Abstract
This work evaluated the effect of grinding and sieving process of green tea leaves on particle size distribution and antioxidant activity. Granulometric classes ranging from 20 μm to 500 μm were studied. Hydroalcoholic extracts of green tea powders (GTPs) were analysed for total phenolic, total flavonoid, and catechin contents. The fraction of 100–180 μm ground at 6000 rpm showed the maximum catechin content with 33.5 mg/g dry matter (DM) and, the best antioxidant activity with IC50 values of 0.28 μg/mL and 0.13 μg/mL using DPPH and ABTS radical scavenging assays, respectively. However, antioxidant properties and catechin content decreased for particle of sizes less than 50 μm. Catechin content was higher for those ground at 6000 rpm as compared to at 18,000 rpm. The best grinding conditions and particle size were 6000 rpm and 100–180 μm.
Keywords: Green tea powders, Fine grinding, Antioxidant activity, Catechins, LC-MS
Introduction
Tea is viewed as an important member of the antioxidant food group, a source of pharmacologically active molecules, and a functional food endowed with beneficial health properties (Dufresne and Farnworth 2001). Indeed, several studies have reported that green tea extract has antioxidant, antibacterial, antiviral, anticarcinogenic, antimutagenic functions (Higdon and Frei 2003), and also diuretic properties (Jouad et al. 2001). Epidemiological reports show that the percentage of cardiovascular diseases decreases due to green tea consumption (Kuriyama 2008). The antioxidant properties of flavonoids, compounds significantly present in green tea, have been shown by Davis et al. (2009). Moreover, these compounds have anti-inflammatory, vasodilator, antithrombotic, antiatherogenic, antipyretic and analgesic properties (Cook and Samman 1996).
Among the bioactive constituents of green tea, polyphenols account for 25–35 % on dry matter. Important characteristics of tea polyphenols are provided by flavan-3-ols, or catechins, with 14–25 % content of dry tea (Yao et al. 2006). Catechins are the most important functional components of green tea. They present a wide range of functional activities, especially a strong antioxidant activity (Pastore and Fratellone 2006). Epigallocatechin gallate has been described by Murase et al. (2002) as the predominant flavan-3-ol in green tea. Catechin and epicatechin are also present in onion, endives, cruciferous vegetables, black grapes, red wine, grapefruits, apples, cherries and berries (Dufresne and Farnworth 2001). Catechins of green tea showed no toxic effects in animals (Chengelis et al. 2008) and can thus be used in various drinks and foods (Kurita et al. 2004; Lu et al. 2010).
Industrial extraction of polyphenolic compounds from tea is mainly based on maceration combined with agitation, circulation, ultrasonic, microwave or enzymatic treatment (Lee and Lee 2008). A way to enhance compound extraction could be to grind plants. Indeed, fine grinding reduces particle size, thickens particle size distribution, and increases specific surface area, which lead to the improvement of material functionalities (Zhao et al. 2009; Zhang et al. 2012; Barnwal et al. 2015). However, grinding conditions have to be chosen carefully. Indeed, during grinding, temperature increases biochemical alterations of food materials and may result in modifications of their physicochemical properties (Maaroufi et al. 2000) like color alteration (Singh and Goswami 1999), which lowers their organoleptic properties (Murthy et al. 1999) and may cause bioactivity losses (Pesek et al. 1985).
To that purpose, a differential split method based on a recent patent (Baudelaire E. 2013) using grinding and sieving processes was used to increase the content of some bioactive compounds in powder extracts. Green tea powders (GTPs) were classified according to the following particle sizes: <20 μm, 20–50 μm, 50–100 μm, 100–180 μm, 180–315 μm, and 315–500 μm. Powders were produced at two grinding speeds, 6000 and 18,000 rpm. Total phenolic content, total flavonoid content, and antioxidant activity were assessed for the different granulometric classes and non-ground tea leaves (NGT). The main flavan-3-ols contained in green tea were also quantified by LC-MS. This article aims at evaluating the effect of particle size and grinding speed on the antioxidant activity and catechin content of green tea extracts.
Materials and methods
Plant material and GTP production
Green tea leaves (Camellia sinensis (L.) O. Kuntze) were provided by Gunpowder tea (Zhejiang Province, China). About 1.5 kg of leaves were slowly ground (50 g plant for each batch) with the Ultra Centrifugal Mill ZM 200 (Retsch France), operating by impact and shearing effects. Grinding was performed at room temperature (approximately 20 °C) using a 1 mm sieve with trapezoid holes. The grinding time was fixed to 2 min. Indeed, Hu et al. (2012) showed that concentrations of polyphenols, flavonoids and the antioxidant properties decrease when the grinding time increases. Two speeds were used for milling: 6000 rpm and 18,000 rpm. After grinding, plant powders were sieved with the vibratory sieve shaker Analysette 3 Spartan (Fritsch, Idar-Oberstein, Germany) at 0.5 mm vibration amplitude for 10 min. 20 mm diameter sieves (Fritsch) were employed so as to obtain the following granulometric fractions: <20 μm; 50–100 μm; 100–180 μm; 315–500 μm; >500 μm. Powders were then stored under vacuum in sealed bags at 10 °C.
