Abstract
The presence of tubuloreticular inclusions (TRIs) in native glomerular endothelial cells associates with viral infections and lupus nephritis. However, the associations of TRIs in renal transplant biopsy specimens are not known. We analyzed data from 316 patients who had a transplant biopsy with electron microscopy examination; 41 of 316 (13.0%) patients had TRIs. Patients with TRIs had significantly lower allograft survival rates (50.9%) than patients without TRIs (74.3%; P=0.03). Transplant glomerulopathy–free survival was also inferior in the TRI-positive group (57.5%) compared with the TRI-negative group (87.3%; P=0.002). Serologically, hepatitis C associated with the presence of TRIs (P=0.04) along with donor-specific antibodies (P=0.01). Furthermore, patients who were TRI positive were more likely than patients who were TRI negative to have had a previous rejection episode (P=0.02). On multivariate analysis, TRIs associated with prior rejection, viral infections, and class 1 HLA donor–specific antibodies. These results show that the presence of TRIs in renal allograft biopsy specimens associates with poor allograft outcomes and serologic evidence of viral infections and alloimmunity. The association with alloimmunity is a novel finding that warrants additional investigation.
Keywords: kidney transplantation, transplant pathology, transplant outcomes, rejection, immunology and pathology
Tubuloreticular inclusions (TRIs) are tubular structures composed of phospholipid and glycoprotein that arise from the rough endoplasmic reticulum in a number of cell types and that are visualized using electron microscopy (EM).1,2 TRIs can be induced in endothelial cells both in vitro and in vivo in the context of enhanced type 1 IFN expression.2–7 IFNs are cytokines produced in response to viral infections and in the context of autoimmune disease.2,5,7 In native renal disease, TRIs in glomerular endothelial cells are most commonly encountered in the setting of SLE and viral infections, especially HIV.8–10 To our knowledge, the significance of TRIs in renal allografts has not been previously reported.
The importance of EM in the diagnosis of allograft dysfunction was acknowledged in 2013 with its enhanced role in the Banff definition of antibody-mediated rejection (AMR).11 In addition to identifying recurrent or de novo GN, EM examination can detect early ultrastructural changes in the glomerular and peritubular capillary basement membranes, which predict subsequent development of transplant glomerulopathy (TG).11–13 TG is the hallmark histologic lesion of chronic AMR, a lesion that, to date, has no effective therapy and is, therefore, associated with a poor prognosis.14 However, TG as a histopathologic lesion is not unique to alloimmune injury and may also result from other precipitants, such as hepatitis C, thrombotic microangiopathy, and other potential unidentified etiologic factors.15 Establishing the different causes of TG may help with therapeutic management and improve allograft survival.
In addition to EM, novel diagnostic tests, such as endothelial cell gene transcripts, have also been endorsed to diagnose alloimmune injury in the recent Banff classification, with Sis and colleagues11,16 having shown a strong correlation between high endothelial cell gene transcript expression, histologic features of AMR, and alloantibody. Using such technology, Hayde et al.17 have independently shown that type 1 IFN expression is increased in renal allograft biopsies in patients with donor-specific antibodies (DSAs). From this, it may be hypothesized that the presence of TRIs, a marker of increased IFN expression, may also be associated with the presence of DSAs in recipients of renal transplants.
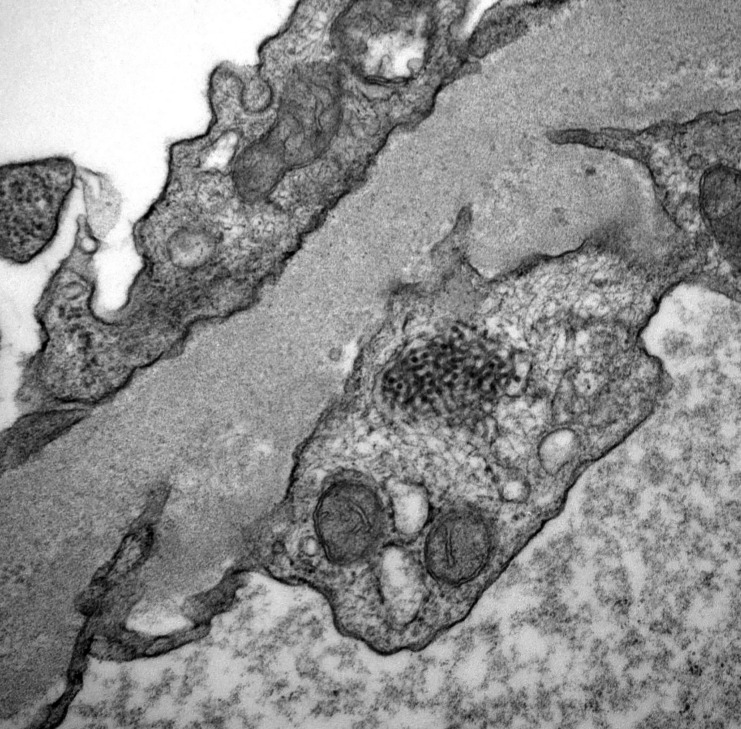
In this first reported study of TRIs in transplant biopsies, we aim to analyze the clinicopathologic associations and outcomes of glomerular endothelial cell TRIs in recipients of transplants; we examine clinical correlations known to exist in native renal biopsies to investigate novel transplant–specific associations. Figure 1 shows a photograph of a TRI in a glomerular endothelial cell of a recipient of a transplant.
Figure 1.
EM photograph showing a TRI in a glomerular endothelial cell.
Results
In total, 316 of 1164 (27.1%) patients transplanted at our center between 2005 and 2014 had at least one biopsy with EM examination, and 41 of 316 (13.0%) patients who had EM examination were found to have TRIs; 82 patients who were TRI negative were used as controls. The mean time to biopsy post-transplantation was 25.6±21.8 and 20.0±20.3 months in the TRI+ and TRI− groups, respectively (P=0.15). The mean follow-up postbiopsy was 37.3±23.1 and 41.5±21.4 months in the TRI+ and TRI− groups, respectively (P=0.33).
