Abstract
Toll-like receptor 9 (TLR9) contributes to the development of polymicrobial septic AKI. However, the mechanisms that activate the TLR9 pathway and cause kidney injury during sepsis remain unknown. To determine the role of mitochondrial DNA (mtDNA) in TLR9-associated septic AKI, we established a cecal ligation and puncture (CLP) model of sepsis in wild-type (WT) and Tlr9-knockout (Tlr9KO) mice. We evaluated systemic circulation and peritoneal cavity dynamics and immune response and tubular mitochondrial dysfunction to determine upstream and downstream effects on the TLR9 pathway, respectively. CLP increased mtDNA levels in the plasma and peritoneal cavity of WT and Tlr9KO mice in the early phase, but the increase in the peritoneal cavity was significantly higher in Tlr9KO mice than in WT mice. Concomitantly, leukocyte migration to the peritoneal cavity increased, and plasma cytokine production and splenic apoptosis decreased in Tlr9KO mice compared with WT mice. Furthermore, CLP-generated renal mitochondrial oxidative stress and mitochondrial vacuolization in the proximal tubules in the early phase were reversed in Tlr9KO mice. To elucidate the effects of mtDNA on immune response and kidney injury, we intravenously injected mice with mitochondrial debris (MTD), including substantial amounts of mtDNA. MTD caused an immune response similar to that induced by CLP, including upregulated levels of plasma IL-12, splenic apoptosis, and mitochondrial injury, but this effect was attenuated by Tlr9KO. Moreover, MTD-induced renal mitochondrial injury was abolished by DNase pretreatment. These findings suggest that mtDNA activates TLR9 and contributes to cytokine production, splenic apoptosis, and kidney injury during polymicrobial sepsis.
Keywords: acute renal failure, Immunology and pathology, renal injury, mitochondria
AKI is often accompanied by multiorgan dysfunction syndromes1 in the intensive care unit. Sepsis and septic shock are the primary causes of AKI in the intensive care unit, accounting for 40%–70% of all cases.2–5 The mortality of septic AKI reaches 60%–70%4,6; nevertheless, little is known regarding the mechanisms and contributing pathologic conditions. Sepsis is characterized by excessive immune reactions caused by bacterial infection. The innate immune response is triggered by the recognition of exogenous molecules from microorganisms known as pathogen-associated molecular patterns. Toll-like receptors (TLRs) are the key receptors that recognize pathogen-associated molecular patterns. TLR4 recognizes LPS from gram-negative bacillus,7 and TLR9 recognizes bacterial DNA with unmethylated CpG motifs.8
Cecal ligation and puncture (CLP) is considered the most relevant model of human sepsis.9 Gram-negative bacilli are the predominant pathologic bacteria that invade after CLP; they can release LPS, which, in turn, induces a systemic inflammatory response via TLR4. Furthermore, AKI was mitigated in Tlr9 knockout (KO) mice but not in Tlr4KO mice after CLP.10,11 These findings compelled us to search for novel ligands of TLR9, which would be released into the systemic circulation in greater amounts than LPS after CLP.
Mitochondria are ancient bacteria that have become endosymbionts throughout evolution. Therefore, their DNA shows similarities to bacterial DNA, and although they escape the immune system because of their intracellular location, they can also be recognized as “nonself” or foreign material when they exist extracellularly. Furthermore, endogenous mitochondrial DNA (mtDNA) was recently shown to activate the TLR9 pathway.12 Therefore, we hypothesized that endogenous mtDNA might be released into the extracellular space in much greater amounts than LPS, which would amplify innate immunity and kidney injury via binding to TLR9 after CLP. To evaluate this hypothesis, we first evaluated the amount of circulating mtDNA after CLP.
Previous studies showed that Tlr9KO improved survival in mice after CLP,11,13,14 suggesting that immune reactions in the peritoneal cavity and spleen are critical for extermination of invading bacteria in polymicrobial peritonitis.11,13,14 TLR9 has also impaired granulocyte trafficking to the focal point of infection.13,14 Given that Tlr9KO promoted leukocyte trafficking to the peritoneal cavity after CLP, we further hypothesized that mtDNA from these damaged and/or dead immune cells would increase in the peritoneal cavity and might play an important role in killing bacteria after CLP. To evaluate this hypothesis, we evaluated the effects of Tlr9KO on free mtDNA in the peritoneal cavity and on the immune response in the systemic circulation, peritoneal cavity, and spleen after CLP.
CLP has been reported to cause very subtle histologic changes in the kidney. Recently, tubular oxidative stress and mitochondrial dysfunction were proposed as important pathologic changes occurring in septic AKI15–17; however, little is known about effects of Tlr9KO on tubular dysfunctions after CLP.11 Therefore, we evaluated the effects of Tlr9KO on tubular mitochondrial dysfunction after CLP.
mtDNA has potential to activate innate immunity and can induce acute lung injury,18 but its effects on kidney injury are unknown. Thus, we also evaluated the effects of mtDNA on the production of inflammatory cytokines and the induction of kidney injury via TLR9.
Results
Dynamics of Circulating mtDNA after CLP and LPS Administration
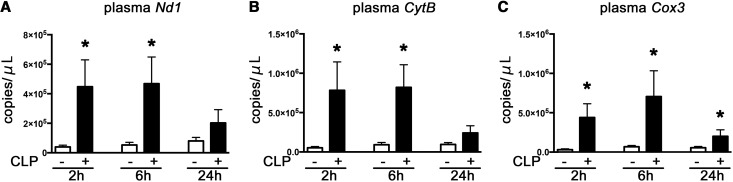
Plasma nicotinamide adenine dinucleotide dehydrogenase 1 (Nd1), cytochrome B (CytB), and cytochrome C oxidase subunit III (Cox3), which are mtDNA markers, increased substantially at 2 hours after CLP, which continued at least until 6 hours after CLP (Figure 1, A–C).
Figure 1.
The systemic circulating mtDNA increased at 2, 6 and 24 hours after CLP of WT mice. (A–C). Plasma Nd1, CytB, and Cox3. *P<0.05 versus sham (n=8–14 per group). All bar graphs represent mean±SEM.
We also evaluated plasma mtDNA concentration after endotoxic AKI induced by LPS. No markers increased at 2 hours after administration of LPS (Supplemental Figure 1, A–C).
Effects of Tlr9KO on Circulating and Local mtDNA Levels after CLP
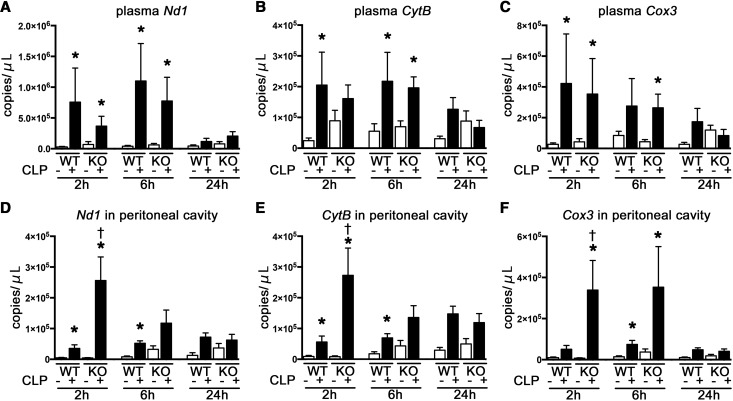
CLP significantly increased plasma three mtDNA markers at 2 and 6 hours to a similar extent in Tlr9KO and wild-type (WT) mice (Figure 2, A–C), although TLR9 deficiency tended to decrease plasma mtDNA levels.
Figure 2.
Tlr9KO increased peritoneal but not circulating mtDNA in the early phase after CLP. (A–C) Plasma Nd1, CytB, and Cox3 (n=7–12 per CLP group; n=4–9 per sham group). (D–F) Nd1, CytB, and Cox3 in the peritoneal cavity. *P<0.05 versus each sham group; †P<0.05 versus WT (n=6–8 per CLP group; n=4–5 per sham group). All bar graphs represent mean±SEM.
The amount of mtDNA in the peritoneal cavity was increased at 2 and 6 hours after CLP in both WT and Tlr9KO mice compared with each sham control. Moreover, the amount of mtDNA was significantly increased in Tlr9KO mice at 2 hours after CLP compared with WT mice (Figure 2, D–F).
