Abstract
Idiopathic retroperitoneal fibrosis (RPF), reviewed herein, is a rare fibro-inflammatory disease that develops around the abdominal aorta and the iliac arteries, and spreads into the adjacent retroperitoneum, where it frequently causes ureteral obstruction and renal failure. The clinical phenotype of RPF is complex, because it can be associated with fibro-inflammatory disorders involving other organs, is considered part of the spectrum of IgG4-related disease, and often arises in patients with other autoimmune conditions. Obstructive uropathy is the most common complication, although other types of renal involvement may occur, including stenosis of the renal arteries and veins, renal atrophy, and different types of associated GN. Environmental and genetic factors contribute to disease susceptibility, whereas the immunopathogenesis of RPF is mediated by different immune cell types that eventually promote fibroblast activation. The diagnosis is made on the basis of computed tomography or magnetic resonance imaging, and positron emission tomography is a useful tool in disease staging and follow-up. Treatment of idiopathic RPF aims at relieving ureteral obstruction and inducing disease regression, and includes the use of glucocorticoids, combined or not with other traditional immunosuppressants. However, biologic therapies such as the B cell–depleting agent rituximab are emerging as potentially efficacious agents in difficult-to-treat cases.
Keywords: retroperitoneal fibrosis, IgG4, hydronephrosis, vasculitis, fibrosis
The term retroperitoneal fibrosis (RPF) is used to describe a condition of variable etiology characterized by a highly fibrotic retroperitoneal mass that frequently causes ureteral obstruction. RPF encompasses the idiopathic form (>75% of the cases) and secondary forms, which include cases secondary to malignancies, infections, drugs, radiotherapy, or other conditions.1,2
Idiopathic RPF is a rare disease, with an estimated incidence of 0.1–1.3 cases/100,000 persons per year, and a prevalence of 1.4 cases/100,000 inhabitants.2,3 The male-to-female ratio is 2:1–3:1, and the mean age at onset ranges between 55 and 60 years.4,5 Although RPF has become the most used name for this disease, it does not adequately reflect its pathology or its exact topography. The disease usually involves the adventitia of the abdominal aorta and the iliac arteries and the surrounding retroperitoneum, and histologically shows a mixture of fibrous tissue and chronic inflammation.6 In addition, it also frequently affects the thoracic aorta.7 For these reasons, the term “chronic periaortitis,” coined in the 1980s, seems to be more appropriate. Chronic periaortitis also includes inflammatory abdominal aortic aneurysms (without ureteral involvement) and perianeurysmal fibrosis (with ureteral involvement); the two latter conditions are clinically and histologically similar to idiopathic RPF except for aortic aneurysmal dilatation.8–10
During the last decade, the concept of IgG4-related disease (IgG4‑RD) has emerged: IgG4‑RD embraces fibro-inflammatory disorders affecting different structures (e.g., pancreas, biliary tract, lymph nodes) and is characterized by lympho-plasmacytic inflammation, irregular and pronounced fibrosis, and infiltration by IgG4+ plasma cells. Idiopathic RPF belongs to this disease spectrum.11,12 Finally, idiopathic RPF may be associated with systemic (e.g., small-vessel vasculitis, rheumatoid arthritis) and organ-specific (e.g., Hashimoto thyroiditis) autoimmune diseases, which makes the puzzle of its nosology even more complex.1
Pathology
Idiopathic RPF is a fibro-inflammatory disease, histologically hallmarked by fibrous tissue and chronic inflammation. The fibrous tissue comprises an extracellular matrix composed of type I collagen fibers organized in thick irregular bundles and often encircling small retroperitoneal vessels (Figure 1). Fibroblasts show signs of activation and transition into myofibroblasts (α‑smooth muscle actin expression), and are probably the major source of collagen production.6 They rarely show mitoses, although they have been shown to undergo clonal proliferation.13
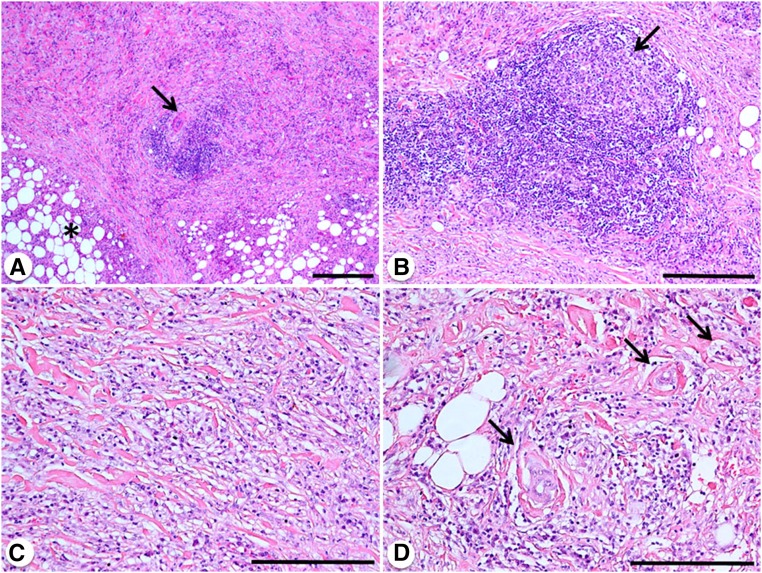
Figure 1.
