Abstract
Home dialysis, which comprises peritoneal dialysis (PD) or home hemodialysis (home HD), offers patients with ESRD greater flexibility and independence. Although ESRD disproportionately affects racial/ethnic minorities, data on disparities in use and outcomes with home dialysis are sparse. We analyzed data of patients who initiated maintenance dialysis between 2007 and 2011 and were admitted to any of 2217 dialysis facilities in 43 states operated by a single large dialysis organization, with follow-up through December 31, 2011 (n=162,050, of which 17,791 underwent PD and 2536 underwent home HD for ≥91 days). Every racial/ethnic minority group was significantly less likely to be treated with home dialysis than whites. Among individuals treated with in-center HD or PD, racial/ethnic minorities had a lower risk for death than whites; among individuals undergoing home HD, only blacks had a significantly lower death risk than whites. Blacks undergoing PD or home HD had a higher risk for transfer to in-center HD than their white counterparts, whereas Asians or others undergoing PD had a lower risk than whites undergoing PD. Blacks irrespective of dialysis modality, Hispanics undergoing PD or in-center HD, and Asians and other racial groups undergoing in-center HD were significantly less likely than white counterparts to receive a kidney transplant. In conclusion, there are racial/ethnic disparities in use of and outcomes with home dialysis in the United States. Disparities in kidney transplantation evident for blacks and Hispanics undergoing home dialysis are similar to those with in-center HD. Future studies should identify modifiable causes for these disparities.
Keywords: end-stage renal disease, hemodialysis, peritoneal dialysis
The treatment of ESRD with maintenance dialysis requires patients to make substantial and sustained changes to their lifestyle to accommodate the dialysis schedule. The magnitude of effect on patients’ lives is amplified by the high burden of disease, because many of these individuals also have multimorbidity, experience frequent care transitions, and have a high pill burden.1,2 The effect can be mitigated by patients selecting a dialysis modality that best meets their lifestyle needs and expectations, because hemodialysis (HD) can be performed either in center or at home and peritoneal dialysis (PD) can be performed at home.3 In the last 5 years, the number of patients undergoing home dialysis in the United States has increased by >50%.4 Given that racial/ethnic minorities comprise a substantially larger proportion of patients with ESRD than the general population, this increasing use of home dialysis makes it imperative to examine racial/ethnic differences in the use of and outcomes with PD and home HD.
Data from the national registry of patients on dialysis indicate that racial/ethnic minorities are less likely to be treated with home dialysis (PD or home HD).1,5 Given the disparate effects of each dialysis modality on patients’ lifestyle, it is important to ensure that this represents informed choice by patients or is dictated by their clinical condition rather than resulting from disparities in access to home dialysis. However, no study has examined whether the lower use of home dialysis by racial/ethnic minorities reflects differences in age, body size, or overall health, each of which is an important determinant of an individuals’ ability to engage in self-care dialysis. Furthermore, there is substantial paucity of data for racial/ethnic differences in outcomes with home dialysis, such as the risk for death or transfer to in-center HD.6–9 There are no such data for Hispanics or Asians treated with PD and outcomes by race/ethnicity for any population of patients treated with home HD.
In addition, racial/ethnic minorities are less likely to receive a kidney transplant.1,10 Patients undergoing home dialysis are healthier, have better social support and socioeconomic status, and assume greater responsibility for their own care.3,11,12 It is, thus, reasonable to postulate that racial/ethnic disparities in transplantation rates may not exist among individuals undergoing PD or home HD. However, this question has thus far not been examined.
Using data from a large dialysis provider in the United States, we undertook this study to test two hypotheses: (1) in patients starting maintenance dialysis, the underuse of home dialysis by racial/ethnic minorities cannot be explained by differences in age and health status; and (2) as with patients undergoing in-center HD, there are significant racial/ethnic differences in all-cause mortality, transfer to in-center HD, and kidney transplantation in individuals undergoing home dialysis.
Results
Study Population