Effects of TLR9 on Defense against Bacterial Infections in Plasma and Peritoneal Cavity after CLP
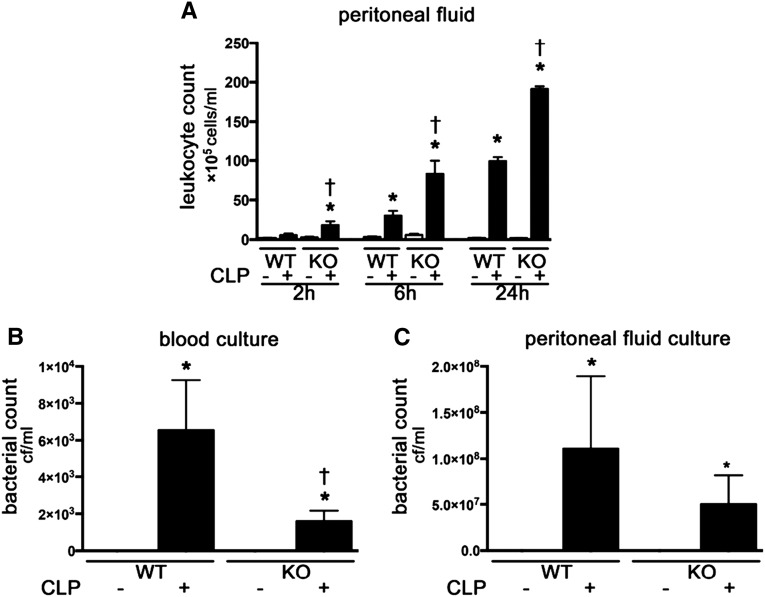
Tlr9KO mice had a higher degree of leukocyte migration to the focal point of infection at 2, 6 and 24 hours after CLP (Figure 3A). Similarly, more dead leukocytes from the peritoneal cavity were identified in Tlr9KO mice compared with WT after CLP (Supplemental Figure 2). Tlr9KO mice also had lower bacteremia at 24 hours after CLP than WT mice (Figure 3B). There was no difference in bacterial counts of the peritoneal fluid after CLP between the two groups (Figure 3C).
Figure 3.
Tlr9KO promoted leukocyte migration to peritoneal cavity from 2 hours and reduced bacteremia at 24 hours after CLP. (A) Leukocytes in peritoneal cavity (n=4–6 per group). (B) Bacterial count in blood. (C) Bacterial count in peritoneal fluid (n=4–7 per group). *P<0.05 versus each sham group; †P<0.05 versus WT. All bar graphs represent mean±SEM.
Effects of TLR9 on Splenic Apoptosis and Cytokine Profiles after CLP
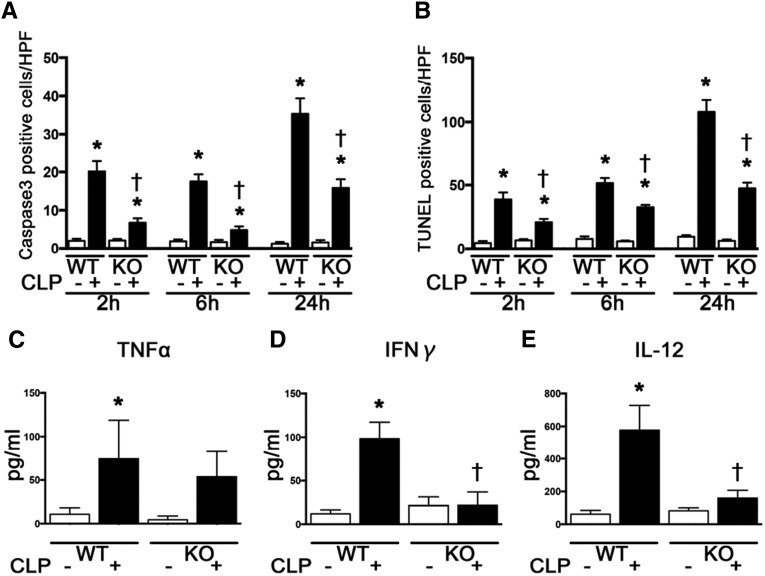
We evaluated the effects of TLR9 on splenic apoptosis because it worsens the outcome of sepsis and contribute to immune depression.19–21 Splenic apoptosis, as detected by active-caspase 3 and terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling (TUNEL) staining, was mostly observed in the white pulp. The numbers of active caspase 3-positive and TUNEL-positive cells in the white pulp of the spleen were markedly increased from 2 hours after CLP, which were significantly reduced at 2 and 6 and 24 hours after CLP in Tlr9KO mice (Figure 4, A and B).
Figure 4.
Tlr9KO attenuated splenic apoptosis from 2 to 24 hours and reduced plasma cytokines at 2 hours after CLP. (A and B) Active caspase 3- and TUNEL-positive cells by immunohistochemical staining (n=3 per group). (C–E) Plasma TNF-α, IFN-γ, and IL-12 (n=8–10 per group). *P<0.05 versus each sham group; †P<0.05 versus WT. HPF, high-power field. All bar graphs represent mean±SEM.
CLP increased plasma TNF-α, IFN-γ, and IL-12 levels at 2 hours in WT mice, whereas plasma IFN-γ and IL-12 levels were attenuated at 2 hours after CLP in Tlr9KO mice (Figure 4, C–E).
Effect of TLR9 on Renal Outcomes after CLP and LPS Administration
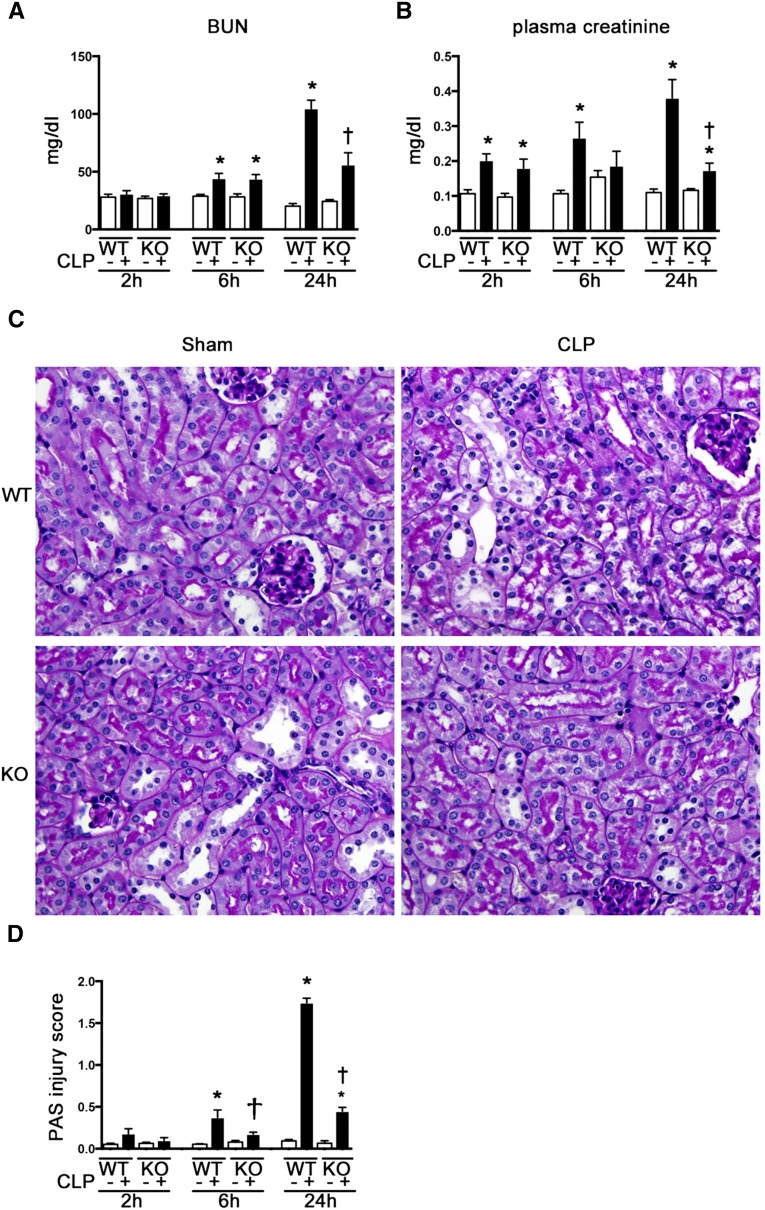
Plasma creatinine levels were significantly increased at 2, 6, and 24 hours after CLP, and BUN levels were increased at 6 and 24 hours after CLP. Increases in plasma creatinine and BUN were reduced at 24 hours after CLP in Tlr9KO mice compared with WT mice (Figure 5A). Renal function at 24 hours after administration of LPS degenerated in WT mice and did not improve in Tlr9KO mice (Supplemental Figure 1D).
