Abstract
The close relationship between endothelial and hematopoietic precursors during early development of the vascular system suggested the possibility of a common yet elusive precursor for both cell types. Whether similar or related progenitors for endothelial and hematopoietic cells are present during organogenesis is unclear. Using inducible transgenic mice that specifically label endothelial and hematopoietic precursors, we performed fate-tracing studies combined with colony-forming assays and crosstransplantation studies. We identified a progenitor, marked by the expression of helix-loop-helix transcription factor stem cell leukemia (SCL/Tal1). During organogenesis of the kidney, SCL/Tal1+ progenitors gave rise to endothelium and blood precursors with multipotential colony-forming capacity. Furthermore, appropriate morphogenesis of the kidney vasculature, including glomerular capillary development, arterial mural cell coating, and lymphatic vessel development, required sphingosine 1-phosphate (S1P) signaling via the G protein–coupled S1P receptor 1 in these progenitors. Overall, these results show that SCL/Tal1+ progenitors with hemogenic capacity originate and differentiate within the early embryonic kidney by hemovasculogenesis (the concomitant formation of blood and vessels) and underscore the importance of the S1P pathway in vascular development.
Keywords: vascular, renal development, renal vascular precursors
A common progenitor for blood and vessels, the hemangioblast, was proposed about 100 years ago.1 Recent studies provided evidence supporting the existence of hemangioblasts and identified transcription factors involved in their differentiation.2–6 However, their presence and contribution in vivo to the development of blood and vessels is still controversial.7 Further, the contribution of such progenitor to blood and blood vessel formation during early organogenesis remains to be tested. Helix-loop-helix transcription factor stem cell leukemia (SCL/Tal1) not only identifies putative hemangioblasts but is also essential for their differentiation.8,9 Within the SCL locus, a 5′ enhancer directs its expression to the endothelium and a 3′ enhancer guides expression to early hematopoietic precursors.9 Using mice with tamoxifen-inducible Cre expression driven by either enhancer,10,11 we designed experiments to trace the fate of SCL+ progenitors and define their role in hemovasculogenesis in the embryonic kidney.
Interestingly, kidney vascular development initiates, in mice, around embryonic day (E) 12.5, before it connects to the systemic circulation. On the basis of embryonic kidney crosstransplantation studies and single-cell microaspiration followed by RT-PCR, we suggested that the embryonic kidney possesses all the necessary precursors for the development of the kidney vasculature.12 In addition, we observed hematopoietic cells budding from the endothelium in embryonic mouse kidneys grown in culture,13 suggesting that the developing kidney generates its own vessels and blood during early development. Although we recently identified the earliest precursor for the mural cell of the kidney arterioles (i.e., smooth muscle cells [SMCs]),14,15 the origin of the renal endothelial cells (ECs) is still not clear.
Sphingosine 1-phosphate (S1P) is a bioactive sphingolipid crucial in many biologic processes, including angiogenesis.16 It activates a family of five G protein–coupled receptors. The variety of responses mediated by S1P receptors depends on the receptor type and their coupled downstream effectors expressed in a given cell. The S1P1 receptor is highly expressed in precursors of the kidney arterioles and later in ECs and vascular SMCs.17 S1P1 functions autonomously in ECs because the phenotype observed in S1P1fl/fl;Tie2Cre mice mimics the one found in S1P1 knockout (S1P1KO) mice.18 S1P1KO mice seem to develop normally until E11.5, but at E12.5–E13.5 they develop severe edema and hemorrhages and die in utero by E14.5, presumably because of a role of S1P1 in vascular maturation.19 Because the aforementioned knockout mice die before the kidney develops its vasculature, the role of S1P1 in renal vascular development is unknown.
We therefore designed a series of experiments to define whether (1) the prevascular embryonic kidney possesses hemovascular precursors, (2) SCL+ precursors give rise to vascular endothelium and hematopoietic progenitor cells, (3) hemovasculogenesis occurs in the embryonic kidney, and (4) S1P-S1P1 signaling controls the development of the kidney vasculature.
Results
SCL+ Precursors Give Rise to Renal Vascular ECs
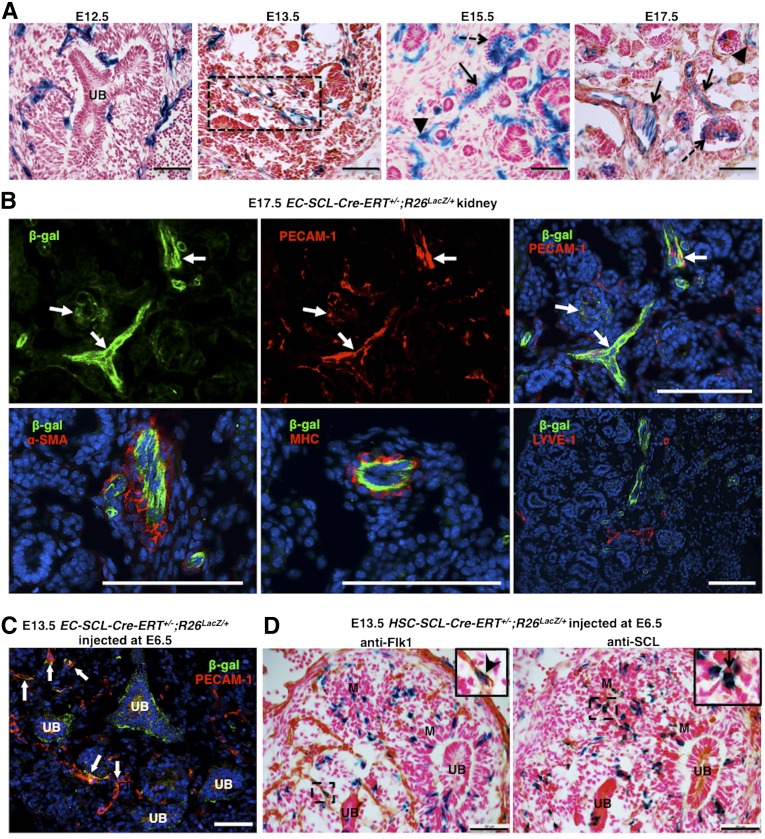
To label the EC-SCL and hematopoietic stem cell (HSC)-SCL precursors generated during definitive hematopoiesis, which begins around E10.5 in mice,20 we performed daily maternal tamoxifen injections from E9.5 to the day before harvesting EC-SCL-Cre-ERT+/−;R26LacZ/+ and HSC-SCL-Cre-ERT+/−;R26LacZ/+ embryos. As shown in Figure 1, in the EC-SCL-Cre-ERT+/−;R26LacZ/+ embryo, β-gal+ cells from the EC-SCL lineage were widely spread within the metanephric mesenchyme around the dividing ureteric bud (at E12.5) and formed nascent vascular endothelial tubes with hemoglobin+ erythroblasts in their lumen (at E13.5). By E15.5, the kidney had developed glomerular and peritubular capillaries, arterioles with SMC coating, and veins. ECs of all types of blood vessels were β-gal+ in kidneys of EC-SCL-Cre-ERT+/−;R26LacZ/+ mice at E15.5 and E17.5, suggesting their EC-SCL origin (Figure 1A). Interestingly, kidneys from HSC-SCL-Cre-ERT+/−;R26LacZ/+ mice (at E12.5 to E17.5) showed a small population of vascular ECs in addition to the expected putative blood cells derived from the HSC-SCL precursors (Supplemental Figure 1A). Further, costaining by immunofluorescence showed that EC-SCL and HSC-SCL lineage cells expressed the EC marker platelet endothelial cell adhesion molecule (PECAM-1/CD31) but did not express α-smooth muscle actin (α-SMA), myosin heavy chain, or the lymphatic endothelial marker lymphatic vessel endothelial hyaluronic acid receptor 1 (LYVE-1) (Figure 1B, Supplemental Figure 1B), suggesting that kidney ECs have a distinct origin from the mural cells of arteries and veins and from the lymphatic endothelium. In addition to the early metanephric mesenchyme and typical embryonic hematopoietic sites (yolk sac, aorta-gonad-mesonephros [AGM], and fetal liver), HSC-SCL lineage cells were observed in multiple developing organs and structures, such as heart, brain, lung, gut and limbs (not shown).
Figure 1.
