Abstract
To reduce lithium–induced nephrogenic diabetes insipidus (lithium-NDI), patients with bipolar disorder are treated with thiazide and amiloride, which are thought to induce antidiuresis by a compensatory increase in prourine uptake in proximal tubules. However, thiazides induced antidiuresis and alkalinized the urine in lithium-NDI mice lacking the sodium-chloride cotransporter, suggesting that inhibition of carbonic anhydrases (CAs) confers the beneficial thiazide effect. Therefore, we tested the effect of the CA–specific blocker acetazolamide in lithium-NDI. In collecting duct (mpkCCD) cells, acetazolamide reduced the cellular lithium content and attenuated lithium-induced downregulation of aquaporin-2 through a mechanism different from that of amiloride. Treatment of lithium-NDI mice with acetazolamide or thiazide/amiloride induced similar antidiuresis and increased urine osmolality and aquaporin-2 abundance. Thiazide/amiloride-treated mice showed hyponatremia, hyperkalemia, hypercalcemia, metabolic acidosis, and increased serum lithium concentrations, adverse effects previously observed in patients but not in acetazolamide-treated mice in this study. Furthermore, acetazolamide treatment reduced inulin clearance and cortical expression of sodium/hydrogen exchanger 3 and attenuated the increased expression of urinary PGE2 observed in lithium-NDI mice. These results show that the antidiuresis with acetazolamide was partially caused by a tubular-glomerular feedback response and reduced GFR. The tubular-glomerular feedback response and/or direct effect on collecting duct principal or intercalated cells may underlie the reduced urinary PGE2 levels with acetazolamide, thereby contributing to the attenuation of lithium-NDI. In conclusion, CA activity contributes to lithium-NDI development, and acetazolamide attenuates lithium-NDI development in mice similar to thiazide/amiloride but with fewer adverse effects.
Keywords: cell and transport physiology, diabetes insipidus, diuretics, osmolality, pathophysiology of renal disease and progression, water transport
Lithium is the drug of choice for the treatment of bipolar disorders, and it is also regularly used to treat schizoaffective disorders and depression. Lithium is a frequently prescribed drug, because it is provided to 0.1% of the Western population. Unfortunately, in 2%–85% of patients and depending on age, lithium usage leads to nephrogenic diabetes insipidus (NDI), a disorder characterized by an impaired response of the kidney to vasopressin (arginine vasopressin [AVP]), leading to polyuria and polydipsia.1–3 Patients with lithium–induced nephrogenic diabetes insipidus (Li-NDI) are at risk for dehydration–induced lithium toxicity, and prolonged lithium treatment might lead to cyst formation and ESRD.4 However, cessation of lithium therapy is not an option for most patients with NDI, because bipolar disorder symptoms have a larger effect on the patient’s quality of life.
From studies in rats, it became clear that Li-NDI develops in two stages. In the short term (10 days), Li-NDI coincides with downregulation of aquaporin-2 (AQP2) water channels, which is caused by a reduced AQP2 transcription.5–7 Despite an increased proliferation of the AQP2–expressing principal cells of the collecting duct, long–term lithium treatment (4 weeks) also results in a severe loss of AQP2–expressing principal cells, which might be attributed to a lithium–induced G2/M–phase cell cycle arrest.8,9 This principal cell loss is compensated for by an increased number of α-intercalated cells, which are involved in acid secretion.8
To reduce polyuria in patients receiving lithium, a low-sodium diet together with thiazide and amiloride diuretics are prescribed.10 Amiloride acts on the principal cell epithelial sodium channel (ENaC), and we and others found that amiloride blocks principal cell lithium entry through ENaC, thereby attenuating polyuria in rodents and humans.11–13 Thiazides are known to block sodium and chloride reabsorption through the NaCl cotransporter (NCC) in the renal distal convoluted tubule, and the antidiuretic effect has been ascribed to a hypovolemia-induced activation of the renin-angiotensin-aldosterone system and a compensatory increased uptake of sodium and water in proximal tubules. Recently, however, we discovered that thiazide also has an NCC-independent effect, because NCC knockout mice with Li-NDI showed a clear antidiuretic response on treatment with thiazide.14
Because urine of our thiazide-treated mice was alkalinized and thiazides are derived from carbonic anhydrase (CA) inhibitors,15 our data indicated that the antidiuretic effect of thiazide in Li-NDI may involve CA inhibition. CAs catalyze the hydration of carbon dioxide to form carbonic acid, which then rapidly dissociates to form protons and bicarbonate, and they play major roles in pH balance regulation. Here, we show that CAs are, indeed, involved in lithium–induced AQP2 downregulation and that, by inducing a tubular glomerular feedback response and through direct action on collecting duct cells, the CA–selective drug acetazolamide not only attenuates Li-NDI but yields superior in vivo effects compared with the presently used treatment for Li-NDI.
Results
The Clinically Used Drug Acetazolamide Attenuates Lithium-Induced Downregulation of AQP2 in mpkCCD Cells
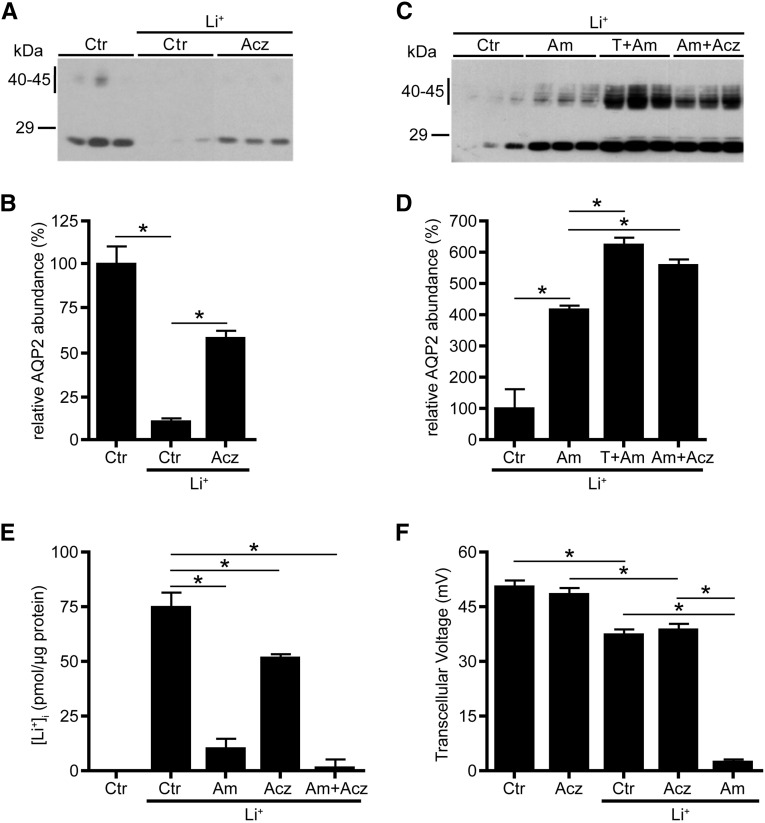
mpkCCD cells are mouse collecting duct cells showing dDAVP-dependent expression of endogenous AQP2, and we have shown that thiazide reduces lithium-induced downregulation of AQP2 in these cells, whereas they lack NCC expression.7,11,14 Because our previous animal studies suggested that thiazides reduced polyuria in our NCC knockout mice by inhibiting CAs, we wanted to test whether acetazolamide, a stable CA inhibitor that is commonly used in patients, could also rescue lithium–induced AQP2 downregulation in mpkCCD cells. Indeed, whereas lithium again reduced the AQP2 abundance in mpkCCD cells, acetazolamide significantly attenuated this downregulation (Figure 1, A and B). Because our data suggest that both thiazide and acetazolamide influence lithium–reduced AQP2 abundances through CAs, we assessed whether the action mechanism of acetazolamide differs from that of amiloride. If so, we anticipated that acetazolamide and amiloride together should attenuate the lithium–induced AQP2 downregulation better than cells treated with amiloride only. Indeed, immunoblotting revealed a significantly higher AQP2 abundance in cells treated with amiloride and acetazolamide compared with amiloride only (Figure 1, C and D). We and others discovered that ENaC is the main cellular entry site for lithium and that amiloride strongly reduced the intracellular lithium levels in mpkCCD cells.11,14 Determination of the intracellular lithium concentrations revealed that amiloride, indeed, reduced the intracellular lithium concentration by 87%, whereas this was only 30% with acetazolamide (Figure 1E). The mean intracellular lithium concentration was nominally lower with amiloride/acetazolamide; however, there was no significant difference (P=0.23). Transcellular transport of sodium and potassium through ENaC, renal outer medullary K+, and the Na/K-ATPase is electrogenic and therefore, generates a transcellular voltage (Tv) over mpkCCD cell monolayers. Lithium slightly reduced the Tv, which was not further decreased with acetazolamide (Figure 1F). In contrast, amiloride completely blocked the Tv in lithium–treated mpkCCD cells. Together, these data reveal that the CA–specific inhibitor acetazolamide attenuates lithium-induced downregulation of AQP2 in vitro and that its mechanism of action is different from that of amiloride.