Chemicals
Chemicals Folin & Ciocalteu’s phenol reagent, gallic acid, ABTS as diammonium salt, 2,2′-Azobis(2-amidino-propane) dihydrochloride (AAPH), standards of (+)-catechin (C), (−)-epicatechin (EC), (−)-epigallocatechin (EGC), (−)-epicatechin gallate (ECG), (−)-epigallocatechin gallate (EGCG) were acquired from Sigma-Aldrich Corporation (Saint Quentin Fallavier, France). HPLC-grade acetonitrile, water, methanol and formic acid were purchased from Sigma Chemicals Co. (Saint Louis, MO). Sodium carbonate (Na2CO3), aluminum chloride (AlCl3), sodium nitrite (NaNO2) and sodium hydroxide (NaOH) from Acros Organics (Geel, Belgium). Each standard (C, EC, EGC, ECG, and EGCG) was prepared in a methanol-water solvent 70–30 (v/v) at a concentration of 0.5 mg/mL.
Preparation of extracts
Extraction of polyphenols from GTPs were carried out according to the method of Kim et al. (2002), with some modifications. 2 g of dried leaves and 2 g of powdered dried leaves were macerated during 24 h under stirring, in 20 mL methanol/water 70/30 (v/v). Then, the methanol/water extract was centrifuged at 20,000 rpm for 20 min. The supernatant layer was filtered, brought to 15 mL and stored at 4 °C before analysis.
Total phenolic content
Total phenolic compounds (TPC) were determined according the protocol used by Muanda et al. (2011). 40 μL of samples was added to test tubes containing 3.16 mL of distilled water followed by an addition of 200 μL of Folin-Ciocalteu reagent (1 N) and 600 μL of sodium carbonate (Na2CO3, 20 %). Samples and blank were thoroughly mixed and vortexed. After 40 min of incubation at room temperature, the absorbance was measured at 725 nm. For the calibration curves, diluted solutions of gallic acid (solutions at 1 mg/mL, 1.5 mg/mL, 2 mg/mL, 2.5 mg/mL and 3 mg/mL) were used and total phenolic content was expressed in terms of equivalent amounts of gallic acid per 100 g of dry matter (GAE/100 g DM). Absorbance measurements were carried out with UV–visible spectrophotometer Cary 50 Scan (Agilent, Santa Clara, California, USA).
Total flavonoid content
Total flavonoid compounds (TFC) were dosed by a colorimetric assay described by Chen and Chen (2011). 250 μL of sample were added to a volumetric flask containing 1 mL of distilled water. At 0 min, 75 μL of sodium nitrite (NaNO2, 5 %) were added to the flask. After 5 min, 75 μL of aluminum chloride (AlCl3, 10 %) were added. After 6 min, 500 μL of sodium hydroxide (NaOH, 1 N) were added to the mixture. At this time the mixture was diluted with 2.5 mL of distilled water, then the vortexed samples were kept at room temperature. The absorbance was directly measured at 510 nm. For use as calibration curve, diluted solutions of catechin were used and total flavonoids were expressed as mg of catechin equivalent per 100 g of dry matter (mg CE/100 g DM). As for TPCs, absorbance measurements were performed with UV–visible spectrophotometer Cary 50 Scan (Agilent, Santa Clara, California, USA).
Antioxidant activity
ABTS tests
The method developed by Muanda et al. (2010) was used in this experiment; 1 mM of AAPH solution was mixed with 2.5 mM ABTS as diammonium salt in phosphate buffered saline (PBS) solution (100 mM, pH 7.4) containing 150 mM NaCl. After incubation in a water bath at 68 °C for 20 min, the resulting ABTS solution was diluted with the methanol to get blue-green coloration with an absorbance of 0.70 ± 0.02 at 734 nm, then 2.94 mL of ABTS radical anion solution was added. The decrease of absorbance was measured at 734 nm. The radical stock was prepared daily and all the tests were triplicated. The half maximal inhibitory concentration (IC50), which refers to the smallest concentration of antioxidants required to scavenge 50 % of the ABTS radical cation, was calculated.
DPPH tests
The DPPH radical scavenging activity was evaluated according to the method described by Sharififar et al. (2009). 1 mL of 100 μM DPPH solution in methanol was mixed with 1 mL of plant extract. The reaction mixture was incubated in the dark for 20 min, and the optical density was recorded at 517 nm against the blank. For the control, 1 mL of DPPH solution in methanol was mixed with 1 mL of methanol, and optical density of solution was recorded after 20 min. IC50 values related to the DPPH radical scavenging activity were calculated.