Figure 5.
Tlr9KO reduced renal dysfunction and histologic change at 24 hours after CLP. (A) BUN. (B) Plasma creatinine (n=8–12 per group). (C) Histologic features of the cortex; original magnification, ×400. (D) Tubular damage score in the cortex (n=4–8 per group). *P<0.05 versus each sham group; †P<0.05 versus WT. PAS, periodic acid-Schiff. All bar graphs represent mean±SEM.
Renal histologic features in the cortex worsened at 6 and 24 hours after CLP, and this tubular damage was significantly prevented by Tlr9KO in the late phase of CLP (Figure 5, B and C).
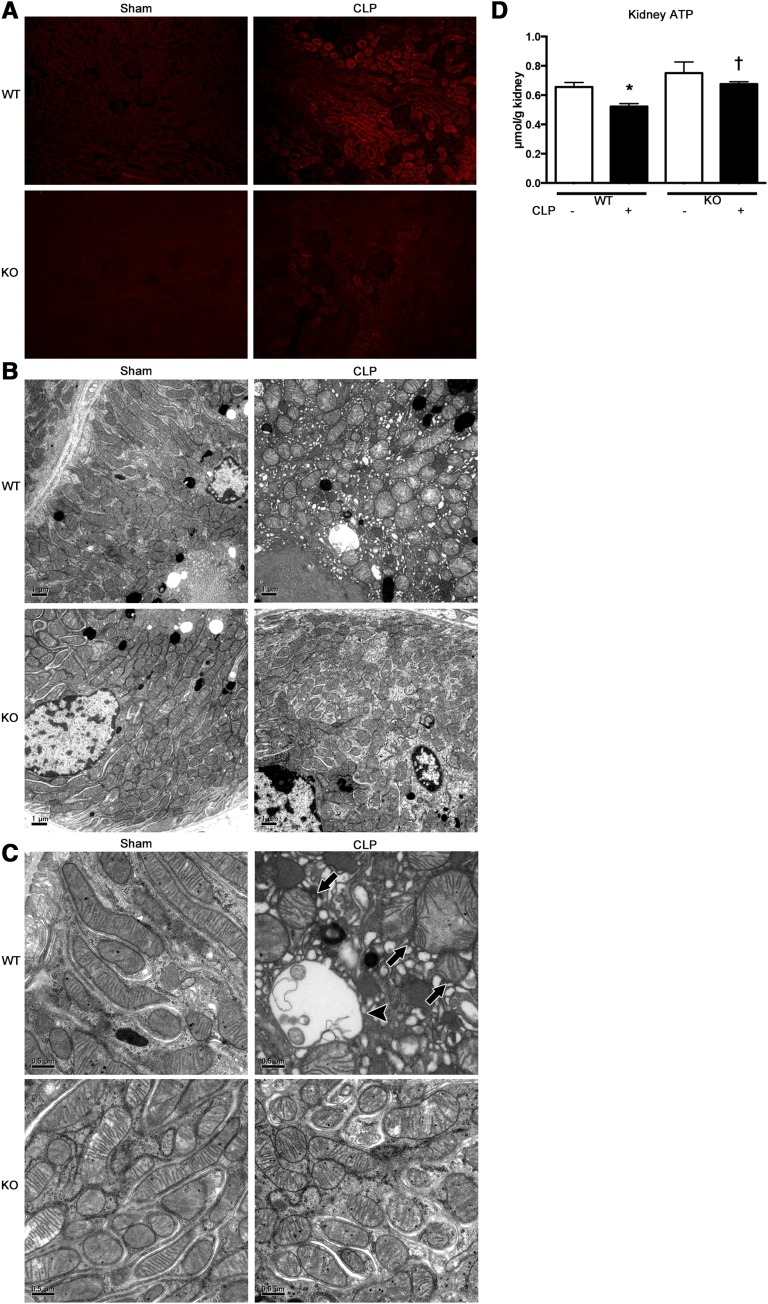
Effects of TLR9 on Mitochondrial Oxidative Stress and Injury in Proximal Tubules and Kidney ATP Levels after CLP
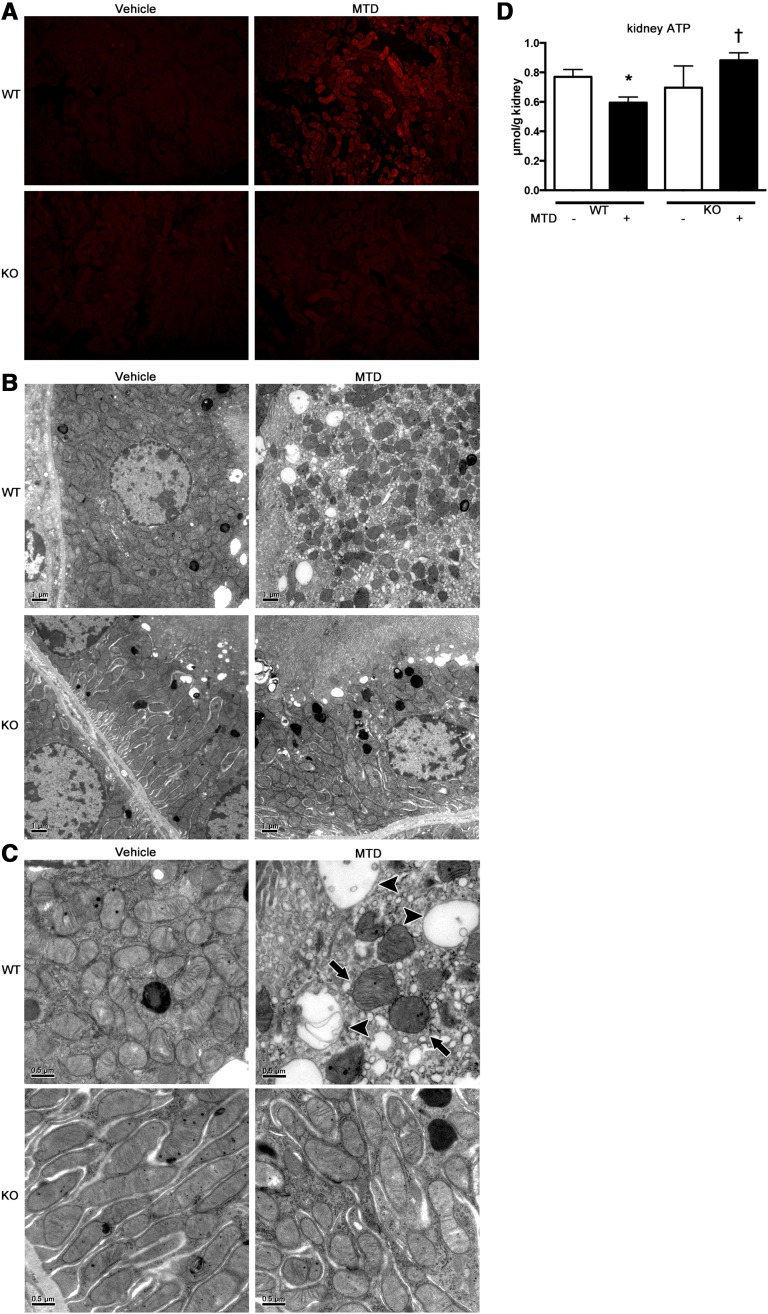
Production of superoxide in the proximal tubular cells was attenuated in Tlr9KO mice, as indicated by incorporation of MitoSOX, at 2 hours after CLP (Figure 6A). Furthermore, the mitochondria in the proximal tubular cells underwent dramatic morphologic changes at 2 hours after CLP, which were almost completely reversed by Tlr9KO. Representative electron microscopy images revealed that in WT mice, the mitochondria were commonly scattered, swollen, and rounded, and some mitochondria showed vacuolization as matrix swelling with loss of cristae membranes after CLP, but these effects were not observed in the Tlr9KO mice (Figure 6, B and C). Kidney ATP levels were significantly decreased at 2 hours after CLP and reversed in Tlr9KO (Figure 6D).
