Abstract
Innovation in kidney diseases is not commensurate with the effect of these diseases on human health and mortality or innovation in other key therapeutic areas. A primary cause of the dearth in innovation is that kidney diseases disproportionately affect a demographic that is largely disenfranchised, lacking sufficient advocacy, public attention, and funding. A secondary and likely consequent cause is that the existing infrastructure supporting nephrology research pales in comparison with those for other internal medicine specialties, especially cardiology and oncology. Citing such inequities, however, is not enough. Changing the status quo will require a coordinated effort to identify and redress the existing deficits. Specifically, these deficits relate to the need to further develop and improve the following: understanding of the disease mechanisms and pathophysiology, patient engagement and activism, clinical trial infrastructure, and investigational clinical trial designs as well as coordinated efforts among critical stakeholders. This paper identifies potential solutions to these barriers, some of which are already underway through the Kidney Health Initiative. The Kidney Health Initiative is unique and will serve as a current and future platform from which to overcome these barriers to innovation in nephrology.
Keywords: kidney disease, clinical trial, end stage kidney disease, Pathophysiology of Renal Disease and Progression, Patient satisfaction
Kidney disease is a global health care epidemic. However, the kidney community has generated fewer randomized, controlled trials than any internal medicine specialty, produced a limited number of innovative drugs and devices, and received less funding from public and private sources compared with other diseases.1–3 A significant proportion of the United States adult population has CKD,4 individuals with CKD are 16–40 times more likely to die from cardiovascular disease than to reach ESRD,5 ESRD is responsible for the death of more Americans than breast and prostate cancer combined,6 and blacks, Native Americans, and Latinos are at significantly greater risk for CKD, resulting in serious health disparity issues.7–9 These realities have failed to sufficiently drive public awareness, industry innovation, and research funding. Additionally, Medicare costs for ESRD exceeded $34 billion in 2013, whereas patients with ESRD represent approximately 1% of the Medicare population; also, costs associated with the care of beneficiaries with ESRD constituted >6% of the Medicare budget in 2013.10 Why has the discipline of nephrology not kept pace with other internal medicine and surgical specialties in the development of novel therapies?
We must break away from the status quo with a call for action that supports several transformative initiatives, including elucidation of the pathophysiology of kidney diseases that develops biomarkers and identifies “drugable” targets; increased patient engagement to advance awareness and increased research funding to ensure the conduct of relevant trials; creation of infrastructure that fosters an environment conducive to the development of medical devices and drugs that improve the lives of people with kidney diseases; improved clinical trial design and execution (including the elucidation of relevant and feasible clinical trial end points); and improved coordination among all stakeholders.
The Kidney Health Initiative (KHI), a public-private partnership to enhance patient safety and foster innovation in kidney disease, is a community comprised of >75 member organizations (listed in Table 1; including patient groups, medical societies, pharmaceutical and device companies, clinical research organizations, dialysis companies, and government organizations) committed to working together to effect such change. The KHI must help create a reality where research priorities mirror the effect of kidney diseases on patient quality of life and cost to society.
Table 1.
Members of the KHI (as of February of 2016)
| KHI Stakeholder Type | Organization |
|---|---|
| Device manufacturers | C. R. Bard, Inc.a |
| Medtronic, Inc.a | |
| NxStage Medical Inc.a | |
| PuraCath Medical, Inc. | |
| TVA Medical | |
| W. L. Gore & Associatesa | |
| Dialysis providers | DaVita Healthcare Partnersa |
| Dialysis Clinic, Inc. (DCI)a | |
| Fresenius Medical Carea | |
| Peace Health Dialysis Centera | |
| The Rogosin Institute | |
| Foundations | Institute Kidney Foundation of Delhia |
| JDRF Internationala | |
| NephCure Kidney Internationala | |
| Oxalosis and Hyperoxaluria Foundation Polycystic Kidney Disease Foundationa | |
| Vasculitis Foundationa | |
| Patient organizations | American Association of Kidney Patients (AAKP)a |
| Home Dialyzors Uniteda | |
| National Kidney Foundation (NKF)a | |
| Renal Support Network (RSN)a | |
| Research institutions | Arbor Research Collaborative for Healtha |
| Collaborative Study Group Duke Clinical Research Institute (DCRI)a | |
| The George Institutea | |
| Karolinska Institutea | |