Histopathology of idiopathic RPF. (A) Low-power magnification view of a retroperitoneal biopsy showing abundant and irregular fibrosis replacing normal retroperitoneal soft tissues (asterisk), and an inflammatory infiltrate organized in a lymphoid aggregate that is centered around a small retroperitoneal artery (arrow). (B) A lymphoid nodular aggregate with a clear (germinal) center (arrow) is visible. (C) Diffuse pattern of the inflammatory infiltrate, mainly consisting of lymphocytes and plasma cells that are diffusely interspersed within collagen bundles. (D) These collagen bundles form rinds around small retroperitoneal vessels (arrows). Hematoxylin and eosin (A–D). Original magnification, ×4 in A (bar is 0.5 mm); ×10 in B (bar is 300 μm); ×20 in C and D (bar is 300 μm).
The inflammatory infiltrate consists of numerous lymphocytes, plasma cells, and macrophages. Neutrophil infiltration is rare, and so are granulomas. The inflammatory cells are interspersed within the collagen bundles (“diffuse” pattern), but are also organized in nodular aggregates, usually around small vessels. Such aggregates have a B cell core surrounded by T cells, which are predominantly CD4+. In some cases, these lymphoid follicles have the structure of germinal centers (Figure 1), which reveals ectopic lymphoneogenesis, a process typical of chronic autoimmune diseases.6,14 Plasma cells account for a significant proportion of the inflammatory cells, and when the IgG4+/total IgG+ plasma cell ratio is >40%, RPF is classified as “IgG4-related” if other features such as storiform fibrosis, eosinophil infiltration, and obliterative phlebitis are also present.11,15 Mast cells are also found. They are identified using tryptase immunostaining, which also demonstrates their degranulating state, a finding consistent with their active participation in the fibro-inflammatory reaction.16
The aforementioned lesions involve not only the periaortic retroperitoneum, but also the aortic wall. In typical cases with periaortic RPF distribution, the fibro-inflammatory reaction mainly involves the aortic adventitia, whereas the other aortic layers may show atherosclerotic changes; this is the basic notion behind the concept of chronic periaortitis, which was initially described as an exaggerated fibro-inflammatory, adventitial, and peri-adventitial reaction to atherosclerotic plaque components.9,17,18 Such aortic wall lesions may also occur in the thoracic aorta and its major branches in patients whose periaortitis extends to involve these vascular territories; thoracic aorta involvement can be seen in both IgG4+ and IgG4– cases.7
Clinical Manifestations and Laboratory Findings
Clinical Signs and Symptoms
Systemic symptoms (e.g., fatigue, anorexia, weight loss), possible expression of an inflammatory status, often herald the disease onset (Table 1). They usually coexist with back, flank, or abdominal pain. Pain is usually dull, does not modify with position, and transiently responds to nonsteroidal anti-inflammatory drugs; in cases of ureteral involvement, it may mimic a ureteral colic. Constipation may be another disease-related manifestation, although it is rarely severe. Other urologic manifestations are frequent: they range from testicular pain, often accompanied by hydrocele and/or varicocele– due to spermatic vein encasement by RPF– to retrograde ejaculation and erectile dysfunction. Other less common manifestations include frequency, hematuria, and dysuria.2,4,5,19
Table 1.
Main demographic, clinical and laboratory findings of patients with idiopathic RPF in four different clinical series
| Mayo Clinic, Rochester (n=185)4 | Johns Hopkins University, Baltimore (n=48)19 | A. Schweitzer Hospital, Dordrecht (n=53)2 | University Hospital, Parma (n=210) | |
|---|---|---|---|---|
| Mean age at diagnosis, years | 58 | 54 | 64 | 58 |
| Male gender, % | 61 | 54 | 77 | 70 |
| Systemic symptoms, %a | 27 | 60 | 92 | 66 |
| Pain (flank, abdominal), % | 38 | 94 | 92 | 81 |
| Testicular manifestations (pain, varicocele, hydrocele), % | 13 | 27 | 46 | 51 |
| Constipation, % | 12 | NA | 30 | 28 |
| Lower extremity edema, % | 13 | 23 | 8 | 15 |
| Lower extremity claudication, % | 2 | NA | 11 | 12 |
| Hydronephrosis, % | 57 | 67 | 55 | 72 |
| Unilateral, % | 25 | 21 | 40 | 29 |
| Bilateral, % | 32 | 46 | 15 | 43 |
| Renal atrophy, % | 8 | NA | 21 | 30 |
| Impaired renal function, %b | 42 | NA | 66 | 57 |
| Mean ESR, mm/h | 32 | 40 | 45 | 63 |
| Mean CRP, mg/L | 20.7 | NA | 23 | 32 |
| Mean serum creatinine, mg/dL | 1.3 | NA | 1.4 | 3.9c |
| Mean Hb, g/dL | 12.6 | 11.6 | 12.4 | 12.5 |
| Increased ESR, % | 53 | NA | 74 | 85 |
| Increased CRP, % | 47 | NA | 62 | 78 |
NA, not available; ESR, erythrocyte sedimentation rate; CRP, C‑reactive protein; Hb, hemoglobin. Normal CRP values are <5 mg/L.