SCL+ endothelial precursors give rise to renal endothelium but not mural cells in the embryonic kidney. (A) Kidneys of EC-SCl-Cre-ERT+/−;R26LacZ/+ mice treated with tamoxifen at E9.5 show EC-SCL progenitor–derived cells labeled by X-gal staining in blue (β-gal+) distributed within the metanephric mesenchyme surrounding the branching ureteric bud (UB) at E12.5, in a nascent vessel (dashed rectangle) at E13.5 and in ECs of renal arteries and arterioles (arrows), peritubular capillaries (arrowheads), and glomerular capillaries (dashed arrows) at E15.5 and E17.5. (B) β-gal+ (green) cells coexpress the EC marker PECAM-1 (red) (arrows, upper panels), but not the mural markers α-SMA (red) and myosin heavy chain (red), or the lymphatic EC marker LYVE-1 (red) (lower panels). Nuclei were stained with Hoechst (blue). (C) E13.5 EC-SCl-Cre-ERT+/−;R26LacZ/+ mice treated with tamoxifen at E6.5 show several β-gal+ cells (green) costained with PECAM-1 (red) in the developing vessels (arrows) of the kidney. Nuclei were stained with Hoechst (blue). (D) Immunostaining of E13.5 kidney consecutive sections from HSC-SCl-Cre-ERT+/−;R26LacZ/+ mice treated with tamoxifen at E6.5 showed that only few β-gal+ cells coexpressed the early EC marker Flk1(inset, arrow head). Instead, most of them were distributed in the undeveloped mesenchyme (M) and coexpressed SCL/Tal1 (inset, arrow). Scale bars: 50 μm (A, C, and D) and 100 μm (B).
To trace the fate of EC-SCL and HSC-SCL precursors generated before E9.5, we injected tamoxifen at E6.5 to allow the Cre-LacZ expression span from E6.5 to E8.5, encompassing the period of yolk sac hematopoiesis (E7.5). In E13.5 embryos, β-gal+ cells derived from both precursors were present in the kidney (Figure 1, C and D). Similar to the EC-SCL-Cre-ERT+/−;R26LacZ/+ mice injected at E9.5, the labeled EC-SCL–derived cells were positive for PECAM-1 (Figure 1C). However, in the kidney of HSC-SCL-Cre-ERT+/−;R26LacZ/+ mice, cells coexpressing β-gal and SCL were mainly distributed in the undeveloped mesenchyme and did not overlap with Flk1+ endothelium (Figure 1D). It is possible that these cells are from early progenitors before the commitment to the EC lineage.
SCL+ Hematopoietic and Hemogenic Endothelial Progenitors in the Early Embryonic Kidney
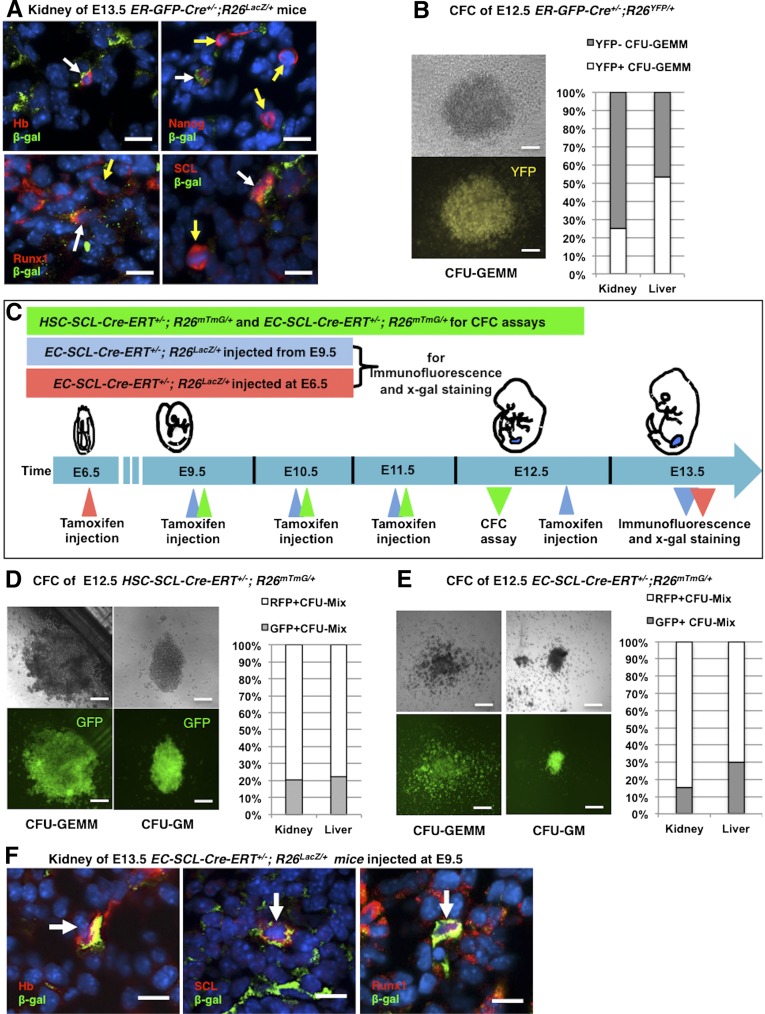
To investigate in vivo the presence of erythroid progenitors in the early embryonic kidney, we crossed ER-GFP-Cre mice (harboring a GFP-Cre knockin into the erythropoietin receptor locus) to R26LacZ/LacZ mice. Figure 2 shows that in the E13.5 kidney, β-gal+ erythroid cells are also positive for hemoglobin, Nanog, and hematopoietic precursor markers, such as Runx1 and SCL/Tal1 (Figure 2A). At this age, the kidney is undergoing formation of nascent endothelial tubes. Note that Nanog is a stem cell marker and hemoglobin is widely expressed in the hematopoietic lineage, including long term repopulating HSCs, suggesting that some of these cells within the embryonic kidney may not be erythroblasts but earlier hematopoietic precursors.
Figure 2.
The early embryonic kidney possesses hematopoietic precursors. (A) Double immunofluorescence staining of E13.5 kidney sections from ER-GFP-Cre+/−;R26LacZ/+ mice showed that β-gal expressing erythroblasts, in green, also expressed hemoglobin (Hb), Nanog, Runx1, and SCL/Tal1 (white arrows). However, some Nanog+, Runx1+, and SCL/Tal1+ cells did not coincide with labeled erythroblasts (yellow arrows). Nuclei were stained with Hoechst (blue). (B) CFC assay of cells from E12.5 kidneys and livers of ER-GFP-Cre+/−;R26LacZ/+ mice. A representative photograph of a YFP+ CFU-GEMM grown from kidney cells is shown (left). The chart shows the percentage of YFP+ CFU-GEMM from kidneys (25%, n=3) and livers (53.4%, n=3). (C) Timeline of tamoxifen administration in HSC-SCL-Cre-ERT+/−;R26mTmG/+ and EC-SCL-Cre-ERT+/−;R26mTmG/+ mice. For CFC assays shown in D and E, HSC-SCL-Cre-ERT+/−;R26mTmG/+ and EC-SCL-Cre-ERT+/−;R26mTmG/+ embryos were treated with tamoxifen from E9.5 to E11.5 by intraperitoneal maternal injection (green triangles). To label EC-SCL precursors generated after E9.5 (shown in F), EC-SCL-Cre-ERT+/−;R26LacZ/+ embryos were treated with tamoxifen daily from E9.5 to E12.5 and harvested at E13.5 (blue triangles). To label EC-SCL precursors present before E9.5 (Supplemental Figure 2B), EC-SCL-Cre-ERT+/−;R26LacZ/+ embryos were treated with a single injection of tamoxifen at E6.5. The embryos were harvested at E13.5 (red triangles). (D) CFC assay of cells from E12.5 kidneys (n=4) and livers (n=4) of HSC-SCL-Cre-ERT+/−;R26mTmG/+mice. CFU-GEMM (left) and CFU-GM (right) colonies grew from all the samples. Bar graph shows the percentage of GFP+ colonies out of total CFU-Mixs grown from kidneys (21%) and livers (22.2%). E CFC assay of cells from E12.5 kidneys (n=6) and livers (n=6) of EC-SCL-Cre-ERT+/−;R26mTmG/+ mice. CFU-GEMM (left) and CFU-GM (right) colonies grew from all the samples. Bar graph shows that 15.4% of the colonies grown from kidney cells and 30% of those from liver cells express GFP. Data were collected from two independent experiments. (F) E13.5 kidney of EC-SCL-Cre-ERT+/−;R26LacZ/+ mice treated with tamoxifen from E9.5 to E12.5. Blood cells derived from SCL+ endothelial precursors, in green, coincided by immunofluorescence with Hb, SCL/Tal1, and Runx1 (red, white arrows). Nuclei were stained with Hoechst (blue). Scale bars: 10 μm (A and F) and 50 μm (B, D, and E).