| Kidney Research Institute at the University of Washingtona | |
| University of Oxford Clinical Trial Service Unit and Epidemiologic Studiesa | |
| The University of Tokyo Hospitala | |
| Health professional organizations | American College of Clinical Pharmacy Nephrology Practice and Research Network (ACCP-PRN)a |
| American Nephrology Nurses’ Association (ANNA)a | |
| American Society for Apheresis (ASFA)a | |
| American Society of Diagnostic and Interventional Nephrology (ASDIN)a | |
| American Society of Nephrology (ASN)a | |
| American Society of Pediatric Nephrology (ASPN)a | |
| American Society of Transplantation (AST)a | |
| Association for the Advancement of Medical Instrumentation (AAMI)a | |
| European Renal Association–European Dialysis and Transplant Association (ERA-EDTA)a | |
| National Renal Administrators Association (NRAA)a | |
| Nephrology Nursing Certification Commission (NNCC)a | |
| Renal Physicians Association (RPA)a | |
| Society for Interventional Radiology (SIR) | |
| Vascular Access Society of the Americas (VASA)a | |
| Vascular Access Societya | |
| Pharmaceutical and biotechnology companies | AbbVie, Inc.a |
| Amgen, Inc. (Thousand Oaks, CA)a | |
| Angion Biomedica Corp. | |
| Anthera Pharmaceuticals, Inc.a | |
| AstraZeneca Pharmaceuticals (Wilmington, DE)a | |
| Baxter Healthcare Corporation (Deerfield, IL)a | |
| Blood Purification Technologies, Inc. | |
| The Binding Sitea | |
| ChemoCentryx Inc. | |
| Clinical Metabolomics Inc. (d/b/a ClinMet)a | |
| Eli Lilly (Indianapolis, IN)a | |
| FAST BioMedicala | |
| Frenova Renal Research | |
| Hospira, Inc.a | |
| Humacyte, Inc. | |
| Keryx Biopharmaceuticals, Inc. | |
| Mallinckrodt Pharmaceuticalsa | |
| MediBeacon, LLCa | |
| Nephropath | |
| OPKO Health, Inc.a | |
| Pfizer, Inc.a | |
| Pharmalink Proteon Therapeutics, Inc.a | |
| Quintiles Global Clinical Research Organization (Morrisville, NC)a | |
| Relypsa, Inc. | |
| Rockwell Medical Inc.a | |
| Sanofi US (Bridgewater, NJ; a Sanofi Company)a | |
| Takeda Chemical Industries (Osaka, Japan) | |
| Thrasos, Inc. (Montreal, QC, Canada)a | |
| Vascular Pharmaceuticals Inc. | |
| Vascular Therapies Inc.a | |
| ZS Pharma (Coppell, TX) | |
| Additional Partnership with | Centers for Disease Control (CDC) |
| CMS | |
| FDA | |
| NIH | |
| Veterans Health Administration (VHA) |
d/b/a, doing business as.
Denotes pioneer members.
Elucidate the Pathophysiology of Kidney Disease
Although beyond the KHI’s purview, better elucidation of disease mechanisms is critical to identifying “drugable” targets and affords opportunities for preclinical studies to show that modulation of a pathway meaningfully affects disease. After identified, these pathways and targets must be tested in longitudinal studies using relevant patient cohorts and appropriate disease controls. Patient registries for all forms of kidney disease have the potential to support such studies. Important examples include the Polycystic Kidney Disease Outcomes Consortium,11 the Chronic Renal Insufficiency Cohort Study,12 and the nascent CureGN Registry.13 The development of diverse patient registries, a mechanistic understanding of kidney diseases, and the therapeutic effects of new device and drug candidates are vital.
Molecules that modulate critical target pathways in humans and animal models require testing both in vitro and in vivo. Cell culture experiments can provide valuable information, but target selection and initial validation are often on the basis of pharmacodynamic changes in animal models. When an identified molecule hits the target in vitro, its activity, efficacy, and toxicity are typically confirmed in animal models of human disease. The nephrology community lacks uniform characterization and validation of animal models that reliably mimic human kidney diseases.
A biomarker has been defined as “a characteristic that is objectively measured and evaluated as an indicator of normal biologic processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention.”14 Biomarkers can enhance the understanding of disease pathogenesis and clinical course; help predict and identify unexpected safety concerns during early phases of drug development rather than in later, more costly phase 3 trials; serve as surrogate end points; aggregate subjects at high risk of specific outcomes; and stratify subjects at the time of enrollment. Despite considerable effort, the kidney community has produced few biomarkers that have been adopted into clinical practice or that have been recognized for their potential to inform drug development.15 soluble tumor necrosis factor receptors 1 and soluble tumor necrosis factor receptors 2 have been proposed as potential biomarkers for diabetic nephropathy progression, but a concerted effort to qualify these candidates has not materialized. In cooperation with global regulatory authorities, stakeholders need to build on current efforts, such as the National Institutes of Health (NIH) Biomarker Consortium16 and the Critical-Path initiative,17 to qualify biomarkers and facilitate product development for patients with kidney disease.