Figure 1.
Acetazolamide (Acz) reduces lithium (Li+) -induced downregulation of AQP2 abundance in mpkCCD cells. Native mpkCCD cells were grown to confluence for 4 days and subsequently exposed to 1 nM dDAVP for another 4 days. During the last 2 days, cells were incubated in the absence (control [Ctr]) or presence of Li+ only or with Li+ and 100 μM Acz, 10 μM amiloride (Am), 100 μM hydrochlorothiazide and Am (T+Am), or Am and Acz (Am+Acz). At the basolateral and apical sides, final concentrations of 1 and 10 mM Li+ were used, respectively. (F) After measurements of Tv, (A and C) cells were lysed and subjected to AQP2 immunoblotting. Molecular masses (in kilodaltons) are indicated. (B and D) The signals for nonglycosylated (29 kD) and complex-glycosylated (40–45 kD) AQP2 were densitometrically quantified. Mean values±SEMs of normalized AQP2 abundance are given relative to Ctr. (E) Intracellular Li+ concentrations were determined, corrected for contamination with extracellular Li+, and normalized for the amount of protein ([Li+]±SEM in picomoles per microgram protein). Data from three independent experiments (one-way ANOVA and Bonferroni multiple comparison test). *P<0.05.
Acetazolamide Attenuates Development of Li-NDI in Mice
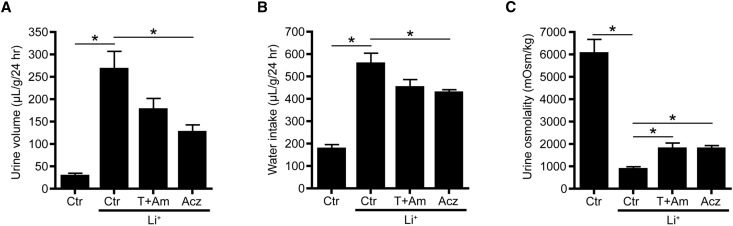
To investigate whether acetazolamide attenuates development of Li-NDI, mice were maintained on lithium chow only or lithium combined with acetazolamide or thiazide/amiloride for 10 days. As reported,11,16 mice treated with lithium developed severe polyuria and polydipsia combined with a significantly reduced urine osmolality (Figure 2). Interestingly, acetazolamide treatment induced a significant antidiuresis and increase in urine osmolality, which was slightly but not significantly better than that in mice treated with thiazide/amiloride. Consistent with the induced antidiuresis, water intake was significantly reduced with the acetazolamide treatment compared with lithium only.
Figure 2.
Acetazolamide (Acz) attenuates lithium (Li+)-induced NDI in mice. (A) Urine volume, (B) water intake, and (C) urine osmolality of untreated mice (control [Ctr]) or mice treated for 10 days with Li+ or Li+ combined with thiazide/amiloride (T+Am) or Acz. During the last 48 hours, mice were housed in metabolic cages; during the last 24 hours, water intake was measured, and urine was collected to determine urine volume and osmolality (n=8 mice per group; one-way ANOVA and Bonferroni multiple comparison test). *P<0.05.
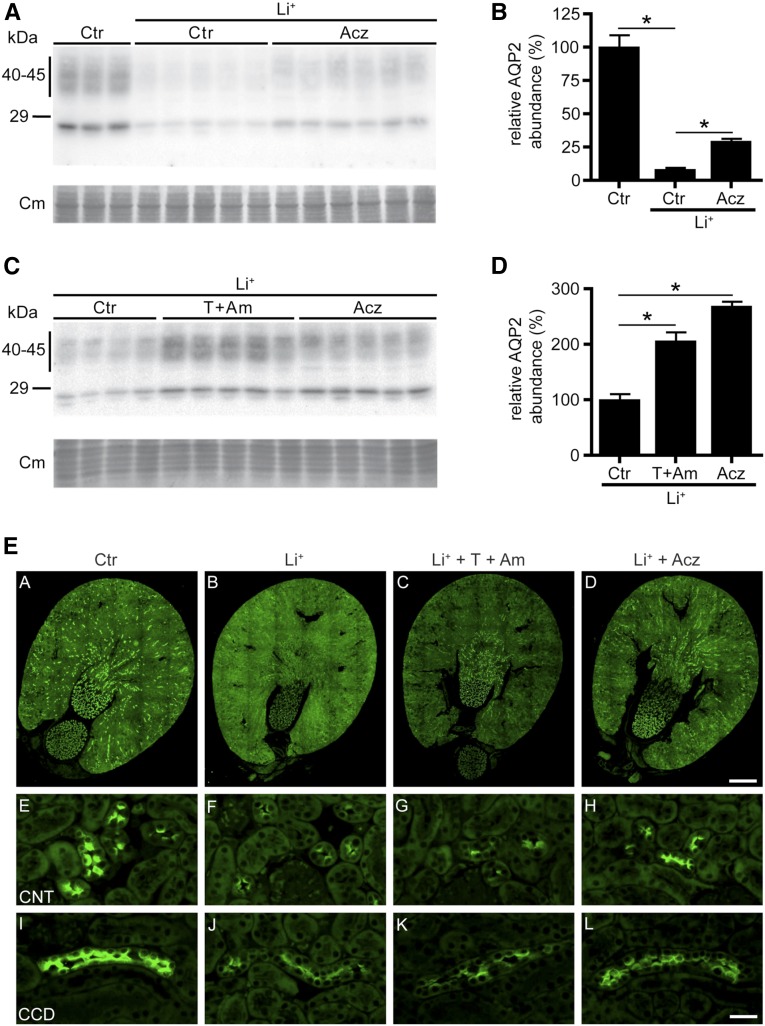
Because long–term lithium treatment coincides with reduced AQP2 and increased H+-ATPase abundance in the kidney,6 we also analyzed their abundances. Immunoblot analysis revealed that lithium reduced AQP2 abundance (Figure 3, A and B), which was significantly attenuated by both acetazolamide and thiazide/amiloride (Figure 3, A–D). With acetazolamide or thiazide/amiloride, however, AQP2 levels did not return to control levels. H+-ATPase levels were similar for all groups (Supplemental Figure 1), which is in line with the notion that lithium–induced collecting duct remodeling is not present after 10 days of lithium treatment in rodents.9,16
Figure 3.