LC-MS analyses
Analysis parameters
LC-MS analyses were performed on a LC-MS 2020 system (Shimadzu, Tokyo, Japan) associated to an electrospray ionization source (ESI). Separation was carried on a 150 × 4.6 mm i.d. C18 reverse phase Gemini column (Phenomenex, Torrance, CA, USA) with particle size of 3 μm and pore size of 130 Å. The column oven was fixed at 30 °C. The mobile phase consisted of (A) 0.5 % formic acid in water and (B) acetonitrile. The injection volume was 20 μL and the flow rate was 0.6 mL/min. The 40 min gradient used was as follows: 0–5 min, 90:10 (A:B); 5–7 min from 90:10 to 86:14 (linear gradient); 7–17 min held at 86:14; 17–19 min from 86:14 to 75:25 (linear gradient); 19–24 min held at 75:25; 24–25 min from 75:25 to 10:90 (linear gradient); 25–31 min held at 10:90; 31–32 min from 10:90 to 90:10, 32–40 min held at 90:10 (re-equilibration step). The electrospray ionization source was operated in negative mode. The nebulization gas flow was set to 1.5 L/min, drying gas flow at 20 L/min, the heat block temperature was fixed at 350 °C, and the desolvation line (DL) temperature at 250 °C. The probe voltage was set to - 4500 V.
Calibration method
For the determination of calibration curves, standards of (+)-catechin, (−)-epicatechin, (−)-epigallocatechin, (−)-epicatechin gallate, (−)-epigallocatechin gallate were prepared in 70/30 methanol/water (v/v) at concentrations comprised between 0.3 mg/mL and 2 mg/mL. Dosages of catechins were performed using the selected ion monitoring (SIM) mode to increase sensitivity compared to the full scan mode.
LOD and LOQ
Solutions of standard compounds were diluted at different concentrations to perform calibration curves in order to dose the main catechins present in GTP extracts. The detector response was linear over a range from 0.005 mg/mL to 0.5 mg/mL for the catechin standards (C, EC, EGC, ECG and EGCG). The method sensitivity was assessed by determining the limit of detection (LOD) and the limit of quantification (LOQ) defined as the concentrations with a signal-to-noise (S/N) of 3 and 10, respectively. Data are expressed in mg of standard per gram of dried matter.
Statistical analyses
Each experiment and measurement was performed in triplicate. Data are expressed as means ± standard deviation. Statistical analysis was conducted using SAS software (v8.2, SAS Institute Inc., Cary, NC, USA) with analysis of variance (ANOVA). Means for significant differences were compared by using Student’s t test. Differences were considered statistically significant at p < 0.05.
Results and discussion
Total phenolic content of GTPs
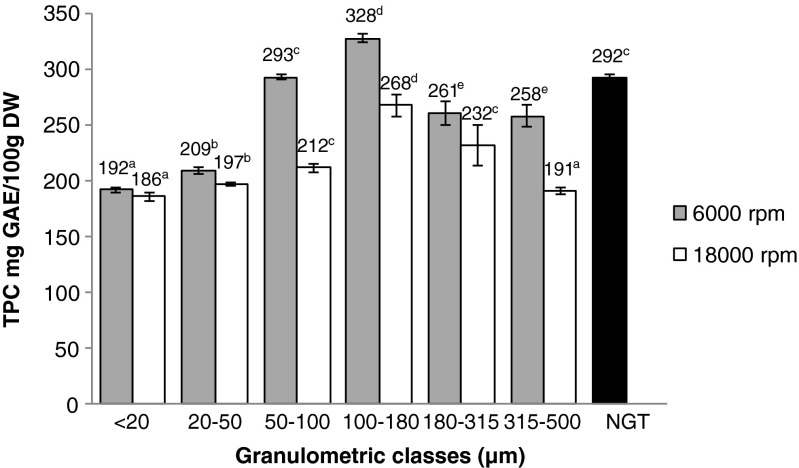
To evaluate polyphenol content in GTPs, Folin & Ciocalteu test is used, results are displayed in Fig. 1. The most important total phenolic content in GTPs was found in the fraction 100–180 μm at 6000 rpm, 328 mg GAE/100 g DM. Values of TPC increased when granulometry decreased from 500 μm to 50 μm. However, between 20 μm and 50 μm, TPCs decreased dramatically to 192 mg GAE/100 g DM (6000 rpm). The non-ground tea extract showed a high TPC, 305 mg GAE/100 g DM, which is superior than every GTPs obtained at 18,000 rpm. This can be explained by the speed increase from 6000 rpm to 18,000 rpm which leads to a temperature rise causing a loss of catechin content, as reported by Chen et al. (2001). Optimal parameters to obtain the highest TPC were the 100–180 μm sieve at 6000 rpm. The use of a faster grinding speed led to lower TPC values.
Fig. 1.