Partner with People with Kidney Diseases
Cancer, heart disease, and HIV/AIDS are diseases with highly motivated, organized, and effective patient advocacy. The kidney community can learn from this experience and build on past efforts to unify the patient voice and drive every aspect of the research enterprise. People with kidney diseases struggle to advocate for their own needs because of sociodemographic variables (including health literacy and economic status), health limitations caused by comorbid conditions, and even recognition of their condition. Awareness of CKD is low even in at-risk populations that have seen a physician in the last year.18
We must foster a culture where people with kidney diseases are empowered to advocate for their own needs. Patient engagement is essential for innovation in nephrology. Ideally, patient needs will inform and direct clinical trials, and patients will participate in clinical studies. Advocates in the cancer, cardiovascular, and HIV/AIDS arenas have shown the powerful effect of the patient voice demanding clinical investigations that advance their agenda. An engaged patient base leads to reducing the costs associated with traditional clinical trials, such as those associated with patient recruitment and study startup times, because more patients want to participate. Innovative trial designs require collaboration with leaders in the patient community to build a patient-supported platform that is best positioned to participate in clinical trial enterprises.
Create an Infrastructure That Increases Clinical Trial Efficiency
The infrastructure needed to support clinical nephrology investigation is expansive and includes a well trained workforce, properly positioned clinical research organizations and trial networks, patient registries and databases that capture relevant data, and efficiently administered institutional review boards (IRBs). Clinical trials are costly; standardizing data collection and shortening device and drug development timelines by improving patient access and selection will significantly reduce expenses. No amount of resources allocated to conducting studies in kidney diseases will satisfy the needs if we do not first create an efficient clinical trial enterprise.
The need to transform our care delivery systems into learning health care systems, where research is embedded into clinical care, has been increasingly recognized.19 The Patient Centered Outcomes Research Institute (PCORI) has taken important steps with the creation of PCORnet, a national health data network that combines data from both clinical data research networks and patient–powered research networks (PPRNs). The clinical data research networks derive their data from hospital and health care systems, whereas the data from the PPRNs are contributed by patient groups.20 The PPRNs include kidney-centered groups, such as NephCure Kidney Network International and the Vasculitis Patient Powered Research Network. The NIH has established its Health Care Systems Research Collaboratory to create a new infrastructure to embed research into health care systems.21 Like the PCORI, the Collaboratory is developing a distributed research network. A critical feature of both PCORnet and the NIH Collaboratory to developing meaningful learning health care systems is the recognition of the critical importance of prospective, randomized, controlled trials in generating meaningful evidence. At present, clinical research in nephrology—particularly in the CKD and ESRD populations—largely consists of observational studies that may generate important hypotheses but frequently do not yield definitive answers. Research networks, like PCORnet and the NIH Collaboratory, provide an attractive alternative: the opportunity to conduct randomized, pragmatic trials at far less prohibitive costs. Although visionary and encouraging, these early efforts will require considerable support to realize their potential.
The development of data standards for trials in kidney diseases could improve data quality and enable analyses of data collected across studies. Government groups, including those at the Food and Drug Administration (FDA) and the NIH, and nongovernment groups, such as the Clinical Data Interchange Standards Consortium (CDISC) and TransCelerate BioPharma, Inc., have begun work in this area. The kidney community needs to work in tandem to establish broadly accepted common data elements for maximal leverage of evidence generated in such studies and learn to jointly develop common protocol templates that advance critical therapeutic areas in kidney disease. Standardized templates can articulate best practices related to recruitment and retention of subjects to avoid loss to follow-up and missing data as well as clarify study outcome measures and end points.
The adoption of new standard operating procedures could improve the productivity of the clinical trial enterprise. Although the FDA and the Office for Human Research Protections encourage centralized review by a single IRB, most institutions perform internal IRB review because of liability concerns.22 Use of multiple IRBs is resource intensive and may decrease human subject protections and disempower IRBs from making needed protocol changes. Use of central IRBs would eliminate redundancy, thus saving time and money while increasing human subject protections.
Improve the Design of Clinical Trials and Clarify Appropriate End Points
Successful development programs require trials that more efficiently identify and enroll patients who are likely to benefit from the therapeutic intervention. Clinical trial design factors, such as prescreening of patients and clear inclusion/exclusion criteria, must balance the desire to eliminate likely biologic nonresponders who decrease trial efficiency with the need to rapidly accrue patients who are representative of the target population. Such design choices benefit from a sophisticated understanding of the pathophysiology of the disease process. In some circumstances, biomarkers can help drive such study enrichment.
