Abstract
For years, erythropoiesis-stimulating agent (ESA) use among patients on dialysis was much higher in the United States than in Europe or Japan. Sweeping changes to dialysis reimbursement and regulatory policies for ESA in the United States in 2011 were expected to reduce ESA use and hemoglobin levels. We used the Dialysis Outcomes and Practice Patterns Study (DOPPS) data from 7129 patients in 223 in–center hemodialysis facilities (average per month) to estimate and compare time trends in ESA dose and hemoglobin levels among patients on hemodialysis in the United States, Germany, Italy, Spain, the United Kingdom, and Japan. From 2010 to 2013, substantial declines in ESA use and hemoglobin levels occurred in the United States but not in other DOPPS countries. Between August of 2010 and April of 2013, mean weekly ESA dose in the United States decreased 40.4% for black patients and 38.0% for nonblack patients; mean hemoglobin decreased from 11.5 g/dl in black patients and 11.4 g/dl in nonblack patients to 10.6 g/dl in both groups. In 2010 and 2013, adjusted weekly ESA doses per kilogram were 41% and 11% lower, respectively, in patients in Europe and 60% and 18% lower, respectively, in patients in Japan than in nonblack patients in the United States. Adjusted hemoglobin levels in 2010 and 2013 were 0.07 g/dl lower and 0.56 g/dl higher, respectively, in patients in Europe and 0.93 and 0.01 g/dl lower, respectively, in patients in Japan than in nonblack patients in the United States. In conclusion, ESA dosing reductions in the United States likely reflect efforts in response to changes in reimbursement policy and regulatory guidance.
Keywords: erythropoietin, ESRD, epoetin, anemia, hemodialysis
Deficient renal erythropoietin production is a major factor contributing to the anemic status that affects the vast majority of patients on dialysis.1 Treatment with erythropoiesis-stimulating agents (ESAs) and intravenous (iv) iron has been the cornerstone of anemia management for over two decades. Since the late 1990s, the international Dialysis Outcomes and Practice Patterns Study (DOPPS) has shown differences in anemia management among patients on dialysis in the United States compared with 11 other countries, with hemoglobin (Hb) levels equal to or higher than, and ESA doses substantially exceeding those in other countries.2,3 Although these differences might be attributable, in part, to greater ESA resistance in United States patients on dialysis than in patients elsewhere,3 for many years, ESAs in the United States were reimbursed on a per unit dose basis, thus providing a direct financial incentive for higher ESA doses. Historically higher ESA doses among for–profit United States dialysis centers also supported the possibility that financial incentives influenced ESA dose.4 Conversely, no direct financial or regulatory incentives were present in other countries.3,5
Clinical trial evidence published in 2006 (e.g., the Correction of Hemoglobin and Outcomes in Renal Insufficiency [CHOIR] Trial 6 and the Cardiovascular Risk Reduction by Early Anemia Treatment with Epoetin-β [CREATE] Trial7; later, the Trial to Reduce Cardiovascular Events with Aranesp Therapy (TREAT)8 in 2009) raised serious concerns about ESA safety. During a 2007 Congressional subcommittee hearing9 on the matter, data from the US Renal Data System were shared that indicated that more than one half of United States patients on dialysis had Hb levels above the US Food and Drug Administration10 (FDA) target range of 10–12 g/dl, and the US Government Accounting Office11 (GAO) presented a 2006 report recommending implementation of an expanded bundled payment for dialysis services including renal medications and laboratory services. Subsequent regulatory action culminated in three important changes that occurred in 2011. In January of 2011, the US Centers for Medicare and Medicaid Services (CMS) introduced a major modification to the ESRD Prospective Payment System (PPS) that was fully implemented nearly 90% of United States dialysis units, with the remainder opting to phase in over 4 years. The revised PPS added approximately $3 billion worth of separately billable services to the bundled payment, of which dose-based reimbursement for ESAs and iv iron accounted for approximately 67% and 8%, respectively.12 In June of 2011, the FDA approved a revision to the ESA prescribing information that removed the Hb target range of 10–12 g/dl for patients with CKD, instead advising initiation of ESA therapy at Hb<10 g/dl and reduction or interruption of treatment in patients on dialysis when Hb nears or exceeds 11 g/dl.13 The updated label cited three studies assessing the effect of different Hb targets, which showed higher mortality rates in dialysis (Normal Hematocrit Trial; target 14 versus 10 g/dl) and CKD (CHOIR; target 13.5 versus 11.3 g/dl) and higher stroke rates in CKD (TREAT; target 13 versus ≥9 g/dl) among patients randomized to the higher targets.6,8,14,15 Finally, in July of 2011, the CMS proposed a modification to the Quality Incentive Payment (QIP), which was approved in November of 2011, that removed the payment penalty for Hb<10 g/dl but retained the penalty for Hb>12 g/dl.16,17
The GAO, the American Society of Nephrology, and others anticipated that financial incentives resulting from the PPS would favor lower ESA dosing and greater use of less expensive iv iron.18–20 Combined with the bundled reimbursement for ESA, the removal of specific floor Hb levels in the updated ESA label and QIP proposed rule increased the likelihood that Hb levels would also decline.21 Indeed, the Dialysis Outcomes and Practice Patterns Study Practice Monitor (DPM) reported substantial declines in average Hb levels and ESA doses in the United States between 2010 and 2012 along with a temporary increase in iv iron use and a sustained rise in clinical measures of iron stores.22
Because no significant anemia–related policy changes were implemented in other DOPPS countries during this same time period, in this study, we compare recent trends in anemia practices and outcomes in the United States, Europe, and Japan. We also evaluate whether and to what extent international differences in ESA doses over time are explained by differences in patient characteristics known to influence ESA dose and other aspects of anemia management.
Results
Study Sample
Mean monthly patient (facility) counts were 2023 (70), 1664 (57), and 3442 (96) for Europe, Japan, and the United States, respectively (Supplemental Table 1). During the study period, modest declines were observed for mean vintage (Europe and Japan), ESA delivery by iv route (Japan), and catheter use (United States black patients compared with arteriovenous fistula or graft) (Table 1). The European sample also tended over time toward older age and proportionally more men, whereas United States black patients tended toward higher serum albumin levels and predialysis weight.
Table 1.
