Abstract
Dietary protein restriction may improve determinants of CKD progression. However, the extent of improvement and effect of ketoanalogue supplementation are unclear. We conducted a prospective, randomized, controlled trial of safety and efficacy of ketoanalogue–supplemented vegetarian very low–protein diet (KD) compared with conventional low–protein diet (LPD). Primary end point was RRT initiation or >50% reduction in initial eGFR. Nondiabetic adults with stable eGFR<30 ml/min per 1.73 m2, proteinuria <1 g/g urinary creatinine, good nutritional status, and good diet compliance entered a run-in phase on LPD. After 3 months, compliant patients were randomized to KD (0.3 g/kg vegetable proteins and 1 cps/5 kg ketoanalogues per day) or continue LPD (0.6 g/kg per day) for 15 months. Only 14% of screened patients patients were randomized, with no differences between groups. Adjusted numbers needed to treat (NNTs; 95% confidence interval) to avoid composite primary end point in intention to treat and per-protocol analyses in one patient were 4.4 (4.2 to 5.1) and 4.0 (3.9 to 4.4), respectively, for patients with eGFR<30 ml/min per 1.73 m2. Adjusted NNT (95% confidence interval) to avoid dialysis was 22.4 (21.5 to 25.1) for patients with eGFR<30 ml/min per 1.73 m2 but decreased to 2.7 (2.6 to 3.1) for patients with eGFR<20 ml/min per 1.73 m2 in intention to treat analysis. Correction of metabolic abnormalities occurred only with KD. Compliance to diet was good, with no changes in nutritional parameters and no adverse reactions. Thus, this KD seems nutritionally safe and could defer dialysis initiation in some patients with CKD.
Keywords: nutrition, progression of chronic renal failure, phosphate binders, renal function decline
Dietary proteins are the source of nitrogen, phosphate, and acid load, and sodium intake parallels protein ingestion. A reduction in protein consumption was shown to result in better control of BP and a decrease in proteinuria,1–4 the major determinants of the progression of CKD.5 Moreover, certain complications of advanced CKD, such as mineral metabolism disorders, acidosis, and oxidative stress, also involved in accelerating its progression5 were favorably influenced by the low-protein diets.6
Diet was a key component of management in nephrology. Dietary intervention was the mainstay approach in kidney failure in the first one-half of the 20th century, and diet manipulation was tried in its last decades.6 Different dietary protein regimens have been proposed: conventional low–protein diets (LPDs; 0.6 g/kg per day), very low–protein diets (VLPDs; 0.3–0.4 g/kg per day), and practically vegetarian diets supplemented with either essential amino acids or a mixture of essential amino acids and nitrogen-free ketoanalogues (keto diet [KD]).6,7
Supplementation of a VLPD with ketoanalogues seems to have some advantages above and beyond the protein restriction. If enough energy is provided, ketoanalogues could be converted to essential amino acids by urea recycling, allowing for a nutritionally safe, more severe reduction in protein intake.7 Furthermore, the calcium content of ketoanalogues preparations and their phosphate binder capabilities allow for even better correction of mineral metabolism abnormalities.8 Although the favorable metabolic effects of KD were shown in many observational studies,7–11 the few controlled studies investigating its influence on CKD progression using hard end points were underpowered and gave variable results.12–18 However, the risk ratio was in favor of VLPD when studies were pooled together in a recent systematic review (0.63; 95% confidence interval [95% CI], 0.48 to 0.83).19
The largest study addressing KD, the Modification of Diet in Renal Disease (MDRD) Study 2, provided conflicting results: the protein-restricted diet only marginally reduced the decline in GFR; the advantage was small and apparently caused by the protein restriction, not the ketoanalogues supplementation. Information on hard end point outcome was not published so far.20 Moreover, not only did long–term follow-up of this study not support the delay of CKD progression on LPD, but it even suggested an increase in the risk of death.21 Notably, patients’ adherence to LPD was also a problem.21
Because the efficacy of protein-restricted regimens seemed uncertain,20 their feasibility was repeatedly questioned; because the adherence and compliance of patients to the diet were reported as poor and the risk of malnutrition was frequently invoked,22–25 the use of KD and VLPDs was not extended.
Nevertheless, the interest in dietary management resurged, because the high prevalence of CKD revealed a major effect on not only morbidity, mortality, social activities, and patients’ quality of life but also, health budget. Recent studies focused on the advantages of the LPD in certain groups of patients with CKD (the elderly, in whom ketoanalogue-supplemented VLPD seems a better alternative to dialysis),26 patients’ selection aiming to increase compliance,17 or modification of diets to make them more acceptable.27 Moreover, the dietary approaches to stop hypertension diet is now largely accepted in cardiology,28,29 and diet is the first step recommended in correction of acidosis and hyperkalemia associated to CKD.5
Accordingly, we aimed to evaluate the effectiveness and safety of a vegetarian VLPD supplemented with ketoanalogues of essential amino acids in reducing CKD progression.
Results
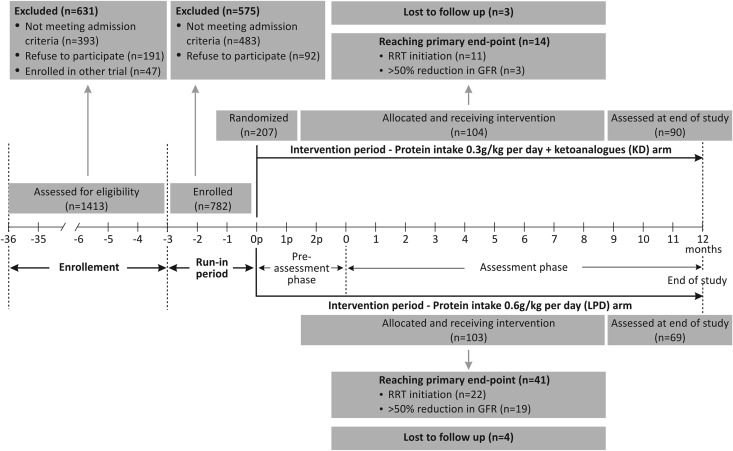
All of the 1413 consecutive patients with CKD admitted to a large nephrology clinic during an enrolment period of 36 months (March of 2006 to April of 2009) have been assessed for eligibility. A number of 1206 patients were excluded, because they either did not meet the eligibility criteria (mainly the compliance to the diet) or refused to participate. To note, only 42% of patients fulfilling all of the other selection criteria accepted to potentially follow a vegetarian diet. Finally, only 207 (14% of the screened patients) met all of the eligibility criteria and could be randomized (Figure 1).
Figure 1.
Study phases and patients’ flowchart. The enrolled patients entered a run-in phase; the compliant ones were randomized for the intervention.
There were no significant differences between KD and LPD groups in age (median age, 55.2 and 53.6 years old, respectively), sex (63% and 59% men, respectively), or primary renal disease (glomerular nephropathies, 59% and 57%, respectively; tubulointerstitial nephropathies, 27% and 27%, respectively) at baseline. The eGFR, proteinuria, and BP control as well as the parameters of nutritional status were also similar in both arms (Tables 1 and 2).
Table 1.