Patient demographics are shown in Table 1. There was no significant difference in sex, ethnicity, and age at transplantation between the TRI+ and TRI− groups. Four of 41 (9.8%) and three of 82 (3.8%) patients had reached end stage renal failure secondary to SLE in the TRI+ and TRI− groups, respectively (P=0.22). There was no difference in the mean HLA mismatch between the groups. However, there were significantly more sensitized patients in the TRI+ group compared with the TRI− group, with 20 of 41 (48.8%) and 18 of 82 (22.0%) being sensitized, respectively (P=0.01). Nine of 41 (22.0%) and five of 82 (6.1%) patients who were TRI+ and TRI−, respectively, had performed DSA at the time of transplantation (P=0.02).
Table 1.
Patient demographics
| Characteristic | TRI+ (n=41; %) | TRI− (n=82; %) | P Value |
|---|---|---|---|
| Sex | |||
| Women | 16 (39.0) | 25 (30.5) | 0.46 |
| Men | 25 (61.0) | 57 (69.5) | |
| Age at Tx, yr | 51.9±11.7 | 48.2±12.7 | 0.46 |
| Age at ESRD, yr | 46.9±12.6 | 44.8±13.0 | 0.40 |
| Cause of ESRD | |||
| APKD | 3 (7.3) | 7 (8.5) | 0.97 |
| GN [SLE] | 11 (26.8) [4] | 24 (29.3) [3] | |
| DM | 11 (26.8) | 18 (22.0) | |
| Urologic | 2 (4.9) | 3 (3.7) | |
| Other | 4 (9.8) | 6 (7.3) | |
| Unknown | 10 (24.4) | 24 (29.3) | |
| Ethnicity | |||
| Black | 4 (9.8) | 13 (15.9) | 0.64 |
| White | 20 (48.8) | 25 (30.5) | |
| Indoasian | 14 (34.1) | 38 (46.3) | |
| Other | 3 (7.3) | 6 (7.3) | |
| Tx type | |||
| DD | 24 (58.5) | 40 (48.8) | 0.43 |
| LD | 14 (34.1) | 35 (42.7) | |
| SPK | 3 (7.3) | 7 (8.5) | |
| Graft number | |||
| 1 | 32 (78.0) | 71 (86.6) | 0.34 |
| ≥2 | 9 (22.0) | 11 (13.4) | |
| Preemptive Tx | |||
| Yes | 9 (22.0) | 14 (17.1) | 0.68 |
| No | 32 (78.0) | 68 (82.9) | |
| Induction agent | |||
| Alemtuzumab | 33 (80.4) | 68 (82.9) | 0.93 |
| IL2RA | 8 (19.5) | 14 (17.1) | |
| HLA mismatch, mean | 3.37±1.36 | 3.34±1.60 | 0.93 |
| Sensitization at time of Tx | |||
| Yes [DSAs] | 20 (48.8) [9] | 18 (22.0) [5] | 0.02 [<0.01] |
| No | 21 (51.2) | 64 (78.0) | |
| Timing of biopsy post-Tx, mo | 25.9±21.8 | 20.3±20.3 | 0.15 |
| Length of follow-up postbiopsy, mo | 37.3±23.1 | 41.5±21.4 | 0.33 |
Tx, transplant; APKD, autosomal dominant polycystic kidney disease; DM, diabetic nephropathy; DD, deceased donor; LD, living donor; SPK, simultaneous pancreas and kidney; IL2RA, IL-2 receptor antagonist.
Patient and Allograft Outcomes
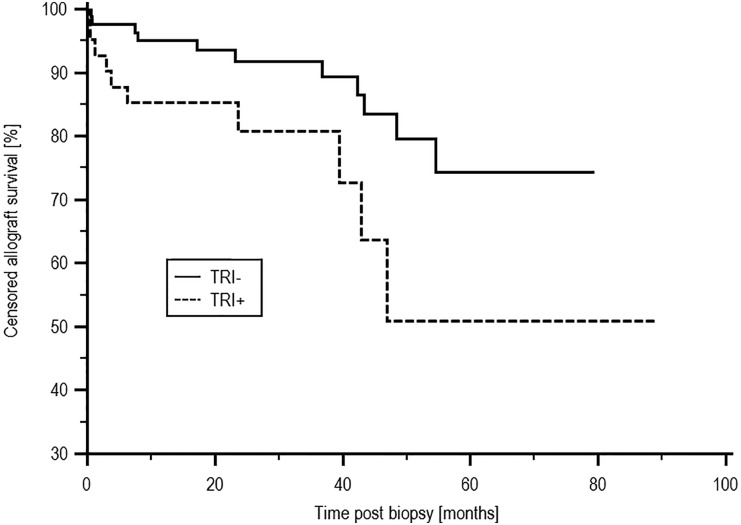
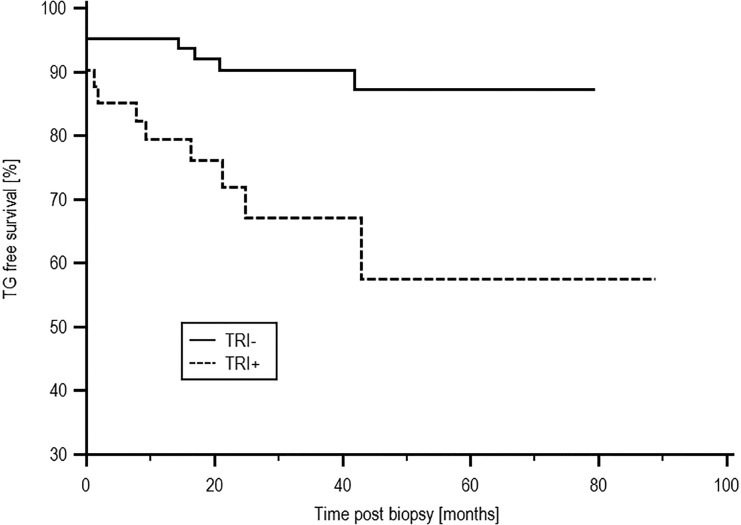
There was no difference in patient survival between the TRI+ and TRI− groups, with survival of 66.4% and 81.5%, respectively (P=0.21). Death–censored allograft survival was inferior in the TRI+ compared with the TRI− group, with an allograft survival of 50.9% and 74.3%, respectively (P=0.03), as shown in Figure 2. Of 10 causes of allograft loss in the TRI+ group, three were caused by rejection alone, one was caused by recurrent/de novo disease alone (FSGS), five were caused by rejection with evidence of recurrent/de novo disease (three FSGS, one atypical haemolytic uraemic syndrome, and one membranoproliferative glomerulonephritis), and one was caused by involved progressive scarring in a marginal graft. In 11 patients who were TRI− and lost their allograft, four losses were secondary to rejection, three were caused by a combination of rejection and recurrent disease (one lupus nephritis, one IgA, and one complement factor H-related protein 5 nephropathy), and four failures were because of other causes. Patients who were TRI+ were more likely to have had a biopsy–proven rejection episode before the index biopsy; 14 of 41 (34.1%) patients who were TRI+ and 12 of 82 (14.6%) patients who were TRI− had experienced rejection previously (P=0.02). There