Abstract
CKD prevalence estimation is central to CKD management and prevention planning at the population level. This study estimated CKD prevalence in the European adult general population and investigated international variation in CKD prevalence by age, sex, and presence of diabetes, hypertension, and obesity. We collected data from 19 general-population studies from 13 European countries. CKD stages 1–5 was defined as eGFR<60 ml/min per 1.73 m2, as calculated by the CKD-Epidemiology Collaboration equation, or albuminuria >30 mg/g, and CKD stages 3–5 was defined as eGFR<60 ml/min per 1.73 m2. CKD prevalence was age- and sex-standardized to the population of the 27 Member States of the European Union (EU27). We found considerable differences in both CKD stages 1–5 and CKD stages 3–5 prevalence across European study populations. The adjusted CKD stages 1–5 prevalence varied between 3.31% (95% confidence interval [95% CI], 3.30% to 3.33%) in Norway and 17.3% (95% CI, 16.5% to 18.1%) in northeast Germany. The adjusted CKD stages 3–5 prevalence varied between 1.0% (95% CI, 0.7% to 1.3%) in central Italy and 5.9% (95% CI, 5.2% to 6.6%) in northeast Germany. The variation in CKD prevalence stratified by diabetes, hypertension, and obesity status followed the same pattern as the overall prevalence. In conclusion, this large-scale attempt to carefully characterize CKD prevalence in Europe identified substantial variation in CKD prevalence that appears to be due to factors other than the prevalence of diabetes, hypertension, and obesity.
Keywords: chronic kidney disease, clinical epidemiology, creatinine
CKD reduces lifespan significantly.1 Individuals with CKD have an increased risk of cardiovascular disease and may develop ESRD.1,2 Fortunately, the development of these complications can be delayed or prevented.1
CKD prevalence estimation is central to CKD management and prevention planning at the population level.3 Identification of countries with a relatively low or high CKD prevalence will guide the medical community and policy makers where to focus prevention and disease management strategies. To date, international comparisons have been hampered by differences in national age and sex distributions and in definitions of CKD.4 Moreover, prevalence estimates are influenced by the use of different creatinine determination methods.5,6 Because diabetes, hypertension, and obesity are important risk factors for CKD,2 the prevalence of these diseases should be taken into account when comparing CKD prevalence. Whether disparities in CKD prevalence are explained by these risk factors will guide policy makers to focus on secondary or primary prevention.
Therefore the purpose of our study was (1) to estimate the CKD prevalence in the adult general population across Europe, and (2) to investigate variation in prevalence across countries by age, sex, and the presence of diabetes, hypertension, and obesity. We collected data from 19 general population-based studies from 13 European countries and estimated CKD prevalence using one definition of CKD.
Results
Study Characteristics
Study Populations
We included data from 19 general population-based studies from 13 European countries (Table 1). Table 2 presents study population characteristics and laboratory methods for the nine studies using isotope dilution mass spectrometry (IDMS) traceable creatinine, which included a large spectrum of the adult population (≥45 years). Supplemental Appendix 1, Table 1 presents these data for all 19 studies for subjects aged ≥65 years.
Table 1.
Description of included studies and study sampling methods
| Country | Region(s)/Cities | Study | Age Range | Sampling Frame | Sample Selection | Response (%) | Representativenessb |
|---|---|---|---|---|---|---|---|
| Finland | All | FINRISK | 25–74 | Population register | Age and sex stratified sample | 70 | Yes |
| France | Bordeaux, Dijon and Montpellier | Three City | 65+ | Electoral rolls | Random sample of noninstitutionalized individuals | 37 | Yes |
| Lille, Bas-Rhin and Haute-Garonne | MONALISA | 35–74 | Electoral rolls | Age and sex stratified random sample | 51 | Yes | |
| Germany | South | ActiFE | 65–91 | Population register | Age and sex stratified random sample of noninstitutionalized individuals | 20 | Yes |
| Southwest | ESTHER | 50–74 | General practitioners lists | Recruitment during biannually health examination for older adults | Not given | Yesc | |
| Northeast | SHIP | 20–79 | Population registers | Two stages: | 69 | Yes | |
| (1) Stratification based on N. of residents per municipality | |||||||
| (2) Age and sex stratified random sample selection per community | |||||||
| Ireland | All | SLAN | 45+ | Postal residential lists | Age and urban/rural location and social class stratified sample | 66 | Yesd |
| Italy | Northeast | INCIPE | 40+ | General practitioners lists | Random selection of participants from 62 random selected practices | 62 | Yes |
| Central | MATISS | 20–79 | Electoral rolls | Age and sex stratified random sample of four municipalities | 60 | Yes | |
| South | VIP | 25–74 | Electoral rolls | Age and sex stratified random sample | 72 | Yes | |
| Netherlands | North | LifeLines | 20+ | General practitioners lists | Two stages: | Not given | Yes |
| (1) All subjects aged 25–50 years registered with general practitionera | |||||||
| (2) Family members of first sample | |||||||
| Groningen | PREVEND | 28–75 | Population register | Three stages: | 48 | Yes | |
| (1) All inhabitants of city | |||||||
| (2) Selection of subjects based on albuminuria level | |||||||
| (3) Correction for oversampling of albuminuria | |||||||
| Norway | Central | HUNT | 20+ | Census data | All residents in region | 71 | Yes |
| Poland | All | PolSenior | 65+ | Population register | Three stages: | 42 | Yes |
| (1) Stratification based on N. of residents per municipality | |||||||
| (2) Stratification based on streets/towns | |||||||
| (3) Age stratified random sample | |||||||
| Portugal | All | PREVADIAB | 20–79 | Universal health card (held by 99% of population) | Two stages: | 84 | Yesd |
| (1) Age and sex stratified sample | |||||||
| (2) Correction for unintentional oversampling of elderly females | |||||||
| Spain | All | EPIRCE | 20+ | Census data | Age and sex and habitat stratified random sample | 43 | Yes |
| Sweden | Uppsala | PIVUS | 70–70 | Population register | Random selection of all (70-year-old) residents | 50 | n/a |
| Switzerland | Southwest | Bus Santé | 35–74 | Population register | Age and sex stratified sample | 62 | Yese |
| UK | All | MRC | 75+ | General practitioners lists | Patients registered in a representative sample of general practices | 73 | Yes |
Study acronyms: FINRISK, Finland Cardiovascular Risk Study; MONALISA, MOnitoring NAtionaL du rISque Arteriel; ActiFE Ulm, Activity and Function in the Elderly in Ulm study; ESTHER, Epidemiologische Studie zu Chancen der Verhütung, Früherkennung und optimierten THerapie chronische ERkrankungen in der älteren Bevolkerung; SHIP, Study of Health in Pomeranzia; SLAN, Survey of Lifestyle and Attitudes & Nutrition in Ireland; INCIPE, Initiative on Nephropathy, of relevance to public health, which is Chronic, possibly in its Initial stages, and carries a Potential risk of major clinical Endpoints; MATISS, Malattie cardiovascolari ATerosclerotiche Istituto Superiore di Sanita; VIP, Valle dell’Irno Prevenzione; LifeLines, LifeLines Cohort and Study Biobank; PREVEND, Prevention of Renal and Vascular End-stage Disease; HUNT, Nord-Trøndelag Health Study; PolSenior, Medical, psychologic, sociological and economical aspects of aging of people in Poland; PREVADIAB, Prevalence of Diabetes and Risk Factors in Portugal; EPIRCE, Estudio Epidemiológico de la Insuficiencia Renal en España; PIVUS, Prospective Investigation of the Vasculature in Uppsala Seniors Study; MRC, Medical Research Council trial of assessment and management of older people in the community.