Means or percentages of selected patient characteristics by region and calendar time
| Month/Yeara | Europeb | Japan | United States Black | United States Nonblack | ||||
|---|---|---|---|---|---|---|---|---|
| N | Mean (%) | N | Mean (%) | N | Mean (%) | N | Mean (%) | |
| Age, yr | ||||||||
| 08/2010 | 2186 | 65.8 | 1708 | 65.0 | 1053 | 58.6 | 2451 | 65.6 |
| 12/2011 | 2103 | 66.3 | 1672 | 65.5 | 1261 | 59.0 | 2794 | 64.8 |
| 04/2013 | 2011 | 66.6 | 1681 | 65.6 | 1099 | 58.5 | 2195 | 64.6 |
| P value for trend | 0.07 | 0.14 | 0.95 | 0.18 | ||||
| Vintage, yr | ||||||||
| 08/2010 | 2160 | 5.2 | 1702 | 8.4 | 1046 | 4.6 | 2433 | 3.5 |
| 12/2011 | 2084 | 5.4 | 1664 | 8.8 | 1252 | 4.8 | 2777 | 3.8 |
| 04/2013 | 1995 | 5.2 | 1672 | 8.0 | 1082 | 4.7 | 2167 | 3.6 |
| P value for trend | 0.001 | <0.001 | 0.10 | 0.18 | ||||
| Men, % | ||||||||
| 08/2010 | 2186 | 59.5 | 1707 | 64.1 | 1053 | 55.5 | 2451 | 56.1 |
| 12/2011 | 2103 | 59.6 | 1671 | 64.0 | 1261 | 56.1 | 2794 | 55.4 |
| 04/2013 | 2003 | 61.8 | 1678 | 64.5 | 1098 | 54.7 | 2196 | 56.3 |
| P value for trend | 0.05 | 0.56 | 0.87 | 0.88 | ||||
| Predialysis weight, kg | ||||||||
| 08/2010 | 2040 | 73.9 | 1606 | 56.7 | 1024 | 86.6 | 2304 | 81.4 |
| 12/2011 | 1316 | 74.3 | 1595 | 57.7 | 1179 | 87.0 | 2508 | 81.8 |
| 04/2013 | 1577 | 75.4 | 1569 | 58.2 | 1050 | 88.2 | 2051 | 81.0 |
| P value for trend | 0.13 | 0.04 | 0.02 | 0.96 | ||||
| Diabetes, % | ||||||||
| 08/2010 | 2154 | 34.7 | 1698 | 38.9 | 1042 | 61.5 | 2385 | 66.3 |
| 12/2011 | 1370 | 35.7 | 1659 | 38.9 | 1213 | 62.1 | 2610 | 67.4 |
| 04/2013 | 1657 | 35.0 | 1596 | 41.8 | 1081 | 60.0 | 2135 | 63.0 |
| P value for trend | 0.83 | 0.11 | 0.76 | 0.10 | ||||
| Gastrointestinal bleeding in prior 12 mo | ||||||||
| 08/2010 | 2142 | 3.8 | 1681 | 4.3 | 991 | 3.4 | 2298 | 2.9 |
| 12/2011 | 1360 | 4.6 | 1642 | 4.0 | 1123 | 3.3 | 2466 | 2.8 |
| 04/2013 | 1627 | 4.1 | 1573 | 3.9 | 931 | 2.9 | 1888 | 2.8 |
| P value for trend | 0.67 | 0.62 | 0.33 | 0.48 | ||||
| Serum albumin, g/dl | ||||||||
| 08/2010 | 1662 | 3.81 | 1470 | 3.67 | 1034 | 3.86 | 2369 | 3.82 |
| 12/2011 | 1221 | 3.74 | 1533 | 3.74 | 1177 | 3.94 | 2623 | 3.87 |
| 04/2013 | 1301 | 3.72 | 1518 | 3.72 | 970 | 3.90 | 1869 | 3.87 |
| P value for trend | 0.24 | 0.65 | 0.06 | 0.31 | ||||
| Serum creatinine, mg/dl | ||||||||
| 08/2010 | 1511 | 8.28 | 1536 | 10.93 | 1009 | 9.76 | 2253 | 7.54 |
| 12/2011 | 1194 | 8.02 | 1577 | 10.65 | 1072 | 9.52 | 2438 | 7.64 |
| 04/2013 | 1366 | 8.16 | 1500 | 10.56 | 915 | 9.64 | 1707 | 7.74 |
| P value for trend | 0.89 | 0.16 | 0.70 | 0.20 | ||||
| Catheter, % | ||||||||
| 08/2010 | 2126 | 19.1 | 1564 | 0.2 | 982 | 16.8 | 2270 | 16.8 |
| 12/2011 | 1640 | 16.9 | 1550 | 0.2 | 1157 | 18.0 | 2546 | 16.5 |
| 04/2013 | 1841 | 19.8 | 1584 | 0.2 | 1069 | 11.4 | 2149 | 12.3 |
| P value for trend | 0.46 | 0.40 | 0.001 | 0.30 | ||||
| ESA route, % iv | ||||||||
| 08/2010 | 1792 | 90.1 | 1271 | 99.8 | 849 | 99.4 | 1939 | 98.8 |
| 12/2011 | 1624 | 87.5 | 1276 | 99.6 | 945 | 99.0 | 2000 | 94.9 |
| 04/2013 | 1445 | 90.6 | 1348 | 96.1 | 898 | 99.9 | 1660 | 97.4 |
| P value for trend | 0.53 | 0.004 | 0.53 | 0.72 | ||||
P values for trend obtained from models adjusted for monthly seasonality.
Facility sample transitioned from the DOPPS 4 to the DOPPS 5 during January to April of 2012.
Europe includes the DOPPS facility samples in Germany, Italy, Spain, and the United Kingdom.
Long-Term Trends: 2002–2013
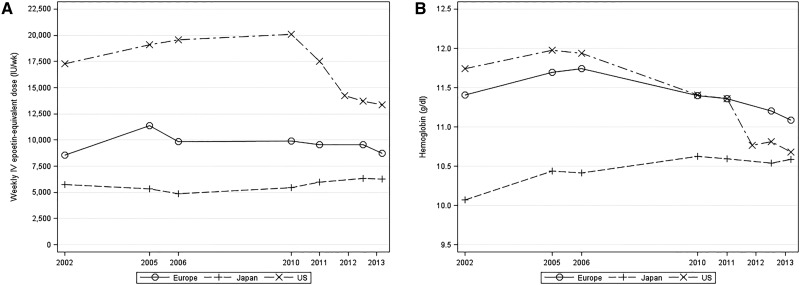
Mean prescribed ESA dose among ESA-treated patients in the United States rose steadily from 2002 to 2005, reached a plateau between 2006 and 2010, and then, sharply declined from 2010 to 2013 (Figure 1A). By contrast, mean prescribed ESA dose in Europe rose from 2002 to 2005 but was stable between 2006 and 2012. In Japan, mean prescribed ESA dose declined between 2002 and 2006 but then, increased slightly through 2013.
Figure 1.
Temporal trends (2002–2013) in prescribed ESA dose and Hb level among patients managed with ESAs by region. (A) Prescribed ESA dose and (B) Hb level among patients managed with ESAs by region. Values were reported at seven indicated time points using data from the DOPPS 2 (2002), the DOPPS 3 (2005 and 2006), the DOPPS 4 (2010 and 2011), and the DOPPS 5 (2012 and 2013).
Mean Hb levels among ESA-treated patients in the United States and Europe reached their peaks in 2005 and 2006, respectively (Figure 1B). Although mean Hb levels in the United States were substantially higher than those in Europe from 2002 to 2006, Hb levels declined in both regions and reached parity in 2010–2011. Hb levels in Japan increased steadily from 2002 to 2010, and by 2013, they nearly reached the mean Hb level in the United States.
ESA Prescription and Dose Trends: August of 2010 to April of 2013
ESA prescription in a 3-month period increased from 87.4% to 90.8% in Japan but fell from 89.6% to 88.0% in Europe, from 94.5% to 92.3% in United States black patients, and from 95.5% to 89.9% in United States nonblack patients (Supplemental Figure 1A). Seasonality–adjusted absolute differences during this period were 3.7% (95% confidence interval [95% CI], 1.2 to 6.2) in Japan, −1.7% (95% CI, −4.3 to 1.0) in Europe, −2.2% (95% CI, −4.9 to 0.4) in United States black patients, and −3.5% (95% CI, −6.1 to −1.0) in United States nonblack patients.