Metabolic parameters, renal function, BP, and requirements for antihypertensive treatment in study arms
| Parameter and Study Moment | KD (n=104) | LPD (n=103) | P Valuec |
|---|---|---|---|
| Renal function | |||
| eGFR (ml/min) | |||
| Baseline | 18.0 (15.5 to 20.1) | 17.9 (14.3 to 19.3) | 0.68 |
| End of study | 15.1 (13.2 to 17.4) | 10.8 (9.0 to 12.2) | <0.01 |
| Proteinuria (g/d)a | |||
| Baseline | 0.88 (0.79 to 0.96) | 0.88 (0.82 to 0.96) | 0.73 |
| End of study | 0.78 (0.67 to 0.85) | 0.67 (0.57 to 0.81) | 0.06 |
| BPb | |||
| Mean arterial BP (mmHg) | |||
| Baseline | 86 (78 to 96) | 93 (86 to 96) | 0.06 |
| End of study | 90 (86 to 92) | 87 (83 to 93) | 0.42 |
| Patients with optimal BP controlc (%) | |||
| Baseline | 91 | 89 | 0.67 |
| End of study | 85 | 84 | 0.95 |
| Patients on antihypertensive drugs (%) | |||
| Baseline | 86 | 89 | 0.55 |
| End of study | 88 | 93 | 0.24 |
| Patients receiving ACEIs and/or ARBs (%) | |||
| Baseline | 71 | 71 | 0.96 |
| End of study | 71 | 70 | 0.84 |
| Nitrogen waste products | |||
| Serum urea (mg/dl)d | |||
| Baseline | 187 (164 to 225) | 213 (183 to 248) | 0.73 |
| End of study | 122 (114 to 127) | 226 (191 to 252) | <0.01 |
| Serum uric acid (mg/dl)e | |||
| Baseline | 6.5 (6.5 to 6.6) | 6.3 (6.2 to 6.3) | 0.06 |
| End of study | 6.0 (5.8 to 6.1) | 6.3 (6.1 to 6.4) | 0.01 |
| Acid-base balance | |||
| Serum bicarbonate (mEq/L) | |||
| Baseline | 16.7 (15.8 to 17.6) | 16.8 (15.9 to 17.8) | 0.98 |
| End of study | 22.9 (21.7 to 24.1) | 16.2 (15.4 to 16.9) | <0.01 |
| Sodium bicarbonate supplementation | |||
| Patients treated (%) | |||
| Baseline | 36 | 40 | 0.53 |
| End of study | 29 | 51 | <0.01 |
| Prescribed dose (g/d) | |||
| Baseline | 2.0 (2.0 to 2.4) | 2.0 (1.6 to 2.4) | 0.63 |
| End of study | 2.2 (2.0 to 2.6) | 3.2 (2.4 to 3.6) | 0.03 |
| Calcium-phosphorus metabolism | |||
| Serum calcium (mg/dl) | |||
| Baseline | 3.8 (3.7 to 3.9) | 3.8 (3.7 to 4.0) | 0.61 |
| End of study | 4.4 (4.3 to 4.5) | 3.9 (3.7 to 3.9) | <0.01 |
| Serum phosphates (mg/dl) | |||
| Baseline | 5.9 (5.3 to 6.2) | 5.8 (5.2 to 6.1) | 0.68 |
| End of study | 4.4 (4.3 to 4.5) | 6.2 (5.8 to 6.5) | <0.01 |
| Calcium supplementation | |||
| Patients treated (%) | |||
| Baseline | 46 | 40 | 0.35 |
| End of study | 49 | 51 | 0.72 |
| Prescribed dose (g/d) | |||
| Baseline | 6.1 (5.7 to 6.5) | 6.0 (5.6 to 6.5) | 0.91 |
| End of study | 6.3 (6.0 to 6.6) | 6.9 (6.6 to 7.3) | <0.01 |
| Vitamin D therapy (patients %) | |||
| Baseline | 22 | 30 | 0.19 |
| End of study | 22 | 54 | <0.01 |
Values at randomization were compared with those at the end of the study (i.e., reach of the EPP or end of the follow-up period). Data are shown as median and 95% CI. To convert eGFR in milliliters per minute to milliliters per second, multiply by 0.01667. To convert serum urea in milligrams per deciliter to millimoles per liter, multiply by 0.357. To convert serum urea in milligrams per deciliter to BUN in milligrams per deciliter, divide by 2.14. To convert serum creatinine in milligrams per deciliter to ± moles per liter, multiply by 88.4. To convert serum bicarbonate in equivalents per liter to millimoles per liter, multiply by 1. To convert serum calcium in milligrams per deciliter to millimoles per liter, multiply by 0.2495. To convert serum phosphates in milligrams per deciliter to millimoles per liter, multiply by 0.3229. To convert serum albumin in grams per deciliter to grams per liter, multiply by 10.
See also Supplementary Figure 1.
See also Supplementary Figure 2.
Arterial BP<130/75 mmHg.
See also Supplementary Figure 3.
See also Supplementary Figure 4.
Table 2.
Safety and compliance parameters in the study arms
| Parameter and Study Moment | KD (n=104) | LPD (n=103) | P Valueb |
|---|---|---|---|
| SGA A, % | |||
| Baseline | 86 | 90 | 0.29 |
| End of study | 83 | 82 | 0.83 |
| BMI (kg/m2) | |||
| Baseline | 23.6 (23.1 to 24.2) | 23.2 (22.7 to 23.7) | 0.20 |
| End of study | 23.3 (22.9 to 23.7) | 23.1 (22.6 to 23.5) | 0.45 |
| TSF (cm) | |||
| Baseline | 20.0 (19.6 to 20.4) | 19.9 (19.8 to 20.3) | 0.82 |
| End of study | 19.8 (19.3 to 20.1) | 19.7 (19.5 to 20.1) | 0.94 |
| MAMC (cm) | |||
| Baseline | 23.4 (23.1 to 23.6) | 23.2 (22.9 to 23.4) | 0.46 |
| End of study | 23.1 (22.8 to 23.5) | 22.8 (22.7 to 23.2) | 0.26 |
| CRP (mg/L)a | |||
| Baseline | 4.6 (4.1 to 5.1) | 4.4 (3.9 to 4.8) | 0.45 |
| End of study | 4.8 (4.3 to 5.4) | 6.4 (5.7 to 7.0) | <0.01 |
| Serum albumin (g/dl)b | |||
| Baseline | 4.1 (4.1 to 4.2) | 4.1 (4.1 to 4.2) | 0.51 |
| End of study | 4.1 (4.0 to 4.1) | 4.1 (4.1 to 4.2) | 0.65 |
| Serum total cholesterol (mg/dl) | |||
| Baseline | 225.5 (218.0 to 232.0) | 217.0 (214.0 to 222.0) | 0.06 |
| End of study | 198.4 (190.8 to 206.0) | 197.7 (192.0 to 203.4) | 0.87 |
| Serum potassium (mEq/L)c | |||
| Baseline | 4.7 (4.4 to 5.0) | 4.2 (4.1 to 4.4) | 0.001 |
| End of study | 4.9 (4.7 to 5.0) | 4.6 (4.5 to 4.7) | 0.12 |
| Protein intake (g/kg per day) | |||
| Baseline | 0.61 (0.58 to 0.62) | 0.60 (0.58 to 0.61) | 0.84 |
| End of study | 0.29 (0.29 to 031) | 0.58 (0.57 to 0.59) | <0.01 |
| Energy intake (kcal/kg per day) | |||
| Baseline | 31.0 (30.2 to 31.6) | 30.5 (30.0 to 31.0) | 0.26 |
| End of study | 30.5 (29.5 to 31.5) | 30.2 (29.8 to 30.8) | 0.99 |
Values at randomization were compared with those at the end of the study (i.e., reach of the EPP or end of the follow-up period). Data are shown as median and 95% CI. BMI, body mass index; TSF, tricipital skinfolds; MAMC, midarm muscular circumference.
See also Supplementary Figure 5.
See also Supplementary Figure 6.
See also Supplementary Figure 7.
Efficacy: Progression of CKD
In the intention to treat (ITT) analyses, 55 patients (28% of the cohort) reached the primary composite efficacy end point (i.e., RRT initiation or a >50% reduction in the initial GFR). A significantly lower percentage of patients in the KD group reached the primary end point: 13% versus 42% in the LPD group (P<0.001); the difference between arms was >10%. Thus, the predefined primary efficacy criteria were met.
