Abstract
Store-operated calcium entry (SOCE) is the mechanism by which extracellular signals elicit prolonged intracellular calcium elevation to drive changes in fundamental cellular processes. Here, we investigated the role of SOCE in the regulation of renal water reabsorption, using the inbred rat strain SHR-A3 as an animal model with disrupted SOCE. We found that SHR-A3, but not SHR-B2, have a novel truncating mutation in the gene encoding stromal interaction molecule 1 (STIM1), the endoplasmic reticulum calcium (Ca2+) sensor that triggers SOCE. Balance studies revealed increased urine volume, hypertonic plasma, polydipsia, and impaired urinary concentrating ability accompanied by elevated circulating arginine vasopressin (AVP) levels in SHR-A3 compared with SHR-B2. Isolated, split-open collecting ducts (CD) from SHR-A3 displayed decreased basal intracellular Ca2+ levels and a major defect in SOCE. Consequently, AVP failed to induce the sustained intracellular Ca2+ mobilization that requires SOCE in CD cells from SHR-A3. This effect decreased the abundance of aquaporin 2 and enhanced its intracellular retention, suggesting impaired sensitivity of the CD to AVP in SHR-A3. Stim1 knockdown in cultured mpkCCDc14 cells reduced SOCE and basal intracellular Ca2+ levels and prevented AVP-induced translocation of aquaporin 2, further suggesting the effects in SHR-A3 result from the expression of truncated STIM1. Overall, these results identify a novel mechanism of nephrogenic diabetes insipidus and uncover a role of SOCE in renal water handling.
Keywords: calcium, water transport, diabetes insipidus, vasopressin, collecting ducts
The conservation of bodily fluids is of key importance for the growth and survival of all terrestrial organisms. Kidneys control water balance via the regulation of urinary volume production. Elevations in plasma osmolality of less than 1% elicit release of the antidiuretic hormone, arginine vasopressin (AVP) from the posterior pituitary gland.1,2 AVP acts on type 2 vasopressin receptors (V2R) to increase water permeability in the collecting duct (CD), the major site for regulation of renal water handling.3–5 AVP promotes synthesis and trafficking of aquaporin 2 (AQP2) water channels to the apical membrane of the CD principal cells to drive water transport by osmotic gradient.4,6 Inability of the CD to adequately respond to augmented AVP levels results in production of large volumes of dilute urine with secondary polydipsia, as found during congenital or acquired forms of nephrogenic diabetes insipidus (NDI) in humans.3,6,7
At the molecular level, stimulation of Gs-protein–coupled V2R at the CD basolateral plasma membrane by AVP causes increase in cyclic-AMP (cAMP) levels and activation of protein kinase A-dependent cascades.1,8,9 This in turn elicits a series of serine phosphorylation events in AQP2, among which phosphorylation of Ser256 and Ser269 are critical for trafficking and retaining of the channel at the CD apical plasma membrane.1,10–12 In addition, AVP induces a prolonged elevation of intracellular calcium ([Ca2+]i) in CD principal cells by stimulating calcium (Ca2+) release from the ryanodine-sensitive endoplasmic reticulum (ER) stores and subsequent Ca2+ influx via unidentified plasma membrane cation channels through a mechanism resembling store-operated calcium entry (SOCE).13–15 While the importance of cAMP-protein kinase A signaling is certain, the role of AVP-dependent [Ca2+]i elevations in AQP2-mediated water transport and renal water conservation remains largely unknown.
SOCE is the process by which depletion of ER calcium stores causes influx of Ca2+ across the plasma membrane.16–18 This mechanism is widespread in eukaryotic cells and is involved in many cellular functions ranging from gene expression to regulation of proliferation.19–23 SOCE is triggered by the stromal interaction molecule 1 (STIM1), an ER Ca2+ sensor that detects the depletion of ER Ca2+ stores and conveys this information to the plasma membrane. STIM1 protein architecture reflects its function. The N-terminal EF domain residing in the ER senses the decrease of Ca2+ levels, while the polybasic domain at the STIM1 C-terminus is involved in the activation of plasma membrane Ca2+-permeable channels, such as Ca2+-selective calcium release-activated calcium channel 1 (ORAI1) or nonselective transient receptor potential-canonical (TRPC) channels 24–28. Little is known about the role of SOCE in normal renal function or disease.
In this study, we identified the first animal model with dysfunctional AVP-induced [Ca2+]i signaling and used it to probe the physiologic relevance of this mechanism and to determine the pathophysiologic implications for whole-body fluid homeostasis when it is disrupted. We found that the stroke-prone spontaneously hypertensive rat line (SHR-A3) possesses a truncation mutation in the Stim1 gene. Our investigation demonstrates that dysfunctional SOCE results from Stim1 mutation which attenuates the sustained elevation of [Ca2+]i in response to AVP in freshly isolated, split-open CDs. This in turn leads to decreased AQP2 expression and translocation to the apical plasma membrane. Targeted silencing of Stim1 in cultured immortalized mouse cortical collecting duct (mpkCCDc14) cells also supports this conclusion. Furthermore, CDs from SHR-A3 exhibit resistance to exogenously applied AVP. At the systemic level, these animals manifest polyuria, polydipsia, and diminished urinary concentrating ability in the presence of augmented circulating AVP and plasma hypertonicity. Overall, we provide evidence that SOCE plays an important role in renal AVP function and that defective SOCE causes NDI in rats.
Results
SHR-A3 Have Impaired Renal Water Handling
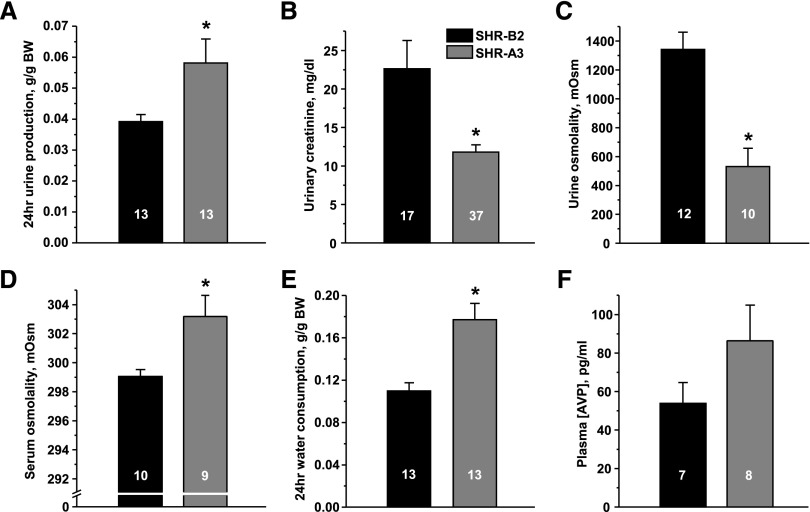
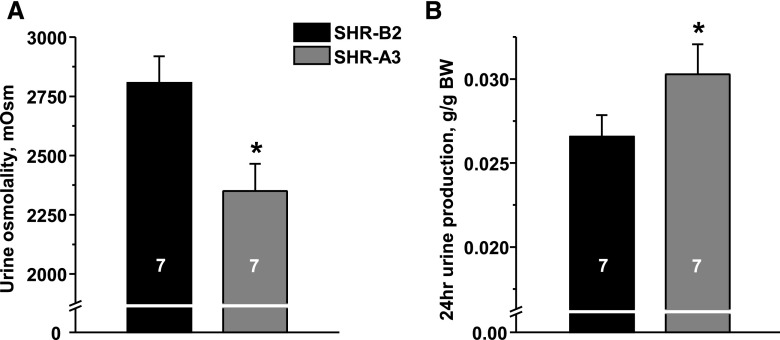
Balance studies identified a markedly altered systemic water homeostasis in SHR-A3. We found that SHR-A3 exhibit significantly greater 24-hour urinary production than SHR-B2 (Figure 1A). This is associated with decreased urinary creatinine concentration (U[creat]) (Figure 1B) and reduced osmolality (Figure 1C), indicating substantial dilution. Importantly, serum osmolarity is increased (Figure 1D) and water intake is elevated in SHR-A3 (Figure 1E). Increased plasma AVP levels (Figure 1F) suggest that the observed defect is primarily attributed to the kidney. At the tested age of 6–12 weeks, both strains had not yet developed noticeable kidney injury.29 Serum aldosterone, as an indicator of renin-angiotensin-aldosterone system (RAAS) status, was within the normally observed range,30 while SHR-A3 had greater values than SHR-B2. Further assessment of spot urine and serum electrolytes in two SHR lines revealed that urinary potassium excretion was increased in SHR-A3 and, consistently, these animals were hypokalemic. Urine sodium excretion was increased in SHR-A3, while serum sodium was not different across the two SHR lines (Supplemental Figure 1). Overall, we conclude that SHR-A3 develop polyuria, plasma hypertonicity with secondary polydipsia in the presence of elevated circulating AVP, therefore demonstrating an NDI phenotype during basal (unstressed) conditions.
