Abstract
The negative effect of donor-specific antibodies on the success of solid transplant is now clearly established. However, the lack of effective treatment to prevent the development of antibody-mediated lesions deepens the need for clinicians to focus on primary prevention of de novo humoral allosensitization. Among the factors associated with the risk of developing de novo donor–specific antibodies, therapeutic immunosuppression is the most obvious parameter in which improvement is possible. Beyond compliance and the overall depth of immunosuppression, it is likely that the nature of the drugs is also crucial. Here, we provide an overview of the molecular effect of the various immunosuppressive drugs on B cell biology. Clinical data related to the effect of these drugs on de novo humoral allosensitization are also examined, providing a platform from which clinicians can optimize immunosuppression for prevention of de novo donor–specific antibody generation at the individual level.
Keywords: renal transplantation, immunosuppression, immunology
Antibody-mediated rejection (AMR) is widely recognized as the leading cause of late transplant failure and accounts for approximately two thirds of renal allograft losses.1,2 In 2003, AMR was added to the Banff classification for interpretation of renal allograft biopsy,3 sparking intense interest in the transplant community. Clinical studies over the last 10 years have established that antibodies generated de novo post-transplantation against donor-specific antigens (DSAs) are strongly associated and may be an important cause of allograft loss.1,4,5
Experimental studies have shed light on the natural history of AMR.6,7 The sequence starts with the generation of antibodies directed against the graft. Although highly polymorphic mismatched HLA molecules represent the most documented targets for DSAs, it is clear that DSAs can also be directed against other kinds of molecular targets, including polymorphic minor histocompatibility antigens and after a breakdown of B cell tolerance,8 nonpolymorphic autoantigens.9 DSAs are massive proteins that are largely sequestrated in the circulation.10 Binding of circulating DSAs to directly accessible graft endothelial cells can trigger the activation of a classic complement pathway, a central process in the pathophysiology of acute AMR (i.e., AMR with acute graft dysfunction).11 Complement activation by antibodies depends on the recruitment and activation of the component C1q. After antigen binding on cells, IgGs establish specific noncovalent interactions between Fc segments, resulting in the formation of ordered antibody hexamers with exquisite ability to recruit and activate C1q.12 The probability that a sufficient number of IgGs binds close enough to form such hexameric complexes is strongly influenced by antibody titer. In line with this idea is the fact that the ability of DSAs to bind C1q or C3d in a solid-phase assay correlates with their MFI.11,13 Another clue that DSA titer is an important factor in their ability to activate complement is in the study by Yell et al.14 which shows that most C1q binding DSAs can be converted into non–C1q binding DSAs when diluted. However, DSAs with similar MFI do not always activate the complement,11 which indicates that, beyond the quantity, some qualitative features of antibodies also affect their ability to activate the complement. It is well known that IgG4 is much less effective than IgG1 and IgG3 in triggering the complement cascade. Strikingly, a recent study reported that DSA subclasses identify distinct phenotypes of kidney allograft AMR. In this study, IgG3 DSAs were associated with a shorter rejection time, increased microcirculation injury, and more C4d capillary deposition; in contrast, IgG4 correlated with later allograft injury.15
In contrast to acute AMR, activation of the classic complement pathway does not seem to be critical for the development of chronic AMR lesions. The first evidence supporting this concept was in the study by Colvin and coworkers,16 which transplanted immunodeficient RAG knockout mice with allogenic heart. Colvin and coworkers16 observed that a passive transfer of noncomplement binding DSAs was sufficient enough to promote allograft vasculopathy. Innate immune cells, including neutrophils, macrophages, and NK cells, can bind to Fc fragments of antibodies and release lytic enzymes (a mechanism called antibody–dependent, cell–mediated cytotoxicity), which mediate smoldering endothelial cell damages. In turn, chronic vascular inflammation promotes the progressive development of typical vascular lesions (i.e., transplant glomerulopathy, allograft vasculopathy, and lamination of the peritubular capillary basement membrane).
Current treatment protocols for AMR use permutations of a multipronged approach that include DSA removal with plasmapheresis, B or plasma cell depletion with anti-CD20 and proteasome inhibitors (PIs), respectively, and the blockade of antibody–mediated effector functions with intravenous Igs (IVIgs) or terminal complement pathway inhibitors.7 The 3-year graft survival rate with these protocols is <50%.17 Given the detrimental effect of DSAs on transplant survival and the lack of effective treatment to prevent the development of antibody-mediated lesions, the priority of clinicians in charge of recipients of solid organs should be primary prevention of DSA generation.
Incidence and Risk Factors for Sensitization
The prevalence of de novo donor–specific antigens (dnDSAs) is generally between 5% and 10% at 1 year postrenal transplantation and slowly increases thereafter to 20% at 5 years.18–20 However, these percentages should be interpreted with caution. During the last 10 years, assays for the detection of anti-HLA antibodies have evolved from complement-dependent cytotoxicity to the highly sensitive microbead–based Luminex assay. Therefore, it is possible that DSAs identified in the post-transplant period could, in fact, be preformed DSAs that were not previously detected by the complement–dependent cytotoxicity assay. Furthermore, a negative pretransplant serum does not completely rule out the possibility that DSAs might be expressed in the context of B cell memory response. Indeed, using a novel HLA B Cell Enzyme-Linked ImmunoSpot Assay, Lúcia et al.21 showed that, in 30% of patients, memory B cells could be detected in highly sensitized patients in the absence of corresponding anti–HLA antibodies in the circulation.
Risk factors for sensitization fall into two categories: either they increase graft immunogenicity or they increase the recipient immune system’s ability to respond to allogeneic epitopes. Graft immunogenicity depends on both the quantity of allogeneic epitopes expressed by the graft (i.e., the number of mismatched amino acid polymorphisms)22 and their nature.23,24 It is also possible that graft inflammation (either preexisting in extended criteria donors or secondary to ischemia-reperfusion injuries) could increase immunogenicity.25 The ability of recipients’ immune systems to respond to allogeneic epitopes is reflected in their pretransplant immunization status (i.e., responses to previous challenges), acute cellular rejection episodes, and younger age, all of which have been consistently associated with increased risk of sensitization.19,26,27 Among the factors associated with post-transplant sensitization, therapeutic immunosuppression represents the most obvious modifiable risk factor. To optimize immunosuppressive strategies, it is fundamental to take into account drug exposure evaluated by a pretransplant pharmagenomics analysis and post–transplant pharmacokinetic monitoring. Indeed, the incidence of sensitization is dramatically increased in patients who are nonadherent.2,19 Moreover, strict monitoring of DSAs should be performed when clinical situations, such as infectious episodes, BK virus viremia,21 diarrhea, or cancer,22 lead to a reduction of immunosuppression. It is also noteworthy that death with a functional allograft is currently an important cause of graft loss. All recommendations regarding antirejection therapy must, therefore, take into account the related risk of overimmunosuppression and the benefit-to-risk ratio of all therapeutic decisions.28 Finally, defining the best drug combination for primary prevention requires an understanding of B cell biology and the molecular mechanisms by which each drug interferes with dnDSA generation.