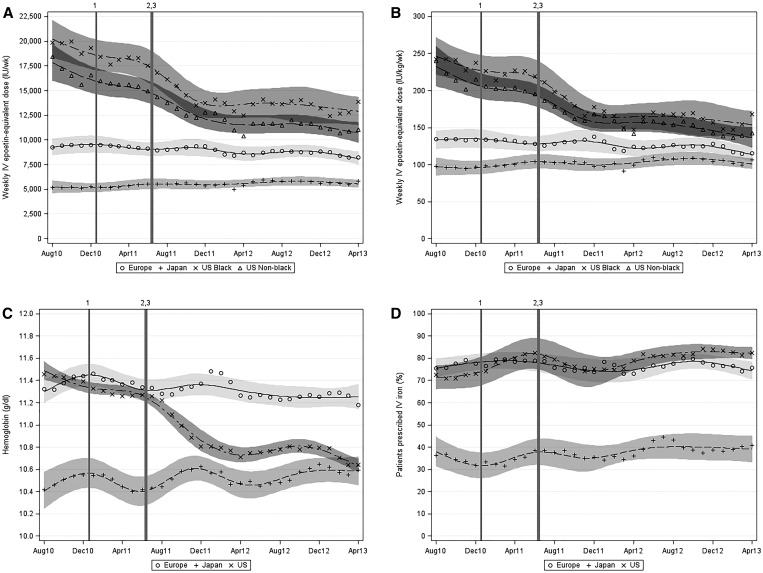
The mean (median) prescribed ESA dose in a 3-month period increased from 5171 (3933) to 5848 (5000) units/wk in Japan but fell from 9272 (7500) to 8216 (6249) units/wk in Europe, from 19,865 (14,250) to 13,834 (8655) units/wk in United States black patients, and from 18,392 (12,467) to 11,046 (7311) units/wk in United States nonblack patients (Figure 2A, Table 2, Supplemental Table 2). Seasonality–adjusted relative differences during this period were 19.4% (95% CI, 5.9 to 34.7) in Japan, −6.4% (95% CI, −17.7 to 6.5) in Europe, −40.4% (95% CI, −50.6 to −28.0) in United States black patients, and −38.0% (95% CI, −44.9 to −30.2) in United States nonblack patients. Results were similar in analyses restricted to iv epoetin alfa doses, analyses expressing ESA dose per unit of Hb (Supplemental Figure 1B) or per kilogram predialysis body weight (Figure 2B), and analyses using alternate conversion ratios for darbepoetin (200:1) and subcutaneous epoetin alfa (1.3:1).
Figure 2.
Temporal trends (by region and month [August 2010 to April 2013]) in weekly prescribed ESA dose, weekly prescribed ESA dose per kilogram, Hb level among ESA-treated patients, and proportion of patients prescribed iv iron. (A) Weekly prescribed ESA dose, (B) weekly prescribed ESA dose per kilogram, (C) Hb level among ESA-treated patients, and (D) proportion of patients prescribed iv iron. Markers indicate monthly observed means. Regional trend lines were estimated using a restricted cubic spline model with seven knots. Vertical lines indicate significant changes to United States dialysis reimbursement policy or regulatory updates. PPS indicates the January of 2011 New Medicare ESRD PPS. QIP indicates the Medicare ESRD QIP program proposed rule removing Hb<10 g/dl as a measure. ESA dose conversions: subcutaneous epoetin, ×1.15; darbepoetin (iv or subcutaneous), ×250 units/μg; and pegylated epoetin-β (iv or subcutaneous), ×208 units/μg. 1Introduction of the revised PPS in January of 2011. 2ESA label revision in June of 2011. 3ESRD QIP proposed rule in July of 2011.
Table 2.
Prescribed ESA dose quantiles by region
| Month/Yeara | Scaled by Predialysis Weight | Not Scaled | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | 10th | 25th | 50th | 75th | 90th | N | 10th | 25th | 50th | 75th | 90th | |
| Europeb | ||||||||||||
| 08/2010 | 1528 | 33 | 56 | 101 | 175 | 274 | 1628 | 2500 | 4000 | 7500 | 11,997 | 19,663 |
| 12/2011 | 881 | 31 | 52 | 101 | 173 | 303 | 1419 | 2236 | 4000 | 6944 | 11,854 | 20,000 |
| 04/2013 | 1158 | 30 | 54 | 90 | 144 | 232 | 1338 | 2297 | 3900 | 6249 | 10,000 | 15,000 |
| Japan | ||||||||||||
| 08/2010 | 832 | 26 | 41 | 76 | 124 | 193 | 872 | 1500 | 2500 | 3933 | 6525 | 10,000 |
| 12/2011 | 973 | 24 | 43 | 76 | 131 | 190 | 1020 | 1404 | 2500 | 4315 | 7500 | 10,000 |
| 04/2013 | 1092 | 30 | 51 | 88 | 136 | 192 | 1111 | 1774 | 2924 | 5000 | 7500 | 10,000 |
| United States black | ||||||||||||
| 08/2010 | 825 | 49 | 88 | 167 | 317 | 554 | 833 | 4230 | 7229 | 14,250 | 25,230 | 46,087 |
| 12/2011 | 872 | 37 | 64 | 131 | 227 | 396 | 916 | 2933 | 4769 | 9523 | 17,415 | 30,782 |
| 04/2013 | 813 | 35 | 56 | 107 | 222 | 401 | 839 | 2918 | 4604 | 8655 | 18,262 | 31,751 |
| United States nonblack | ||||||||||||
| 08/2010 | 1835 | 42 | 88 | 159 | 292 | 549 | 1883 | 3299 | 6522 | 12,467 | 23,077 | 43,786 |
| 12/2011 | 1744 | 36 | 61 | 115 | 214 | 361 | 1912 | 2933 | 4597 | 8416 | 16,025 | 27,526 |
| 04/2013 | 1481 | 24 | 46 | 94 | 176 | 328 | 1540 | 1983 | 3640 | 7311 | 13,735 | 24,334 |
Values expressed as iv epoetin–equivalent units per week were calculated using the following conversions: subcutaneous epoetin (×1.15), darbepoetin (×250 units/μg), and pegylated epoetin-β (×208 units/μg).
Facility sample transitioned from the DOPPS 4 to the DOPPS 5 during January to April of 2012.
Europe includes the DOPPS facility samples in Germany, Italy, Spain, and the United Kingdom.
Between August of 2010 and April of 2013, adjusted differences in prescribed ESA dose scaled by predialysis body weight compared with United States nonblack patients (Table 3, scaled by predialysis body weight) declined from −41% (95% CI, −51% to −30%) to −11% (95% CI, −25% to 6%) in Europe, declined from −60% (95% CI, −66% to −51%) to −18% (95% CI, −33% to 0%) in Japan, and increased from 2% (95% CI, −11% to 16%) to 17% (95% CI, 4% to 32%) in United States black patients. These differences were slightly larger in unscaled analyses (Table 3, not scaled) and analyses expressing ESA dose per unit of Hb (not shown).
Table 3.