Between January 1, 2007 and December 31, 2011, 162,664 patients started maintenance dialysis in 2217 facilities operated by a single dialysis provider in 43 states and the District of Columbia. The analytic cohort comprised 162,050 patients with known race/ethnicity; this included 17,791 patients who underwent PD in 953 dialysis facilities and 2536 patients who underwent home HD in 423 dialysis facilities (Supplemental Figure 1). This also included 131 patients who were treated with PD and home HD at separate time points. Patient characteristics for the cohort stratified by race/ethnicity are summarized in Table 1. White patients were older and more likely to be men than other racial/ethnic groups. Black patients were more likely to have hypertension, and Hispanics were more likely to have diabetes.
Table 1.
Characteristics of patients who initiated dialysis between 2007 and 2011 in participating dialysis facilities at the time of initiation of dialysis stratified by race/ethnicity
| Variable | White | Black | Hispanic | Asian | Other |
|---|---|---|---|---|---|
| No. of subjects | 79,546 | 48,089 | 23,147 | 5538 | 5730 |
| Initial modality, % | |||||
| In-center HD | 90 | 94 | 92 | 89 | 91 |
| PD | 9 | 6 | 7 | 10 | 8 |
| Home HD | 1 | <1 | <1 | <1 | <1 |
| Ever treated, % | |||||
| PD | 13 | 9 | 10 | 13 | 10 |
| Home HD | 2 | 1 | 1 | 1 | 1 |
| Age, yr | 65±15 | 57±15 | 58±15 | 62±16 | 60±15 |
| Men, % | 59 | 52 | 58 | 56 | 56 |
| Primary health insurance, % | |||||
| Medicare | 58 | 51 | 47 | 46 | 50 |
| Medicaid | 4 | 7 | 13 | 9 | 7 |
| Initially uninsured | 3 | 4 | 5 | 4 | 5 |
| Veterans Affairs | 1 | 1 | 1 | <1 | 1 |
| Othera | 35 | 37 | 34 | 41 | 37 |
| Cause of ESRD, % | |||||
| Diabetes | 42 | 42 | 59 | 47 | 55 |
| Hypertension | 26 | 39 | 22 | 29 | 23 |
| Glomerular disease | 12 | 10 | 8 | 11 | 9 |
| Other | 20 | 10 | 11 | 13 | 13 |
| H/o previous transplant, % | 2 | 1 | 1 | 2 | 1 |
| Comorbidities, % | |||||
| Diabetes | 54 | 56 | 66 | 56 | 64 |
| Hypertension | 49 | 61 | 44 | 47 | 44 |
| Congestive heart failure | 34 | 35 | 35 | 32 | 35 |
| Atherosclerotic heart disease | 17 | 13 | 12 | 10 | 12 |
| Other cardiovascular | 18 | 13 | 12 | 9 | 12 |
| Dyslipidemia | 29 | 25 | 24 | 29 | 26 |
| Hospitalized in first 91 d, % | 31 | 30 | 27 | 25 | 27 |
| Access type at start of dialysis | |||||
| Central venous catheter | 59 | 63 | 66 | 58 | 63 |
| AV fistula | 13 | 10 | 10 | 12 | 12 |
| AV graft | 3 | 5 | 2 | 4 | 3 |
| PD catheter | 7 | 4 | 6 | 8 | 6 |
| Unknown | 18 | 17 | 16 | 18 | 16 |
| Body mass index, kg/m2 | 28±7 | 29±8 | 27±6 | 24±5 | 28±7 |
| Laboratory variables from first 91-d period from date of first dialysis | |||||
| Hemoglobin, g/dl | 11.2±1.2 | 10.9±1.2 | 11.3±1.2 | 11.2±1.2 | 11.2±1.1 |
| Iron saturation, % | 22 [17–27] | 22 [18–27] | 22 [18–28] | 24 [19–29] | 22 [18–27] |
| Serum ferritin, ng/ml | 267 [155–457] | 290 [165–502] | 254 [146–432] | 340 [195–589] | 288 [161–493] |
| Serum albumin, g/dl | 3.5±0.5 | 3.5±0.5 | 3.5±0.5 | 3.6±0.5 | 3.5±0.5 |
| Serum calcium, mg/dl | 8.7±0.6 | 8.7±0.7 | 8.5±0.6 | 8.6±0.6 | 8.6±0.6 |
| Serum phosphorous, mg/dl | 4.9±1.2 | 4.9±1.1 | 5.1±1.2 | 5.0±1.2 | 5.1±1.2 |
| Parathyroid hormone, pg/ml | 262 [163–403] | 406 [260–619] | 330 [217–491] | 297 [194–436] | 316 [203–469] |
| Alkaline phosphatase, IU/L | 84 [67–110] | 86 [68–114] | 94 [75–123] | 81 [65–104] | 88 [69–115] |
| Hemoglobin A1C, % | 6.2 [5.6–7.0] | 6.2 [5.6–7.1] | 6.4 [5.7–7.2] | 6.3 [5.7–7.0] | 6.3 [5.7–7.2] |
| Potassium, mEq/L | 4.4±0.5 | 4.3±0.5 | 4.6±0.5 | 4.5±0.5 | 4.4±0.5 |
| Bicarbonate, mEq/L | 24±3 | 24±3 | 23±3 | 23±3 | 23±3 |
| iv Medications in first 91-d period from date of first dialysis | |||||
| Cumulative iron, mg/mo | 650 [0–1300] | 700 [0–1300] | 700 [0–1200] | 500 [0–1100] | 650 [0–1200] |
| ESA median week dose, units per wk | 4714 [1500–12,000] | 4714 [1467–12,000] | 4669 [1506–11,721] | 4809 [1516–11,859] | 4800 [1558–11,776] |
| Geographic location, % | |||||
| Northeast | 15 | 12 | 7 | 9 | 7 |
| Midwest | 26 | 16 | 5 | 9 | 9 |
| West | 22 | 11 | 53 | 65 | 48 |
| South | 38 | 61 | 34 | 17 | 36 |
| Year of incidence, % | |||||
| 2007 | 21 | 22 | 21 | 20 | 23 |
| 2008 | 21 | 21 | 21 | 20 | 23 |
| 2009 | 22 | 22 | 21 | 22 | 19 |
| 2010 | 21 | 20 | 21 | 22 | 20 |
| 2011 | 15 | 15 | 16 | 17 | 15 |
Data are presented as means±SDs, medians [interquartile ranges], or proportions where appropriate. H/o, history of; AV, arteriovenous; ESA, eryhtropoiesis stimulating agents.
Includes Medicare Advantage plans, managed care Medicaid, and employer–based health insurance.
Racial/Ethnic Differences in Treatment with PD or Home HD
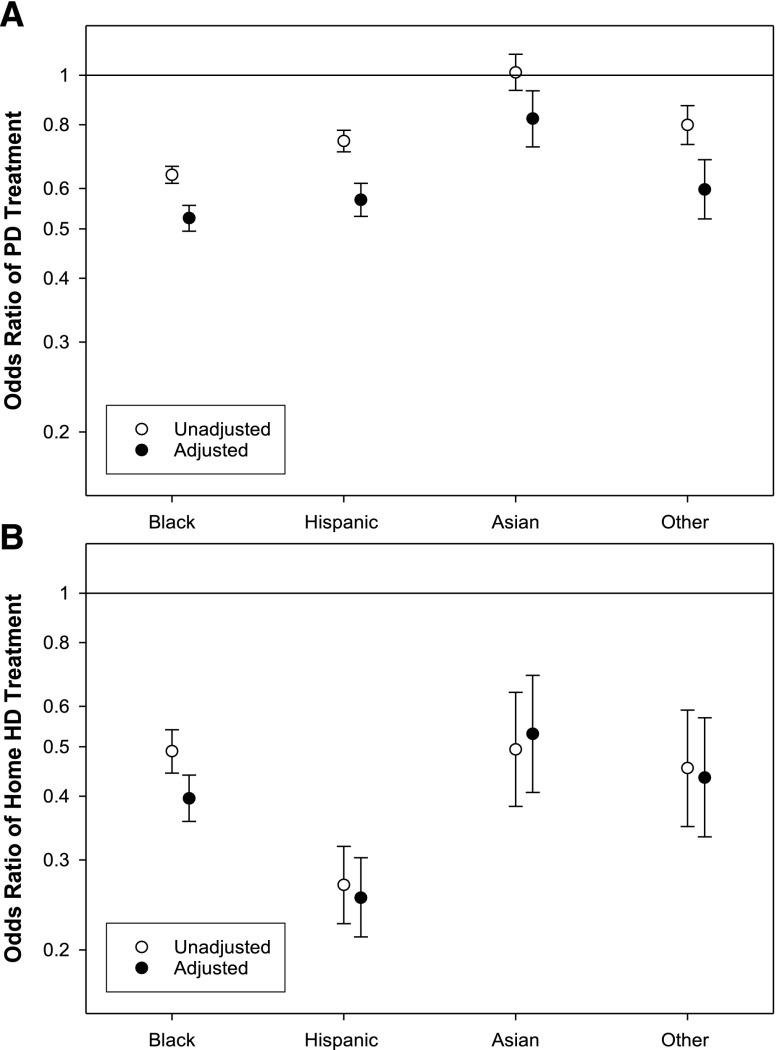
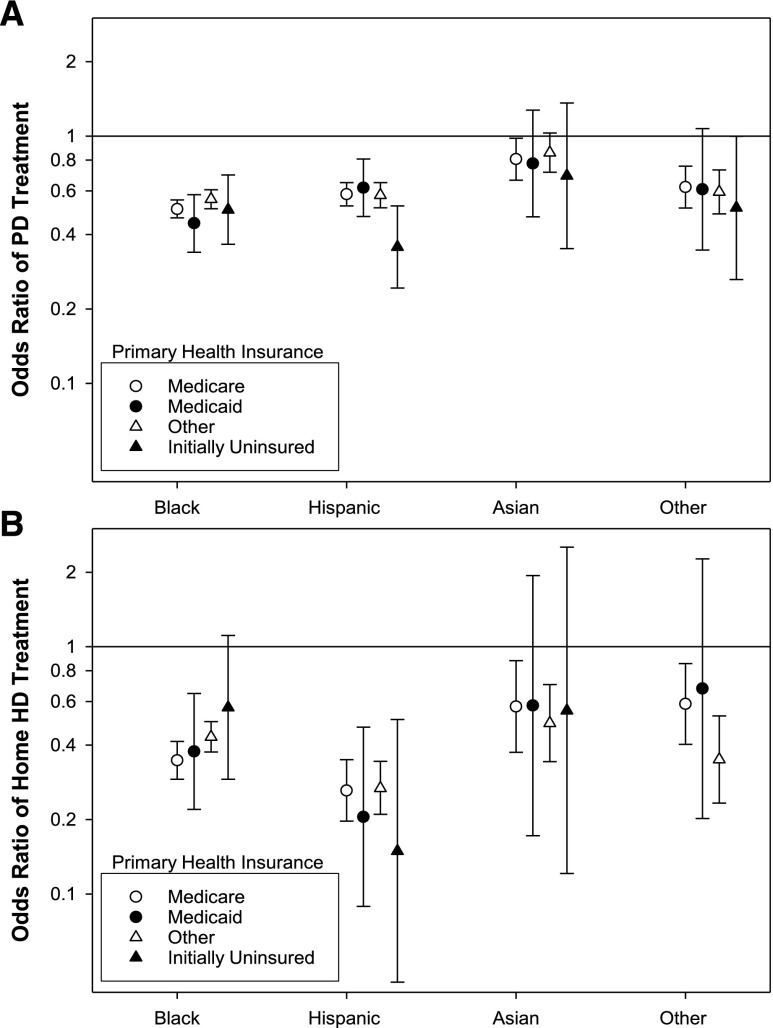
Compared with whites, the adjusted odds for treatment with PD were significantly lower for every racial/ethnic group (Figure 1A, Supplemental Table 1). The difference was least pronounced for Asians, who had 18% lower odds of being treated with PD, and most marked for blacks. The adjusted odds for treatment with home HD was also significantly lower for every racial/ethnic group compared with whites (Figure 1B, Supplemental Table 1). The largest difference was for Hispanics, with 75% lower odds for treatment with home HD, and the smallest difference was for Asians. The racial/ethnic differences in use of home dialysis were similar irrespective of initial primary health insurance at the time of admission to the dialysis facility (Figure 2).