LifeLines additionally included participants which actively volunteered to participate; however, the vast majority of the LifeLines cohort presented in this paper was recruited through their general practitioner.
Representative = age and sex distribution of the study sample is nonsignificantly different from regional/national age and sex distribution.
Under sampling of males in age group 55–59 years, the remaining age ranges were nonsignificantly different from Saarland population of 2000.
After the study population was weighted to census data, there was no significant difference.
Under sampling of age group 35–39 years, the remaining age ranges were nonsignificantly different from the Geneva population of 2005.
Table 2.
Study population characteristics and laboratory methods of IDMS studies covering age range 45–74 years
| Country | Finland | Germany | Italy | Netherlands | Norway | Spain | Italy | Ireland | Switzerland |
|---|---|---|---|---|---|---|---|---|---|
| Study | FINRISK | SHIP | MATISS | LifeLines | HUNT | EPIRCE | INCIPE | SLAN | Bus Santé |
| Age range | Ages 20+ | Ages 45+ | |||||||
| N study population | 4228 | 4308 | 3780 | 94,133 | 65,252 | 2746 | 3868 | 1160 | 4748 |
| Sample collection years | 2007 | 1997–2001 | 1993–1996 | 2007–2013 | 1995–1997 | 2004–2008 | 2006–2007 | 2007 | 2005–2008 |
| Mean age, years (SD) | 49.7 (12.5) | 49.8 (16.4) | 49.2 (14.1) | 44.6 (12.5) | 50.3 (17.3) | 49.3 (16.3) | 59.8 (11.4) | 59.7(9.9) | 58.4 (11.0) |
| Females (%) | 54.3 | 50.9 | 48.8 | 58.7 | 53.22 | 58.2 | 52,0 | 56,0 | 48.9 |
| DM (%) | 8.4 | 11.0 | 5.1 | 2.4 | 3.4 | 10.3 | 7.42 | 7.7 | 4.2 |
| HT (%) | 43.3 | 52.5 | 15.6 | 18.2 | 43.7 | 41.2 | 35.89 | 57,0 | 23,0 |
| Smokers (%) | 18.8 | 30.3 | 27.6 | 18.8 | 29.2 | 25.3 | 15.32 | 18.3 | 13.6 |
| Mean SBP, mmHg (SD) | 132 (19) | 136 (21) | 140 (23) | 126 (15) | 138 (22) | 131 (22) | 138 (20) | 140 (20) | 127 (23) |
| Mean DBP, mmHg (SD) | 79 (11) | 83 (11) | 85 (13) | 74 (9) | 80 (12) | 79 (12) | 85 (10) | 82 (13) | 76 (11) |
| Mean BMI (kg/m2) (SD) | 27.1 (4.9) | 27.3 (4.8) | 27.7 (4.5) | 26.1 (4.3) | 26.4 (4.10) | 27.5 (5.3) | 26.7 (5.2) | 27.98 (4.58) | 25.6(4.17) |
| ARB use (%) | n/a | 1.7 | n/a | n/a | n/a | 5,0 | n/a | n/a | n/a |
| ACEi use (%) | n/a | 12.8 | n/a | n/a | n/a | 6.4 | n/a | n/a | n/a |
| ACEi/ARB use (%) | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| Creatinine method | Enzymatic | Jaffe | Enzymatic | Enzymatic | Jaffe | Jaffe | Jaffe | Jaffe | Jaffe |
| IDMS traceable creatinine? | Yes | Yes | Yes | Yes | Yes | Yes | Standardized | no | yes |
| Albuminuria method | n/a | Immunoassay | n/a | Immunoassay | Immunoassay | Immunoassay | Immunoassay | Bromocresol green | n/a |
| Mean eGFR by CKD-EPI (SD) | 89.5 (15.0) | 82.2 (17.0) | 100.4 (14.8) | 96.5 (15.0) | 97.6 (19.5) | 86.6 (18.0) | 85.7 (16) | 80.7(16.9) | 89.5(13.4) |
| Mean eGFR by MDRD (SD) | 86.9 (15.0) | 79.4 (15.1) | 103.4 (19.7) | 93.4 (17.1) | 100.1 (24.2) | 84.1 (17.7) | 87.8 (20) | 81.1(18.3) | 90.6(15.8) |
| Median ACR mg/g (25–75 p) | n/a | 8.4 (5.1–17.3) | n/a | 2.2 (1.3–4.2) | 10.3 (7.4–14.5) | 4.6 (2.5–8.6) | Available in dip+ | 9.45(4.46–18.42) | n/a |
DM, diabetes mellitus; HT, hypertension; SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; ARB, angiotensin receptor blockers; ACEi, angiotensin-converting enzyme inhibitors; ARB/ACEi, use of either ARB or ACEi; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; MDRD, Modification of Diet in Renal Disease; ACR, albumin-to-creatinine ratio; FINRISK, Finland Cardiovascular Risk Study; n/a, not applicable; MATISS, Malattie cardiovascolari ATerosclerotiche Istituto Superiore di Sanita; LifeLines, LifeLines Cohort and Study Biobank; HUNT, Nord-Trøndelag Health Study; EPIRCE, Estudio Epidemiológico de la Insuficiencia Renal en España; INCIPE, Initiative on Nephropathy, of relevance to public health, which is Chronic, possibly in its Initial stages, and carries a Potential risk of major clinical Endpoints; dip+, ACR was calculated in participants with positive results on the dipstick test for proteinuria; SLAN, Survey of Lifestyle and Attitudes & Nutrition in Ireland.
Representativeness of Study Populations
Thirteen (68%) studies used a population register/electoral rolls as sampling frame to identify eligible participants (Table 1). Four (21%) studies used general practitioner lists and two studies used other sampling frames. The response varied between 20% and 84%, with two studies not providing a response percentage. The response was at least 60% in ten (59%) studies. Five studies independently performed nonresponse analyses to test representativeness of study populations.7–11 Two studies (Survey of Lifestyle and Attitudes & Nutrition in Ireland, and Prevalence of Diabetes and Risk Factors in Portugal) weighted the study population to correct for oversampling of females and older individuals in comparison to their respective national population. In the nine studies that covered the entire adult population, the age and sex distribution was not significantly different from the age and sex distribution of their respective target populations (Table 1).
Creatinine Measurements
Serum creatinine was determined by Jaffe assays in the majority of studies (n=14; 74%) and four studies used enzymatic assays (21%). The Medical Research Council trial of assessment and management of older people in the community study used both Jaffe and enzymatic assays, with the majority of laboratories using Jaffe assays. IDMS standardization was used in 13 studies (68%), of which the CKD prevalence estimates are presented in the body of this paper; the CKD prevalence estimates of studies using non–IDMS-standardized creatinine (n=6, 32%) are presented exclusively in Supplemental Appendix 1.
Albuminuria Measurements
Of the 11 studies that collected albuminuria data, ten (91%) used immunoassays to measure urinary albumin. Two studies (18%) used dipsticks to assess albuminuria presence, which was confirmed by immunoassay in the Initiative on Nephropathy, of relevance to public health, which is Chronic, possibly in its Initial stages, and carries a Potential risk of major clinical Endpoints study.