was a trend toward increased incidence of subsequent acute rejection episodes in patients who were TRI+, with a rejection-free survival of 40.8% and 70.9% in the TRI+ and TRI− groups, respectively (P=0.09). TG-free survival was inferior in the TRI+ group at 57.5% compared with 87.3% in patients who were TRI− (P=0.002) as shown in Figure 3. There was no difference in the median serum creatinine at the time of biopsy between the TRI+ (166.0 μmol/L; interquartile range [IQR], 101.5–219.0 μmol/L) and TRI− groups (151.0 μmol/L; IQR, 124.0–191.0 μmol/L; P=0.96). Subsequent allograft function measured at yearly time points postindex biopsy also did not differ between the two groups and is shown in Supplemental Material. There was no difference in proteinuria at the time of index biopsy between the groups, with median protein creatinine ratios of 48.0 (IQR, 13.75–134.75) units and 82.0 (IQR, 23.0–167.0) units in the TRI+ and TRI− groups, respectively (P=0.25).
Figure 2.
Censored allograft survival post-EM by presence of TRIs. Death–censored allograft survival from the time of index biopsy was inferior in the TRI+ compared with the TRI− group, with allograft survival of 50.9% and 74.3%, respectively (P=0.03).
Figure 3.
TG-free survival post-EM by presence of TRIs. TG-free survival was inferior in the TRI+ group at 57.5% compared with 87.3% in patients who were TRI− (P=0.002).
Serologic and Histologic Risk Factors for TRIs
Twelve of 41 (29.3%) patients who were TRI+ had evidence of viral infection compared with 12 of 82 (14.6%) patients who were TRI− (P=0.09). Table 2 shows the distribution of viral infections. There was a significantly higher number of cases of hepatitis C in patients who were TRI+, with three of 41 [7.3%] patients who were TRI+ and no patients who were TRI− having hepatitis C, respectively (P=0.04). There was no difference in the prevalence of malignancy or serologic evidence of autoimmunity between the groups. However, a statistically higher number of patients who were TRI+ had serologic evidence of alloimmunity, because 20 of 41 (48.8%) patients who were TRI+ had a DSA at the time of biopsy compared with 20 of 82 (24.4%) patients who were TRI− (P=0.01); 13 of 41 (31.2%) patients who were TRI+ had class 1 HLA DSAs (five combined with class 2) compared with 11 of 82 (13.4%) patients who were TRI− (three combined with class 2; P=0.03). In total, 12 of 41 (29.3%) patients who were TRI+ had class 2 HLA DSAs compared with 12 of 82 (14.6%) patients who were TRI− (P=0.09).
Table 2.
Risk factors for TRIs
| Risk Factor | TRI+ (n=41; %) | TRI− (n=82; %) | P Value |
|---|---|---|---|
| Traditional risk factors | |||
| Viral infection | |||
| Total | 12 (29.3) | 12 (13.4) | 0.09 |
| EBV | 4 (9.8) | 4 (4.9) | |
| Hepatitis C | 3 (7.3) | 0 | |
| Hepatitis E | 1 (2.4) | 0 | |
| CMV | 1 (2.4) | 2 (2.4) | |
| EBV + CMV | 1 (2.4) | 0 | |
| BK | 2 (4.9) | 5 (6.1) | |
| HIV | 0 | 1 (1.2) | |
| Malignancy, total | 2 (4.9) | 2 (2.4) | 0.60 |
| Autoimmune | |||
| Total | 8 (19.5) | 10 (12.2) | 0.42 |
| Low C3 or C4 | 4 (9.8) | 4 (4.9) | |
| dsDNA | 2 (4.9) | 2 (2.4) | |
| PR3/ACA | 1 (2.4) | 0 | |
| MPO | 1 (2.4) | 1 (1.2) | |
| PR3 | 0 | 3 (3.7) | |
| Infection | |||
| Total | 3 (7.3) | 4 (4.9) | 0.68 |
| UTI | 3 (7.3) | 4 (4.9) | |
| Alloimmune risk factors | |||
| DSAs | |||
| Yes | 20 (48.8) | 20 (24.4) | 0.01 |
| No | 21 (51.2) | 62 (75.6) | |
| HLA class | |||
| Class 1 alone | 8 (19.5) | 8 (9.8) | 0.03 |
| Class 2 alone | 7 (17.1) | 9 (11.0) | 0.09 |
| Classes 1 and 2 | 5 (12.2) | 3 (3.7) |
Epstein-Barr virus; BK, BK virus; dsDNA, double stranded DNA; PR3, proteinase-3 antibodies; ACA, anticardiolipin antibodies; MPO, myeloperoxidase antibodies; UTI, urinary tract infection.