Thiazide/amiloride (T+Am) and acetazolamide (Acz) reduce lithium (Li+) -induced downregulation of AQP2 in Li-NDI mice. (A–D) Immunoblot and corresponding densitometric analyses of AQP2 of mouse kidneys that are untreated (control [Ctr]), treated with Li+ only, or treated with Li+ together with Acz or T+Am. (B and D) The signals for AQP2 are densitometrically quantified. Mean values±SEMs of normalized AQP2 abundance are given relative to Ctr. Equal loading of the samples was confirmed by staining of the blots with Coomassie blue (Cm). One-way ANOVA and Bonferroni multiple comparison test. *Significant differences (P<0.05) from Ctr. Paraffin sections of immersion-fixed kidneys from (A, E, and I) Ctr, (B, F, and J) Li+-treated, (C, G, and K) Li++T+Am-treated, and (D, H, and L) Li++Acz-treated mice were incubated with a rabbit polyclonal AQP2 antibody followed by a Cy3–coupled goat anti–rabbit IgG. (A–D) Overviews and high magnifications of representative (E–H) connecting tubules (CNTs) and (I–L) cortical collecting ducts (CCDs).
To examine segment-specific effects of the different therapies on AQP2 abundance, immunohistochemistry was performed. Consistent with our immunoblot data, lithium treatment strongly reduced AQP2 staining, which was clearly attenuated in kidneys of mice treated with lithium and acetazolamide or thiazide/amiloride (Figure 3E). Interestingly, although lithium abolished AQP2 expression in the entire kidney, the increased AQP2 abundance in the thiazide/amiloride-treated group mainly localized to the inner medulla of the kidney, whereas in the acetazolamide-treated mice, AQP2 abundance was increased along the connecting tubule and entire collecting duct. Consistent with our immunoblot data, immunohistochemistry revealed no clear changes in H+-ATPase labeling between the different groups (Supplemental Figure 2).
Acetazolamide Shows an Improved Overall Electrolyte Balance over Thiazide/Amiloride
Some patients on thiazide/amiloride therapy have been reported to develop hyponatremia, hyperkalemia, metabolic acidosis, and/or hypercalcemia.17–20 Moreover, initiating thiazide/amiloride treatment in patients with Li-NDI often leads to elevated blood lithium levels, necessitating adjustment of the lithium dose.21 Therefore, to assess and compare the effect of acetazolamide and thiazide/amiloride on these parameters, we analyzed blood and urine for lithium and electrolyte levels (Table 1). Indeed, although mice treated with thiazide/amiloride had a reduced body weight and developed hyponatremia, hyperkalemia, and a metabolic acidosis, these parameters were not affected in acetazolamide-treated mice. Also, thiazide/amiloride treatment induced hypercalcemia, which was significantly reduced with acetazolamide, although not to control levels. Moreover and consistent with patients, serum lithium concentrations were significantly increased in our thiazide/amiloride mice but unchanged in our acetazolamide mice compared with lithium controls.
Table 1.
Metabolic parameters of mice treated for 10 days with standard chow only or together with lithium; lithium, thiazide, and amiloride; or lithium and acetazolamide
| Metabolic Parameters | Ctr | Li+ | Li+ + Am + T | Li+ + Acz |
|---|---|---|---|---|
| Serum osmolality (mosM/kg) | 320±1 | 319±1 | 311±0.5a | 321±3b |
| Serum sodium (mmol/L) | 150±0.3 | 149±0.4 | 139±0.8a | 150±0.5b |
| Serum potassium (mmol/L) | 5.3±0.1 | 5.6±0.2 | 7.6±0.5a | 5.4±0.2b |
| Serum lithium (mmol/L) | — | 0.63±0.04c | 2.11±0.12a | 0.69±0.04b |
| Serum creatinine (mg/dl) | 0.08±0.01 | 0.09±0.00 | 0.06±0.01a | 0.04±0.01a,b |
| Blood ionized calcium (mmol/L) | 1.24±0.00 | 1.32±0.01c | 1.34±0.01 | 1.27±0.01a,b |
| Blood pH | 7.34±0.01 | 7.32±0.01 | 7.24±0.02a | 7.35±0.02b |
| Urine sodium (mmol/L) | 352±40 | 46±6c | 484±68a | 112±12b |
| Urine potassium (mmol/L) | 870±83 | 131±15c | 154±19 | 271±31a,b |
| Urine lithium (mmol/L) | — | 20±3c | 24±3 | 38±8 |
| Urine creatinine (mg/dl) | 70±7 | 10±1c | 14±2 | 18±2a |
| Total sodium excretion (mmol/24 h) | 0.17±0.02 | 0.16±0.01 | 1.26±0.12a | 0.22±0.02b |
| Total potassium excretion (mmol/24 h) | 0.42±0.05 | 0.55±0.02c | 0.40±0.03a | 0.54±0.05 |
| Total lithium excretion (mmol/24 h) | — | 84±3c | 64±6 | 72±11 |
| Body weight (g) | 18.9±0.4 | 18.0±0.2c | 16.0±0.5a | 17.4±0.4b |
| Food intake (mg/g per 24 h) | 214±8 | 198±10 | 188±5 | 228±5a,b |
| Feces production (mg/g per 24 h) | 111±7 | 98±8 | 82±2 | 123±9b |
Values are means±SEMs. Ctr, control (standard chow only); Li+, lithium; Am, amiloride; T, thiazide; Acz, acetazolamide; —, below detection limit.
P<0.05 compared with lithium treatment.
P<0.05 compared with lithium, thiazide, and amiloride treatment.
P<0.05 compared with control treatment.
The Antidiuretic Effect of Acetazolamide Coincides with a Lowered GFR and a Reduced Prostaglandin E2 Release
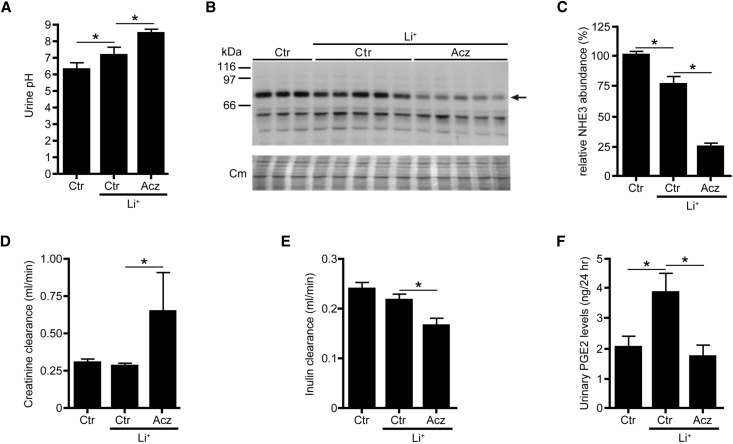
Paradoxically, the clear antidiuresis found in our acetazolamide group (Figure 2) coincided with a significantly increased creatinine clearance (Figure 4A). However, because the creatinine clearance (especially in mice) highly depends on both creatinine secretion and reabsorption in the proximal tubule, the segment mainly influenced by acetazolamide,22,23 we hypothesized that acetazolamide may affect proximal tubular creatinine reabsorption/secretion in our mice and thus, that the creatinine clearance did not properly reflect the GFR. Therefore, we used FITC-inulin to determine the GFR in an identically performed animal experiment. Although acetazolamide again significantly attenuated Li-NDI (not shown), the clearance of FITC-inulin was significantly reduced with acetazolamide (Figure 4B), indicating that acetazolamide reduced the GFR. Urinary prostaglandin E2 (PGE2), which extensively contributes to AQP2 downregulation in Li-NDI,24 was significantly increased in our Li-NDI mice but fully attenuated in our mice treated with lithium/acetazolamide (Figure 4C). Moreover and consistent with its CA inhibitory action in proximal tubules, acetazolamide further increased urinary pH (Figure 4D) and strongly reduced the abundance of NHE3 in the renal cortex compared the cortex of mice treated with lithium only (Figure 4, E and F).
Figure 4.