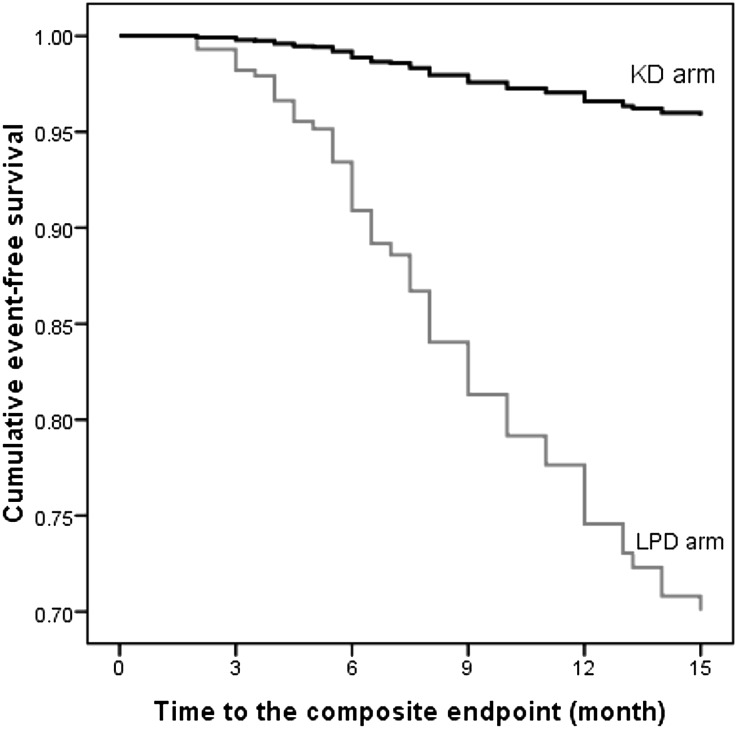
In Kaplan–Meier analysis, the cumulative probability to reach the end point during 1 year was also lower in the KD group (12% versus 39%).
The probability to reach the end point was even lower in the KD group when adjusted for the other significant predictors of outcome (eGFR, body mass index, C-reactive protein [CRP], and angiotensin–converting enzyme inhibitor [ACEI] /angiotensin receptor blocker [ARB] therapy) in a Cox proportional hazard model: adjusted hazard ratio, 0.10; 95% CI, 0.05 to 0.20 (Figure 2, Table 3). The adjusted number of patients needed to treat for 1 year to avoid reaching the composite primary efficacy end point by one patient was 4.4 (95% CI, 4.2 to 5.1).
Figure 2.
Adjusted event–free survival rates of patients assigned to the KD or the LPD. The probability to reach the end-point was even lower in KD group when adjusted for the other significant predictors of outcome in a Cox proportional hazard model.
Table 3.
Determinants of the primary efficacy end point (halving of initial eGFR or dialysis initiation)
| Determinant | B | SEM | Exp(B) (95% CI) | P Value |
|---|---|---|---|---|
| Arm (KD versus LPD) | −2.29 | 0.35 | 0.10 (0.05 to 0.20) | <0.001 |
| Body mass index | −4.28 | 1.46 | 0.01 (0.00 to 0.24 | 0.003 |
| ACEI/ARB (yes versus no) | −0.68 | 0.29 | 0.51 (0.28 to 0.90) | 0.02 |
| CRP | 1.55 | 0.38 | 4.72 (2.25 to 9.90) | <0.001 |
| eGFR | −5.79 | 0.74 | <0.01 (<0.01 to 0.01) | <0.001 |
Backward stepwise Cox regression: variables present in the last step. Significant predictors of outcome5 were considered at the first step: ACEI/ARB, age, body mass index, CRP, diet, initial eGFR, sex, midarm muscular circumference, mean arterial pressure, primary renal disease, proteinuria, serum albumin, serum bicarbonate, serum calcium, serum phosphate, and urinary creatinine. B, regression coefficient; SEM, standard error of the mean; Exp(B), hazard ratio.
Moreover, RRT initiation was required in a lower proportion in the KD group (11% versus 30%; P<0.001). The number of patients needed to treat for 1 year to avoid dialysis initiation in one patient was 22.4 (95% CI, 21.5 to 25.1).
Seven patients discontinued the diets: three in the intervention group and four in the control group. The per-protocol (PP) analyses were performed on 101 and 99 patients in the KD and LPD groups, respectively.
The adjusted numbers needed to treat (NNTs) to avoid reaching the primary composite end point and dialysis initiation in the PP group by one patient were 4.0 (95% CI, 3.9 to 4.4) and 23.7 (95% CI, 22.8 to 26.2), respectively. Thus, comparable results were observed in both ITT and PP analyses.
Additional ITT and PP analyses were performed using various eGFR cutoff levels (Table 4).
Table 4.
Adjusted number needed to treat according to eGFR level to avoid the primary composite end point and dialysis initiation in one patient (ITT and PP analyses)
| eGFR (ml/min) | ITT | PP | ||
|---|---|---|---|---|
| Primary End Point | Dialysis Initiation | Primary End Point | Dialysis Initiation | |
| <30 | 4.4 (4.2 to 5.1) | 22.4 (21.5 to 25.1) | 4.0 (3.9 to 4.4) | 23.7 (22.8 to 26.2) |
| <25 | 2.7 (2.5 to 3.1) | 8.0 (7.6 to 9.2) | 2.5 (2.3 to 2.9) | 6.3 (6.0 to 7.0) |
| <20 | 1.9 (1.7 to 2.2) | 2.7 (2.6 to 3.1) | 1.8 (1.6 to 2.1) | 2.6 (2.4 to 2.9) |
| <15 | 1.3 (1.1 to 1.6) | 1.3 (1.2 to 1.7) | 1.2 (1.1 to 1.5) | 1.2 (1.1 to 1.5) |
Data are presented as the numbers of patients needed to treat and 95% CI. ITT, intention-to-treat analysis; PP, per protocol analysis.
Although the KD was effective starting from an eGFR<30 ml/min and at the level suggested by the manufacturer (eGFR<25 ml/min), its efficacy was higher at eGFR<20 ml/min when NNTs to prevent the primary composite end point and dialysis initiation were 2 and 3, respectively (Table 4).
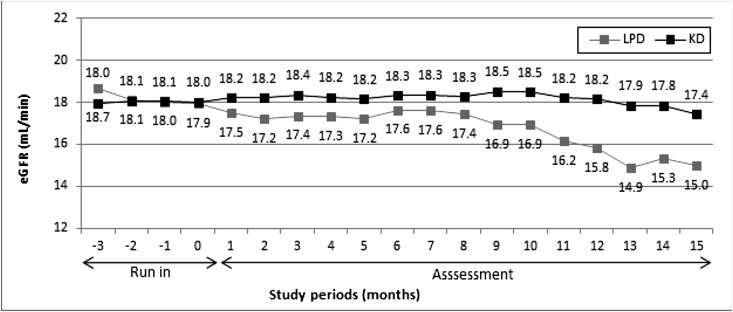
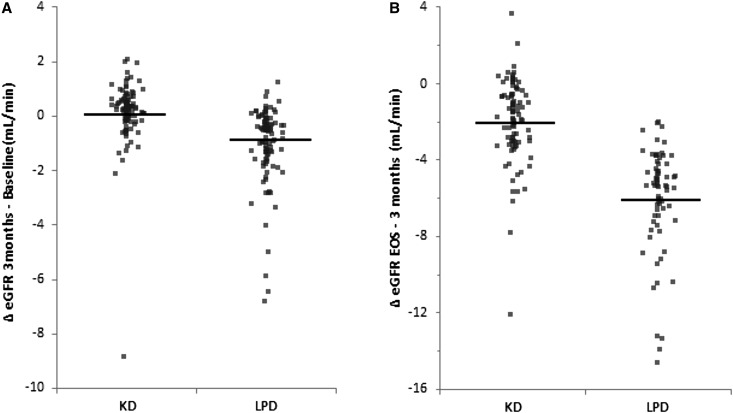
The median eGFR was similar in both arms at any study moment, but a trend to higher levels (P=0.08) was observed at end of study only in the KD group (Figure 3, Table 1). In the first 3 months after randomization, the eGFR increased in the KD arm (median change at 3 months versus baseline, 0.2 ml/min; 95% CI, 0.1 to 0.3 ml/min; P=0.001) and decreased in the LPD arm (−0.8 ml/min; 95% CI, −1.1 to −0.6 ml/min; P<0.001), supporting the effect of the KD on the eGFR (Figure 4).
Figure 3.
Median eGFR throughout the study. There were no significant differences in eGFR between LPD and KD groups at any moment.
Figure 4.
Median changes in eGFR (95% CI) between study moments (Δ implies statistically significant difference between the two groups). EOS, end of study. (A) In the first 3 months after randomization, eGFR significantly increased in KD arm and decreased in LPD. (B) The decrease in eGFR was lower in KD when considering the interval between 3 months after randomization and the end of study.