The series included in this table were selected essentially on the basis of their sample size and the accuracy of data reporting.
In the first series (Mayo Clinic) data were collected retrospectively, whereas in the remaining series they were collected prospectively. Data included in the Parma series are unpublished.
For testicular manifestations, the percentage was calculated on male patients only.
Systemic symptoms include: fatigue, anorexia, weight loss and low-grade fever.
Impaired renal function indicates a serum creatinine level >1.2 mg/dL.
In this series, the distribution of serum creatinine was not normal, therefore we also report median (range) serum creatinine levels, which are 1.4 (0.5–23) mg/dL.
Ureteral and Renal Complications
Ureteral involvement is the most common disease-related complication; the disease usually causes medial ureteral deviation, and frequently obstruction of the pelvic ureteral tract.20 This is why RPF limited to the periaortic space rarely causes ureteral obstruction, whereas RPF with peri-iliac extension frequently does. Ureteral encasement can be unilateral or bilateral, and in the latter case ARF is frequent; in cases with unilateral involvement, contralateral progression can occur weeks to years after the initial presentation.4,5 An interesting finding at diagnosis is renal hypoplasia/atrophy (diameter <8.5 cm), at a frequency of 8%–30% (Table 1). Whether this is due to previous ureteral obstruction, renal artery stenosis by RPF, or other causes, is still unclear. Indeed, idiopathic RPF can extend to the renal vascular peduncle:10 this may cause compression of renal veins (which is often slowly progressive and allows the formation of collateral circles),21 and renal arteries with resulting reno-vascular hypertension. New-onset hypertension or worsening of preexisting hypertension is found in up to one-third of patients at diagnosis.19
Vascular Complications
Aneurysmal forms of RPF must be carefully followed for timely repair of the aortic aneurysm; interestingly, both surgical and endovascular repair have been associated with regression of perianeurysmal RPF, although RPF may also develop following endovascular treatment of atherosclerotic aortic aneurysms.22 Idiopathic RPF typically arises around the aorta and iliac arteries, but stenosis of these vessels is quite rare.2,4 Conversely, venous compression (mainly of the inferior vena cava) is common, and can cause lower limb edema, to whose pathogenesis lymphatic compression may contribute. Again, probably due to the slow progression of venous encasement, collateral circles develop, therefore inferior vena cava syndrome, deep vein thrombosis, and pulmonary embolism are uncommon.4,19,23
Other vascular districts may also be involved. The periaortic tissue can extend to the mesenteric and celiac arteries, causing stenosis and ischemic complications resembling mesenteric vasculitis.24 Up to one-third of patients with abdominal RPF also have thoracic aorta involvement, most of them presenting with thoracic aorta aneurysm, the progression of which must also be carefully monitored.7
Laboratory Findings
Acute-phase reactants such as erythrocyte sedimentation rate and C‑reactive protein levels are increased in the majority of patients at presentation and are routinely used to monitor disease activity. Although high baseline acute-phase reactants are associated with a more symptomatic disease, these parameters poorly predict response to therapy and do not correlate with mass regression.25,26 Additionally, relapses commonly occur when acute-phase reactants are still normal.27 Serum IL‑6 is also high, reflecting an acute-phase response; its correlation with disease activity or prognosis is unexplored.28 High IgG4 levels have been linked to IgG4-related RPF, but a systematic assessment of IgG4 in idiopathic RPF is lacking. However, the exact proportion of patients with high serum IgG4, as well as the prognostic significance of this biomarker are still unknown. Experimental studies on small cohorts showed that serum chemokines such as chemokine (C-C motif) ligand 11 (CCL11)/eotaxin‑116 and CCL1829 are increased during active disease; CCL18 correlates with RPF thickness variations after therapy.29
Association with Autoimmune or Fibro-Inflammatory Diseases
One intriguing aspect of idiopathic RPF is its association with autoimmune disorders, which highlights the pathogenic relevance of autoimmune mechanisms. Autoimmune thyroiditis is the most frequently associated autoimmune condition: in a recent case–control study, idiopathic RPF patients had a prevalence of anti-thyroperoxidase antibodies of 24.7% (versus 10.6% in healthy controls) and ultrasound evidence of thyroiditis; after a median follow-up of 45 months, 25% of RPF patients developed hypothyroidism requiring l‑thyroxine. Where available, histology showed typical Hashimoto thyroiditis or its fibrous variant.30 However, cases of Riedel thyroiditis were also described.31
Other associations include rheumatoid arthritis,32 ankylosing spondylitis,33 ANCA-associated vasculitis,34–36 systemic lupus erythematosus,37 and psoriasis.38 Idiopathic RPF has also been linked to different types of GN, particularly membranous nephropathy (MN).39,40 This association is interesting, because MN is an IgG4-mediated disease;41 however, target antigens in MN associated with RPF or IgG4‑RD probably differ from those (e.g., phospholipase A2 receptor) detected in classic MN.39,42–45
Idiopathic RPF can also be associated with fibro-inflammatory conditions involving other structures, especially sclerosing pancreato-cholangitis, fibrosing mediastinitis, orbital pseudotumor, and sclerosing sialoadenitis; this multiorgan disorder was once referred to as multifocal fibrosclerosis.46 The recognition of a shared histopathologic background between these lesions– with main features being irregular (“storiform”) fibrosis, lymphoplasmacytic infiltrates and abundance of IgG4+ plasma cells– provided the rationale for their inclusion in the spectrum of IgG4‑RD.47 Recent studies have demonstrated that, based on histologic findings (e.g., IgG4+ plasma cell infiltration), ≤50% of idiopathic RPF can be histologically classified as “IgG4-related” even when the disease is not associated with other IgG4RD lesions;11,15 however, systematic analyses of large cohorts are lacking. IgG4-related and -unrelated RPF do not appear to differ clinically, except for a higher frequency of extra-retroperitoneal manifestations in the former group; in particular, they have similar demographic and laboratory characteristics, comparable mass location and thickness, and almost identical rates of ureteral involvement.11 Therefore, it is likely that they represent different ends of the same disease spectrum. Additionally, how often RPF overlaps with other IgG4‑RD manifestations is still unclear.