Figure 1.
Truncation of STIM1 results in abnormal systemic water homeostasis. (A) Summary graph showing average 24-hour urine production in SHR-B2 (black) and SHR-A3 (gray). (B) Summary graph comparing U[creat] in SHR-B2 and SHR-A3. (C) Summary graph demonstrating averaged urine osmolality in SHR-B2 and SHR-A3. (D) Summary graph showing serum osmolality in SHR-B2 and SHR-A3. (E) Summary graph comparing water consumption by SHR-A3 and SHR-B2 within a 24-hour interval. (F) Summary graph showing averaged AVP levels in plasma samples taken from SHR-A3 and SHR-B2. *P<0.05 versus SHR-B2, estimated with one-way ANOVA test. The number of animals in each group is indicated on top of the respective bars.
Genome Sequencing Identifies a Major Genetic Variation between SHR-A3 and SHR-B2 which Affects STIM1 Function that Segregates with U[creat]
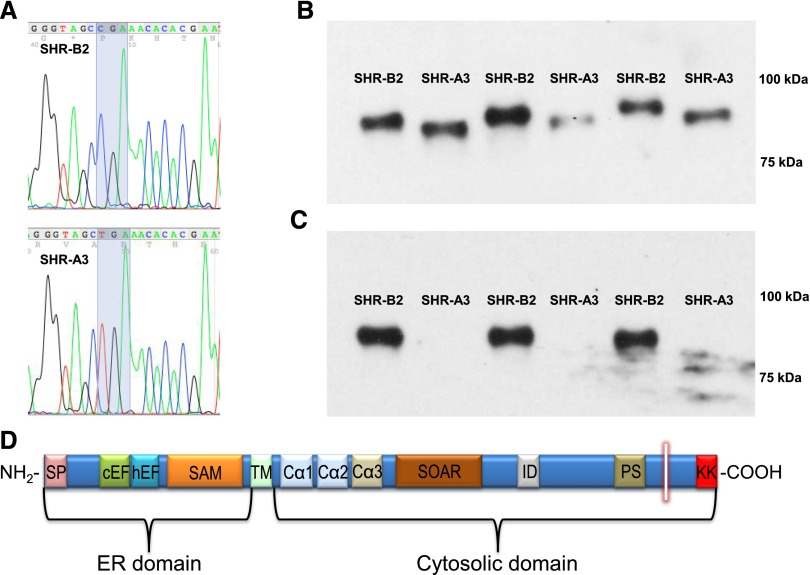
We have previously shown that shared ancestry between inbred SHR lines has produced extensive genetic identity between these lines. Single nucleotide polymorphism markers (approximately 10,000) indicated that 87% of the genomes of SHR-A3 and SHR-B2 arose from an ancestor common to both lines. We used genotypes from approximately 200 polymorphic nucleotides to determine whether a locus of major effect on U[creat] could be identified in the F2 progeny of an SHR-A3×SHR-B2 intercross, but none was localized. This suggested the possibility that a new mutation arising in the 87% of the genome shared identical by descent, and therefore not targeted by mapping markers, might be related to altered renal water handling. To investigate the identical-by-descent genome we examined the whole genome sequence for these two SHR lines and observed a truncating mutation resulting from a premature stop codon affecting Stim1 in SHR-A3. Stim1 lies in the 87% of the genome that is inherited from a single shared ancestor. We found only ten genes that had major mutations including start lost (1), stop gained (2), stop lost (1), and frame-shift (7). None of these genes are expected to be involved in renal function or the regulation of water reabsorption or mechanisms involved in its control, except for the mutation in Stim1. We further verified the mutation by targeted resequencing (Figure 2A). We examined the publically available whole-genome sequence for 28 inbred rat strains. The only other strain possessing this mutation is the SHR-A3 strain maintained in Glasgow, Scotland. We infer that this mutation is a recent event likely occurring after the separation of SHR into distinct lines.31 Premature stop codons can result in nonsense-mediated decay of mRNA, preventing protein expression. We investigated whether STIM1 protein was present in SHR-A3 and SHR-B2 animals and detected STIM1 by Western blotting in both SHR lines. Migration of the protein indicated a smaller apparent molecular weight in SHR-A3 than in SHR-B2. This protein was detected using an antiserum directed to the N-terminus of STIM1 (Figure 2B). A STIM1 antiserum raised against amino acid residues encoded downstream of the SHR-A3 premature stop codon failed to detect STIM1 protein in SHR-A3 only (Figure 2C), as expected from the position of the premature stop codon (Figure 2D). In the F2 progeny of a cross between SHR-A3 and SHR-B2, inheritance of the SHR-A3 Stim1 mutant allele was correlated with reduced U[creat]. In F2 animals inheriting only SHR-B2 Stim1 alleles, U[creat] was 25.8±3.81 mg/dl (n=53), while in F2 animals possessing one or two copies of the SHR-A3 Stim1 allele U[creat] was 18.2±1.45 mg/dl (n=144; P=0.02).
Figure 2.
A novel nonsense mutation of Stim1 gene truncates C-terminal domain of STIM1 protein. (A) Targeted resequencing to verify next-generation sequence variant in Stim1. SHR-B2 possesses the wild-type codon 640 encoding Arg, in SHR-A3 the cytosine residue is mutated to thymidine creating a stop codon. (B) Western blot using N-terminal–directed antibodies (antigen includes human STIM1 residues 61–74) detects STIM1 protein in both SHR-A3 and SHR-B2. (C) Western blot using C-terminal–directed antibodies (antigen includes human STIM1 residues 657–683) detects STIM1 protein in SHR-B2 but not in SHR-A3. (D) Domain structure of STIM1. A short transmembrane spans the ER membrane. The ER resident portion contains two EF hand domains including a Ca2+-binding canonical cEF domain and a non-Ca2+–binding hidden domain (hEF) and a sterile α-motif (SAM). The cytosolic domain contains coiled-coil regions with the CC1 region divided into Ca1, Ca2, and Ca3. Ca2 and Ca3 comprise part of the STIM1-ORAI (SOAR) activating region. An acidic inhibitory domain (ID) mediates calcium-dependent ORAI1 inactivation. A Pro/Ser-rich domain (PS) is followed by a poly-basic lysine-rich C-terminus (KK). The stop codon created in SHR-A3 terminates the STIM1 protein between the PS and KK domains (shown by the vertical white bar). The C-terminal polybasic region may serve in spatial coordination between STIM1 anchored in the ER membrane and ORAI1 in the plasma membrane. The KK domain has also been proposed to serve as a gating region of STIM1 controlling TRPC channel activation (see Discussion).
Mutation in Stim1 Gene Compromises SOCE in the CD
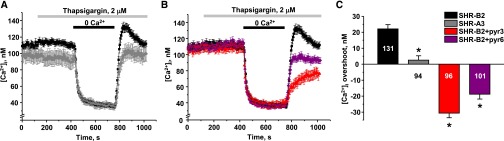
Because STIM1 mediates SOCE in multiple cell types (reviewed in Cahalan32), we directly monitored [Ca2+]i dynamics in freshly isolated split-open CDs from SHR-B2 and SHR-A3. The CD is the site at which AVP acts to determine renal water clearance. A standard protocol of extracellular Ca2+ removal/readdition upon depletion of ER stores with thapsigargin was used to stimulate SOCE.33 This experimental maneuver elicits transient elevations of [Ca2+]i above the baseline ([Ca2+]i “overshoot”) in CD cells from SHR-B2 having full-length STIM1 (Figure 3A, black trace). In contrast, no transient elevation of [Ca2+]i is detected in CD cells from SHR-A3 (Figure 3A, light gray trace). In addition, STIM1 truncation also results in significantly lower basal [Ca2+]i levels in SHR-A3, pointing to perturbed [Ca2+]i homeostasis.
Figure 3.
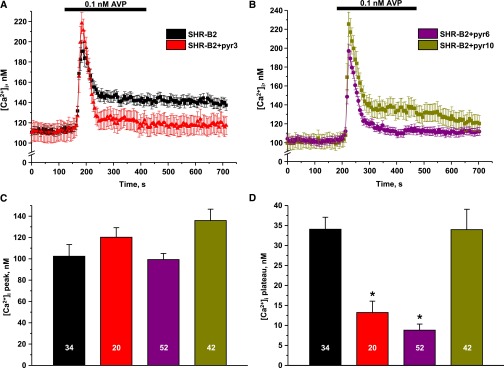
Defect in STIM1 or inhibition of ORAI1 disrupts SOCE in CD cells. (A) The average time courses of [Ca2+]i changes in response to Ca2+ removal–Ca2+ readdition protocol upon depletion of [Ca2+]i stores with thapsigargin recorded in CD cells from SHR-B2 (black) and SHR-A3 (gray). (B) The average time courses of [Ca2+]i changes in response to Ca2+ removal–Ca2+ readdition protocol upon depletion of [Ca2+]i stores with thapsigargin recorded in CD cells from SHR-B2 in control (black, reproduced from A), on the background of nonspecific TRPC3/ORAI1 inhibitor pyr3 (6 μM, shown in red) and specific ORAI1 inhibitor pyr6 (5 μM, shown in purple). (C) Summary graph comparing average magnitudes of [Ca2+]i overshoots observed in SHR-B2 in control, SHR-A3, SHR-B2 treated with pyr3 and pyr6. *P<0.05 versus [Ca2+]i overshoot in SHR-B2, estimated with one-way ANOVA test. The number of cells in each group is indicated on top of the respective bars. At least six CDs from three different animals were used for each treatment.