Immune Mechanisms Underlying DSA Generation
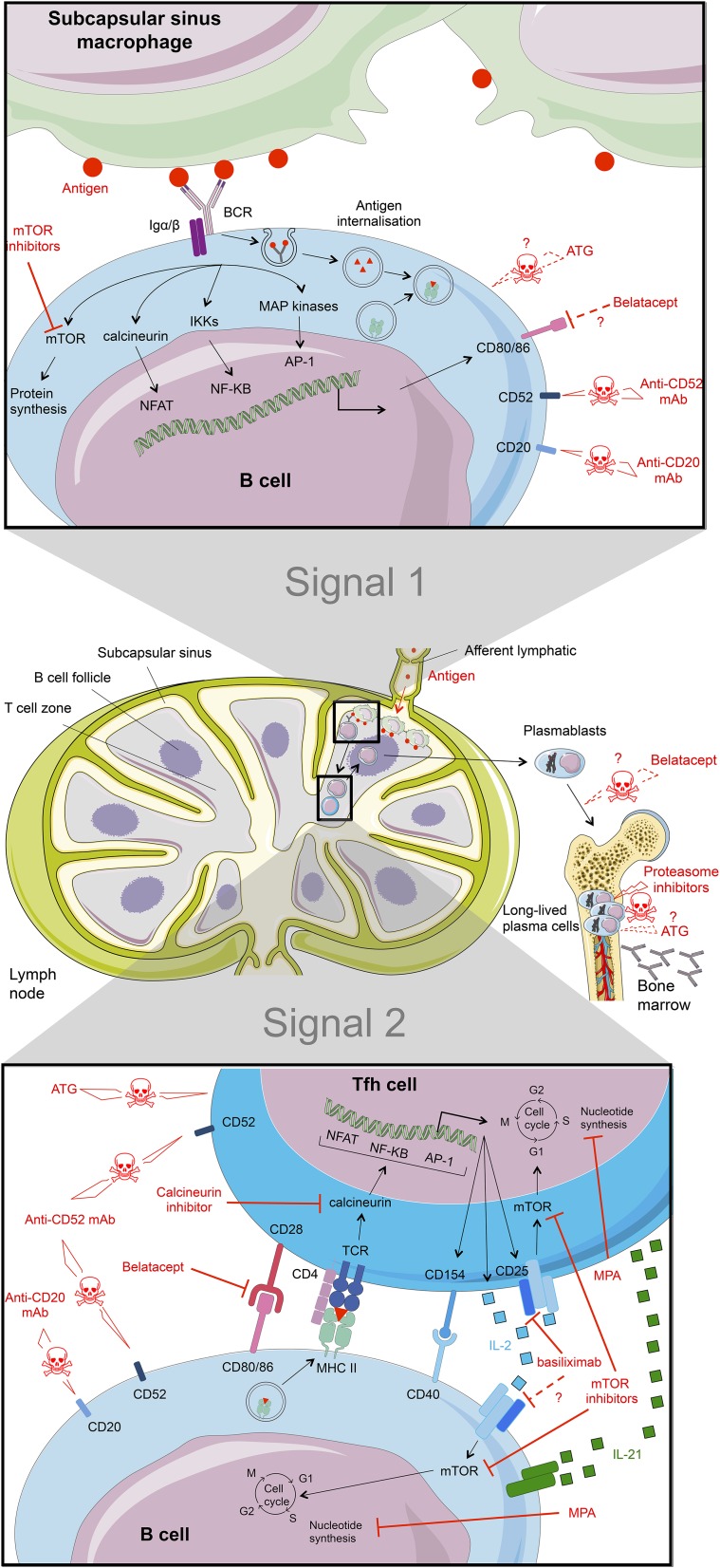
DSAs are high-affinity IgGs directed against protein antigen. These characteristics imply that DSA generation results from a thymo–dependent B cell response. This type of humoral response, which develops within secondary lymphoid organs (spleen or lymph nodes), involves two distinct events that are separated both spatially and temporally29 (Figure 1). B cell activation is initiated in the B cell area of secondary lymphoid organs by the binding of cognate antigen to surface B cell receptors (BCRs). This step is strengthened with the help of innate immune effectors. Macrophages of the subcapsular sinus capture lymph-borne antigens,30 and the binding of these antigens to the surface Ig of the cognate B cells contributes to the establishment of the immunologic synapse between the B cells and the subcapsular sinus macrophages30 (Figure 1, top panel). BCR crosslinking leads to the phosphorylation of immunoreceptor tyrosine–based activation motifs by the Src family kinase Lyn. This initiates the formation of the signalosome, an assembly of intracellular signaling molecules that, together with adaptor molecules, are responsible for the coordinated regulation of downstream signaling pathways, including calcineurin and mammalian target of rapamycin (mTOR) pathways, and ultimately, modify the gene expression. Importantly, signaling BCR microclusters also associate with molecular motor dynein, which allows for the coordinated movement of antigen into the intracytoplasmic endosomal compartment of the B cell.29,31 Internalized antigen is processed and subsequently exposed on the B cell surface in association with MHC molecules.32 B cells that have received this first activation signal migrate toward the B zone-T zone boundary,33 where they pair with a particular subset of cognate CD4+ T cells called T follicular helper (Tfh) cells34 (Figure 1, bottom panel). This provides the second activation signal for B cells through cell surface costimulatory ligand interactions (especially CD40L) and directional cytokine production (in particular, IL-21).34 The activated B cells enter the germinal center, where they proliferate. Their progenies go through repeated cycles of random somatic hypermutation of Ig variable regions driven by the enzyme activation–induced cytidine deaminase followed by selection of progressively higher–affinity mutants. Differentiation of some of these high-affinity mutants into plasma cells or memory B cells leads to the increased affinity of serum antibodies and the high affinity of the recall response.35 Interestingly, recent evidence has shown that plasma cells and memory B cells generated by the germinal center reaction require additional helper signals from innate immune effectors, including eosinophils and basophils, to extend their lifespan in postgerminal center niches (summarized in ref. 36).
Figure 1.
Key molecular steps of B cell activation and sites of action of immunosuppressive drugs. B cell activation is a two-step process. (Top panel) Binding of the cognate antigen to the surface Ig of B cells serves as the first signal of activation. BCR signaling drives the internalization of bound antigens (donor’s HLA molecules) to endosomal compartments, where they are processed. Selected peptides are then loaded onto recipient MHC class 2 molecules and presented on the B cell surface. (Bottom panel) B cells interact with cognate Tfh cells that provide the second costimulation signal for the completion of B cell activation. Therapies with proven efficiency are shown as red lines. Putative mechanisms of action are dashed red lines with question marks. TCR, T cell receptor.
Recent data suggest that B cell functions are not restricted to the deleterious role of secreting dnDSAs.37 In the inflammatory context, some B cell subsets have shown regulatory properties that rely on either secretion of anti-inflammatory cytokines (IL-10) and/or contact-dependent mechanisms. The fine equilibrium between these two roles of B cells should also be taken into account when discussing immunosuppressive strategies.38
Determining the actions of each immunosuppressive drug on B cell biology and their consequent effect on dnDSA production represents the first step in defining practical clinical immunosuppressive strategies.
Effect of Induction Therapy on dnDSA Generation
Rabbit antithymocyte globulin (ATG) is a mixture of polyclonal IgGs recovered from the sera of rabbits immunized with human thymocytes. The binding of antibodies to recipient’s T lymphocytes results in complement– and/or antibody–dependent, cell–mediated cytotoxicity and an interaction with T cell surface antigens, possibly resulting in apoptosis or anergy (Figure 1). Interestingly, because certain epitopes are shared between T and B cells and because pediatric thymi also contain CD20+ B cells and CD138+ plasma cells, ATG contain IgGs directed against these latter subsets.39 As a result, in vitro studies have shown that ATG induces apoptosis of naive, activated B cells and bone marrow resident plasma cells at clinically relevant concentrations (1–100 ng/ml).39 However, the importance of ATG–induced plasma and/or B cell depletion in vivo has not been clearly shown.40
An alternative to ATG as a depleting agent for induction is alemtuzumab, a humanized mAb directed against CD52, which is a protein widely distributed on the surface of lymphocytes and monocytes (but with an unknown function). Alemtuzumab induces rapid and profound B (and T) lymphocyte depletion41 (Figure 1). A recent study showed that, after this initial depletion, B cells repopulate the peripheral blood from 6 weeks onward and exceed baseline levels after 6 months.42 Moreover, the composition of B cell subsets is altered compared with that found pretransplant; the levels of naïve and transitional B cells increase, whereas the absolute number of memory B cells decreases significantly.42 Interestingly, in organ transplantation, the composition of the B cell compartment is repeatedly identified as an important determinant for graft outcome.43,44 Heidt et al.42 suggested that lymphocyte depletion with alemtuzumab may skew the B cell compartment toward a protolerogenic profile. This hypothesis has been further strengthened by another work that showed that a significant expansion of regulatory–type B cells (defined as CD19+CD5+CD1dhigh) is associated with superior graft function and that this pattern is more common after alemtuzumab induction.45 However, although this hypothesis is interesting, it has been challenged by recent conflicting reports from two independent groups showing that alemtuzumab–induced B cell depletion/reconstitution is associated with a higher incidence of AMR compared with induction with ATG.46,47
Basiliximab is a chimeric mouse-human nondepleting mAb targeting the α-chain (CD25) of the IL-2 receptor (Figure 1). Basiliximab was developed for induction therapy on the basis of the concept that it would block IL-2 binding, thereby inhibiting T cell proliferation. Interestingly, activated B cells also express CD25,48 and IL-2 has a crucial role in driving the differentiation of activated B cells toward plasma cells in vitro.48
In practice, ATG should be preferred in sensitized patients without DSAs, because two randomized clinical trials showed beneficial short–term effects on acute rejection, graft function, and survival (however, the relationship between induction immunosuppression, dnDSAs, and AMR was not specifically addressed).49,50 In a more recent trial, Brokhof et al.51 reported in a cohort of 114 moderately sensitized recipients of deceased donor renal transplants that induction with ATG is associated with a reduction in the occurrence of dnDSAs and AMR compared with basiliximab. However, it should be noted that, in this study, (1) the difference became statistically different only at 3 years, (2) only a few patients assigned to basiliximab had a follow-up at 36 months, and (3) the patients who received ATG also received more plasmapheresis and IVIg than the patients who received rituximab. Finally, if ATG should be considered as the first–line induction therapy in previously sensitized patients, no data are available concerning a potential benefit of this induction strategy in nonsensitized recipients.