To identify multipotential and lineage-committed hematopoietic precursors, we performed an in vitro methylcellulose based colony-forming cell (CFC) assay using cells from E12.5 kidneys and livers (as controls) of ER-GFP-Cre+/−;R26YFP/+ mice. During culture, the progenitors differentiated into different types of colonies characterized by their size, morphology, and cellular composition. The results showed that cells within these organs formed two types of early-stage hematopoietic colonies (CFU-GEMM, containing granulocytes, erythrocytes, monocytes/macrophages, and megakaryocytes; CFU-GM, containing granulocytes and macrophages). The YFP reporter was found homogeneously expressed in 25% of the CFU-GEMM derived from kidney cells (Figure 2B), but not in the CFU-GM colonies. In addition, Nanog+, Runx1+, and SCL+ hematopoietic precursors without β-gal expression were also found in the metanephric mesenchyme or in forming renal vessels (Figure 2A). These results indicated the existence of at least two different early hematopoietic precursors within the embryonic kidney.
To further investigate the contribution of the HSC-SCL precursors to multipotent hematopoietic precursors in early embryonic kidney, we performed CFC assays using kidney and liver cells from E12.5 HSC-SCL-Cre-ERT+/−;R26mTmG/+ embryos treated with tamoxifen from E9.5 to E11.5 (Figure 2C). After 12 days in culture, CFU-GEMM and CFU-GM formed at a rate of 30 colonies per 105 kidney cells (Figure 2D), suggesting that the developmental stage of hematopoietic precursor cells in the early prevascular kidney spans from early multipotent to later myeloid precursors. In this model, green fluorescent protein (GFP) reporter expression was observed in approximately 20% of the colonies from the kidney and liver (Figure 2D). Overall, this experiment demonstrates that the embryonic kidney possesses multipotent hematopoietic precursors derived from HSC-SCL+ cells.
By E13.5, the EC-SCL precursors (labeled at E6.5 or from E9.5 to E12.5) gave rise to a subset of blood-like cells in the kidney (Supplemental Figure 2A). Thus, we investigated whether these cells were hematopoietic precursors. Within the kidney, cells derived from the EC-SCL progenitors labeled from E9.5 to E12.5 showed expression of hemoglobin, SCL and Runx1 (Figure 2F). When the progenitors were labeled at E6.5, the E13.5 kidneys showed β-gal+ cells coexpressing SCL and angiotensin-converting enzyme (ACE) (Supplemental Figure 2B). These results suggest that the EC-SCL progenitors in the early embryonic kidney generate hematopoietic cells.
Next, to study the potency of the hematopoietic cells derived from renal EC-SCL precursors, we performed CFC assays from kidneys and liver of E12.5 EC-SCL-Cre-ERT+/−;R26mTmG/+ mice treated with tamoxifen (E9.5 to E11.5). CFU-GM and CFU-GEMM formed at a rate of 25.3±4 colonies per 105 kidney cells, and 15.4% of the colonies expressed GFP (Figure 2E).
Hemovasculogenesis in the Embryonic Kidney
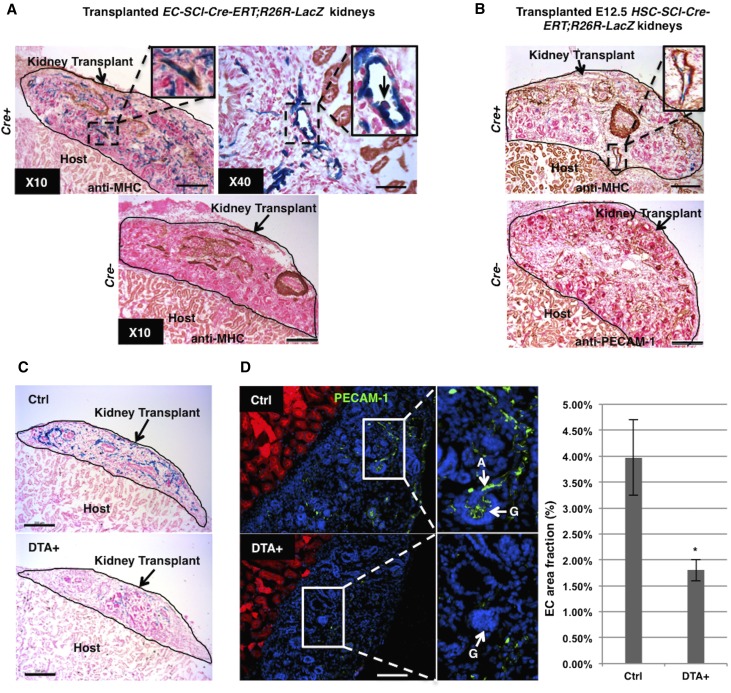
While the aforementioned data indicates the existence of hematopoietic precursors in the early embryonic kidney, to further assess whether those precursors originate and differentiate in situ, we used an in vivo crosstransplantation model, which allows the development of the vasculature in the embryonic kidney. Prevascular kidneys from E12.5 EC-SCL-Cre-ERT+/−;R26LacZ/+ mice and their Cre− siblings were transplanted under the kidney capsule of adult wild-type hosts. Then the hosts were treated with tamoxifen for 7 days to induce Cre expression in the EC-SCL precursors present at E12.5 in the transplanted embryonic kidneys. As shown in Figure 3, the transplanted kidneys developed vessels with β-gal+ ECs and vascular SMCs coating (Figure 3A). Hematopoietic cells derived from EC-SCL precursors were also identified in the vessels of the transplanted kidney (Figure 3A), confirming that those hematopoietic precursors originated in situ. In transplanted kidneys from E12.5 HSC-SCL-Cre-ERT+/−;R26LacZ/+ mice, HSC-SCL precursors gave rise to a small population of renal vascular ECs (Figure 3B). The transplanted kidneys from Cre− control mice of both strains developed mature arterioles and nephrons similar to the Cre+ ones but, as expected, lacked β-gal+ cells. These results further supported the notion that the embryonic kidney has the capability to establish its own vasculature and blood cells at an early stage.
Figure 3.
SCL+ precursors are required for hemovasculogenesis in the transplanted embryonic kidneys. (A) EC-SCL-Cre-ERT+/−;R26LacZ/+ (Cre+) prevascular embryonic kidney transplanted under the kidney capsule of an adult host for 7 days with tamoxifen treatment, developed β-gal+ vascular endothelium (in blue), with SMC coating, in brown (×10 inset, anti-myosin heavy chain). Inset in ×40 (right) shows a blood cell budding from an EC (arrow) in the transplanted embryonic kidney at higher magnification. The transplanted kidneys from Cre− control siblings (EC-SCL-Cre-ERT+/−;R26LacZ/+) developed similar to the Cre+ ones after tamoxifen treatment, but were negative for X-gal staining. (B) Scattered β-gal+ cells in the developing vessels of transplanted Cre+ (HSC-SCL-Cre-ERT+/−;R26LacZ/+) embryonic kidneys (inset). Lower panel shows the Cre- (HSC-SCL-Cre-ERT−/−;R26LacZ/+) transplanted kidneys immunostained for PECAM-1. (C) Transplanted E12.5 DTA+ (EC-SCL-Cre-ERT+/−;R26LacZ/+;R26DTA/+) and control (EC-SCL-Cre-ERT+/−;R26LacZ/+;R26+/+) kidneys. DTA+ transplanted kidneys developed fewer β-gal+ ECs compared with their respective controls. (D) Immunofluorescence for PECAM-1 (green) in frozen sections from transplanted control (top panel) and DTA+ (bottom panel) kidneys. Nuclei were stained with Hoechst (blue). Cells from host kidney express RFP (red). Inset of top panel shows at higher magnification the presence of well developed ECs of renal arterioles (A, arrow) and a normal glomerular capillary network (G, arrow) in a control transplanted kidney. Inset of bottom panel shows lack of ECs inside a glomerulus (G, arrow) in DTA+ transplanted kidneys. Note that ECs (green) in transplanted kidneys do not express RFP. Bar graph shows measurement of EC area fractions in frozen sections from transplanted control (n=3) and DTA+ (n=3) kidneys. Values are expressed as mean±SEM. *P<0.05. Scale bars: 50 μm (A, ×40), 200 μm (A, ×10, B and C), and 100 μm (D).
To investigate whether intrinsic renal EC-SCL precursors are required for the development of the kidney vasculature, we designed experiments to ablate renal EC-SCL precursors in the early developing kidney. We crossed EC-SCL-Cre-ERT mice with R26DTA/DTA mice, which express diphtheria toxin subunit A (DTA) in the presence of Cre recombinase, resulting in specific cell ablation. To overcome the embryonic lethality resulting from ablation of ECs, we took advantage of the crosstransplantation technique described earlier. We transplanted E12.5 kidneys of EC-SCL-Cre-ERT+/−;R26LacZ/+; R26DTA/+ mice (and DTA-controls) under the kidney capsule of adult R26mTmG/mTmG host mice (which expresses ubiquitously cell membrane-localized red fluorescence) and induced DTA expression in EC-SCL precursors after transplantation. In the DTA+ transplanted kidneys, there were significantly reduced numbers of β-gal+ ECs (Figure 3C). Measurement of area fraction (percentage) of PECAM-1+ cells in frozen sections of transplanted kidneys showed that the EC area fraction of DTA+ kidneys (1.8%) was reduced compared with controls (3.98%) (Figure 3D). Importantly, cells from the host (expressing red fluorescent protein [RFP]) do not contribute to the EC endowment in control or DTA+ transplanted kidneys (Figure 3D). These experiments underscore the requirement of intrinsic EC-SCL precursors within the early embryonic kidney for normal organogenesis.