Total phenolic content of green tea powders at 6000 and 18,000 rpm; DM dry matter, GAE gallic acid equivalent, NGT non-grinded tea leaves. All values are expressed as means ± SD (n = 3). For a same grinding speed, values followed by different letters were statistically different (p < 0.05)
Total flavonoid content of GTPs
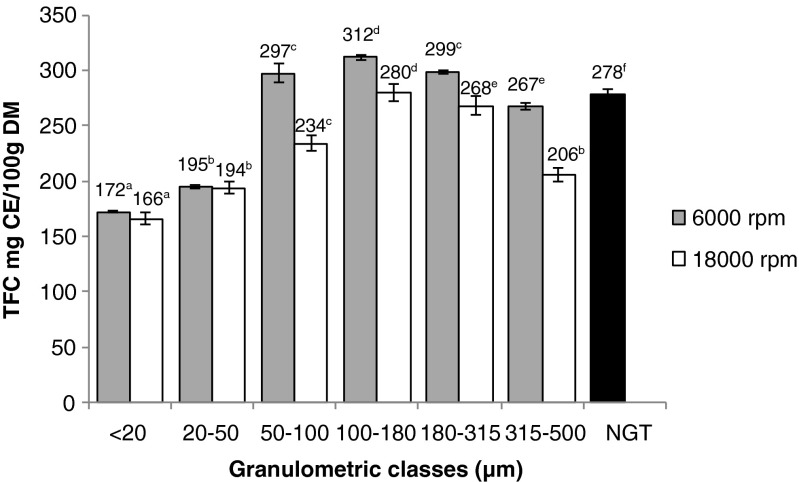
This test was performed to assay more specifically flavan-3-ols, compounds predominantly present in green tea (Oliveira 2012). Total flavonoid contents are reported in Fig. 2. At 6000 rpm, TFCs tended to increase when particle size decreased to 50 μm, then, for 20 μm at 6000 rpm, the amount of flavonoids fell down to 172 mg CE/100 g DM. Here also, the highest TFC is reached with the 100–180 μm fraction at 6000 rpm with 312 mg CE/100 g DM. As for total phenolic content, the non-ground tea TFC was higher than GTPs at 18,000 rpm with 278 mg CE/100 g DM, confirming the hypothesis previously raised of catechin degradation during grinding at higher speed.
Fig. 2.
Total flavonoid content of green tea powders at 6000 and 18,000 rpm; DM dry matter, CE catechin equivalent, NGT non-grinded tea leaves. All values are expressed as means ± SD (n = 3). For a same grinding speed, values followed by different letters were statistically different (p < 0.05)
Antioxidant activity of GTPs
DPPH radical scavenging activity
Antioxidant activity is defined as the ability of a compound to inhibit oxidative degradation (Roginsky and Lissi 2005). The DPPH method has been extensively used to measure the radical scavenging abilities of various antioxidant substances. The antioxidant activity of GTPs and NGT assessed by DPPH is reported in Table 1. Values are expressed as mean ± standard deviation from triplicate. The IC50 is the concentration of antioxidant required to reduce the original amount of radical by 50 %.
Table 1.
Evaluation of the antioxidant activities of extracts from green tea powders with different particle sizes obtained at grinding speeds of 6000 rpm and 18,000 rpm, and non-grinded tea leaves (NGT) as determined by DPPH radical scavenging effect and ABTS radical scavenging effect
| GTPs (μm) | IC 50 values of radical scavenging activity (μg/mL) | |||
|---|---|---|---|---|
| DPPH test | ABTS test | |||
| 6000 rpm | 18,000 rpm | 6000 rpm | 18,000 rpm | |
| <20 | 0.53 ± 0.02a | 0.70 ± 0.02a | 0.24 ± 0.02a | 0.54 ± 0.02a |
| 20–50 | 0.44 ± 0.03b | 0.61 ± 0.03b | 0.22 ± 0.02a | 0.44 ± 0.04b |
| 50–100 | 0.31 ± 0.01c | 0.51 ± 0.02c | 0.16 ± 0.03b | 0.32 ± 0.03c |
| 100–180 | 0.28 ± 0.01d | 0.48 ± 0.01c | 0.13 ± 0.02b | 0.28 ± 0.04d |
| 180–315 | 0.31 ± 0.02d | 0.53 ± 0.01c | 0.14 ± 0.03 b | 0.33 ± 0.03c |
| 315–500 | 0.44 ± 0.02b | 0.64 ± 0.03b | 0.21 ± 0.02a | 0.46 ± 0.05b |
| NGT | 0.39 ± 0.02d | 0.35 ± 0.03c | ||
The data were mean ± standard deviation of triplicate (n = 3). Means in the same column followed by different letters were statistically different (p < 0.05)
At both grinding speed, IC50 values were not significantly different among 50–100 μm, 100–180 μm and 180–315 μm fractions (p > 0.05). These granulometric classes showed thus the highest DPPH scavenging activity with a maximum IC50 of 0.28 μg/mL for the 100–180 μm GTP. In comparison, the IC50 value of the non-ground tea was evaluated at 0.39 μg/mL which constitutes a significant difference (p < 0.05). Similar variations were observed at 18,000 rpm: intermediate fractions led to the highest activity. However, as for the total phenolic content and total flavonoid content, the best results were obtained at 6000 rpm. A positive correlation was also observed between the TPC (r2 = 0.801) and TFC (r2 = 0.840) of the GTP extracts and their antioxidant activity assessed by the DPPH test. This shows the relation between antioxidant activity and polyphenols content, as described by Burin et al. (2014).