Effect of Maintenance Immunosuppression on dnDSA Generation
Corticosteroids
Corticosteroids are the oldest immune response–modifying drugs. Their mechanism of action is diverse and includes interference with intracellular transcription factors and signaling pathways of several surface receptors, including the T cell antigen receptor52 and downstream kinases.53
High doses of corticosteroids (>1 mg/kg), used for induction therapy in transplantation, also induce lymphocyte apoptosis (including B cells)46,54 and can prevent in vitro differentiation of B cells into plasma cells.55 High concentrations of corticosteroids are also likely to interfere with Fc γ–receptor signaling, including the inhibitory Fc γ–RIIb, which acts as a key negative modulator of B cell function and controls bone marrow plasma cell apoptosis.56 The effect of a standard maintenance dose of corticosteroid therapy on human B cells was studied in a seminal in vitro study by Cupps et al.57 in the mid-1980s. Early events in B cell activation, such as anti-IgM–induced B cell activation and proliferation, are profoundly suppressed by corticosteroids.57 To be effective, the corticosteroids have to be present in the culture during the first 24 hours, suggesting a drug-induced modulation of an early event.57
Over the last decades, several attempts have been made to develop steroid-sparing strategies to avoid their important deleterious side effects. No increased risk in sensitization has been reported in patients without corticosteroids in a prospective single–center study on kidney transplantation58 and two retrospective studies on liver transplantation.59,60 These encouraging results are also supported by the conclusions in the work by Delgado et al.,61 which randomized 37 recipients of kidney transplants with ATG induction, tacrolimus, and mycophenolic acid (MPA) for corticosteroid withdrawal 1 week post-transplantation. Delgado et al.61 did not observe any difference in dnDSA incidence at 5 years. However, because early steroid withdrawal is associated with a higher incidence of subclinical rejection and chronic graft injury in recipients with high immunologic risk, such as blacks,62 a steroid-sparing strategy should only be considered in selected recipients with low immunologic risk.
Calcineurin Inhibitors
It has been known since the mid-1990s that, similar to what happens in T cells, the engagement of BCR activates NFAT through a calcineurin-dependent pathway in B lymphocytes (Figure 1). The importance of this pathway for T cell–dependent humoral responses has been studied in mice, in which calcineurin was deleted specifically in B cells.63 These mice had larger germinal centers, reduced plasma cell development, and lower antigen–specific antibody titers, indicating a block in plasma cell differentiation.63 Although interesting, these experimental results cannot be directly translated into patients, because calcineurin inhibitors (CNIs), in contrast with genetic deletion, do not completely suppress calcineurin activity in lymphocytes.64 In fact, when Heidt et al.65 tested the effect of CNIs in T and B cell cocultures, they observed that, whereas cyclosporin A (CsA) and tacrolimus were both capable of inhibiting Ig production when B cells were cultured with T cells that were not preactivated, they failed to do so when preactivated T cells were used to stimulate B cells. These results indicate that CNIs affect the humoral immune response of patients with transplants by interfering with T helper signals and not by targeting B cells directly.65 This hypothesis is supported by the study by Struijk et al.,66 which showed that patients with renal transplants who were stable on prednisolone plus CsA failed to develop antibodies against T cell–dependent model antigens but responded normally to T cell–independent antigens.
Some studies have reported that the use of CsA is associated with an increased risk of dnDSAs compared with tacrolimus, 59,67 but this difference is not always found.5 The effect of the nature of CNIs (CsA or tacrolimus) on dnDSA occurrence is made difficult to assess because of the specific pharmacokinetic features of each molecule (including variance of oral bioavailability, action on enterohepatic circulation of MPA observed with CsA, and genetic polymorphisms of tacrolimus), which are likely to modify drug exposure. Finally, beyond the nature of the CNI, compliance19 and trough levels59 have been established as the most important risk factors for sensitization of patients on CNI.
mTOR Inhibitors
Immunosuppressive drugs targeting the mTOR signaling pathway have been developed to avoid or reduce CNI-associated toxicity. Sirolimus and everolimus engage FK binding proteins, forming a complex that inhibits mTOR. This inhibition prevents the translation of mRNA-encoding proteins needed to enter the cell cycle68 (thus reducing T cell proliferation) and cytokine production40,69 (Figure 1). Interestingly, engagement of the BCR activates PI3K, which beyond activation of NF-κB–dependent transcription, also initiates a distinct signaling pathway involving the Akt and mTOR serine/threonine kinases.70
Surprisingly little is known about the functions of the mTOR complexes in B cells. Homozygous knockin mice for hypomorphic mTOR showed decreased ability to produce antibodies.69 To determine whether these effects were caused by a direct mTOR blockade in the B cells or only reflected T cell deficiency, the same group examined the humoral responses in mTOR conditional B cell knockout mice.71 Mice with mTOR deleted in their B cell lineage produced fewer splenic germinal centers and also, exhibited a decrease in switched high–affinity antibody responses in contrast to their wild-type littermates.71 The fact that mTOR inhibitors are able to prevent the in vitro production of Ig with equal efficiency when human B cells are cultured with preactivated or not preactivated T cells further shows that mTOR inhibitors, in contrast with CNIs, have a direct effect on B cells.65,72 This concept is also validated by the results of a recent study that compared the effects of CNI and mTOR inhibitors on B cells from healthy volunteers. Only sirolimus was able to inhibit the proliferation of B cells and their differentiation into plasma cells. Interestingly, the effect of sirolimus seemed more pronounced on the CD19+CD27+ memory subset.73
Several clinical studies have reported successful conversion to an mTOR inhibitor–based regimen.74–77 Although several of them showed a significant improvement in renal function after conversion to a CNI-free regimen, long-term data on the humoral response are limited. In the ZEUS Trial,74 recipients of kidney transplants on CsA-based immunosuppression were randomized at 4 months post-transplantation to be either maintained on the same regimen or converted to everolimus-based immunosuppression. In a retrospective analysis of a subset of patients, Liefeldt et al.78 reported that 23% of those receiving everolimus had developed dnDSAs compared with only 11% of those who were receiving CsA. This difference was, however, only observed when steroid withdrawal was allowed. In contrast, the 4-year report of the CONCEPT Trial79 did not find significant difference in dnDSA incidence between patients switched to sirolimus at 3 months and the CsA controls (12% versus 21%; P=0.24). In line with these last reassuring results, a case-control study evaluating the effect of late conversion from CNI to everolimus (median of 22 months post-transplantation) also fails to show increased dnDSA incidence.80 Finally, the question of whether mTOR inhibitors favor dnDSA emergence is still controversial. Available data suggest that conversion to mTOR inhibitors after complete CNI withdrawal is a potential risk factor, especially if it is made before the end of the first year and/or associated with steroid withdrawal.
Belatacept
Belatacept is a human fusion protein combining the extracellular portion of cytotoxic T lymphocyte–associated antigen 4 (CTLA4), which has been mutated to confer greater binding avidity to CD80 and CD86, and the constant region fragment of human IgG1. The CTLA4 binds surface costimulatory ligands (CD80 and CD86) of antigen-presenting cells, thus preventing their interaction with CD28 and thereby, blocking T cell activation81 (Figure 1).