Timed Deletion of S1P1 in Renal EC Precursors Results in Abnormal Renal Vascular Development
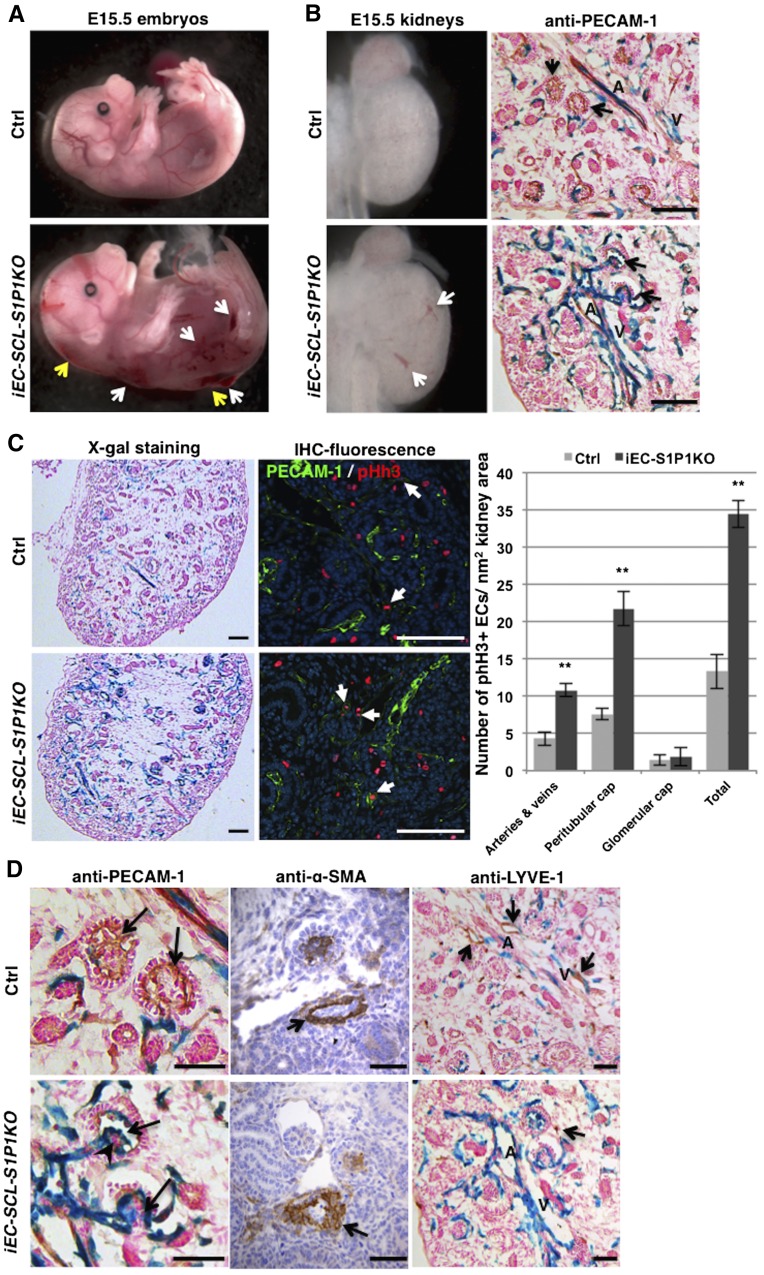
To study the role of S1P1 in the development of the kidney vasculature, we induced the deletion of S1P1 in the SCL+ ECs at E10.5 (just before nephrogenesis starts at E11.5) and at E12.5. The iEC-SCL-S1P1KO embryos showed severe dorsal subcutaneous edema, hemorrhages (Figure 4A), and bradycardia and died around E14.5 to E16.5. To confirm the deletion of S1P1 in ECs, we performed RT-PCR for S1P1 in sorted SCL+ ECs (GFP+) from E15.5 kidneys of iEC-SCL-S1P1KO-mTmG and heterozygous Cre+ control mice. (Supplemental Figure 3).
Figure 4.
Timed deletion of S1P1 in endothelial precursors leads to abnormal renal vascular development. (A) Tamoxifen-treated E15.5 iEC-SCL-S1P1KO (EC-SCLCre-ERT+/−; S1P1Δ/fl; R26LacZ/+, named conditional [c] KO hereafter) embryos showed severe hemorrhages (white arrows) and dorsal subcutaneous edema (yellow arrows). However, the control siblings (EC-SCLCre-ERT+/−; S1P1fl/+; R26LacZ/+) were normal. (B) E15.5 cKO kidneys showed striking dilation of intrarenal arteries (A) and veins (V) evident in the whole kidney (left, white arrows) and in histologic sections subjected to X-gal staining and immunostaining for PECAM-1 (right). Black arrows indicate glomeruli. (C) X-gal staining of kidney sections showed hyperplastic endothelium labeled in blue in the cKO (left). Coimmunofluorescence staining for PECAM-1 (in green) and phosphorylated histone H3 (pHh3) (in red) shows proliferating ECs with coincidence of nuclear RFP with cell membrane GFP (arrows). Bar graph shows the number of proliferating ECs in different vessel types in E14.5 kidneys. The cKO kidneys show significant increase of EC proliferation in renal arteries, veins, and peritubular capillaries (cap) but not in the glomerular capillaries (cap). Data are expressed as mean±SEM. **P<0.01. (D) Immunostaining for PECAM-1 showed that cKO kidneys lacked the normal capillary loops (upper panel, arrows) and that they developed capillary shunts (lower panel, arrows) accompanied by clumps of abnormal mesangial cells (arrowhead). Immunostaining for α-SMA on kidney sections of control and cKO embryos showed abnormal arteriolar development with irregular smooth muscle coating in the cKO embryo (arrow). LYVE-1+ lymphatic vessels (upper panel, arrows) did not overlap with the EC-SCL+ derived cells (in blue) and were almost absent in cKO kidneys (lower panel, arrow points to a single LYVE-1+ cell). Scale bars: 100 μm (B and C) and 50 μm (D).
Because at E15.5 the kidney starts to form mature renal arteries and arterioles with SMCs and pericytes coating the EC tubes, we first characterized the kidneys of iEC-SCL-S1P1KO mice and their Cre+ control siblings at this age. X-gal staining showed striking dilatation of intrarenal arteries and veins in kidneys of iEC-SCL-S1P1KO mice (Figure 4B), accompanied by a marked increase in the number of cells derived from EC-SCL precursors (Figure 4C). Coimmunofluorescence staining for PECAM-1 and phosphorylated histone H3 (pHh3) showed a significant increase of EC proliferation in intrarenal arteries, veins, and peritubular capillaries in kidneys of iEC-SCL-S1P1KO mice (Figure 4C). No difference was found within the glomerular capillaries (Figure 4C).
At E15.5, mature glomeruli appear in the deep renal cortex of both iEC-SCL-S1P1KO mice and control mice. Capillary shunts (single dilated glomerular capillary lumens) were present within the glomeruli of iEC-SCL-S1P1KO mice (Figure 4D). This phenotype may be due to abnormal development of mesangial cells, which normally support and maintain the structure of the glomerular capillaries as clumps of mesangial cells were present within the abnormal glomeruli (Figure 4D).
Unlike previous findings in the aorta of S1P1fl/fl;Tie2-Cre+/− mice and S1P1KO mice, immunostaining for α-SMA showed that SMCs covered the whole circumference of arteries and arterioles (Figure 4D). However, their orientation was disturbed with α-SMA+ cells aligning irregularly outside of the endothelial tubes (Figure 4D).
In addition, lymphatic ECs (LYVE-1+) were absent in the kidneys of iEC-SCL-S1P1KO mice (Figure 4D). Most of the LYVE-1+ cells were not labeled by X-gal staining in control mice, confirming that the lymphatic endothelium originates from a distinct progenitor (Figure 4D).
Kidney Vascular Abnormalities of iEC-SCL-S1P1KO Mice Are Intrinsic and Not Secondary to Extrarenal Defects
In addition to the kidney, we studied abnormalities in other major organs of the iEC-SCL-S1P1KO mice. The most significant abnormalities were found in the heart. The interventricular septum and ventricular myocardium compact layer, especially of the left ventricle, of iEC-SCL-S1P1KO mice were thinner than those in control mice (Supplemental Figure 4, A and B). Intramyocardial vessels of the iEC-SCL-S1P1KO mice were dilated (Supplemental Figure 4C).