Acetazolamide (Acz) reduces the GFR and abolishes the elevated PGE2 levels in lithium-treated mice. Mice were treated for 10 days with control (Ctr) diet or diet containing lithium (Li+) only or Li+ combined with Acz. During the last 48 hours, mice were housed in metabolic cages, and during the last 24 hours, urine was collected to determine (A) urinary pH, (D) creatinine clearance, and (F) PGE2 levels. (C) At day 10, mice were euthanized, and blood and kidneys were isolated, enabling the analysis of renal NHE3 abundance. In B, the arrow indicates the approximately 85-kD band of NHE3. (E) To measure GFR using FITC-inulin, the above-mentioned experiment was repeated; however, at day 4, osmotic minipumps containing FITC-inulin were implanted, and at day 10, FITC-inulin levels were measured in 24-hour urine and serum (n=8 mice per group). One-way ANOVA and Bonferroni multiple comparison test. Cm, Coomassie blue. *P<0.05.
Discussion
Acetazolamide Is Superior to Thiazide/Amiloride to Attenuate Li-NDI
Our mouse studies revealed that acetazolamide attenuates development of Li-NDI to the same extent as thiazide/amiloride, but in contrast to acetazolamide, our thiazide/amiloride-treated mice developed hyponatremia, hyperkalemia, and metabolic acidosis. In humans, hyponatremia is mostly a consequence of upregulated AQP2 expression by high circulating AVP levels. Although AVP levels are elevated in Li-NDI, this cannot explain hyponatremia in our thiazide/amiloride-treated mice, because AQP2 is downregulated here. Instead, our data indicate that the hyponatremia is because of the induced natriuresis caused by thiazide and amiloride in a status of polyuria and polydipsia, because our Li-NDI mice were normonatremic and our thiazide/amiloride mice were highly natriuretic compared with the other groups. Note, however, that part of the increased natriuresis must be because of an increased consumption of salt from the provided salt block, because food intake was not increased. The mice apparently drank water to satiety, because the hematocrit was not different between the groups (data not shown). Similarly, patients with congenital NDI also sometimes develop hyponatremia when treated with thiazide combinations.25,26
The observed hyperkalemia is likely caused by inhibition of ENaC by amiloride, because renal secretion of potassium occurs only in exchange of ENaC–mediated sodium reabsorption.27 Lithium itself can lead to metabolic acidosis,28 which would even be increased with inhibition of bicarbonate uptake by acetazolamide, but we only found metabolic acidosis in our thiazide/amiloride-treated mice. Whereas metabolic acidosis in our acetazolamide group may have been compensated for by increased ventilation, metabolic acidosis in the thiazide/amiloride group may be secondary to the observed hyperkalemia, because mammals attenuate hyperkalemia at the expense of development of metabolic acidosis.29
Our Li-NDI mice developed hypercalcemia, which was sustained in our thiazide/amiloride-treated mice but not in our acetazolamide mice. Hyperparathyroidism is common with lithium-using patients, and the occurrence of hypercalcemia in Li-NDI has been ascribed to inhibition of calcium–sensing receptor signaling by lithium in the parathyroid.30–32 The corrected blood calcium levels with acetazolamide, however, may be unrelated to the parathyroid effect of lithium, because plasma calcium is increased by bone resorption, a process that involves CA2 activity in osteoclast and is strongly inhibited by acetazolamide.33,34
Another important advantage of the use of acetazolamide over thiazide/amiloride is that plasma lithium concentrations remained unchanged with acetazolamide. Blood lithium is mainly set by the amount reabsorbed in proximal tubules, a process in which the apical NHE3 is highly involved, and it is stimulated by thiazide.35,36 Considering this, the unaltered blood lithium levels with acetazolamide are best explained by combinatory effects of the reduced NHE3 abundance in proximal tubules and the reduced GFR.
Acetazolamide Attenuates Li-NDI by a Dual Mode of Action
Our data indicate that the observed antidiuresis and reduced GFR with acetazolamide is because of a tubular glomerular feedback response caused by inhibition of CAs in the proximal tubule.37,38 Ninety percent of renal HCO3− is reabsorbed in proximal tubules, which is strongly facilitated by CA4/14 (luminal/apical), CA2 (intracellular), and CA4/12 (basolateral) hydrating CO2 and dehydrating H2CO3.39 In this process, secretion of protons by NHE3 is important. By blocking these CAs, acetazolamide prevents the intracellular generation of H+, which is needed for NHE3 to reabsorb filtered Na+.40–43 The consequently increased tubular salt and water load in the proximal tubule leads to an increased fluid delivery and tubular [Cl−] at the macula densa, which induces a tubular glomerular feedback response (i.e., a reduced GFR).38 Indeed, acetazolamide in our Li-NDI mice led to an increased urinary pH, reduced NHE3 abundance, and reduced GFR, which is in agreement with reported data on NHE3 knockout mice.38,41,44
Other than intercalated cells (see below), the increased fluid delivery to the macula densa with acetazolamide may also partially explain the observed lower urinary levels of PGE2, thereby attenuating Li-NDI. By acting on EP1/3 receptors, increased urinary PGE2 levels in Li-NDI reduce principal cell AQP2 expression and thus, water reabsorption.45 In Li-NDI, a fraction of the elevated urinary PGE2 levels is thought to be derived from macula densa and surrounding cTAL cells, which produce PGE2 to increase renin synthesis and release in response to a reduced fluid delivery to the TAL/hypovolemia.43,44 As such, the increased fluid delivery to the TAL with acetazolamide will reduce the cortical release of PGE2 and therefore, Li-NDI.
However, our in vitro data indicate that acetazolamide also directly protects collecting duct cells from lithium, but it is at present unclear whether in vivo acetazolamide acts directly on principal cells or indirectly through intercalated cells. Support for the first is that mpkCCD cells endogenously express and show proper regulation of the typical principal cell proteins AQP2 and ENaC. Moreover, mpkCCD cells express high CA2 mRNA levels (http://esbl.nhlbi.nih.gov/mpkCCD-transcriptome/), which are also expressed in principal cells in vivo.39,46–48 Also, the in vivo activity of ENaC, the lithium entry site of principal cells, has been reported to be functionally paired with CA activity, because CA inhibition by acetazolamide reduced the intracellular pH and ENaC activity in sweat duct cells and colon.49,50
However, mpkCCD cells may not fully represent principal cells, and because intercalated cells express abundant levels of CA2, -4, -12, and -15,39 acetazolamide may increase principal cell AQP2 expression and water uptake indirectly by inhibiting ACs in intercalated cells. Indeed, long–term lithium treatment leads to metabolic acidosis, which underlies the increased number of α-intercalated cells,11,51 and because lithium inhibits intercalated cell H+-ATPase and H+/K+-ATPase activity,52,53 it has been suggested that acidosis-induced proliferation of α-intercalated cells may contribute to Li-NDI.16 It is unlikely, however, that attenuation of Li-NDI in our mice is caused by direct action of acetazolamide on α-intercalated cells or collecting duct remodeling for several reasons. First, acetazolamide increases the number of α-intercalated cells in rodents, because it causes acidosis itself.54,55 Second and consistent with the unchanged H+-ATPase expression in our mice, collecting duct remodeling is not observed within 10 days of lithium treatment but only starts at about 4 weeks of treatment.9,56
An effect of acetazolamide on β-intercalated cells, however, may be more likely. Although lithium treatment did not reduce the number of these cells,57 exciting recent studies revealed the existence of extensive cross-talk between β-intercalated and principal cells in the regulating collecting duct function.58–61 Although Eladari and coworkers62 elegantly showed that, in rodents, the sodium–dependent chloride bicarbonate exchanger (NDCBE; SLC4a8) and chloride bicarbonate exchanger protein pendrin allow for NaCl reabsorption through β-intercalated cells, chloride permeation through pendrin also seemed necessary for ENaC–mediated sodium reabsorption and expression. Also, in mice lacking functional intercalated cell–specific H+-ATPase, the observed natriuresis and aquaresis were caused by dysfunctional and lower abundances of ENaC and pendrin/NDCBE and reduced AQP2 levels, respectively.60 Eladari and coworkers62 further showed that the lack/inhibition of β-intercalated cells H+-ATPase led, through flow–stimulated luminal ATP release, to autocrine and paracrine release of PGE2, which reduced the cortical and medullary ENaC activity and AQP2 abundance. Importantly, thiazides inhibited NCC–independent NaCl reabsorption through NDCBE/pendrin,59 and acetazolamide reduced pendrin abundance,63 drugs that we showed to attenuate Li-induced downregulation of AQP2 and Li-NDI through a similar mechanism. As such, the attenuated Li-NDI with acetazolamide, which was given during the entire lithium treatment, may be caused by an impaired functioning of pendrin, resulting in increased ATP/PGE2 release, reduced ENaC activity in principal cells, and thus, reduced influx of lithium from prourine. The finding that acetazolamide is beneficial chiefly in the cortical segments that contain intercalated cells is consistent with the possibility that intercalated cell CAs could be involved. A prime candidate here is CA12, because it is highly sensitive to acetazolamide, and patients with reduced CA12 activity have a preponderance to hyponatremic dehydration.64 Whether one of these mechanisms underlies the beneficial effect of acetazolamide in Li-NDI remains to be studied.