Table 3 shows a comparison of the histologic features between the TRI+ and TRI− groups. There was no difference in the presence of glomerulitis, peritubular capillaritis (ptc), or chronic glomerulopathy between the TRI+ and TRI− groups. Fifteen of 41 (36.59%) patients who were TRI+ compared with 24 of 82 (29.27%) patients who were TRI− had glomerulitis (P=0.54); 10 of 41 (24.39%) patients who were TRI+ compared with 14 of 82 (17.07%) patients who were TRI− had capillaritis (P=0.47), and six of 41 (14.6%) patients who were TRI+ compared with six of 82 (7.32%) patients who were TRI− had chronic glomerulopathy scores ≥1 (P=0.33). There was also no difference between the TRI+ and TRI− groups when considering these lesions in terms of the mean Banff scores. However, there was a trend toward a higher proportion of the TRI− group having a microcirculation inflammation (MI) score of zero compared with the TRI+ group, with 53 of 82 of the TRI− group (64.63%) and 20 of 41 of the TRI+ group (48.78%) having an MI score of zero (P=0.14). There was no difference in the proportions of patients with C4d positivity (C4d 2 or 3) or tubulitis or interstitial inflammation between the TRI+ and TRI− groups.
Table 3.
Comparison of biopsy characteristics
| Variable and Banff score (where applicable) | TRI+ (n=41; %) | TRI− (n=82; %) | P Value |
|---|---|---|---|
| Type of biopsy | |||
| Surveillance | 14 (34.1) | 20 (24.4) | 0.35 |
| Indicative | 27 (65.9) | 62 (75.6) | |
| g Score | |||
| 0a | 26 (63.41) | 58 (70.73) | 0.54 |
| 1 | 8 (19.51) | 18 (21.95) | |
| 2 | 6 (14.63) | 5 (6.10) | |
| 3 | 1 (2.44) | 1 (1.22) | |
| Mean g score | 0.56±0.84 | 0.38±0.66 | 0.19 |
| ptc Score | |||
| 0a | 31 (75.61) | 68 (82.93) | 0.47 |
| 1 | 7 (17.07) | 7 (8.54) | |
| 2 | 3 (7.32) | 7 (8.54) | |
| 3 | 0 | 0 | |
| Mean ptc score | 0.32±0.61 | 0.28±0.62 | 0.73 |
| MI score (ptc + g) | |||
| 0a | 20 (48.78) | 53 (64.63) | 0.14 |
| 1 | 13 (31.71) | 15 (18.29) | |
| 2 | 5 (12.20) | 7 (8.54) | |
| 3 | 0 | 5 (6.10) | |
| 4 | 2 (4.88) | 2 (2.44) | |
| 5 | 1 (2.44) | 0 | |
| 6 | 0 | 0 | |
| Mean MI score | 0.85±1.22 | 0.64±1.04 | 0.32 |
| cg Score | |||
| 0a | 35 (85.37) | 76 (92.68) | 0.33 |
| 1 | 4 (9.76) | 3 (3.66) | |
| 2 | 1 (2.44) | 1 (1.22) | |
| 3 | 1 (2.44) | 2 (2.44) | |
| Mean cg score | 0.22±0.61 | 0.13±0.54 | 0.43 |
| t Score | |||
| 0a | 29 (70.73) | 55 (67.07) | 0.73 |
| 1 | 9 (21.95) | 18 (21.95) | |
| 2 | 2 (4.88) | 6 (7.32) | |
| 3 | 1 (2.44) | 3 (3.66) | |
| Mean t score | 0.39±0.70 | 0.48±0.79 | 0.56 |
| i Score | |||
| 0a | 32 (78.05) | 66 (80.49) | 0.94 |
| 1 | 4 (9.76) | 8 (9.76) | |
| 2 | 4 (9.76) | 5 (6.10) | |
| 3 | 1 (2.44) | 3 (3.66) | |
| Mean i score | 0.37±0.77 | 0.33±0.76 | 0.82 |
| ct Score | |||
| 0a | 14 (34.15) | 28 (34.15) | 0.84 |
| 1 | 14 (34.15) | 40 (48.78) | |
| 2 | 9 (21.95) | 8 (9.76) | |
| 3 | 4 (9.76) | 5 (6.40) | |
| Mean ct score | 1.07±0.98 | 0.88±0.82 | 0.25 |
| ci Score | |||
| 0a | 14 (34.15) | 28 (34.15) | 0.84 |
| 1 | 14 (34.15) | 40 (48.78) | |
| 2 | 9 (21.95) | 8 (9.76) | |
| 3 | 4 (9.76) | 5 (6.40) | |
| Mean ci score | 1.07±0.98 | 0.88±0.82 | 0.25 |
| v Score | |||
| 0a | 41 (100.0) | 76 (92.68) | 0.18 |
| 1 | 0 | 4 (4.88) | |
| 2 | 0 | 2 (2.44) | |
| 3 | 0 | 0 | |
| Mean v score | 0 | 0.10±0.38 | NA |
| cv Score | |||
| 0a | 14 (34.15) | 28 (34.15) | |
| 1 | 15 (36.59) | 34 (41.46) | |
| 2 | 10 (24.39) | 17 (20.73) | |
| 3 | 2 (4.88) | 3 (3.66) | |
| Mean cv score | 1.00±0.89 | 0.94±0.84 | 0.76 |
| PTCBMML | |||
| Yes | 5 (13.16)b | 11 (14.67)c | 0.95 |
| No | 33 (86.84) | 64 (85.33) | |
| C4d | |||
| 0a | 23 (56.10) | 40 (48.78) | 0.57 |
| 1 | 13 (31.71) | 23 (28.05) | |
| 2 | 4 (9.76) | 10 (12.20) | |
| 3 | 1 (2.44) | 9 (10.98) | |
| Evidence of GN | |||
| Yes | 10 (24.4) | 25 (30.5) | 0.62 |
| No | 31 (75.6) | 57 (69.5) | |
| BK nephropathy | |||
| Yes | 2 (4.9) | 5 (6.1) | 0.99 |
| No | 39 (95.1) | 77 (93.9) |
g, Glomerulitis; cg, chronic glomerulopathy; t, tubulitis; i, interstitial inflammation; ct, tubular atrophy; ci, interstitial fibrosis; v, vasculitis; NA, non-applicable; cv, chronic vascular; PTCBMML, peritubular capillary basement membrane multilayering; BK, BK virus.