ABTS radical scavenging activity
ABTS radical scavenging assay can also be applied to test food and natural water-soluble phenolics. The antioxidant activity of GTPs and NGT assessed by ABTS are reported in Table 1. Results showed that the difference between samples come from different GTPs. IC50 values were significantly different among 100–180 μm and 315–500 μm (p < 0.05). The best activity was obtained for the fraction between 100 and 180 μm at 6000 rpm, with an IC50 of 0.13 μg/mL. The scavenging capacity of <20 μm on ABTS decreased significantly with 0.24 μg/mL at 6000 rpm. Antioxidant activity were systematically lower for GTPs ground at 18,000 rpm. The trends were in agreement with those exhibited by the results of DPPH radical scavenging test, confirming that particle size has an important influence on extract antioxidant activity. It can be also noticed that IC50 values obtained with ABTS assay were lower than DPPH assay, this difference between these two tests is consistent with the study of Czyzowska et al. (2015).
LC-MS
Analysis of catechin standards and GTP extracts
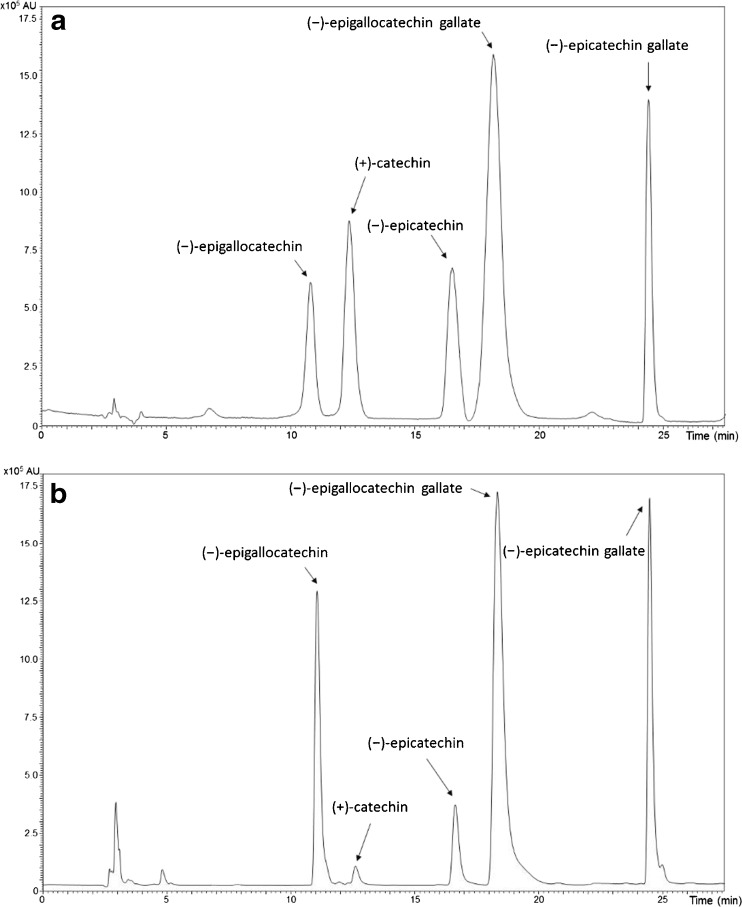
The LC-MS profile of standard compound analysis is displayed in Fig. 3a. This initial experiment allows us to know the retention times of each catechin. Retention times and m/z ratios of the selected catechins are given in Table 2. The same settings were applied for the analysis of GTP and NGT extracts. Before their analysis, extracts were filtrated and diluted ten times in order to prevent the electrospray source from contamination. The LC-MS profile of this experiment is presented in Fig. 3b. The chromatographic peaks found in tea extracts showed the same retention times and m/z ratios than standard compounds, enabling to identify the main catechins in green tea in the following elution order: C, EC, ECG, EGC, and EGCG. The major signals were peaks at 18.2 and 24.4 min, which correspond to the (−)-epigallocatechin gallate and the (−)-epicatechin gallate, respectively (Fig. 3b).
Fig. 3.
LC-MS profiles of a a mixture of standard compounds (+)-catechin (C), (−)-epicatechin (EC), (−)-epigallocatechin (EGC), (−)-epicatechin gallate (ECG), (−)-epigallocatechin gallate (EGCG) prepared at a concentration 0.015 g/L and b green tea extract
Table 2.