Considering the changes in eGFR levels between 3 months after randomization and the end of study, the decrease in eGFR was lower in KD compared with LPD (median difference of changes between groups, 3.2 ml/min; 95% CI, 2.6 to 3.8 ml/min) (Figure 4, Table 1). Thus, a 3.2 ml/min per year lower decline in eGFR was observed in patients following the KD.
Proteinuria was similarly <1 g/d in both groups during the study (Table 1).
There were no differences between groups in achieving and maintaining BP control as well as the percentage of patients receiving ACEIs and/or ARBs throughout the study (Table 1).
Efficacy: Correction of CKD Metabolic Complications
Although similar at baseline in both arms, clinically significant lower serum urea and uric acid levels were seen at the end of study in patients following the KD (Table 1). Specifically, serum urea significantly decreased only in the KD group (Table 1).
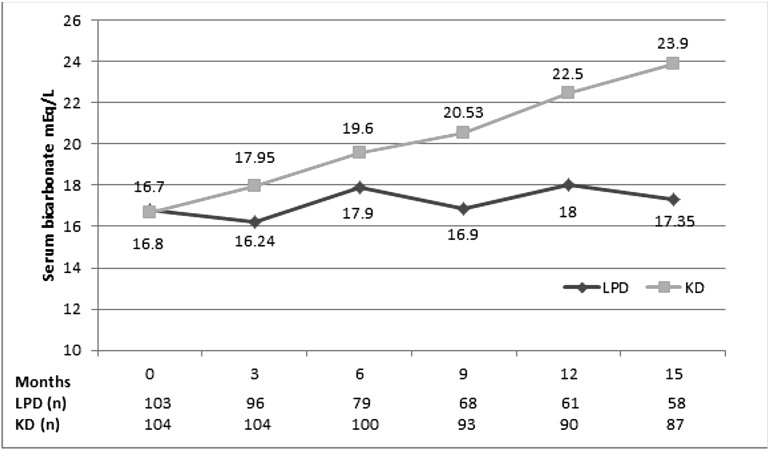
Serum bicarbonate was lower at baseline in KD but increased significantly in this arm (Figure 5) and became higher at the end of study compared with LPD, whereas the need for bicarbonate supplementation was higher in the control group (51% versus 29%; P<0.01), with higher doses of sodium bicarbonate in patients on the LPD (Table 1).
Figure 5.
Serum bicarbonate (milliequivalents per liter) during the study. Serum bicarbonate was lower at baseline in KD, but increased significantly in this arm.
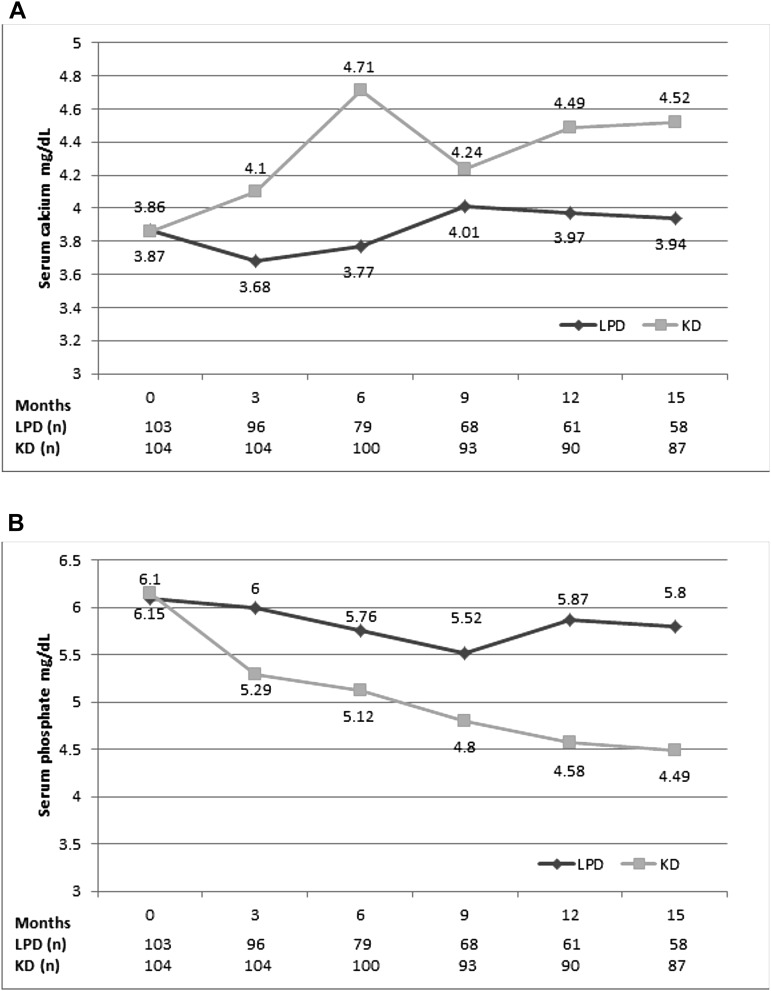
Similarly, calcium-phosphorus metabolism improved only in the KD group: at baseline, patients on the KD had lower calcium but higher phosphates, whereas at the end of study, serum calcium was higher and serum phosphate was lower in this group compared with LPD (Table 1). Moreover, compared with baseline, calcium increased and serum phosphates decreased in the KD arm, whereas opposite variations were seen in the control group (Figure 6). Additionally, although the need for calcium supplementation was similar in both arms, calcium doses were lower in the KD group, and the need for vitamin D supplementation was higher in controls (54% versus 22%; P=0.004) at the end of study (Table 1).
Figure 6.
(A) Serum calcium (milligrams per deciliter) and (B) serum phosphates (milligrams per deciliter) during the study. Serum calcium increased and serum phosphates decreased only in KD arm; opposite variations were seen in the LPD group.
Safety Parameters
The nutritional status, as assessed by the Subjective Global Assessment (SGA), was preserved in both groups during the study.
No significant changes have been noticed in any of the anthropometric or biochemical nutritional parameters in any group at the end of study compared with baseline, suggesting that the two protein–restricted regimens could preserve the nutritional status in patients with advanced CKD (Table 2). Moreover, CRP was lower at the end of the study in the patients on the KD and significantly increased from baseline to end of study only in the LPD arm (Table 2).
Serum potassium level was higher in patients on the KD at randomization. Despite the low-protein intake and even the vegetarian diet, it did not significantly change during the study, and it remained, in both arms, within the normal range (Table 2).
Ketoanalogues supplementation was well tolerated. No adverse reactions to Ketosteril were noted. No patients’ death was registered in any group during the study.
Compliance to the Diet
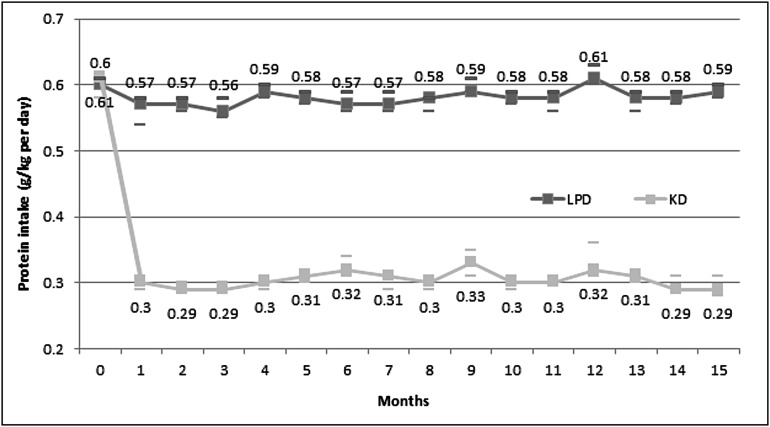
The carefully selected patients for their declared and proved acceptance and compliance to the diet during the run-in phase had, as expected, very good compliance to the restricted protein regimens regarding both protein and energy intake. The achieved protein intake was very close to prescription throughout the study and remained stable at the end of study (median KD and LPD, 0.29 and 0.59 g/kg per day, respectively) (Figure 7, Table 2). The daily achieved energy intake was also close to recommendations (31 kcal/kg per day) in both arms, without differences at any of the study moments (Table 2).
Figure 7.