To elucidate whether the phenotype observed in the iEC-SCL-S1P1KO mice was intrinsic to the kidney and not secondary to heart failure or other extrarenal anomalies, we transplanted E12.5 kidneys of iEC-SCL-S1P1KO and control siblings under the kidney capsule of wild-type host mice. We induced Cre expression and subsequent S1P1 deletion with simultaneous reporter expression after transplantation. iEC-SCL-S1P1KO transplanted kidneys also showed EC hyperplasia, dilated intrarenal arteries and veins, and glomerular capillary shunts (Figure 5), suggesting that the abnormal renal vascular development was not secondary to heart failure and underscoring the intrinsic role of endothelial S1P1 in kidney vascular development.
Figure 5.
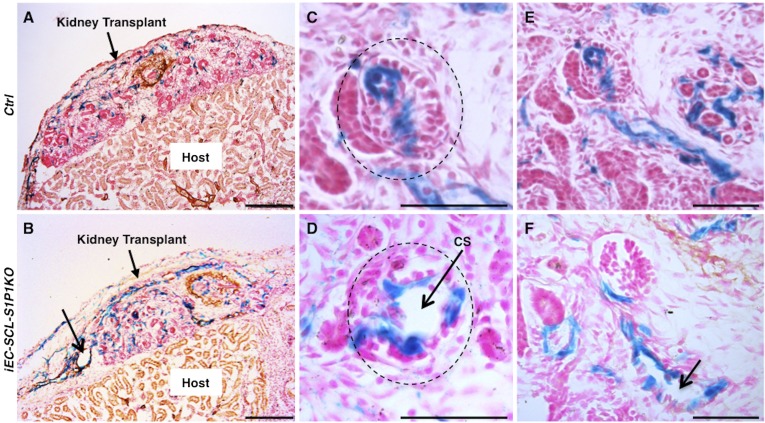
S1P1 functions intrinsically to regulate renal vascular development. (A and B) X-gal staining and immunostaining for myosin heavy chain on sections from transplanted embryonic kidneys of control (A) and iEC-SCL-S1P1KO (B) mice showed increased number of β-gal+ ECs and dilated vessels (thin arrow) in the iEC-SCL-S1P1KO kidneys. (C and D) Control kidneys under transplantation showed normal development of glomerular capillaries (C, dotted circle) whereas iEC-SCL-S1P1KO kidneys developed capillary shunts (CS) (D, arrow). (E and F) Control kidneys under transplantation developed normal arterioles (E), whereas iEC-SCL-S1P1KO kidneys showed dilated arteriolar lumen (F, arrow). Scale bars: 200 μm (A and B) and 50 μm (C–F).
Kidney Vascular Development Ex Vivo Requires S1P-S1P1 Signaling
Embryonic kidneys under ex vivo culture conditions are able to develop a normal tubular epithelial compartment (i.e., ureteric bud branches and glomerular epithelial differentiation) but not a normal vasculature. To test whether renal vascular development is regulated by the S1P-S1P1 signaling, we performed embryonic kidney cultures exposed to synthetic S1P and S1P1 inhibitor 2-Amino-N-(3-octylphenyl)-3-(phosphonooxy)-propanamaide (VPC23019, named VPC hereafter) treatment. Four groups of E12.5 kidneys were cultured and treated with vehicle, S1P, S1P and VPC, and VPC alone, respectively, for 72 hours.
Immunostaining for PECAM-1, as expected, showed that untreated (vehicle) embryonic kidneys did not develop an organized vasculature. Similarly, treatment with VPC alone resulted in abnormal disorganized endothelium of intrarenal vessels. However, the kidneys with S1P treatment developed a normal thin layer of ECs, which was attenuated by adding VPC (Figure 6, A and B). All the cultured kidneys lacked small vessels, including arterioles, peritubular capillaries, and glomerular capillaries (Figure 6, A and B) and mesangial cells (Figure 6C).
Figure 6.
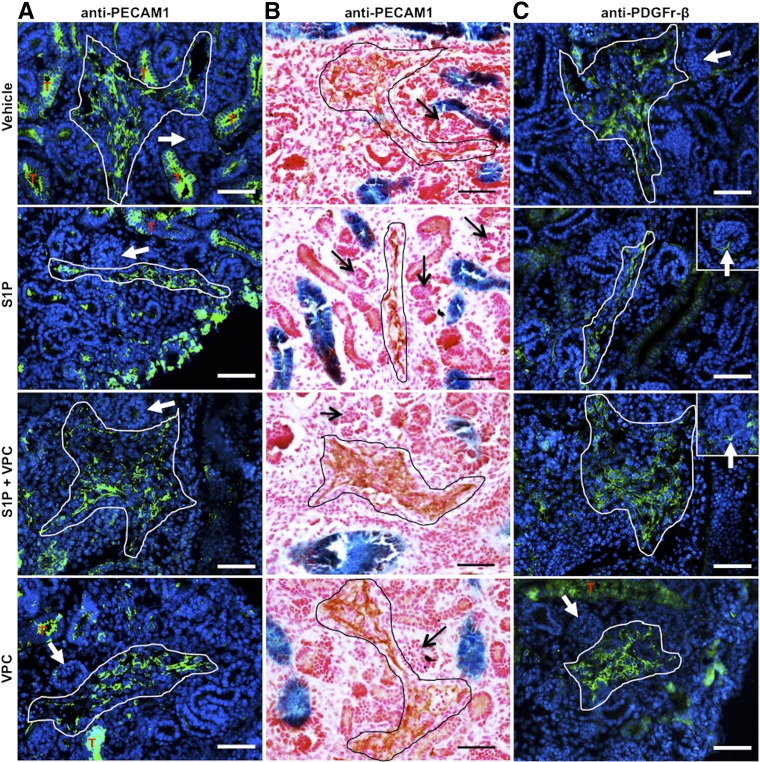
Kidney vascular development ex vivo requires S1P-S1P1 signaling. E12.5 Hoxb7Cre+/−;R26LacZ/+ kidneys treated with vehicle, S1P, S1P+VPC, and VPC in culture for 72 hours and subjected to immunostaining for PECAM-1 and PDGFR-β. (A) Immunofluorescence for PECAM-1 (in green) showed that control kidneys (vehicle) and kidneys treated with VPC developed thickened EC layer (white lined area), whereas those treated with S1P showed a thin layer of ECs. Kidneys treated with S1P+VPC developed even thicker EC layers in the renal vessels. Note that tubular (T, in red) staining in green was due to background signal (also found in control sections without the PECAM-1 antibody). (B) Similar results (without tubular background staining) were observed by immunohistochemistry using 3,3′-diaminobenzidine as a substrate (black lined area). Glomerular capillaries did not develop in vitro (A and B, arrows). (C) Immunofluorescence for PDGFR-β showed that a large number of PDGFR-β+ mural cells (white lined area) were closely associated with vessels in kidneys exposed to all treatments. Kidneys from all the groups lacked mesangial cells (arrows). Scale bar: 50 μm.
Moreover, abundant platelet-derived growth factor receptor (PDGFR)-β+ cells, presumably SMC precursors, were identified in close association with ECs in kidneys of the four groups (Figure 6C). The most α-SMA+ cells were found widely distributed in the kidneys with S1P treatment (not shown), compared with the few found in the other groups, in which PDGFR-β+ cells do not acquire the α-SMA marker, indicating lack of maturation of the SMC coating of the vessels in culture.
Discussion
In this study we show that the early embryonic kidney possesses SCL+ hemovascular precursors that contribute to the intrinsic generation of endothelium concomitant with blood generation, a process that was previously defined as hemovasculogenesis.13,21,22 We found that within the kidneys, SCL+ precursors give rise to hemogenic ECs of renal arteries, veins, arterioles, peritubular capillaries, and glomerular capillaries. Furthermore, we show that S1P1 expressed in SCL+ derived ECs controls the development and assembly of the renal vasculature (Figure 7).
Figure 7.
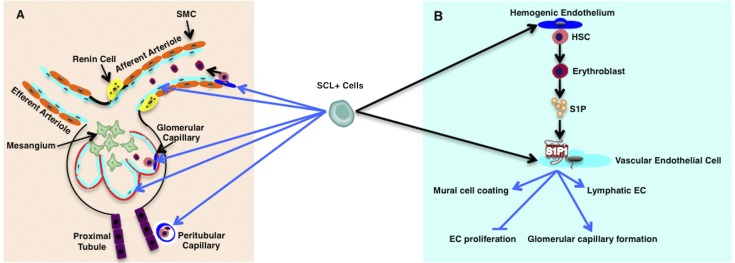
SCL+ precursors contribute to hemovasculogenesis in the kidney. Summary of findings on the lineage relationship between the SCL+ precursors and the renal vascular endothelium and blood cells in the early embryo. Blue arrows indicate the lineage relationship from our results, and black arrows show published data from other groups. (A) The SCL+ precursor gives rise to definitive (light blue cells) and hemogenic (dark blue cells) vascular endothelium of renal vessels, including renal arteries, veins (not shown), glomerular capillaries, and peritubular capillaries. (B) S1P-S1P1 signaling between blood cells and ECs derived from SCL+ precursors regulates kidney vascular development. Endothelial S1P1 is involved in mural cell coating of renal arteries and arterioles and development of glomerular capillaries and lymphatic ECs and inhibits renal EC proliferation.
