Bajaj and Kaur 1981).
Enzyme dynamics of peroxidase (POD) and polyphenol oxidase (PPO)
Whole fruit pulp devoid of seed was stirred in 50 mM sodium acetate buffer (pH 5.5) (1:2 w/v) containing 2 mM EDTA, 1 mM MgCl2 and 0.1 % triton for 180 min in ice bath. Solution was then centrifuged at 10,000 rpm 4 °C for 30 min and supernatant used as crude extract for PPO and POD (Aydin and Kadioglu 2001). One unit activity of PPO was defined as change in absorbance of 0.001 units (at 420 nm) per minute (Gawlikdziki et al. 2007). POD activity was estimated using method of Ciou et al. (2011). One unit of POD activity is defined as change in absorbance of 0.001 units (at 475 nm) per minute.
Statistical analysis
All the experiments were conducted in triplicates. The data was subjected to analysis of variance (ANOVA) and Duncan’s multiple range tests were used to compare differences between treatments at the 95 % confidence level of each variable using a computer software IBM SPSS statistics 20.
Results and discussion
HPMC-PO coating was well taken up by guava fruits giving a lustrous appearance to fruits. Coating was non-sticky, non-smelly after complete drying of coat. Control fruit could only survive till day 9, on day 12 control fruit showed complete loss of tissue firmness and skin darkening rendering it with no value and hence not studied.
Physical analysis of fruits
Coating proved its effectiveness on day 1 itself which can be deduced from the observation that physiological weight loss of control fruit sample (3.69 ± 1.39 %) was more as compared with coated sample (1.94 ± 0.30 %) on day 1. Figure 1a details physiological weight loss of control and coated fruits during the storage period. Weight loss of 16.03 ± 1.05 % was observed in control fruit on day 6 whereas significantly similar weight loss (16.65 ± 2.08 %) was observed in coated fruit on day 9. Composite coating having hydrophobic particle in hydrophilic matrix gives water-soluble coating with good water vapour barrier properties (Baldwin 1994). Results showed that edible coating controlled the physiological weight loss of guavas. Results are similar to various other research done with coating using lipid and HPMC (Hagenmaier and Shaw 1990), methyl cellulose (MC) and lipid (Greener and Fennema 1989), MC and fatty acid (Sapru and Labuza 1994).
Fig. 1.

Changes in a physiological weight loss b fruit firmness c O2 consumption and CO2 release and d ethylene release in Lalit guava fruits stored at 24 ± 1 °C and 65 ± 5%RH for coated and control samples
Skin brightness (L* value) increased more gradually in case of coated guava fruits (Table 1). Soares et al. (2007) showed that for white guava fruits the green colour decreased along with the decrease of chlorophyll level and yellow colour increased with the increase of carotenoid level. In the present study L value of 60.81 ± 4.18 was observed for control fruit on day 6 whereas similar value of 60.86 ± 6.29 was observed in coated fruit on day 12 of storage. The loss of green colour and increase of yellow colour is evidenced by the increase of chroma values from 35.99 ± 5.09 to 50.82 ± 2.34 in control fruit. Coated fruit showed the same trend but the process of colour change was significantly (p < 0.05) delayed by edible coating. Pink colouration in the skin was visible on day 6 in control fruit and on day 9 in case of coated fruit which can be confirmed from the positive a* values (Table 1). In case of fruit flesh brightness decreased with increase in red colour of flesh. L value of 43.27 ± 0.55 was observed on day 6 in control fruits whereas L value of 43.62 ± 0.56 was observed on day 9 in coated fruits.
Table 1.