Comparator group.
Data available in 38 of 41 patients.
Data available in 75 of 82 patients.
Ten of 41 (24.4%) patients who were TRI+ and 25 of 82 (30.5%) patients who were TRI− had evidence of recurrent or de novo GN (P=0.62). In the TRI+ group, eight of 41 (19.5%) had FSGS, and two of 41 (4.9%) had IgA. In the TRI− group, 12 of 82 (14.6%) had FSGS, eight of 82 (9.8%) had IgA, three of 82 (3.7%) had membranous glomerulonephritis, one of 82 (1.2%) had lupus nephritis, and one of 82 (1.2%) had complement factor H-related protein 5 nephropathy.
Multivariate Analyses of Risk Factors Associated with TRIs and Allograft Loss
Multivariate analysis of factors associated with the presence of TRIs was performed by including all variables found to be significant by univariate analysis (P<0.15). Variables associated with TRIs included previous rejection, class 1 HLA DSAs, class 2 HLA DSAs, viral infection, and MI score ≥1. Factors found to be significant by multivariate analysis included class 1 HLA DSAs (odds ratio [OR], 3.16; 95% confidence interval [95% CI], 1.20 to 8.36; P=0.02), viral infection (OR, 2.66; 95% CI, 1.01 to 7.00; P=0.05), and previous rejection (OR, 2.90; 95% CI, 1.13 to 7.44; P=0.03). There was a trend toward significance for an MI score ≥1 (OR, 2.04; 95% CI, 0.90 to 4.66; P=0.08) as shown in Table 4.
Table 4.
Multivariate analysis of factors associated with TRIs
| Variable | OR | 95% CI | P Value |
|---|---|---|---|
| CI DSAs | 3.16 | 1.20 to 8.36 | 0.02 |
| Prior rejection | 2.90 | 1.13 to 7.44 | 0.03 |
| Viral infection | 2.66 | 1.01 to 7.00 | 0.05 |
| Microcirculation score ≥1 | 2.04 | 0.90 to 4.66 | 0.08 |
A Cox regression analysis of factors associated with poor allograft survival was performed looking at the clinical and histologic variables present at the time of index biopsy. Variables included in the model were Banff scores for g, ptc, arteritis, tubulitis, interstitial inflammation, and glomerular double contours together with the presence of active viral infection, malignancy, positive autoimmune screen, TRIs, and DSAs. Independent factors found to be associated with graft loss in the short term were TRIs (hazard ratio, 3.19; 95% CI, 1.24 to 8.21; P=0.02) and glomerular double contours (hazard ratio, 9.54; 95% CI, 2.06 to 44.21; P=0.004).
Discussion
In this first report of the significance of TRIs in renal allografts, we have made a number of important observations. First, TRIs are not common, but their presence is associated with inferior allograft outcomes. Second and comparable with native renal biopsies, viral infections are significantly associated with glomerular endothelial cell TRIs. Third, a novel observation from this study is that TRIs are seen at an increased frequency in patients with DSAs and evidence of alloimmune injury.
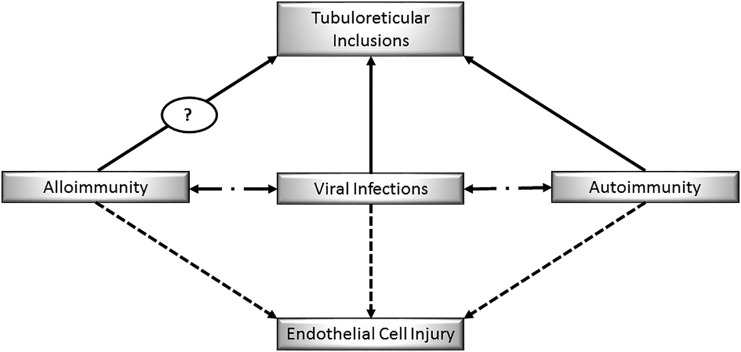
The potential novel association between TRIs and alloimmune injury adds to the intriguing relationship between infection and rejection.18–22 An analogy can be made to the relationship in native renal disease between infection and autoimmune GN.23 Figure 4 is a pictorial representation of these relationships. The premise of our discussion is that, knowing that endothelial cell injury and TRIs are seen in both viral infection and autoimmunity in native disease, we propose that, in transplantation, endothelial cell injury and TRIs may be associated with viral infections and alloimmunity.2,5,7,10
Figure 4.
Theoretical association between viral infection, autoimmunity, and alloimmunity causing endothelial cell injury in native kidneys and renal transplants.
Endothelial cell TRIs may be induced in vitro and in vivo by type 1 IFNs.2–7 There are three main types of IFNs, which collectively play an important role in both innate and adaptive immunity. IFN-α and IFN-β are the clinically relevant type 1 IFNs, and they are produced predominantly by plasmacytoid dendritic cells and fibroblasts. After production, IFN-α/β can mediate antiviral and proapoptotic properties and lead to upregulation of proteins involved in the adaptive immune response.3,5–7 In addition to bacteria and viruses, endogenous RNA or DNA is also a potent inducer of type 1 IFNs, and IFN-α is regarded as the primary cytokine involved in SLE and other autoimmune diseases.2,3,24,25
Infection remains an important cause of morbidity and mortality post-transplantation.26 In this study, we have shown that serologic evidence of viral infection is associated with the presence of renal allograft TRIs. Both primary infection and reactivation of latent viral disease have been associated with allograft rejection; however, the correlation is complex, and the cause and effect relationship has still not been established.18–22,27 Analysis of the histology from patients with rejection and coexisting acute viral infection, of which cytomegalovirus (CMV) and Epstein-Barr virus (EBV) have both been studied, shows a correlation with C4d positivity and the development of chronic allograft nephropathy, but these reports do not include ultrastructural examination for TRIs.27 Independently, it has been shown that infection with CMV can lead to the development of antiendothelial cell antibodies in recipients of renal transplants and recipients of cardiac transplants.28 However, it is not known whether viral infections can lead to the development of de novo HLA DSAs.