Figure 1.
Association of race/ethnicity with treatment with PD or home HD. Unadjusted and adjusted odds ratio for blacks, Hispanics, Asians, and others (reference: whites) to be treated for at least one 91-day period with (A) PD or (B) home HD for 162,050 patients who started dialysis between January 1, 2007 and December 31, 2011. Compared to whites, the adjusted odds ratio and 95% confidence interval for (A) PD: black, 0.53 (0.50, 0.56); Hispanic, 0.57 (0.53, 0.61); Asian, 0.82 (0.72, 0.93); and Other, 0.60 (0.52, 0.68); and (B) Home HD: black, 0.40 (0.36, 0.44); Hispanic, 0.25 (0.21, 0.30); Asian, 0.53 (0.41, 0.69); and Other, 0.44 (0.33, 0.57).
Figure 2.
Association of race/ethnicity and initial primary health insurance with treatment with PD or home HD. Adjusted odds for blacks, Hispanics, Asians, and others (reference: whites) to be treated for at least one 91-day period with (A) PD or (B) home HD for 162,050 patients who started dialysis between January 1, 2007 and December 31, 2011, stratified by initial primary health insurance. The group ‘other’ includes patients with Medicare Advantage plans, managed care Medicaid, and employer–based health insurance.
Racial/Ethnic Differences in Risk of Death by Modality
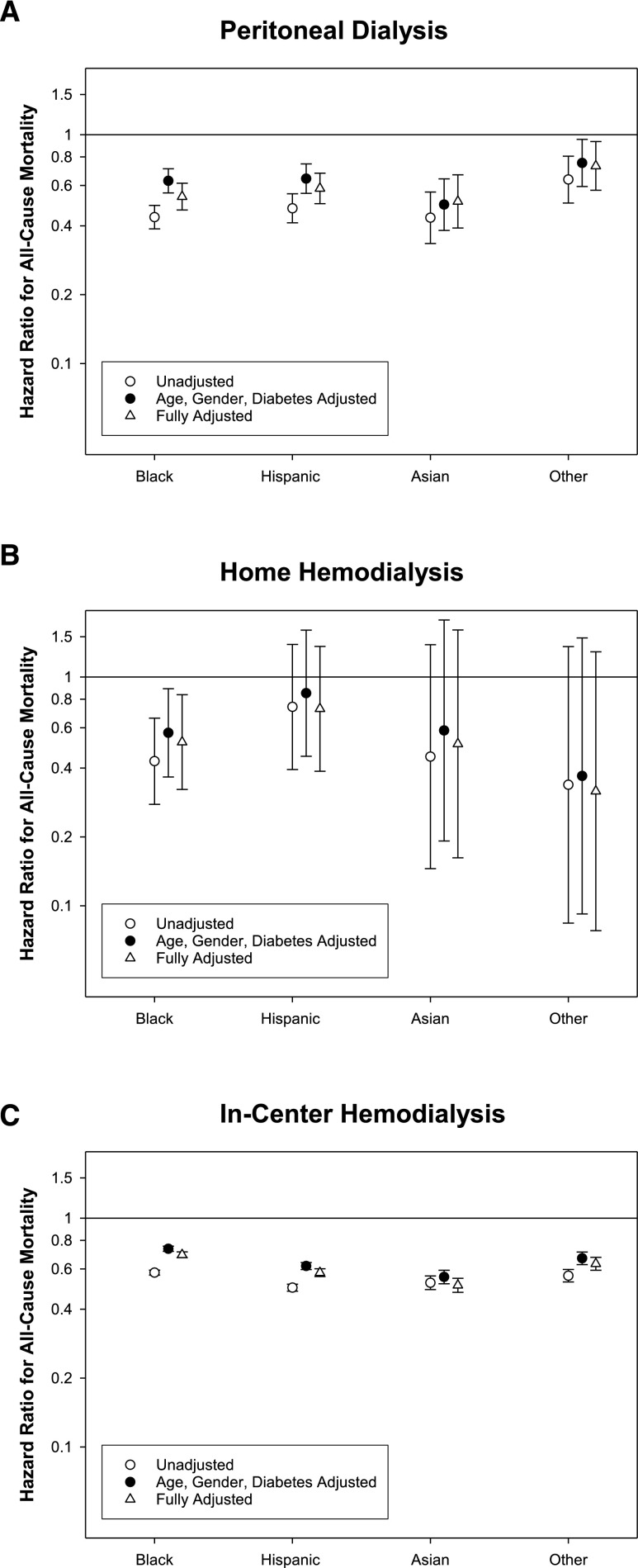
The differences by race/ethnicity for patients treated with PD or home HD at the time of start of treatment with the respective home dialysis modality are summarized in Tables 2 and 3, respectively; the differences in characteristics of patients by race/ethnicity treated with in-center HD were similar to the entire cohort and are summarized in Supplemental Table 2. Over a period of 13 (interquartile range, 7–23) months, 2326 patients (13%) died while being treated with PD (10 deaths per 100 patient-years). Compared with whites, the adjusted risk of death was significantly lower in every racial/ethnic group (Figure 3, Supplemental Table 3). Of 2536 patients treated with home HD, 215 individuals (8%) died over the follow-up period of 11 (interquartile range, 6–17) months (7 deaths per 100 patient-years). Compared with whites, the risk for death was significantly lower among blacks but similar for other racial/ethnic groups treated with home HD (Figure 2, Supplemental Table 3). Among 140,389 patients treated with in-center HD, 33,937 patients (24%) died over the follow-up period of 16 (interquartile range, 7–30) months (15 deaths per 100 patient-years). Compared with whites, the adjusted risk for death was significantly lower in every racial/ethnic group (Figure 2, Supplemental Table 3).
Table 2.