Percentage differences (with 95% CIs) in mean prescribed ESA dose in Europe, Japan, and United States black patients compared with United States nonblack patients by calendar time and model used to adjust for covariates
| Month/Yeara | Scaled by Predialysis Body Weight | Not Scaled | |||||
|---|---|---|---|---|---|---|---|
| Unadjusted | Model 1b | Model 2c | Unadjusted | Model 1b | Model 2c | Model 3d | |
| Europee | |||||||
| 08/2010 | −37% (−46% to −28%) | −41% (−49% to −32%) | −41% (−51% to −30%) | −43% (−50% to −35%) | −44% (−51% to −36%) | −46% (−54% to −37%) | −45% (−53% to −36%) |
| 12/2011 | −17% (−29% to −2%) | −28% (−39% to −15%) | −21% (−35% to −6%) | −22% (−31% to −11%) | −27% (−38% to −14%) | −25% (−37% to −10%) | −22% (−35% to −8%) |
| 04/2013 | −4% (−17% to 10%) | −13% (−24% to 0%) | −11% (−25% to 6%) | −11% (−22% to 2%) | −16% (−26% to −4%) | −17% (−29% to −4%) | −15% (−27% to −2%) |
| Japan | |||||||
| 08/2010 | −54% (−61% to −47%) | −58% (−64% to −51%) | −60% (−66% to −51%) | −68% (−72% to −63%) | −69% (−73% to −65%) | −71% (−75% to −66%) | −70% (−75% to −65%) |
| 12/2011 | −36% (−45% to −26%) | −41% (−49% to −31%) | −39% (−50% to −26%) | −53% (−60% to −46%) | −55% (−62% to −48%) | −56% (−64% to −47%) | −56% (−63% to −46%) |
| 04/2013 | −9% (−20% to 4%) | −13% (−25% to 1%) | −18% (−33% to −0%) | −34% (−42% to −26%) | −36% (−44% to −26%) | −43% (−52% to −31%) | −41% (−51% to −29%) |
| United States black | |||||||
| 08/2010 | 4% (−9% to 20%) | 2% (−11% to 17%) | 2% (−11% to 16%) | 13% (−1% to 28%) | 7% (−6% to 22%) | 7% (−5% to 21%) | 6% (−6% to 20%) |
| 12/2011 | 8% (−4% to 20%) | 5% (−8% to 19%) | 3% (−10% to 18%) | 10% (−2% to 23%) | 4% (−8% to 19%) | 3% (−10% to 18%) | 2% (−10% to 17%) |
| 04/2013 | 21% (7% to 37%) | 19% (6% to 34%) | 17% (4% to 32%) | 30% (14% to 48%) | 25% (11% to 42%) | 23% (9% to 40%) | 23% (8% to 39%) |
Doses expressed as iv epoetin–equivalent units per week were calculated using the following conversions: subcutaneous epoetin (×1.15), darbepoetin (×250 units/μg), and pegylated epoetin-β (×208 units/μg).
Facility sample transitioned from the DOPPS 4 to the DOPPS 5 during January to April of 2012.
Model 1 includes age, years on dialysis, black race (United States only), sex, catheter use, diabetes, history of gastrointestinal bleeding, creatinine, and most recent albumin level in the prior 3 months.
Model 2 includes model 1 adjustments plus iv iron use in the prior 3 months (yes or no), most recent transferrin saturation and ferritin levels in the prior 3 months, and facility–reported upper Hb target.
Model 3 includes model 2 adjustments plus predialysis body weight.
Europe includes the DOPPS facility samples in Germany, Italy, Spain, and the United Kingdom.
Hb Trends: August of 2010 to April of 2013
The mean Hb level among ESA-treated patients changed little from 10.4 to 10.6 g/dl in Japan and from 11.3 to 11.2 g/dl in Europe but fell from 11.5 to 10.6 g/dl in United States black patients and from 11.4 to 10.6 g/dl in United States nonblack patients (Figure 2C). Seasonality–adjusted absolute differences during this period were 0.10 g/dl (95% CI, −0.06 to 0.25) in Japan, −0.19 g/dl (95% CI, −0.30 to −0.09) in Europe, −0.91 g/dl (95% CI, −1.02 to −0.79) in United States black patients, and −0.84 g/dl (95% CI, −0.94 to −0.74) in United States nonblack patients.
Between August of 2010 and April of 2013, the unadjusted mean Hb differences compared with United States nonblack patients increased from −0.10 g/dl (95% CI, −0.23 to 0.03) to 0.53 g/dl (95% CI, 0.41 to 0.65) in Europe; it decreased from −1.01 g/dl (95% CI, −1.20 to −0.82) to −0.05 g/dl (95% CI, −0.19 to 0.09) in Japan and from 0.11 g/dl (95% CI, 0.01 to 0.21) to −0.03 g/dl (95% CI, −0.13 to 0.06) in United States black patients (Table 4).
Table 4.
Differences in mean Hb in Europe, Japan, and United States black patients compared with United States nonblack patients by calendar time and model used to adjust for covariates
| Month/Yeara | Unadjusted | 95% CI | Model 1b | 95% CI | Model 2c | 95% CI | Model 3d | 95% CI |
|---|---|---|---|---|---|---|---|---|
| Europee | ||||||||
| 08/2010 | −0.10 | −0.23 to 0.03 | −0.08 | −0.21 to 0.05 | −0.08 | −0.23 to 0.08 | −0.07 | −0.22 to 0.09 |
| 12/2011 | 0.55 | 0.39 to 0.70 | 0.60 | 0.45 to 0.75 | 0.57 | 0.39 to 0.75 | 0.58 | 0.40 to 0.76 |
| 04/2013 | 0.53 | 0.41 to 0.65 | 0.58 | 0.44 to 0.72 | 0.56 | 0.38 to 0.74 | 0.56 | 0.38 to 0.74 |
| Japan | ||||||||
| 08/2010 | −1.01 | −1.20 to −0.82 | −0.94 | −1.14 to −0.74 | −0.97 | −1.19 to −0.75 | −0.93 | −1.15 to −0.72 |
| 12/2011 | −0.20 | −0.35 to −0.05 | −0.15 | −0.32 to 0.02 | −0.17 | −0.38 to 0.03 | −0.14 | −0.34 to 0.06 |
| 04/2013 | −0.05 | −0.19 to 0.09 | −0.01 | −0.17 to 0.14 | −0.04 | −0.25 to 0.16 | −0.01 | −0.21 to 0.19 |
| United States black | ||||||||
| 08/2010 | 0.11 | 0.01 to 0.21 | 0.14 | 0.04 to 0.24 | 0.13 | 0.04 to 0.23 | 0.13 | 0.04 to 0.22 |
| 12/2011 | −0.06 | −0.23 to 0.10 | −0.05 | −0.21 to 0.12 | −0.03 | −0.19 to 0.13 | −0.03 | −0.19 to 0.12 |
| 04/2013 | −0.03 | −0.13 to 0.06 | −0.01 | −0.09 to 0.08 | 0.00 | −0.09 to 0.08 | −0.01 | −0.09 to 0.07 |
Facility sample transitioned from the DOPPS 4 to the DOPPS 5 during January to April of 2012.
Model 1 includes age, years on dialysis, black race (United States only), sex, catheter use, diabetes, history of gastrointestinal bleeding, creatinine, and most recent albumin level in the prior 3 months.
Model 2 includes model 1 adjustments plus iv iron use in the prior 3 months (yes or no), most recent transferrin saturation and ferritin levels in the prior 3 months, and facility–reported upper Hb target.
Model 3 includes model 2 adjustments plus predialysis body weight.
Europe includes the DOPPS facility samples in Germany, Italy, Spain, and the United Kingdom.