L, a*, b* and chroma* values of coated and control fruits of Lalit guava variety during post-harvest storage of guava at 24 ± 1 °C and 65 ± 5%RH
| L | a* | b* | Chroma* | Hue angle* | |
|---|---|---|---|---|---|
| Skin colour values | |||||
| Control | |||||
| Day 1 | 49.72 ± 4.83a | −8.06 ± 1.98a | 34.96 ± 5.57a | 35.99 ± 5.09a | −1.00 ± 0.00a |
| Day 3 | 58.38 ± 4.10a | −2.12 ± 3.17a | 44.64 ± 4.15a | 44.81 ± 3.97a | −0.47 ± 0.92a |
| Day 6 | 60.81 ± 4.18b | 3.30 ± 5.47b | 47.87 ± 4.36b | 48.23 ± 4.70b | 0.20 ± 1.03b |
| Day 9 | 63.24 ± 3.05c | 10.14 ± 2.64c | 49.73 ± 2.29c | 50.82 ± 2.34c | 1.00 ± 0.00c |
| Coated | |||||
| Day 1 | 46.84 ± 3.55a | −8.91 ± 2.14a | 32.47 ± 4.29a | 33.80 ± 3.67a | −1.00 ± 0.00a |
| Day 3 | 51.10 ± 4.47a | −5.34 ± 1.67a | 35.02 ± 4.45a | 35.45 ± 4.46a | −1.00 ± 0.00a |
| Day 6 | 57.46 ± 5.59b | −2.55 ± 5.09b | 42.57 ± 7.25b | 42.95 ± 7.04b | −0.40 ± 0.97b |
| Day 9 | 56.81 ± 4.70c | 1.92 ± 2.51c | 43.20 ± 4.30c | 43.30 ± 4.36c | 0.60 ± 0.84c |
| Day 12 | 60.86 ± 6.29d | 1.97 ± 4.22c | 34.51 ± 6.17c | 54.77 ± 6.01b | 0.00 ± 1.05c |
| Flesh colour values | |||||
| Control | |||||
| Day 1 | 51.01 ± 0.19a | −0.95 ± 0.20a | 23.53 ± 0.12a | 23.55 ± 0.13a | −1.00 ± 0.00a |
| Day 3 | 46.42 ± 0.62b | 13.90 ± 0.16b | 18.55 ± 0.21b | 23.18 ± 0.26a | 0.87 ± 0.00b |
| Day 6 | 43.27 ± 0.55c | 23.19 ± 0.40c | 20.74 ± 0.15c | 31.11 ± 0.35b | 0.71 ± 0.01c |
| Day 9 | 33.40 ± 1.18d | 21.58 ± 0.51d | 18.30 ± 0.60b | 28.30 ± 0.72c | 0.69 ± 0.01d |
| Coated | |||||
| Day 1 | 52.53 ± 0.93a | −6.06 ± 0.17a | 27.52 ± 0.63a | 28.18 ± 0.64a | −1.00 ± 0.00a |
| Day 3 | 49.28 ± 1.55b | −3.04 ± 0.28b | 24.20 ± 0.87b | 24.39 ± 0.84b | 1.00 ± 0.00b |
| Day 6 | 49.91 ± 0.87b | −2.50 ± 0.10b | 27.53 ± 0.48a | 27.64 ± 0.47a | −1.00 ± 0.00a |
| Day 9 | 47.34 ± 1.15b | 5.26 ± 0.21c | 24.82 ± 0.51b | 25.37 ± 0.53b | 1.00 ± 0.00b |
| Day 12 | 43.62 ± 0.56c | 8.93 ± 0.09c | 28.95 ± 0.48a | 28.97 ± 0.48a | −1.00 ± 0.00a |
Note: (i) Mean ± S.D. of 15 determinations; (ii) Mean among each set of data labelled by the same letter are not significantly different (P < 0.05) by Duncan’s multiple range test
Skin firmness decreased very rapidly in case of control fruits as compared to coated fruits. Skin firmness on day 0 was 8.34 ± 0.15 N and 8.43 ± 0.13 N for control and coated fruits which eventually decreased. Skin firmness on day 6 was 5.42 ± 0.10 N and 6.36 ± 0.15 N for control and coated sample respectively (Fig. 1b). Flesh firmness of control fruit was 1.88 ± 0.44 N on day 6 whereas coated fruit showed flesh firmness of 1.60 ± 0.18 N on day 12. Edible coating of HPMC-PO maintained the fruit firmness of guava for 12 days. These results corroborate with the findings of Pandey et al. (2010) who studied influence of gamma-irradiation, growth retardants and coatings on the shelf life of winter guava fruits.