Protein intake (median and 95% CI) during the study. The achieved protein intake was very close to prescription and stable throughout the study.
Only seven patients (3%) abandoned the diet, without difference between arms.
Discussion
This study is the first to support the efficacy of a vegetarian VLPD supplemented with ketoanalogues in compliant patients. Five patients with eGFR<30 ml/min need to be treated to avoid a >50% reduction in eGFR or dialysis initiation in one patient and 23 patients with eGFR<30 ml/min need to be treated to avoid dialysis in one patient without detrimental effects on the nutritional status. However, when patients with eGFR<20 ml/min were considered, the NNTs are two for the composite end point and three for dialysis initiation. The favorable effects of the KD seem to be mediated more by the correction of metabolic complications of advanced CKD, notably the improvement in nitrogen balance, mineral metabolism disturbances, metabolic acidosis, and inflammation, than by reduction in GFR decline. Despite the poor acceptance of LPDs, excellent compliance could be obtained by intensive nutritional counseling and monitoring.
Although various reports support the improvement in metabolic complications of renal failure by restricting protein intake, the effects of LPDs on hard end points are still controversial.
For instance, in a recent Cochrane review,19 zero of three controlled studies13,15,16 comparing the effects of low protein (0.6/kg per day) with those of more liberal protein restriction (0.8–1.3 g/kg per day) published so far and their pooled data significantly influenced the outcome (risk ratio, 0.76; 95% CI, 0.54 to 1.05; P=0.10), which could suggest that protein restriction should be <0.6 g/kg per day to effectively ameliorate CKD progression.
Furthermore, data have been published on seven prospective, randomized studies comparing the effects of VLPDs (0.3–0.4 g/kg per day) in three of them supplemented with ketoanalogues3,15,16 with those of LPDs (0.6–0.8 g/kg per day).12,14,17,18 In these studies, a clear–cut positive effect of low-protein intake on outcome could not be proved. However, when pooled together, the relative risk was significantly lower in patients on VLPDs (relative risk, 0.63; 95% CI, 0.47 to 0.86; NNT=8), suggesting that protein intake should be reduced below 0.6 g protein to obtain a beneficial effect. Notably, analysis of pooled data revealed a trend to better outcomes in patients on VLPDs (relative risk, 0.69; 95% CI, 0.46 to 1.03; P=0.07)19 in studies investigating VLPD without ketoanalogues supplementation, whereas a significantly lower relative risk in the KD arm (risk ratio, 0.57; 95% CI, 0.38 to 0.85; NNT=4) was seen only for studies on VLPD supplemented with ketoanalogues. Thus, the ketoanalogues supplementation of a VLPD seems to have some advantages itself.
In our data, the risk to reach the composite end point (halving initial eGFR or dialysis initiation) was significantly lower in patients on a VLPD supplemented with ketoanalogues than in those on a conventional LPD, with an adjusted NNT of five in ITT and four in PP analyses, similar to the aforementioned analysis of studies on the KD.13,15,16 However, the KD allowed avoidance of dialysis initiation in a lower proportion of patients (NNT=23). Nevertheless, when examining the outcomes of patients according to the eGFR at baseline (<30, <25 [as suggested by the manufacturer], or <20 ml/min) the adjusted NNTs at 1 year to avoid dialysis in one patient were 23, 8, and 3, respectively.
Consequently, if prescribed to avoid dialysis initiation, vegetarian VLPD supplemented with ketoanalogues seems to be more beneficial in patients with more advanced CKD.
However, the effects of ketoanalogues supplementation are difficult to discern from those of a lower protein intake, which was highlighted in MDRD post hoc analyses23; the association of ketoanalogues with a VLPD seems more efficient than the LPD alone, probably because it permits an additional decrease in the protein intake <0.6 g/kg per day, which could affect the outcome. Moreover, in our study, the quality of proteins was different between groups (i.e., vegetables only in KD versus conventional mixed LPD in controls); therefore, the better metabolic control on the KD could be attributed to the vegetarian content of the diet itself rather than to the more severe protein restriction or supplementation with ketoanalogues.
The GFR was similar in both arms at all study moments, with a trend to lower values at the end of study in the LPD group. However, eGFR increased from baseline to 3 months in the KD group but decreased in the control group; the decrease from 3 months to the end of study was lower in the KD group, with a difference between arms of 3 ml/min per year. Thus, the KD could perform better than ACEIs, because the estimated effect of a 10-mmHg decrease in BP by ACEIs is a reduction in eGFR of 1.5 ml/min.30 Nonetheless, we used eGFR and not measured GFR, which could be biased, because the severe reduction in protein intake could induce changes in glomerular hemodynamics31 and decrease creatine intake31 and endogenous creatinine production by a potential decline in muscle mass.9
However, the LPDs could improve BP control and proteinuria, the main determinants of CKD progression.1–5 For instance, Bellizzi et al.1 found an antihypertensive effect of supplemented VLPD, possibly attributed to reduction in salt intake, to the type of dietary proteins and ketoanalogues supplementation but independent of the actual protein intake.1 Similarly, reduction in proteinuria was suggested under the KD, mainly in patients with nephrotic syndrome.3,10,32,33 In our patients treated in both groups with ACEIs/ARBs in similar percentages (>70%), an optimal BP control was observed in >85% at all study moments, and we did not find significant changes in urinary protein excretion in any group. Because poor BP control and proteinuria >1 g/d were exclusion criteria, protein intake was low in both arms, and monitoring was intensive, the effects of the diet on BP and proteinuria could have been mitigated. However, the absence of ACEI/ARB therapy was an independent predictor of a poor outcome, supporting an additive renoprotective effect of the KD to ACEIs/ARBs as suggested by Gansevoort et al.2 Thus, the dietary intervention seems to be an effective adjunct to ACEI therapy.
The metabolic complications of CKD were clearly improved by the KD, in line with older and more recent reports,7–11,34 even if the patients in the control arm were on an LPD. As expected, serum urea and uric acid were lower at the end of study in patients on the KD. Acidosis was corrected, whereas the need of bicarbonate supplementation was lower in the KD arm. An impressive improvement in mineral metabolism parameters (higher calcium and lower serum phosphates and reduction in calcium supplements and the proportion of patients treated with vitamin D) was obtained in this arm at the end of study. Moreover, KD ameliorated inflammation. These effects could be related to not only the reduction in protein intake and ketoanalogues supplementation, which is a source of calcium and effective phosphate binders,35 but also, the vegetable sources of proteins. Vegetable-source proteins are base producing rather than acid producing, which happens with animal-source proteins. Vegetable proteins generate fewer acids compared with animal ones, contributing to better acid-base control with the KD. With vegetable proteins, there is also a lower phosphate content, lower production of p-cresyl sulfate, lower oxidative stress,22 and lower phosphorus content, allowing for better control of acid-base and calcium-phosphorus disorders, crucial for not only mineral metabolism but also, proteinuria and CKD progression.33,36 There was a slight increase in serum potassium within the normal range.
Recent data support the role of vegetarian diet in correction of metabolic acidosis and preservation of eGFR.37 Similarly, a high percentage of vegetables in the diet seems efficient and safe in ameliorating calcium-phosphorus metabolism through a reduction in urine phosphorus excretion.38
Our data could support vegetarian VLPD supplemented with ketoanalogues as a possible therapeutic intervention in CKD mineral metabolism disorders, and it is clearly superior to a moderate restriction in protein intake.
The dialysis deferral observed in our patients on the KD could be explained by a reduction in eGFR decline, an improvement in uremic symptoms, or both as initially suggested by Walser and Hill.39 According to our data, the difference in the rate of decline in renal function between the arms was important; however, the role of a slower decrease in GFR is uncertain, but the correction of metabolic disturbances was obvious, favoring the alleviation of uremic symptoms by the KD but not by the conventional LPD as the cause of dialysis deferral.4,6
Concerns about the nutritional safety of the KD have been raised,20–22,40 more recently fueled by long–term follow-up of the MDRD Study results.21 Similar to most of the groups,6,10,26,27 we found no significant alteration in any of the nutritional parameters in any group, despite the very low–protein content of the KD, hence preserving the recommended energy intake. Ketoanalogues supplementation and a proper energy intake achieved by motivated patients under close dietary counseling and nutritional monitoring seem to be the clues.