Pathogenesis
Idiopathic RPF, as part of the spectrum of chronic periaortitis, was initially viewed as a localized reaction to antigens contained in the atherosclerotic plaques of the abdominal aorta such as oxidized low-density lipoproteins.48 Such antigens would be presented by plaque macrophages to lymphoid cells residing in the adventitia, where they would elicit a fibro-inflammatory response.8,9,17,18 This theory, however, cannot explain the complex clinical spectrum of idiopathic RPF, particularly the associations with autoimmune or fibro-inflammatory diseases involving other organs. Additionally, the disease can develop in patients without atherosclerotic lesions or involve vascular territories spared by atherosclerosis.7,10 These findings raised the question whether idiopathic RPF is a manifestation of a systemic condition rather than a localized reaction to atherosclerosis.
The pathogenesis of the disease is multifactorial. Environmental agents play a definite role: an association with asbestos exposure has been postulated, and anecdotal cases of pleural asbestosis in RPF patients have been described.3,49 A recent case–control study confirmed the predisposing role of asbestos exposure and also identified smoking as a risk factor. Interestingly, smoking and asbestos had a multiplicative effect on disease risk, with an odds ratio of 12.04 (95% confidence interval, 4.32 to 38.28) in co-exposed subjects.23 The role of other environmental or infectious agents remains elusive. Genetic determinants also contribute to disease susceptibility. Idiopathic RPF is associated with HLA‑DRB1*03, a risk factor for other autoimmune diseases such as lupus erythematosus and type 1 diabetes.50 Other genetic associations include the Δ32 polymorphism of the gene encoding CCR5, a chemokine receptor,51 and the TTCCAT haplotype of the gene encoding CCL11/eotaxin‑1.16
CD4+ T cells are abundant in idiopathic RPF biopsies;6 in IgG4‑RD lesions they show a T-helper 2 (Th2)-polarization and produce IL‑4, IL‑5, and IL‑13, although regulatory T cells are also found.52 In idiopathic RPF, it has been shown that T cells also locally produce IL‑6, which can activate B cells and fibroblasts. B lymphocytes account for a high proportion of infiltrating cells and may be precursors of plasma cells; Th2 cytokines may induce the enrichment of the IgG4+ plasma cell subset, although this has not yet been proven in RPF.47 The pathogenic importance of the IL‑6–mediated axis and of B cells was confirmed in vivo by the efficacy of therapies targeting the IL‑6 receptor (tocilizumab)28 and the B cell marker CD20 (rituximab),53 but it must be acknowledged that these data are limited to small case series. Th2 responses are often characterized by tissue eosinophilia, which is also observed in idiopathic RPF and IgG4‑RD. Tissue recruitment of eosinophils can be driven by chemokines such as CCL11/eotaxin‑1, whose tissue expression and serum levels are high in idiopathic RPF. Eotaxin‑1 also induces recruitment of mast cells, which have been found in idiopathic RPF lesions. Notably, in idiopathic RPF biopsies, eosinophils and mast cells strongly express CCR3, the receptor for CCL11/eotaxin‑1.16 Eosinophil and mast cell products (e.g., eosinophil granule proteins, tryptase) stimulate fibroblast proliferation and collagen production.16 Fibroblasts can also be activated by CCL18, a chemokine whose serum levels are increased in idiopathic RPF.29 The immunopathogenesis of idiopathic RPF is summarized in Figure 2.
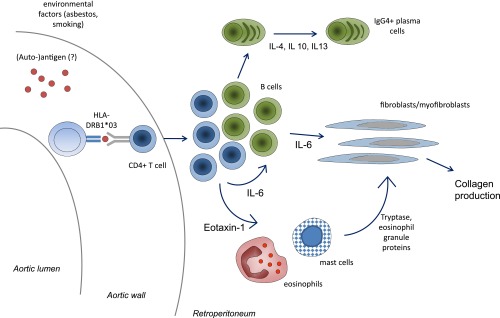
Figure 2.