Retention times, m/z ratios, validation (r2) and LOD-LOQ values for flavan-3-ols quantified
| Compound | m/z | Retention time (min) | LOD (ppm) | LOQ (ppm) | Coefficient of determination (r2) |
|---|---|---|---|---|---|
| (+)-catechin (C) | 289 | 12.4 | 2 | 8 | 0.998 |
| (−)-epicatechin (EC) | 289 | 16.5 | 2 | 6 | 0.999 |
| (−)-epigallocatechin (EGC) | 305 | 10.8 | 4 | 13 | 0.999 |
| (−)-epicatechin gallate (ECG) | 441 | 24.4 | 5 | 18 | 0.996 |
| (−)-epigallocatechin gallate (EGCG) | 457 | 18.2 | 3 | 10 | 0.997 |
Quantification of catechins
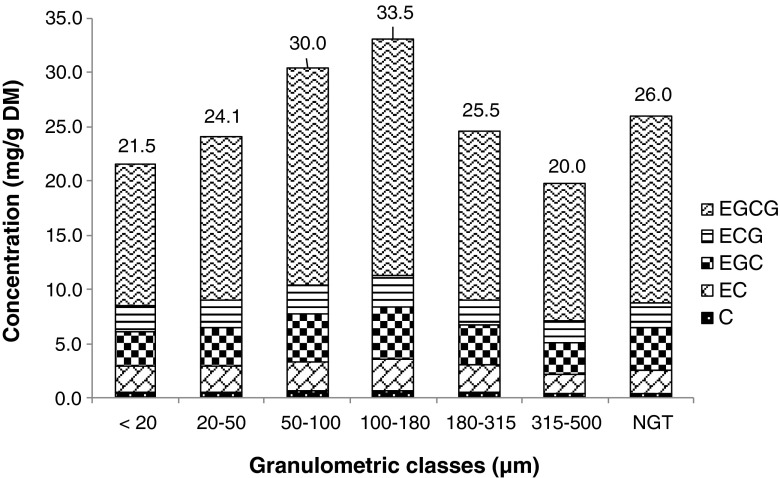
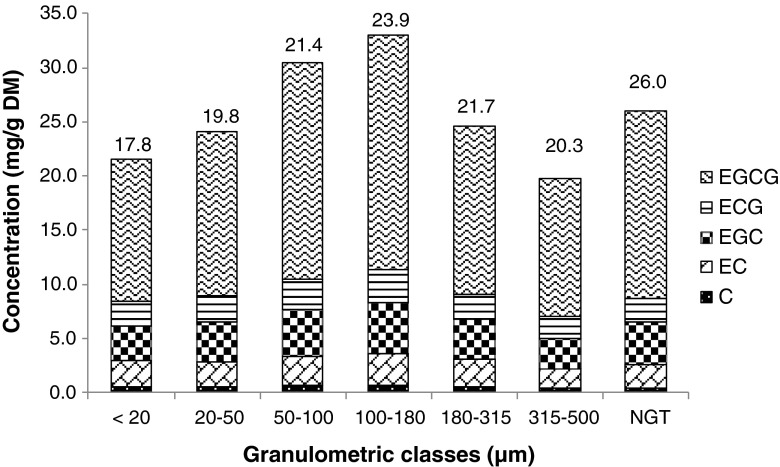
Coefficients of determination (r2) from the calibration curves obtained with the external method are displayed in the Table 2. The catechin signals (S/N ratios) at the studied concentrations were equal or 10 times higher than the limits of the quantification (LOQs). These calibration curves were used to quantify catechins in GTP extracts. The catechin peaks in chromatograms obtained from extracts were quantified in terms of equivalents of external standards. Figures 4 and 5 display catechin contents calculated for GTPs at 6000 rpm and 18,000 rpm, respectively, and NGT.
Fig. 4.
LC-MS dosages of flavan-3-ols: (+)-catechin (C), (−)-epicatechin (EC), (−)-epigallocatechin (EGC), (−)-epicatechin gallate (ECG), (−)-epigallocatechin gallate (EGCG) from tea powder extracts and the unground leaves extract at 6000 rpm. Total content of the catechins quantified are displayed above each bar in mg/g of dried matter. Variations between granulometric classes were statistically significant (p < 0.05)
Fig. 5.
LC-MS dosages of flavan-3-ols (+)-catechin (C), (−)-epicatechin (EC), (−)-epigallocatechin (EGC), (−)-epicatechin gallate (ECG), (−)-epigallocatechin gallate (EGCG) from tea powder extracts and the unground leaves extract at 18,000 rpm. Total content of the catechins quantified are displayed above each bar in mg/g of dried matter. Variations between granulometric classes were statistically significant (p < 0.05)
Concentrations of EGCG were much higher than other catechins, from five to twenty times higher according to the compound. The order of relative concentrations of catechins (EGCG > EGC > ECG > EC > C) is similar to the one described by Suzuki et al. (2013). Nevertheless, another article showed a different ranking (Khokhar and Magnusdottir 2002). This may depend on the green tea variety. Variations of concentration of the five catechins followed the same trend according to particle size. For all catechins, maximum concentrations were found for granulometric classes between 100 and 180 μm. The lowest catechin contents were obtained for the <20 μm, 20–50 μm and 315–500 μm granulometric classes. The concentration for the non-ground leaf extract (26.0 mg/g DM) was lower than 100–180 μm (33.5 mg/g DM) at 6000 rpm. A positive correlation was calculated between antioxidant activity and catechin content (r2 = 0.893).
Even if variations of catechin concentrations were similar at both speeds, NGT extract led to a higher catechin content at 18,000 rpm (Figs. 5). Indeed, as Hu et al. (2012) mentioned, it is crucial to avoid excessive heat during grinding, and operating at 18,000 rpm may increase heat. Catechin contents increased when particle size decreased except for smaller particle size, <20 μm and 20–50 μm. In that case, the larger surface area in finer GTPs could increase the contact surface between O2 and particles, thus leading to the oxidation of catechins (Neilson et al. 2007).