Iron Prescription Trends: August of 2010 to April of 2013
The proportion of patients prescribed iv iron in a 3-month period increased from 75.4% to 75.8% in Europe, from 36.3% to 40.7% in Japan, from 73.5% to 82.9% in United States black patients, and from 72.0% to 82.1% in United States nonblack patients (Figure 2D). Seasonality–adjusted absolute differences during this period were −1.1% (95% CI, −4.5 to 2.2) in Europe, 7.9% (95% CI, −2.2 to 18.0) in Japan, 7.4% (95% CI, 1.4 to 13.3) in United States black patients, and 10.7% (95% CI, −1.6 to 22.9) in United States nonblack patients. Median monthly iv iron doses (among treated patients) in the United States remained fairly stable during this period (approximately 50 mg/wk), and oral iron use was <5% in all regions (not shown).
Anemia Treatment Targets: 2010–2013
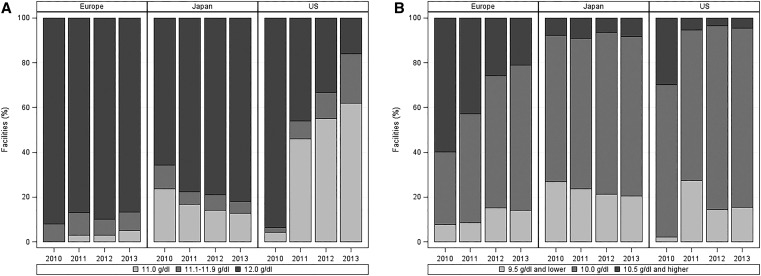
Among United States study sites, 94% reported an upper Hb target of 12 g/dl in 2010, but they were evenly split between 11 and 12 g/dl (46% each) in 2011. In 2013, targets of 11, 11.1–11.9, and 12 g/dl were reported by 62%, 22%, and 16% of facilities, respectively (Figure 3A). During the same period, Hb upper targets of 12 g/dl declined slightly from 92% to 87% in Europe and increased gradually from 66% to 82% in Japan. Hb lower targets ≤10 g/dl increased from 70% to 96% in the United States and from 40% to 79% in Europe but remained relatively stable at 91%–94% in Japan (Figure 3B).
Figure 3.
Temporal trends in upper and lower Hb target levels reported by the DOPPS site medical directors by region and year (2010–2013). (A) Upper and (B) lower Hb target levels reported by the DOPPS site medical directors by region and year (2010–2013).
Discussion
Since the introduction of significant United States dialysis payment and regulatory changes in 2011, there have been substantial decreases in mean ESA dose and Hb levels and a modest increase in the proportion of patients receiving iv iron. Observed ESA and Hb trends in the United States from 2010 to 2013 exceed prior trends from 2002 to 2010 or trends in other DOPPS countries from 2002 to 2013. Preliminary data for 2014 and 2015 suggest that Hb levels have achieved a relative stability, whereas ESA doses have continued to decline slowly (data not shown). Notably, the most rapid decline occurred soon after the ESA label revision (in June of 2011) rather than after the PPS bundling (in January of 2011). We infer from this that the United States dialysis community did not primarily change anemia practice for cost reasons alone but changed in the context of supportive clinical guidance from the FDA–approved ESA label change. In contrast, we observed rising ESA dose, iv iron prescription, and Hb levels in Japan and mostly flat trends in four European countries.
Before the US PPS revision, the maximum Medicare–reimbursable ESA dose was 400,000 units/mo, and doses of that magnitude were not uncommon in highly ESA–resistant patients. With the inclusion of ESAs into the bundled payment, the DPM indicates that many dialysis facilities in the United States may be voluntarily limiting prescribed ESA doses at much lower maximum levels, with 90% percentile doses now around 30,000 units/wk, suggesting a dramatic change in treatment strategy for these patients.22 However, ESA doses at or approaching even this reduced ceiling dose remain uncommon in Europe, where many countries allow separate billing for ESA therapy, and are absent in Japan, where the maximum reimbursable dose (determined from prescribing information on the ESA label) is 9000 units/wk.
Similar changes to reimbursement and payment–linked quality initiatives in other countries seem to have had a more modest effect on anemia management than in the United States. In Germany, an expanded, weekly flat–rate dialysis payment was introduced in 2002, and a quality assurance program—proposed in 2006 and implemented in 2009—included administrative penalties for facilities failing to achieve targets for Hb levels and three other nonanemia measures.23,24 Kleophas et al.24 reported that the proportion with Hb<10 g/dl remained steady at approximately 10% from 2006 to 2011 along with an approximately 17% decline in ESA dose and an approximately 20% increase in iv iron dose. However, these results should be viewed in the context of declining ESA use trends in Europe because of a changing balance of clinical evidence after publication of results from the CHOIR and CREATE clinical trials in 2006.6,7
In 2006, Japan introduced a bundled payment for ESA within the overall reimbursement for dialysis services. Hasegawa et al.25 showed that this shift in reimbursement policy was associated with an increase of 9.6% of patients receiving iv iron and an 11.8% reduction in mean EPO dose between January of 2006 and January of 2007. The percentage reduction in mean ESA dose in Japan was much less than in the United States after ESA bundling in 2011; a possible explanation is that prebundle ESA doses in Japan were already quite low and could be reduced little to still maintain an erythropoietic effect.
ESA doses, which had been twice as high in the United States than elsewhere to support a given Hb level, are now only modestly higher than in some European countries, indicating that, in contrast to what may have been previously inferred, clinical ESA requirements for patients differ little across these regions. Turenne et al.26 reported comparable relative reductions in mean ESA doses adjusted for Hb among black and nonblack patients in the United States subsequent to the 2011 policy changes, indicating that ESA doses to support a given Hb level could be reduced in both groups. This study extends this result, showing that adjustment for an array of common patient case–mix factors (including iv iron use and ferritin levels) did not explain much of the observed differences in prescribed ESA dose among the DOPPS regions at several time points (Table 3). Together, these data support the speculation by McFarlane et al.3 that between-country differences in anemia management have been related primarily to clinical guidelines and reimbursement policies rather than specific physiologic requirement.
Indeed, inter-regional differences in treatment targets for Hb level have been shrinking. Before 2004, anemia treatment targets in Japan were based informally on insurance pricing tables that suggested maintaining hematocrit levels at 30% (approximately 10.0 g/dl). However, the Japanese Society for Dialysis Therapy (JSDT) published anemia management guidelines in 2004 that recommended an Hb target level of 10–11 g/dl for most patients and 11–12 g/dl for younger, more active patients.27 A 2008 update to the JSDT guidelines added 12 g/dl as a criterion for reducing or interrupting ESA treatment.28 Although the distribution of lower Hb targets reported by Japanese DOPPS facilities has not changed much since 2010 (Figure 3B), in recent years, we have observed a higher proportion of Japanese DOPPS facilities reporting upper Hb targets of 12 g/dl (Figure 3A). In contrast, nonguideline position statements regarding anemia treatment in European patients with CKD released by the European Renal Best Practice (ERBP) group through 2010 generally suggested maintaining Hb levels between 11 and 12 g/dl.29–31 In 2012, responding to new guidelines from the Kidney Disease Improving Global Outcomes group,32 the ERBP group cited a lack of evidence of benefit or harm for maintaining Hb values in the range of 11.5 and 13.5 g/dl and suggested that Hb levels in European populations should be individualized between 10 and 12 g/dl.33 Between 2010 and 2013, we have shown a steady increase in the proportion of European DOPPS facilities reporting a lower Hb target of 10 g/dl compared with 10.5 or 11 g/dl (Figure 3B), with relatively little change in the proportion of facilities reporting an upper Hb target of 12 g/dl (Figure 3A).