Figure 6.
Tlr9KO reduced kidney ATP depletion, mitochondrial oxidative stress and disruption in tubules at 2 hours after CLP. (A) Representative images (original magnification, ×200) of MitoSOX fluorescence microscopy of the proximal tubules after sham or CLP in WT or Tlr9KO mice. (B and C) Electron microscope images of the proximal tubules in sham-operated WT and KO mice showing elongated mitochondria with densely packed cristae membranes. The proximal tubules in WT mice after CLP showing scattered and swollen mitochondria (arrows) with damaged cristae or without cristae (arrowheads). These findings are representative of four mice per group. (D) Kidney ATP (n=4–8 per group). *P<0.05 versus each sham group; †P<0.05 versus WT. All bar graphs represent mean±SEM.
Renal Function and Cytokine Profiles after Administration of Mitochondrial Debris
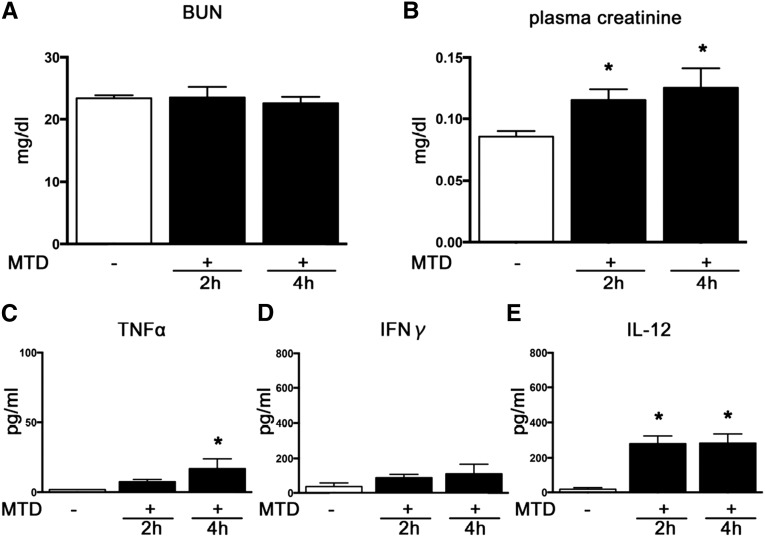
Administration of mitochondrial debris (MTD) increased plasma creatinine, but not BUN, over 2 and 4 hours (Figure 7, A and B). Plasma IL-12 levels increased at 2 and 4 hours, and plasma TNF-α levels increased at 4 hours after MTD administration (Figure 7, C–E). Five-fold concentrated MTD did not significantly increase plasma levels of creatinine, IL-12, TNF-α, and IFN-γ at 2 hours compared with one-fold concentrated MTD (data not shown).
Figure 7.
MTD caused slight but significant renal dysfunction and production of plasma IL-12 and TNFα. (A and B) BUN and plasma creatinine. (C–E) Plasma TNF-α, IFN-γ, and IL-12 (n=4–8 per group). *P<0.05 versus vehicle.
Effects of TLR9 on Cytokine Profiles and Splenic Apoptosis after Administration of MTD
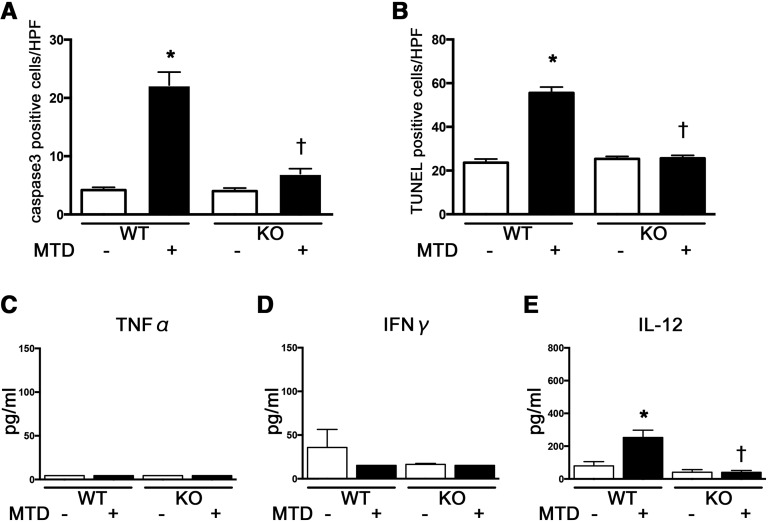
To confirm that circulating mtDNA directly contributes to the immune response during sepsis through TLR9, we intravenously injected mice with MTD that included mtDNA, which resulted in a profound increase in the number of active caspase 3-positive and TUNEL-positive cells in the white pulp of the spleen of WT mice at 2 hours; these increases in apoptotic cells were attenuated in Tlr9KO mice (Figure 8, A and B). We also evaluated the effects of TLR9 on the level of proinflammatory cytokines after MTD administration, which showed that plasma levels of IL-12, but not TNF-α and IFN-γ, were upregulated at 2 hours in WT mice. The increase in plasma IL-12 was reduced in Tlr9KO mice compared with that in WT mice (Figure 8, C–E).
Figure 8.
Tlr9KO reduced splenic apoptosis and production of plasma IL-12 at 2 hours after MTD administration. (A and B) active caspase 3- and TUNEL-positive cells by immunohistochemistry (n=4 per group). (C–E) Plasma TNF-α, IFN-γ, and IL-12 (n=4–8 per group). *P<0.05 versus each vehicle group; †P<0.05 versus WT. HPF, high-power field. All bar graphs represent mean±SEM.
Effects of TLR9 on Mitochondrial Oxidative Stress and Damage in Proximal Tubules and Kidney ATP Levels after MTD Administration
MTD induced mitochondrial oxidative stress, as detected by incorporation of MitoSOX (Figure 9A), and morphologic alterations, as detected by electron microscopy (Figure 9, B and C), in proximal tubular cells at 2 hours in WT mice. MTD also induced kidney ATP depletion (Figure 8D). These mitochondrial disorders were similar to the tubular injuries observed after CLP. Tlr9KO attenuated the renal mitochondrial disorders induced by MTD administration (Figure 9, A–D).
Figure 9.
MTD administration caused kidney ATP depletion, mitochondrial oxidative stress and disruption in tubules at 2 hours, which were reduced by Tlr9KO. (A) Representative images (original magnification, ×200) of MitoSOX fluorescence microscopy of the proximal tubules are shown. (B and C) Electron microscopy images of the proximal tubules after vehicle administration to WT or Tlr9KO mice, showing elongated mitochondria with densely packed cristae membranes. Proximal tubules in WT mice after MTD showed scattered and swollen mitochondria (arrows) with damaged cristae or without cristae (arrowheads). This mitochondrial damage was not observed in Tlr9KO mice after MTD administration. These findings are representative of four mice per group. (D) Kidney ATP (n=4–8 per group). *P<0.05 versus each vehicle group; †P<0.05 versus WT. All bar graphs represent mean±SEM.