Existing experimental evidence suggests that, upon ER Ca2+ depletion, STIM1 physically interacts with Ca2+-permeable ORAI1 and TRPC3 channels at the plasma membrane to trigger SOCE.24,28,34,35 Pretreatment with the pyrazole drugs, pyr3 (a combined TRPC3 and ORAI1 inhibitor) and pyr6 (ORAI1 inhibitor)36 abolishes [Ca2+]i overshoot in split-open CD from SHR-B2 (Figure 3B), similarly to that observed in STIM1-deficient SHR-A3. Pyr3 applied to CD cells from SHR-A3 has a mild inhibitory effect (Supplemental Figure 2), indicating a small residual activity of the truncated STIM1. Figure 3C summarizes the amplitude of SOCE in all tested conditions. Thus, we concluded that truncation of the C-terminus of STIM1 drastically diminishes SOCE in CD of SHR-A3.
Abnormal AVP-Induced [Ca2+]i Responses in CD of SHR-A3
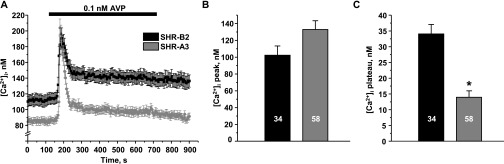
Previous studies have shown that AVP causes an elevation in [Ca2+]i, suggesting the possible involvement of SOCE in CD cells.13–15 Thus, we next probed how STIM1 dysfunction in SHR-A3 affects [Ca2+]i responses to AVP in split-open CD. AVP (0.1 nM) produces a biphasic response consisting of a transient initial peak and a sustained phase (plateau) in CD cells of SHR-B2 (Figure 4, black trace). Whereas no differences in the initial AVP-induced [Ca2+]i peak are detected, the sustained plateau phase is virtually absent in CD cells from SHR-A3 (Figure 4A, gray trace). Figure 4, B and C compare the absolute values of the initial peak and plateau in SHR-B2 and SHR-A3, respectively. We have not detected significant changes in expression of V2R in the kidney of SHR-B2 and SHR-A3, arguing against the possibility that variations in V2R levels might underlie the observed differences in Ca2+ responses (Supplemental Figure 3). The application of higher concentrations of AVP (<10 nM) also does not significantly change the magnitudes of the initial peak and plateau for each group (Supplemental Figure 4). We concluded that STIM1 truncation drastically diminishes the sustained elevation of [Ca2+]i in response to AVP in CD cells.
Figure 4.
The sustained phase of AVP-activated [Ca2+]i response is significantly impaired in CD cells with defective STIM1. (A) The average time courses of [Ca2+]i responses to application of 0.1 nM AVP (shown with a black bar on top) recorded in CD cells from SHR-B2 (black) and SHR-A3 (gray). (B,C) Summary graphs comparing the average magnitudes of (B) transient and (C) sustained phases of AVP-induced response in SHR-B2 and SHR-A3. *P<0.05 versus [Ca2+]i plateau in SHR-B2, estimated with one-way ANOVA test. The number of cells in each group is indicated on top of the respective bars. At least six CDs from three different experimental animals were used for each treatment.
We next tested whether the lack of the plateau phase in the AVP-induced [Ca2+]i response in the CD cells from SHR-A3 is due to dysfunctional SOCE. Inhibition of SOCE-mediated Ca2+ entry with pyr3 has no effect on the initial [Ca2+]i peak in response to AVP, but greatly diminishes the sustained phase (Figure 5A). We then aimed to discriminate whether the AVP-induced [Ca2+]i plateau is mediated by ORAI1 or TRPC3, both of which are known to interact with STIM1.24,28,34,35 The selective inhibitor of ORAI1, pyr6, diminishes the plateau, whereas TRPC3 blockade with a specific antagonist (pyr10) has no measurable effect on AVP-induced elevation of [Ca2+]i (Figure 5B). Figure 5, C and D summarize the effects of the tested inhibitors on the initial [Ca2+]i peak and the sustained plateau phase in response to AVP, respectively. Overall, the results suggest that the inability of the truncated STIM1 to properly activate the Ca2+-permeable channel ORAI1 underlies the lack of the sustained [Ca2+]i elevation in response to AVP in CD cells.
Figure 5.
Ca2+ entry via ORAI1 channel sustains AVP-induced [Ca2+]i response in CD cells. (A) The average time course of [Ca2+]i elevation after application of AVP (shown with a black bar) in CD cells from SHR-B2 in control (shown in black) and on the background of nonspecific TRPC3/ORAI1 channel inhibitor pyr3 (6 μM, shown in red). (B) The average time course of [Ca2+]i responses to AVP in CD cells from SHR-B2 on the background of selective TRPC3 inhibitor pyr10 (5 μM, shown in dark yellow), and ORAI1 inhibitor pyr6 (5 μM, shown in purple). Summary graphs comparing the average magnitudes of (C) transient and (D) sustained phases of AVP-induced responses recorded in CD cells from SHR-B2 in control and upon treatment with pyr3, pyr6, and pyr10. CDs were preincubated with TRPC3/ORAI1 inhibitors for 5 minutes before AVP application. *P<0.05 versus [Ca2+]i plateau in SHR-B2, estimated with one-way ANOVA test. The number of cells in each group is indicated on top of the respective bars. At least six CDs from three different rats were tested for each treatment.
SHR-A3 Have Reduced AQP2 Expression in the Kidney
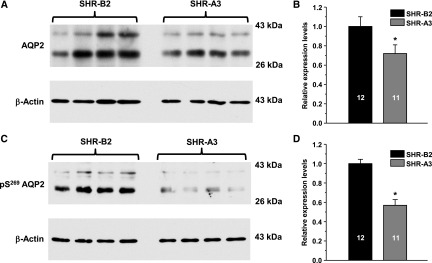
We examined whether abnormalities in AVP-induced [Ca2+]i signaling lead to changes in AQP2 levels in SHR-A3. Because the CD is the only renal site of AQP2 expression, we quantified levels of the protein in whole-kidney homogenates (Figure 6). As demonstrated in the representative Western blot (Figure 6A) and the summary graph (Figure 6B), SHR-A3 have significantly lower total expression of AQP2 than that detected in SHR-B2. Because the majority of the AQP2 is stored in intracellular vesicles unless an AVP signal is present, we also assessed the expression of the phosphorylated Ser269 AQP2 (pS269AQP2), which was shown to be localized exclusively on the apical plasma membrane.11,12 We detected a much greater reduction of the membrane portion of AQP2 in SHR-A3 compared with that in SHR-B2, pointing to a compromised subcellular distribution of the channel, as shown in Figure 6, C and D. The decrease in the membrane-associated pS269AQP2 fraction observed in SHR-A3 could be caused merely by the reduction of total AQP2 expression, while the trafficking rates of the protein remained intact. To disqualify this possibility, relative abundance of pS269AQP2 in SHR-A3 was normalized to the relative expression level of total AQP2 in this line. Consistently, the adjusted value of pS269AQP2 abundance, estimating AQP2 trafficking rate, is still significantly lower in the kidneys from SHR-A3 (Supplemental Figure 5). Overall, we conclude that STIM1 truncation decreases the expression and apical localization of AQP2, which is likely contributing to increased urinary volume production in SHR-A3.
Figure 6.
Compromised SOCE markedly diminishes AQP2 abundance in the kidney. (A) Representative Western blot from whole-kidney lysates of SHR-B2 and SHR-A3 probed with anti-AQP2 and anti-actin antibodies. AQP2 is present as a duplet of the upper glycosylated (approximately 37 kDa) and lower nonglycosylated (approximately 29 kDa) bands. (B) Summary graph comparing total renal AQP2 expression (both glycosylated and nonglycosylated forms) in SHR-B2 (black) and SHR-A3 (gray) from Western blots similar to that shown in (A). (C) Representative Western blot from whole-kidney lysates of SHR-B2 and SHR-A3 probed with anti–phospho-Ser269 AQP2 (retained in the plasma membrane) and anti-actin antibodies. (D) Summary graph comparing renal expression of phospho-Ser269 AQP2 (both glycosylated and nonglycosylated forms) in SHR-B2 (black) and SHR-A3 (gray) from Western blots similar to that shown in (C). Samples were run in duplicates. Intensities of AQP2-reporting bands were normalized to the intensities of the respective actin bands. *P<0.05 versus SHR-B2, estimated with one-way ANOVA test. The number of animals in each group is indicated on top of the respective bars.