CKD Stage 1–5 Prevalence
Adult Population
Prevalence estimates were adjusted to the age and sex distribution of the population of the 27 Member States of the European Union (EU27) in 200512 to correct for differences in national age and sex distributions. The adjusted CKD stages 1–5 prevalence in the adult population, including subjects aged 20–74 years, for studies using IDMS-standardized creatinine varied between 3.31% (95% confidence interval [95% CI], 3.30 to 3.33) in Norway and 17.3% (95% CI, 16.5 to 18.1) in the Northeast German Study of Health in Pomeranzia (SHIP) study (Supplemental Appendix 1, Figure 1).
Supplemental Appendix 1, Table 2 shows the unadjusted and adjusted CKD stages 1–5 prevalence in the adult population, for both IDMS and non-IDMS studies. For studies using non–IDMS-standardized creatinine, the unadjusted and adjusted CKD stages 1–5 prevalence in the adult population is graphically presented in Supplemental Appendix Figure 1.
Across Age Strata
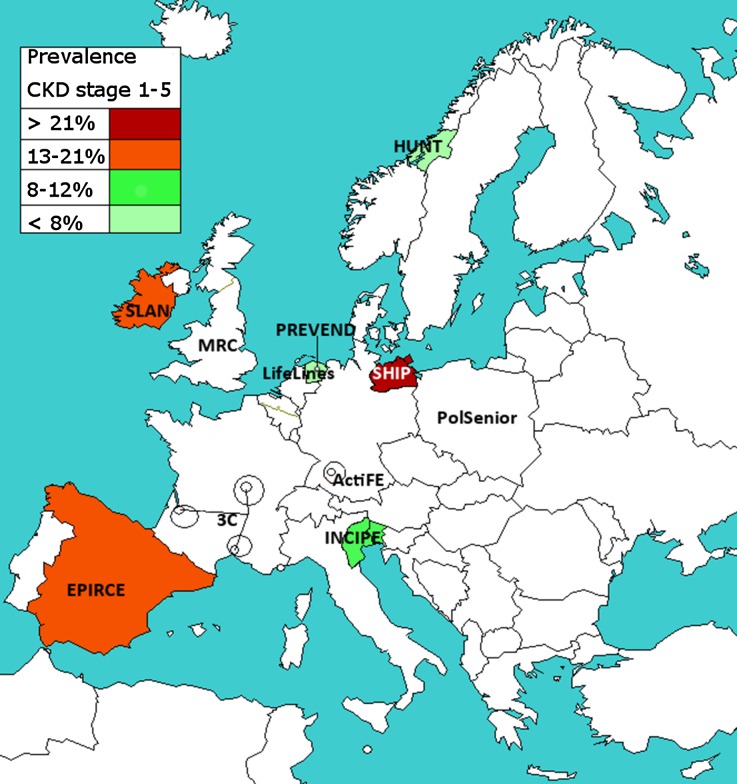
Figure 1 shows the geographic variation in the adjusted CKD stages 1–5 prevalence in the population aged 45–74 years, for studies using IDMS-standardized creatinine. Figure 2A shows this adjusted CKD stages 1–5 prevalence, including 95% CI. This prevalence varied between 6.3% (95% CI, 6.0 to 6.5) in Norway and 25.6% (95% CI, 23.7 to 27.5) in the Northeast German SHIP study.
Figure 1.
Adjusted CKD stages 1–5 prevalence in the population aged 45–74 years, in IDMS studies. Prevalence was age- and sex-adjusted to the EU27 population of 2005. The study names in uncolored regions are studies which used non–IDMS-standardized creatinine or studies which recruited subjects aged ≥50 years: the CKD prevalence results of these studies are shown in Supplemental Appendix 1. 3C, Three City Study; ActiFE, Activity and Function in the Elderly in Ulm study; EPIRCE, Estudio Epidemiológico de la Insuficiencia Renal en España; HUNT, Nord-Trøndelag Health Study; INCIPE, Initiative on Nephropathy, of relevance to public health, which is Chronic, possibly in its Initial stages, and carries a Potential risk of major clinical Endpoints; LifeLines, LifeLines Cohort and Study Biobank; MRC, Medical Research Council trial of assessment and management of older people in the community; PolSenior, Medical, psychological, sociological and economical aspects of aging of people in Poland; PREVEND, Prevention of Renal and Vascular End-stage Disease; SLAN, Survey of Lifestyle and Attitudes & Nutrition in Ireland.
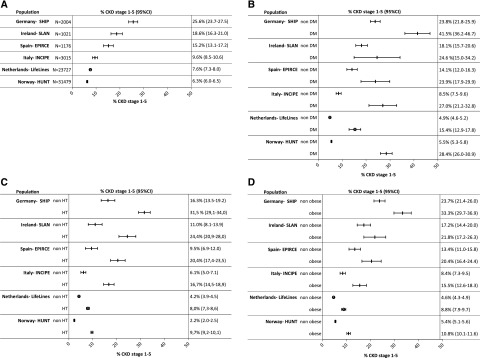
Figure 2.
(A) Adjusted CKD prevalence stages 1–5 (95% CI) in the population aged 45–74 years, in IDMS studies. (B) CKD prevalence stages 1–5 (95% CI) in the population aged 45–74 years, in IDMS studies, by diabetic status. (C) CKD prevalence stages 1–5 (95% CI) in the population aged 45–74 years, in IDMS studies, by hypertensive status. (D) CKD prevalence stages 1–5 (95% CI) in the population aged 45–74 years, in IDMS studies, by obesity status. Prevalence was age- and sex-adjusted to the EU27 population of 2005. N, the number of study subjects aged 45–74 years with creatinine and albuminuria measurement. Studies not covering the entire age range are not included in this figure. DM, diabetes mellitus; EPIRCE, Estudio Epidemiológico de la Insuficiencia Renal en España; HUNT, Nord-Trøndelag Health Study; HT, hypertension; INCIPE, Initiative on Nephropathy, of relevance to public health, which is Chronic, possibly in its Initial stages, and carries a Potential risk of major clinical Endpoints; LifeLines, LifeLines Cohort and Study Biobank; SLAN, Survey of Lifestyle and Attitudes & Nutrition in Ireland; Ɵ, studies using enzymatic method; | studies using Jaffe method.
The prevalence of CKD stages 1–5 for age categories 20–44 years, 45–64 years, 65–74 years, and 75–84 years is shown in Supplemental Appendix 1, Figure 2, 1–4, separately for studies using IDMS and non–IDMS-standardized creatinine assays. The CKD stages 1–5 prevalence was lowest in the age group 20–44 years and increased with every consecutive age group.
Across Risk Strata
Figure 2, B–D presents the CKD stages 1–5 prevalence in the population aged 45–74 years of studies using IDMS-standardized creatinine, stratified by diabetic, hypertension, and obesity status. The variation in CKD stages 1–5 prevalence stratified by risk factors followed the pattern of the overall adjusted prevalence across regions. Supplemental Appendix 1, Figure 1, B–D, show this same pattern in the adult population, separately for studies using IDMS- and non–IDMS-standardized creatinine.
CKD Stage 3–5 Prevalence
Adult Population
Supplemental Appendix 1, Figure 3 shows the CKD stages 3–5 prevalence including 95% CI for the adult population, separately for IDMS and non-IDMS studies. This CKD stages 3–5 prevalence varied between 1.0% (95% CI, 0.7 to 1.3) in the Italian Malattie cardiovascolari ATerosclerotiche Istituto Superiore di Sanita study and 5.9% (95% CI, 5.2 to 6.6) in the Northeast German SHIP study.