Baseline characteristics of patients stratified by race/ethnicity treated with PD at the time of initiation of PD
| Variable | White | Black | Hispanic | Asian | Other |
|---|---|---|---|---|---|
| No. of subjects | 10,123 | 4094 | 2263 | 713 | 598 |
| Initial modality, %a | |||||
| In-center HD | 32 | 37 | 30 | 25 | 27 |
| PD | 68 | 63 | 70 | 75 | 73 |
| Home HD | <1 | <1 | 0 | 0 | 0 |
| Interval from start of dialysis to treatment with modality, d | 31 [9–165] | 41 [11–214] | 27 [9–137] | 26 [9–136] | 23 [6–128] |
| Age, yr | 59±15 | 51±14 | 50±16 | 55±16 | 54±15 |
| Men, % | 60 | 48 | 56 | 53 | 57 |
| Primary health insurance, % | |||||
| Medicare | 49 | 42 | 45 | 38 | 43 |
| Medicaid | 3 | 4 | 11 | 7 | 6 |
| Initially uninsured | 3 | 4 | 5 | 4 | 4 |
| Veterans Affairs | 1 | 1 | <1 | <1 | <1 |
| Otherb | 44 | 50 | 39 | 51 | 47 |
| Cause of ESRD, % | |||||
| Diabetes | 39 | 36 | 50 | 35 | 49 |
| Hypertension | 24 | 38 | 22 | 28 | 24 |
| Glomerular disease | 16 | 16 | 14 | 23 | 15 |
| Other | 21 | 10 | 13 | 14 | 12 |
| H/o previous transplant, % | 3 | 2 | 2 | 3 | 2 |
| Comorbidities, % | |||||
| Diabetes | 61 | 63 | 70 | 60 | 68 |
| Hypertension | 51 | 63 | 47 | 51 | 46 |
| Congestive heart failure | 19 | 23 | 18 | 14 | 18 |
| Atherosclerotic heart disease | 19 | 14 | 13 | 14 | 16 |
| Other cardiovascular | 17 | 13 | 12 | 9 | 14 |
| Dyslipidemia | 48 | 43 | 44 | 51 | 42 |
| Baseline body mass index, kg/m2 | 28±6 | 29±7 | 27±6 | 24±4 | 28±6 |
| Laboratory variables from first 91-d period of treatment with PD | |||||
| Hemoglobin, g/dl | 11.6±1.3 | 11.2±1.4 | 11.4±1.3 | 11.5±1.3 | 11.4±1.4 |
| Iron saturation, % | 27 [21–35] | 28 [22–35] | 28 [22–36] | 30 [24–40] | 27 [21–35] |
| Serum ferritin, ng/ml | 286 [146–536] | 327 [165–612] | 273 [136–530] | 353 [183–637] | 294 [142–581] |
| Serum albumin, g/dl | 3.7±0.5 | 3.6±0.5 | 3.6±0.5 | 3.7±0.5 | 3.7±0.5 |
| Weekly total Kt/V | 2.5±0.8 | 2.4±0.8 | 2.5±0.8 | 2.6±0.8 | 2.6±0.8 |
| Residual renal Kt/V | 1.1±0.8 | 0.9±0.8 | 0.9±0.8 | 1.0±0.8 | 1.0±0.8 |
| Serum calcium, mg/dl | 8.9±0.6 | 8.7±0.7 | 8.6±0.7 | 8.8±0.7 | 8.7±0.7 |
| Serum phosphorous, mg/dl | 5.0±1.3 | 5.0±1.3 | 5.2±1.3 | 5.1±1.2 | 5.0±1.2 |
| Parathyroid hormone, pg/ml | 251 [152–397] | 414 [260–665] | 335 [220–511] | 284 [173–458] | 290 [194–456] |
| Alkaline phosphatase, IU/L | 80 [64–104] | 82 [65–107] | 89 [70–115] | 74 [59–96] | 80 [65–105] |
| Hemoglobin A1C, % | 6.8 [6.0–7.7] | 6.8 [5.9–7.8] | 6.9 [6.1–7.9] | 6.6 [5.9–7.6] | 6.8 [6.1–8.1] |
| Potassium, mEq/L | 4.2±0.5 | 4.1±0.5 | 4.3±0.6 | 4.3±0.6 | 4.2±0.5 |
| Bicarbonate, mEq/L | 25±3 | 25±3 | 24±3 | 24±3 | 24±3 |
| 4-h D/P creatinine ratio | 0.65±0.12 | 0.65±0.12 | 0.65±0.13 | 0.64±0.13 | 0.66±0.11 |
| iv Medication from first 91-d period of treatment with PD | |||||
| Cumulative iron, mg/mo | 0 [0–325] | 0 [0–300] | 100 [0–400] | 0 [0–300] | 0 [0–350] |
| ESA median week dose, units | 4992 [1650–11,971] | 5648 [1886–13,514] | 5067 [1703–11,622] | 4840 [1400–11,000] | 4950 [1800–11,000] |
| PD modality, % | |||||
| Initial treatment with APD | 58 | 58 | 60 | 58 | 47 |
| Ever treated with APD | 86 | 86 | 88 | 84 | 84 |
| Geographic location, % | |||||
| Northeast | 13 | 11 | 6 | 8 | 7 |
| Midwest | 25 | 16 | 6 | 9 | 10 |
| West | 20 | 10 | 48 | 60 | 48 |
| South | 42 | 63 | 40 | 23 | 36 |
| Year of incidence, % | |||||
| 2007 | 19 | 21 | 19 | 22 | 19 |
| 2008 | 20 | 20 | 19 | 19 | 22 |
| 2009 | 22 | 21 | 21 | 21 | 21 |
| 2010 | 23 | 23 | 23 | 22 | 21 |
| 2011 | 16 | 15 | 17 | 17 | 16 |
Data are presented as means±SDs, medians [interquartile ranges], or proportions where appropriate. H/o, history of; D/P, 4-hour dialysate to plasma ratio; ESA, eryhtropoiesis stimulating agents; APD, auotmoated peritoneal dialysis.
Of patients with an assigned modality during the first 91-day period of dialysis (n=15,706).
Includes Medicare Advantage plans, managed care Medicaid, and employer–based health insurance.
Table 3.
Baseline characteristics of patients stratified by race/ethnicity treated with home HD at the time of initiation of home HD
| Variable | White | Black | Hispanic | Asian | Other |
|---|---|---|---|---|---|
| No. of subjects | 1,753 | 525 | 139 | 61 | 58 |
| Initial modality, %a | |||||
| In-center HD | 65 | 74 | 71 | 58 | 67 |
| PD | 3 | 5 | 3 | 0 | 0 |
| Home HD | 31 | 21 | 26 | 42 | 33 |
| Interval from start of dialysis to treatment with modality, d | 243 [85–489] | 375 [158–697] | 291 [112–789] | 224 [45–459] | 320 [111–648] |
| Age, yr | 55±14 | 47±12 | 47±15 | 48±15 | 51±15 |
| Men, % | 65 | 68 | 67 | 62 | 72 |
| Primary health insurance, % | |||||
| Medicare | 41 | 33 | 39 | 38 | 52 |
| Medicaid | 3 | 4 | 5 | 5 | 5 |
| Initially uninsured | 2 | 3 | 2 | 3 | 0 |
| Veterans Affairs | 1 | 2 | 1 | 0 | 0 |