The efficiency of belatacept in preventing dnDSA generation is generally attributed to its effect on Tfh cells.82 B cells are, indeed, known to lack CD28, which has expression that is specifically repressed by the B cell master regulator gene Pax-5.83 However, several experimental studies have shown the role of reverse signaling of CD80/CD86 in CTLA4-mediated coinhibition.84 CTLA4-Ig binds to CD80/CD86 molecules on dendritic cells and promotes the induction of the immunomodulatory enzyme indoleamine 2,3 dioxygenase85,86 and the tolerogenic molecule HLA-G.87 Experimental data suggest that reverse signaling of CD80/CD86 also exists in B cells.88,89 It is, therefore, tempting to speculate that, when belatacept binds to CD80/CD86, it directly influences the B cell fate. This mechanism could account for the recent observation that the frequency and absolute number of CD19+CD24hiCD38hi B cells (a subset that has previously been associated with operational tolerance43,44) are increased in patients on belatacept compared with controls on CNIs.90
Most of the clinical data where belatacept was used come from two multinational phase 3 studies: the BENEFIT Trial and the BENEFIT-EXT Trial. The BENEFIT Trial studied patients who received a kidney from a living donor or a standard criteria deceased donor. The BENEFIT-EXT Trial, however, studied patients who received a kidney from an extended criteria deceased donor. In both studies, patients received corticosteroids and basiliximab for induction therapy intraoperatively and MPA and tapering doses of the steroid postoperatively. Results from these trials indicate that belatacept may have a paradoxical effect on T versus B cell–mediated alloimmune response. The BENEFIT Trial revealed surprisingly low rates (5%–6%) of dnDSA formation at 3 years, whereas the incidence of acute T cell–mediated rejection was markedly increased.91 Schwarz et al.92 performed a retrospective patient match analysis of 14 patients on belatacept originally enrolled in the phase 2 multicenter trial. Fifty-six patients treated with CsA were matched according to age at transplantation, first transplant/retransplant, and donor type. None of the patients treated with belatacept had DSAs≥10 years post-transplantation compared with 38.5% of tested subjects treated with CsA (P=0.05).92 Although extremely promising, these results need to be confirmed through additional experimentation. It should also be remembered that part of the effect could be related to the fact that, in contrast with all other immunosuppressive drugs, belatacept is given intravenously monthly in an outpatient clinic, which could limit noncompliance, a major risk factor for dnDSA generation.2,19
Purine Inhibitors
After signal 1 (BCR engagement) and signal 2 (provided by cognate Tfh cells), B cells undergo massive proliferation, which is crucial for the expansion of the rare antigen–specific clones and therefore, the development of humoral response. Azathioprine and mycophenolate mofetil (MPA) both block cell division by inhibiting DNA synthesis through two distinct mechanisms (Figure 1). Azathioprine is a prodrug for 6-mercaptopurine; its metabolites block purine synthesis enzymes and impede later steps of DNA synthesis by being incorporated into newly synthetized DNA. In contrast, MPA only acts as a selective inhibitor of inosine-5′-monophosphate dehydrogenase, a key enzyme in the de novo synthesis of purine. Therefore, MPA does not incorporate false purine analogs into DNA.
Adding MPA to primary human B cells stimulated in vitro with IL-21, anti-CD40, and anti-IgM or CpG-B inhibits B cell proliferation and the generation of antibody–producing plasma cells.93 Similar findings have been reported in the T/B coculture model.65,72 As expected, however, the drug has no effect on terminally differentiated plasma cells,93 suggesting that it can only prevent (but not cure) AMR.
Some clinical studies suggest that MPA might perform better than azathioprine in preventing dnDSA formation. For instance, two retrospective studies in kidney transplantation5,26 have reported that azathioprine is more frequently used than MPA among patients who develop dnDSAs. However, this difference, which was not observed in liver transplantation,59 could also be explained by the dosage (or the nature) of other concomitant medications. In a study designed to determine the effect of immunosuppression on both naïve and memory humoral responses, Struijk et al.66 reported that recipients of kidneys on prednisone combined with either CsA, MPA, or everolimus all generated significantly fewer antibodies during primary humoral responses compared with healthy controls. In contrast, when patients were tested for memory responses against the tetanus toxin, only patients on prednisone plus MPA showed impaired secondary humoral responses, suggesting that MPA could be of special interest in preventing the rise of DSA titers in presensitized recipients.66
In Clinical Practice
A maintenance immunosuppression strategy should be defined according to the level of the pretransplant sensitization status. A conventional strategy using CNI plus MPA and avoiding minimization in the absence of specific clinical indications should be chosen in sensitized recipients. In recipients with low immunologic risk, belatacept can now be considered as a promising alternative strategy in association with MPA and steroids. Regarding the other CNI–sparing strategies, mTOR inhibitors with MPA should not be considered as the first choice in either highly or moderately sensitized recipients. In recipients with low immunologic risk, the effect of both mTOR inhibitors (with or without low-dose CNI) as steroid-sparing strategies has to be clearly defined by additional analysis.
Maintenance immunosuppression should also be adapted to the results of clinical, pharmacologic, and immunologic monitoring. DSA monitoring should be performed at least once between 3 and 12 months after transplantation. An evaluation of DSAs should also be performed when a change of immunosuppressive regimen is considered and when nonadherence or graft rejection is suspected.94 Detection of a significant level of dnDSAs should prompt verification of drug compliance, and pharmacokinetic analyses should be performed to ensure that drug exposure is within therapeutic ranges (in particular, in patients with therapeutic minimization).
The Effect of B or Plasma Cell–Targeted Therapy on DSAs
Data on the effect of B or plasma cell–targeted therapy on the prevention de novo humoral allosensitization after kidney transplantation are scarce. These drugs have, indeed, mainly been used in the context of desensitization protocols or AMR treatment (two situations that are beyond the scope of this review, because DSAs are present before drug administration). Another problem to determine their effect is the fact that these drugs were mostly used in combination with plasma exchanges and/or IVIg.95
Rituximab is a chimeric depleting antibody directed against the B cell surface molecule CD20 that is not found on mature plasma cells (Figure 1). Thus, rituximab should not affect serum antibody titers directly. However, some studies have reported that prolonged B cell depletion with rituximab (in association with IVIg) could lead to a decrease in DSA titers in patients diagnosed with chronic AMR.96,97 A recent study also found significantly less HLA antibody rebound in patients treated with rituximab compared with a control cohort desensitized and transplanted without rituximab.98 Compared with controls, patients treated with rituximab had a significantly greater mean reduction in DSA MFI but a similar rate of DSA persistence.98 Some patients sensitized to HLA antigens do not have antibody present in serum before transplantation.21,97 Such patients are at risk for an anamnestic response resulting from a proinflammatory response to the trauma of transplant surgery. Elimination of HLA–specific memory B cells with rituximab in these patients successfully abrogated anamnestic response.99 Regarding prevention of dnDSA generation with rituximab, a Japanese group has recently reported that dnDSA incidence is significantly lower in ABO–incompatible renal transplantations induced with rituximab.100 Incidence of chronic AMR was also significantly reduced in the group of ABO-incompatible recipients who received induction therapy with rituximab or splenectomy compared with a control group of ABO-compatible recipients (without rituximab nor splenectomy). However, this beneficial effect of rituximab should be carefully interpreted, because it was not observed in a randomized, double–blind trial of rituximab induction for ABO–compatible renal transplantations.101
PIs deplete plasma cells in human transplant recipients and animal models (Figure 1). In the context of AMR, PI seems to reduce the titers of immunodominant DSAs by 50%, especially in early AMR.102 A similar but transient reduction rate has also been observed for preformed anti–HLA antibodies in a prospective trial of PI-based desensitization.103 Interestingly, Woodle et al.103 also reported a low rate of dnDSA formation (12.5%) in patients who were desensitized.