Supplemental Appendix 1, Table 2 shows the unadjusted and adjusted CKD stages 3–5 prevalence in the adult population, for both IDMS and non-IDMS studies. For studies using non–IDMS-standardized creatinine, the unadjusted and adjusted CKD stages 3–5 prevalence in the adult population is graphically presented in Supplemental Appendix 1, Figure 3A.
Across Age Strata
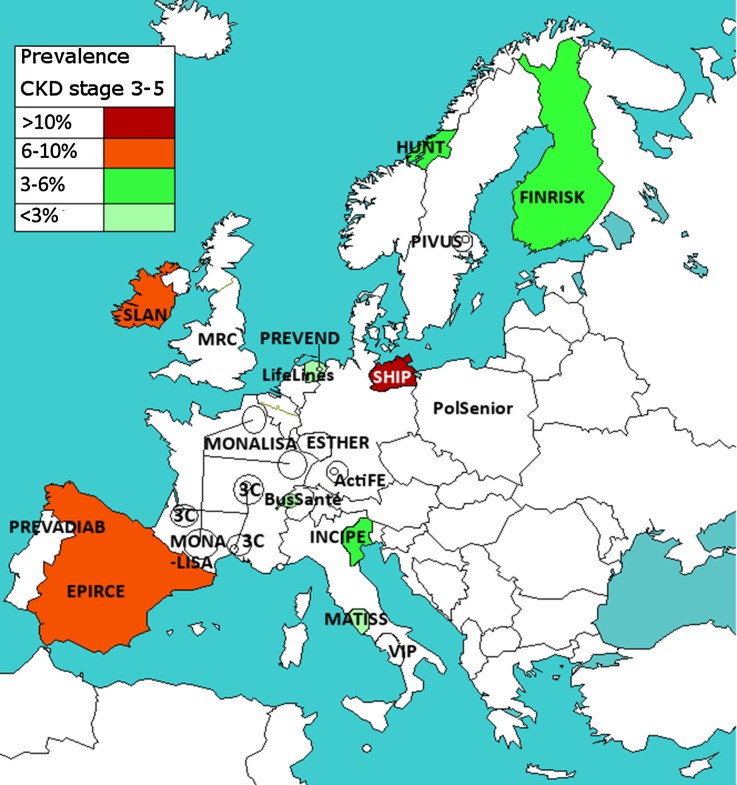
Figure 3 shows the geographic variation of the adjusted CKD stages 3–5 prevalence in the population aged 45–74 years, for studies using IDMS-standardized creatinine. Figure 4A shows the overall adjusted CKD stages 3–5 prevalence in these studies, including 95% CI. This CKD prevalence varied between 1.7% (95% CI, 1.3 to 2.1) in the Swiss Bus Santé study and 11.5% (95% CI, 10.2 to 12.8) in the Northeast German SHIP study.
Figure 3.
Adjusted CKD stages 3–5 prevalence in the population aged 45–74 years, in IDMS studies. Prevalence was age- and sex-adjusted to the EU27 population of 2005. The study names in uncolored regions are studies which used non–IDMS-standardized creatinine or studies which recruited subjects aged ≥50 years; the CKD prevalence results of these studies are shown in Supplemental Appendix 1. 3C, Three City Study; ActiFE, Activity and Function in the Elderly in Ulm study; EPIRCE, Estudio Epidemiológico de la Insuficiencia Renal en España; ESTHER, Epidemiologische Studie zu Chancen der Verhütung, Früherkennung und optimierten THerapie chronische ERkrankungen in der älteren Bevolkerung; FINRISK, Finland Cardiovascular Risk Study; HUNT, Nord-Trøndelag Health Study; INCIPE, Initiative on Nephropathy, of relevance to public health, which is Chronic, possibly in its Initial stages, and carries a Potential risk of major clinical Endpoints; LifeLines, LifeLines Cohort and Study Biobank; MATISS, Malattie cardiovascolari ATerosclerotiche Istituto Superiore di Sanita; MONA LISA, MOnitoring NAtionaL du rISque Arterie; MRC, Medical Research Council trial of assessment and management of older people in the community; PIVUS, Prospective Investigation of the Vasculature in Uppsala Seniors Study; PolSenior, Medical, psychological, sociological and economical aspects of aging of people in Poland; PREVADIAB, Prevalence of Diabetes and Risk Factors in Portugal; PREVEND, Prevention of Renal and Vascular End-stage Disease; SLAN, Survey of Lifestyle and Attitudes & Nutrition in Ireland; VIP, Valle dell'Irno Prevenzione
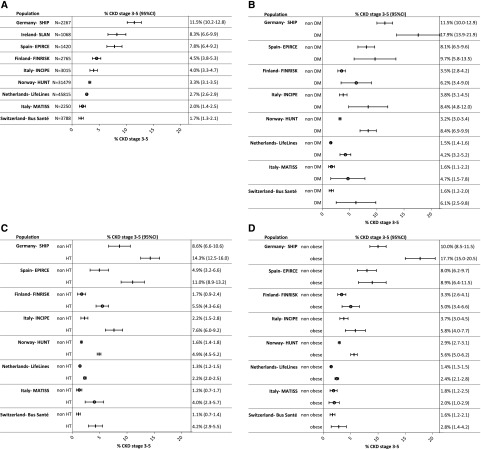
Figure 4.
(A) Adjusted CKD prevalence stages 3–5 (95% CI) in the population aged 45–74 years, in IDMS studies. (B) CKD prevalence stages 3–5 (95% CI) in the population aged 45–74 years, in IDMS studies, by diabetic status. (C) CKD prevalence stages 3–5 (95% CI) in the population aged 45–74 years, in IDMS studies, by hypertensive status. (D) CKD prevalence stages 3–5 (95% CI) in the population aged 45–74 years, in IDMS studies, by obesity status. Prevalence was age- and sex-adjusted to the EU27 population of 2005. N, the number of study subjects aged 45–74 years with creatinine measurement. Studies not covering the entire age range are not included in this figure. DM, diabetes mellitus; EPIRCE, Estudio Epidemiológico de la Insuficiencia Renal en España; FINRISK, Finland Cardiovascular Risk Study; HUNT, Nord-Trøndelag Health Study; INCIPE, Initiative on Nephropathy, of relevance to public health, which is Chronic, possibly in its Initial stages, and carries a Potential risk of major clinical Endpoints; LifeLines, LifeLines Cohort and Study Biobank; MATISS, Malattie cardiovascolari ATerosclerotiche Istituto Superiore di Sanita. Ɵ studies using enzymatic method; | studies using Jaffe method.
The prevalence of CKD stages 3–5 for age categories 20–44 years, 45–64 years, 65–74 years, and 75–84 years is shown in Supplemental Appendix 1, Figure 4, 1–4, separately for studies using IDMS and non–IDMS-standardized creatinine assays. The CKD stages 3–5 prevalence was lowest in the youngest age group and increased with every consecutive age group.
Across Risk Strata
Figure 4, B–D presents the CKD stages 3–5 prevalence in the population aged 45–74 years of studies using IDMS-standardized creatinine, stratified by diabetic, hypertension, and obesity status. The variation in CKD stages 3–5 prevalence stratified by risk factor followed the pattern of the overall adjusted prevalence across regions. Supplemental Appendix 1, Figure 3, B–D, show this same pattern in the adult population, separately for studies using IDMS- and non–IDMS-standardized creatinine.