Patient selection criteria are not always driven by science (e.g., routine age exclusion for those >65 years old for kidney transplant trials) and may needlessly result in high screen failure and dropout rates. Overall recruitment rate for a diabetic kidney disease trial, for example, is approximately 0.25 patients per site per month (approximately one fourth the number of patients enrolled per month for a diabetes trial at one patient per site per month).23 Although the size of clinical trials may be similar for diabetes studies, the rate at which patients enroll into diabetic kidney disease trials significantly delays timelines, increases cost, and stifles drug development. Greater attention to these design features would improve the success of trials in kidney diseases.
Oncology provides a great example by sometimes using a master protocol to evaluate multiple drugs from multiple companies. This innovation reduces the costs and time associated with the development of new cancer drugs. The investigation of serial studies to predict the therapeutic response with imaging and molecular analysis-2 adaptive trial in breast cancer evaluated multiple products in the setting of neoadjuvant chemotherapy.24 The trial is supported through the Biomarkers Consortium, a public-private partnership that includes the FDA, the NIH, and major pharmaceutical companies. The use of such standing protocols in nephrology becomes feasible with the development of new, rapidly reactive biomarkers that predict meaningful clinical response to enable adaptive trials.
The nephrology community has a unique opportunity to capitalize on the fact that the vast majority of patients with ESRD are seen several times each week for provision of clinical care by two large dialysis providers. With appropriate informed consent, dialysis unit electronic health records could be used to collect clinical information and generate high–quality clinical data in almost real time, providing a snapshot of current standards of care. Efforts to expand research into clinical practices and other nontraditional research sites could be accomplished with pragmatic trials that align with clinical practice. A new model for generating critically valuable evidence is the NIH Research Collaboratory’s Time to Reduce Mortality in End-Stage Renal Disease (TIME) Study.25 A large, cluster–randomized trial, the TIME Study evaluates the effect of longer treatment sessions and uses the electronic data systems of Fresenius Medical Care and DaVita Healthcare Partners to help evaluate interventions. Ideally, the nephrology community will coordinate with regulatory authorities to maximize this valuable data source.
Clarifying appropriate end points for pivotal therapeutic trials addresses a significant barrier to medical product development in nephrology. Potential sponsors face the burden of establishing defined end points where none may currently exist in the context of their development program. This burden increases the assumed developmental risk, discouraging potential sponsors who may instead choose to study a candidate product in the context of other disease states, where end points are more clearly defined. The kidney community needs to be thoughtful in establishing clinically meaningful end points across disparate therapeutic areas. A clinically meaningful end point measures how a patient feels, functions, or survives. Patient-reported outcomes that capture critical evidence about quality of life can be as important and valid as traditional clinical end points, such as survival and hospitalization. Collaboration with people with kidney diseases will increase the understanding of how trials can best incorporate patient preferences and capture their experiences. The FDA Center for Devices and Radiologic Health (CDRH) has recently begun efforts through guidance and a public-private partnership called the Medical Device Innovation Consortium to develop strategies for incorporating patient preference data into device applications.26,27
Surrogate end points (laboratory measures or physical signs intended to be used as substitutes for clinically meaningful end points) may be used to develop some types of therapeutics.28 Typically more feasible to study compared with clinical outcomes, surrogate end points have the potential to accelerate delivery of new, effective, and safe therapies. Many products currently used by patients with kidney diseases were approved on the basis of surrogate end points, including laboratory tests (like hemoglobin, parathyroid hormone, phosphorus, and potassium). Surrogate end points can be used for drug approval when established by definitive studies, or they can be used when deemed “reasonably likely, based on epidemiologic, therapeutic, pathophysiologic, or other evidence, to predict clinical benefit” to secure early “accelerated approval” of a drug to treat “serious or life-threatening diseases, where products provide meaningful therapeutic advantages over existing treatment.”28 This second approach presumes an understanding that additional postapproval studies confirming benefit will be required.28
Improve Coordination among All Stakeholders
A platform is needed where patients, investigators, caregivers, industry, and government can work together to increase understanding of pathophysiology, patient engagement, research infrastructure and efficiency, and trial design and conduct.