Effects of edible coatings on respiratory gases released by guava in headspace during storage was studied. Respiratory peak was observed on day 9 of storage in all fruits with 10.00 ± 0.16, 10.34 ± 0.00 mmol/g.hr. of CO2 release and 18.16 ± 0.00, 25.29 ± 0.36 mmol/g.hr. of O2 uptake in control and coated fruits respectively. HPMC-PO coated fruit respired at lower rate (Fig. 1c). Coatings provided an excellent semi-permeable film around the fruit, modifying the internal atmosphere. Ethylene release by fruits gradually increased till day 3 in case of control fruit and till day 6 for coated fruit, which eventually decreased after that (Fig. 1d). Ethylene release in control fruit on day 3 was 0.50 ± 0.06 nmol/g.hr and that of coated fruit on day 6 was 0.95 ± 0.01 nmol/g.hr. The delayed increase in ethylene production of HPMC-PO coated fruit as compared to the control fruit suggests that edible coating exerted a barrier to the gaseous exchange. The delayed ethylene production can be correlated with delayed senescence (Ali et al. 2010) and a reduced susceptibility to decay (Maqbool et al. 2010).
Chemical analysis of fruits
TSS of control and coated fruit sample increased during the storage (Fig. 2a). Initial TSS of both control and coated fruit on day 1 was 12.35 ± 0.00 °Brix, which eventually increased to highest of 23.35 ± 0.00 °Brix on day 6 in case of control fruit and on day 9 of storage for coated fruits. The increase in TSS can be because of moisture loss by the fruits and conversion of organic acids to sugars (Gorny and Kader 1998). Edible coating could delay the increase in TSS to 9th day and maintain the TSS content till 12th day of storage. Ascorbic acid, citric acid, tartaric acid and malic acid are the principle organic acids produced in guava during ripening (Mahmood et al. 2012). While acidity usually tends to decrease during fruit ripening of several fruits, increase in TA has been reported in few varieties of guava (Bashir and Abu-Goukh, 2003; Mahmood et al. 2012). Similar results were obtained from present study, TA of both coated and uncoated guava fruit increased as a function of ripening. However, in coated fruit, the increase in TA was suppressed possibly due to reduction in the respiration rate. TA of 0.87 ± 0.01 % of control fruit on day 6 was comparable to 0.89 ± 0.02 % of coated fruit observed on day 9 (Fig. 2b). Results show that edible coating delayed the TA changes in the guava fruit.