CD Cells of SHR-A3 Exhibit Resistance to AVP
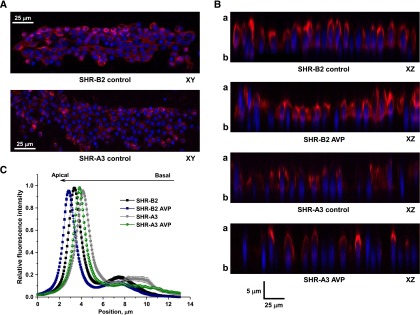
A stronger decrease in apical AQP2 levels compared with the total channel expression may indicate compromised trafficking of the channel in response to AVP. To test this possibility, we employed fluorescent confocal microscopy to monitor subcellular AQP2 localization in freshly isolated split-open CD from SHR-A3 and SHR-B2 in the control and after treatment with saturating concentration of AVP (0.1 nM) for 30 minutes (Figure 7). Consistent with Western blot data (Figure 6), we observed a weaker fluorescent signal in CD cells of SHR-A3 than in SHR-B2 using the same laser settings (see representative XY plane images in Figure 7A and XZ plane images in Figure 7B). To perform a semiquantitative estimation of the observed differences in subcellular AQP2 localization, we employed line-scan analysis of the AQP2-reporting fluorescent signal distribution along the Z-axis in cross-sections of three-dimensional stacks similar to that shown in Figure 7, A and B. We found that AQP2 distribution is shifted away from the apical plasma membrane in SHR-A3, when compared with SHR-B2 (Figure 7C, gray and black traces, respectively). Pretreatment with AVP causes apically directed translocation of AQP2 in SHR-B2 (Figure 7, B and C, blue trace). We also detected a small shift of fluorescent signal in response to AVP in SHR-A3 (Figure 7, B and 7C, green trace). However, AQP2 remains chiefly cytosolic in AVP-treated SHR-A3 and does not even reach the apical distribution observed in the absence of AVP pretreatment in SHR-B2 (compare green and black traces). Therefore, we conclude that CD cells from SHR-A3 fail to properly translocate AQP2 to the apical plasma membrane, thus demonstrating resistance to AVP treatment.
Figure 7.
CD cells with impaired SOCE have a drastically reduced sensitivity to AVP. (A) Representative confocal plane micrographs of split-opened CDs from SHR-B2 and SHR-A3 showing AQP2 localization (pseudocolor red). Nuclear staining with 4′,6-diamidino-2-phenylindole is shown in pseudocolor blue. (B) Representative micrographs of XZ planes reconstructed from three-dimensional stacks of confocal images, visualizing AQP2 distribution along apical-basal axis in SHR-B2 and SHRA3 in control and after treatment with AVP. (C) The distribution of averaged relative fluorescent signals representing AQP2 localization along Z-axis in CD cells from SHR-B2 and SHR-A3 in control and after treatment with AVP. For each individual cell, the fluorescent signal was normalized to its corresponding maximal value. Position of the apical and basal sides is shown with “a” and “b,” respectively. At least six CDs from three different rats were used to obtain statistics for any given treatment.
STIM1 Modulates AVP-Induced AQP2 Translocation to the Plasma Membrane
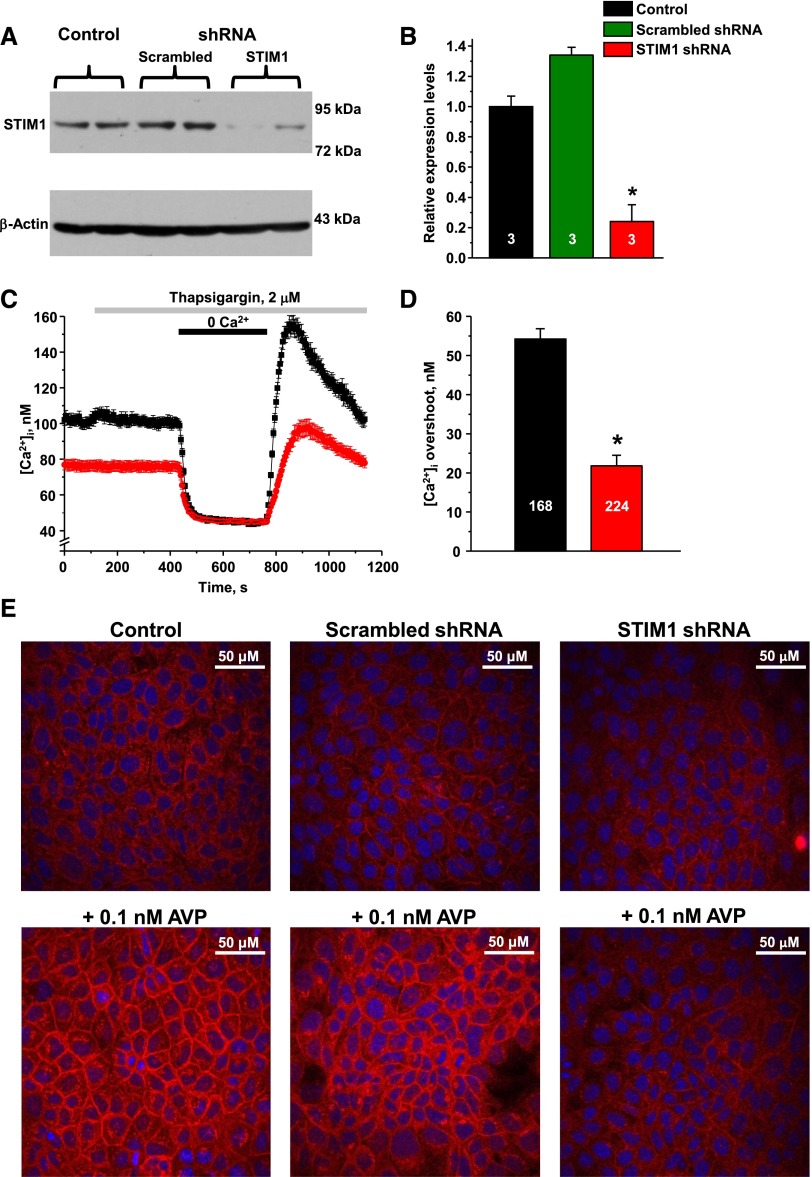
To determine whether STIM1 dysfunction underlies compromised AQP2 trafficking, we next performed shRNA-mediated knockdown of Stim1 in cultured mpkCCDc14 cells, a well established model of the CD principal cells.37 Representative Western blot (Figure 8A) and summary graph (Figure 8B) demonstrate a successful decrease of STIM1 expression by approximately 80% after transfection with STIM1-targeting but not with scrambled shRNA. Stim1 knockdown drastically reduces SOCE in mpkCCDc14 cells and decreases basal [Ca2+]i levels (Figure 8, C and D), as was similarly observed in CD cells from SHR-A3 having truncated (Figure 3A). Treatment with 0.1 nM of AVP for 30 minutes elicits a prominent translocation of AQP2 to the plasma membrane in nontransfected (control) mpkCCDc14 cells and in cells transfected with scrambled shRNA (Figure 8E, left and middle panels, respectively). In contrast, Stim1 knockdown impairs AQP2 redistribution mpkCCDc14 cells (Figure 8E, right panel). These results provide direct evidence that STIM1 is an important component of AVP-induced AQP2 trafficking in CD cells.
Figure 8.
Stim1 knockdown interferes with AVP-induced AQP2 trafficking in mpkCCDc14 cells. (A) Representative Western blot from mpkCCDc14 cell lysates probed with anti-STIM1 and anti-β-actin antibodies in the control, upon stable transfection with scrambled shRNA, and shRNA targeting Stim1, respectively. (B) Summary graph comparing total STIM1 expression from Western blots similar to that shown in (A). (C) The average time courses of [Ca2+]i changes in response to Ca2+ removal–Ca2+ readdition protocol upon depletion of [Ca2+]i stores with thapsigargin recorded in nontransfected controls (black) and cells transfected with shRNA targeting Stim1 (red). (D) Summary graph comparing average magnitudes of [Ca2+]i overshoots observed in nontransfected controls (black) and cells transfected with Stim1-targeting shRNA (red). (E) Representative micrographs of confocal planes monitoring AQP2 (pseudocolor red) distribution in control (upper row) and after 30 minutes of treatment with AVP (bottom row) for nontransfected control (left column), transfected with scrambled shRNA (central column), and shRNA targeting Stim1 (right column), respectively. Nuclear staining with 4′,6-diamidino-2-phenylindole is shown in pseudocolor blue. *P<0.05 versus control, estimated with one-way ANOVA test. The number of samples/cells in each group is indicated on top of the respective bars. At least three different culture plates were used for each treatment.