Conclusion
The prevention of dnDSA generation is critical to the success of modern renal transplant practice. The depth of immunosuppression together with the nature of the drugs used play an important role in the success of the transplant. The data provided by clinical trials regarding the effect of therapeutic immunosuppression on the risk of developing dnDSAs are still limited, relatively recent, and considerably varied. This because of the rapid evolution of methods used to detect anti-HLA antibodies as well as the heterogeneity of monitoring and follow-up across centers. Nevertheless, these data together with the basic advances in our understanding of B cell biology provide a platform from which clinicians in charge of patients with transplants can now design immunosuppressive strategies tailored to the specific immunologic risk of each recipient.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Einecke G, Sis B, Reeve J, Mengel M, Campbell PM, Hidalgo LG, Kaplan B, Halloran PF: Antibody-mediated microcirculation injury is the major cause of late kidney transplant failure. Am J Transplant 9: 2520–2531, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Sellarés J, de Freitas DG, Mengel M, Reeve J, Einecke G, Sis B, Hidalgo LG, Famulski K, Matas A, Halloran PF: Understanding the causes of kidney transplant failure: The dominant role of antibody-mediated rejection and nonadherence. Am J Transplant 12: 388–399, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Racusen LC, Colvin RB, Solez K, Mihatsch MJ, Halloran PF, Campbell PM, Cecka MJ, Cosyns J-P, Demetris AJ, Fishbein MC, Fogo A, Furness P, Gibson IW, Glotz D, Hayry P, Hunsickern L, Kashgarian M, Kerman R, Magil AJ, Montgomery R, Morozumi K, Nickeleit V, Randhawa P, Regele H, Seron D, Seshan S, Sund S, Trpkov K: Antibody-mediated rejection criteria - an addition to the Banff 97 classification of renal allograft rejection. Am J Transplant 3: 708–714, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Terasaki PI, Cai J: Human leukocyte antigen antibodies and chronic rejection: From association to causation. Transplantation 86: 377–383, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Terasaki PI, Ozawa M: Predicting kidney graft failure by HLA antibodies: A prospective trial. Am J Transplant 4: 438–443, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Smith RN, Kawai T, Boskovic S, Nadazdin O, Sachs DH, Cosimi AB, Colvin RB: Four stages and lack of stable accommodation in chronic alloantibody-mediated renal allograft rejection in Cynomolgus monkeys. Am J Transplant 8: 1662–1672, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pouliquen E, Koenig A, Chen CC, Sicard A, Rabeyrin M, Morelon E, Dubois V, Thaunat O: Recent advances in renal transplantation: Antibody-mediated rejection takes center stage. F1000Prime Rep 7: 51, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thaunat O, Graff-Dubois S, Fabien N, Duthey A, Attuil-Audenis V, Nicoletti A, Patey N, Morelon E: A stepwise breakdown of B-cell tolerance occurs within renal allografts during chronic rejection. Kidney Int 81: 207–219, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Li L, Wadia P, Chen R, Kambham N, Naesens M, Sigdel TK, Miklos DB, Sarwal MM, Butte AJ: Identifying compartment-specific non-HLA targets after renal transplantation by integrating transcriptome and “antibodyome” measures. Proc Natl Acad Sci U S A 106: 4148–4153, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olszewski WL, Engeset A, Lukasiewicz H: Immunoglobulins, complement and lysozyme in leg lymph of normal men. Scand J Clin Lab Invest 37: 669–674, 1977 [DOI] [PubMed] [Google Scholar]
- 11.Sicard A, Ducreux S, Rabeyrin M, Couzi L, McGregor B, Badet L, Scoazec JY, Bachelet T, Lepreux S, Visentin J, Merville P, Fremeaux-Bacchi V, Morelon E, Taupin J-L, Dubois V, Thaunat O: Detection of C3d-binding donor-specific anti-HLA antibodies at diagnosis of humoral rejection predicts renal graft loss. J Am Soc Nephrol 26: 457–467, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diebolder CA, Beurskens FJ, de Jong RN, Koning RI, Strumane K, Lindorfer MA, Voorhorst M, Ugurlar D, Rosati S, Heck AJR, van de Winkel JGJ, Wilson IA, Koster AJ, Taylor RP, Saphire EO, Burton DR, Schuurman J, Gros P, Parren PWHI: Complement is activated by IgG hexamers assembled at the cell surface. Science 343: 1260–1263, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loupy A, Lefaucheur C, Vernerey D, Prugger C, Duong van Huyen J-P, Mooney N, Suberbielle C, Frémeaux-Bacchi V, Méjean A, Desgrandchamps F, Anglicheau D, Nochy D, Charron D, Empana J-P, Delahousse M, Legendre C, Glotz D, Hill GS, Zeevi A, Jouven X: Complement-binding anti-HLA antibodies and kidney-allograft survival. N Engl J Med 369: 1215–1226, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Yell M, Muth BL, Kaufman DB, Djamali A, Ellis TM: C1q binding activity of de novo donor-specific HLA antibodies in renal transplant recipients with and without antibody-mediated rejection. Transplantation 99: 1151–1155, 2015 [DOI] [PubMed] [Google Scholar]
- 15.Lefaucheur C, Viglietti D, Bentlejewski C, Duong van Huyen JP, Vernerey D, Aubert O, Verine J, Jouven X, Legendre C, Glotz D, Loupy A, Zeevi A: IgG donor-specific anti-human HLA antibody subclasses and kidney allograft antibody-mediated injury. J Am Soc Nephrol 27: 293–304, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirohashi T, Uehara S, Chase CM, DellaPelle P, Madsen JC, Russell PS, Colvin RB: Complement independent antibody-mediated endarteritis and transplant arteriopathy in mice. Am J Transplant 10: 510–517, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta G, Abu Jawdeh BG, Racusen LC, Bhasin B, Arend LJ, Trollinger B, Kraus E, Rabb H, Zachary AA, Montgomery RA, Alachkar N: Late antibody-mediated rejection in renal allografts: Outcome after conventional and novel therapies. Transplantation 97: 1240–1246, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Everly MJ, Rebellato LM, Haisch CE, Ozawa M, Parker K, Briley KP, Catrou PG, Bolin P, Kendrick WT, Kendrick SA, Harland RC, Terasaki PI: Incidence and impact of de novo donor-specific alloantibody in primary renal allografts. Transplantation 95: 410–417, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Wiebe C, Gibson IW, Blydt-Hansen TD, Karpinski M, Ho J, Storsley LJ, Goldberg A, Birk PE, Rush DN, Nickerson PW: Evolution and clinical pathologic correlations of de novo donor-specific HLA antibody post kidney transplant. Am J Transplant 12: 1157–1167, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Rebellato LM, Everly MJ, Haisch CE, Ozawa M, Briley KP, Parker K, Catrou PG, Bolin P, Kendrick WT, Kendrick SA, Harland RC: A report of the epidemiology of de novo donor-specific anti-HLA antibodies (DSA) in “low-risk” renal transplant recipients. Clin Transplant 2011: 337–340, 2011 [PubMed] [Google Scholar]
- 21.Lúcia M, Luque S, Crespo E, Melilli E, Cruzado JM, Martorell J, Jarque M, Gil-Vernet S, Manonelles A, Grinyó JM, Bestard O: Preformed circulating HLA-specific memory B cells predict high risk of humoral rejection in kidney transplantation. Kidney Int 88: 874–887, 2015 [DOI] [PubMed] [Google Scholar]
- 22.Duquesnoy RJ: HLA epitope based matching for transplantation. Transpl Immunol 31: 1–6, 2014 [DOI] [PubMed] [Google Scholar]