In addition to clinical guidelines, reimbursement policies may account for differences between United States and European Hb levels in the most recent years. Reimbursement to United States dialysis providers is reduced if there is an excess of patients with Hb>12 g/dl under the CMS QIP in place during the analysis time period17 (although this measure is now to be phased out for QIP Program Year 2017), and thus, this may explain, in part, the rapid reduction of Hb upper targets to 11.0 or 11.5 g/dl to avoid penalties. By contrast, reimbursement generally is not conditioned on achievement of specific Hb levels in many European countries.34 Thus, European providers may be less concerned, for reimbursement reasons, with occasional excursions of Hb levels into the ERBP gray area of 11.5–13.5 g/dl.
United States ESA dosing guidance before 2011 recommended treating patients with CKD to an Hb level between 10 and 12 g/dl (well above common thresholds for using red blood cell transfusions to correct severe anemia)35,36 and may have led to administration of higher doses than necessary in ESA-resistant patients. The revised ESA label approved by the FDA in June of 2011 did not specify a particular Hb target, instead recommending using the lowest dose required to avoid transfusion. Dialysis provider efforts to modify treatment protocols under this new guidance and the resulting reductions in ESA doses may partly explain a short-term increase during the study period in the proportion of Medicare ESRD beneficiaries receiving a transfusion (from 2.5%–3.0% per month in 2010 to 3.2%–3.6% per month in 2012) reported elsewhere.37 Concerns regarding ESA safety (e.g., cancer progression and increased stroke risk) may have influenced the use of transfusions. Transfusions incur a risk of allosensitization, which may reduce the likelihood of a suitable renal transplant match. Although the proportion of patients on dialysis undergoing kidney transplant each year is low, careful monitoring of transfusion rates, particularly among transplant candidates, remains an important priority.
This study has several strengths. The DOPPS includes a wide range of data collected in standardized fashion internationally since 2002 (or earlier), enabling the regional comparisons presented in this paper. Much of our contemporary data collection is performed by direct electronic health record download, which allows routine collection of anemia management measures and improved data fidelity. The decline in United States Hb and ESA doses after implementation of the revised PPS was first described using the DOPPS data and has since been confirmed with national data sources.37,38
We acknowledge several study limitations. The DPM extends backward only to August of 2010, precluding a broader analysis of anticipatory practice changes in response to the PPS proposed rule during 2010. Our trend models used a discontinuity parameter to account for the low retention of United States facilities from the DOPPS 4 to the DOPPS 5; this may result in a slight underestimation of actual trends. ESA dose comparisons by region are additionally dependent on global conversion factors for subcutaneous ESA or nonepoetin alfa preparations, which may not be appropriate for all patients. However, our results were robust to a variety of plausible scenarios related to these conversions. Other dialysis practices, which are not consistently captured in the DPM data, may partially explain residual differences in ESA dose or Hb between study regions.
In summary, we report dramatic trends in United States anemia management practices and laboratory outcomes between August of 2010 and April of 2013 after updates to United States regulatory guidance and reimbursement policy in 2011. Compared with the observed trends in the United States, changes in anemia management in other DOPPS countries during this period were minimal. United States ESA dosing reductions likely reflect efforts to limit high ESA doses and accept lower Hb levels in response to changes in reimbursement policy and regulatory guidance. Despite adjusted ESA dosing levels that have recently converged, Hb levels in the United States are now lower than in Europe for the first time in more than a decade; residual differences may reflect higher prevalence or severity of ESA resistance in United States patients. Ongoing observation and research are needed to estimate any lasting effects on the patient experience and clinical outcomes.
Concise Methods
Data Sources
The DOPPS is an international, multistage prospective cohort study of patients on in-center hemodialysis and practices ongoing since 1996. Hemodialysis facilities are randomly selected in each country according to factors, such as geographic location and facility setting or dialysis organization. Basic demographic information (age, sex, race, and years on dialysis) is collected for all facility patients, and a random sample of patients on hemodialysis is selected for DOPPS enrollment with expanded longitudinal data collection, including medical history, monthly laboratory values, vascular access history, and medication lists with dosing information (details of the study design are in the works by Young et al.39 and Pisoni et al.40).
On the basis of the DOPPS 4 (2009–2011) and the DOPPS 5 (2012–2015) samples, the DPM is designed to inform the community of contemporary trends in United States dialysis treatment practices before and after implementation of the revised PPS in January of 2011. Sampling design and analytic methods have been published,41 and the DPM results have been consistent with published national data.37,38,42 This study extends the DPM framework to other DOPPS countries with complete follow-up during the analysis period (Germany, Italy, Spain, the United Kingdom, and Japan) to describe and contrast regional trends in anemia practices and outcomes between August of 2010 and April of 2013. Cross-sections from the DOPPS 2 (2002) and the DOPPS 3 (2005 and 2006) are also provided for historical context.
Treatment practices included prescription of ESA or iv iron during the prior 3 months and prescribed ESA dose. ESAs include epoetin (epoetin alfa in the United States and epoetin alfa and other forms elsewhere), darbepoetin (available in the United States but primarily used in other countries), and pegylated epoetin-β (not available in the United States). ESA prescribed doses were obtained at the end of each study month and scaled to a 7-day weekly dose. Administered ESA doses were not available in all countries during the study period and were not analyzed. On average, the ESA dose required to maintain a given Hb level tends to be higher in black patients than in patients of other races (nonblack).16,43,44 To limit confounding by the large proportion of black patients in the United States, results are presented separately for black and nonblack patients in the United States.
Facility–reported Hb targets were obtained from annual surveys of the DOPPS site medical directors in September to November of 2010, 2011, 2012, and 2013.
Statistical Analyses
The DOPPS facility sample transitioned from the DOPPS 4 to the DOPPS 5 (January of 2012 to April of 2012) during the analysis period; we used the DOPPS 5 sample frame as the common reference population for weighting purposes. The proportions of facilities retained from the DOPPS 4 to the DOPPS 5 were 55% in the United States, 90% in Europe, and 88% in Japan.
Subcutaneous epoetin (×1.15)3 and darbepoetin (×250 units/μg)45 doses were converted to iv epoetin–equivalent values, and pegylated epoetin-β (×208 units/μg) doses were converted by calculating a darbepoetin-equivalent dose (dividing by 1.2)46 and applying the darbepoetin conversion. Last observation carry–forward imputation (up to 3 months) was used to populate missing values for serum albumin, which is not always measured monthly, particularly in Europe.
Observed monthly means and proportions are overlaid with smoothed trends estimated by a restricted cubic spline with knots at August of 2010, January of 2011, June of 2011, November of 2011, April of 2012, October of 2012, and March of 2013.47 Seasonality-adjusted differences between August of 2010 and April of 2013 with 95% CIs were estimated separately using a continuous variable for calendar year, indicator variables for each nominal calendar month (e.g., January and December), and a discontinuity allowed at January of 2012 to account for potential differences resulting from the facility sample transition from the DOPPS 4 to the DOPPS 5.
Linear mixed models to investigate regional differences in prescribed ESA dose and mean Hb at three time points (August of 2010, December of 2011, and April of 2013) were estimated using progressive levels of adjustment and a random facility intercept to account for within-facility clustering. Because body weight may influence ESA dosing practices differently across regions, separate models are shown using ESA dose scaled per kilogram predialysis body weight and covariate adjustment for predialysis body weight. A natural log transform for ESA dose was used in trend analyses where it was the outcome. Sensitivity analyses for ESA dose included restriction to iv epoetin doses only, ESA dose expressed per unit Hb, and use of alternate conversion ratios for subcutaneous epoetin (×1.3)48 and darbepoetin (×200 units/μg). Dataset development and analyses were performed with SAS 9.3 (SAS Institute Inc., Cary, NC).