Fig. 2.
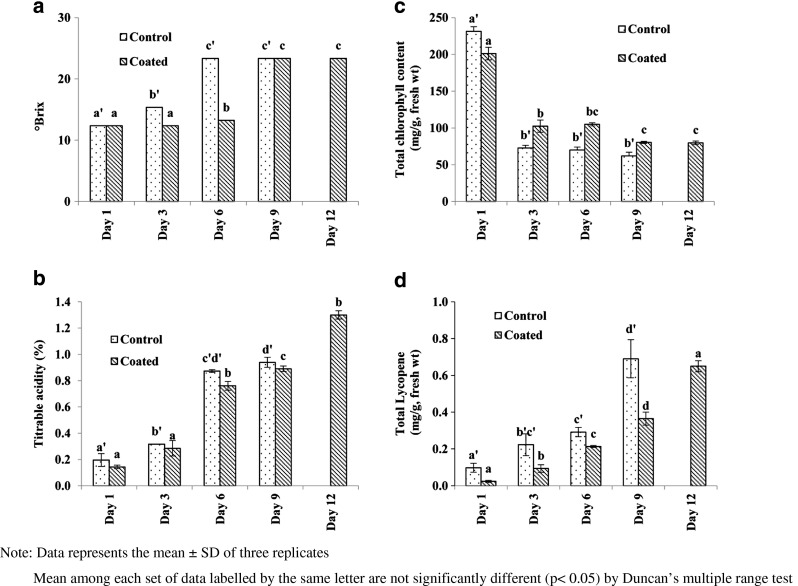
Changes in a TSS b TA c total chlorophyll content and d total lycopene content in Lalit guava fruits stored at 24 ± 1 °C and 65 ± 5%RH for coated and control samples
Chlorophyll content decreased while carotenoid content increased during ripening as reported in guava fruits cv. Banarsi Surkha (Jain et al. 2003). In the present study, total chlorophyll content of skin decreased as the fruit ripened and attained an edible appeal. In case of control fruits 231.18 ± 6.46 mg/g, fresh weight of total chlorophyll value reduced rapidly to 71.77 ± 3.77 mg/g, fresh weight on day 3 itself and then it showed insignificant gradual decrease. Coated fruit on other hand showed slow decrease in total chlorophyll content which reduced from 201.17 ± 8.60 mg/g, fresh weight on day 1 to 80.30 ± 1.37 mg/g, fresh weight on day 9 (Fig. 2c). Total lycopene content, a characteristic of pink guava increased eventually in both control and coated fruits. Highest content of lycopene of 0.69 ± 0.10 mg/g, fresh weight was observed on day 9 for control fruit whereas similar highest value of 0.65 ± 0.03 mg/g, fresh weight was seen in case of coated fruit 3 days later i.e., on 12th day of storage (Fig. 2d). Results indicate that the edible coating can delay the degradation of chlorophyll and carotenoid synthesis as also demonstrated by Chitravathi et al. (2014).
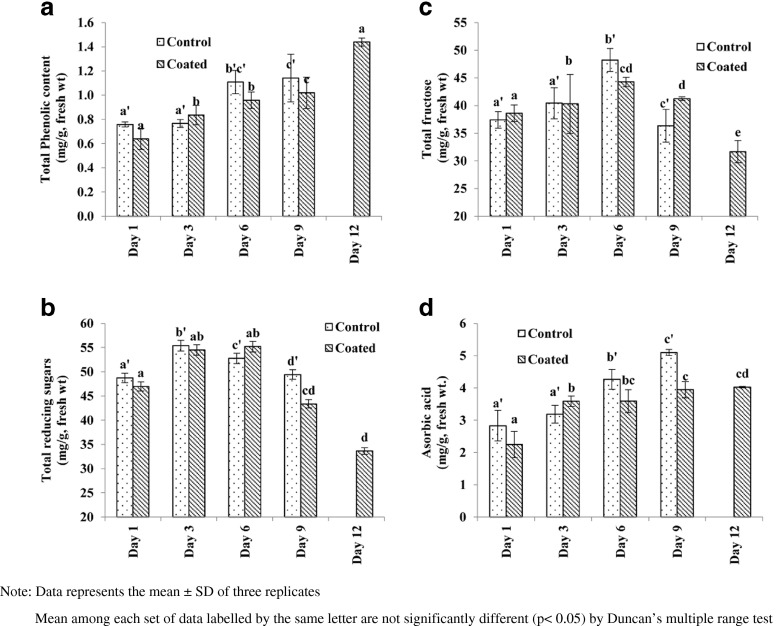
Total reducing sugars on day 1 of storage was 48.73 ± 0.97 and 46.96 ± 0.94 mg/g, fresh weight for control and coated fruit, respectively. Total reducing sugars in control fruit reached a highest value (55.37 ± 1.11 mg/g, fresh weight) on day 3 and then it decreased to 49.42 ± 0.99 mg/g, fresh weight on day 9 (Fig. 3a). Highest total reducing sugars (55.20 ± 1.10 mg/g, fresh weight) were estimated on day 6 for coated fruit which decreased thereafter. Fructose comprising a substantial part of total reducing sugars showed similar changes during ripening of coated and control fruits (Fig. 3b). Fructose concentration was highest on day 6, viz.48.21 ± 2.08 and 44.27 ± 0.85 mg/g, fresh weight for control and coated fruits respectively. Edible coating delayed overall process of synthesis and degradation of total reducing sugars. Changes in fructose content of coated fruit was similar that of coated fruit but changes were slow and insignificant.