Effect of Pretreatment with DNase on Circulating IL-12 and Renal Mitochondrial Injury after MTD Administration
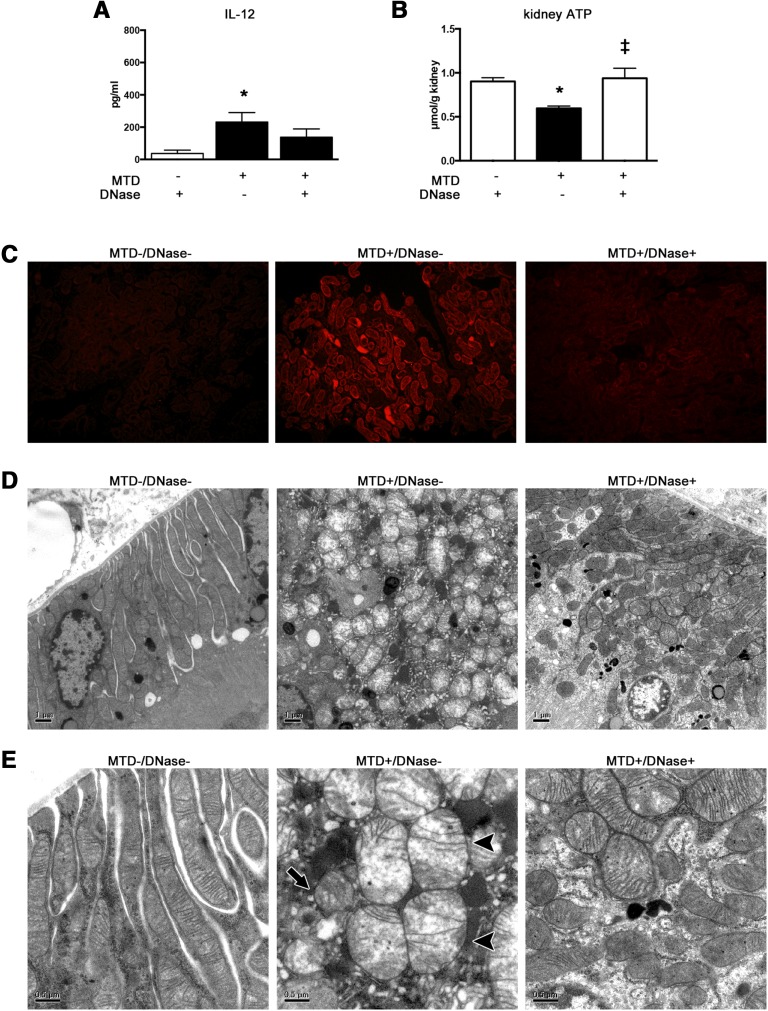
Plasma IL-12 tended to be decreased by pretreatment with deoxyribonuclease (DNase) for MTD, but there was no statistically significant difference between MTD and MTD plus DNase (Figure 10A). Increase of MitoSOX incorporation, mitochondrial deformities, and depletion of kidney ATP, which was induced by MTD, were improved by pretreatment with DNase (Figure 10, B–F).
Figure 10.
Pretreatment with DNase reduced production of plasma IL-12 and kidney ATP depletion, mitochondrial oxidative stress and disruption in tubules, which were caused by MTD. (A) Plasma IL-12. (B) Kidney ATP (n=4–8 per group). (C) Representative images (original magnification, ×200) of MitoSOX fluorescence microscopy of the proximal tubules. (D and E) Electron microscopy images of the proximal tubules after vehicle with DNase pretreatment. Proximal tubules after MTD showed scattered and swollen mitochondria (arrows) with damaged cristae or without cristae (arrowheads). This mitochondrial damage was hardly observed after administration of MTD with DNase pretreatment. These findings are representative of 4–6 mice per group. *P<0.05 versus vehicle; ‡P<0.05 versus MTD. All bar graphs represent mean±SEM.
Discussion
We evaluated the roles of mtDNA and TLR9 during the development of septic AKI. Endogenous mtDNA was released into the systemic circulation and peritoneal cavity in the early phase of the development of CLP-induced AKI. Tlr9KO increased mtDNA and leukocyte infiltration in the peritoneal cavity, with consequent reduction of bacteremia after CLP. Tlr9KO also attenuated CLP-induced proinflammatory cytokines and splenic apoptosis. CLP increased mitochondrial damage in the early phase of AKI, which were attenuated by Tlr9KO. Finally, administration of MTD, including substantial amounts of mtDNA, caused renal mitochondrial dysfunction and immune responses similar to those induced by CLP, which were also attenuated by Tlr9KO. These findings indicate that mtDNA could play important roles in dysregulation of immune response and cause renal mitochondrial dysfunction via the TLR9 pathway after CLP.
Endogenous mtDNA, which was released into the systemic circulation and peritoneal cavity at 2 hours after CLP, was maintained at high levels at least until 6 hours, and then reduced at 24 hours when the mice started to die.11,22,23 These findings indicate that mtDNA may play a role in the development of systematic inflammatory response syndrome. Plasma levels of mtDNA are elevated in patients with trauma, and mtDNA released from shock-injured tissues induced neutrophil activation and contributed to systematic inflammatory response syndrome, probably via TLR9, as revealed in trauma models.12,18,24 However, it remains controversial as to whether the level of mtDNA is elevated and related to death in patients with sepsis.24–27
Here, we first showed that the levels of mtDNA are elevated in the systemic circulation and peritoneal cavity from the early phase of polymicrobial peritonitis but not endotoxemia. Our findings suggest that the circulating mtDNA originated, at least in part, from the inflammatory cells that migrated to the peritoneal cavity to attack the invading bacteria. More dead leukocytes were identified in the peritoneal cavity after CLP in Tlr9KO mice compared with sham. The amount of dead cells in the peritoneal cavity are compatible with mtDNA levels in the peritoneal cavity, suggesting that mtDNA in peritoneal cavity could be released from infiltrated dead leukocytes. In addition to the inflammatory site, the free mtDNA might also be provided from injured organs, as indicated by splenic apoptosis and mitochondrial disorders in renal tubular cells. Indeed, a transient decrease in kidney mtDNA was found during sepsis.28 Thus, the origin and supply route of circulating mtDNA in septic AKI might be multifocal and complicated.
Our study also provides insight into the effects of TLR9 on the dynamics of mtDNA in the plasma and peritoneal cavity and on the immune response after CLP. Plasma mtDNA levels tended to be decreased in Tlr9KO mice compared with WT mice, but the difference was not statistically significant. mtDNA in the peritoneal cavity was significantly increased in Tlr9KO mice compared with WT mice at 2 hours after CLP. As mentioned earlier, this accumulation of mtDNA might have been caused by a concentrated attack of immunocytes to the localized infection site. The total and dead leukocyte counts in the peritoneal cavity and bacterial clearance after CLP were also increased in Tlr9KO mice.
These findings are consistent with those of previous studies.13,14 In particular, Plitas et al. demonstrated that TLR9-deficient dendritic cells (DCs) played an important role in increasing granulocyte trafficking to the peritoneal fluid and in promoting survival after CLP,13 although TLR9 is exclusively expressed in immune cells, such as DCs and B cells.29 Direct activation of their own TLR9 by circulating neutrophils contributes to chemokine receptor desensitization and thus impairs their ability to migrate to the site of infection during severe sepsis,14 Tlr9KO also attenuated the burst of proinflammatory cytokines in the early phase of AKI development and splenic apoptosis, which were upregulated after CLP. These findings are also in line with previous evidence11 indicating that reduction of proinflammatory cytokines and splenic apoptosis by inhibition of the TLR9 pathway contributed to improved survival after CLP. A high degree of splenic apoptosis is a hallmark of immune dysfunction with resultant mortality during sepsis.29–32 These data taken together indicate that Tlr9KO contributes to leukocyte trafficking to site of infection and reduction of splenic apoptosis, with resultant removal of bacteria, and appears to harmonize the sepsis-induced immune response.
Renal dysfunction was improved in Tlr9KO mice at 24 hours after CLP. We also found evidence of mitochondrial oxidative stress by incorporation of MitoSOX, morphologic abnormality by electron microscopy in the proximal tubules, and kidney ATP depletion at 2 hours after CLP. The mitochondrial deformities, such as mitochondrial matrix swelling and unfolding of cristae membranes, have also been reported in an LPS-treated model33 and a renal ischemia-reperfusion model.34 These mitochondrial damages were attenuated by Tlr9KO. Mitochondrial oxidant generation in septic AKI plays an important role in renal tubular injury during sepsis, manifested as ischemic injury.15,16,35–37 Interestingly, mitochondria-targeted antioxidants could attenuate CLP-induced AKI, independent of systemic blood pressure.17 Our findings showed that TLR9 deficiency helped to prevent renal mitochondrial injury and the subsequent decrease in renal function after CLP. However, it is not clear whether the TLR9 pathway induced tubular dysfunction directly. Under normal conditions, TLR9 is detected on DCs in the interstitium, but not on tubular epithelial cells.8,38,39 A local immune response induced by resident immune cells expressing TLR9 such as DCs, or systemic cytokines induced by the TLR9 pathway in the peritoneal or systemic reticuloendothelial system, might contribute to renal mitochondrial dysfunction. On the other hand, Liu et al. recently reported that TLR9 was slightly expressed in tubular epithelial and glomerular cells at 24 hours after CLP.40 A recent study showed that TLR9 reduced mitochondrial ATP synthesis by alteration of mitochondrial calcium ion handling in cardiomyocytes.41 This mechanism is consistent with our findings that Tlr9KO prevented kidney ATP from its depletion after CLP. However, it remains unknown whether this late and slight expression of TLR9 on tubular cells after CLP is related to early tubular mitochondrial dysfunction. There are at least two possible approaches for investigating whether these cells can transduce the signal from MTD to the tubular epithelial cells. One is to administer MTD to bone-marrow chimeric mice to rule out a role for nonimmune TLR9. Another is to compare the effects of WT and Tlr9KO DCs in Tlr9KO recipients.