The FDA, the NIH, and the Centers for Medicare and Medicaid Services (CMS) have critical but distinct roles in creating an environment conducive to innovation in nephrology. The FDA and the CMS have different legal authorities and standards for marketing and Medicare coverage approvals, respectively. The FDA is concerned with safety and efficacy of products, whereas the CMS determines whether new therapies are reasonable and necessary for diagnosis or treatment in the Medicare population. The CMS could be involved early in the development process to reduce uncertainty among innovators about evidentiary expectations for coverage determinations as well as payment pathways and rates. Parallel rather than sequential reviews by the FDA and the CMS could improve trial efficiency, reduce regulatory burden, and expedite patient access to safe, effective treatments. In a pilot program, the two agencies have established a process for overlapping evaluations of premarket FDA–regulated medical products when the product sponsor and both agencies agree to such parallel review.29 This process will serve the public interest by reducing the time between the FDA marketing approval or clearance decisions and the CMS national coverage determinations. In August of 2014, the pilot program led to the first simultaneous FDA approval and CMS–proposed national coverage determination for Cologuard for colorectal cancer screening.30
The CDRH has conducted a separate pilot program for innovative devices that address ESRD called the ESRD Innovation Challenge.31 Three device developers at different stages of development engaged in earlier and more collaborative discussions with the FDA, and each developer also met with the CMS for prospective input on coverage and reimbursement. This pilot led to the development of the new pathway: Expedited Access for Pre-Market Approval and De Novo Medical Devices Intended for Unmet Medical Need for Life Threatening or Irreversibly Debilitating Diseases or Conditions.32 This pathway includes use of surrogate end points and the opportunity to better balance data collection between pre- and postmarket.32
Aim to Overcome Barriers to Innovation in Nephrology
To enhance the development of novel medical products for meaningful patient outcomes, every stakeholder must embrace five interconnected goals.
Support the development of diagnostic biomarkers
Increase patient engagement to advance awareness and funding of kidney disease
Expand the infrastructure supporting clinical trials, including the development of patient registries
Optimize end points and design to enhance clinical trial efficiency
Enhance collaboration with the government to overcome obstacles that inhibit innovation and advances in care
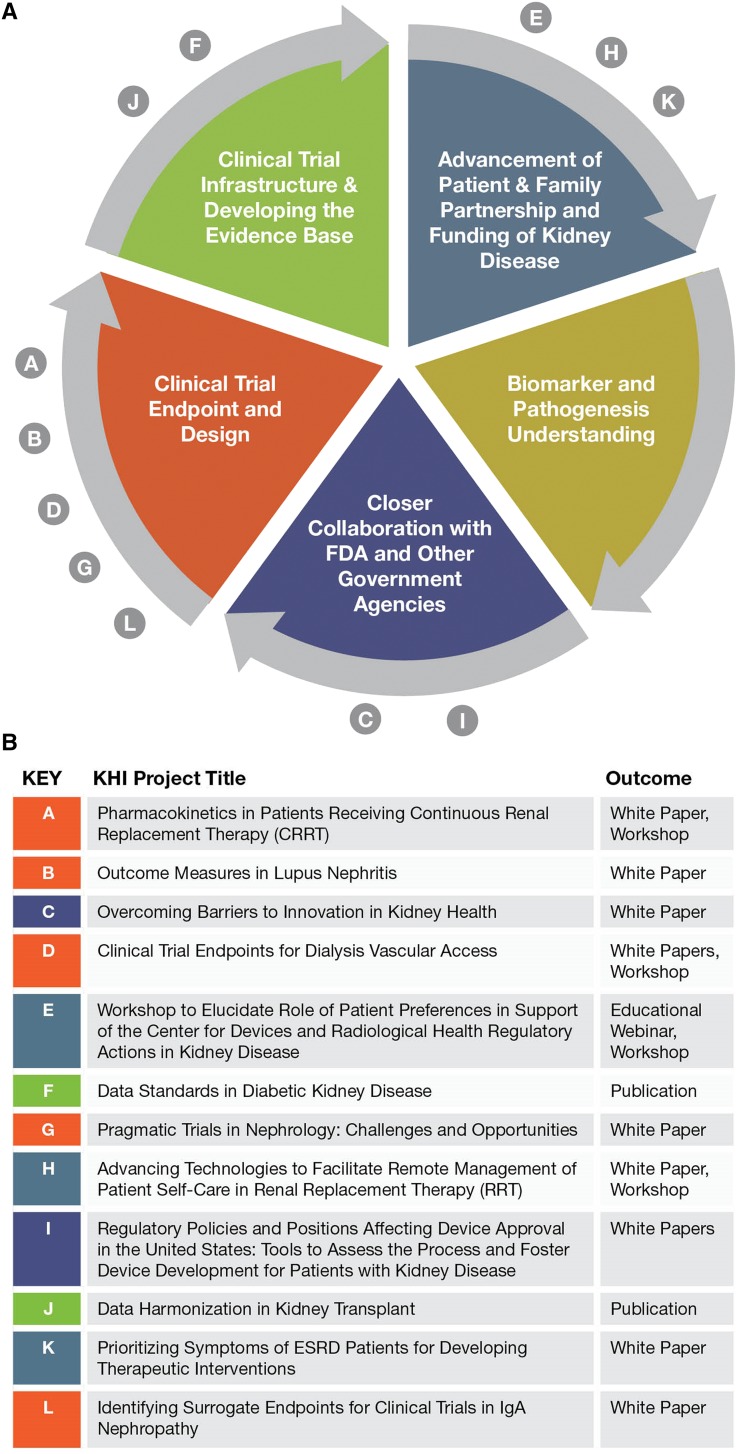
The KHI is a unique platform to bring together diverse but critical stakeholders to address the barriers that impede innovation in nephrology. The mission of the KHI is to enhance patient safety and foster innovation in kidney disease.33 This collaborative partnership is helping the FDA become more responsive to public health needs identified by patients, health professionals, scientists, and industry. The KHI is poised to harness the cooperative energy and earnest efforts of its diverse membership, which includes >75 organizations and companies. A number of member-initiated projects are currently underway or in development to address barriers to innovation in kidney diseases. As detailed in Figure 1, the KHI is committed to finding and implementing specific ways to augment patient engagement and facilitate creation of new infrastructure and databases that will enable use of clinical end points and improve clinical trial design. This organization has taken on the bold mission of breaking the continuous cycle of obstacles that have held back nephrology for decades.