- 23.Kosmoliaptsis V, Chaudhry AN, Sharples LD, Halsall DJ, Dafforn TR, Bradley JA, Taylor CJ: Predicting HLA class I alloantigen immunogenicity from the number and physiochemical properties of amino acid polymorphisms. Transplantation 88: 791–798, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Kosmoliaptsis V, Sharples LD, Chaudhry AN, Halsall DJ, Bradley JA, Taylor CJ: Predicting HLA class II alloantigen immunogenicity from the number and physiochemical properties of amino acid polymorphisms. Transplantation 91: 183–190, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Fuquay R, Renner B, Kulik L, McCullough JW, Amura C, Strassheim D, Pelanda R, Torres R, Thurman JM: Renal ischemia-reperfusion injury amplifies the humoral immune response. J Am Soc Nephrol 24: 1063–1072, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hourmant M, Cesbron-Gautier A, Terasaki PI, Mizutani K, Moreau A, Meurette A, Dantal J, Giral M, Blancho G, Cantarovich D, Karam G, Follea G, Soulillou JP, Bignon JD: Frequency and clinical implications of development of donor-specific and non-donor-specific HLA antibodies after kidney transplantation. J Am Soc Nephrol 16: 2804–2812, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Chemouny J-M, Suberbielle C, Rabant M, Zuber J, Alyanakian M-A, Lebreton X, Carmagnat M, Pinheiro N, Loupy A, Van Huyen J-P, Timsit M-O, Charron D, Legendre C, Anglicheau D: De novo donor-specific human leukocyte antigen antibodies in nonsensitized kidney transplant recipients after T cell-mediated rejection. Transplantation 99: 965–972, 2015 [DOI] [PubMed] [Google Scholar]
- 28.Thaunat O: Finding the safe place between the hammer and the anvil: Sounding the depth of therapeutic immunosuppression. Kidney Int 88: 1226–1228, 2015 [DOI] [PubMed] [Google Scholar]
- 29.Thaunat O, Granja AG, Barral P, Filby A, Montaner B, Collinson L, Martinez-Martin N, Harwood NE, Bruckbauer A, Batista FD: Asymmetric segregation of polarized antigen on B cell division shapes presentation capacity. Science 335: 475–479, 2012 [DOI] [PubMed] [Google Scholar]
- 30.Harwood NE, Batista FD: Early events in B cell activation. Annu Rev Immunol 28: 185–210, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Schnyder T, Castello A, Feest C, Harwood NE, Oellerich T, Urlaub H, Engelke M, Wienands J, Bruckbauer A, Batista FD: B cell receptor-mediated antigen gathering requires ubiquitin ligase Cbl and adaptors Grb2 and Dok-3 to recruit dynein to the signaling microcluster. Immunity 34: 905–918, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Lanzavecchia A: Antigen-specific interaction between T and B cells. Nature 314: 537–539, 1985 [DOI] [PubMed] [Google Scholar]
- 33.Okada T, Miller MJ, Parker I, Krummel MF, Neighbors M, Hartley SB, O’Garra A, Cahalan MD, Cyster JG: Antigen-engaged B cells undergo chemotaxis toward the T zone and form motile conjugates with helper T cells. PLoS Biol 3: e150, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crotty S: A brief history of T cell help to B cells. Nat Rev Immunol 15: 185–189, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Victora GD: SnapShot: The germinal center reaction. Cell 159: 700–700.e1, 2014 [DOI] [PubMed] [Google Scholar]
- 36.Cerutti A, Puga I, Cols M: New helping friends for B cells. Eur J Immunol 42: 1956–1968, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stolp J, Turka LA, Wood KJ: B cells with immune-regulating function in transplantation. Nat Rev Nephrol 10: 389–397, 2014 [DOI] [PubMed] [Google Scholar]
- 38.Thaunat O, Morelon E, Defrance T: Am“B”valent: Anti-CD20 antibodies unravel the dual role of B cells in immunopathogenesis. Blood 116: 515–521, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Zand MS, Vo T, Huggins J, Felgar R, Liesveld J, Pellegrin T, Bozorgzadeh A, Sanz I, Briggs BJ: Polyclonal rabbit antithymocyte globulin triggers B-cell and plasma cell apoptosis by multiple pathways. Transplantation 79: 1507–1515, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Morelon E, Lefrançois N, Besson C, Prévautel J, Brunet M, Touraine J-L, Badet L, Touraine-Moulin F, Thaunat O, Malcus C: Preferential increase in memory and regulatory subsets during T-lymphocyte immune reconstitution after Thymoglobulin induction therapy with maintenance sirolimus vs cyclosporine. Transpl Immunol 23: 53–58, 2010 [DOI] [PubMed] [Google Scholar]
- 41.Lee F, Luevano M, Veys P, Yong K, Madrigal A, Shaw BE, Saudemont A: The effects of CAMPATH-1H on cell viability do not correlate to the CD52 density on the cell surface. PLoS One 9: e103254, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heidt S, Hester J, Shankar S, Friend PJ, Wood KJ: B cell repopulation after alemtuzumab induction-transient increase in transitional B cells and long-term dominance of naïve B cells. Am J Transplant 12: 1784–1792, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sagoo P, Perucha E, Sawitzki B, Tomiuk S, Stephens DA, Miqueu P, Chapman S, Craciun L, Sergeant R, Brouard S, Rovis F, Jimenez E, Ballow A, Giral M, Rebollo-Mesa I, Le Moine A, Braudeau C, Hilton R, Gerstmayer B, Bourcier K, Sharif A, Krajewska M, Lord GM, Roberts I, Goldman M, Wood KJ, Newell K, Seyfert-Margolis V, Warrens AN, Janssen U, Volk H-D, Soulillou J-P, Hernandez-Fuentes MP, Lechler RI: Development of a cross-platform biomarker signature to detect renal transplant tolerance in humans. J Clin Invest 120: 1848–1861, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pallier A, Hillion S, Danger R, Giral M, Racapé M, Degauque N, Dugast E, Ashton-Chess J, Pettré S, Lozano JJ, Bataille R, Devys A, Cesbron-Gautier A, Braudeau C, Larrose C, Soulillou J-P, Brouard S: Patients with drug-free long-term graft function display increased numbers of peripheral B cells with a memory and inhibitory phenotype. Kidney Int 78: 503–513, 2010 [DOI] [PubMed] [Google Scholar]
- 45.Cherukuri A, Salama AD, Carter C, Smalle N, McCurtin R, Hewitt EW, Hernandez-Fuentes M, Clark B, Baker RJ: An analysis of lymphocyte phenotype after steroid avoidance with either alemtuzumab or basiliximab induction in renal transplantation. Am J Transplant 12: 919–931, 2012 [DOI] [PubMed] [Google Scholar]
- 46.Todeschini M, Cortinovis M, Perico N, Poli F, Innocente A, Cavinato RA, Gotti E, Ruggenenti P, Gaspari F, Noris M, Remuzzi G, Casiraghi F: In kidney transplant patients, alemtuzumab but not basiliximab/low-dose rabbit anti-thymocyte globulin induces B cell depletion and regeneration, which associates with a high incidence of de novo donor-specific anti-HLA antibody development. J Immunol 191: 2818–2828, 2013 [DOI] [PubMed] [Google Scholar]
- 47.Noureldeen T, Albekioni Z, Machado L, Muddana N, Marcus RJ, Hussain SM, Sureshkumar KK: Alemtuzumab induction and antibody-mediated rejection in kidney transplantation. Transplant Proc 46: 3405–3407, 2014 [DOI] [PubMed] [Google Scholar]
- 48.Le Gallou S, Caron G, Delaloy C, Rossille D, Tarte K, Fest T: IL-2 requirement for human plasma cell generation: Coupling differentiation and proliferation by enhancing MAPK-ERK signaling. J Immunol 189: 161–173, 2012 [DOI] [PubMed] [Google Scholar]
- 49.Thibaudin D, Alamartine E, de Filippis JP, Diab N, Laurent B, Berthoux F: Advantage of antithymocyte globulin induction in sensitized kidney recipients: A randomized prospective study comparing induction with and without antithymocyte globulin. Nephrol Dial Transplant 13: 711–715, 1998 [DOI] [PubMed] [Google Scholar]