Immunopathogenetic mechanisms of idiopathic RPF. Susceptibility to idiopathic RPF is conferred by exposure to environmental agents (asbestos, smoking) and by genetic factors such as HLA class II alleles (HLA‑DRB1*03). The presence of a restricted HLA class II repertoire makes it likely that the disease is antigen-driven, although the triggering antigens are as yet unknown. Antigen-presenting cells present such hypothetical antigens to CD4+ cells within the aortic wall or the surrounding retroperitoneum. CD4+ T cells expand, secrete IL‑6, which is able to activate B cells and fibroblasts. CD4+ T cells also secrete Th2 cytokines such as IL‑4, IL‑10 and IL‑13, which drive B-cell expansion and maturation into plasma cells, and may lead to preferential expansion of IgG4-producing plasma cells. Lymphoid cells also secrete eotaxin‑1, which drives recruitment of eosinophils and mast cells, whose products are also able to activate fibroblasts. Once activated, fibroblasts mature into myofibroblasts and secrete collagen. This pathogenetic hypothesis and the resulting cartoon have been generated on the basis of the available evidence on the immunopathogenetic mechanisms of the disease. See text for further details.
Diagnosis
Imaging Studies and Role of Biopsy
Idiopathic RPF is usually diagnosed by computed tomography (CT) or magnetic resonance imaging (MRI). On CT, it appears as a homogeneous plaque surrounding the anterolateral sides of the abdominal aorta and encircling the common iliac arteries (Figure 3); medial ureteral deviation and/or obstruction and inferior vena cava encasement are common. The tissue is muscle-isodense and has varying degrees of contrast enhancement.54,55 On MRI, its intensity is low in T1-weighted images and variable (high in active stages) in T2-weighted images. Contrast enhancement and diffusion coefficient values are useful in differentiating active and inactive lesions.56 When RPF has a bulky appearance, inhomogeneous intensity on MRI, extends above the origin of the renal arteries, or tends to displace the aorta anteriorly, it is more likely to be malignant. Also, malignant RPF causes medial ureteral deviation less frequently than idiopathic RPF.57
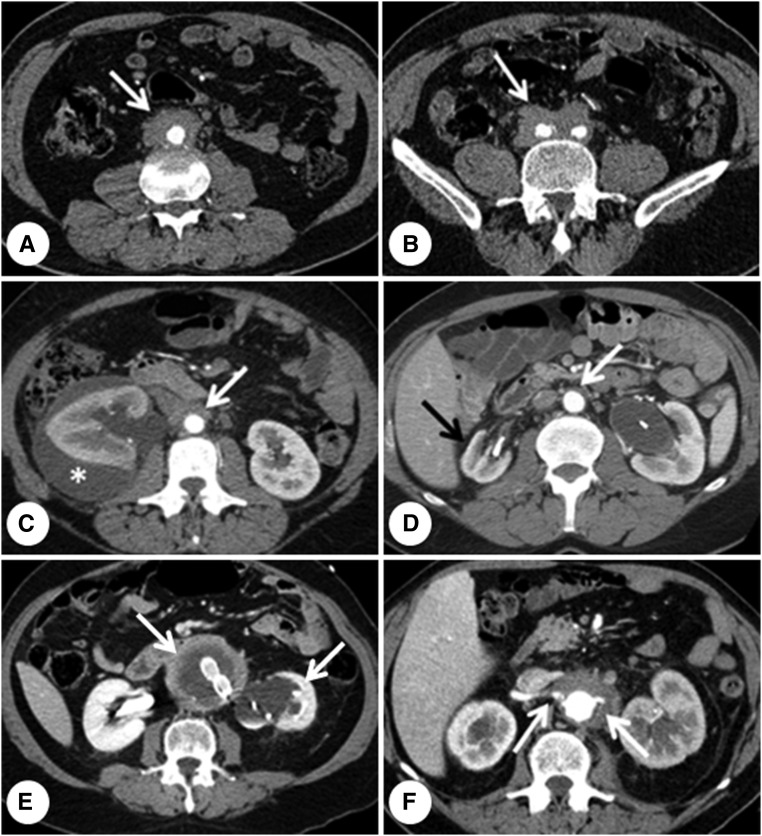
Figure 3.
Computed tomography findings in idiopathic RPF. (A, B) Typical idiopathic RPF that develops around the anterior and lateral sides of the abdominal aorta (A, arrow) and both common iliac arteries (B, arrow). (C) A rare complication of idiopathic RPF, that is a perirenal urinoma (asterisk) due to severe right-hand ureteral obstruction; the arrow indicates the periaortic tissue. (D) In a patient with periaortic (white arrow) and peri-iliac (not shown) RPF, marked hypoplasia of the right kidney (black arrow) as well as severe left-hand hydronephrosis. Indwelling ureteral stents can be seen bilaterally in the renal pelvis. (E) Typical case of perianeurysmal RPF, where the periaortic tissue surrounds an aneurysmal aorta (left-hand arrow) and causes hydronephrosis of the left kidney (arrow), which appears atrophic. Endovascular aorto-iliac prosthesis can be seen in the aortic lumen. (F) Periaortic idiopathic RPF encasing the origin of both renal arteries (arrows).