Figure 1.
Interrupting a vicious cycle in nephrology. Major barriers to innovating new kidney therapies and the KHI projects to address them. (A) Mutually interactive barriers to innovation in nephrology. (B) Current KHI projects underway to break the barrier cycle.
Since its inception in 2012, the KHI has undertaken more than a dozen discrete projects to address the five interconnected goals. The KHI is conducting projects to elucidate the evidence underpinning appropriate end point selection for lupus nephritis, vascular access, and IgA nephropathy trials. The KHI is also examining opportunities that broaden approaches, developed through an NIH Collaboratory trial, evaluating the effects of longer hemodialysis sessions on patient outcomes to support pragmatic drug trials based in dialysis centers. Additionally, the KHI is bringing together nephrology trialists and the CDISC data standards experts to develop data standards for diabetic nephropathy and kidney transplant trials, and the KHI has outlined feasible study designs to generate clinically useful pharmacokinetic data to guide drug dosing in critically ill patients on continuous RRT. Inherent to all of these projects is safety-related data for later use in clinical trials and patient care.
For each of the five goals (other than the development of new biomarkers), the KHI has completed or is conducting at least one project (Figure 1). Even more transformative than the valuable white papers, workshops, and webinars produced by these projects are their effects through the participating KHI members. On the basis of the work of the continuous RRT project, the NIH is considering the creation of a data coordinating center to facilitate the conduct of pharmacokinetics trials as outlined by the workgroup. The data standards generated by the diabetic nephropathy and kidney transplant trial projects can be considered by the FDA for addition to its Data Standards Catalog and implementation in guidance.34 Each KHI project is expected to move the field of nephrology forward through both communicating deliverables broadly and informing the activities of individual KHI members.
We must reject the status quo, emphasize discovery and innovation, and make a collective commitment to curing kidney diseases. The collaborative coordinated efforts of a diverse array of stakeholders can effectively remove existing barriers to innovation, while forging a platform for delivering critically needed therapies for the most important stakeholders—the millions of people with kidney disease. If we fail, then we fail our patients who will continue to suffer unnecessarily while observing the cascade of cures for other diseases and wonder why not them and why not now.