SHR-A3 Have Impaired Urinary Concentrating Ability
Finally, we probed whether an impaired capacity of CD cells to induce AQP-mediated water reabsorption in response to AVP affects urinary concentrating ability. For this, we subjected SHR-A3 and SHR-B2 to a 24-hour water deprivation test. While we detected a prominent increase in urinary osmolality compared with the control unstressed condition (Figure 1), this value is significantly lower in SHR-A3 compared with that in SHR-B2 (Figure 9A). In accordance, urinary volume tends to be greater in SHR-A3 (Figure 9B). We conclude that STIM1 dysfunction in SHR-A3 compromises the ability of the kidney to conserve water, which is consistent with the NDI phenotype observed in these animals at the baseline condition.
Figure 9.
STIM1 truncation impairs urinary concentrating ability. Summary graphs comparing averaged (A) urine osmolality and (B) volume in SHR-B2 (black) and SHR-A3 (gray), which were deprived of water for 24 hours. *P<0.05 versus SHR-B2, estimated with one-way ANOVA test. The number of animals in each group is indicated on top of the respective bars.
Discussion
Defective regulation of the urinary concentrating mechanism can arise centrally from AVP deficiency (central diabetes insipidus) or due to insensitivity of the kidney to AVP (NDI). Both forms of diabetes insipidus can be caused by mutations affecting AVP production and AVP to AQP2 signaling, or by the action of environmental agents that disrupt the normal control of the concentrating mechanism.7,38 A portion of individuals with NDI do not have mutations in the two genes (AQP2 and V2R) known to be disrupted in heritable cases of NDI and lack a recognized environmental mechanism of disease.38 This introduces the possibility that novel mechanisms linking AVP signaling in the kidney to renal water reabsorption can become defective in a way that compromises the capacity of AVP to drive water reabsorption. It has been recognized for some time that activation of the V2R in the distal nephron induces calcium signaling,13–15,39,40 but the functional implications of this signaling have not been resolved. The data we present here reveal a physiologically pertinent role for calcium signaling in control of the renal concentrating mechanism and shows how impairment of calcium signaling can create NDI.
In the present study, we revealed the presence of a disturbance of the renal concentrating mechanism in the SHR-A3 strain that is apparent when urine creatinine levels in spot urine samples are compared between SHR-A3 and SHR-B2 (Figure 1). Whole-genome sequencing uncovered a mutation producing a premature termination codon in the Stim1 gene that is essential for SOCE. We crossed SHR-A3 animals with a very closely related line, SHR-B2, which shares 87% of its genome from a single common ancestor with SHR-A3, but lacks the Stim1 mutation present in SHR-A3. We tracked the inheritance of the mutant Stim1 allele in the freely segregating F2 progeny of this intercross and observed that there was a significant relationship between inheritance of the Stim1 SHR-A3 mutant allele and the production of dilute urine. This provides evidence that Stim1 mutation may affect renal function and indicates the potential relevance of calcium signaling to the urinary concentrating mechanism.
There have been reports in the clinical literature of humans who have inherited homozygous premature termination codons in Stim1. These individuals have a complex phenotype in which severe combined immunodeficiency is the predominant disease, reflecting the essential role that lymphocyte calcium signaling plays in immune mechanisms.41 The SHR-A3 line does not present evidence of severe combined immunodeficiency. Loss of STIM1 protein in humans homozygous for premature termination codons has been attributed to nonsense-mediated decay.41 Examination of STIM1 protein in SHR-A3 and SHR-B2 indicated the presence of protein in both strains, but SHR-A3 lacks amino acid residues in the C-terminus of STIM1 that can be recognized by a C-terminal–directed antibody. The capacity of the SHR-A3 STIM1 mutation to escape nonsense-mediated decay likely reflects the location of the premature stop codon in the last coding exon. Terminal coding exons are not looped in the spliceosome and this may permit escape from nonsense-mediated decay and allow translation of a truncated protein. The presence of a C-terminal–deleted STIM1 protein raises interesting questions regarding the function of the C-terminal domain of STIM1. This domain contains a polybasic region that has been implicated in two vital STIM1 functions. Lysine residues in this region have been proposed to interact with acidic residues in TRPC channels to provide a gating function, allowing ER calcium depletion to result in increased cellular calcium entry through these channels.34,42 In the cell membrane, the polybasic STIM1 C-terminus has been proposed to provide spatial coordination between the ER and the inner leaflet of the plasma membrane by interaction of its poly-lysine residues with membrane phospholipids critical for activation of ORAI1.43 Thus, SHR-A3 presents an interesting opportunity to uncover the functional implications of loss of these C-terminal interactions. The use of calcium entry inhibitors specific for the TRPC and ORAI1 pathways revealed that SOCE in response to ER calcium depletion is largely inhibited by blocking ORAI1 in the CD of SHR-B2 (Figure 3), while in SHR-A3, SOCE is markedly impaired and ORAI1 inhibition consequently has a much smaller effect.
In order to implicate defective SOCE in polyuria and other indicators of NDI in SHR-A3, we examined the capacity of AVP to initiate calcium signaling in the isolated CD and compared responses in SHR-A3 and SHR-B2. In both rat lines, AVP produces an initial calcium release (Figure 4), which has been previously demonstrated and proposed to reflect activation of ER ryanodine receptors.13 Sustained calcium signaling may be necessary for both the activation of mechanisms leading to AQP2 insertion into the apical membrane as well as nuclear responses that drive expression of AQP2 and other cellular mediators of urine concentration.14,40,44 The source of calcium for sustained elevation of [Ca2+]i is extracellular and depends on ER store depletion to drive STIM1-mediated control of extracellular calcium entry. In SHR-A3 CD, this sustained Ca2+ entry in response to AVP was attenuated compared with SHR-B2.
Our data are in good agreement with the previous studies showing that AVP elicits a prolonged elevation of [Ca2+]i, critical for AQP2 trafficking and water permeability in CD.14,40,45 In our experiments CD cells from SHR-A3 had lower basal [Ca2+]i levels, indicating that altered basal cellular [Ca2+]i balance may also affect AQP2 abundance and/or trafficking. Consistently, AQP2 expression was shown to be regulated by [Ca2+]i-dependent calcineurin–nuclear factor of activated T cells cascade.44 Of note, recent studies suggest the existence of a positive feedback control between STIM1/SOCE and nuclear factor of activated T cells in myoblasts.46 On the other hand, clamping of [Ca2+]i below the normally observed resting levels inhibits AQP2 exocytosis.47 Taken together with the systemic manifestations such as diminished urinary concentrating ability, polyuria, and polydipsia observed in SHR-A3, these data unequivocally demonstrate that disruption of SOCE signaling is one of the cellular mechanisms capable of creating NDI.
AVP is known to also evoke changes in cAMP levels in the CD and, in the presence of normal SOCE, to induce AQP2 phosphorylation and apical insertion as a result.1,9,12,15,39 This implies that parallel and coordinated pathways of cellular signaling between adenyl-cyclase–driven mechanisms and signals originating from altered cellular calcium interact to provide control of renal water reabsorption. Defects arising from the mutation of genes encoding proteins operating in both pathways are now revealed to be capable of disrupting body water homeostasis by impairing renal urine concentration.
The Stim1 mutation in SHR-A3 provides a unique opportunity to uncover other aspects of the coordination of calcium signaling by ER and plasma membrane interactions. There is clearly some STIM1 residual function in SHR-A3 so that ORAI1 regulation is preserved, although at a much reduced level. This is congruent with the existing evidence from overexpressed transfected protein studies that truncation of the STIM1 C-terminus attenuates protein redistribution to the plasma membrane48 and delays the activation of calcium release activated current.49 The residual STIM1 function of the C-terminal truncated protein may account for the absence of severe combined immunodeficiency in SHR-A3. Interestingly, studies of the evolution of STIM1 indicate that the acquisition of a C-terminal polybasic module occurred around the time of the emergence of chordates. Invertebrates lack this adaptation; however, SOCE is present and invertebrate STIM participates in this process.50–54 This is consonant with the retention of STIM1 function, although at a reduced level, in SHR-A3 lacking the same C-terminal domain absent in invertebrates. Our findings do not implicate the TRPC channel regulation in the control of renal water handling in the CD. However, in other tissues, STIM1 may have an important function in TRPC gating that may be uncovered in SHR-A3 and which may contribute to other phenotypes present in this model of hypertension and stroke.