| Otherb | 53 | 58 | 53 | 54 | 43 |
| Cause of ESRD, % | |||||
| Diabetes | 35 | 30 | 41 | 39 | 43 |
| Hypertension | 18 | 38 | 19 | 18 | 28 |
| Glomerular disease | 18 | 20 | 19 | 28 | 7 |
| Other | 29 | 12 | 21 | 15 | 22 |
| H/o previous transplant, % | 6 | 7 | 5 | 7 | 2 |
| Comorbidities, % | |||||
| Diabetes | 60 | 60 | 66 | 62 | 60 |
| Hypertension | 70 | 85 | 68 | 75 | 69 |
| Congestive heart failure | 50 | 50 | 51 | 41 | 48 |
| Atherosclerotic heart disease | 27 | 25 | 31 | 30 | 19 |
| Other cardiovascular | 23 | 20 | 23 | 25 | 14 |
| Dyslipidemia | 44 | 46 | 41 | 48 | 33 |
| Access type at start of home HD | |||||
| Central venous catheter | 25 | 19 | 23 | 18 | 19 |
| AV fistula | 59 | 61 | 63 | 64 | 67 |
| AV graft | 8 | 13 | 8 | 11 | 5 |
| Unknown | 8 | 7 | 6 | 7 | 9 |
| Baseline body mass index, kg/m2 | 30±8 | 30±8 | 29±7 | 26±5 | 29±7 |
| Laboratory variables from first 91-d period of treatment with home HD | |||||
| Hemoglobin, g/dl | 11.2±1.3 | 11.0±1.3 | 11.4±1.3 | 11.1±1.0 | 11.0±1.3 |
| Iron saturation, % | 25 [20–33] | 26 [21–32] | 27 [23–32] | 25 [21–33] | 25 [22–31] |
| Serum ferritin, ng/ml | 351 [192–594] | 403 [244–644] | 370 [206–640] | 343 [167–662] | 316 [140–678] |
| Serum albumin, g/dl | 3.9±0.5 | 4.0±0.4 | 4.1±0.4 | 4.0±0.4 | 4.0±0.4 |
| Urea reduction ratio, % | 42±9 | 40±8 | 42±8 | 44±10 | 42±11 |
| Serum calcium, mg/dl | 8.9±0.6 | 8.9±0.6 | 8.9±0.6 | 8.7±0.6 | 8.9±0.6 |
| Serum phosphorous, mg/dl | 5.1±1.2 | 5.0±1.1 | 5.2±1.2 | 5.5±1.2 | 5.1±1.3 |
| Parathyroid hormone, pg/ml | 305 [191–477] | 502 [319–742] | 346 [195–493] | 425 [259–644] | 290 [189–443] |
| Alkaline phosphatase, IU/L | 79 [62–106] | 78 [63–103] | 77 [65–105] | 80 [61–118] | 73 [60–107] |
| Hemoglobin A1C, % | 6.7 [5.8–7.8] | 6.8 [5.8–8.4] | 6.5 [5.7–7.8] | 6.5 [5.7–7.9] | 6.1 [5.7–7.0] |
| Potassium, mEq/L | 4.4±0.6 | 4.3±0.6 | 4.4±0.6 | 4.6±0.5 | 4.4±0.5 |
| Bicarbonate, mEq/L | 24±2 | 24±3 | 23±2 | 23±3 | 23±3 |
| iv Medications in first 91-d period of treatment with home HD | |||||
| Cumulative iron, mg/mo | 0 [0–300] | 50 [0–400] | 0 [0–200] | 100 [0–200] | 200 [0–400] |
| ESA median week dose, units | 5097 [1650–12,099] | 4872 [1941–11,880] | 5371 [2200–11,611] | 2438 [0–8800] | 4180 [1532–11,313] |
| Geographic location, % | |||||
| Northeast | 17 | 15 | 13 | 13 | 21 |
| Midwest | 28 | 24 | 12 | 20 | 9 |
| West | 20 | 8 | 40 | 48 | 47 |
| South | 35 | 53 | 35 | 20 | 24 |
| Year of incidence, % | |||||
| 2007 | 24 | 30 | 36 | 16 | 28 |
| 2008 | 27 | 22 | 24 | 36 | 26 |
| 2009 | 22 | 27 | 15 | 25 | 17 |
| 2010 | 18 | 14 | 14 | 13 | 17 |
| 2011 | 10 | 7 | 11 | 10 | 12 |
Data are presented as means±SDs, medians [interquartile ranges], or proportions where appropriate. H/o, history of; AV, arteriovenous; ESA, eryhropoiesis stimulating agents.
Of patients with an assigned modality during the first 91-day period of dialysis (n=1896).
Includes Medicare Advantage plans, managed care Medicaid, and employer–based health insurance.
Figure 3.
Association of race/ethnicity with mortality among patients undergoing PD or home HD. Unadjusted and adjusted hazards ratio for blacks, Hispanics, Asians, and others for all-cause mortality among those treated with (A) PD, (B) home HD, and (C) in-center HD. Hazard ratios are presented for models that are (1) unadjusted; (2) adjusted for age, sex, and diabetes; and (3) fully adjusted. Compared to whites, the hazards ratios, adjusted for age, gender, and diabetes mellitus, and 95% confidence interval for all-cause mortality among those treated with (A) PD: black, 0.63 (0.56, 0.71); Hispanic, 0.64 (0.55, 0.75); Asian, 0.49 (0.38, 0.64); and other, 0.75 (0.59, 0.95); (B) home HD: black, 0.57 (0.37, 0.89); Hispanic, 0.85 (0.45, 1.60); Asian, 0.58 (0.19, 1.77); and other, 0.37 (0.09, 1.48); and (C) in-center HD: black, 0.73 (0.72, 0.75); Hispanic, 0.62 (0.60, 0.64); Asian, 0.55 (0.52, 0.59); and other, 0.67 (0.63, 0.71).
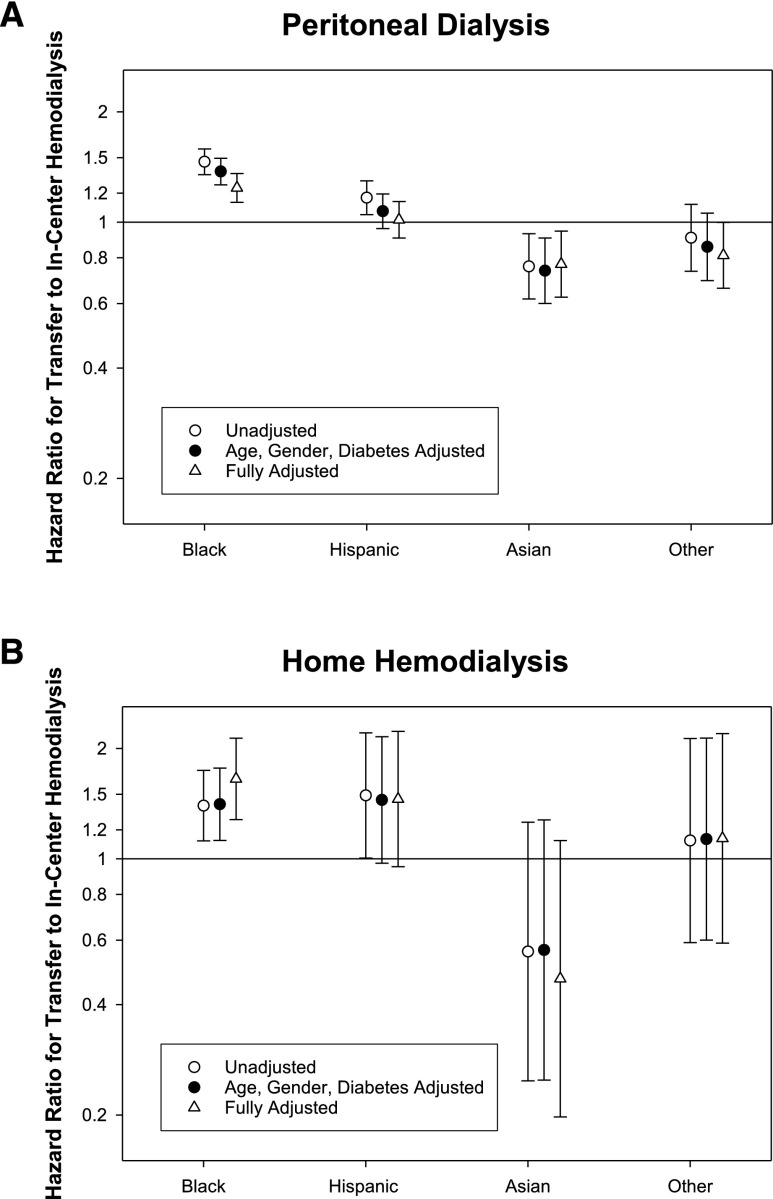
Racial/Ethnic Differences in Transfer to In-Center HD for Patients Treated with PD or Home HD
Of the patients treated with PD or home HD, 3138 (18%) and 436 (17%) patients, respectively, transferred to in-center HD (13 and 15 transfers per 100 patient-years, respectively). Black patients treated with PD or home HD had a significantly higher risk for transfer to in-center HD (Figure 3, Supplemental Table 4). There was no significant difference in adjusted hazards for Hispanics treated with PD or home HD to transfer to in-center HD. Although Asians and other racial groups treated with PD had lower risks for transfer to in-center HD, there was no significant difference in risk for these groups treated with home HD compared with whites (Figure 4, Supplemental Table 4).