In our data, we show that a significantly higher number of patients who are TRI+ had hepatitis C infection. Chronic viral infection post-transplantation, specifically hepatitis C, has recently been associated with TG.15 The delineation between recurrent or de novo hepatitis C–associated membranoproliferative GN and TG is not always clear, with immunofluorescence and/or EM required to show more immune complex deposition in the former.15 TRIs can be seen in native glomerular endothelial cells in patients with hepatitis C; therefore, the presence of TRIs in transplant biopsies will not help differentiate between a hepatitis C–associated membranoproliferative GN and TG. Instead, it may be that their presence will provide additional evidence of ongoing endothelial cell injury.29 Furthermore, not only can the presence of hepatitis C be associated with alloimmune injury, but also, its treatment is also linked with rejection. There are well documented reports of the use of exogenous IFN after solid organ transplantation for the treatment of hepatitis C leading to rejection, which suggests a causal link between IFN-α and alloimmune response.30,31 Outside of transplantation, it has also been shown that treatment with exogenous IFN-α can produce autoantibodies, leading to a catalog of different autoimmune diseases with native renal pathology.32 In one case series, Markowitz et al.6 reported 11 patients with collapsing FSGS after treatment with IFN, and 10 of these patients had TRIs. Of interest, a relatively large proportion of our patients who were TRI+ (eight of 41; 19.5%) had FSGS in their biopsy as well.
Although the importance of EM in determining causes of allograft dysfunction is increasingly apparent, to our knowledge, there has been no dedicated study of TRIs in renal transplant allografts. The finding of TRIs in renal transplant biopsies has been studied in a subanalysis of recurrent lupus post-transplantation.33 In their study, Meehan et al.33 looked at 19 patients who had evidence of recurrent lupus nephritis post-transplant, and 14 of 19 patients had TRIs. Not all of the patients had serologic markers of active lupus, but of note, 10 of 19 patients studied had thrombotic microangiopathy, rejection, or polyoma virus as a coexisting pathology.34 In our study, we have shown a highly significant correlation between DSA and TRIs. However, although there was a trend toward statistical significance between the presence of TRIs and MI on multivariate analysis, there did not seem to be any difference in any other histologic features of rejection between patients who were TRI+ and patients who were TRI−, which fails to support an association of alloimmune injury and TRIs. Interestingly, Hayde et al.17 found that rejection–associated gene transcripts were increased in patients with DSAs who had no evidence of AMR on light microscopy (LM). Taking into account the postulated four–stage model of AMR by Colvin,34 the first two stages represent a period of graft accommodation, where there is evidence of DSAs in the absence of graft pathology.33 Whether endothelial cell transcripts and TRIs may predict escape from the accommodated state and progression to the later stages of AMR, which include graft pathology and dysfunction, is not known. The increased risk of TG and allograft failure in patients who were TRI+ in our study, despite a lack of other features of AMR at the time of index biopsy, would support this theory. However, this hypothesis will need to be tested in the form of a prospective study incorporating analysis of gene transcripts in patients who are TRI+.
Although the precise significance of TRIs in renal allografts is still to be determined, clinically, their presence should not be ignored given the deleterious effect that they have on allograft survival. After the incorporation of EM findings into the Banff classification, additional evidence should emerge to either validate or refute our findings. We should note that there are a number of limitations to our study, which largely result from its retrospective nature. Most importantly, not all patients were routinely screened for viral infections and autoimmune disease at the time of biopsy, and testing was only performed when clinically indicated. Also, because the patients in this study had EM performed for a variety of clinical and/or histologic reasons, the population studied consisted of a cohort at high risk of glomerular injury, whether it be de novo GN or alloimmune injury, and as such, does not represent our overall patient population.
In conclusion, we have reported on the clinicopathologic associations of TRIs in renal allografts in a selective group of patients undergoing indicative EM examination. TRIs are associated with viral infections, which follows intuitively from our knowledge of TRIs in native renal disease. However, the finding of a correlation between TRIs, DSA, and an alloimmune response is novel and warrants additional investigation. Although we acknowledge that we have not conclusively proven such a relationship, we have shown that the presence of TRIs is associated with significant allograft pathology. We suggest that the finding of TRIs in a transplant biopsy should instigate screening for viral infections and DSA.
Concise Methods
A retrospective analysis of prospectively collected data was performed on all patients transplanted at Imperial College Renal and Transplant Centre between 2005 and 2013. Included were recipients of kidneys from both deceased and living donors and recipients of simultaneous pancreas and kidney. We excluded all patients who lost their allograft within the first week post-transplant. All patients had negative T and B cell complement dependent cytotoxicity and T cell flow cytometry cross–match at the time of transplantation. We included patients with low–level preformed DSAs detected by luminex only. We excluded patients who were blood group ABO incompatible or HLA incompatible and underwent antibody removal therapy pretransplantation.
In total, 2946 transplant biopsies were performed in 1164 patients, of which 316 of 1164 (27.1%) patients had EM examination on at least one biopsy. Indications for EM examination at our center include proteinuria, abnormal glomeruli by LM, patient at risk for or with previous features of AMR, and patient at risk for or with previous histologic features of recurrent or de novo GN. As such, patients who have EM examination tend to have worse allograft outcomes than patients not requiring EM. Data comparing the outcomes of patients who underwent EM examination with those who did not can be found in Supplemental Material. Our patients were those who were found to have TRIs on EM. Controls were the first patients to be transplanted immediately before and after each patient who also underwent EM examination. Thus, the ratio of patients to controls was 1:2.