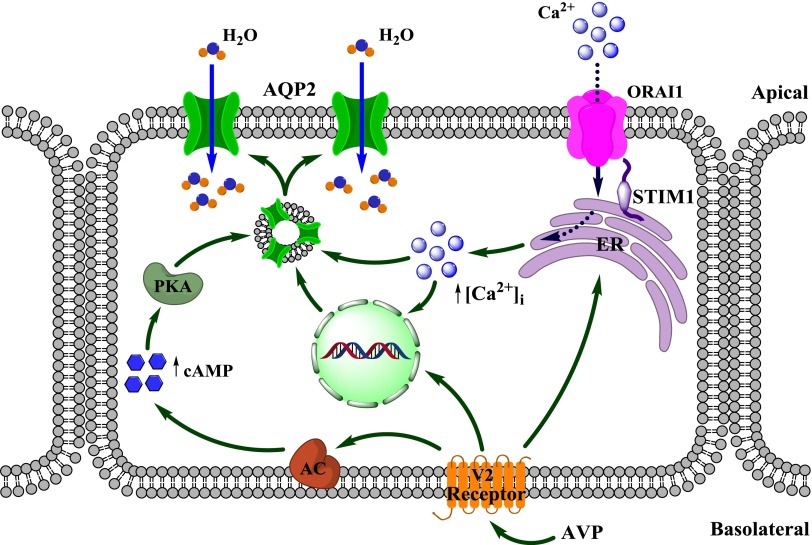
In conclusion, we found a novel pathway critical for AVP-dependent water handling by the kidney. We propose that AVP-induced sustained [Ca2+]i elevations via SOCE are pivotal for adequate AQP2 trafficking and expression in the CD (Figure 10). Disruption of SOCE causes NDI with no apparent effect on V2R or AQP2 genes. From a clinical perspective, our study implicates SOCE-related genes in congenital NDI when the genetic etiology is unknown. Furthermore, pharmacologic interventions or environmental factors affecting SOCE function may be causative for some forms of acquired NDI. Of interest, numerous genetic variants of Stim1 are known to exist in the human population at relatively high frequency (the 1000 Genomes Project indicates 577 missense variants and 17 premature stop codon variants detected in Stim1 sequences from the project sample set). It is possible that some of these mutations may also cause mild to moderate disturbances in renal water handling, which could be easily overlooked in the clinic.
Figure 10.
AVP-induced signal transduction in the CD. AVP stimulates the expression and apical translocation of AQP2 in CD cells via cAMP- and Ca2+-dependent pathways. Ca2+ release from ER cannot maintain a prolonged [Ca2+]i mobilization required for an adequate response of CD cells to AVP. The Ca2+ necessary to sustain cellular response to AVP is provided by means of SOCE via ORAI1 channel. AC, adenylate cyclase.
Concise Methods
Materials and Animals
All chemicals and materials were from Sigma-Aldrich (St. Louis, MO), VWR (Radnor, PA), and Tocris (Ellisville, MO) unless noted otherwise and were of reagent grade. Experimental animals were housed in an Association for Assessment and Accreditation of Laboratory Animal Care-approved facility. All animal use was prospectively reviewed and approved by the University of Texas Health Science Center at Houston Animal Welfare Committee in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. Studies were performed on male rats of the stroke-prone spontaneously hypertensive-A3 (SHR-A3, SHRSP/Bbb) line and the injury-resistant SHR-B2 line. Unless noted otherwise, 6–12-week-old animals were used for experiments. The two inbred lines are derived from a single pair of founders common to both lines.29,31,55 The approximately 87% of the genome shared identical by descent across these lines is partitioned into discrete haplotype blocks.55 An F2 intercross was generated by crossing male SHR-A3 and female SHR-B2 animals and further crossing the resulting F1 progeny, as previously described,56 and was used to test the association between inheritance of Stim1 alleles and urine creatinine. Animals were provided a standard rodent chow diet and drinking water ad libitum unless subjected to 24-hour water deprivation test in metabolic cages using established protocols.57,58
Genome Sequencing, Bioinformatic Analysis, and Stim1 Genotyping
Genomic DNA was extracted from samples of liver tissue obtained from male representatives of SHR-A3 and SHR-B2 and quantified by ultraviolet spectroscopy. DNA samples were submitted to a commercial genome sequencing company (Axeq Technologies, Rockville, MD) for Illumina sequencing using the HiSEquation 2500 platform. An average of 1.37 billion reads of 100 bps were obtained from each DNA sample, providing genome coverage of approximately 45×. We mapped the paired-end reads to the rat assembly (RGSC3.4, Ensembl release 69) using Novoalign software (Novocraft.com, version v2.08.01 using default settings).39 After generating BAM files, the mapped reads were sorted by coordinate location using SamTools40 and Picard tools.41 Finally, we used the Genome Analysis Tool Kit (GATK version v2.3–9-ge5ebf34)59 to perform local realignment, recalibration, and variant calling. We removed false positive calls by using a postcalling filter that enforces that each variant has a mapping quality >30, a base quality >20, and a coverage ≥10, with at least a 3:7 ratio of variant to reference and the presence of the variant in reads from both orientations. The resulting variant call format files (vcf version 4.1) were annotated using Annovar software60 together with the rat genome annotations (RGSC3.4.69). We visually inspected the paired-end read data in the integrative genomics viewer (Integrative Genomics Viewer, Broad Institute).61 Inheritance of the wild-type and mutant Stim1 alleles in the freely segregating F2 progeny was determined by TaqMan allelic discrimination assay. The relationship between inheritance of 0, 1, or 2 SHR-A3 Stim1 alleles and urinary creatinine levels was investigated by regression analysis.
Determination of Sodium, Potassium, Creatinine, and Aldosterone in Bodily Fluids
Urinary and serum concentrations for sodium and potassium were measured using a PFP7 Flame photometer (Techne, Burlington, NJ). Urinary sodium and potassium excretion were calculated as the ratio of urinary concentration for the respective cation to creatinine concentration in the same sample. U[creat] was assessed with the Hitachi 7000 HPLC System (Pleasanton, CA). Aldosterone was purified from serum samples with chloroform extraction and measured using an enzymatic immunoassay kit (Cat. No. 501090, Cayman Chemical), following the vendor’s protocol.
Cell Culturing and Targeted Stim1 Silencing
mpkCCDc14 principal cells were grown to confluence in DMEM/F12 medium (Cat. No. 10–092-CM, Cellgro), supplemented with 3% FBS, 100 IU/ml penicillin, and 100 μg/ml streptomycin and 50 nM dexamethasone. Stim1 silencing was achieved using specific shRNA plasmids, carrying the puromycin resistance gene (Cat. No. 336314KM24556P, Qiagen). In brief, mpkCCDc14 cells seeded at low density (approximately 30% confluence) were transfected with Stim1-specific or negative control shRNA plasmids. A mixture of Attractene transfection reagent (Cat. No. 301004, Qiagen) and appropriate shRNA (4 μl of Attractene per 1 μg of plasmid) was used. Cells were selected for stable transfection with 1 μg/ml of puromycin (Cat. No. 13384, Cayman Chemical) for 1–2 passages and the established stably transfected cultures were further employed for experimental assessment.
[Ca2+]i Measurements
Intracellular calcium levels were measured in individual cells of the split-open CD or confluent mpkCCDc14 monolayers using Fura-2 fluorescence ratiometric imaging as described previously.62–66 Briefly, split-open CDs were loaded with Fura-2 by incubation with 2 μM Fura-2/AM in a bath solution for 45 minutes at room temperature. Subsequently, the tissue samples were washed and incubated for an additional 10–15 minutes prior to experimentation. After loading, the CD were placed in an open-top imaging study chamber (RC-10; Warner Instruments) with a bottom coverslip viewing window and the chamber attached to the microscope stage of an InCa Imaging Workstation (Intracellular Imaging Inc.). Cells were imaged with a 20× Nikon Super Fluor objective and regions of interest drawn for individual cells. The Fura-2 fluorescence intensity ratio was determined by excitation (an average for approximately 100 ms) at 340 and 380 nm and calculating the ratio of the emission intensities at 511 nm in the usual manner every 5 seconds. We observed no significant Fura-2 bleaching and minimal Fura-2 leakage at both wavelengths during experiments. The changes in the ratio were converted to intracellular Ca2+ concentrations using the calibration methods as we have done before.62,64–66 At least three individual CD from three rats or three monolayers from two different passages of mpkCCDc14 were used for each experimental set.