Disclosures
The Dialysis Outcomes and Practice Patterns Study Program is supported by Amgen, Inc., Kyowa Hakko Kirin, AbbVie Inc., Sanofi Renal, Baxter Healthcare, and Vifor Fresenius Medical Care Renal Pharma Ltd. Additional support for specific projects and countries is also provided by Amgen, Inc., BHC Medical, Janssen Biotech, Takeda Chemical Industries, and Kidney Foundation of Canada (for logistics support) in Canada; Hexal, DGfN, Shire, and WiNe Institute in Germany; and the Japanese Society for Peritoneal Dialysis for Peritoneal Dialysis Outcomes and Practice Patterns Study in Japan. All support is provided without restrictions on publications. R.L.P. has received speaker’s fees from Amgen, Inc., Kyowa Hakko Kirin, and Vifor Fresenius Medical Care Renal Pharma Ltd. and has served on an advisory panel for Merck GmbH. B.M.R. has received a speaker’s fee from Kyowa Hakko Kirin. The other authors declare that they have no other relevant financial interests.
Supplementary Material
Acknowledgments
The authors thank Shauna Leighton, a medical editor with Arbor Research Collaborative for Health, for providing editorial assistance.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015060673/-/DCSupplemental.
References
- 1.Babitt JL, Lin HY: Mechanisms of anemia in CKD. J Am Soc Nephrol 23: 1631–1634, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pisoni RL, Bragg-Gresham JL, Young EW, Akizawa T, Asano Y, Locatelli F, Bommer J, Cruz JM, Kerr PG, Mendelssohn DC, Held PJ, Port FK: Anemia management and outcomes from 12 countries in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 44: 94–111, 2004 [DOI] [PubMed] [Google Scholar]
- 3.McFarlane PA, Pisoni RL, Eichleay MA, Wald R, Port FK, Mendelssohn D: International trends in erythropoietin use and hemoglobin levels in hemodialysis patients. Kidney Int 78: 215–223, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Thamer M, Zhang Y, Lai D, Kshirsagar O, Cotter D: Influence of safety warnings on ESA prescribing among dialysis patients using an interrupted time series. BMC Nephrol 14: 172, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirth RA: The organization and financing of kidney dialysis and transplant care in the United States of America. Int J Health Care Finance Econ 7: 301–318, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, Reddan D CHOIR Investigators : Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 355: 2085–2098, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Drüeke TB, Locatelli F, Clyne N, Eckardt KU, Macdougall IC, Tsakiris D, Burger HU, Scherhag A CREATE Investigators : Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 355: 2071–2084, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Pfeffer MA, Burdmann EA, Chen C-Y, Cooper ME, de Zeeuw D, Eckardt K-U, Feyzi JM, Ivanovich P, Kewalramani R, Levey AS, Lewis EF, McGill JB, McMurray JJ, Parfrey P, Parving HH, Remuzzi G, Singh AK, Solomon SD, Toto R TREAT Investigators : A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 361: 2019–2032, 2009 [DOI] [PubMed] [Google Scholar]
- 9.United States Congress, House Committee on Ways and Means, Subcommittee on Health: Hearing on Ensuring Kidney Patients Receive Safe and Appropriate Anemia Management Care [Electronic Resource]: Hearing before the Subcommittee on Health of the Committee on Ways and Means, US House of Representatives, One Hundred Tenth Congress, First Session, June 26, 2007. Available at: http://purl.access.gpo.gov/GPO/LPS122225. Accessed June 5, 2015
- 10.Saran R, Li Y, Robinson B, Ayanian J, Balkrishnan R, Bragg-Gresham J, Chen JT, Cope E, Gipson D, He K, Herman W, Heung M, Hirth RA, Jacobsen SS, Kalantar-Zadeh K, Kovesdy CP, Leichtman AB, Lu Y, Molnar MZ, Morgenstern H, Nallamothu B, O’Hare AM, Pisoni R, Plattner B, Port FK, Rao P, Rhee CM, Schaubel DE, Selewski DT, Shahinian V, Sim JJ, Song P, Streja E, Kurella Tamura M, Tentori F, Eggers PW, Agodoa LY, Abbott KC: US Renal Data System 2014 annual data report: Epidemiology of kidney disease in the United States. Am J Kidney Dis 66[Suppl 1]: S1–S305, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.US Government Accountability Office : End-Stage Renal Disease: Medicare Should Pay a Bundled Rate for All ESRD Items and Services, Washington, DC, US Government Accountability Office, 2007 [Google Scholar]
- 12.Centers for Medicare & Medicaid Services (CMS) HHS : Medicare program; end-stage renal disease prospective payment system. Final rule. Fed Regist 75: 49029–49214, 2010 [PubMed] [Google Scholar]
- 13.US Food and Drug Administration; US Department of Health and Human Services: FDA Drug Safety Communication: Modified Dosing Recommendations to Improve the Safe Use of Erythropoiesis-Stimulating Agents (ESAs) in Chronic Kidney Disease. Safety Announcement 6/24/2011. Available at: http://www.fda.gov/drugs/drugsafety/ucm259639.htm. Accessed June 15, 2015
- 14.Besarab A, Bolton WK, Browne JK, Egrie JC, Nissenson AR, Okamoto DM, Schwab SJ, Goodkin DA: The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med 339: 584–590, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Coyne DW: The health-related quality of life was not improved by targeting higher hemoglobin in the Normal Hematocrit Trial. Kidney Int 82: 235–241, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Medicare & Medicaid Services (CMS) HHS : Medicare program; end-stage renal disease prospective payment system and quality incentive program; ambulance fee schedule; durable medical equipment; and competitive acquisition of certain durable medical equipment, prosthetics, orthotics and supplies. Proposed rule. Fed Regist 76: 40498–40550, 2011 [PubMed] [Google Scholar]
- 17.Centers for Medicare & Medicaid Services (CMS) HHS : Medicare program; end-stage renal disease prospective payment system and quality incentive program; ambulance fee schedule; durable medical equipment; and competitive acquisition of certain durable medical equipment prosthetics, orthotics and supplies. Final rule. Fed Regist 76: 70228–70316, 2011 [PubMed] [Google Scholar]
- 18.US Government Accountability Office : End-Stage Renal Disease: CMS Should Monitor Access to and Quality of Dialysis Care Promptly After Implementation of New Bundled Payment System, Washington, DC, US Government Accountability Office, 2010 [Google Scholar]
- 19.American Society of Nephrology: ASN Comments on ESRD PPS and QIP Final Rule November 2010. Available at: http://www.asn-online.org/policy/webdocs/nov2010guidanceonfinalrule.pdf. Accessed August 7, 2014
- 20.Wish JB: Anemia management under a bundled payment policy for dialysis: A preview for the United States from Japan. Kidney Int 79: 265–267, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Manns BJ, Tonelli M: The new FDA labeling for ESA--implications for patients and providers. Clin J Am Soc Nephrol 7: 348–353, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arbor Research Collaborative for Health: DOPPS Practice Monitor. Available at: http://www.dopps.org/DPM/Default.aspx. Accessed February 3, 2015