Taken together, we have shown that CA activity contributes to Li-NDI development, that acetazolamide attenuates Li-NDI by inducing a tubular glomerular feedback response and through a direct action on the collecting duct, and that acetazolamide attenuates Li-NDI development similar to thiazide/amiloride but with fewer side effects.
Concise Methods
Cell Culture
mpkCCD cells were cultured as described.65 Cells were seeded at a density of 1.5×105 cells per centimeter2 on semipermeable filters (Transwell; 0.4-μm pore size; Corning Costar, Cambridge, MA) and cultured for 8 days. Unless stated otherwise, the cells were exposed to 1 nM dDAVP at the basolateral side for the last 96 hours to induce AQP2 expression. Lithium and compounds were administered as indicated. At the end of the experiment, transcellular electrical resistance and voltage were measured using a Millicell-ERS Meter (Millipore Corp., Bedford, MA). On day 8, cells were harvested and lysed in Laemmli buffer for Western blotting or stored in Trizol Reagent (Invitrogen, Carlsbad, CA) at −80°C for RNA isolation.
Lithium Assays
Determination of intracellular lithium concentrations was done as described.11 Shortly, mpkCCDcl4 cells were grown on 4.7-cm2 filters. To determine the extent of lithium contamination from the extracellular side, FITC-dextran was added to the lithium-containing medium to a final concentration of 10 μM just before harvesting, after which the medium was mixed. Then, the filters were washed three times with iso-osmotic sucrose (pH 7.3) at 4°C, and cells were lysed by sonication in 1 ml milli-Q Water. Of the 800-μl sample, the amount of lithium was determined by flame photometry, from which the total amount of lithium in the sample was calculated.
Of the 100-μl sample, the amount of FITC-dextran was measured using spectrofluorophotometry (RF-5301; Shimadzu, Tokyo, Japan) at 492-nm (excitation) and 518-nm (emission) wavelengths. By comparing the obtained values with a 2-fold FITC-dextran dilution series, the FITC-dextran concentration in each sample was determined, from which the extent of extracellular lithium contamination was calculated. This was subtracted from the total amount to obtain the intracellular lithium amount. With the used FITC-dextran concentration, a contamination >1:5000 would be detected. To correct for differences in cellular yield, the intracellular lithium amounts were normalized for the protein amount in each sample, which was determined using the Bio-Rad Protein Assay (Bio-Rad, Munich, Germany).
Experimental Animals
Eight- to 10-week-old female C57Bl6/JOlaHsd mice (Harlan Laboratories Inc., Frederick, MD) were maintained in a temperature-controlled room with lights on from 8:00 AM to 8:00 PM. They received normal diet (ssniff R/M-H V1534; ssniff Spezialdiaten GmbH, Soest, Germany) with additions (see below) and water ad libitum for 10 days. For the experiments, mice were divided into four groups (n=8), which were treated as follows: group 1: control mice given a normal diet; group 2: normal diet with 40 mmol LiCl per 1 kg dry food66; group 3: diet of group 2 with 200 mg amiloride11 and 350 mg hydrochlorothiazide per 1 kg dry food36; and group 4: diet of group 2 with 180 mg acetazolamide per 1 kg dry food.67 LiCl, amiloride, hydrochlorothiazide, and acetazolamide were solubilized in water and mixed with the chow, after which it was dried. All mice had free access to water, food, and a sodium-chloride block.
For the last 48 hours of the experiment, mice were housed in metabolic cages to measure water intake and urine output during the last 24 hours. Mice were anesthetized with isofluorothane, after which their blood was removed by orbita extraction. Then, mice were killed by cervical dislocation, and the kidneys were rapidly removed. One kidney was processed for immunohistochemistry, whereas the other kidney was used for immunoblotting, both as described below. For immunoblotting, the tissue was homogenized using a Polytron Homogenizer (VWR International, Amsterdam, The Netherlands) in 1 ml ice–cold homogenization buffer A (20 mM Tris, 5 mM MgCl2, 5 mM Na2HPO4, 1 mM EDTA, 80 mM sucrose, and protease inhibitors [1 mM PMSF, 5 µg/ml pepstatin A, 5 µg/ml leupeptin, and 5 µg/ml a-protinin]), cleared from nuclei and unbroken cells by centrifugation at 4000×g for 15 minutes, and diluted in Laemmli buffer to a final protein concentration of 1 μg/μl.
Determination of GFR Using FITC-Inulin
To determine the GFR by the FITC-inulin clearance method,68,69 we used mice as described above, and these mice were also treated as before (n=8 per group). Four days after the start of the diet, minipumps (Model 2001; Alzet, Cupertino, CA) containing 3% FITC-inulin were subcutaneously implanted in the isofluorane-anesthetized mice. At treatment days 9 and 10, mice were housed in metabolic cages, and 24-hour urine was collected in amber tubes at day 10. During this 24 hours, metabolic cages and urine collection tubes were covered with aluminum foil to prevent exposure to light. Traces of left FITC-inulin urine in metabolic cages were added to the collected urine by washing the cage with 5 ml 500 mM HEPES buffer. On day 10, mice were anesthetized with isofluorane, blood was collected by retro-orbital bleeding, and mice were killed by cervical dislocation. Urine fluorescence was determined using a Cytofluor II Fluorescence Multiwell Plate Reader (PerSeptive Biosystems, Framingham, MA) with 485-nm excitation and 538-nm emission. The excretion rate of inulin (24-hour urinary fluorescence counts/plasma fluorescence counts per ml) was taken as the GFR.
Blood and Urine Analyses
Whole blood was analyzed immediately for sodium, potassium, hematocrit, and pH using the EG7+ Cartridge and the I-Stat Clinical Analyzer (Abbott BV, Hoofddorp, The Netherlands). The remaining blood was collected in a BD Microtainer SST Tube (REF 365968; Becton Dickinson BV, Breda, The Netherlands) for serum and centrifuged at 10,000×g for 3 minutes to sediment the red blood cells. Serum and urine samples were analyzed for osmolality using an osmometer (Fiske, Needham Heights, MA), and electrolyte concentrations were measured on a Synchron CX5 Analyzer (Beckman Coulter, Inc., Brea, CA) following the manufacturer’s protocols. Urine PGE2 levels were determined by measuring stable prostaglandin E2 metabolite (PGEM) after chemical derivation of PGE2 and its primary metabolites 13,14-dihydro-15-keto PGE2 and 13,14-dihydro-15-keto PGA2 to the single PGEM compound. PGEM concentrations were determined with the Prostaglandin E Metabolite EIA Kit (Cayman Chemicals, Ann Arbor, MI) according to the manufacturer’s instructions.