18F‑Fluorodeoxyglucose (18F‑FDG) positron emission tomography (PET) has emerged as a useful tool for the assessment of RPF activity (Figure 4);27,58,59 this technique also detects the metabolic activity of post-treatment residual disease and thus guides subsequent therapy.60 Notably, 18F‑FDG PET allows whole-body imaging and can help identify extra-retroperitoneal lesions to which RPF may be associated (e.g., thoracic periaortitis, IgG4‑RD) or secondary lesions (e.g., malignancies).7,61 However, 18F‑FDG PET has little diagnostic utility because many infectious, inflammatory, or neoplastic lesions also accumulate 18F‑FDG.
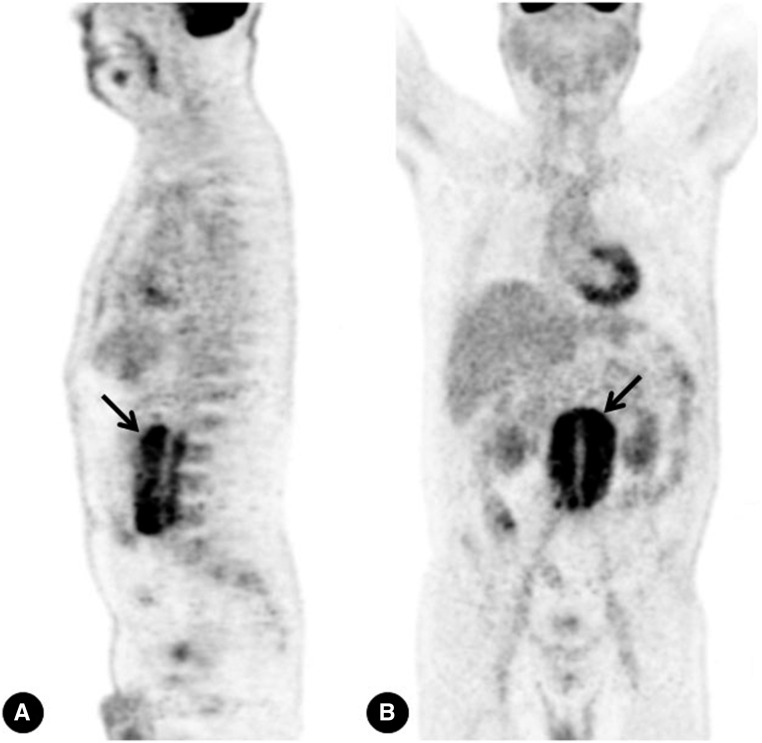
Figure 4.
PET findings in idiopathic RPF. 18F‑FDG PET scans in a patient with idiopathic RPF show intense accumulation of 18F‑FDG around the abdominal aorta (arrows) (A, sagittal view, B, coronal view).
Although no guidelines exist, retroperitoneal biopsy is usually performed (via open, laparoscopic, or CT-guided approaches) in cases with atypical localization (e.g., periureteral, perirenal),54,62,63 or with clinical or imaging findings consistent with neoplastic RPF.57 The percentage of biopsy-proven cases varies widely among series, with a range of 24%–77%.4,5,64
Differential Diagnosis
Malignancies and infections are the major challenges for the diagnosis of RPF. Malignancies that can mimic RPF on CT/MRI include retroperitoneal lymphomas and sarcomas, and retroperitoneal metastases from various types of carcinomas.54,55 Carcinoids can cause RPF– through as yet unclear mechanisms– even without metastasizing to the retroperitoneum.65 Among infections, tuberculosis should always be considered: it can spread into the retroperitoneum from neighboring foci or be disease-triggering when located distantly.66 Pelvic actinomycosis may mimic pelvic RPF and should be suspected particularly in women with a history of intrauterine device use.67
RPF has traditionally been linked to the use of drugs, particularly ergot alkaloids (e.g., methysergide, ergotamine) and dopamine agonists (e.g., pergolide); intriguingly, recent reports described RPF developing during anti-TNFα therapy for rheumatoid arthritis.68 Radiotherapy, major abdominal surgery, and trauma are uncommon causes of RPF. RPF also develops in Erdheim–Chester disease, a non-Langerhans histiocytosis that – unlike idiopathic RPF – tends to infiltrate the perirenal space.69 Finally, Takayasu and giant-cell arteritis can cause diffuse aortic thickening and thus mimic RPF, but they lack retroperitoneal diffusion and ureteral involvement.7
Treatment and Outcome
The first goal of treatment is the relief of ureteral obstruction. Surgical ureterolysis with intraperitonealization and omental wrapping of the ureters is no longer the first-line approach, and conservative procedures (e.g., double-J stent or nephrostomy placement) followed by medical therapy are preferred.70 Ureteral stenting allows better quality of life than does nephrostomy and is usually successful; however, stents and nephrostomies have comparable complication rates (e.g., infection, obstruction).71 Although no guidelines exist, when ureteral obstruction is mild and there is no kidney function impairment, it seems advisable to start medical therapy without urinary drainage.