Conclusion
This study showed the advantage of grinding and sieving green tea leaves to increase content of polyphenols. Our results revealed that antioxidant activity was dependent on particle size. However, it is important to control grinding speed and particle size to avoid catechin degradation by heat and oxidation. The highest antioxidant activity and catechin content were obtained with the particle size of 100–180 μm at 6000 rpm, which then determine the best conditions for the grinding of green tea leaves. This procedure may be extended to other food applications, for example to improve nutritive values.
Acknowledgments
The authors are thankful to Extrapole and LIBio which produced the green tea powders.
References
- Barnwal P, Singh KK, Sharma A, et al. (2015) Influence of pin and hammer mill on grinding characteristics, thermal and antioxidant properties of coriander powder. J Food Sci Technol:1–12. doi:10.1007/s13197-015-1975-0 [DOI] [PMC free article] [PubMed]
- Baudelaire E. (2013) Brevet WO2013057379A1 - Procede Ptc Pour l’extraction Par Voie Seche Des Principes Actifs Naturels - Google Brevets http://www.google.com/patents/WO2013057379A1?cl=fr.
- Burin VM, Ferreira-Lima NE, Panceri CP, Bordignon-Luiz MT. Bioactive compounds and antioxidant activity of Vitis vinifera and Vitis labrusca grapes: evaluation of different extraction methods. Microchem J. 2014;114:155–163. doi: 10.1016/j.microc.2013.12.014. [DOI] [Google Scholar]
- Chen G, Chen H. Extraction and deglycosylation of flavonoids from sumac fruits using steam explosion. Food Chem. 2011;126:1934–1938. doi: 10.1016/j.foodchem.2010.12.025. [DOI] [PubMed] [Google Scholar]
- Chengelis CP, Kirkpatrick JB, Regan KS, et al. 28-day oral (gavage) toxicity studies of green tea catechins prepared for beverages in rats. Food Chem Toxicol. 2008;46:978–989. doi: 10.1016/j.fct.2007.10.027. [DOI] [PubMed] [Google Scholar]
- Chen Z, Zhu QY, Tsang D, Huang Y. Degradation of green tea catechins in tea drinks. J Agric Food Chem. 2001;49:477–482. doi: 10.1021/jf000877h. [DOI] [PubMed] [Google Scholar]
- Cook NC, Samman S. Flavonoids—chemistry, metabolism, cardioprotective effects, and dietary sources. J Nutr Biochem. 1996;7:66–76. doi: 10.1016/0955-2863(95)00168-9. [DOI] [Google Scholar]
- Czyzowska A, Klewicka E, Pogorzelski E, Nowak A. Polyphenols, vitamin C and antioxidant activity in wines from Rosa canina L. and Rosa rugosa thunb. J Food Compos Anal. 2015;39:62–68. doi: 10.1016/j.jfca.2014.11.009. [DOI] [Google Scholar]
- Davis JM, Murphy EA, Carmichael MD, Davis B. Quercetin increases brain and muscle mitochondrial biogenesis and exercise tolerance. Am J Phys Regul Integr Comp Phys. 2009;296:R1071–R1077. doi: 10.1152/ajpregu.90925.2008. [DOI] [PubMed] [Google Scholar]
- Dufresne CJ, Farnworth ER. A review of latest research findings on the health promotion properties of tea. J Nutr Biochem. 2001;12:404–421. doi: 10.1016/S0955-2863(01)00155-3. [DOI] [PubMed] [Google Scholar]
- Higdon JV, Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr. 2003;43:89–143. doi: 10.1080/10408690390826464. [DOI] [PubMed] [Google Scholar]
- Hu J, Chen Y, Ni D. Effect of superfine grinding on quality and antioxidant property of fine green tea powders. LWT Food Sci Technol. 2012;45:8–12. doi: 10.1016/j.lwt.2011.08.002. [DOI] [Google Scholar]
- Jouad H, Lacaille-Dubois MA, Lyoussi B, Eddouks M. Effects of the flavonoids extracted from Spergularia purpurea pers. on arterial blood pressure and renal function in normal and hypertensive rats. J Ethnopharmacol. 2001;76:159–163. doi: 10.1016/S0378-8741(01)00209-4. [DOI] [PubMed] [Google Scholar]
- Khokhar S, Magnusdottir SGM. Total phenol, catechin, and caffeine contents of teas commonly consumed in the United Kingdom. J Agric Food Chem. 2002;50:565–570. doi: 10.1021/jf010153l. [DOI] [PubMed] [Google Scholar]
- Kim D-O, Lee KW, Lee HJ, Lee CY. Vitamin C Equivalent antioxidant capacity (VCEAC) of phenolic phytochemicals. J Agric Food Chem. 2002;50:3713–3717. doi: 10.1021/jf020071c. [DOI] [PubMed] [Google Scholar]