Immunoblotting
mpkCCD cells from 1.13-cm2 filters were lysed in 200 μl Laemmli buffer and sonicated. mpkCCD lysate and 5–10 μg kidney material in Laemmli were denatured for 30 minutes at 37°C. Protein concentration was determined using the Bio-Rad Protein Assay (Bio-Rad) according to manufacturer’s instructions. SDS-PAGE, blotting, and blocking of the PVDF membranes were done as described.70 Membranes were incubated for 16 hours at 4°C with 1:2000 affinity–purified rabbit pre–C tail AQP2 antibody recognizing amino acids 236–25571 in Tris-Buffered Saline Tween-20 supplemented with 1% nonfat dried milk. In an identical way, other blots were incubated with a rabbit CA12 antibody (gift from William S. Sly, St. Louis University School of Medicine, St. Louis, MO) and a rabbit CA2 antibody (Abcam, Inc., Cambridge, MA). After washing in Tris-Buffered Saline Tween-20, all blots were incubated for 1 hour with 1:5000-diluted goat anti–rabbit IgGs (Sigma-Aldrich, St. Louis, MO) as secondary antibody coupled to horseradish peroxidase. Proteins were visualized using enhanced chemiluminescence (ECL; Pierce, Rockford, IL). Densitrometric analyses were performed using Bio-Rad quantification equipment (Bio-Rad 690c Densitometer; Chemidoc XRS) and software (QuantityOne; Bio-Rad). Equal loading of the samples was confirmed by staining of the blots with Coomassie blue.
Immunohistochemistry
Kidneys were fixed by immersion for 24 hours in 4% paraformaldehyde in 0.1M phosphate buffer at 4°C, embedded in paraffin, and cut into 3- to 4-μm-thick sections. After deparaffinization, sections were placed into a microwave oven and heated for 10 minutes at 98°C in 0.01 M sodium-citrate buffer (pH 6.0) for antigen retrieval. Subsequently, sections were incubated overnight at 4°C with 1:80,000–diluted rabbit polyclonal AQP2 antibodies or 1:2000–diluted rabbit polyclonal H+-ATPase antibodies as described.72,73 The bound primary antibodies were revealed with Cy3–coupled goat anti–rabbit IgG (Jackson ImmunoResearch Laboratories, West Grove, PA). To check for unspecific binding of primary or secondary antibodies, incubations with nonimmune sera or without any primary antibodies were performed. All control experiments were negative. Cryosections were studied by epifluorescence using a Leica Microscope (Leica Microsystems, Buffalo Grove, IL). Connecting tubules and cortical collecting ducts were distinguished on the basis of their specific localization in the cortical labyrinth and the medullary rays, respectively. Images were acquired with a charge–coupled device camera. For overviews, single images were taken with the automated scanning mode of the microscope and afterward, stitched using the Leica Application Suite. Digital images were processed electronically with Adobe Photoshop (Adobe Systems, Inc., San Jose, CA) and Microsoft Powerpoint (Microsoft, Redmond, WA) software. Adjustments for brightness and contrast were kept constant for each kidney section.
Statistical Analyses
One-way ANOVA with Bonferroni correction was applied. A P value of <0.05 was considered significant. Data are presented as means and SEMs.
Study Approval
All animal studies (DEC no. 2011–010) were approved by the Animal Ethical Committee of the Radboud University Medical Center.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Marthe Minderman, Marcel Jaklofsky, and Monique Carrel for their expert help. The H+-ATPase antibody was a gift from Dr. Carsten Wagner.
This project received support from a Niels Stensen Fellowship (to T.d.G.), Marie Curie Fellowship PIOF-GA-2012-332395 (to T.d.G.), grants from the European Community’s Seventh Framework Programme FP7/2007-2013 (agreement 305608 [EURenOmics]; to O.D.), Swiss National Science Foundation Grants 310030_146490 (to O.D.) and 310030_143929/1 (to J.L.), grants from the Rare Disease Initiative Zurich from the University of Zurich (to O.D.), VICI Grant 865.07.002 (to P.M.T.D.) from The Netherlands Organization for Scientific Research, Radboud University Medical Center Grant 2004.55 (to P.M.T.D.), and a grant from the Society of Experimental Laboratory Medicine (to P.M.T.D.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Water, Water Everywhere: A New Cause and a New Treatment for Nephrogenic Diabetes Insipidus,” on pages 1872–1874.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015070796/-/DCSupplemental.
References
- 1.Grünfeld JP, Rossier BC: Lithium nephrotoxicity revisited. Nat Rev Nephrol 5: 270–276, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Walker RJ, Weggery S, Bedford JJ, McDonald FJ, Ellis G, Leader JP: Lithium-induced reduction in urinary concentrating ability and urinary aquaporin 2 (AQP2) excretion in healthy volunteers. Kidney Int 67: 291–294, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Rej S, Herrmann N, Shulman K: The effects of lithium on renal function in older adults--a systematic review. J Geriatr Psychiatry Neurol 25: 51–61, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Farres MT, Ronco P, Saadoun D, Remy P, Vincent F, Khalil A, Le Blanche AF: Chronic lithium nephropathy: MR imaging for diagnosis. Radiology 229: 570–574, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Laursen UH, Pihakaski-Maunsbach K, Kwon TH, Østergaard Jensen E, Nielsen S, Maunsbach AB: Changes of rat kidney AQP2 and Na,K-ATPase mRNA expression in lithium-induced nephrogenic diabetes insipidus. Nephron, Exp Nephrol 97: e1–e16, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Marples D, Christensen S, Christensen EI, Ottosen PD, Nielsen S: Lithium-induced downregulation of aquaporin-2 water channel expression in rat kidney medulla. J Clin Invest 95: 1838–1845, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, Shaw S, Kamsteeg EJ, Vandewalle A, Deen PM: Development of lithium-induced nephrogenic diabetes insipidus is dissociated from adenylyl cyclase activity. J Am Soc Nephrol 17: 1063–1072, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Christensen BM, Marples D, Kim YH, Wang W, Frøkiaer J, Nielsen S: Changes in cellular composition of kidney collecting duct cells in rats with lithium-induced NDI. Am J Physiol Cell Physiol 286: C952–C964, 2004 [DOI] [PubMed] [Google Scholar]
- 9.de Groot T, Alsady M, Jaklofsky M, Otte-Höller I, Baumgarten R, Giles RH, Deen PM: Lithium causes G2 arrest of renal principal cells. J Am Soc Nephrol 25: 501–510, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Batlle DC, von Riotte AB, Gaviria M, Grupp M: Amelioration of polyuria by amiloride in patients receiving long-term lithium therapy. N Engl J Med 312: 408–414, 1985 [DOI] [PubMed] [Google Scholar]