Glucocorticoids are the first-line therapy, with initial doses of 0.75–1 mg/kg per day of prednisone gradually tapered to 5–7.5 mg/day within 6–9 months.5,72 Remission generally indicates symptom and hydronephrosis resolution, together with acute-phase reactant normalization and radiographic regression. Remission rates after steroid therapy range between 75% and 95%;5,72 mean mass thickness reduction is around 50%.5 Glucocorticoids are rapidly effective, and most of the radiographic response is seen in the first weeks of treatment. However, they can also fail to induce mass regression, and chronic residual hydronephrosis may require surgical ureterolysis.
Tamoxifen, an anti-estrogen agent with potential antifibrotic activity, has been proposed as an alternative to glucocorticoids73 particularly in patients experiencing steroid-related toxicity or when there are contraindications to glucocorticoids.64,74 However, in a recent randomized controlled trial, an 8‑month treatment with tamoxifen was significantly less effective than a treatment with prednisone of equal duration in maintaining remission in patients treated with prednisone induction (1 mg/kg per day for 1 month).5 Therefore, to date, the efficacy of tamoxifen is not supported by controlled trials and its superiority to other agents is unproven.
Immunosuppressants have been used in combination with glucocorticoids; however, it is still debated whether they actually potentiate glucocorticoid efficacy or function as steroid-sparing agents. Mycophenolate mofetil is widely used, also given its good tolerability and lack of contraindications in patients with renal insufficiency.75,76 Cyclophosphamide has also been effectively used as initial therapy followed by maintenance with other immunosuppressants, but is currently not recommended as first-line therapy.77,78
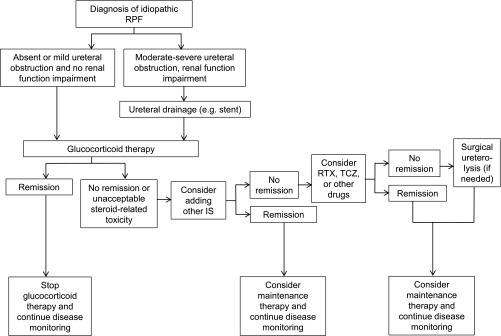
The patients who achieve remission must be carefully followed using laboratory examinations, periodic ultrasound (to monitor hydronephrosis and aneurysmal dilatation) and CT/MRI studies (able to accurately define size and morphologic changes of RPF) to allow early detection of relapses. Long-term maintenance therapy must be considered, particularly in patients with aggressive disease. Idiopathic RPF is indeed a chronic-relapsing disorder, with relapse rates of up to 72%.72 Importantly, relapsing patients often experience multiple relapses and are thus exposed to high cumulative glucocorticoid doses. In such cases, methotrexate has been successfully used as a steroid-sparing agent.79 Figure 5 shows a proposed therapeutic algorithm for idiopathic RPF.
Figure 5.
Proposed therapeutic algorithm for idiopathic RPF. IS, immunosuppressants (particularly mycophenolate mofetil, methotrexate, azathioprine, cyclophosphamide); RTX, rituximab; TCZ, tocilizumab.
Although rare, refractory cases also occur. In such patients, anecdotal reports demonstrated the efficacy of biologic agents, namely the anti–IL‑6 receptor tocilizumab,28 and rituximab,53 a B cell–depleting agent also effective in IgG4‑RD.80 Notably, rituximab was reported as efficacious in both IgG4+ and IgG4– cases, although studies comparing the response to treatment in these two subgroups are lacking.53,80
Despite its chronic-relapsing course, idiopathic RPF shows good patient and renal outcomes. Studies with long-term follow-up (median, 48–61 months) provide mortality rates of 3.3%–7.3%.4,5 Varying degrees of chronic renal insufficiency occur in up to 32% of the patients,4 but end-stage renal disease is exceedingly rare.
Conclusions
Idiopathic RPF is an idiopathic disease that sits in the spectrum of fibro-inflammatory disorders and should be viewed as a potentially systemic condition. Genetic and environmental agents confer disease susceptibility and represent active areas of research. Immune-mediated and autoimmune mechanisms play relevant pathogenic roles; new therapies targeting specific pathways will complement traditional immunosuppressive approaches.
Disclosures
None.