Figure 4.
Association of race/ethnicity with transfer to in-center HD among patients undergoing PD or home HD. Unadjusted and adjusted hazards ratio for blacks, Hispanics, Asians, and others for transferring to in-center HD among those treated with (A) PD and (B) home HD. Hazard ratios are presented for models that are (1) unadjusted; (2) adjusted for age, sex, and diabetes; and (3) fully adjusted. Compared to whites, the hazards ratios, adjusted for age, gender, and diabetes mellitus, and 95% confidence interval for transfer to in-center HD among those treated with (A) PD: black, 1.37 (1.26, 1.49); Hispanic, 1.07 (0.96, 1.19); Asian, 0.74 (0.60, 0.91); and other, 0.86 (0.69, 1.06); and (B) home HD: black, 1.41 (1.12, 1.77); Hispanic, 1.45 (0.97, 2.16); Asian, 0.56 (0.25, 1.28); and other, 1.13 (0.60, 2.14).
Racial/Ethnic Differences in Receiving a Kidney Transplant by Modality
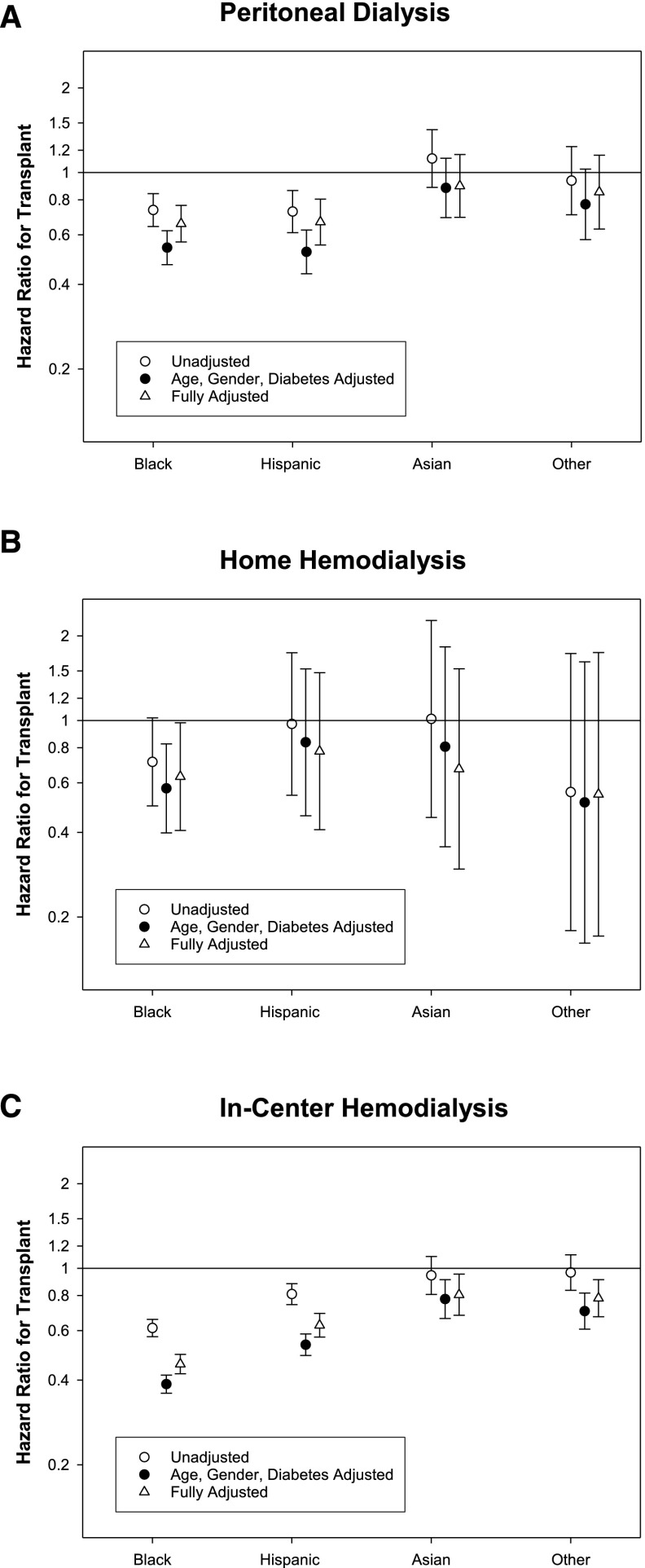
During the follow-up period, 1435 (8%), 221 (9%), and 4580 (3%) patients undergoing PD, home HD, and in-center HD, respectively, received a kidney transplant (yielding six, eight, and two transplants per 100 patient-years, respectively). Compared with whites, the probability of receiving a kidney transplant was significantly lower among blacks irrespective of dialysis modality, Hispanics treated with PD or in-center HD, and Asians and others undergoing in-center HD (Figure 5, Supplemental Table 5).
Figure 5.
Association of race/ethnicity with probability of receiving kidney transplantation among patients undergoing PD or home HD. Unadjusted and adjusted hazards ratios for blacks, Hispanics, Asians, and others for receiving a kidney transplant among those treated with (A) PD, (B) home HD, and (C) in-center HD. Hazard ratios are presented for models that are (1) unadjusted; (2) adjusted for age, sex, and diabetes; and (3) fully adjusted. Compared to whites, the hazards ratios, adjusted for age, gender, and diabetes mellitus, and 95% confidence interval for undergoing kidney transplantation among those treated with (A) PD: black, 0.54 (0.47, 0.62); Hispanic, 0.52 (0.44, 0.62); Asian, 0.88 (0.69, 1.12); and other, 0.77 (0.58, 1.03); (B) home HD: black, 0.57 (0.40, 0.83); Hispanic, 0.84 (0.46, 1.53); Asian, 0.81 (0.36, 1.83); and other, 0.51 (0.16, 1.62); and (C) in-center HD: black, 0.39 (0.36, 0.42); Hispanic, 0.52 (0.49, 0.58); Asian, 0.78 (0.66, 0.91); and other, 0.70 (0.61, 0.82).
Discussion
Analysis of this large, contemporary, and nationally representative cohort of patients initiating maintenance dialysis provides the first comprehensive assessment of racial/ethnic differences in use of and outcomes with home dialysis and allows us to make a few key conclusions. First, every racial/ethnic minority group in the United States is significantly less likely to be treated with home dialysis than whites, and demographic and clinical characteristics are insufficient to explain this differential use. Second, as for patients treated with in-center HD, racial/ethnic minorities treated with PD have a significantly lower risk of death than whites and a significantly lower probability of receiving a kidney transplant. Third, blacks treated with home HD had a lower risk of death and a lower probability of undergoing kidney transplantation compared with whites. Fourth, the risk for transfer to in-center HD was significantly higher for blacks treated with PD or home HD and lower for Asians and others treated with PD compared with whites.