- 50.Noël C, Abramowicz D, Durand D, Mourad G, Lang P, Kessler M, Charpentier B, Touchard G, Berthoux F, Merville P, Ouali N, Squifflet J-P, Bayle F, Wissing KM, Hazzan M: Daclizumab versus antithymocyte globulin in high-immunological-risk renal transplant recipients. J Am Soc Nephrol 20: 1385–1392, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brokhof MM, Sollinger HW, Hager DR, Muth BL, Pirsch JD, Fernandez LA, Bellingham JM, Mezrich JD, Foley DP, D’Alessandro AM, Odorico JS, Mohamed MA, Vidyasagar V, Ellis TM, Kaufman DB, Djamali A: Antithymocyte globulin is associated with a lower incidence of de novo donor-specific antibodies in moderately sensitized renal transplant recipients. Transplantation 97: 612–617, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van Laethem F, Baus E, Smyth LA, Andris F, Bex F, Urbain J, Kioussis D, Leo O: Glucocorticoids attenuate T cell receptor signaling. J Exp Med 193: 803–814, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Löwenberg M, Tuynman J, Bilderbeek J, Gaber T, Buttgereit F, van Deventer S, Peppelenbosch M, Hommes D: Rapid immunosuppressive effects of glucocorticoids mediated through Lck and Fyn. Blood 106: 1703–1710, 2005 [DOI] [PubMed] [Google Scholar]
- 54.Lill-Elghanian D, Schwartz K, King L, Fraker P: Glucocorticoid-induced apoptosis in early B cells from human bone marrow. Exp Biol Med (Maywood) 227: 763–770, 2002 [DOI] [PubMed] [Google Scholar]
- 55.Haneda M, Owaki M, Kuzuya T, Iwasaki K, Miwa Y, Kobayashi T: Comparative analysis of drug action on B-cell proliferation and differentiation for mycophenolic acid, everolimus, and prednisolone. Transplantation 97: 405–412, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xiang Z, Cutler AJ, Brownlie RJ, Fairfax K, Lawlor KE, Severinson E, Walker EU, Manz RA, Tarlinton DM, Smith KGC: FcgammaRIIb controls bone marrow plasma cell persistence and apoptosis. Nat Immunol 8: 419–429, 2007 [DOI] [PubMed] [Google Scholar]
- 57.Cupps TR, Gerrard TL, Falkoff RJ, Whalen G, Fauci AS: Effects of in vitro corticosteroids on B cell activation, proliferation, and differentiation. J Clin Invest 75: 754–761, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lachmann N, Terasaki PI, Schönemann C: Donor-specific HLA antibodies in chronic renal allograft rejection: A prospective trial with a four-year follow-up. Clin Transpl 2006: 171–199, 2006 [PubMed] [Google Scholar]
- 59.Kaneku H, O’Leary JG, Banuelos N, Jennings LW, Susskind BM, Klintmalm GB, Terasaki PI: De novo donor-specific HLA antibodies decrease patient and graft survival in liver transplant recipients. Am J Transplant 13: 1541–1548, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Del Bello A, Congy-Jolivet N, Muscari F, Lavayssière L, Esposito L, Cardeau-Desangles I, Guitard J, Dörr G, Suc B, Duffas JP, Alric L, Bureau C, Danjoux M, Guilbeau-Frugier C, Blancher A, Rostaing L, Kamar N: Prevalence, incidence and risk factors for donor-specific anti-HLA antibodies in maintenance liver transplant patients. Am J Transplant 14: 867–875, 2014 [DOI] [PubMed] [Google Scholar]
- 61.Delgado JC, Fuller A, Ozawa M, Smith L, Terasaki PI, Shihab FS, Eckels DD: No occurrence of de novo HLA antibodies in patients with early corticosteroid withdrawal in a 5-year prospective randomized study. Transplantation 87: 546–548, 2009 [DOI] [PubMed] [Google Scholar]
- 62.Anil Kumar MS, Irfan Saeed M, Ranganna K, Malat G, Sustento-Reodica N, Kumar AMS, Meyers WC: Comparison of four different immunosuppression protocols without long-term steroid therapy in kidney recipients monitored by surveillance biopsy: Five-year outcomes. Transpl Immunol 20: 32–42, 2008 [DOI] [PubMed] [Google Scholar]
- 63.Winslow MM, Gallo EM, Neilson JR, Crabtree GR: The calcineurin phosphatase complex modulates immunogenic B cell responses. Immunity 24: 141–152, 2006 [DOI] [PubMed] [Google Scholar]
- 64.Jørgensen KA, Koefoed-Nielsen PB, Karamperis N: Calcineurin phosphatase activity and immunosuppression. A review on the role of calcineurin phosphatase activity and the immunosuppressive effect of cyclosporin A and tacrolimus. Scand J Immunol 57: 93–98, 2003 [DOI] [PubMed] [Google Scholar]
- 65.Heidt S, Roelen DL, Eijsink C, Eikmans M, van Kooten C, Claas FHJ, Mulder A: Calcineurin inhibitors affect B cell antibody responses indirectly by interfering with T cell help. Clin Exp Immunol 159: 199–207, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Struijk GH, Minnee RC, Koch SD, Zwinderman AH, van Donselaar-van der Pant KAMI, Idu MM, ten Berge IJM, Bemelman FJ: Maintenance immunosuppressive therapy with everolimus preserves humoral immune responses. Kidney Int 78: 934–940, 2010 [DOI] [PubMed] [Google Scholar]
- 67.Huang Y, Ramon D, Luan FL, Sung R, Samaniego M: Incidences of preformed and de novo donor-specific HLA antibodies and their clinicohistological correlates in the early course of kidney transplantation. Clin Transpl 2012: 247–256, 2012 [PubMed] [Google Scholar]
- 68.Kahan BD, Chang JY, Sehgal SN: Preclinical evaluation of a new potent immunosuppressive agent, rapamycin. Transplantation 52: 185–191, 1991 [DOI] [PubMed] [Google Scholar]
- 69.Zhang S, Readinger JA, DuBois W, Janka-Junttila M, Robinson R, Pruitt M, Bliskovsky V, Wu JZ, Sakakibara K, Patel J, Parent CA, Tessarollo L, Schwartzberg PL, Mock BA: Constitutive reductions in mTOR alter cell size, immune cell development, and antibody production. Blood 117: 1228–1238, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Limon JJ, Fruman DA: Akt and mTOR in B cell activation and differentiation. Front Immunol 3: 228, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang S, Pruitt M, Tran D, Du Bois W, Zhang K, Patel R, Hoover S, Simpson RM, Simmons J, Gary J, Snapper CM, Casellas R, Mock BA: B cell-specific deficiencies in mTOR limit humoral immune responses. J Immunol 191: 1692–1703, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Matz M, Lehnert M, Lorkowski C, Fabritius K, Unterwalder N, Doueiri S, Weber UA, Mashreghi M-F, Neumayer H-H, Budde K: Effects of sotrastaurin, mycophenolic acid and everolimus on human B-lymphocyte function and activation. Transpl Int 25: 1106–1116, 2012 [DOI] [PubMed] [Google Scholar]
- 73.Traitanon O, Mathew JM, La Monica G, Xu L, Mas V, Gallon L: Differential effects of tacrolimus versus sirolimus on the proliferation, activation and differentiation of human B cells. PLoS One 10: e0129658, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Budde K, Becker T, Arns W, Sommerer C, Reinke P, Eisenberger U, Kramer S, Fischer W, Gschaidmeier H, Pietruck F ZEUS Study Investigators : Everolimus-based, calcineurin-inhibitor-free regimen in recipients of de-novo kidney transplants: An open-label, randomised, controlled trial. Lancet 377: 837–847, 2011 [DOI] [PubMed] [Google Scholar]
- 75.Lebranchu Y, Thierry A, Toupance O, Westeel PF, Etienne I, Thervet E, Moulin B, Frouget T, Le Meur Y, Glotz D, Heng A-E, Onno C, Buchler M, Girardot-Seguin S, Hurault de Ligny B: Efficacy on renal function of early conversion from cyclosporine to sirolimus 3 months after renal transplantation: Concept study. Am J Transplant 9: 1115–1123, 2009 [DOI] [PubMed] [Google Scholar]
- 76.Weir MR, Mulgaonkar S, Chan L, Shidban H, Waid TH, Preston D, Kalil RN, Pearson TC: Mycophenolate mofetil-based immunosuppression with sirolimus in renal transplantation: A randomized, controlled Spare-the-Nephron trial. Kidney Int 79: 897–907, 2011 [DOI] [PubMed] [Google Scholar]