All patients received mAb induction with alemtuzumab (Campath-1H, Cambridge, MA), Daclizumab (Zenax; Roche, Basel, Switzerland), or Basiliximab (Simulect; Novartis Pharma Corp., Basel, Switzerland). Maintenance immunosuppression in the alemtuzumab group consisted of tacrolimus monotherapy. Patients induced with an IL-2 receptor antagonist received tacrolimus with the addition of mycophenolate mofetil. All patients receive 500 mg methylprednisolone perioperatively followed by 1 week of corticosteroids only. Patients on maintenance steroids at the time of transplantation were maintained at their baseline dose post-transplant.
Histologic Examination
All biopsy specimens were examined by LM and classified using the Banff 07 Classification of Renal Allograft Pathology.11 Immunohistochemistry for C4d was performed on paraffin sections from all biopsies. C4d was classified as negative (ptc staining <10%), focal (11%–50%), or diffuse (>50%). For EM examination, fresh renal biopsy tissue was fixed in 3% glutaraldehyde in 0.1 M cacodylate buffer, postfixed in osmium tetroxide, block stained in 2% aqueous uranyl acetate, dehydrated through ascending grades of ethanol, and embedded in Spurr resin. Ultrathin sections were stained with Reynold lead citrate. Peritubular basement membrane multilayering is defined as more than five layers in three or more peritubular capillaries of 25 examined.
For analysis purposes, prior rejection refers to patients who had histologic evidence of AMR, T cell–mediated rejection, or both on an indicative or surveillance biopsy before their index biopsy. Patients with histologic evidence of AMR had indication or surveillance biopsies with features of active or chronic active AMR (C4d positive or negative) as defined by Banff 2013. TG was defined as the presence of double contours by LM in the absence of immune complex deposition by immunofluorescence and/or EM.
Serologic Examination
Patients are routinely screened for DSAs at 1, 3, 6, and 12 months post-transplant and then yearly thereafter and also, at times of allograft dysfunction. Patients are screened initially using LABScreen Mixed Beads (One Lambda, Inc., Canoga Park, CA) if nonsensitized and subsequently or primarily screened using LABScreen Single-Antigen Beads if sensitized. We include DSAs to HLA-A, -B, -Cw, -DR, -DQ, and -DP antigens in our study. A mean fluorescence index of >500 by single-antigen beads on two separate occasions was taken as positive.
Autoantibody and virology screening were requested only when clinically indicated. Epstein-Barr virus and CMV viremia was defined as detectable viral DNA by PCR in proximity to the transplant biopsy. Patients who are HIV positive with no viral load were included, because TRIs have been reported in such patients in native kidneys.5,8,9
Statistical Analyses
All analyses were performed using Medcalc, version 10.4.3. Comparisons of means and frequencies of normally distributed variables were calculated using t tests and chi-squared/Fisher exact tests. Kaplan–Meier survival analysis was used to calculate time of event from index biopsy, and statistical significance was determined by log rank testing. Multivariate analyses were performed using logistic and Cox proportional regression methods. A P value of <0.05 was deemed statistically significant.
Disclosures
A.G.M. has received research funding from Astellas Pharma UK Ltd. (Surrey, UK). D.T. has received consultation fees from Sandoz Ltd. (Surrey, UK). C.R. is supported by Roche (Basel, Switzerland) Organ Transplantation Research Foundation Project Grant CI 857917463.
Supplementary Material
Acknowledgments
The authors thank the staffs of the Transplant Clinic, Histocompatibility and Immunogenetics Laboratory, Histopathology Department, and Planned Investigation Unit at Hammersmith Hospital for contributed work. Tissue samples were provided by the Imperial College Healthcare National Health Service Trust Tissue Bank.
The research was supported by the National Institute for Health Research Biomedical Research Centre based at the Imperial College Healthcare NHS Trust and Imperial College London.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015050478/-/DCSupplemental.
References
- 1.Anders HJ, Lichtnekert J, Allam R: Interferon-alpha and -beta in kidney inflammation. Kidney Int 77: 848–854, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Meyer O: Interferons and autoimmune disorders. Joint Bone Spine 76: 464–473, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Baccala R, Kono DH, Theofilopoulos AN: Interferons as pathogenic effectors in autoimmunity. Immunol Rev 204: 9–26, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Hammar SP, Luu JY, Bockus DE, Remington FL, Luu JW, Friedman S, Bean MA: Induction of tubuloreticular structures in cultured human endothelial cells by recombinant interferon alfa and beta. Ultrastruct Pathol 16: 211–218, 1992 [DOI] [PubMed] [Google Scholar]
- 5.Malmgaard L: Induction and regulation of IFNs during viral infections. J Interferon Cytokine Res 24: 439–454, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Markowitz GS, Nasr SH, Stokes MB, D’Agati VD: Treatment with IFN-alpha, -beta, or -gamma is associated with collapsing focal segmental glomerulosclerosis. Clin J Am Soc Nephrol 5: 607–615, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Münz C, Lünemann JD, Getts MT, Miller SD: Antiviral immune responses: Triggers of or triggered by autoimmunity? Nat Rev Immunol 9: 246–258, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee CJ, Suh KS, Kim KH, Chang YK, Na KR, Lee KW: The clinicopathologic significance of endothelial tubuloreticular inclusions in glomerular diseases. Ultrastruct Pathol 37: 386–394, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Singh HK, Nickeleit V: Kidney disease caused by viral infections. Curr Diagn Pathol 10: 11–21, 2004 [Google Scholar]