The national registry of patients on dialysis, the US Renal Data System, has long reported that racial/ethnic minorities are significantly less likely to use PD or home HD.1 Our analysis indicates that demographic and clinical differences are insufficient to explain the lower use of home dialysis by racial/ethnic minorities. Each minority group was significantly younger and had a lower prevalence of coexisting cardiovascular illnesses, factors generally associated with a greater use of home dialysis.5,12–14 Moreover, there were no meaningful differences in laboratory parameters as surrogate measures of health to explain the lower use of home dialysis. In the absence of demographic and clinical variables explaining the differential use, it is likely that these differences may arise from patient preferences, social differences (such as sufficient space at home and ability to afford the incremental expense of utilities), or factors associated with health care delivery (such as availability of predialysis nephrology care or home dialysis in communities where minorities live) or physician or provider perception of patients’ ability to perform home dialysis. Most of these issues have never been systematically examined with two exceptions. First, in an analysis of the period from 1995 to 2003, the availability of PD services was demonstrably incongruent with the population density of ESRD.15 Second, two separate studies have shown that patients who live in black majority communities or are treated in facilities located in such communities are significantly less likely to have received predialysis nephrology care.16,17 No such data are available for other racial/ethnic groups. The need to ensure equitable access to home dialysis is even more urgent now as the use of both PD and home HD are seeing unprecedented and rapid growth, and these racial/ethnic differences need additional investigation.
Our analysis is the first multicenter and nationally representative cohort study to compare the racial/ethnic differences in mortality among patients treated with PD or home HD in the United States. The lower risk for death in racial minorities treated with PD compared with whites is consistent with what has been previously reported using data from the US Renal Data System, one single center of patients on PD in the United States, and a multicenter cohort study from Brazil.1,6,18–21 Minority populations treated with PD in our cohort were younger and had fewer coexisting illnesses; hence, it is possible that the differences reflect residual confounding. Several hypotheses have been put forth to explain the better health status of minorities starting dialysis, such as higher risk for death in minorities in early stages of kidney disease or renal-limited disease, particularly in blacks. However, there is no direct evidence to support any of these hypotheses. It is also possible that the lower transplantation rate in racial/ethnic minorities may have made it more likely for the minority group to be enriched with healthier patients over longer follow-up. However, our analytic plan using competing risk regression took into account this potential source of bias. To our knowledge, the lower risk for death among blacks treated with home HD compared with whites has heretofore not been reported. The number of deaths among other racial/ethnic groups was very small, and hence, our analyses did not have sufficient statistical power for us to definitively conclude if their survival differs from that of whites treated with home HD.
We also observed a significantly higher likelihood of blacks treated with PD or home HD to transfer to in-center HD. Although a higher risk for blacks has been reported in several but not all studies of patients treated with PD, our investigation is the first to show this for patients undergoing home HD.6–9 In contrast, Asians and others treated with PD had a lower risk for transfer to in-center HD compared with whites. Transfer to in-center HD, also referred to as technique failure, often results from an intercurrent therapy–related complication (such as peritonitis with PD), change in health status or social circumstances that precludes self-care, or patient burnout. Some studies have shown a higher risk for peritonitis among blacks22,23; there are no such studies that have examined differential risk for any of these events for any other racial group treated with PD or among those treated with home HD. It is also possible that some of these differences reflect biologic responses to disease and/or treatment. There is a need to understand these issues better, because transfer of patients on home dialysis to in-center HD often results in significant morbidity for patients and incurs considerable expense to the health care system.24 There is also a compelling need to develop and test interventions that mitigate the racial differences in risk for transfer to in-center HD.
Our study also shows that, even among patients treated with PD, racial/ethnic minorities are significantly less likely to receive a kidney transplant. Although racial disparities in access to transplantation are well established and also evident in our cohort of patients undergoing in-center HD, the persistence of this difference in the subgroup of patients undergoing PD is surprising. In the United States, patients treated with PD are significantly more likely to receive kidney transplant, probably because these individuals are younger and healthier, have a higher socioeconomic status, and are motivated to engage in self-care—medical and social characteristics that are all associated with a higher probability of receiving a kidney transplant.11 Furthermore, minorities treated with PD were significantly younger than whites. Future studies need to examine racial differences in probability of being referred for kidney transplant, effect of insurance coverage, particularly for Hispanics, on such referral, and time to listing. Among patients undergoing home HD, disparities were evident only for blacks and not for other racial/ethnic groups. This is likely to represent the relatively small number of events in these other groups, making our analyses underpowered to make definitive conclusions.
There are several reasons why our study has considerable external validity. The study cohort included patients from >2000 dialysis facilities in 43 states and the District of Columbia and included substantial granularity of data on clinical characteristics, including results of laboratory tests and medication use that have heretofore not been available for patients undergoing home dialysis. In that context, this is the largest cohort of patients on home HD ever described. Notwithstanding the strength, our findings have to be interpreted in light of their limitations, which primarily stem from the lack of availability of data on socioeconomic characteristics or predialysis care that could have shed light on the reasons for racial/ethnic differences described herein.
In conclusion, there are substantial racial/ethnic differences in use of and outcomes with home dialysis in the United States. Because ESRD disproportionately afflicts racial/ethnic minorities, understanding the reasons for the racial/ethnic differences in patient-centered outcomes is imperative to make sustainable improvements for the entire population of patients undergoing maintenance dialysis. With an unprecedented growth in the number of patients undergoing home dialysis in the United States, ensuring equitable access and improving health outcomes for patients undergoing PD and home HD have become even more important.
Concise Methods
Study Population and Data Source
This cohort study comprised all patients ≥18 years of age who initiated maintenance dialysis in 2007–2011, had data on race/ethnicity available, and received care in facilities operated by DaVita Inc. for at least 60 days. Follow-up data were available through December 31, 2011. The study cohort was divided into five groups on the basis of race/ethnicity as white, black, Hispanic, Asian, or other.
The entire follow-up period for each patient was divided into successive 91-day periods from the date of first dialysis, and follow-up was available for ≤5 years. Each patient was assigned the dialysis modality (PD, home HD, or in-center HD) with which they were treated for >45 days of any given 91-day period as long as the patient was exposed to the modality for 60 consecutive days (which may have spanned more than one 91-day period).12 Patients were labeled as having ever been treated with PD or home HD if they were assigned to the treatment modality for at least one 91-day period during follow-up. The analyses for patients treated with in-center HD were limited to individuals who were not treated with any other dialysis modality at any point during follow-up. The dialysis access with which the patient was treated for >45 days was assigned as the access for each 91-day period. Each patient was also assigned a dialysis facility where the patient received care for >45 days in a given 91-day period. All routine blood and dialysate samples were shipped to a central laboratory and analyzed within 24 hours of collection; the results were summarized for each 91-day period as arithmetic means. Similarly, all hemodynamic parameters and doses of parenteral medications were summarized for each 91-day period.
Outcomes
The association of race/ethnicity with treatment with PD or home HD at any follow-up period was examined for all patients starting maintenance dialysis. The racial/ethnic differences in the following three treatment outcomes were examined separately for each dialysis modality (PD, home HD, and in-center HD): (1) all-cause mortality, (2) transfer to in-center HD (among patients treated with PD or home HD), and (3) kidney transplantation. The follow-up period comprised the interval from the date of first treatment with the modality to the occurrence of one of the following events (whichever occurred first): kidney transplantation, transfer to a facility operated by another dialysis provider, end of administrative follow-up, or death. In addition, for the analyses of death or kidney transplantation among patients treated with PD or home HD, individuals were also censored at the time of transfer to in-center HD.
Statistical Analyses
Data are presented as means±SDs, medians and interquartile ranges, or proportions where appropriate. Logistic regression analyses were used to determine the odds of treatment with PD or home HD of blacks, Hispanics, Asians, or others compared with whites. These analyses were performed (1) unadjusted and (2) adjusted with inclusion of age, sex, primary health insurance, geographic region (northeast, south, midwest, or west) at the time of first dialysis, incidence year, cause of ESRD, history of previous kidney transplant, diabetes, hypertension, congestive heart failure, atherosclerotic heart disease, other cardiovascular disease, and vascular access type at the time of first dialysis as additional covariates.