Because LPDs impose, for most of the patients, serious changes in personal behavior and family habits, acceptance and compliance to protein-restricted diets were reported as poor in most clinical trials.18,21,23 Less than one half of our patients fulfilling the selection criteria accepted to potentially follow a vegetarian diet, and only 14% proved compliant during the run-in phase and could be, therefore, randomized, a proportion that is higher than that recently reported by Piccoli et al.27 (1.5%). Compliance to LPDs could also be a problem. Only 3% of our patients, equally distributed in the study arms, discontinued the diet, but those continuing the study showed excellent compliance to both protein and energy intake. This could be a center effect, because in other reports, 16% of patients chose to discontinue the KD diet,27 and the compliance to an even less protein–restricted diet (0.55 g/kg per day) was only 27% and inversely related to the severity of protein restriction.17 Nevertheless, our experience suggests that appropriate selection of patients, intensive counseling, and close monitoring could improve compliance. However, because patients’ training is time consuming, studies addressing identification of patients with the most fitted profiles of acceptance are necessary to increase efficacy. Another possibility to increase compliance is diet liberalization, which was suggested by Piccoli et al.27
In a very recently published observational study focusing on feasibility and efficacy of protein-restricted diets, the same group of Piccoli et al.27 used a multiple-choice approach to LPDs (0.6 g protein/kg per day either vegan supplemented with ketoanalogues or on the basis of commercial aproteic food) and revealed high acceptance (95%) and compliance (90%), whereas both LPDs led to similar results in terms of the composite end point (death or dialysis), suggesting at least survival equivalence with dialysis at lower cost.41
Finally, probably, as in other fields of medicine, the dietetic approach is “a story of believers and nonbelievers.”42,43
There are several limitations of our study. We enrolled only white, nondiabetic, relatively young subjects with good nutritional status, without severe proteinuria, and with well controlled BP and proved dietary compliance. Additionally, the comparator was a diet with 0.6 g protein/kg per day, which is unusual in many parts of the world. Therefore, the wide applicability of the results could be questioned. However, these restrictions were necessary to conform to current practice guidelines and allow an accurate evaluation of ketoanalogues efficiency. Although the study was powered enough for the primary composite end point and the predefined efficacy criteria were met, the study sample size is still relatively small but yet, one of the largest reported so far. We used eGFR and not measured GFR, which could be biased by the diet-induced alteration in creatinine metabolism. However, the primary end point was composite and less dependent on the accuracy of GFR measurement, and analyses pertaining to GFR were performed in a period when the creatinine was supposed to be equilibrated. The single-center setting could be a limitation but also, one of the study strengths considering the experience in nutritional monitoring and counseling as well as the possibility to spread it.
This study comes with some strong points: the randomized, controlled design; the properly powered sample size; and the hard end point to evaluate CKD progression. Furthermore, the strict control of the protein intake at two distinct levels allowed for a clear evaluation of the efficacy and safety of ketoanalogues supplementation in conjunction with a VLPD in delaying CKD progression. Our results draw attention to the role of nutritional intervention, particularly of the ketoanalogues–supplemented severe protein–restricted diet as an effective, safe, and feasible link in the predialysis care of certain groups of patients with CKD. The KD could be used not only as a palliative management in elderly patients with CKD as suggested by Brunori et al.26 but also, in patients with advanced CKD with good nutritional status waiting for angioaccess maturation or who have a preemptive transplantation planned.
Vegetarian VLPD supplemented with ketoanalogues seems nutritionally safe and could defer dialysis initiation in patients with eGFR<20 ml/min by ameliorating CKD–associated metabolic disturbances.
Concise Methods
Study Design
This is a prospective, single–center, open–label, randomized, controlled trial with a total duration of 18 months.
All eligible patients who gave informed consent were screened. Those meeting the selection criteria were enrolled and entered a 3-month run–in phase, during which a conventional LPD was prescribed.
At the end of the run-in phase, the subjects still fulfilling the selection criteria were randomized in a 1:1 ratio to receive the KD or continue the conventional LPD for 15 months (intervention phase). All of the parameters were assessed at baseline, throughout the intervention phase, and at end of the study (Figure 1). To avoid the effect of reduction in protein intake on GFR and serum creatinine,31 which would bias the eGFR, the analyses pertaining to eGFR were conducted from 3 months after randomization until the end of study.
Results of the pilot phase of the study after the first year of enrollment have already been published.34
The trial was conducted with the provisions of the Declaration of Helsinki and Tokyo as amended in Venice (1983). The protocol was approved by the local Hospital Ethics Committee and registered at National Clinical Trials (NCT02031224).
Subjects
Adult patients with stage 4+ CKD (eGFR calculated using the MDRD four–variable equation44 of <30 ml/min per year) were enrolled. They had stable renal function for at least 12 weeks before enrollment (defined as a reduction in eGFR<4 ml/min per year24, proteinuria <1 g/g urinary creatinine, good nutritional status as indicated by an SGA score A/B, and serum albumin ≥3.5 g/dl). Only the patients who declared a potentially good compliance with an LPD and agreed to follow the monitoring schedule were considered. The decline in eGFR before inclusion was assessed using at least two serum creatinine values measured at 1-month intervals, with the last measurement within 1 month before enrolment.
Before enrolment, the patients were informed that it would be necessary to follow a vegetarian VLPD and receive a nutritional supplement. Only those agreeing to follow such a diet and take the number of tablets according to their dry body weight were included into the run-in phase.
Patients with poorly controlled arterial BP (≥145/85 mmHg), relevant comorbidities (diabetes mellitus, heart failure, active hepatic disease, digestive diseases with malabsorption, or inflammation/anti-inflammatory therapy), uremic complications (pericarditis or polyneuropathy), or feeding inability (anorexia or nausea) were excluded.
Selection criteria were evaluated at screening, monthly during the run-in phase, and at randomization.
During the run-in phase, conventional LPD was recommended to all of the enrolled patients: it contained a daily protein intake of 0.6 g/kg dry ideal body wt and total daily energy intake of 30 kcal/kg dry ideal body wt. We appreciated compliance to the diet if both the achieved protein and energy intakes were in the range of ±10% of the recommended values. The adherence to the monitoring schedule was considered good in patients following the initial visit date ±7 days. After compliance was established and other eligibility criteria were met, patients were randomized.
In all enrolled patients, the BP and cholesterol levels were controlled with antihypertensive medication (including ACEIs, sartans, and diuretics) and statins or fibrates according to Romanian Best Practice Guidelines—CKD.45 Iron therapy and erythropoietic-stimulating agents administration were continued according to Romanian Best Practice Guidelines—Anaemia of Chronic Kidney Disease.46 Mineral metabolism abnormalities and metabolic acidosis were monitored and corrected with calcium, phosphate binders, vitamin D, and sodium bicarbonate according to Romanian Best Practice Guidelines for CKD—Mineral Metabolism and Bone Disorders.45,47 The enrolled patients also received water–soluble vitamin supplementation as required.
Parameters
The primary composite end point was set as the need for RRT initiation or a >50% reduction in the initial eGFR any time during the assessment phase. The decision to initiate RRT was made by the Ethical Committee of the Hospital on the basis of the clinical and laboratory data. Uremic symptoms, acute pulmonary edema, feeding inability, and uncontrolled acid-base and/or hydroelectrolyte disturbances were considered for the decision to start RRT and registered at the patient’s last study visit (See Supplementary Table I). The members of the Ethical Committee were aware of the patients’ inclusion in the clinical trial but unaware of the arm to which the patients had been assigned.
The need of RRT initiation, the decline in GFR, and the correction of metabolic complications of CKD (e.g., serum urea, calcium and phosphate disorders, and acidosis) were predefined as secondary efficacy parameters.
Parameters of nutritional status were set as safety variables. A panel of nutritional parameters was used: SGA,48 anthropometric markers (body mass index, tricipital skinfold, and midarm muscular circumference), and biochemical parameters (serum albumin, serum CRP, and serum total cholesterol).