To elucidate the effects of mtDNA on kidney injury and the systemic immune system, MTD including mtDNA was administered to WT and Tlr9KO mice. MTD increased plasma IL-12 levels and splenic apoptosis, which were attenuated by Tlr9KO. These findings indicated that circulating mtDNA could induce proinflammatory cytokines and splenic apoptosis via the TLR9 pathway. MTD increased plasma IL-12 at 2 hours and slightly increased TNF-α at 4 hours but not plasma IFN-γ, indicating that IL-12 might play a central role in MTD-induced innate immunity. IL-12 is produced by DCs soon after TLR9 stimulation.28 The phase of protective innate immune status starts a few days after the initial TLR9 activation by natural killer cells and certain DC subsets to induce IFN-γ secretion.28,42,43 In addition, IL-12 causes T cells to differentiate into T helper type-1 cells, which are recruited to the sites of inflammation and produce IFN-γ.44 It is controversial whether MTD-induced upregulation of IL-12 caused renal injury. Renal IL-12 contributes in part to ischemia-reperfusion tubular injury45 and has the potential to be critical for renal injury. Moreno et al.46 showed that IL-12–deficient mice were susceptible to sublethal CLP. IL-12 also has beneficial effects on survival in the face of infections.47,48 Thus, IL-12 might be required to some degree for survival during infections. The role of IL-12 on MTD-induced kidney injury should be clarified with further studies.
MTD induced mitochondrial dysfunction in the tubules and kidney ATP depletion, which were attenuated by Tlr9KO. These findings indicate that circulating mtDNA could cause renal mitochondrial dysfunction via the TLR9 pathway. Zhang et al. also demonstrated that administration of MTD caused oxidative lung injury with leukocyte infiltration at 1 hour.18 Together, these findings suggest that mtDNA might be a key factor contributing to multiorgan dysfunction syndromes. MTD includes another damage-associated molecular pattern, formyl peptide, to activate PMNs in addition to mtDNA.18 The pretreatment with DNase abolished MTD-induced renal mitochondrial injury. These results support the theory that mtDNA could be the main damage-associated molecular pattern in MTD, as well as that immune responses, including elevated plasma IL-12 and splenic apoptosis, and the tubular mitochondrial dysfunction observed after administration of MTD, depend on TLR9.
In conclusion, circulating mtDNA is released from the early phase of sepsis and contributes to cytokine production, splenic apoptosis, and tubular mitochondrial disorders via TLR9. Thus, mtDNA could be a therapeutic target and useful biomarker for determining disease severity in septic AKI.
Concise Methods
Animals
All animal protocols were approved by the Ethics Review Committee for Animal Experimentation of Hamamatsu University School of Medicine. C57BL/6J mice were obtained from CLEA Japan Inc. (Tokyo, Japan). Tlr9KO mice were obtained from Oriental BioService, Inc. (Kyoto, Japan).
CLP
CLP was performed on 10- to 11-week-old male C57BL/6 and Tlr9KO mice on 37°C heated pads under 5% sevoflurane for induction and 3% sevoflurane for maintenance of anesthesia. After laparotomy, a 2-0 silk ligature was placed 1 cm from the cecal tip. The cecum was punctured twice with a 21-gauge needle and gently squeezed to express a 1-mm column of cecal material. Sham operations were identical to the surgery, except for CLP, respectively. Prewarmed normal saline (NS; 1.5 ml) was injected intraperitoneally (ip) just after CLP and sham surgery. Animals were euthanized at 2, 6, and 24 hours after CLP for collecting blood and tissue specimens and peritoneal fluid.
LPS Administration
LPS from Escherichia coli O111:B4 (Sigma-Aldrich, St. Louis, MO) dissolved in NS (1 mg/ml, ip) was administered at a concentration of 10 mg/kg. Animals were euthanized at 2 or 24 hours after administration of LPS for collecting blood.
MTD Administration
We modified the method of Zhang et al18 to prepare MTD. The Mitochondria Isolation Kit for Tissue (Pierce, Rockford, IL) was used to isolate the mitochondria from the mouse liver. Mitochondria were isolated under sterile conditions at 4°C. Isolated mitochondrial pellets from the liver (200 mg) were suspended in 500 μl of HBSS with protease inhibitor cocktail (1:100) and sonicated on ice. The disrupted mitochondrial suspensions were centrifuged and used for the experiments. The 10- to 11-week-old male C57BL/6 and Tlr9KO mice were intravenously given MTD (200 μl) 0 and 1 hour later. Animals were euthanized at 2 or 4 hours after administration of MTD for collecting blood and tissue specimens.
DNase Treatment
Recombinant DNase 1 (TAKARA Bio Inc.; Shiga, Japan) was added to MTD for degradation of mtDNA and incubated for 1 hour at 37°C. ND1, CytB, and COX3 were hardly detected by PCR in MTD with DNase pretreatment.
Quantitation of Plasma and peritoneal mtDNA
Plasma and peritoneal DNA was prepared using the QIAamp DNA Mini and Blood Mini kit (Qiagen, Germantown, MD). Primers for mouse Nd1 (forward 5′- ggatccgagcatcttatcca-3′ and reverse 5′- ggtggtactcccgctgtaaa-3′) and mouse CytB (forward 5′-tgagggggcttctcagtaga-3′ and reverse 5′-ctgtttcgtggaggaagagg-3′), and mouse COX3 (forward 5′- cgtgaaggaaactacccagg-3′ and reverse 5′- cgctcagaagaatcctgcaa-3′) were synthesized by Biologica Co. (Nagoya, Japan). The primer sequences have no significant homology with DNA found in any bacterial species published on basic local alignment search tool. Real-time PCR standard curves were created and used for spectrophotometric determination of the concentration of mtDNA extracted from the isolated mitochondria of liver tissues using DNeasy Blood & Tissue kit (Qiagen) and purified by QIA quick PCR Purification Kit (Qiagen) after PCR by each primer. Real-time quantitative PCR was carried out in an Applied Biosystems StepOnePlus system using the following program: 95°C for 10 minutes, followed by 40 cycles at 95°C for 15 seconds, and 60 seconds at the primer-specific annealing temperature (58°C for Nd1 and CytB, 56°C for Cox3). The concentration of mtDNA is expressed as copies per microliter.
Leukocytes Count and Viability in the Peritoneal Cavity
The animals were euthanized at 2, 6, and 24 hours after CLP, NS (2 ml) was ip injected, and peritoneal fluid was collected. The peritoneal fluid was centrifuged, and the pellet was collected. Total cells were counted on a hemocytometer in the presence of 0.4% trypan blue (Sigma-Aldrich). In terms of viability experiment, total cells and dead cells were counted by Luna (Logos Biosystems, Annandale, VA).
Bacterial Count
Peritoneal exudate after NS (2 ml) was injected into the peritoneal cavity, and blood specimens were collected under sterile conditions. Serial dilutions of these samples were plated on Drigalski agar, modified dishes (Japan BD, Tokyo, Japan) and incubated at 37°C. The number of CFU was recorded after 18 hours of culture.