Using graft studies and multiple markers, including Flk1, Flt1, and Tie1, other groups have identified early EC precursors for the glomerular microvessels in the rodent kidney.23–25 Whereas our studies suggest that SCL+ precursors originate locally in the kidney, the fact that we found cells derived from the SCL+ precursors labeled at E6.5 or E9.5, before the kidney starts to form, indicates the possibility that precursors from the yolk sac and AGM may have seeded the intermediate mesoderm before the condensation and development of the “avascular” metanephric mesenchyme.
Our fate-tracing studies identified SCL+ derived hemogenic ECs and hematopoietic precursors in E12.5 and E13.5 kidneys by immunostaining, CFC assay, and crosstransplantation studies. During early embryogenesis, hematopoiesis was found to occur in organs other than the yolk sac or the AGM region. A recent study showed that the mouse embryonic head also functions as a hemogenic organ to a similar extent as the AGM region, which suggests broader potential hemogenic areas in the mouse embryo; this is in agreement with previous studies showing that hemovasculogenesis also occurs in the head mesenchyme of early embryos.13,26 The kidney is a major hemogenic organ in teleosts, such as zebrafish. Our results suggest that the mouse kidney also functions as a temporary hemogenic organ via hemovasculogenesis during early embryogenesis. Although we did not identify SCL+ lineage–derived blood cells in the embryonic kidneys at later stages (E15.5 and E17.5; data not shown), they might directly join the blood circulation at earlier stages and home to the classic hematopoietic sites, such as fetal liver, spleen, and bone marrow. In fact, the connection between the embryonic kidney and peripheral circulation is already established at E15.5. In our CFC assays, not all colonies were labeled by reporter expression. The unlabeled colonies may originate from earlier hematopoietic precursors or may have escaped the induction by tamoxifen. These results agree with our fate-tracing studies of EC-SCL and HSC-SCL precursors using X-gal staining and immunostaining. Interestingly, we found that the HSC-SCL precursors give rise not only to blood cells but also to a small population of ECs within the embryonic kidney, suggesting the early embryonic presence of endothelial heterogeneity within the same organ.
The temporary hemogenic function of the embryonic kidney may serve as a smooth transition before the establishment of the connection to the general circulation, as the formation of a local vascular system also requires blood to generate regulatory factors, such as S1P. A recent study has shown that deletion of red blood cell–specific sphingosine kinases 1 and 2 in mice results in abnormal vascular development, which can be rescued by treatment with an S1P1 receptor agonist.27 Thus, the SCL+ hemogenic precursors in the embryonic kidney give rise to a small population of blood cells, which in turn may function as crucial regulators for the concomitant development of the kidney vasculature. Interestingly, the development of the renal vascular tree is hindered in the culture system. The smaller vessels and glomerular capillaries in the nephrogenic zone are missing in the cultured kidneys even with S1P treatment, suggesting that the development of these vessels requires other factors, such as blood flow, angiogenic factors (such as vascular endothelial growth factor A), and a hypoxic microenvironment.
Recent studies show that S1P1 inhibits sprouting angiogenesis of retinal vessels in mice by regulating interactions between VE-Cadherin and VEGFR2 during vascular development.28,29 Our study indicates that S1P1 functions as an inhibitor of angiogenesis in a cell autonomous manner within the kidney. E14.5-E15.5 iEC-SCL-S1P1KO embryonic kidneys and crosstransplanted kidneys displayed increased EC proliferation and EC hyperplasia. In culture, the embryonic kidneys treated with VPC and S1P+VPC developed hyperplastic ECs of intrarenal vessels, whereas in those treated with S1P the EC layer was thin. Because S1P is mainly generated by erythrocytes and ECs, and the plasma level of S1P is higher than that in the tissue, lack of S1P normally supplied by the circulating blood may lead to the EC hyperplasia in cultured kidneys.
Although proliferation in the developing glomerular capillaries of iEC-SCL-S1P1KO kidneys did not significantly increase, they developed capillary shunts with abnormal mesangial cells, suggesting a distinct role of S1P1 in the development of glomerular capillaries compared with other renal blood vessels. Mesangial cells play an important role in the development and stabilization of the glomerular capillary loops.30–32 It has been hypothesized that the mesangial cell splits the single vessel into multiple parallel branches, which form the capillary tufts.32 However, exactly how the capillary loops form and the molecular cues guiding this process are unknown. Previous studies showed that knockout of several transcription factors expressed in podocytes (Pod1, Foxc2, Limx1b, and Krm1/MafB) resulted in a decreased number of capillary loops and abnormal mesangium similar to what we observed in our study.33–36 Therefore, S1P1 may be involved in the interactions among podocytes, ECs, and mesangium to regulate the development of the glomerular capillary loops. Whether S1P1 operates through the transcription factors mentioned earlier requires further investigation.
Deletion of S1P1 in EC precursors did not result in absence of mural cells of the renal vessels; however, the SMC layer was disorganized, and the vessels of cultured kidneys lacked SMC coating. Although S1P treatment to the kidneys in culture increased the number of α-SMA+ cells, the kidneys still showed vascular SMC recruitment defects. Although this result could be due to the culture system, the overall agreement between the in vivo and in vitro studies suggests that in fact S1P-S1P1 signaling controls the recruitment of mural cells during vascular development.
Interestingly, the iEC-SCL-S1P1KO mice showed abnormal heart development. S1P1 is ubiquitously expressed in multiple cardiac cell types, including cardiomyocytes, fibroblasts, SMCs, and ECs.37 Furthermore, S1P1, S1P2, and S1P3 are expressed in cardiomyocytes and mediate cardioprotection from hypoxia.37–39 Until now, the role of endothelial S1P1 in the heart was unclear. Our study suggests that S1P1 expressed in cardiac ECs may not only regulate vascular development but also play an important overall role in heart development.
An additional finding from our study is the lack of lymphatic vessels in the kidneys of iEC-SCL-S1P1KO mice. Previous studies have shown that lymphatic ECs start to appear in the mouse kidney at E13.540 and that multiple cell types contribute to the their development, including venous derived ECs, mesenchymal cells, and hematopoietic cells.41–43 Lack of reporter expression in LYVE-1+ lymphatic ECs of EC-SCL-Cre-ERT+/−;R26LacZ/+ and HSC-SCL-Cre-ERT+/−;R26LacZ/+ mice suggests a distinct origin of lymphatic ECs from non-SCL+ precursors. However, S1P1 expressed in vascular ECs still seems to play a key role in development of the renal lymphatic vessels. The mechanism involved remains to be investigated.
We showed that iEC-SCL-S1P1KO mice developed severe edema. The accumulated interstitial fluid caused by absence of lymphatic vessels, the abnormal SMC coating of blood vessels, and abnormal heart function may have increased hydrostatic pressure and therefore contributed to prominent vessel dilation, hemorrhages, and dorsal edema.
In summary, as depicted in Figure 7, our data showed that vascular endothelium and blood cells originate in situ from SCL+ precursors in the mouse prevascular embryonic kidney. The S1P-S1P1 signaling pathway controls the development and assembly of the kidney vasculature during mouse early embryogenesis.
Concise Methods
Mice
The generation of ER-GFP-Cre,44 EC-SCL-Cre-ERT,10 HSC-SCL-Cre-ERT,11 and Hoxb7-Cre45 mice has been previously described. B6.129S4-Gt(ROSA)26Sortm1Sor/J (R26LacZ/LacZ),46 B6.129(Cg)-Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J (R26mTmG/mTmG)47 and B6.Cg-Gt(ROSA)26Sortm3(CAG-EYFP)Hze/J (R26YFP/YFP)48 reporter mice were used to trace the fate of erythroblasts, EC-SCL, and HSC-SCL precursors. EC-SCL-Cre-ERT mice were crossed to B6.129-GT(ROSA)26SSortm1(DTA)Lky/J mice (R26DTA/DTA)49 and R26LacZ/LacZ mice to investigate whether ablation of EC precursors affects kidney development. Pregnant mice were injected with tamoxifen (2 mg/30 g body wt) intraperitoneally. Inducible endothelial S1P1KO mice (iEC-SCL-S1P1KO) were generated by crossing the EC-SCL-Cre-ERT mice to S1P1fl/fl (Jackson Laboratory #019141)19 and R26LacZ/LacZ mice. For details, see the Supplemental Material and Supplemental Table 1.