Compliance to the prescribed diet, the occurrence of any adverse event, and the number of patients withdrawing from the study were used as safety variables and evaluated monthly during the run-in phase, weekly for the first month after randomization, every 4 weeks during the next 6 months, and every 3 months thereafter. Protein intake was evaluated by urinary urea nitrogen excretion and the Maroni formula49; energy intake was assessed by 3-day food diaries.50 The compliance to the use of ketoanalogues supplementation was assessed at each visit by the number of tablets removed from the blisters since the previous visit.
Therapeutic Intervention
The patients in the intervention arm (KD group) received a vegetarian VLPD (0.3 g protein/kg ideal body wt per day) supplemented with ketoanalogues of essential amino acids (Ketosteril; Fresenius Kabi, Bad Homburg, Germany; see Supplementary Table II) at 0.125 g/kg ideal dry body wt per day as recommended by the manufacturer (http://www.anm.ro/_/_RCP/RCP_1028_25.09.08.pdf?anmOrder=Sorter_cod_atc&anmPage=1347&ID=11966).
The patients in the control arm (LPD group) continued the conventional LPD with 0.6 g/kg per day, including high–biologic value proteins.
The total recommended energy intake was 30 kcal/kg ideal dry body wt per day in both arms.
Nutritional interventions and monitoring schedule followed Romanian Best Practice Guidelines—Nutrition and Nutritional Intervention in Chronic Kidney Disease.50
Patients in both arms received intensive nutritional counseling and monitoring monthly during the run-in phase, which was further intensified to once every other week in the first month, monthly until 3 months, and every 3 months thereafter.
Monitoring Schedule
Nineteen blood and urine samplings were scheduled to be drawn monthly for each patient. The laboratory reports included the nitrogen compounds, calcium-phosphorus metabolism parameters, acid-base balance, biochemical nutritional markers, serum CRP, hemoglobin, blood cell count, and biochemical safety parameters (sodium, potassium, liver enzymes, and bilirubin).
The anthropometric measurements and SGA were assessed at enrollment, at randomization, and every 3 months thereafter by a single examiner.
The compliance with the prescribed diet (protein and energy intake) and the supplement intake were assessed monthly during the run-in phase, weekly for the first month after randomization, every 4 weeks during the next 6 months, and every 3 months thereafter.
The BP level, the requirements of drugs for hypertension, acidosis, and mineral metabolism disorders, and the occurrence of adverse events were monthly recorded.
Statistical Analyses
Data have been processed with descriptive methods: mean or median and 95% CIs according to their distribution. Differences and 95% CIs in values and proportions between groups were calculated at study moments. Changes within each arm from baseline to end of study were similarly evaluated. Pearson chi-squared and Mann–Whitney tests were used for comparison. Wilcoxon and paired t tests were also used to evaluate pairs of values. A P value of <0.05 was considered statistically significant.
Kaplan–Meier analysis was conducted to determine the occurrence of the composite end point, and the log-rank test was used for comparison. To evaluate the variables related to outcome and adjust the chances of event-free survival, multivariable Cox proportional hazard ratio models were built using logarithmic transformation of skewed variables to normalize distribution. The associated risk was presented as adjusted hazard ratio and adjusted NNTs.51
Analyses pertaining to the primary end point were made in both the ITT and PP populations. All of the other analyses were performed per protocol. The patients who experienced a >50% reduction in the initial eGFR during the study were censored for reaching primary end point; however, their follow-up continued until dialysis initiation.
A sample size of ≥96 patients per arm was required for a probability of 95% and a power of 80%, considering a 25% probability to achieve the predefined primary end point during the follow-up and a 10% difference between groups to be significant.
Analyze-it (Analyze-it Software, Ltd., Leeds, United Kingdom), and SPSS version 14 (SPSS Inc., Chicago, IL) software were used.
Disclosures
No financial support was received for the study. L.G. participated in scientific meetings (as a speaker) and at the international advisory board meetings for Ketosteril. Speaker fees and consultancy fees were received from Fresenius Kabi. A.S. has no known conflicts of interest. D.D. has no known conflicts of interest. G.S. participated as a speaker in a national scientific meeting. A speaker fee was received from Fresenius Kabi. G.M. has no known conflicts of interest.
Supplementary Material
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Dietary Protein as Kidney Protection: Quality or Quantity?,” on pages 1877–1879.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015040369/-/DCSupplemental.
References
- 1.Bellizzi V, Di Iorio BR, De Nicola L, Minutolo R, Zamboli P, Trucillo P, Catapano F, Cristofano C, Scalfi L, Conte G ERIKA Study-group : Very low protein diet supplemented with ketoanalogs improves blood pressure control in chronic kidney disease. Kidney Int 71: 245–251, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Gansevoort RT, de Zeeuw D, de Jong PE: Additive antiproteinuric effect of ACE inhibition and a low-protein diet in human renal disease. Nephrol Dial Transplant 10: 497–504, 1995 [DOI] [PubMed] [Google Scholar]
- 3.Chauveau P, Combe C, Rigalleau V, Vendrely B, Aparicio M: Restricted protein diet is associated with decrease in proteinuria: Consequences on the progression of renal failure. J Ren Nutr 17: 250–257, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Ikizler TA: Dietary protein restriction in CKD: The debate continues. Am J Kidney Dis 53: 189–191, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Kidney Disease: Improving Global Outcomes : KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int 3: 5–10, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Mitch WE: Dietary protein restriction in chronic renal failure: Nutritional efficacy, compliance, and progression of renal insufficiency. J Am Soc Nephrol 2: 823–831, 1991 [DOI] [PubMed] [Google Scholar]
- 7.Mitch WE: Are supplements of ketoacids and amino acids useful in treating patients with chronic renal failure? Wien Klin Wochenschr 112: 863–864, 2000 [PubMed] [Google Scholar]
- 8.Combe C, Deforges-Lasseur C, Caix J, Pommereau A, Marot D, Aparicio M: Compliance and effects of nutritional treatment on progression and metabolic disorders of chronic renal failure. Nephrol Dial Transplant 8: 412–418, 1993 [PubMed] [Google Scholar]
- 9.Aparicio M, Fouque D, Chauveau P: Effect of a very low-protein diet on long-term outcomes. Am J Kidney Dis 54: 183, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Aparicio M, Chauveau P, De Précigout V, Bouchet JL, Lasseur C, Combe C: Nutrition and outcome on renal replacement therapy of patients with chronic renal failure treated by a supplemented very low protein diet. J Am Soc Nephrol 11: 708–716, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Barsotti G, Cupisti A, Morelli E, Meola M, Cozza V, Barsotti M, Giovannetti S: Secondary hyperparathyroidism in severe chronic renal failure is corrected by very-low dietary phosphate intake and calcium carbonate supplementation. Nephron 79: 137–141, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Rosman JB, Donker AJ: The response to a low-protein diet to retard the progression of renal failure is sex-dependent (abstract). Nephrol Dial Transplant 4: 457, 1989 [Google Scholar]
- 13.Ihle BU, Becker G, Whithworth JA, Charlwood RA, Kincaid-Smith PS: The effect of protein restriction on the progression of renal insufficiency. N Engl J Med 321: 1773–1777, 1989 [DOI] [PubMed] [Google Scholar]
- 14.Jungers P, Chauveau P, Ployard F, Lebkiri B, Ciancioni C, Man NK: Comparison of ketoacids and low protein diet on advanced chronic renal failure progression. Kidney Int Suppl 22: S67–S71, 1987 [PubMed] [Google Scholar]