- 11.Kortenoeven ML, Li Y, Shaw S, Gaeggeler HP, Rossier BC, Wetzels JF, Deen PM: Amiloride blocks lithium entry through the sodium channel thereby attenuating the resultant nephrogenic diabetes insipidus. Kidney Int 76: 44–53, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Bedford JJ, Leader JP, Jing R, Walker LJ, Klein JD, Sands JM, Walker RJ: Amiloride restores renal medullary osmolytes in lithium-induced nephrogenic diabetes insipidus. Am J Physiol Renal Physiol 294: F812–F820, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Bedford JJ, Weggery S, Ellis G, McDonald FJ, Joyce PR, Leader JP, Walker RJ: Lithium-induced nephrogenic diabetes insipidus: Renal effects of amiloride. Clin J Am Soc Nephrol 3: 1324–1331, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sinke AP, Kortenoeven ML, de Groot T, Baumgarten R, Devuyst O, Wetzels JF, Loffing J, Deen PM: Hydrochlorothiazide attenuates lithium-induced nephrogenic diabetes insipidus independently of the sodium-chloride cotransporter. Am J Physiol Renal Physiol 306: F525–F533, 2014 [DOI] [PubMed] [Google Scholar]
- 15.Pickkers P, Garcha RS, Schachter M, Smits P, Hughes AD: Inhibition of carbonic anhydrase accounts for the direct vascular effects of hydrochlorothiazide. Hypertension 33: 1043–1048, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Christensen BM, Kim YH, Kwon TH, Nielsen S: Lithium treatment induces a marked proliferation of primarily principal cells in rat kidney inner medullary collecting duct. Am J Physiol Renal Physiol 291: F39–F48, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Fidler HM, Goldman J, Bielawska CA, Rai GS, Hoffbrand BI: A study of plasma sodium levels in elderly people taking amiloride or triamterene in combination with hydrochlorothiazide. Postgrad Med J 69: 797–799, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mathew TH, Boyd IW, Rohan AP: Hyponatraemia due to the combination of hydrochlorothiazide and amiloride (Moduretic): Australian spontaneous reports 1977-1988. Med J Aust 152: 308–309, 1990 [DOI] [PubMed] [Google Scholar]
- 19.Bayer AJ, Farag R, Browne S, Pathy MS: Plasma electrolytes in elderly patients taking fixed combination diuretics. Postgrad Med J 62: 159–162, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaffey L, Martin A: Malignant hyperkalaemia after amiloride/hydrochlorothiazide treatment. Lancet 1: 1272, 1981 [DOI] [PubMed] [Google Scholar]
- 21.Boton R, Gaviria M, Batlle DC: Prevalence, pathogenesis, and treatment of renal dysfunction associated with chronic lithium therapy. Am J Kidney Dis 10: 329–345, 1987 [DOI] [PubMed] [Google Scholar]
- 22.Eisner C, Faulhaber-Walter R, Wang Y, Leelahavanichkul A, Yuen PS, Mizel D, Star RA, Briggs JP, Levine M, Schnermann J: Major contribution of tubular secretion to creatinine clearance in mice. Kidney Int 77: 519–526, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Musso CG, Michelángelo H, Vilas M, Reynaldi J, Martinez B, Algranati L, Macías Núñez JF: Creatinine reabsorption by the aged kidney. Int Urol Nephrol 41: 727–731, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Kwon TH: Dysregulation of renal cyclooxygenase-2 in rats with lithium-induced nephrogenic diabetes insipidus. Electrolyte Blood Press 5: 68–74, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anton-Gamero M, Garcia-Martinez E, Fernandez-Ramos J, Rodríguez-Salas M, Gil-Campos M: Nephrogenic diabetes insipidus: The key element of paradoxical hyponatremia. Pediatr Nephrol 24: 2277–2278, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Boussemart T, Nsota J, Martin-Coignard D, Champion G: Nephrogenic diabetes insipidus: Treat with caution. Pediatr Nephrol 24: 1761–1763, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Christensen BM, Perrier R, Wang Q, Zuber AM, Maillard M, Mordasini D, Malsure S, Ronzaud C, Stehle JC, Rossier BC, Hummler E: Sodium and potassium balance depends on αENaC expression in connecting tubule. J Am Soc Nephrol 21: 1942–1951, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nascimento L, Rademacher DR, Hamburger R, Arruda JA, Kurtzman A: On the mechanism of lithium-induced renal tubular acidosis. J Lab Clin Med 89: 455–462, 1977 [PubMed] [Google Scholar]
- 29.Palmer BF: Metabolic complications associated with use of diuretics. Semin Nephrol 31: 542–552, 2011 [DOI] [PubMed] [Google Scholar]
- 30.McHenry CR, Racke F, Meister M, Warnaka P, Sarasua M, Nemeth EF, Malangoni MA: Lithium effects on dispersed bovine parathyroid cells grown in tissue culture. Surgery 110: 1061–1066, 1991 [PubMed] [Google Scholar]
- 31.Brown EM: Lithium induces abnormal calcium-regulated PTH release in dispersed bovine parathyroid cells. J Clin Endocrinol Metab 52: 1046–1048, 1981 [DOI] [PubMed] [Google Scholar]
- 32.McHenry CR, Stenger DB, Racke F: Investigation of calcium-induced hydrolysis of phosphoinositides in normal and lithium-treated parathyroid cells. Am J Surg 170: 484–487, 1995 [DOI] [PubMed] [Google Scholar]
- 33.Brown GM, Morris CA, Mitnick MA, Insogna KL: Treatment of humoral hypercalcemia of malignancy in rats with inhibitors of carbonic anhydrase. J Bone Miner Res 5: 1037–1041, 1990 [DOI] [PubMed] [Google Scholar]
- 34.Lehenkari P, Hentunen TA, Laitala-Leinonen T, Tuukkanen J, Väänänen HK: Carbonic anhydrase II plays a major role in osteoclast differentiation and bone resorption by effecting the steady state intracellular pH and Ca2+. Exp Cell Res 242: 128–137, 1998 [DOI] [PubMed] [Google Scholar]
- 35.Timmer RT, Sands JM: Lithium intoxication. J Am Soc Nephrol 10: 666–674, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Nijenhuis T, Vallon V, van der Kemp AW, Loffing J, Hoenderop JG, Bindels RJ: Enhanced passive Ca2+ reabsorption and reduced Mg2+ channel abundance explains thiazide-induced hypocalciuria and hypomagnesemia. J Clin Invest 115: 1651–1658, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cogan MG, Maddox DA, Warnock DG, Lin ET, Rector FC Jr.: Effect of acetazolamide on bicarbonate reabsorption in the proximal tubule of the rat. Am J Physiol 237: F447–F454, 1979 [DOI] [PubMed] [Google Scholar]
- 38.Leyssac PP, Karlsen FM, Holstein-Rathlou NH, Skøtt O: On determinants of glomerular filtration rate after inhibition of proximal tubular reabsorption. Am J Physiol 266: R1544–R1550, 1994 [DOI] [PubMed] [Google Scholar]
- 39.Purkerson JM, Schwartz GJ: The role of carbonic anhydrases in renal physiology. Kidney Int 71: 103–115, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Schnermann J: Sodium transport deficiency and sodium balance in gene-targeted mice. Acta Physiol Scand 173: 59–66, 2001 [DOI] [PubMed] [Google Scholar]
- 41.Amlal H, Ledoussal C, Sheriff S, Shull GE, Soleimani M: Downregulation of renal AQP2 water channel and NKCC2 in mice lacking the apical Na+-H+ exchanger NHE3. J Physiol 553: 511–522, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maren TH: Carbonic anhydrase: Chemistry, physiology, and inhibition. Physiol Rev 47: 595–781, 1967 [DOI] [PubMed] [Google Scholar]
- 43.Deng A, Miracle CM, Lortie M, Satriano J, Gabbai FB, Munger KA, Thomson SC, Blantz RC: Kidney oxygen consumption, carbonic anhydrase, and proton secretion. Am J Physiol Renal Physiol 290: F1009–F1015, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Brooks HL, Sorensen AM, Terris J, Schultheis PJ, Lorenz JN, Shull GE, Knepper MA: Profiling of renal tubule Na+ transporter abundances in NHE3 and NCC null mice using targeted proteomics. J Physiol 530: 359–366, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Olesen ET, Fenton RA: Is there a role for PGE2 in urinary concentration? J Am Soc Nephrol 24: 169–178, 2013 [DOI] [PubMed] [Google Scholar]