Western Blotting
Immediately after dissection, kidneys were placed on ice, decapsulated and homogenized in three volumes of ice-cold hypotonic lysis buffer containing 50 mM Tris, 1% Triton X-100, 5 mM EDTA (pH=7.4) supplemented with protease inhibitor cocktail (Complete mini, Roche Diagnostics, Germany) and phosphatase inhibitor cocktail (PhosSTOP, Roche Diagnostics), both at 1 tablet/10 ml final concentration. mpkCCDc14 cell culture lysates were obtained by scraping cells off 100×20 mm Petri dishes and homogenizing in 500 μl of hypotonic lysis buffer with protease and phosphatase inhibitors. Protein concentration was determined with a Bradford assay using IgG as a standard. The samples were diluted with hypotonic lysis buffer, denatured, and reduced in Laemmli buffer supplemented with β-mercaptoethanol at 5% final concentration for 10 minutes at +100°C to obtain the final protein concentration of 1 μg/ml. The samples (10–40 μg/lane) were separated on 12% polyacrylamide gels at 150 V for 1 hour 30 minutes and transferred to a nitrocellulose membrane for 1.5 hours at 100 V. Subsequently, the nitrocellulose membrane was incubated with primary antibodies for 2 hours at room temperature. We used the primary antibodies against N- and C-termini of STIM1 (1:5000 dilution, Sigma-Aldrich; Cat. # S6072 and S6197), anti-AQP2 (1:1500, Alomone Labs, Israel; Cat. # AQP2-002), anti–phospho-Ser269 AQP2 (1:5000, Phosphosolutions, Aurora, CO; Cat. # p112–269), anti-V2R (1:1000, Alomone Labs; AVR-012) and anti–β-actin (1:5000, Abcam, UK; Cat. # ab8227). Upon washout (three times for 10 minutes in TBS-Tween) the membrane was incubated with peroxidase-conjugated goat anti-rabbit secondary antibodies (1:10000, Bio-Rad) for 1 hour at room temperature. Blots were quantified using ImageJ 1.48 software (NIH). The intensities of the studied protein bands were normalized to the intensities of the corresponding actin bands, used as a loading control. All experiments were repeated three times.
Immunofluorescent Microscopy
Freshly isolated split-open CD or confluent monolayers of mpkCCDc14 cells were fixed with 4% paraformaldehyde in PBS (pH=7.4) for 15 minutes at room temperature. After fixation, the samples were permeabilized by addition of 0.1% Triton X-100 in PBS for 10 minutes and washed in PBS three times for 5 minutes. Nonspecific staining was blocked with 10% normal goat serum (NGS, Jackson Immunoresearch) in PBS for 30 minutes at room temperature. After washing with PBS (three times for 5 minutes) the samples were incubated for 1.5 hours at room temperature in the dark with anti-AQP2 tagged with ATTO 550 (1:200, Alomone Labs; Cat. # AQP2-002-AO) in 1% serum + 0.1% Triton X-100 in PBS. After washing with PBS (three times for 5 minutes) the samples were stained with 1.5 μM 4′,6-diamidino-2-phenylindole, Calbiochem, San Diego, CA) to visualize nuclei. Subsequently, the samples were dehydrated and mounted with permanent mounting medium (SouthernBiotech, Birmingham, AL). Labeled tissue samples were examined with an inverted Nikon A1R confocal laser microscope using a 40× Plan-Fluor oil-immersion (1.3 NA) objective. Samples were excited with 405 and 561.7 nm laser diodes and emission captured with a 16-bit Cool SNAP HQ2 camera (Photometrics, Tucson, AZ) interfaced to a PC running NIS 4.13 elements software. Three-dimensional stacks of split-open CD were generated from series of confocal plane images with 0.25 μm step. The focal plane for the shown XY projections was chosen to be within one step from the tight junction area, visualized by higher fluorescent signal intensity lines along the cell borders. The position of the apical and basolateral membranes was estimated under bright field illumination.
Solutions and Data Analyses
Typical bath solution was (in mM): 150 NaCl, 5 mM KCl, 1 CaCl2, 2 MgCl2, 5 glucose, and 10 HEPES (pH=7.4). Reagents were applied by perfusing the experimental chamber at 1.5 ml/min. All summarized data are reported as mean±SEM. All statistical comparisons were made using one-way ANOVA. A P value less than 0.05 was considered significant.
Disclosures
None.
Supplementary Material
Acknowledgments
This research was supported by grants from the National Institutes of Health (NIH)-the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) DK095029 (to O.P.), NIH-NIDDK DK069632 (to P.A.D.), and NIH-NIDDK DK081866 (to P.A.D.), and grants from the American Heart Association (AHA)-GIA-409-13GRNT16220002 (to O.P.), and AHA-14POST20380979 and AHA-15SDG25550150 (to M.M.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Water, Water Everywhere: A New Cause and a New Treatment for Nephrogenic Diabetes Insipidus,” on pages 1872–1874.
References
- 1.Moeller HB, Fenton RA: Cell biology of vasopressin-regulated aquaporin-2 trafficking. Pflugers Arch 464: 133–144, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Robertson GL: Physiology of ADH secretion. Kidney Int Suppl 21: S20–S26, 1987 [PubMed] [Google Scholar]
- 3.Nielsen S, Frøkiaer J, Marples D, Kwon TH, Agre P, Knepper MA: Aquaporins in the kidney: from molecules to medicine. Physiol Rev 82: 205–244, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Staruschenko A: Regulation of transport in the connecting tubule and cortical collecting duct. Compr Physiol 2: 1541–1584, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson JL, Miranda CA, Knepper MA: Vasopressin and the regulation of aquaporin-2. Clin Exp Nephrol 17: 751–764, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schrier RW: Body water homeostasis: clinical disorders of urinary dilution and concentration. J Am Soc Nephrol 17: 1820–1832, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Moeller HB, Rittig S, Fenton RA: Nephrogenic diabetes insipidus: essential insights into the molecular background and potential therapies for treatment. Endocr Rev 34: 278–301, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fenton RA, Brønd L, Nielsen S, Praetorius J: Cellular and subcellular distribution of the type-2 vasopressin receptor in the kidney. Am J Physiol Renal Physiol 293: F748–F760, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Roos KP, Strait KA, Raphael KL, Blount MA, Kohan DE: Collecting duct-specific knockout of adenylyl cyclase type VI causes a urinary concentration defect in mice. Am J Physiol Renal Physiol 302: F78–F84, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fushimi K, Sasaki S, Marumo F: Phosphorylation of serine 256 is required for cAMP-dependent regulatory exocytosis of the aquaporin-2 water channel. J Biol Chem 272: 14800–14804, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Moeller HB, Knepper MA, Fenton RA: Serine 269 phosphorylated aquaporin-2 is targeted to the apical membrane of collecting duct principal cells. Kidney Int 75: 295–303, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moeller HB, Praetorius J, Rützler MR, Fenton RA: Phosphorylation of aquaporin-2 regulates its endocytosis and protein-protein interactions. Proc Natl Acad Sci U S A 107: 424–429, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chou CL, Yip KP, Michea L, Kador K, Ferraris JD, Wade JB, Knepper MA: Regulation of aquaporin-2 trafficking by vasopressin in the renal collecting duct. Roles of ryanodine-sensitive Ca2+ stores and calmodulin. J Biol Chem 275: 36839–36846, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Yip KP: Coupling of vasopressin-induced intracellular Ca2+ mobilization and apical exocytosis in perfused rat kidney collecting duct. J Physiol 538: 891–899, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yip KP, Sham JS: Mechanisms of vasopressin-induced intracellular Ca2+ oscillations in rat inner medullary collecting duct. Am J Physiol Renal Physiol 300: F540–F548, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoth M, Penner R: Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature 355: 353–356, 1992 [DOI] [PubMed] [Google Scholar]
- 17.Hoth M, Penner R: Calcium release-activated calcium current in rat mast cells. J Physiol 465: 359–386, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zweifach A, Lewis RS: Mitogen-regulated Ca2+ current of T lymphocytes is activated by depletion of intracellular Ca2+ stores. Proc Natl Acad Sci U S A 90: 6295–6299, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan Z, Brotto M, Ma J: Store-operated Ca2+ entry in muscle physiology and diseases. BMB Rep 47: 69–79, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Umemura M, Baljinnyam E, Feske S, De Lorenzo MS, Xie LH, Feng X, Oda K, Makino A, Fujita T, Yokoyama U, Iwatsubo M, Chen S, Goydos JS, Ishikawa Y, Iwatsubo K: Store-operated Ca2+ entry (SOCE) regulates melanoma proliferation and cell migration. PLoS One 9: e89292, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feske S, Skolnik EY, Prakriya M: Ion channels and transporters in lymphocyte function and immunity. Nat Rev Immunol 12: 532–547, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prakriya M: The molecular physiology of CRAC channels. Immunol Rev 231: 88–98, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prakriya M: The theory, operation, and roles of store-operated calcium. Curr Top Membr 71: xi–xii, 2013 [DOI] [PubMed] [Google Scholar]