Fig. 3.
Changes in a total phenolic content b total reducing sugars c total fructose content and d ascorbic acid content in Lalit guava fruits stored at 24 ± 1 °C and 65 ± 5%RH for coated and control samples
Decrease in phenolic content with increase in softness is reported in pink guava by Bashir and Abu-Goukh (2003). In our studies total phenolic contents increased in both control and coated guava fruits (Fig. 3c). Concentrations of 1.14 ± 0.20 and 1.44 ± 0.03 mg/g, fresh weight was obtained on day 9 and day 12 of studies for control and coated guava fruits, respectively. Increase in phenolic contents may be related to increased antioxidant activity (Nadeesha et al. 2007). Ascorbic acid concentration increased during the storage of Lalit guava fruit in both control and coated samples (Fig. 3d). Increase in ascorbic acid levels during the ripening of guava was also verified by other authors (El-Bulk et al. 1997; Mercado-Silva et al. 1998). The level of ascorbic acid can vary with genotypic differences, pre-harvest climatic conditions, maturity and postharvest handling procedures (Chitravathi et al. 2014). Ascorbic acid concentration was 5.10 ± 0.09 mg/g, fresh weight on 9th day of storage in control samples whereas 4.02 ± 0.02 mg/g, fresh weight in coated samples on 12th day of storage. The synthesis of ascorbic acid was delayed due to coating but coated fruit failed to achieve the ascorbic acid content as that of the control fruit.
Enzyme dynamics of POD and PPO
POD specific activity on day 1 shows difference in case of control and coated fruits. Increased activity on day 1 may be due to handling and processing during coating procedure. The activity further increased in control fruit to a highest of 44.89 ± 3.08 U/mg proteins, fresh weight on day 3 and then decreased thereafter (Fig. 3a). Such increase in case of coated fruit was observed on day 9 showing an activity of 35.86 ± 1.17 U/mg proteins, fresh weight. Specific activity of PPO was low in initial days of storage. Control fruits showed highest activity on day 6 whereas coated fruits showed the increased activity on day 9 (Fig. 4b). Activity in coated fruits on day 9 is nearly double the activity of control fruit activity on day 6.
Fig. 4.
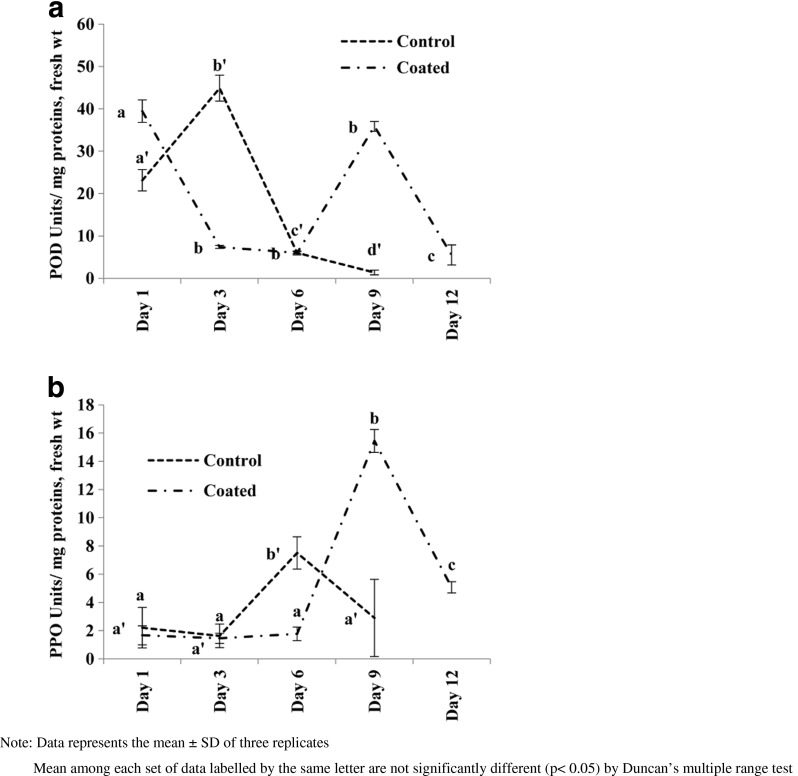
Changes in a POD and b PPO activity in Lalit guava fruits stored at 24 ± 1 °C and 65 ± 5%RH for coated and control samples
Postharvest browning of fruit pericarp is a major problem, resulting in lowered shelf life and reduced commercial value of the fruits. The browning was generally thought to be a rapid degradation of phenolic compounds caused by PPO and POD (Zhang et al. 2005). Changes in specific activity of POD are similar to observations on tomato (Andrews et al. 2004) and papaya (Silva et al. 1990) in which these activities were low at greenish stage, increased during ripening and then gradually fell as the fruit turned to over ripe stage. PPO activity within fruit has been observed in many other fruits, and has been correlated with the spatial occurrence of browning (Underhill and Critchley 1995). HPMC-PO coating delayed the activities of POD and PPO.