Discussion
Our study suggests a substantial variation in CKD prevalence across Europe. Stratification by risk factors further suggests that this variation in CKD prevalence is remarkably consistent across high- and low-risk populations, implying that the difference in overall prevalence of CKD is at least in part due to other factors than the prevalence of diabetes, hypertension, and obesity in the general population.
Global Perspective
Regional differences in CKD prevalence have been documented around the world, even when comparing age- and sex-adjusted prevalence estimates using standardized creatinine methods. For example, in the adult general population of the United States, the adjusted CKD stages 3–5 prevalence varied from 4.8% in the Northeast to 11.8% in the Midwest.13 In contrast, in China the adjusted CKD stages 3–5 prevalence showed lower prevalence estimates, from 1.1% in East China to 3.8% in Southwest China.14 This variation is similar to that in Europe, where the adjusted CKD stages 3–5 prevalence varied from 1.0% to 5.9%. The adjusted CKD stages 1–5 prevalence in China showed larger variation, from 6.7% in South China to 18.3% in Southwest China.10 In Europe, this adjusted CKD stages 1–5 prevalence varied from 3.3% to 17.3%.
Explanatory Factors in Europe
There are many factors which potentially contribute to the observed differences in CKD prevalence across countries and regions. We argue that these differences are possibly due to true differences in the prevalence of CKD as well as to heterogeneity of studies. In the following we will discuss five possible explanations.
Human and Environmental Factors
Dietary habits across European regions vary substantially.15 Dietary protein intake is known to influence serum creatinine and thus eGFR.16,17 This may not only be an artifact, but also a true effect, because studies have shown that protein-rich diets are associated with accelerated eGFR decline.18 Also, the Mediterranean diet has been suggested to reduce the risk of development of CKD.19 Therefore, regional differences in dietary habits could lead to a difference in observed CKD prevalence through both a direct effect on serum creatinine and through reno-damaging or reno-protective influences. Additionally, there are multiple other factors associated with CKD prevalence, such as smoking, physical activity,20 socioeconomic status,21 and birth weight.22 These factors may vary between regions and may therefore contribute to the observed CKD prevalence variation.
Public Health Policies
European regions differ greatly with regard to healthcare policies.23 Public health initiatives may both prevent diseases and their complications by primary and secondary prevention, respectively.24 National and regional public health initiatives may therefore contribute to differences in the prevalence of underlying causes of CKD, like diabetes, hypertension, and obesity, as well as to the prevalence of CKD itself.
The consistently higher prevalence of CKD in high- compared with low-risk groups implies that the focus of public health initiatives should, indeed, lie with prevention of CKD in patients with underlying diseases. However, the remarkable consistency of international variation in CKD prevalence, irrespective of the presence of risk factors, emphasizes that the focus of public health initiatives should also lie with primary prevention of CKD, through the promotion of a healthy lifestyle in the entire population.
Genetic Factors
In studies which collected ethnicity data, almost all participants were white. However, even within the white European populations there are substantial genetic differences.25 Studies have shown that the development of CKD and the incidence of RRT are associated with multiple genetic loci.26,27 The regional differences in CKD prevalence could therefore be influenced by genetic differences across the various regions. This has been shown for the geographic pattern in the prevalence of RRT for IgA nephropathy.28 Because 7.1% of all RRT is provided for hereditary nephropathies (European Renal Association–European Dialysis and Transplant Association Registry, unpublished data), it is to be expected that some of the variation in CKD prevalence is also due to genetic differences.
Heterogeneity in Laboratory Methods
In addition to reflecting true differences in CKD prevalence, our results may also vary due to the heterogeneity of methodology used to measure creatinine5 and albuminuria,29 including differences in assays but also in handling and storage conditions (e.g., duration as well as number of freeze and thaw cycles until analysis).29,30
Differences in creatinine assays will likely contribute to the variation in CKD prevalence, as most Jaffe assays overestimate serum creatinine.5 The resulting bias may vary depending on the creatinine concentration, specific assay, manufacturer, and calibration material used.31,32 Fortunately, the IDMS calibration standardization has reduced the bias and improved the interlaboratory comparability.5,32 Despite the use of IDMS standardization, some interlaboratory variability still exists,31 being lowest in IDMS-standardized enzymatic assays.5 Notably, our study shows substantial differences in CKD stages 3–5 prevalence across studies using enzymatic IDMS-standardized assays. In these studies, the adjusted CKD stages 3–5 prevalence in the age group 65–74 years varied from 4.8% in central Italy to 11.4% in Finland.
Moreover, CKD stages 1–5 prevalence may additionally be influenced by albuminuria assays.29 As recommended by Kidney Disease Improving Global Outcomes (KDIGO), urinary albumin was measured by immunoassays in 91% of studies and we used urinary albumin-to-creatinine ratio for the definition of CKD.1 Importantly, there is no standardization available to enhance comparability of immunoassays across laboratories.29
Heterogeneity in Study Populations
Finally, differences in prevalence may have resulted from differences in population sample selections. As in the United States, CKD prevalence in Europe may have changed over time, and this might explain some of the observed differences.33 Nevertheless, we found both high and low CKD prevalence in older and in more recent studies. For example, in studies performed in the period 2005–2010 using the IDMS-standardized Jaffe method, the adjusted CKD stages 3–5 prevalence in the age group 65–74 years ranged from 4.1% in Switzerland to 20.8% in South Germany. This suggests that differences in time periods cannot fully explain the observed differences in CKD prevalence.
Although all studies were designed to be representative of the respective regional or national general population, their sample selections varied substantially as outlined in Table 1. Sample selection methods influence the coverage of the population investigated and influence the response, which both influence the ultimate representativeness of the sample.34,35 We have checked the representativeness of the included studies by comparing the age and sex distribution of the study populations to the relevant census data. Although overall the study populations appear to be representative for the age and sex distribution of their target population, we cannot exclude a selection bias based on unmeasured factors, such as the presence of (unmeasured) comorbidities.
Studies with high response are less likely to suffer from nonresponse bias, yet the impact of nonresponse bias on representativeness is not solely determined by the response.34 Even with low response a sample may be highly representative by chance alone. To date, there is no validated method to measure the influence of nonresponse bias on the representativeness of study results.34,36 However, nonresponse analyses can provide some insight into the likely direction of a possible nonresponse bias.34 All nonresponse analyses performed by the individual studies suggested that recruited participants were similar or healthier in comparison to nonparticipants.7–11 This might have led to an underestimation of the true CKD prevalence in these studies.
Strengths and Limitations
The new European CKD Burden Consortium enabled this first large-scale study to describe CKD prevalence across Europe. The comparability of CKD prevalence across studies was increased by using the same definition based on one eGFR equation. Additionally, we only compared studies using IDMS-standardized creatinine to increase interlaboratory comparability of creatinine results. Moreover, the comparability of study populations across European countries was enhanced by removing the influence of differences in national age and sex distributions through age and sex standardization to the EU27 population.12 Finally, to avoid the influence of international differences in the prevalence of diabetes, hypertension, and obesity, we determined CKD prevalence in subgroups with and without these risk factors.