- 24.Huang GN, Zeng W, Kim JY, Yuan JP, Han L, Muallem S, Worley PF: STIM1 carboxyl-terminus activates native SOC, I(crac) and TRPC1 channels. Nat Cell Biol 8: 1003–1010, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE Jr, Meyer T: STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol 15: 1235–1241, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Veliçelebi G, Stauderman KA: STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol 169: 435–445, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu MM, Buchanan J, Luik RM, Lewis RS: Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J Cell Biol 174: 803–813, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang SL, Yu Y, Roos J, Kozak JA, Deerinck TJ, Ellisman MH, Stauderman KA, Cahalan MD: STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature 437: 902–905, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Braun MC, Herring SM, Gokul N, Monita M, Bell R, Hicks MJ, Wenderfer SE, Doris PA: Hypertensive renal disease: susceptibility and resistance in inbred hypertensive rat lines. J Hypertens 31: 2050–2059, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ronzaud C, Loffing J, Bleich M, Gretz N, Gröne HJ, Schütz G, Berger S: Impairment of sodium balance in mice deficient in renal principal cell mineralocorticoid receptor. J Am Soc Nephrol 18: 1679–1687, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Okamoto K, Aoki K: Development of a strain of spontaneously hypertensive rats. Jpn Circ J 27: 282–293, 1963 [DOI] [PubMed] [Google Scholar]
- 32.Cahalan MD: STIMulating store-operated Ca(2+) entry. Nat Cell Biol 11: 669–677, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bird GS, DeHaven WI, Smyth JT, Putney JW Jr: Methods for studying store-operated calcium entry. Methods 46: 204–212, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeng W, Yuan JP, Kim MS, Choi YJ, Huang GN, Worley PF, Muallem S: STIM1 gates TRPC channels, but not Orai1, by electrostatic interaction. Mol Cell 32: 439–448, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee KP, Choi S, Hong JH, Ahuja M, Graham S, Ma R, So I, Shin DM, Muallem S, Yuan JP: Molecular determinants mediating gating of Transient Receptor Potential Canonical (TRPC) channels by stromal interaction molecule 1 (STIM1). J Biol Chem 289: 6372–6382, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schleifer H, Doleschal B, Lichtenegger M, Oppenrieder R, Derler I, Frischauf I, Glasnov TN, Kappe CO, Romanin C, Groschner K: Novel pyrazole compounds for pharmacological discrimination between receptor-operated and store-operated Ca(2+) entry pathways. Br J Pharmacol 167: 1712–1722, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duong Van Huyen J, Bens M, Vandewalle A: Differential effects of aldosterone and vasopressin on chloride fluxes in transimmortalized mouse cortical collecting duct cells. J Membr Biol 164: 79–90, 1998 [DOI] [PubMed] [Google Scholar]
- 38.Fujiwara TM, Bichet DG: Molecular biology of hereditary diabetes insipidus. J Am Soc Nephrol 16: 2836–2846, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Ecelbarger CA, Chou CL, Lolait SJ, Knepper MA, DiGiovanni SR: Evidence for dual signaling pathways for V2 vasopressin receptor in rat inner medullary collecting duct. Am J Physiol 270: F623–F633, 1996 [DOI] [PubMed] [Google Scholar]
- 40.Yip KP: Epac-mediated Ca(2+) mobilization and exocytosis in inner medullary collecting duct. Am J Physiol Renal Physiol 291: F882–F890, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Picard C, McCarl CA, Papolos A, Khalil S, Lüthy K, Hivroz C, LeDeist F, Rieux-Laucat F, Rechavi G, Rao A, Fischer A, Feske S: STIM1 mutation associated with a syndrome of immunodeficiency and autoimmunity. N Engl J Med 360: 1971–1980, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yuan JP, Kim MS, Zeng W, Shin DM, Huang G, Worley PF, Muallem S: TRPC channels as STIM1-regulated SOCs. Channels (Austin) 3: 221–225, 2009 [DOI] [PubMed] [Google Scholar]
- 43.Walsh CM, Chvanov M, Haynes LP, Petersen OH, Tepikin AV, Burgoyne RD: Role of phosphoinositides in STIM1 dynamics and store-operated calcium entry. Biochem J 425: 159–168, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li SZ, McDill BW, Kovach PA, Ding L, Go WY, Ho SN, Chen F: Calcineurin-NFATc signaling pathway regulates AQP2 expression in response to calcium signals and osmotic stress. Am J Physiol Cell Physiol 292: C1606–C1616, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Balasubramanian L, Sham JS, Yip KP: Calcium signaling in vasopressin-induced aquaporin-2 trafficking. Pflugers Arch 456: 747–754, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Phuong TT, Yun YH, Kim SJ, Kang TM: Positive feedback control between STIM1 and NFATc3 is required for C2C12 myoblast differentiation. Biochem Biophys Res Commun 430: 722–728, 2013 [DOI] [PubMed] [Google Scholar]
- 47.Lorenz D, Krylov A, Hahm D, Hagen V, Rosenthal W, Pohl P, Maric K: Cyclic AMP is sufficient for triggering the exocytic recruitment of aquaporin-2 in renal epithelial cells. EMBO Rep 4: 88–93, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liou J, Fivaz M, Inoue T, Meyer T: Live-cell imaging reveals sequential oligomerization and local plasma membrane targeting of stromal interaction molecule 1 after Ca2+ store depletion. Proc Natl Acad Sci U S A 104: 9301–9306, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Z, Lu J, Xu P, Xie X, Chen L, Xu T: Mapping the interacting domains of STIM1 and Orai1 in Ca2+ release-activated Ca2+ channel activation. J Biol Chem 282: 29448–29456, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Lorin-Nebel C, Xing J, Yan X, Strange K: CRAC channel activity in C. elegans is mediated by Orai1 and STIM1 homologues and is essential for ovulation and fertility. J Physiol 580: 67–85, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Strange K, Yan X, Lorin-Nebel C, Xing J: Physiological roles of STIM1 and Orai1 homologs and CRAC channels in the genetic model organism Caenorhabditis elegans. Cell Calcium 42: 193–203, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yan X, Xing J, Lorin-Nebel C, Estevez AY, Nehrke K, Lamitina T, Strange K: Function of a STIM1 homologue in C. elegans: evidence that store-operated Ca2+ entry is not essential for oscillatory Ca2+ signaling and ER Ca2+ homeostasis. J Gen Physiol 128: 443–459, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dziadek MA, Johnstone LS: Biochemical properties and cellular localisation of STIM proteins. Cell Calcium 42: 123–132, 2007 [DOI] [PubMed] [Google Scholar]
- 54.Venkiteswaran G, Hasan G: Intracellular Ca2+ signaling and store-operated Ca2+ entry are required in Drosophila neurons for flight. Proc Natl Acad Sci U S A 106: 10326–10331, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bell R, Herring SM, Gokul N, Monita M, Grove ML, Boerwinkle E, Doris PA: High-resolution identity by descent mapping uncovers the genetic basis for blood pressure differences between spontaneously hypertensive rat lines. Circ Cardiovasc Genet 4: 223–231, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Braun MC, Herring SM, Gokul N, Monita M, Bell R, Zhu Y, Gonzalez-aray ML, Wenderfer SE, Doris PA: Hypertensive renal injury is associated with gene variation affecting immune signaling. Circ Cardiovasc Genet 7: 903–910, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gabbi C, Kong X, Suzuki H, Kim HJ, Gao M, Jia X, Ohnishi H, Ueta Y, Warner M, Guan Y, Gustafsson JA: Central diabetes insipidus associated with impaired renal aquaporin-1 expression in mice lacking liver X receptor β. Proc Natl Acad Sci U S A 109: 3030–3034, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kato A, Naruse M, Knepper MA, Sands JM: Long-term regulation of inner medullary collecting duct urea transport in rat. J Am Soc Nephrol 9: 737–745, 1998 [DOI] [PubMed] [Google Scholar]
- 59.McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M, DePristo MA: The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res 20: 1297–1303, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang K, Li M, Hakonarson H: ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res 38: e164, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thorvaldsdóttir H, Robinson JT, Mesirov JP: Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief Bioinform 14: 178–192, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mamenko M, Zaika O, Ilatovskaya DV, Staruschenko A, Pochynyuk O: Angiotensin II increases activity of the epithelial Na+ channel (ENaC) in distal nephron additively to aldosterone. J Biol Chem 287: 660–671, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mamenko M, Zaika O, Jin M, O’Neil RG, Pochynyuk O: Purinergic activation of Ca2+-permeable TRPV4 channels is essential for mechano-sensitivity in the aldosterone-sensitive distal nephron. PLoS One 6: e22824, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mamenko M, Zaika O, O’Neil RG, Pochynyuk O: Ca2+ Imaging as a tool to assess TRP channel function in murine distal nephrons. Methods Mol Biol 998: 371–384, 2013 [DOI] [PubMed] [Google Scholar]
- 65.Mamenko M, Zaika OL, Boukelmoune N, Berrout J, O’Neil RG, Pochynyuk O: Discrete control of TRPV4 channel function in the distal nephron by protein kinases A and C. J Biol Chem 288: 20306–20314, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zaika O, Mamenko M, Berrout J, Boukelmoune N, O’Neil RG, Pochynyuk O: TRPV4 dysfunction promotes renal cystogenesis in autosomal recessive polycystic kidney disease. J Am Soc Nephrol 24: 604–616, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.