- 23.Kleophas W, Reichel H: International study of health care organization and financing: Development of renal replacement therapy in Germany. Int J Health Care Finance Econ 7: 185–200, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Kleophas W, Karaboyas A, Li Y, Bommer J, Reichel H, Walter A, Icks A, Rump LC, Pisoni RL, Robinson BM, Port FK: Changes in dialysis treatment modalities during institution of flat rate reimbursement and quality assurance programs. Kidney Int 84: 578–584, 2013 [DOI] [PubMed] [Google Scholar]
- 25.Hasegawa T, Bragg-Gresham JL, Pisoni RL, Robinson BM, Fukuhara S, Akiba T, Saito A, Kurokawa K, Akizawa T: Changes in anemia management and hemoglobin levels following revision of a bundling policy to incorporate recombinant human erythropoietin. Kidney Int 79: 340–346, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Turenne MN, Cope EL, Porenta S, Mukhopadhyay P, Fuller DS, Pearson JM, Dahlerus C, Lantz B, Tentori F, Robinson BM: Has dialysis payment reform led to initial racial disparities in anemia and mineral metabolism management? J Am Soc Nephrol 26: 754–764, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gejyo F, Saito A, Akizawa T, Akiba T, Sakai T, Suzuki M, Nishi S, Tsubakihara Y, Hirakata H, Bessho M Japanese Society for Dialysis Therapy : 2004 Japanese Society for Dialysis Therapy guidelines for renal anemia in chronic hemodialysis patients. Ther Apher Dial 8: 443–459, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Tsubakihara Y, Nishi S, Akiba T, Hirakata H, Iseki K, Kubota M, Kuriyama S, Komatsu Y, Suzuki M, Nakai S, Hattori M, Babazono T, Hiramatsu M, Yamamoto H, Bessho M, Akizawa T: 2008 Japanese Society for Dialysis Therapy: Guidelines for renal anemia in chronic kidney disease. Ther Apher Dial 14: 240–275, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Locatelli F, Aljama P, Bárány P, Canaud B, Carrera F, Eckardt KU, Hörl WH, Macdougal IC, Macleod A, Wiecek A, Cameron S European Best Practice Guidelines Working Group : Revised European best practice guidelines for the management of anaemia in patients with chronic renal failure. Nephrol Dial Transplant 19[Suppl 2]: ii1–ii47, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Locatelli F, Covic A, Eckardt KU, Wiecek A, Vanholder R ERA-EDTA ERBP Advisory Board : Anaemia management in patients with chronic kidney disease: A position statement by the Anaemia Working Group of European Renal Best Practice (ERBP). Nephrol Dial Transplant 24: 348–354, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Locatelli F, Aljama P, Canaud B, Covic A, De Francisco A, Macdougall IC, Wiecek A, Vanholder R Anaemia Working Group of European Renal Best Practice (ERBP) : Target haemoglobin to aim for with erythropoiesis-stimulating agents: A position statement by ERBP following publication of the Trial to reduce cardiovascular events with Aranesp therapy (TREAT) study. Nephrol Dial Transplant 25: 2846–2850, 2010 [DOI] [PubMed] [Google Scholar]
- 32.Kidney Disease Improving Global Outcomes : Kidney Disease Improving Global Outcomes clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl 2: 279–335, 2012 [Google Scholar]
- 33.Locatelli F, Bárány P, Covic A, De Francisco A, Del Vecchio L, Goldsmith D, Hörl W, London G, Vanholder R, Van Biesen W ERA-EDTA ERBP Advisory Board : Kidney Disease: Improving Global Outcomes guidelines on anaemia management in chronic kidney disease: A European Renal Best Practice position statement. Nephrol Dial Transplant 28: 1346–1359, 2013 [DOI] [PubMed] [Google Scholar]
- 34.Vanholder R, Davenport A, Hannedouche T, Kooman J, Kribben A, Lameire N, Lonnemann G, Magner P, Mendelssohn D, Saggi SJ, Shaffer RN, Moe SM, Van Biesen W, van der Sande F, Mehrotra R Dialysis Advisory Group of American Society of Nephrology : Reimbursement of dialysis: A comparison of seven countries. J Am Soc Nephrol 23: 1291–1298, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Fuller DS, Pisoni RL, Bieber BA, Gillespie BW, Robinson BM: The DOPPS Practice Monitor for US dialysis care: Trends through December 2011. Am J Kidney Dis 61: 342–346, 2013 [DOI] [PubMed] [Google Scholar]
- 36.Whitman CB, Shreay S, Gitlin M, van Oijen MGH, Spiegel BMR: Clinical factors and the decision to transfuse chronic dialysis patients. Clin J Am Soc Nephrol 8: 1942–1951, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Centers for Medicare and Medicaid Services (CMS): ESRD Payment Spotlight: ESRD Prospective Payment System (ESRD PPS) Overview of 2013 Claims-Based Monitoring Program. Available at: http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ESRDpayment/Spotlight.html. Accessed June 15, 2015
- 38.US Renal Data System : USRDS 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2013 [Google Scholar]
- 39.Young EW, Goodkin DA, Mapes DL, Port FK, Keen ML, Chen K, Maroni BL, Wolfe RA, Held PJ: The Dialysis Outcomes and Practice Patterns Study (DOPPS): An international hemodialysis study. Kidney Int 57[Suppl 74]: S74–S81, 2000 [Google Scholar]
- 40.Pisoni RL, Gillespie BW, Dickinson DM, Chen K, Kutner MH, Wolfe RA: The Dialysis Outcomes and Practice Patterns Study (DOPPS): Design, data elements, and methodology. Am J Kidney Dis 44[Suppl 2]: 7–15, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Robinson B, Fuller D, Zinsser D, Albert J, Gillespie B, Tentori F, Turenne M, Port F, Pisoni R: The Dialysis Outcomes and Practice Patterns Study (DOPPS) Practice Monitor: Rationale and methods for an initiative to monitor the new US bundled dialysis payment system. Am J Kidney Dis 57: 822–831, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.ESRD Network 11: National 2010 and Trends Elab Report, 2011. Available at: http://www.esrdnet11.org/Elab/elab_national_2010_and_trends_report.pdf. Accessed November 8, 2011
- 43.Lea JP, Norris K, Agodoa L: The role of anemia management in improving outcomes for African-Americans with chronic kidney disease. Am J Nephrol 28: 732–743, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Derebail VK, Lacson EK Jr., Kshirsagar AV, Key NS, Hogan SL, Hakim RM, Mooney A, Jani CM, Johnson C, Hu Y, Falk RJ, Lazarus JM: Sickle trait in African-American hemodialysis patients and higher erythropoiesis-stimulating agent dose. J Am Soc Nephrol 25: 819–826, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bock HA, Hirt-Minkowski P, Brünisholz M, Keusch G, Rey S, von Albertini B Swiss EFIXNES trial investigators : Darbepoetin alpha in lower-than-equimolar doses maintains haemoglobin levels in stable haemodialysis patients converting from epoetin alpha/beta. Nephrol Dial Transplant 23: 301–308, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Choi P, Farouk M, Manamley N, Addison J: Dose conversion ratio in hemodialysis patients switched from darbepoetin alfa to PEG-epoetin beta: AFFIRM study. Adv Ther 30: 1007–1017, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harrell FE, Jr.: Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis, New York, Springer, 2001 [Google Scholar]
- 48.Kaufman JS, Reda DJ, Fye CL, Goldfarb DS, Henderson WG, Kleinman JG, Vaamonde CA Department of Veterans Affairs Cooperative Study Group on Erythropoietin in Hemodialysis Patients : Subcutaneous compared with intravenous epoetin in patients receiving hemodialysis. N Engl J Med 339: 578–583, 1998 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.