There are also limitations to this study. First, the prevalence of CKD might have been slightly overestimated using single creatinine and albuminuria measurements. However, this will not have influenced the variation of CKD prevalence across studies, as all estimations will be equally affected. Ethnicity status was not included in the eGFR equation because this was not available for all studies, which might have led to a slight overestimation of CKD prevalence in some studies. However, in studies which did collect ethnicity data, at least 96% were white; therefore, the expected impact on the overall prevalence estimation is negligible. Other limitations relate to the heterogeneity of included studies with regard to laboratory methods and sample selection. This heterogeneity might have influenced the variation of CKD prevalence as discussed. The first may be solved by central measurement of serum creatinine and albuminuria in a reference laboratory. The effect of response, however, is inherent to population surveys and cannot be avoided.
In conclusion, this is the first study which carefully characterizes CKD prevalence across Europe. Our results suggest substantial variation in CKD prevalence across population samples in Europe. These differences are possibly due to true differences in the prevalence of CKD as well as to heterogeneity of the laboratory and sample selection methods. The effect of the variation in European regions with regard to human and environmental factors, public health policies, and genetics on CKD prevalence needs further investigation.
Our results may be used to guide future projections of the CKD burden in Europe and thereby help estimate the growing demand for CKD services that the ageing population will likely create. Our results are also a first step in monitoring the impact of strategies designed to reduce the burden of CKD in Europe. This monitoring may assist the medical community and policy makers in the further development of these strategies.
Concise Methods
Data Collection
Study Selection
We first systematically searched scientific publications, to identify European studies with data on CKD prevalence in the general population (details described in Supplemental Appendix 2).37 Additionally, the representatives of national kidney foundations, renal registries, and expert nephrologists in 39 European countries were asked to provide contact details for any relevant unpublished studies. Studies were included if they were designed to select a representative sample of the adult general population and CKD prevalence could be calculated. Studies that ended recruitment prior to 1996 were excluded. Eligible studies were invited to participate in an online questionnaire, assessing general study information (e.g., period of participant inclusion), collected data, and regional healthcare system characteristics. Answers regarding collected data and healthcare system characteristics are shown in Supplemental Appendix 2, Tables 1 and 2, respectively. Finally, studies that agreed to contribute data were sent a statistical analysis syntax to collect aggregated data. All studies were approved by local ethical committees and all participants gave consent.
All studies which contributed data were included in the European CKD Burden Consortium. The European CKD Burden Consortium, including nephrologists and epidemiologists, was established to characterize CKD prevalence and progression of CKD across Europe.
Collected Data
Data were collected for the total study population and for subgroups by age (≤>65 years), sex, and by diabetes, hypertension, and obesity (body mass index ≥30 kg/m2) status. For the continuous variables age, systolic and diastolic blood pressure, body mass index, eGFR, and urinary albumin-to-creatinine ratio, we collected the mean (SD) and median (25th and 75th percentiles). In addition, we collected the following factors known to influence kidney function: the proportion of current smokers and individuals using angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. Furthermore, we collected the laboratory methods for the measurement of serum creatinine and detection of albuminuria. Serum creatinine was measured by a Jaffe or enzymatic method, both of which can be standardized to IDMS.32 Diabetes was defined as self-reported diabetes and/or the use of glucose lowering medication. Hypertension was defined as a systolic blood pressure of ≥140 mmHg, a diastolic blood pressure of ≥90 mmHg, or the use of antihypertensive drugs.
Definition of CKD
CKD stages 1–5: eGFR<60 ml/min per 1.73 m2 calculated by the CKD-Epidemiology Collaboration equation1,38 and/or uACR≥30 mg/g.
CKD stages 3–5: eGFR<60 ml/min per 1.73 m2 calculated by the CKD-Epidemiology Collaboration equation.
These definitions were based on the KDIGO practice guideline.1 As no studies repeated measurements after three months, the chronicity criterion (symptoms ≥3 months) was not applied to the definition.
Statistical Analyses
Normally distributed variables are presented as means with SD, and non-normally distributed data as medians with interquartile ranges. Dichotomous data are given in percentages. The representativeness of study populations was tested by comparing the age and sex distribution of the study population to the distribution of the relevant regional/national population using the chi-squared test. The prevalence of CKD stages 1–5 and CKD stages 3–5 with 95% CI is presented as unadjusted rates and weighted averages using the age and sex distribution of the 2005 EU27 population.12 To limit the influence of random variation, this adjustment was only applied to studies with a minimum of 100 participants per included age stratum. Consequently, (sub)groups with insufficient numbers were excluded from this adjustment. Additionally, we stratified study populations into the following age groups: 45–74, 20–44, 45–64, 65–74, and 75–84 years, and whenever possible adjusted for the effect of age within strata. The age-stratified CKD prevalence is given for the overall study population as well as by diabetic, hypertension, and obesity status.
Disclosures
None.
Supplementary Material
Acknowledgments
K.B. and V.S.S. were responsible for the literature cited. K.B., V.S.S., G.G., S.H., C.T., W.V.B., C.Z., C.W., and K.J.J. were responsible for the study design. K.B., G.G., S.H., H.V., J.Ä., M.K., I.G., J.V., B.S., H.B., J.C., A.O.G., A.K.B., J.F., L.P., G.B., V.C., R.G., G.N., D.R., P.M.F., and D.N. were responsible for data collection. K.B., V.S.S., G.G., S.H., H.V., J.Ä., M. K., I.G., J.V., B.S., H.B., J.C., S.R., C.T., A.O.G., A.K.B., J.F., L.P., G.B., V.C., W.V.B., C.Z., R.G., G.N., D.R., P.M.F., D.N., C.W., and K.J.J. were responsible for data interpretation and revision of the manuscript. K.B., V.S.S., and K.J.J. were responsible for drafting the first manuscript. K.B. was responsible for the creation of figures and data analyses. All authors approved final submission of the manuscript.
The research leading to these results has received funding from (1) the European Community’s Seventh Framework Programme under grant agreement number HEALTH-F2-2009-241544 (Systems Biology towards Novel Chronic Kidney Disease Diagnosis and Treatment), and (2) the European Renal Association–European Dialysis and Transplant Association (ERA-EDTA) under the Quality European Studies initiative. This article was written by K. Brück et al. on behalf of the ERA-EDTA Registry which is an official body of the ERA-EDTA. The Activity and Function in the Elderly in Ulm (ActiFE) study was funded by grants from the German Ministry of Science, Research and Arts, State of Baden-Wuerttemberg, Germany, as part of the Geriatric Competence Center, Ulm University. The Survey of Lifestyle and Attitudes & Nutrition in Ireland (SLAN) 2007 study was originally funded by the Department of Health and Children. Work on this project was also in part funded by the Irish Health Research Board, Health Research Board (HRB) Grant file reference HRC/2007/13 HRB Centre for Health & Diet Research. The Prevention of Renal and Vascular End-stage Disease (PREVEND) study was funded by the Dutch Kidney Foundation. The medical, psychologic, sociological and economical aspects of aging of people in Poland study (PolSenior; project no. PBZ-MEIN-9/2/2006) was funded by Polish Ministry of Science and Higher Education. The Prevalence of Diabetes and Risk Factors in Portugal (PREVADIAB) study was funded by the Portuguese Ministry of Health.