- 46.Brown D, Kumpulainen T, Roth J, Orci L: Immunohistochemical localization of carbonic anhydrase in postnatal and adult rat kidney. Am J Physiol 245: F110–F118, 1983 [DOI] [PubMed] [Google Scholar]
- 47.Lönnerholm G, Wistrand PJ, Bárány E: Carbonic anhydrase isoenzymes in the rat kidney. Effects of chronic acetazolamide treatment. Acta Physiol Scand 126: 51–60, 1986 [DOI] [PubMed] [Google Scholar]
- 48.Parkkila S, Parkkila AK, Saarnio J, Kivelä J, Karttunen TJ, Kaunisto K, Waheed A, Sly WS, Türeci O, Virtanen I, Rajaniemi H: Expression of the membrane-associated carbonic anhydrase isozyme XII in the human kidney and renal tumors. J Histochem Cytochem 48: 1601–1608, 2000 [DOI] [PubMed] [Google Scholar]
- 49.Spicer Z, Clarke LL, Gawenis LR, Shull GE: Colonic H(+)-K(+)-ATPase in K(+) conservation and electrogenic Na(+) absorption during Na(+) restriction. Am J Physiol Gastrointest Liver Physiol 281: G1369–G1377, 2001 [DOI] [PubMed] [Google Scholar]
- 50.Reddy MM, Wang XF, Quinton PM: Effect of cytosolic pH on epithelial Na+ channel in normal and cystic fibrosis sweat ducts. J Membr Biol 225: 1–11, 2008 [DOI] [PubMed] [Google Scholar]
- 51.Trepiccione F, Capasso G, Nielsen S, Christensen BM: Evaluation of cellular plasticity in the collecting duct during recovery from lithium-induced nephrogenic diabetes insipidus. Am J Physiol Renal Physiol 305: F919–F929, 2013 [DOI] [PubMed] [Google Scholar]
- 52.Eiam-Ong S, Dafnis E, Spohn M, Kurtzman NA, Sabatini S: H-K-ATPase in distal renal tubular acidosis: Urinary tract obstruction, lithium, and amiloride. Am J Physiol 265: F875–F880, 1993 [DOI] [PubMed] [Google Scholar]
- 53.Dafnis E, Kurtzman NA, Sabatini S: Effect of lithium and amiloride on collecting tubule transport enzymes. J Pharmacol Exp Ther 261: 701–706, 1992 [PubMed] [Google Scholar]
- 54.Bagnis C, Marshansky V, Breton S, Brown D: Remodeling the cellular profile of collecting ducts by chronic carbonic anhydrase inhibition. Am J Physiol Renal Physiol 280: F437–F448, 2001 [DOI] [PubMed] [Google Scholar]
- 55.Welsh-Bacic D, Nowik M, Kaissling B, Wagner CA: Proliferation of acid-secretory cells in the kidney during adaptive remodelling of the collecting duct. PLoS One 6: e25240, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Christensen BM, Zuber AM, Loffing J, Stehle JC, Deen PM, Rossier BC, Hummler E: alphaENaC-mediated lithium absorption promotes nephrogenic diabetes insipidus. J Am Soc Nephrol 22: 253–261, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim YH, Kwon TH, Christensen BM, Nielsen J, Wall SM, Madsen KM, Frøkiaer J, Nielsen S: Altered expression of renal acid-base transporters in rats with lithium-induced NDI. Am J Physiol Renal Physiol 285: F1244–F1257, 2003 [DOI] [PubMed] [Google Scholar]
- 58.Roy A, Al-bataineh MM, Pastor-Soler NM: Collecting duct intercalated cell function and regulation. Clin J Am Soc Nephrol 10: 305–324, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leviel F, Hübner CA, Houillier P, Morla L, El Moghrabi S, Brideau G, Hassan H, Parker MD, Kurth I, Kougioumtzes A, Sinning A, Pech V, Riemondy KA, Miller RL, Hummler E, Shull GE, Aronson PS, Doucet A, Wall SM, Chambrey R, Eladari D: The Na+-dependent chloride-bicarbonate exchanger SLC4A8 mediates an electroneutral Na+ reabsorption process in the renal cortical collecting ducts of mice. J Clin Invest 120: 1627–1635, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gueutin V, Vallet M, Jayat M, Peti-Peterdi J, Cornière N, Leviel F, Sohet F, Wagner CA, Eladari D, Chambrey R: Renal β-intercalated cells maintain body fluid and electrolyte balance. J Clin Invest 123: 4219–4231, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eladari D, Chambrey R, Peti-Peterdi J: A new look at electrolyte transport in the distal tubule. Annu Rev Physiol 74: 325–349, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jacques T, Picard N, Miller RL, Riemondy KA, Houillier P, Sohet F, Ramakrishnan SK, Büsst CJ, Jayat M, Cornière N, Hassan H, Aronson PS, Hennings JC, Hübner CA, Nelson RD, Chambrey R, Eladari D: Overexpression of pendrin in intercalated cells produces chloride-sensitive hypertension. J Am Soc Nephrol 24: 1104–1113, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hafner P, Grimaldi R, Capuano P, Capasso G, Wagner CA: Pendrin in the mouse kidney is primarily regulated by Cl- excretion but also by systemic metabolic acidosis. Am J Physiol Cell Physiol 295: C1658–C1667, 2008 [DOI] [PubMed] [Google Scholar]
- 64.Feldshtein M, Elkrinawi S, Yerushalmi B, Marcus B, Vullo D, Romi H, Ofir R, Landau D, Sivan S, Supuran CT, Birk OS: Hyperchlorhidrosis caused by homozygous mutation in CA12, encoding carbonic anhydrase XII. Am J Hum Genet 87: 713–720, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hasler U, Mordasini D, Bens M, Bianchi M, Cluzeaud F, Rousselot M, Vandewalle A, Feraille E, Martin PY: Long term regulation of aquaporin-2 expression in vasopressin-responsive renal collecting duct principal cells. J Biol Chem 277: 10379–10386, 2002 [DOI] [PubMed] [Google Scholar]
- 66.Wood AJ, Goodwin GM, De Souza R, Green AR: The pharmacokinetic profile of lithium in rat and mouse; an important factor in psychopharmacological investigation of the drug. Neuropharmacology 25: 1285–1288, 1986 [DOI] [PubMed] [Google Scholar]
- 67.Nijenhuis T, Renkema KY, Hoenderop JG, Bindels RJ: Acid-base status determines the renal expression of Ca2+ and Mg2+ transport proteins. J Am Soc Nephrol 17: 617–626, 2006 [DOI] [PubMed] [Google Scholar]
- 68.Qi Z, Whitt I, Mehta A, Jin J, Zhao M, Harris RC, Fogo AB, Breyer MD: Serial determination of glomerular filtration rate in conscious mice using FITC-inulin clearance. Am J Physiol Renal Physiol 286: F590–F596, 2004 [DOI] [PubMed] [Google Scholar]
- 69.Fenton RA, Flynn A, Shodeinde A, Smith CP, Schnermann J, Knepper MA: Renal phenotype of UT-A urea transporter knockout mice. J Am Soc Nephrol 16: 1583–1592, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kamsteeg EJ, Wormhoudt TA, Rijss JPL, van Os CH, Deen PMT: An impaired routing of wild-type aquaporin-2 after tetramerization with an aquaporin-2 mutant explains dominant nephrogenic diabetes insipidus. EMBO J 18: 2394–2400, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kamsteeg EJ, Bichet DG, Konings IB, Nivet H, Lonergan M, Arthus MF, van Os CH, Deen PM: Reversed polarized delivery of an aquaporin-2 mutant causes dominant nephrogenic diabetes insipidus. J Cell Biol 163: 1099–1109, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wagner CA, Lükewille U, Valles P, Breton S, Brown D, Giebisch GH, Geibel JP: A rapid enzymatic method for the isolation of defined kidney tubule fragments from mouse. Pflugers Arch 446: 623–632, 2003 [DOI] [PubMed] [Google Scholar]
- 73.Wagner CA, Loffing-Cueni D, Yan Q, Schulz N, Fakitsas P, Carrel M, Wang T, Verrey F, Geibel JP, Giebisch G, Hebert SC, Loffing J: Mouse model of type II Bartter’s syndrome. II. Altered expression of renal sodium- and water-transporting proteins. Am J Physiol Renal Physiol 294: F1373–F1380, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.