Conclusion
‘Lalit’ guava variety is not much studied fruit. The physicochemical and physiological changes of fruits were evaluated for 12 days of storage. Edible coating made up of HPMC and PO showed significant delay in post-harvest changes occurring in guava fruit, when fruits were stored at 24 ± 1 °C and 65 ± 5%RH. Fruits coated with 1 % of HPMC and 0.3 % of PO showed decreased weight loss, retention of fruit firmness as well as slower colour change. Edible coating formulation also affected the physiology of the fruit showing slower increase in total reducing sugars and ascorbic acid content. Coating delayed the PPO and POD enzyme activities of the fruit. Overall quality of coated fruit was maintained by edible coating formulation extending the shelf life of fruit up to 12 days as compared to that of 9 days of uncoated fruit.
Acknowledgments
The authors are grateful to University Grants Commission (UGC) for providing the financial support for carrying out this work.
Footnotes
Research highlights
Edible coating emulsion of HPMC and palm oil was prepared
Post-harvest changes in Lalit guava fruit studied after coating
1 % of HPMC and 0.3 % of palm oil gave significant delay in ripening
Coated fruit showed lower weight loss & delayed sugar metabolism
Coating delayed activities of peroxidase & polyphenol oxidase of the fruit
References
- Ali A, Maqbool M, Ramachandran S, Alderson PG. Gum Arabic as a novel edible coating for enhancing shelf-life and improving postharvest quality of tomato (Solanum lycopersicum L.) fruit. Postharvest Biol Technol. 2010;58:42–47. doi: 10.1016/j.postharvbio.2010.05.005. [DOI] [Google Scholar]
- Andrews PK, Fahy DA, Foyer CH (2004) Relationship between fruit exocarp antioxidants in the tomato (Lycopersicon esculentum) high pigment -1 mutant during development. Physiol Plant 120:519–528 [DOI] [PubMed]
- Antoniali SA, Paulo ML, Anna Maria M, Rogerio TF, Juliana S. Physicochemical characterization of ‘zarco hs’yellow bell pepper for different ripeness stages. J Sci Food Agric. 2007;64:19–22. [Google Scholar]
- Ashwell G. In: Methods in enzymology. Colowick SJ, Kaplan NO, editors. New York: Academic Press; 1957. p. 75. [Google Scholar]
- Aydin N, Kadioglu A. Changes in the chemical composition, polyphenol oxidase and peroxidase activities during development and ripening of Medlar fruits (Mespilus germanica L.) Bulg J Plant Physiol. 2001;27:85–92. [Google Scholar]
- Bajaj KL, Kaur G. Spectrophotometric determination of L-ascorbic acid in vegetables and fruits. Analyst. 1981;106(1258):117–120. doi: 10.1039/an9810600117. [DOI] [PubMed] [Google Scholar]
- Baldwin EA. Edible coatings and films to improve food quality. Lancaster: Technomic Publ. Co. Inc; 1994. Edible coatings for fresh fruits and vegetables: past, present, and future; pp. 25–64. [Google Scholar]
- Bashir HA, Abu-Goukh AA. Compositional changes during guava fruit ripening. Food Chem. 2003;80:557–563. doi: 10.1016/S0308-8146(02)00345-X. [DOI] [Google Scholar]