All procedures were performed in accordance with the Guiding Principles for Research Involving Animals and Human Beings by the American Physiologic Society and were approved by the University of Virginia Animal Care Committee.
Immunohistochemistry and X-gal Reaction
Sections (7-μm thick) of 4% paraformaldehyde fixed and cryoembedded tissues were subjected to X-gal staining and immunostained following standard protocols.50 Antibodies are described in Supplemental Material.
CFC Assays
Kidney and liver cells were harvested from E12.5 mouse embryos and disaggregated with collagenase (M7902, StemCell Technologies) at 37°C. The single cells were diluted in Iscove’s Modified Dulbecco’s Medium with 5% FBS and grown in MethoCult GF (M3434, StemCell Technologies) for assessment of mouse hematopoietic progenitors. Cells in MethoCult were plated in duplicate samples at 1×105 cell per 30-mm dish. After incubation for 12 days at 37°C in 5% humidified CO2, the dishes were scored for CFUs according to standard criteria. CFU-GEMM colonies are large colonies containing >500 cells, with a highly dense core (red or brown) and indistinct border between the core and peripheral cells. CFU-GM colonies contain >30 cells with dense cores (without red or brown). Individual cells can be distinguished at the edge of the colonies (described in the manual for mouse CFU assays using MethoCult, StemCell Technologies).
Embryonic Kidney Crosstransplantation
The crosstransplantation of embryonic kidneys under the kidney capsule of adult hosts were performed as described previously.12 After surgery, host mice were injected with tamoxifen (1 mg/30 g body wt) intraperitoneally daily for 7 days. Detailed procedures are described in Supplemental Material.
Measurement of Endothelial Area Fraction in the Transplanted Kidneys
To measure the EC area fraction, we performed immunofluorescence for PECAM-1 (green) on frozen sections (six sections/animal) of transplanted kidneys from control (EC-SCL-Cre-ERT+/−;R26LacZ/+) (n=3) and DTA+ (EC-SCL-Cre-ERT+/−;R26LacZ/+; R26DTA/+) (n=3) mice. Nuclei were costained with 4′,6-diamidino-2-phenylindole (blue). Images were processed with ImageJ software (National Institutes of Health) to select stained endothelial areas occupied by stained pixels and whole transplanted kidney area. Background staining was manually excluded. The EC area fraction was expressed as a percentage:
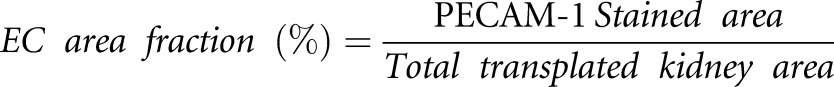 |
Measurement of Endothelial Proliferation
Endothelial proliferation was quantified on kidney sections from E14.5 iEC-SCL-S1P1KO mice and control siblings by scoring number of cells with phosphorylated histone H3 (pHh3) (RFP+) expression in the nucleus and PECAM-1 (GFP+) expression on the cell membrane. The total area of kidney sections was measured using ImageJ software.
Embryonic Kidney Culture
E12.5 kidneys from Hoxb7-Cre+/−;R26LacZ/+ mice were dissected in DMEM-Ham’s F-12 medium with 1% FBS and cultured on filters in 24-well plates with 800 μl of culture medium (DMEM/Ham’s F-12 medium with 1% FBS, 1% HEPES, 1% antibiotic antimycotic, and 0.5% insulin-transferrin-selenium media supplement) plus the following: (1) vehicle (3% fatty acid free BSA + 5 mmol/L HCl + 4.75% DMSO) (n=7); (2) 1 μmol/L S1P (1370, Tocris) (n=7); (3) 1 μmol/L S1P + 1 μmol/L VPC 23019 (857360P, Avanti Polar Lipids Inc.) (n=7); and (4) 1 μmol/L VPC23019 (n=6). Medium was changed daily. Kidneys were harvested after 72 hours and processed for whole-mount X-gal staining followed by postfixation, embedding, sectioning, and immunostaining as previously described.50
Statistical Analyses
Data are shown as mean±SEM. Statistical analyses were carried out by t test using Microsoft Excel 2011 version 14.5.3 (Microsoft Corporation). P<0.05 was considered to represent statistically significant differences.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Dr. Ursula Klingmüller for providing the ER-GFP-Cre mice. We would also like to thank Dr. Silvia Medrano, Dr. Brian C. Belyea, and Dr. Eugene Lin for helping to revise this manuscript. We are grateful to Danielle Stumbo for excellent mouse work and Maria Florencia Martínez for excellent technical advice.
This work was supported by National Institutes of Health grants DK091330 to M.L.S.S.L., HL066242 to R.A.G., the Center of Excellence in Pediatric Nephrology DK096373 to R.A.G. and M.L.S.S.L., the University of Virginia Children’s Hospital Grant-in-Aid to M.L.S.S.L., and the University of Virginia Child Health Research Center and American Heart Association Predoctoral Fellowship 14PRE20000006 to Y.H.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015060610/-/DCSupplemental.
References
- 1.Sabin FR: Origin and development of the primitive vessels of the chick and of the pig. Contrib Embryol 6: 61–124, 1917 [Google Scholar]
- 2.Lancrin C, Sroczynska P, Serrano AG, Gandillet A, Ferreras C, Kouskoff V, Lacaud G: Blood cell generation from the hemangioblast. J Mol Med (Berl) 88: 167–172, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Liu F, Bhang SH, Arentson E, Sawada A, Kim CK, Kang I, Yu J, Sakurai N, Kim SH, Yoo JJ, Kim P, Pahng SH, Xia Y, Solnica-Krezel L, Choi K: Enhanced hemangioblast generation and improved vascular repair and regeneration from embryonic stem cells by defined transcription factors. Stem Cell Rep 1: 166–182, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kennedy M, Firpo M, Choi K, Wall C, Robertson S, Kabrun N, Keller G: A common precursor for primitive erythropoiesis and definitive haematopoiesis. Nature 386: 488–493, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Zambidis ET, Park TS, Yu W, Tam A, Levine M, Yuan X, Pryzhkova M, Péault B: Expression of angiotensin-converting enzyme (CD143) identifies and regulates primitive hemangioblasts derived from human pluripotent stem cells. Blood 112: 3601–3614, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tavian M, Biasch K, Sinka L, Vallet J, Péault B: Embryonic origin of human hematopoiesis. Int J Dev Biol 54: 1061–1065, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Nishikawa S: Hemangioblast: An in vitro phantom. Wiley Interdiscip Rev Dev Biol 1: 603–608, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Gering M, Rodaway AR, Göttgens B, Patient RK, Green AR: The SCL gene specifies haemangioblast development from early mesoderm. EMBO J 17: 4029–4045, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bloor AJ, Sánchez MJ, Green AR, Göttgens B: The role of the stem cell leukemia (SCL) gene in hematopoietic and endothelial lineage specification. J Hematother Stem Cell Res 11: 195–206, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Göthert JR, Gustin SE, van Eekelen JA, Schmidt U, Hall MA, Jane SM, Green AR, Göttgens B, Izon DJ, Begley CG: Genetically tagging endothelial cells in vivo: Bone marrow-derived cells do not contribute to tumor endothelium. Blood 104: 1769–1777, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Göthert JR, Gustin SE, Hall MA, Green AR, Göttgens B, Izon DJ, Begley CG: In vivo fate-tracing studies using the Scl stem cell enhancer: Embryonic hematopoietic stem cells significantly contribute to adult hematopoiesis. Blood 105: 2724–2732, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Sequeira Lopez ML, Pentz ES, Robert B, Abrahamson DR, Gomez RA: Embryonic origin and lineage of juxtaglomerular cells. Am J Physiol Renal Physiol 281: F345–F356, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Sequeira Lopez ML, Chernavvsky DR, Nomasa T, Wall L, Yanagisawa M, Gomez RA: The embryo makes red blood cell progenitors in every tissue simultaneously with blood vessel morphogenesis. Am J Physiol Regul Integr Comp Physiol 284: R1126–R1137, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Lin EE, Sequeira-Lopez ML, Gomez RA: RBP-J in FOXD1+ renal stromal progenitors is crucial for the proper development and assembly of the kidney vasculature and glomerular mesangial cells. Am J Physiol Renal Physiol 306: F249–F258, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sequeira-Lopez ML, Lin EE, Li M, Hu Y, Sigmund CD, Gomez RA: The earliest metanephric arteriolar progenitors and their role in kidney vascular development. Am J Physiol Regul Integr Comp Physiol 308: R138–R149, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kono M, Allende ML, Proia RL: Sphingosine-1-phosphate regulation of mammalian