Splenic Apoptosis
The presence of apoptosis was assessed by activated caspase 3 and TUNEL staining as described previously.10,11,49 Immunohistochemical staining of 3-μm paraffin sections was performed with anti-caspase 3 antibody (Cell Signaling Technology, Danvers, MA). The TUNEL technique was performed with ApopTag Plus Peroxidase In Situ Apoptosis Kit (EMD Millipore, Billerica, MA). The number of positive cells was determined from the mean of five randomly selected 400×-magnified fields in each section.
Renal Function
Plasma creatinine and BUN levels were measured by enzymatic assays conducted by FALCO Biosystems Ltd. (Kyoto, Japan).
Cytokines
Plasma TNF-α, IFN-γ, and IL-12 levels were measured by ELISA (R&D Systems, Minneapolis, MN).
Renal Histologic Analysis by Light Microscopy
Whole kidney samples were fixed in 10% formalin and embedded in paraffin. Three-micrometer sections were stained with periodic acid-Schiff reagent. Because the predominant marker of tubular damage is vacuolization of the cortex, histologic damage was defined as tubular vacuolar degeneration in the cortex. We adopted the following evaluation method as reported previously.22 The degree of kidney damage was estimated at 400× magnification using >50 randomly selected tubules for each animal by the following the criteria: 0, normal; 1, area of damage 25% of tubules; 2, damage involving 25%–50% of tubules; 3, damage involving 50%–75% of tubules; 4, 75%–100% of tubules affected.
MitoSOX Fluorescence
Following incubation with MitoSOX as reported previously,50 kidney slices were rinsed in PBS, fixed in 10% formalin, and embedded in paraffin; 3-μm sections were observed under a fluorescence microscope.
Electron Microscopy
Small portions of cortical kidney tissues were fixed in 2% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) at 4°C for 2 hours, postfixed in 1% osmium tetroxide resolved in the same buffer for 1 hour, and then embedded in Quetol 812. Ultrathin sections including proximal tubular cells observed by semithin sections were counterstained with uranyl acetate and lead citrate and then examined under an electron microscope (JEM-1220, JEOL, Tokyo, Japan).
ATP Assay
A tissue ATP assay kit (Toyo Ink Co., Ltd., Tokyo, Japan) was used to measure ATP levels in the kidney tissue lysates. Luminescence was measured using a Lumicounter NU-700 (Microtec Co., Ltd., Chiba, Japan).
Statistical Analyses
The results are expressed as mean±SEM. Differences among groups were analyzed by the t test or ANOVA. These calculations were performed using GraphPad Prism 5 (GraphPad Software Inc., San Diego, CA). A P value <0.05 was considered as representing a statistically significant difference.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Dr. Kunihiro Yamagata and Dr. Masahiro Hagiwara (University of Tsukuba) and Dr. Moriya Iwaizumi (Internal Medicine 1, Hamamatsu University School of Medicine) for technical support. We thank Dr. Robert A. Star and Dr. Peter S.T. Yuen (National Institute of Diabetes and Digestive and Kidney Disease, National Institutes of Health) for their comments and suggestions.
This work was supported by Grant-in-Aid for Scientific Research (C) grant 24591194 (to H.Y.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Mitochondria in Kidney Injury: When the Power Plant Fails,” on pages 1869–1872.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015040376/-/DCSupplemental.
References
- 1.Okusa MD: The changing pattern of acute kidney injury: From one to multiple organ failure. Contrib Nephrol 165: 153–158, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators : Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA 294: 813–818, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Bagshaw SM, Laupland KB, Doig CJ, Mortis G, Fick GH, Mucenski M, Godinez-Luna T, Svenson LW, Rosenal T: Prognosis for long-term survival and renal recovery in critically ill patients with severe acute renal failure: A population-based study. Crit Care 9: R700–R709, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yasuda H, Kato A, Fujigaki Y, Hishida A Shizuoka Kidney Disease Study Group : Incidence and clinical outcomes of acute kidney injury requiring renal replacement therapy in Japan. Ther Apher Dial 14: 541–546, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Bagshaw SM, Lapinsky S, Dial S, Arabi Y, Dodek P, Wood G, Ellis P, Guzman J, Marshall J, Parrillo JE, Skrobik Y, Kumar A Cooperative Antimicrobial Therapy of Septic Shock (CATSS) Database Research Group : Acute kidney injury in septic shock: Clinical outcomes and impact of duration of hypotension prior to initiation of antimicrobial therapy. Intensive Care Med 35: 871–881, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Bagshaw SM, Uchino S, Bellomo R, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Oudemans-van Straaten HM, Ronco C, Kellum JA Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators : Septic acute kidney injury in critically ill patients: Clinical characteristics and outcomes. Clin J Am Soc Nephrol 2: 431–439, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B: Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: Mutations in Tlr4 gene. Science 282: 2085–2088, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S: A Toll-like receptor recognizes bacterial DNA. Nature 408: 740–745, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Doi K, Leelahavanichkul A, Yuen PST, Star RA: Animal models of sepsis and sepsis-induced kidney injury. J Clin Invest 119: 2868–2878, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dear JW, Yasuda H, Hu X, Hieny S, Yuen PST, Hewitt SM, Sher A, Star RA: Sepsis-induced organ failure is mediated by different pathways in the kidney and liver: Acute renal failure is dependent on MyD88 but not renal cell apoptosis. Kidney Int 69: 832–836, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yasuda H, Leelahavanichkul A, Tsunoda S, Dear JW, Takahashi Y, Ito S, Hu X, Zhou H, Doi K, Childs R, Klinman DM, Yuen PST, Star RA: Chloroquine and inhibition of Toll-like receptor 9 protect from sepsis-induced acute kidney injury. Am J Physiol Renal Physiol 294: F1050–F1058, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Q, Itagaki K, Hauser CJ: Mitochondrial DNA is released by shock and activates neutrophils via p38 map kinase. Shock 34: 55–59, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Plitas G, Burt BM, Nguyen HM, Bamboat ZM, DeMatteo RP: Toll-like receptor 9 inhibition reduces mortality in polymicrobial sepsis. J Exp Med 205: 1277–1283, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trevelin SC, Alves-Filho JC, Sônego F, Turato W, Nascimento DC, Souto FO, Cunha TM, Gazzinelli RT, Cunha FQ: Toll-like receptor 9 activation in neutrophils impairs chemotaxis and reduces sepsis outcome. Crit Care Med 40: 2631–2637, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Pathak E, MacMillan-Crow LA, Mayeux PR: Role of mitochondrial oxidants in an in vitro model of sepsis-induced renal injury. J Pharmacol Exp Ther 340: 192–201, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quoilin C, Mouithys-Mickalad A, Lécart S, Fontaine-Aupart MP, Hoebeke M: Evidence of oxidative stress and mitochondrial respiratory chain dysfunction in an in vitro model of sepsis-induced kidney injury. Biochimica et Biophysica Acta (BBA) Bioenergetics 1837: 1790–1800, 2014 [DOI] [PubMed] [Google Scholar]