- Kurita T, Miyagishima A, Nozawa Y, et al. A dosage design of mitomycin C tablets containing finely powdered green tea. Int J Pharm. 2004;275:279–283. doi: 10.1016/j.ijpharm.2004.01.037. [DOI] [PubMed] [Google Scholar]
- Kuriyama S. The relation between green tea consumption and cardiovascular disease as evidenced by epidemiological studies. J Nutr. 2008;138:1548S–1553S. doi: 10.1093/jn/138.8.1548S. [DOI] [PubMed] [Google Scholar]
- Lee KJ, Lee SH. Extraction behavior of caffeine and EGCG from green and black tea. Biotechnol Bioprocess Eng. 2008;13:646–649. doi: 10.1007/s12257-008-0034-3. [DOI] [Google Scholar]
- Lu T-M, Lee C-C, Mau J-L, Lin S-D. Quality and antioxidant property of green tea sponge cake. Food Chem. 2010;119:1090–1095. doi: 10.1016/j.foodchem.2009.08.015. [DOI] [Google Scholar]
- Maaroufi C, Melcion J-P, de Monredon F, et al. Fractionation of pea flour with pilot scale sieving. I. physical and chemical characteristics of pea seed fractions. Anim Feed Sci Technol. 2000;85:61–78. doi: 10.1016/S0377-8401(00)00127-9. [DOI] [Google Scholar]
- Muanda F, Koné D, Dicko A, et al. Phytochemical composition and antioxidant capacity of Three Malian Medicinal Plant parts. Evid Based Complement Alternat Med. 2011;2011:1–8. doi: 10.1093/ecam/nep109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muanda FN, Bouayed J, Djilani A, et al (2010) Chemical composition and, cellular evaluation of the antioxidant activity of Desmodium adscendens leaves. Evid Based Complement Alternat Med 2011:e620862. [DOI] [PMC free article] [PubMed]
- Murase T, Nagasawa A, Suzuki J, et al. Beneficial effects of tea catechins on diet-induced obesity: stimulation of lipid catabolism in the liver. Int J Obes. 2002;26:1459–1464. doi: 10.1038/sj.ijo.0802141. [DOI] [PubMed] [Google Scholar]
- Murthy Ct, Rani M, Rao P n. S. Optimal grinding characteristics of black pepper for essential oil yield. J Food Process Eng. 1999;22:161–173. doi: 10.1111/j.1745-4530.1999.tb00478.x. [DOI] [Google Scholar]
- Neilson AP, Hopf AS, Cooper BR, et al. Catechin degradation with concurrent formation of homo- and heterocatechin dimers during in Vitro digestion. J Agric Food Chem. 2007;55:8941–8949. doi: 10.1021/jf071645m. [DOI] [PubMed] [Google Scholar]
- Oliveira RMMD. Quantification of catechins and caffeine from green tea (Camellia sinensis) infusions, extract, and ready-to-drink beverages. Food Sci Technol Camp. 2012;32:163–166. [Google Scholar]
- Pastore RL, Fratellone P. Potential health benefits of green tea (Camellia sinensis): A narrative review. Explore J Sci Heal. 2006;2:531–539. doi: 10.1016/j.explore.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Pesek CA, Wilson LA, Hammond EG (1985) Spice quality: effect of cryogenic and ambient grinding on volatiles. J Food Sci 50:599–601. doi:10.1111/j.1365-2621.1985.tb13753.x
- Roginsky V, Lissi EA. Review of methods to determine chain-breaking antioxidant activity in food. Food Chem. 2005;92:235–254. doi: 10.1016/j.foodchem.2004.08.004. [DOI] [Google Scholar]
- Sharififar F, Dehghn-Nudeh G, Mirtajaldini M. Major flavonoids with antioxidant activity from teucrium polium L. Food Chem. 2009;112:885–888. doi: 10.1016/j.foodchem.2008.06.064. [DOI] [Google Scholar]
- Singh Kk, Goswami TK (1999) Studies on cryogenic grinding of cumin seed. J Food Process Eng 22:175–190. doi:10.1111/j.1745-4530.1999.tb00479.x
- Suzuki T, Kumazoe M, Kim Y, et al. Green tea extract containing a highly absorbent catechin prevents diet-induced lipid metabolism disorder. Sci Rep. 2013 doi: 10.1038/srep02749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L, Liu X, Jiang Y, et al. Compositional analysis of teas from Australian supermarkets. Food Chem. 2006;94:115–122. doi: 10.1016/j.foodchem.2004.11.009. [DOI] [Google Scholar]
- Zhang Z, Song H, Peng Z, et al. Characterization of stipe and cap powders of mushroom (lentinus edodes) prepared by different grinding methods. J Food Eng. 2012;109:406–413. doi: 10.1016/j.jfoodeng.2011.11.007. [DOI] [Google Scholar]
- Zhao X, Yang Z, Gai G, Yang Y. Effect of superfine grinding on properties of ginger powder. J Food Eng. 2009;91:217–222. doi: 10.1016/j.jfoodeng.2008.08.024. [DOI] [Google Scholar]