Disclosures
P.G.L. has ownership interest in Abbott Laboratories, AbbVie, Inc., and Fibrogen, Inc. and is a scientific advisor or member of the Kidney Health Initiative (KHI) Board of Directors (American Society of Nephrology [ASN]). P.A. is a Food and Drug Administration Cochair of the KHI Board of Directors. M.D.B. has ownership interest in and received research funding from Eli Lilly (Indianapolis, IN). M.D.B. has patents and inventions with Vanderbilt University and is a scientific advisor or member of the KHI Board of Directors (ASN). T.I. is a scientific advisor or member of The Word Works Board of Directors and the American Society of Microbiology Audit Committee. J.I. has ownership interest in Quintiles Global Clinical Research Organization (Morrisville, NC) and received honoraria from the ASN and the National Kidney Foundation (NKF). J.I. is a scientific advisor or member of Kidney International and the KHI Board of Directors (ASN). R.K. has ownership interest in Amgen, Inc. (Thousand Oaks, CA) and is a scientific advisor or member of the KHI Board of Directors (ASN) and the Clinical Trial Transformation Initiative Steering Committee. C.C.L. is a scientific advisor or member of the KHI Board of Directors (ASN) and the Chair of KHI Patient and Family Partnership Council. C.Y.N. is a scientific advisor or member of the KHI Board of Directors (ASN). P.R.-C. has consultancy agreements with Bioconnect, W.L. Gore & Associates, Proteon, Medtronic, Inc., Bard Peripheral Vascular, Cook, and Abbott Vascular; received research funding from W. L. Gore & Associates, Bard Peripheral Vascular (Lutonix); and received honoraria from Bioconnect, WL Gore, Medtronic, Inc., Bard Peripheral Vascular, Abbott Vascular, and Cook. P.R.-C. is a scientific advisor or member of the American Society of Diagnostic and Interventional Nephrology, the ASN Interventional Nephrology Advisory Group, the ASN Postgraduate Education Committee, the ASN Cochair of the KHI, the Medical Advisory Board of the Cincinnati Region NKF, the Board of Trustees of the Cincinnati Region NKF, the Vascular Access Advisory Panel Renal Network, the Medical Review Board Renal Network (Chair), the is International Society of Nephrology- American Nephrologists of Indian Origin (ISN-ANIO) Transplant Subcommittee, Alternate Scientific Board Member, Schulman Associates (Secretary ANIO), the Medical Advisory Board of the Life Center (Cincinnati, OH) (Chief Scientific Officer), and Inovasc. J.A.S. has ownership interest in Baxter Healthcare Corporation (Deerfield, IL) and is a scientific advisor or member of the KHI Board of Directors (ASN). J.S. has ownership interest in Intercept Pharmaceuticals. R.J.F. received honoraria from the university organization for visiting professorships and is a scientific advisor or member of the KHI Board of Directors (ASN).
Acknowledgments
This work was supported by the Kidney Health Initiative (KHI), a public-private partnership between the American Society of Nephrology, the US Food and Drug Administration, and >75 member organizations and companies to enhance patient safety and foster innovation in kidney disease. The KHI funds were used to defray costs incurred during the conduct of the project, including project management support. However, there was no honorarium or other financial support provided to the KHI workgroup members. The KHI workgroup, including the authors of this paper, had final review authority and is fully responsible for its content. The KHI makes every effort to avoid actual, potential, or perceived conflicts of interest that may arise as a result of industry relationships or personal interests among the members of the writing committee and workgroup. More information on the KHI, the workgroup, or the conflict of interest policy can be found at www.kidneyhealthinitiative.org.
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of any agency of the US Government.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Strippoli GF, Craig JC, Schena FP: The number, quality, and coverage of randomized controlled trials in nephrology. J Am Soc Nephrol 15: 411–419, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Palmer SC, Sciancalepore M, Strippoli GF: Trial quality in nephrology: How are we measuring up? Am J Kidney Dis 58: 335–337, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Deo A, Schmid CH, Earley A, Lau J, Uhlig K: Loss to analysis in randomized controlled trials in CKD. Am J Kidney Dis 58: 349–355, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.US Renal Data System : 2014 USRDS Annual Data Report: An Overview of the Epidemiology of Kidney Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2014 [Google Scholar]
- 5.National Institutes of Health : Fact Sheet: Chronic Kidney Disease and Kidney Failure, Bethesda, MD, National Institutes of Health, 2010 [Google Scholar]
- 6.National Center for Health Statistics, Centers for Disease Control : 2013 Mortality Multiple Cause Micro-Data Files, Deaths: Final Data for 2013. National Vital Statistics Report, Hyattsville, MD, National Center for Health Statistics, Centers for Disease Control, 2013 [Google Scholar]