The following institutions and investigators participated in this study: Pekka Jousilahti (Finland Cardiovascular Risk Study); Catherine Helmer, Marie Metzger (Three City); Jean Bernard Ruidavets, Vanina Bongard (Monitoring National du Risque Arteriel); Wolfgang Koenig, Michael D. Denkinger (ActiFE); Ben Schöttker, Kai-Uwe Saum (Epidemiologische Studie zu Chancen der Verhütung, Früherkennung und optimierten Therapie chronische Erkrankungen in der älteren Bevolkerung); Matthias Nauck, Sylvia Stracke (Study of Health in Pomeranzia); Ivan J. Perry, Joseph Eustace (SLAN); Antonio Lupo (Initiative on Nephropathy, of relevance to public health, which is Chronic, possibly in its Initial stages, and carries a Potential risk of major clinical Endpoints); Chiara Donfrancesco, Simonetta Palleschi (Malattie cardiovascolari ATerosclerotiche Istituto Superiore di Sanita); Norman Lamaida, Ernesto Capuano (Valle dell’Irno Prevenzione); Steef Sinkeler, B.H.R. Wolffenbuttel (LifeLines); Stephan J.L. Bakker, Michel Joosten (PREVEND); Knut Aasarød, Jostein Holmen (Nord-Trøndelag Health Study); Malgorzata Mossakowska, Andrzej Wiecek (PolSenior); Luis Gardete-Correia, João F. Raposo (PREVADIAB); A.L. Martin de Francisco, P. Gayoso Diz (Estudio Epidemiológico de la Insuficiencia Renal en España); Elisabet Nerpin, Lars Lind (Prospective Investigation of the Vasculature in Uppsala Seniors Study); Murielle Bochud, Jean-Michel Gaspoz (Bus Santé); Astrid Fletcher, Paul Roderick (Medical Research Council Trial of Assessment and Management of Older People in the Community); Gijs Van Pottelbergh (Belgian cohort of the very elderly + Intego Project); Arjan Van Der Tol (Unreferred Renal Insufficiency Study); Samy Hadjadj (Survie, Diabete de type 2 et Genetique); Olivera Stojceva-Taneva (Screening for Early Detection of Kidney Disease).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Unlocking the Value of Variation in CKD Prevalence,” on pages 1874–1877.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015050542/-/DCSupplemental.
Contributor Information
Collaborators: Pekka Jousilahti, Catherine Helmer, Marie Metzger, Jean Bernard Ruidavets, Vanina Bongard, Wolfgang Koenig, Michael D. Denkinger, Ben Schöttker, Kai-Uwe Saum, Matthias Nauck, Sylvia Stracke, Ivan J. Perry, Joseph Eustace, Antonio Lupo, Chiara Donfrancesco, Simonetta Palleschi, Norman Lamaida, Ernesto Capuano, Steef Sinkeler, B.H.R. Wolffenbuttel, Stephan J.L. Bakker, Michel Joosten, Knut Aasarød, Jostein Holmen, Malgorzata Mossakowska, Andrzej Wiecek, Luis Gardete-Correia, João F. Raposo, A.L. Martin de Francisco, P. Gayoso Diz, Elisabet Nerpin, Lars Lind, Murielle Bochud, Jean-Michel Gaspoz, Astrid Fletcher, Paul Roderick, Gijs Van Pottelbergh, Arjan Van Der Tol, Samy Hadjadj, and Olivera Stojceva-Taneva
References
- 1.Kidney Disease. Improving Global Outcomes (KDIGO) CKD Work Group : KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl 3: 1–150, 2013 [Google Scholar]
- 2.Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJ, Mann JF, Matsushita K, Wen CP: Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet 382: 339–352, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 4.McCullough K, Sharma P, Ali T, Khan I, Smith WC, MacLeod A, Black C: Measuring the population burden of chronic kidney disease: a systematic literature review of the estimated prevalence of impaired kidney function. Nephrol Dial Transplant 27: 1812–1821, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Drion I, Cobbaert C, Groenier KH, Weykamp C, Bilo HJ, Wetzels JF, Kleefstra N: Clinical evaluation of analytical variations in serum creatinine measurements: why laboratories should abandon Jaffe techniques. BMC Nephrol 13: 133, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheuiche AV, Soares AA, Camargo EG, Weinert LS, Camargo JL, Silveiro SP: Comparison between IDMS-traceable Jaffe and enzymatic creatinine assays for estimation of glomerular filtration rate by the CKD-EPI equation in healthy and diabetic subjects. Clin Biochem 46: 1423–1429, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Holmen J, Midthjell K, Krüger O, Langhammer A, Holmen TL, Bratberg GH, Vatten L, Lund-Larsen PG: The Nord-Trøndelag Health Study 1995-97 (HUNT 2): Objectives, contents, methods and participation. Norsk Epidemiologi 13: 19–32, 2002 [Google Scholar]
- 8.Nerpin E, Ingelsson E, Risérus U, Helmersson-Karlqvist J, Sundström J, Jobs E, Larsson A, Lind L, Ärnlöv J: Association between glomerular filtration rate and endothelial function in an elderly community cohort. Atherosclerosis 224: 242–246, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Guessous I, Bochud M, Theler JM, Gaspoz JM, Pechère-Bertschi A: 1999-2009 Trends in prevalence, unawareness, treatment and control of hypertension in Geneva, Switzerland. PLoS One 7: e39877, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nitsch D, Nonyane BA, Smeeth L, Bulpitt CJ, Roderick PJ, Fletcher A: CKD and hospitalization in the elderly: a community-based cohort study in the United Kingdom. Am J Kidney Dis 57: 664–672, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dallmeier D, Klenk J, Peter RS, Denkinger M, Peter R, Rapp K, Koenig W, Rothenbacher D: A prospective assessment of cardiac biomarkers for hemodynamic stress and necrosis and the risk of falls among older people: the ActiFE study. Eur J Epidemiol 2015, in press [DOI] [PubMed] [Google Scholar]
- 12.Eurostat: Table: Average population by sex and five-year age groups, 2009. Available at: http://ec.europa.eu/eurostat/data/database. Accessed July 15, 2009
- 13.Tanner RM, Gutiérrez OM, Judd S, McClellan W, Bowling CB, Bradbury BD, Safford MM, Cushman M, Warnock D, Muntner P: Geographic variation in CKD prevalence and ESRD incidence in the United States: results from the reasons for geographic and racial differences in stroke (REGARDS) study. Am J Kidney Dis 61: 395–403, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang L, Wang F, Wang L, Wang W, Liu B, Liu J, Chen M, He Q, Liao Y, Yu X, Chen N, Zhang JE, Hu Z, Liu F, Hong D, Ma L, Liu H, Zhou X, Chen J, Pan L, Chen W, Wang W, Li X, Wang H: Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet 379: 815–822, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Nöthlings U, Boeing H, Maskarinec G, Sluik D, Teucher B, Kaaks R, Tjønneland A, Halkjaer J, Dethlefsen C, Overvad K, Amiano P, Toledo E, Bendinelli B, Grioni S, Tumino R, Sacerdote C, Mattiello A, Beulens JW, Iestra JA, Spijkerman AM, van der A DL, Nilsson P, Sonestedt E, Rolandsson O, Franks PW, Vergnaud AC, Romaguera D, Norat T, Kolonel LN: Food intake of individuals with and without diabetes across different countries and ethnic groups. Eur J Clin Nutr 65: 635–641, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, Saran R, Wang AY, Yang CW: Chronic kidney disease: global dimension and perspectives. Lancet 382: 260–272, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Jacobsen FK, Christensen CK, Mogensen CE, Andreasen F, Heilskov NS: Pronounced increase in serum creatinine concentration after eating cooked meat. BMJ 1: 1049–1050, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedman AN: High-protein diets: potential effects on the kidney in renal health and disease. Am J Kidney Dis 44: 950–962, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Chrysohoou C, Panagiotakos DB, Pitsavos C, Skoumas J, Zeimbekis A, Kastorini CM, Stefanadis C: Adherence to the Mediterranean diet is associated with renal function among healthy adults: the ATTICA study. J Ren Nutr 20: 176–184, 2010 [DOI] [PubMed]