- 15.Malvy D, Maingourd C, Pengloan J, Bagros P, Nivet H: Effects of severe protein restriction with ketoanalogues in advanced renal failure. J Am Coll Nutr 18: 481–486, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Di Iorio BR, Minutolo R, De Nicola L, Bellizzi V, Catapano F, Iodice C, Rubino R, Conte G: Supplemented very low protein diet ameliorates responsiveness to erythropoietin in chronic renal failure. Kidney Int 64: 1822–1828, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Cianciaruso B, Pota A, Pisani A, Torraca S, Annecchini R, Lombardi P, Capuano A, Nazzaro P, Bellizzi V, Sabbatini M: Metabolic effects of two low protein diets in chronic kidney disease stage 4-5--a randomized controlled trial. Nephrol Dial Transplant 23: 636–644, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Locatelli F, Alberti D, Graziani G, Buccianti G, Redaelli B, Giangrande A Northern Italian Cooperative Study Group : Prospective, randomised, multicentre trial of effect of protein restriction on progression of chronic renal insufficiency. Lancet 337: 1299–1304, 1991 [DOI] [PubMed] [Google Scholar]
- 19.Fouque D, Laville M: Low protein diets for chronic kidney disease in non diabetic adults. Cochrane Database Syst Rev 3: CD001892, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Adler S, Caggiula AW, England BK, Greene T, Hunsicker LG, Kusek JW, Rogers NL, Teschan PE Modification of Diet in Renal Disease Study Group : Effects of dietary protein restriction on the progression of advanced renal disease in the Modification of Diet in Renal Disease Study. Am J Kidney Dis 27: 652–663, 1996 [DOI] [PubMed] [Google Scholar]
- 21.Menon V, Kopple JD, Wang X, Beck GJ, Collins AJ, Kusek JW, Greene T, Levey AS, Sarnak MJ: Effect of a very low-protein diet on outcomes: Long-term follow-up of the Modification of Diet in Renal Disease (MDRD) Study. Am J Kidney Dis 53: 208–217, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Thilly N: Low-protein diet in chronic kidney disease: From questions of effectiveness to those of feasibility. Nephrol Dial Transplant 28: 2203–2205, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Klahr S, Levey AS, Beck GJ, Caggiula AW, Hunsicker L, Kusek JW, Striker G Modification of Diet in Renal Disease Study Group : The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. N Engl J Med 330: 877–884, 1994 [DOI] [PubMed] [Google Scholar]
- 24.Johnson DW: Dietary protein restriction as a treatment for slowing chronic kidney disease progression: The case against. Nephrology (Carlton) 11: 58–62, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Friedman AN: New evidence for an old strategy to help delay the need for dialysis. Am J Kidney Dis 49: 563–565, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Brunori G, Viola BF, Parrinello G, De Biase V, Como G, Franco V, Garibotto G, Zubani R, Cancarini GC: Efficacy and safety of a very-low-protein diet when postponing dialysis in the elderly: A prospective randomized multicenter controlled study. Am J Kidney Dis 49: 569–580, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Piccoli GB, Ferraresi M, Deagostini MC, Vigotti FN, Consiglio V, Scognamiglio S, Moro I, Clari R, Fassio F, Biolcati M, Porpiglia F: Vegetarian low-protein diets supplemented with keto analogues: A niche for the few or an option for many? Nephrol Dial Transplant 28: 2295–2305, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, Lin PH, Karanja N DASH Collaborative Research Group : A clinical trial of the effects of dietary patterns on blood pressure. N Engl J Med 336: 1117–1124, 1997 [DOI] [PubMed] [Google Scholar]
- 29.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Roccella EJ; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee: Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 42: 1206–1252, 2003 [DOI] [PubMed]
- 30.Bakris GL, Williams M, Dworkin L, Elliott WJ, Epstein M, Toto R, Tuttle K, Douglas J, Hsueh W, Sowers J National Kidney Foundation Hypertension and Diabetes Executive Committees Working Group : Preserving renal function in adults with hypertension and diabetes: A consensus approach. Am J Kidney Dis 36: 646–661, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Poggio ED, Wang X, Greene T, Van Lente F, Hall PM: Performance of the modification of diet in renal disease and Cockcroft-Gault equations in the estimation of GFR in health and in chronic kidney disease. J Am Soc Nephrol 16: 459–466, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Teplan V, Schück O, Racek J, Mareckova O, Stollova M, Hanzal V, Malý J: Reduction of plasma asymmetric dimethylarginine in obese patients with chronic kidney disease after three years of a low-protein diet supplemented with keto-amino acids: A randomized controlled trial. Wien Klin Wochenschr 120: 478–485, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Di Iorio BR, Bellizzi V, Bellasi A, Torraca S, D’Arrigo G, Tripepi G, Zoccali C: Phosphate attenuates the anti-proteinuric effect of very low-protein diet in CKD patients. Nephrol Dial Transplant 28: 632–640, 2013 [DOI] [PubMed] [Google Scholar]
- 34.Mircescu G, Gârneaţă L, Stancu SH, Căpuşă C: Effects of a supplemented hypoproteic diet in chronic kidney disease. J Ren Nutr 17: 179–188, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Kalantar-Zadeh K, Cano NJ, Budde K, Chazot C, Kovesdy CP, Mak RH, Mehrotra R, Raj DS, Sehgal AR, Stenvinkel P, Ikizler TA: Diets and enteral supplements for improving outcomes in chronic kidney disease. Nat Rev Nephrol 7: 369–384, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group: KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 113: S1–S130, 2009 [DOI] [PubMed]
- 37.Goraya N, Simoni J, Jo C-H, Wesson DE: Treatment of metabolic acidosis in patients with stage 3 chronic kidney disease with fruits and vegetables or oral bicarbonate reduces urine angiotensinogen and preserves glomerular filtration rate. Kidney Int 86: 1031–1038, 2014 [DOI] [PubMed] [Google Scholar]
- 38.Moorthi RN, Armstrong CL, Janda K, Ponsler-Sipes K, Asplin JR, Moe SM: The effect of a diet containing 70% protein from plants on mineral metabolism and musculoskeletal health in chronic kidney disease. Am J Nephrol 40: 582–591, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walser M, Hill S: Can renal replacement be deferred by a supplemented very low protein diet? J Am Soc Nephrol 10: 110–116, 1999 [DOI] [PubMed] [Google Scholar]
- 40.Filipowicz R, Beddhu S: Optimal nutrition for predialysis chronic kidney disease. Adv Chronic Kidney Dis 20: 175–180, 2013 [DOI] [PubMed] [Google Scholar]
- 41.Piccoli GB, Deagostini MC, Vigotti FN, Ferraresi M, Moro I, Consiglio V, Scognamiglio S, Mongilardi E, Clari R, Aroasio E, Versino E, Porpiglia F: Which low-protein diet for which CKD patient? An observational, personalized approach. Nutrition 30: 992–999, 2014 [DOI] [PubMed] [Google Scholar]
- 42.El Nahas M: Blog commentary. Nephrol Dial Transplant 28: 2305, 2013 [Google Scholar]
- 43.Lameire N, Van Biesen W: Epidemiology of peritoneal dialysis: A story of believers and nonbelievers. Nat Rev Nephrol 6: 75–82, 2010 [DOI] [PubMed] [Google Scholar]
- 44.Froissart M, Rossert J, Jacquot C, Paillard M, Houillier P: Predictive performance of the modification of diet in renal disease and Cockcroft-Gault equations for estimating renal function. J Am Soc Nephrol 16: 763–773, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Covic A, Mircescu G, Gluhovschi G, Schiller A: Romanian Best Practice Guidelines—CKD, Bucarest, Romania, CurteaVeche Publishing House, 2007 [Google Scholar]
- 46.Mircescu G, Covic A, Gluhovschi G, Schiller A: Romanian Best Practice Guidelines—Anaemia of Chronic Kidney Disease, 2nd Ed., Bucharest, Romania, CurteaVeche Publishing House, 2008 [Google Scholar]
- 47.Covic A, Mircescu G, Gherman-Căprioară M, Schiller A: Romanian Best Practice Guidelines for CKD—Mineral Metabolism and Bone Disorders, Iaşi, Romania, Demiurg Publishing House, 2010 [Google Scholar]
- 48.Enia G, Sicuso C, Alati G, Zoccali C: Subjective global assessment of nutrition in dialysis patients. Nephrol Dial Transplant 8: 1094–1098, 1993 [PubMed] [Google Scholar]
- 49.Maroni BJ, Steinman TI, Mitch WE: A method for estimating nitrogen intake of patients with chronic renal failure. Kidney Int 27: 58–65, 1985 [DOI] [PubMed] [Google Scholar]
- 50.Mircescu G, Covic A, Gluhovschi G, Schiller A: Romanian Best Practice Guidelines—Nutrition and Nutritional Intervention in Chronic Kidney Disease, Bucharest, Romania, CurteaVeche Publishing House, 2010 [Google Scholar]
- 51.Altman DG, Andersen PK: Calculating the number needed to treat for trials where the outcome is time to an event. BMJ 319: 1492–1495, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.