development. Biochim Biophys Acta 1781: 435–441, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kono M, Mi Y, Liu Y, Sasaki T, Allende ML, Wu YP, Yamashita T, Proia RL: The sphingosine-1-phosphate receptors S1P1, S1P2, and S1P3 function coordinately during embryonic angiogenesis. J Biol Chem 279: 29367–29373, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Allende ML, Yamashita T, Proia RL: G-protein-coupled receptor S1P1 acts within endothelial cells to regulate vascular maturation. Blood 102: 3665–3667, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Liu Y, Wada R, Yamashita T, Mi Y, Deng CX, Hobson JP, Rosenfeldt HM, Nava VE, Chae SS, Lee MJ, Liu CH, Hla T, Spiegel S, Proia RL: Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J Clin Invest 106: 951–961, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Bruijn MF, Speck NA, Peeters MC, Dzierzak E: Definitive hematopoietic stem cells first develop within the major arterial regions of the mouse embryo. EMBO J 19: 2465–2474, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zovein AC, Hofmann JJ, Lynch M, French WJ, Turlo KA, Yang Y, Becker MS, Zanetta L, Dejana E, Gasson JC, Tallquist MD, Iruela-Arispe ML: Fate tracing reveals the endothelial origin of hematopoietic stem cells. Cell Stem Cell 3: 625–636, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hasegawa T, McLeod DS, Bhutto IA, Prow T, Merges CA, Grebe R, Lutty GA: The embryonic human choriocapillaris develops by hemo-vasculogenesis. Dev Dyn 236: 2089–2100, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hyink DP, Tucker DC, St John PL, Leardkamolkarn V, Accavitti MA, Abrass CK, Abrahamson DR: Endogenous origin of glomerular endothelial and mesangial cells in grafts of embryonic kidneys. Am J Physiol 270: F886–F899, 1996 [DOI] [PubMed] [Google Scholar]
- 24.Loughna S, Hardman P, Landels E, Jussila L, Alitalo K, Woolf AS: A molecular and genetic analysis of renalglomerular capillary development. Angiogenesis 1: 84–101, 1997 [DOI] [PubMed] [Google Scholar]
- 25.Robert B, St John PL, Hyink DP, Abrahamson DR: Evidence that embryonic kidney cells expressing flk-1 are intrinsic, vasculogenic angioblasts. Am J Physiol 271: F744–F753, 1996 [DOI] [PubMed] [Google Scholar]
- 26.Li Z, Lan Y, He W, Chen D, Wang J, Zhou F, Wang Y, Sun H, Chen X, Xu C, Li S, Pang Y, Zhang G, Yang L, Zhu L, Fan M, Shang A, Ju Z, Luo L, Ding Y, Guo W, Yuan W, Yang X, Liu B: Mouse embryonic head as a site for hematopoietic stem cell development. Cell Stem Cell 11: 663–675, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Xiong Y, Yang P, Proia RL, Hla T: Erythrocyte-derived sphingosine 1-phosphate is essential for vascular development. J Clin Invest 124: 4823–4828, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ben Shoham A, Malkinson G, Krief S, Shwartz Y, Ely Y, Ferrara N, Yaniv K, Zelzer E: S1P1 inhibits sprouting angiogenesis during vascular development. Development 139: 3859–3869, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Gaengel K, Niaudet C, Hagikura K, Laviña B, Muhl L, Hofmann JJ, Ebarasi L, Nyström S, Rymo S, Chen LL, Pang MF, Jin Y, Raschperger E, Roswall P, Schulte D, Benedito R, Larsson J, Hellström M, Fuxe J, Uhlén P, Adams R, Jakobsson L, Majumdar A, Vestweber D, Uv A, Betsholtz C: The sphingosine-1-phosphate receptor S1PR1 restricts sprouting angiogenesis by regulating the interplay between VE-cadherin and VEGFR2. Dev Cell 23: 587–599, 2012 [DOI] [PubMed] [Google Scholar]
- 30.Kikkawa Y, Virtanen I, Miner JH: Mesangial cells organize the glomerular capillaries by adhering to the G domain of laminin alpha5 in the glomerular basement membrane. J Cell Biol 161: 187–196, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindahl P, Hellström M, Kalén M, Karlsson L, Pekny M, Pekna M, Soriano P, Betsholtz C: Paracrine PDGF-B/PDGF-Rbeta signaling controls mesangial cell development in kidney glomeruli. Development 125: 3313–3322, 1998 [DOI] [PubMed] [Google Scholar]
- 32.Vaughan MR, Quaggin SE: How do mesangial and endothelial cells form the glomerular tuft? J Am Soc Nephrol 19: 24–33, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Takemoto M, He L, Norlin J, Patrakka J, Xiao Z, Petrova T, Bondjers C, Asp J, Wallgard E, Sun Y, Samuelsson T, Mostad P, Lundin S, Miura N, Sado Y, Alitalo K, Quaggin SE, Tryggvason K, Betsholtz C: Large-scale identification of genes implicated in kidney glomerulus development and function. EMBO J 25: 1160–1174, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quaggin SE, Schwartz L, Cui S, Igarashi P, Deimling J, Post M, Rossant J: The basic-helix-loop-helix protein pod1 is critically important for kidney and lung organogenesis. Development 126: 5771–5783, 1999 [DOI] [PubMed] [Google Scholar]
- 35.Dreyer SD, Zhou G, Baldini A, Winterpacht A, Zabel B, Cole W, Johnson RL, Lee B: Mutations in LMX1B cause abnormal skeletal patterning and renal dysplasia in nail patella syndrome. Nat Genet 19: 47–50, 1998 [DOI] [PubMed] [Google Scholar]
- 36.Chen H, Lun Y, Ovchinnikov D, Kokubo H, Oberg KC, Pepicelli CV, Gan L, Lee B, Johnson RL: Limb and kidney defects in Lmx1b mutant mice suggest an involvement of LMX1B in human nail patella syndrome. Nat Genet 19: 51–55, 1998 [DOI] [PubMed] [Google Scholar]
- 37.Means CK, Brown JH: Sphingosine-1-phosphate receptor signalling in the heart. Cardiovasc Res 82: 193–200, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang J, Honbo N, Goetzl EJ, Chatterjee K, Karliner JS, Gray MO: Signals from type 1 sphingosine 1-phosphate receptors enhance adult mouse cardiac myocyte survival during hypoxia. Am J Physiol Heart Circ Physiol 293: H3150–H3158, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Means CK, Xiao CY, Li Z, Zhang T, Omens JH, Ishii I, Chun J, Brown JH: Sphingosine 1-phosphate S1P2 and S1P3 receptor-mediated Akt activation protects against in vivo myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 292: H2944–H2951, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Lee HW, Qin YX, Kim YM, Park EY, Hwang JS, Huo GH, Yang CW, Kim WY, Kim J: Expression of lymphatic endothelium-specific hyaluronan receptor LYVE-1 in the developing mouse kidney. Cell Tissue Res 343: 429–444, 2011 [DOI] [PubMed] [Google Scholar]
- 41.Srinivasan RS, Dillard ME, Lagutin OV, Lin FJ, Tsai S, Tsai MJ, Samokhvalov IM, Oliver G: Lineage tracing demonstrates the venous origin of the mammalian lymphatic vasculature. Genes Dev 21: 2422–2432, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buttler K, Kreysing A, von Kaisenberg CS, Schweigerer L, Gale N, Papoutsi M, Wilting J: Mesenchymal cells with leukocyte and lymphendothelial characteristics in murine embryos. Dev Dyn 235: 1554–1562, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Sebzda E, Hibbard C, Sweeney S, Abtahian F, Bezman N, Clemens G, Maltzman JS, Cheng L, Liu F, Turner M, Tybulewicz V, Koretzky GA, Kahn ML: Syk and Slp-76 mutant mice reveal a cell-autonomous hematopoietic cell contribution to vascular development. Dev Cell 11: 349–361, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Heinrich AC, Pelanda R, Klingmüller U: A mouse model for visualization and conditional mutations in the erythroid lineage. Blood 104: 659–666, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Yu J, Carroll TJ, McMahon AP: Sonic hedgehog regulates proliferation and differentiation of mesenchymal cells in the mouse metanephric kidney. Development 129: 5301–5312, 2002 [DOI] [PubMed] [Google Scholar]
- 46.Soriano P: Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet 21: 70–71, 1999 [DOI] [PubMed] [Google Scholar]
- 47.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L: A global double-fluorescent Cre reporter mouse. Genesis 45: 593–605, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H: A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 13: 133–140, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu S, Wu Y, Capecchi MR: Motoneurons and oligodendrocytes are sequentially generated from neural stem cells but do not appear to share common lineage-restricted progenitors in vivo. Development 133: 581–590, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Sequeira López ML, Pentz ES, Nomasa T, Smithies O, Gomez RA: Renin cells are precursors for multiple cell types that switch to the renin phenotype when homeostasis is threatened. Dev Cell 6: 719–728, 2004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.