- 20.Stengel B, Tarver-Carr ME, Powe NR, Eberhardt MS, Brancati FL: Lifestyle factors, obesity and the risk of chronic kidney disease. Epidemiology 14: 479–487, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Vart P, Gansevoort RT, Coresh J, Reijneveld SA, Bültmann U: Socioeconomic measures and CKD in the United States and The Netherlands. Clin J Am Soc Nephrol 8: 1685–1693, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silverwood RJ, Pierce M, Hardy R, Sattar N, Whincup P, Ferro C, Savage C, Kuh D, Nitsch D: Low birth weight, later renal function, and the roles of adulthood blood pressure, diabetes, and obesity in a British birth cohort. Kidney Int 84: 1262–1270, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mackenbach JP, Karanikolos M, McKee M: The unequal health of Europeans: successes and failures of policies. Lancet 381: 1125–1134, 2013 [DOI] [PubMed] [Google Scholar]
- 24.Unal B, Critchley JA, Capewell S: Modelling the decline in coronary heart disease deaths in England and Wales, 1981-2000: comparing contributions from primary prevention and secondary prevention. BMJ 331: 614, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moskvina V, Smith M, Ivanov D, Blackwood D, StClair D, Hultman C, Toncheva D, Gill M, Corvin A, O’Dushlaine C, Morris DW, Wray NR, Sullivan P, Pato C, Pato MT, Sklar P, Purcell S, Holmans P, O’Donovan MC, Owen MJ, Kirov G International Schizophrenia Consortium : Genetic differences between five European populations. Hum Hered 70: 141–149, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Böger CA, Gorski M, Li M, Hoffmann MM, Huang C, Yang Q, Teumer A, Krane V, O’Seaghdha CM, Kutalik Z, Wichmann HE, Haak T, Boes E, Coassin S, Coresh J, Kollerits B, Haun M, Paulweber B, Köttgen A, Li G, Shlipak MG, Powe N, Hwang SJ, Dehghan A, Rivadeneira F, Uitterlinden A, Hofman A, Beckmann JS, Krämer BK, Witteman J, Bochud M, Siscovick D, Rettig R, Kronenberg F, Wanner C, Thadhani RI, Heid IM, Fox CS, Kao WH CKDGen Consortium : Association of eGFR-Related Loci Identified by GWAS with Incident CKD and ESRD. PLoS Genet 7: e1002292, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Köttgen A, Glazer NL, Dehghan A, Hwang SJ, Katz R, Li M, Yang Q, Gudnason V, Launer LJ, Harris TB, Smith AV, Arking DE, Astor BC, Boerwinkle E, Ehret GB, Ruczinski I, Scharpf RB, Chen YD, de Boer IH, Haritunians T, Lumley T, Sarnak M, Siscovick D, Benjamin EJ, Levy D, Upadhyay A, Aulchenko YS, Hofman A, Rivadeneira F, Uitterlinden AG, van Duijn CM, Chasman DI, Paré G, Ridker PM, Kao WH, Witteman JC, Coresh J, Shlipak MG, Fox CS: Multiple loci associated with indices of renal function and chronic kidney disease. Nat Genet 41: 712–717, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kiryluk K, Li Y, Sanna-Cherchi S, Rohanizadegan M, Suzuki H, Eitner F, Snyder HJ, Choi M, Hou P, Scolari F, Izzi C, Gigante M, Gesualdo L, Savoldi S, Amoroso A, Cusi D, Zamboli P, Julian BA, Novak J, Wyatt RJ, Mucha K, Perola M, Kristiansson K, Viktorin A, Magnusson PK, Thorleifsson G, Thorsteinsdottir U, Stefansson K, Boland A, Metzger M, Thibaudin L, Wanner C, Jager KJ, Goto S, Maixnerova D, Karnib HH, Nagy J, Panzer U, Xie J, Chen N, Tesar V, Narita I, Berthoux F, Floege J, Stengel B, Zhang H, Lifton RP, Gharavi AG: Geographic differences in genetic susceptibility to IgA nephropathy: GWAS replication study and geospatial risk analysis. PLoS Genet 8: e1002765, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bachmann LM, Nilsson G, Bruns DE, McQueen MJ, Lieske JC, Zakowski JJ, Miller WG: State of the art for measurement of urine albumin: comparison of routine measurement procedures to isotope dilution tandem mass spectrometry. Clin Chem 60: 471–480, 2014 [DOI] [PubMed] [Google Scholar]
- 30.Cuhadar S, Koseoglu M, Atay A, Dirican A: The effect of storage time and freeze-thaw cycles on the stability of serum samples. Biochem Med (Zagreb) 23: 70–77, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Delanaye P, Cavalier E, Cristol JP, Delanghe JR: Calibration and precision of serum creatinine and plasma cystatin C measurement: impact on the estimation of glomerular filtration rate. J Nephrol 27: 467–475, 2014 [DOI] [PubMed] [Google Scholar]
- 32.Myers GL, Miller WG, Coresh J, Fleming J, Greenberg N, Greene T, Hostetter T, Levey AS, Panteghini M, Welch M, Eckfeldt JH National Kidney Disease Education Program Laboratory Working Group : Recommendations for improving serum creatinine measurement: a report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin Chem 52: 5–18, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Sayday S, Eberhardt M, Rios-Burrows N, Williams D, Geiss L, Dorsey R: Prevalence of CKD and associated risk factors – United States 1999–2004. MMWR Morbid Mortal Wkly Rep, 56: 161–165, 2007 [PubMed] [Google Scholar]
- 34.Groves R, Fowler F, Couper M, Lepkowski J, Singer E, Tourangeau R: Survey Methodology, 2nd Ed., Hoboken, New Jersey, Wiley, 2009 [Google Scholar]
- 35.Tripepi G, Jager KJ, Dekker FW, Zoccali C: Selection bias and information bias in clinical research. Nephron Clin Pract 115: c94–c99, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Halbesleben JR, Whitman MV: Evaluating survey quality in health services research: a decision framework for assessing nonresponse bias. Health Serv Res 48: 913–930, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brück K, Jager KJ, Dounousi E, Kainz A, Nitsch D, Ärnlöv J, Rothenbacher D, Browne G, Capuano V, Ferraro PM, Ferrieres J, Gambaro G, Guessous I, Hallan S, Kastarinen M, Navis G, Gonzalez AO, Palmieri L, Romundstad S, Spoto B, Stengel B, Tomson C, Tripepi G, Völzke H, Wiȩcek A, Gansevoort R, Schöttker B, Wanner C, Vinhas J, Zoccali C, Van Biesen W, Stel VS European CKD Burden Consortium : Methodology used in studies reporting chronic kidney disease prevalence: a systematic literature review. Nephrol Dial Transplant 30[Suppl 4]: iv6–iv16, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.