- 77.Guba M, Pratschke J, Hugo C, Krämer BK, Nohr-Westphal C, Brockmann J, Andrassy J, Reinke P, Pressmar K, Hakenberg O, Fischereder M, Pascher A, Illner W-D, Banas B, Jauch K-W SMART-Study Group : Renal function, efficacy, and safety of sirolimus and mycophenolate mofetil after short-term calcineurin inhibitor-based quadruple therapy in de novo renal transplant patients: One-year analysis of a randomized multicenter trial. Transplantation 90: 175–183, 2010 [DOI] [PubMed] [Google Scholar]
- 78.Liefeldt L, Brakemeier S, Glander P, Waiser J, Lachmann N, Schönemann C, Zukunft B, Illigens P, Schmidt D, Wu K, Rudolph B, Neumayer H-H, Budde K: Donor-specific HLA antibodies in a cohort comparing everolimus with cyclosporine after kidney transplantation. Am J Transplant 12: 1192–1198, 2012 [DOI] [PubMed] [Google Scholar]
- 79.Lebranchu Y, Thierry A, Thervet E, Büchler M, Etienne I, Westeel PF, Hurault de Ligny B, Moulin B, Rérolle JP, Frouget T, Girardot-Seguin S, Toupance O: Efficacy and safety of early cyclosporine conversion to sirolimus with continued MMF-four-year results of the Postconcept study. Am J Transplant 11: 1665–1675, 2011 [DOI] [PubMed] [Google Scholar]
- 80.Kamar N, Del Bello A, Congy-Jolivet N, Guilbeau-Frugier C, Cardeau-Desangles I, Fort M, Esposito L, Guitard J, Gamé X, Rostaing L: Incidence of donor-specific antibodies in kidney transplant patients following conversion to an everolimus-based calcineurin inhibitor-free regimen. Clin Transplant 27: 455–462, 2013 [DOI] [PubMed] [Google Scholar]
- 81.Vincenti F, Larsen C, Durrbach A, Wekerle T, Nashan B, Blancho G, Lang P, Grinyo J, Halloran PF, Solez K, Hagerty D, Levy E, Zhou W, Natarajan K, Charpentier B Belatacept Study Group : Costimulation blockade with belatacept in renal transplantation. N Engl J Med 353: 770–781, 2005 [DOI] [PubMed] [Google Scholar]
- 82.Kim EJ, Kwun J, Gibby AC, Hong JJ, Farris AB 3rd, Iwakoshi NN, Villinger F, Kirk AD, Knechtle SJ: Costimulation blockade alters germinal center responses and prevents antibody-mediated rejection. Am J Transplant 14: 59–69, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Delogu A, Schebesta A, Sun Q, Aschenbrenner K, Perlot T, Busslinger M: Gene repression by Pax5 in B cells is essential for blood cell homeostasis and is reversed in plasma cells. Immunity 24: 269–281, 2006 [DOI] [PubMed] [Google Scholar]
- 84.Chen L: Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol 4: 336–347, 2004 [DOI] [PubMed] [Google Scholar]
- 85.Grohmann U, Orabona C, Fallarino F, Vacca C, Calcinaro F, Falorni A, Candeloro P, Belladonna ML, Bianchi R, Fioretti MC, Puccetti P: CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol 3: 1097–1101, 2002 [DOI] [PubMed] [Google Scholar]
- 86.Munn DH, Sharma MD, Mellor AL: Ligation of B7-1/B7-2 by human CD4+ T cells triggers indoleamine 2,3-dioxygenase activity in dendritic cells. J Immunol 172: 4100–4110, 2004 [DOI] [PubMed] [Google Scholar]
- 87.Bahri R, Naji A, Menier C, Charpentier B, Carosella ED, Rouas-Freiss N, Durrbach A: Dendritic cells secrete the immunosuppressive HLA-G molecule upon CTLA4-Ig treatment: Implication in human renal transplant acceptance. J Immunol 183: 7054–7062, 2009 [DOI] [PubMed] [Google Scholar]
- 88.Rau FC, Dieter J, Luo Z, Priest SO, Baumgarth N: B7-1/2 (CD80/CD86) direct signaling to B cells enhances IgG secretion. J Immunol 183: 7661–7671, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kin NW, Sanders VM: CD86 regulates IgG1 production via a CD19-dependent mechanism. J Immunol 179: 1516–1523, 2007 [DOI] [PubMed] [Google Scholar]
- 90.Leibler C, Matignon M, Pilon C, Montespan F, Bigot J, Lang P, Carosella ED, Cohen J, Rouas-Freiss N, Grimbert P, Menier C: Kidney transplant recipients treated with belatacept exhibit increased naïve and transitional B cells. Am J Transplant 14: 1173–1182, 2014 [DOI] [PubMed] [Google Scholar]
- 91.Vincenti F, Tedesco Silva H, Busque S, O’Connell P, Friedewald J, Cibrik D, Budde K, Yoshida A, Cohney S, Weimar W, Kim YS, Lawendy N, Lan S-P, Kudlacz E, Krishnaswami S, Chan G: Randomized phase 2b trial of tofacitinib (CP-690,550) in de novo kidney transplant patients: Efficacy, renal function and safety at 1 year. Am J Transplant 12: 2446–2456, 2012 [DOI] [PubMed] [Google Scholar]
- 92.Schwarz C, Mayerhoffer S, Berlakovich GA, Steininger R, Soliman T, Watschinger B, Böhmig GA, Eskandary F, König F, Mühlbacher F, Wekerle T: Long-term outcome of belatacept therapy in de novo kidney transplant recipients - a case-match analysis. Transpl Int 28: 820–827, 2015 [DOI] [PubMed] [Google Scholar]
- 93.Karnell JL, Karnell FG 3rd, Stephens GL, Rajan B, Morehouse C, Li Y, Swerdlow B, Wilson M, Goldbach-Mansky R, Groves C, Coyle AJ, Herbst R, Ettinger R: Mycophenolic acid differentially impacts B cell function depending on the stage of differentiation. J Immunol 187: 3603–3612, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tait BD, Süsal C, Gebel HM, Nickerson PW, Zachary AA, Claas FHJ, Reed EF, Bray RA, Campbell P, Chapman JR, Coates PT, Colvin RB, Cozzi E, Doxiadis IIN, Fuggle SV, Gill J, Glotz D, Lachmann N, Mohanakumar T, Suciu-Foca N, Sumitran-Holgersson S, Tanabe K, Taylor CJ, Tyan DB, Webster A, Zeevi A, Opelz G: Consensus guidelines on the testing and clinical management issues associated with HLA and non-HLA antibodies in transplantation. Transplantation 95: 19–47, 2013 [DOI] [PubMed] [Google Scholar]
- 95.Vo AA, Lukovsky M, Toyoda M, Wang J, Reinsmoen NL, Lai C-H, Peng A, Villicana R, Jordan SC: Rituximab and intravenous immune globulin for desensitization during renal transplantation. N Engl J Med 359: 242–251, 2008 [DOI] [PubMed] [Google Scholar]
- 96.Clatworthy MR: B-cell regulation and its application to transplantation. Transpl Int 27: 117–128, 2014 [DOI] [PubMed] [Google Scholar]
- 97.Fehr T, Rüsi B, Fischer A, Hopfer H, Wüthrich RP, Gaspert A: Rituximab and intravenous immunoglobulin treatment of chronic antibody-mediated kidney allograft rejection. Transplantation 87: 1837–1841, 2009 [DOI] [PubMed] [Google Scholar]
- 98.Jackson AM, Kraus ES, Orandi BJ, Segev DL, Montgomery RA, Zachary AA: A closer look at rituximab induction on HLA antibody rebound following HLA-incompatible kidney transplantation. Kidney Int 87: 409–416, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zachary AA, Lucas DP, Montgomery RA, Leffell MS: Rituximab prevents an anamnestic response in patients with cryptic sensitization to HLA. Transplantation 95: 701–704, 2013 [DOI] [PubMed] [Google Scholar]
- 100.Kohei N, Hirai T, Omoto K, Ishida H, Tanabe K: Chronic antibody-mediated rejection is reduced by targeting B-cell immunity during an introductory period. Am J Transplant 12: 469–476, 2012 [DOI] [PubMed] [Google Scholar]
- 101.Tydén G, Ekberg H, Tufveson G, Mjörnstedt L: A randomized, double-blind, placebo-controlled study of single dose rituximab as induction in renal transplantation: A 3-year follow-up. Transplantation 94: e21–e22, 2012 [DOI] [PubMed] [Google Scholar]
- 102.Walsh RC, Alloway RR, Girnita AL, Woodle ES: Proteasome inhibitor-based therapy for antibody-mediated rejection. Kidney Int 81: 1067–1074, 2012 [DOI] [PubMed] [Google Scholar]
- 103.Woodle ES, Shields AR, Ejaz NS, Sadaka B, Girnita A, Walsh RC, Alloway RR, Brailey P, Cardi MA, Abu Jawdeh BG, Roy-Chaudhury P, Govil A, Mogilishetty G: Prospective iterative trial of proteasome inhibitor-based desensitization. Am J Transplant 15: 101–118, 2015 [DOI] [PubMed] [Google Scholar]