- 10.Yang AH, Lin BS, Kuo KL, Chang CC, Ng YY, Yang WC: The clinicopathological implications of endothelial tubuloreticular inclusions found in glomeruli having histopathology of idiopathic membranous nephropathy. Nephrol Dial Transplant 24: 3419–3425, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Haas M, Sis B, Racusen LC, Solez K, Glotz D, Colvin RB, Castro MC, David DS, David-Neto E, Bagnasco SM, Cendales LC, Cornell LD, Demetris AJ, Drachenberg CB, Farver CF, Farris AB 3rd, Gibson IW, Kraus E, Liapis H, Loupy A, Nickeleit V, Randhawa P, Rodriguez ER, Rush D, Smith RN, Tan CD, Wallace WD, Mengel M; Banff meeting report writing committee: Banff 2013 meeting report: Inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant 14: 272–283, 2014 [DOI] [PubMed] [Google Scholar]
- 12.Roufosse CA, Shore I, Moss J, Moran LB, Willicombe M, Galliford J, Chan KK, Brookes PA, de Kort H, McLean AG, Taube D, Cook HT: Peritubular capillary basement membrane multilayering on electron microscopy: A useful marker of early chronic antibody-mediated damage. Transplantation 94: 269–274, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Wavamunno MD, O'Connell PJ, Vitalone M, Fung CL, Allen RD, Chapman JR, Nankivell BJ: Transplant glomerulopathy: Ultrastructural abnormalities occur early in longitudinal analysis of protocol biopsies. Am J Transplant 7: 2757–2768, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Issa N, Cosio FG, Gloor JM, Sethi S, Dean PG, Moore SB, DeGoey S, Stegall MD: Transplant glomerulopathy: Risk and prognosis related to anti-human leukocyte antigen class II antibody levels. Transplantation 86: 681–685, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Baid-Agrawal S, Farris AB 3rd, Pascual M, Mauiyyedi S, Farrell ML, Tolkoff-Rubin N, Collins AB, Frei U, Colvin RB: Overlapping pathways to transplant glomerulopathy: Chronic humoral rejection, hepatitis C infection, and thrombotic microangiopathy. Kidney Int 80: 879–885, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Sis B, Jhangri GS, Bunnag S, Allanach K, Kaplan B, Halloran PF: Endothelial gene expression in kidney transplants with alloantibody indicates antibody-mediated damage despite lack of C4d staining. Am J Transplant 9: 2312–2323, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Hayde N, Broin PO, Bao Y, de Boccardo G, Lubetzky M, Ajaimy M, Pullman J, Colovai A, Golden A, Akalin E: Increased intragraft rejection-associated gene transcripts in patients with donor-specific antibodies and normal biopsies. Kidney Int 86: 600–609, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Babel N, Schwarzmann F, Prang N, Jaeger M, Wolf H, Kern F, Volk HD, Reinke P: Association between Epstein-Barr virus infection and late acute transplant rejection in long-term transplant patients. Transplantation 72: 736–739, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Cainelli F, Vento S: Infections and solid organ transplant rejection: A cause-and-effect relationship? Lancet Infect Dis 2: 539–549, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Reinke P, Fietze E, Ode-Hakim S, Prösch S, Lippert J, Ewert R, Volk HD: Late-acute renal allograft rejection and symptomless cytomegalovirus infection. Lancet 344: 1737–1738, 1994 [DOI] [PubMed] [Google Scholar]
- 21.Reischig T, Jindra P, Svecova M, Kormunda S, Opatrny K Jr., Treska V: The impact of cytomegalovirus disease and asymptomatic infection on acute renal allograft rejection. J Clin Virol 36: 146–151, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Smith C, Khanna R: Immune regulation of human herpesviruses and its implications for human transplantation. Am J Transplant 13[Suppl 3]: 9–23, 2013 [DOI] [PubMed]
- 23.Couser WG, Johnson RJ: The etiology of glomerulonephritis: Roles of infection and autoimmunity. Kidney Int 86: 905–914, 2014 [DOI] [PubMed] [Google Scholar]
- 24.Allam R, Lichtnekert J, Moll AG, Taubitz A, Vielhauer V, Anders HJ: Viral RNA and DNA trigger common antiviral responses in mesangial cells. J Am Soc Nephrol 20: 1986–1996, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hägele H, Allam R, Pawar RD, Reichel CA, Krombach F, Anders HJ: Double-stranded DNA activates glomerular endothelial cells and enhances albumin permeability via a toll-like receptor-independent cytosolic DNA recognition pathway. Am J Pathol 175: 1896–1904, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Snyder JJ, Israni AK, Peng Y, Zhang L, Simon TA, Kasiske BL: Rates of first infection following kidney transplant in the United States. Kidney Int 75: 317–326, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Aiello FB, Calabrese F, Rigotti P, Furian L, Marino S, Cusinato R, Valente M: Acute rejection and graft survival in renal transplanted patients with viral diseases. Mod Pathol 17: 189–196, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Toyoda M, Galfayan K, Galera OA, Petrosian A, Czer LS, Jordan SC: Cytomegalovirus infection induces anti-endothelial cell antibodies in cardiac and renal allograft recipients. Transpl Immunol 5: 104–111, 1997 [DOI] [PubMed] [Google Scholar]
- 29.Cosio FGR, Roche Z, Agarwal A, Falkenhain ME, Sedmak DD, Ferguson RM: Prevalence of hepatitis C in patients with idiopathic glomerulopathies in native and transplant kidneys. Am J Kidney Dis 28: 752–758, 1996 [DOI] [PubMed] [Google Scholar]
- 30.Sharma RK, Bansal SB, Gupta A, Gulati S, Kumar A, Prasad N: Chronic hepatitis C virus infection in renal transplant: Treatment and outcome. Clin Transplant 20: 677–683, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Walter T, Dumortier J, Guillaud O, Hervieu V, Paliard P, Scoazec JY, Boillot O: Rejection under alpha interferon therapy in liver transplant recipients. Am J Transplant 7: 177–184, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Gota C, Calabrese L: Induction of clinical autoimmune disease by therapeutic interferon-α. Autoimmunity 36: 511–518, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Meehan SM, Chang A, Khurana A, Baliga R, Kadambi PV, Javaid B: Pauci-immune and immune glomerular lesions in kidney transplants for systemic lupus erythematosus. Clin J Am Soc Nephrol 3: 1469–1478, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Colvin RB: Antibody-mediated renal allograft rejection: Diagnosis and pathogenesis. J Am Soc Nephrol 18: 1046–1056, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.