- 17.Patil NK, Parajuli N, MacMillan-Crow LA, Mayeux PR: Inactivation of renal mitochondrial respiratory complexes and manganese superoxide dismutase during sepsis: mitochondria-targeted antioxidant mitigates injury. Am J Physiol Renal Physiol 306: F734–F743, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ: Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464: 104–107, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wesche-Soldato DE, Chung C-S, Lomas-Neira J, Doughty LA, Gregory SH, Ayala A: In vivo delivery of caspase-8 or Fas siRNA improves the survival of septic mice. Blood 106: 2295–2301, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hotchkiss RS, Chang KC, Grayson MH, Tinsley KW, Dunne BS, Davis CG, Osborne DF, Karl IE: Adoptive transfer of apoptotic splenocytes worsens survival, whereas adoptive transfer of necrotic splenocytes improves survival in sepsis. Proc Natl Acad Sci U S A 100: 6724–6729, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gigliotti JC, Okusa MD: The spleen: The forgotten organ in acute kidney injury of critical illness. Nephron Clin Pract 127: 153–157, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yasuda H, Yuen PST, Hu X, Zhou H, Star RA: Simvastatin improves sepsis-induced mortality and acute kidney injury via renal vascular effects. Kidney Int 69: 1535–1542, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyaji T, Hu X, Yuen PST, Muramatsu Y, Iyer S, Hewitt SM, Star RA: Ethyl pyruvate decreases sepsis-induced acute renal failure and multiple organ damage in aged mice. Kidney Int 64: 1620–1631, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Yamanouchi S, Kudo D, Yamada M, Miyagawa N, Furukawa H, Kushimoto S: Plasma mitochondrial DNA levels in patients with trauma and severe sepsis: time course and the association with clinical status. J Crit Care 28: 1027–1031, 2013 [DOI] [PubMed] [Google Scholar]
- 25.Puskarich MA, Shapiro NI, Trzeciak S, Kline JA, Jones AE: Plasma levels of mitochondrial DNA in patients presenting to the emergency department with sepsis. Shock 38: 337–340, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakahira K, Kyung S-Y, Rogers AJ, Gazourian L, Youn S, Massaro AF, Quintana C, Osorio JC, Wang Z, Zhao Y, Lawler LA, Christie JD, Meyer NJ, Mc Causland FR, Waikar SS, Waxman AB, Chung RT, Bueno R, Rosas IO, Fredenburgh LE, Baron RM, Christiani DC, Hunninghake GM, Choi AMK: Circulating mitochondrial DNA in patients in the ICU as a marker of mortality: Derivation and validation. PLoS Med 10: e1001577–, discussion e1001577., 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garrabou G, Morén C, López S, Tobías E, Cardellach F, Miró O, Casademont J: The effects of sepsis on mitochondria. J Infect Dis 205: 392–400, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Bartz RR, Fu P, Suliman HB, Crowley SD, MacGarvey NC, Welty-Wolf K, Piantadosi CA: Staphylococcus aureus Sepsis Induces Early Renal Mitochondrial DNA Repair and Mitochondrial Biogenesis in Mice. PLoS ONE 9: e100912, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ishii KJ, Akira S: Innate immune recognition of, and regulation by, DNA. Trends Immunol 27: 525–532, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Qin S, Wang H, Yuan R, Li H, Ochani M, Ochani K, Rosas-Ballina M, Czura CJ, Huston JM, Miller E, Lin X, Sherry B, Kumar A, Larosa G, Newman W, Tracey KJ, Yang H: Role of HMGB1 in apoptosis-mediated sepsis lethality. J Exp Med 203: 1637–1642, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ayala A, Chung CS, Xu YX, Evans TA, Redmond KM, Chaudry IH: Increased inducible apoptosis in CD4+ T lymphocytes during polymicrobial sepsis is mediated by Fas ligand and not endotoxin. Immunology 97: 45–55, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tinsley KW, Grayson MH, Swanson PE, Drewry AM, Chang KC, Karl IE, Hotchkiss RS: Sepsis induces apoptosis and profound depletion of splenic interdigitating and follicular dendritic cells. J Immunol 171: 909–914, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Hotchkiss RS, Tinsley KW, Swanson PE, Schmieg RE Jr, Hui JJ, Chang KC, Osborne DF, Freeman BD, Cobb JP, Buchman TG, Karl IE: Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J Immunol 166: 6952–6963, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Tran M, Tam D, Bardia A, Bhasin M, Rowe GC, Kher A, Zsengeller ZK, Akhavan-Sharif MR, Khankin EV, Saintgeniez M, David S, Burstein D, Karumanchi SA, Stillman IE, Arany Z, Parikh SM: PGC-1α promotes recovery after acute kidney injury during systemic inflammation in mice. J Clin Invest 121: 4003–4014, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szeto HH, Liu S, Soong Y, Birk AV: Improving mitochondrial bioenergetics under ischemic conditions increases warm ischemia tolerance in the kidney. Am J Physiol Renal Physiol 308: F11–F21, 2015 [DOI] [PubMed] [Google Scholar]
- 36.Wang Z, Holthoff JH, Seely KA, Pathak E, Spencer HJ 3rd, Gokden N, Mayeux PR: Development of oxidative stress in the peritubular capillary microenvironment mediates sepsis-induced renal microcirculatory failure and acute kidney injury. Am J Pathol 180: 505–516, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seija M, Baccino C, Nin N, Sánchez-Rodríguez C, Granados R, Ferruelo A, Martínez-Caro L, Ruíz-Cabello J, de Paula M, Noboa O, Esteban A, Lorente JÁ: Role of peroxynitrite in sepsis-induced acute kidney injury in an experimental model of sepsis in rats. Shock 38: 403–410, 2012 [DOI] [PubMed] [Google Scholar]
- 38.Nilakantan V, Liang HL, Rajesh S, Mortensen J, Chandran K: Time-dependant protective effects of mangenese(III) tetrakis (1-methyl-4-pyridyl) porphyrin on mitochondrial function following renal ischemia-reperfusion injury. Free Radic Res 44: 773–782, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Anders H-J, Banas B, Schlöndorff D: Signaling danger: Toll-like receptors and their potential roles in kidney disease. J Am Soc Nephrol 15: 854–867, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Anders H-J, Banas B, Linde Y, Weller L, Cohen CD, Kretzler M, Martin S, Vielhauer V, Schlöndorff D, Gröne H-J: Bacterial CpG-DNA aggravates immune complex glomerulonephritis: Role of TLR9-mediated expression of chemokines and chemokine receptors. J Am Soc Nephrol 14: 317–326, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Liu L, Li Y, Hu Z, Su J, Huo Y, Tan B, Wang X, Liu Y: Small interfering RNA targeting Toll-like receptor 9 protects mice against polymicrobial septic acute kidney injury. Nephron, Exp Nephrol 122: 51–61, 2012 [DOI] [PubMed] [Google Scholar]
- 42.Shintani Y, Drexler HCA, Kioka H, Terracciano CMN, Coppen SR, Imamura H, Akao M, Nakai J, Wheeler AP, Higo S, Nakayama H, Takashima S, Yashiro K, Suzuki K: Toll-like receptor 9 protects non-immune cells from stress by modulating mitochondrial ATP synthesis through the inhibition of SERCA2. EMBO Rep 15: 438–445, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pillarisetty VG, Katz SC, Bleier JI, Shah AB, Dematteo RP: Natural killer dendritic cells have both antigen presenting and lytic function and in response to CpG produce IFN-gamma via autocrine IL-12. J Immunol 174: 2612–2618, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Chan CW, Crafton E, Fan H-N, Flook J, Yoshimura K, Skarica M, Brockstedt D, Dubensky TW, Stins MF, Lanier LL, Pardoll DM, Housseau F: Interferon-producing killer dendritic cells provide a link between innate and adaptive immunity. Nat Med 12: 207–213, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Boehm U, Klamp T, Groot M, Howard JC: Cellular responses to interferon-gamma. Annu Rev Immunol 15: 749–795, 1997 [DOI] [PubMed] [Google Scholar]
- 46.Köken T, Serteser M, Kahraman A, Akbulut G, Dilek ON: Which is more effective in the prevention of renal ischemia-reperfusion-induced oxidative injury in the early period in mice: interleukin (IL)-10 or anti-IL-12? Clin Biochem 37: 50–55, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Moreno SE, Alves-Filho JC, Alfaya TM, da Silva JS, Ferreira SH, Liew FY: IL-12, but not IL-18, is critical to neutrophil activation and resistance to polymicrobial sepsis induced by cecal ligation and puncture. J Immunol 177: 3218–3224, 2006 [DOI] [PubMed] [Google Scholar]
- 48.Göebel A, Kavanagh E, Lyons A, Saporoschetz IB, Soberg C, Lederer JA, Mannick JA, Rodrick ML: Injury induces deficient interleukin-12 production, but interleukin-12 therapy after injury restores resistance to infection. Ann Surg 231: 253–261, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khan AA, Priya S, Saha B: IL-2 regulates SEB induced toxic shock syndrome in BALB/c mice. PLoS One 4: e8473, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsuji T, Kato A, Yasuda H, Miyaji T, Luo J, Sakao Y, Ito H, Fujigaki Y, Hishida A: The dimethylthiourea-induced attenuation of cisplatin nephrotoxicity is associated with the augmented induction of heat shock proteins. Toxicol Appl Pharmacol 234: 202–208, 2009 [DOI] [PubMed] [Google Scholar]
- 51.Huang L, Belousova T, Chen M, DiMattia G, Liu D, Sheikh-Hamad D: Overexpression of stanniocalcin-1 inhibits reactive oxygen species and renal ischemia/reperfusion injury in mice. Kidney Int 82: 867–877, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.