- Che Man YB, Liu JL, Jamilah B, Rahman RA. Quality changes of RBD palm olein, soybean oil and their blends during deep-fat frying. J Food Lipids. 1999;6(3):181–193. doi: 10.1111/j.1745-4522.1999.tb00142.x. [DOI] [Google Scholar]
- Chitravathi K, Chauhan OP, Raju PS. Postharvest shelf-life extension of green chillies (Capsicum annuum L.) using shellac-based edible surface coatings. Postharvest Biol Technol. 2014;92:146–148. doi: 10.1016/j.postharvbio.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciou J, Lin H, Chiang P, Wang C, Charles A. The role of polyphenol oxidase and peroxidase in the browning of water caltrop pericarp during heat treatment. Food Chem. 2011;127:523–527. doi: 10.1016/j.foodchem.2011.01.034. [DOI] [PubMed] [Google Scholar]
- El-Bulk RE, Babiker EFE, Tinay AHE (1997) Changes in chemical composition of guava fruits during development and ripening. Food Chem 59:395–399
- El Ghaouth A, Arul J, Ponnamaplam R, Boulet M. Use of chitosan coating to reduce water loss and maintain quality of cucumber and bell pepper fruits. J Food Process Preserv. 1991;15:359–368. doi: 10.1111/j.1745-4549.1991.tb00178.x. [DOI] [Google Scholar]
- Gawlikdziki U, Szymanowska U, Baraniak B. Characterization of polyphenol oxidase from broccoli (Brassica oleracea var. botrytis italica) florets. Food Chem. 2007;105(3):1047–1053. doi: 10.1016/j.foodchem.2007.05.012. [DOI] [Google Scholar]
- Gontard N, Thibault R, Cuq B, Guilbert S. Influence of relative humidity and film composition on oxygen and carbon dioxide permeabilities of edible films. J Agric Food Chem. 1996;44:1064–1069. doi: 10.1021/jf9504327. [DOI] [Google Scholar]
- Gorny JR, Kader AA (1998) Fresh-cut products: maintaining quality and safety, University of California Davis, Workshop September 15–16 (1998), 4:12–14
- Greener IK, Fennema O. Barrier properties and surface characteristics of edible, bilayer films. J Food Sci. 1989;54:1393–1399. doi: 10.1111/j.1365-2621.1989.tb05120.x. [DOI] [Google Scholar]
- Hagenmaier RD, Shaw PE. Moisture permeability of edible films made with fatty acid and (hydroxypropyl) methyl cellulose. J Agric Food Chem. 1990;38:1799–1803. doi: 10.1021/jf00099a004. [DOI] [Google Scholar]
- Jain N, Dhawan K, Malhotra S, Singh R. Biochemistry of fruit ripening in guava (Psidium guajava L) compositional and enzymatic changes. Plant Food Hum Nutr. 2003;58:309–315. doi: 10.1023/B:QUAL.0000040285.50062.4b. [DOI] [PubMed] [Google Scholar]
- Kong KW, Ismail A. Lycopene content and lipophilic antioxidant capacity of by-products from Psidium guajava fruits produced during puree production industry. Food Bioprod Process. 2011;89:53–61. doi: 10.1016/j.fbp.2010.02.004. [DOI] [Google Scholar]
- Mahmood T, Anwar F, Abbas M, Boyce MC, Saari N. Compositional variation in sugars and organic acids at different maturity stages in selected small fruits from Pakistan. Int J Mol Sci. 2012;13:1380–1392. doi: 10.3390/ijms13021380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maqbool M, Ali A, Ramachandran S, Smith DR, Alderson PG. Control of postharvest anthracnose of banana using a new edible composite coating. Crop Prot. 2010;29:1136–1141. doi: 10.1016/j.cropro.2010.06.005. [DOI] [Google Scholar]