Separate time to event analyses were performed using Cox proportional hazards models to compare the probabilities for all-cause mortality and kidney transplantation among different racial/ethnic groups separately for patients treated with each of three dialysis modalities; for patients treated with PD or home HD, an additional outcome of transfer to in-center HD was examined. Cause–specific hazard ratios for each outcome were computed for patients treated with each dialysis modality using the competing risk model by Fine and Gray.25 The primary analysis examined outcomes with data adjusted for age, sex, and diabetes. In addition, a sensitivity analysis was done with adjustment for age; sex; primary health insurance; geographic location at the time of start of PD, home HD, or in-center HD; incidence year; cause of ESRD; history of previous kidney transplant; diabetes; hypertension; congestive heart failure; atherosclerotic heart disease; other cardiovascular disease; time from the start of first dialysis to the start of PD or home HD; body mass index in the first 91-day period of treatment with the modality for patients treated with in-center or home HD; and vascular access type in the first 91-day period of treatment with the modality for patients treated with in-center or home HD. Laboratory variables from the first 91-day period of treatment with the modality (hemoglobin, serum calcium, phosphorous, parathyroid hormone, ferritin, iron saturation, bicarbonate, and potassium) and modality-specific variables during the first quarter of assigned modality were also included for adjustment (PD: dose of dialysis measured as weekly total Kt/Vurea, residual kidney function measured as weekly renal Kt/Vurea, use of automated PD, and peritoneal solute transport rate measured as 4-hour dialysate-to-plasma ratio of creatinine from the first 6 months of start of PD; home HD: urea reduction ratio).
Data were missing for 8%, 2%, and 7% of patients, on average, at the start of PD, home HD, and in-center HD, respectively. Multiple imputation was used to account for missing data for the competing risk analyses. In logistic regression analyses, missing data categories were used for access type and facility region.
All analyses were performed using SAS, version 9.4 (SAS Institute Inc., Cary, NC) and Stata, version 13.1 (Stata Corporation, College Station, TX).
Disclosures
None.
Supplementary Material
Acknowledgments
The work in this manuscript has been performed with the support of grants from the National Institutes of Health: R01DK95668 (to R.M. and K.K.-Z.), R21AG047306 (to R.M., M.Z.M., and K.K.-Z.), and R01DK099165 (to R.M.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015050472/-/DCSupplemental.
References
- 1.US Renal Data System : Annual Data Report. US Department of Public Health and Human Services, Public Health Service, Bethesda, MD, National Institutes of Health, 2014 [Google Scholar]
- 2.Chiu YW, Teitelbaum I, Misra M, de Leon EM, Adzize T, Mehrotra R: Pill burden, adherence, hyperphosphatemia, and quality of life in maintenance dialysis patients. Clin J Am Soc Nephrol 4: 1089–1096, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morton RL, Tong A, Howard K, Snelling P, Webster AC: The views of patients and carers in treatment decision making for chronic kidney disease: Systematic review and thematic synthesis of qualitative studies. BMJ 340: c112, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rivara MB, Mehrotra R: The changing landscape of home dialysis in the United States. Curr Opin Nephrol Hypertens 23: 586–591, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stack AG: Determinants of modality selection among incident US dialysis patients: Results from a national study. J Am Soc Nephrol 13: 1279–1287, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Korbet SM, Shih D, Cline KN, Vonesh EF: Racial differences in survival in an urban peritoneal dialysis program. Am J Kidney Dis 34: 713–720, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Jaar BG, Plantinga LC, Crews DC, Fink NE, Hebah N, Coresh J, Kliger AS, Powe NR: Timing, causes, predictors and prognosis of switching from peritoneal dialysis to hemodialysis: A prospective study. BMC Nephrol 10: 3, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen JI, Mitani AA, Saxena AB, Goldstein BA, Winkelmayer WC: Determinants of peritoneal dialysis technique failure in incident US patients. Perit Dial Int 33: 155–166, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar VA, Sidell MA, Yang WT, Jones JP: Predictors of peritonitis, hospital days, and technique survival for peritoneal dialysis patients in a managed care setting. Perit Dial Int 34: 171–178, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gill J, Dong J, Rose C, Johnston O, Landsberg D, Gill J: The effect of race and income on living kidney donation in the United States. J Am Soc Nephrol 24: 1872–1879, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehrotra R, Chiu YW, Kalantar-Zadeh K, Bargman J, Vonesh E: Similar outcomes with hemodialysis and peritoneal dialysis in patients with end-stage renal disease. Arch Intern Med 171: 110–118, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Kuttykrishnan S, Kalantar-Zadeh K, Arah OA, Cheung AK, Brunelli S, Heagerty PJ, Katz R, Molnar MZ, Nissenson A, Ravel V, Streja E, Himmelfarb J, Mehrotra R: Predictors of treatment with dialysis modalities in observational studies for comparative effectiveness research. Nephrol Dial Transplant 30: 1208–1217, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vonesh EF, Snyder JJ, Foley RN, Collins AJ: The differential impact of risk factors on mortality in hemodialysis and peritoneal dialysis. Kidney Int 66: 2389–2401, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Walker DR, Inglese GW, Sloand JA, Just PM: Dialysis facility and patient characteristics associated with utilization of home dialysis. Clin J Am Soc Nephrol 5: 1649–1654, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang V, Lee SY, Patel UD, Weiner BJ, Ricketts TC, Weinberger M: Geographic and temporal trends in peritoneal dialysis services in the United States between 1995 and 2003. Am J Kidney Dis 55: 1079–1087, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prakash S, Rodriguez RA, Austin PC, Saskin R, Fernandez A, Moist LM, O’Hare AM: Racial composition of residential areas associates with access to pre-ESRD nephrology care. J Am Soc Nephrol 21: 1192–1199, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall YN, Xu P, Chertow GM, Himmelfarb J: Characteristics and performance of minority-serving dialysis facilities. Health Serv Res 49: 971–991, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kucirka LM, Grams ME, Lessler J, Hall EC, James N, Massie AB, Montgomery RA, Segev DL: Association of race and age with survival among patients undergoing dialysis. JAMA 306: 620–626, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arce CM, Goldstein BA, Mitani AA, Winkelmayer WC: Trends in relative mortality between Hispanic and non-Hispanic whites initiating dialysis: A retrospective study of the US Renal Data System. Am J Kidney Dis 62: 312–321, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan G, Norris KC, Yu AJ, Ma JZ, Greene T, Yu W, Cheung AK: The relationship of age, race, and ethnicity with survival in dialysis patients. Clin J Am Soc Nephrol 8: 953–961, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernandes NM, Hoekstra T, van den Beukel TO, Tirapani L, Bastos K, Pecoits-Filho R, Qureshi AR, Dekker FW, Bastos MG, Divino-Filho JC: Association of ethnicity and survival in peritoneal dialysis: A cohort study of incident patients in Brazil. Am J Kidney Dis 62: 89–96, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Golper TA, Brier ME, Bunke M, Schreiber MJ, Bartlett DK, Hamilton RW, Strife F, Hamburger RJ: Risk factors for peritonitis in long-term peritoneal dialysis: The Network 9 peritonitis and catheter survival studies. Academic Subcommittee of the Steering Committee of the Network 9 Peritonitis and Catheter Survival Studies. Am J Kidney Dis 28: 428–436, 1996 [DOI] [PubMed] [Google Scholar]
- 23.Oo TN, Roberts TL, Collins AJ: A comparison of peritonitis rates from the United States Renal Data System database: CAPD versus continuous cycling peritoneal dialysis patients. Am J Kidney Dis 45: 372–380, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Shih YC, Guo A, Just PM, Mujais S: Impact of initial dialysis modality and modality switches on Medicare expenditures of end-stage renal disease patients. Kidney Int 68: 319–329, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Fine JP, Gray RJ: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Med Assoc 94: 496–509, 1999 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.