- 7.Evans K, Coresh J, Bash LD, Gary-Webb T, Köttgen A, Carson K, Boulware LE: Race differences in access to health care and disparities in incident chronic kidney disease in the US. Nephrol Dial Transplant 26: 899–908, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jurkovitz CT, Li S, Norris KC, Saab G, Bomback AS, Whaley-Connell AT, McCullough PA; KEEP Investigators : Association between lack of health insurance and risk of death and ESRD: Results from the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis 61[Suppl 2]: S24–S32, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jolly SE, Burrows NR, Chen SC, Li S, Jurkovitz CT, Norris KC, Shlipak MG: Racial and ethnic differences in mortality among individuals with chronic kidney disease: Results from the Kidney Early Evaluation Program (KEEP). Clin J Am Soc Nephrol 6: 1858–1865, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.US Renal Data System : USRDS 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2013 [Google Scholar]
- 11.Polycystic Kidney Disease Outcomes Consortium: Available at: http://c-path.org/programs/pkd/. Accessed March 11, 2015
- 12.Feldman HI, Appel LJ, Chertow GM, Cifelli D, Cizman B, Daugirdas J, Fink JC, Franklin-Becker ED, Go AS, Hamm LL, He J, Hostetter T, Hsu CY, Jamerson K, Joffe M, Kusek JW, Landis JR, Lash JP, Miller ER, Mohler ER 3rd, Muntner P, Ojo AO, Rahman M, Townsend RR, Wright JT; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators : The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and methods. J Am Soc Nephrol 14[Suppl 2]: S148–S153, 2003 [DOI] [PubMed] [Google Scholar]
- 13.CureGN: Cure Glomerulopathy Network. Available at: https://curegn.org/. Accessed March 11, 2015
- 14.Biomarkers Definitions Working Group : Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin Pharmacol Ther 69: 89–95, 2001 [DOI] [PubMed] [Google Scholar]
- 15.FDA: Biomarker Qualification Program. Available at: http://www.fda.gov/Drugs/Development ApprovalProcess/DrugDevelopmentToolsQualificationProgram/ucm284076.htm. Accessed February 24, 2016
- 16.The Biomarkers Consortium: Available at: http://www.ckdbiomarkersconsortium.org. Accessed July 8, 2015
- 17.Woodcock J: FDA’s Critical Path Initiative. Drug Discov Today Technol 4: 1, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Saab G, Whaley-Connell AT, McCullough PA, Bakris GL: CKD awareness in the United States: The Kidney Early Evaluation Program (KEEP). Am J Kidney Dis 52: 382–383, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Greene SM, Reid RJ, Larson EB: Implementing the learning health system: From concept to action. Ann Intern Med 157: 207–210, 2012 [DOI] [PubMed] [Google Scholar]
- 20.PCORnet: PCORnet, the National Patient-Centered Clinical Research Network. Available at: http://www. pcornet.org. Accessed February 24, 2016
- 21.NIH: NIH Collaboratory. Available at: http://www.nihcollaboratory.org. Accessed February 24, 2016
- 22.Menikoff J: The paradoxical problem with multiple-IRB review. N Engl J Med 363: 1591–1593, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Clinical Trials.gov: A Service of the U.S. National Institutes of Health. Available at: https://clinicaltrials.gov/. Accessed July 8, 2015
- 24.Barker AD, Sigman CC, Kelloff GJ, Hylton NM, Berry DA, Esserman LJ: I-SPY 2: An adaptive breast cancer trial design in the setting of neoadjuvant chemotherapy. Clin Pharmacol Ther 86: 97–100, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Dember LM: Time to Reduce Mortality in End-Stage Renal Disease (TIME) Study. Available at: https://www.nihcollaboratory.org/demonstration-projects/Pages/TiME.aspx. Accessed April 11, 2016
- 26.US Food and Drug Administration: Patient Preference Information–Submission, Review in PMAs: HDE Applications, and De Novo Requests, and Inclusion in Device Labeling. Draft Guidance for Industry, Food and Drug Administration Staff, and Other Stakeholders, 2015. Available at: http://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM446680.pdf. Accessed July 8, 2015
- 27.Medical Device Innovation Consortium: Available at: https://www.mdic.org/. Accessed July 8, 2015
- 28.Temple R: Are surrogate markers adequate to assess cardiovascular disease drugs? JAMA 282: 790–795, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Federal Reserve Notice: (75 FR 57045)—Parallel Review of Medical Products, 2010. Available at: http://www.gpo.gov/fdsys/pkg/FR-2010-09-17/pdf/2010-23252.pdf. Accessed July 8, 2015
- 30.CMS: Decision Memo for Screening for Colorectal Cancer-Stool DNA Testing: (CAG-00440N), 2014. Available at: http://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=277&bc=AAAAAAAAAAEAAA%3d%3d&. Accessed July 8, 2015
- 31.US Food and Drug Administration: US Food and Drug Administration Innovative Challenge: End-Stage Renal Disease. Available at: http://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDRH/CDRHInnovation/InnovationPathway/ucm286140.htm. Accessed March 11, 2015
- 32.US Food and Drug Administration: Expedited Access for Premarket Approval and De Novo Medical Devices Intended for Unmet Medical Need for Life Threatening or Irreversibly Debilitating Diseases or Conditions—Guidance for Industry and Drug Administration Staff. Available at: http://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM393978.pdf. Accessed July 8, 2015
- 33.Archdeacon P, Shaffer RN, Winkelmayer WC, Falk RJ, Roy-Chaudhury P: Fostering innovation, advancing patient safety: The kidney health initiative. Clin J Am Soc Nephrol 8: 1609–1617, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.US Food and Drug Administration: Providing Regulatory Submissions in Electronic Format—Standardized Study Data Guidance for Industry. Available at: http://www.fda.gov/downloads/Drugs/ GuidanceComplianceRegulatoryInformation/Guidances/UCM292334.pdf. Accessed February 24, 2016