- Mercado-Silva E, Benito-Bautista P, De los A, García-Velasco M (1998) Fruit development, harvest index and ripening changes of guavas produced in central Mexico. Postharvest Biol Technol 13(2):143–150
- Miller GL. Use of DNS reagent for the determination of glucose. Anal Chem. 1972;31:426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- Morillon V, Debeaufort F, Blond G, Capelle M, Voilley A. Factors affecting the moisture permeability of lipid-based edible films: a review. CRC Crit Rev Food Sci. 2002;42(1):67–89. doi: 10.1080/10408690290825466. [DOI] [PubMed] [Google Scholar]
- Nadeesha MKF, Bamunuarachchi, Edirisinghe EMRKBM, Weerasinghe WMSK (2007) Studies on antioxidant activity of Indian gooseberry and seed. J Sci Univ Kelaniya 3:83–92
- Ozden C, Bayindirli L. Effects of combinational use of controlled atmosphere, cold storage and edible coating applications on shelf life and quality attributes of green peppers. Eur Food Res Technol. 2002;214:320–326. doi: 10.1007/s00217-001-0448-z. [DOI] [Google Scholar]
- Pandey SK, Joshua JE, Bisen, Abhay (2010) Influence of gamma-irradiation, growth retardants and coatings on the shelf life of winter guava fruits (Psidium guajava L.). J Food Sci Technol 47(1):124–127 [DOI] [PMC free article] [PubMed]
- Salunkhe DK, Bolin HR, Reddy NR. Storage, processing, and nutritional quality of fruits and vegetables, 2. Boca Raton: CRC Press; 1991. [Google Scholar]
- Sapru V, Labuza TP. Dispersed phase concentration effect on water vapor permeability in composite methyl cellulose-stearic acid edible films. J Food Process Preserv. 1994;18:359–368. doi: 10.1111/j.1745-4549.1994.tb00259.x. [DOI] [Google Scholar]
- Silva ED, Lourenco EJ, Neves VA (1990) Soluble and bound peroxidase from papaya fruit. Phytochem 29:1051–1056
- Soares FD, Pereira T, Marques MM, Monteiro AR. Volatile and non-volatile chemical composition of the white guava fruit (Psidium guajava) at different stages of maturity. Food Chem. 2007;100:15–21. doi: 10.1016/j.foodchem.2005.07.061. [DOI] [Google Scholar]
- Underhill SJR, Critchley C (1995) Cellular localisation of polyphenol oxidase and peroxidase activity in Litchi chinensis Sonn. pericarp. Aust J Plant Physiol 22:627–32
- Wang Z, Duan H, Hu C. Modelling the respiration rate of guava (Psidium guajava L) fruit using enzyme kinetics, chemical kinetics and artificial neural network. Eur Food Res Technol. 2009;229(3):495–503. doi: 10.1007/s00217-009-1079-z. [DOI] [Google Scholar]
- Waskar DP, Kheldar RM, Garande VK. Effect of postharvest treatment on the shelf life and quality of pomegranate in evaporative cooling chamber and ambient conditions. J Food Sci Technol. 2009;2:114–117. [Google Scholar]
- Wolfe K, Wu X, Liu RH. Antioxidant activity of apple peels. J Agric Food Chem. 2003;51:609–614. doi: 10.1021/jf020782a. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Pang X, Xuewu D, Ji Z, Jiang Y (2005) Role of peroxidase in anthocyanin degradation in litchi fruit pericarp. Food Chem 90(1):47–52