Acknowledgments
We are indebted to all of our colleagues that contribute to the clinical management and research on RPF. In particular, we would like to thank Alessandra Palmisano, Maria Letizia Urban, and Lucio Manenti for their help in the care of RPF patients, Stefania Ferretti for her role in the management of urologic complications of RPF patients, Maria Nicastro and Domenico Corradi for their support with histopathologic analysis and for providing histologic figures, Mary Pavia for contributing to data collection, Davide Martorana and Francesco Bonatti for performing genetic studies, Annibale Versari and Rocco Cobelli for imaging studies, Carlo Salvarani, Giacomo Emmi, and Nicolò Pipitone for their helpful clinical insights. A special thanks goes to our former chief Carlo Buzio, who initiated and fostered clinical research on RPF at our center.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Vaglio A, Salvarani C, Buzio C: Retroperitoneal fibrosis. Lancet 367: 241–251, 2006 [DOI] [PubMed] [Google Scholar]
- 2.van Bommel EF, Jansen I, Hendriksz TR, Aarnoudse AL: Idiopathic retroperitoneal fibrosis: prospective evaluation of incidence and clinicoradiologic presentation. Medicine (Baltimore) 88: 193–201, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Uibu T, Oksa P, Auvinen A, Honkanen E, Metsärinne K, Saha H, Uitti J, Roto P: Asbestos exposure as a risk factor for retroperitoneal fibrosis. Lancet 363: 1422–1426, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Kermani TA, Crowson CS, Achenbach SJ, Luthra HS: Idiopathic retroperitoneal fibrosis: a retrospective review of clinical presentation, treatment, and outcomes. Mayo Clin Proc 86: 297–303, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaglio A, Palmisano A, Alberici F, Maggiore U, Ferretti S, Cobelli R, Ferrozzi F, Corradi D, Salvarani C, Buzio C: Prednisone versus tamoxifen in patients with idiopathic retroperitoneal fibrosis: an open-label randomised controlled trial. Lancet 378: 338–346, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Corradi D, Maestri R, Palmisano A, Bosio S, Greco P, Manenti L, Ferretti S, Cobelli R, Moroni G, Dei Tos AP, Buzio C, Vaglio A: Idiopathic retroperitoneal fibrosis: clinicopathologic features and differential diagnosis. Kidney Int 72: 742–753, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Palmisano A, Urban ML, Corradi D, Cobelli R, Alberici F, Maritati F, Versari A, Pipitone N, Salvarani C, Buzio C, Vaglio A: Chronic periaortitis with thoracic aorta and epiaortic artery involvement: a systemic large vessel vasculitis? Rheumatology (Oxford) 54: 2004–2009, 2015 [DOI] [PubMed] [Google Scholar]
- 8.Parums DV: The spectrum of chronic periaortitis. Histopathology 16: 423–431, 1990 [DOI] [PubMed] [Google Scholar]
- 9.Parums DV, Chadwick DR, Mitchinson MJ: The localisation of immunoglobulin in chronic periaortitis. Atherosclerosis 61: 117–123, 1986 [DOI] [PubMed] [Google Scholar]
- 10.Vaglio A, Pipitone N, Salvarani C: Chronic periaortitis: a large-vessel vasculitis? Curr Opin Rheumatol 23: 1–6, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Khosroshahi A, Carruthers MN, Stone JH, Shinagare S, Sainani N, Hasserjian RP, Deshpande V: Rethinking Ormond’s disease: “idiopathic” retroperitoneal fibrosis in the era of IgG4-related disease. Medicine (Baltimore) 92: 82–91, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahajan VS, Mattoo H, Deshpande V, Pillai SS, Stone JH: IgG4-related disease. Annu Rev Pathol 9: 315–347, 2014 [DOI] [PubMed] [Google Scholar]
- 13.Clevenger JA, Wang M, MacLennan GT, Montironi R, Lopez-Beltran A, Cheng L: Evidence for clonal fibroblast proliferation and autoimmune process in idiopathic retroperitoneal fibrosis. Hum Pathol 43: 1875–1880, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Weyand CM, Kurtin PJ, Goronzy JJ: Ectopic lymphoid organogenesis: a fast track for autoimmunity. Am J Pathol 159: 787–793, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zen Y, Onodera M, Inoue D, Kitao A, Matsui O, Nohara T, Namiki M, Kasashima S, Kawashima A, Matsumoto Y, Katayanagi K, Murata T, Ishizawa S, Hosaka N, Kuriki K, Nakanuma Y: Retroperitoneal fibrosis: a clinicopathologic study with respect to immunoglobulin G4. Am J Surg Pathol 33: 1833–1839, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Mangieri D, Corradi D, Martorana D, Malerba G, Palmisano A, Libri I, Bartoli V, Carnevali ML, Goldoni M, Govoni P, Alinovi R, Buzio C, Vaglio A: Eotaxin/CCL11 in idiopathic retroperitoneal fibrosis. Nephrol Dial Transplant 27: 3875–3884, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Ramshaw AL, Parums DV: The distribution of adhesion molecules in chronic periaortitis. Histopathology 24: 23–32, 1994 [DOI] [PubMed] [Google Scholar]
- 18.Ramshaw AL, Roskell DE, Parums DV: Cytokine gene expression in aortic adventitial inflammation associated with advanced atherosclerosis (chronic periaortitis). J Clin Pathol 47: 721–727, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scheel PJ Jr, Feeley N: Retroperitoneal fibrosis: the clinical, laboratory, and radiographic presentation. Medicine (Baltimore) 88: 202–207, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Kottra JJ, Dunnick NR: Retroperitoneal fibrosis. Radiol Clin North Am 34: 1259–1275, 1996 [PubMed] [Google Scholar]
- 21.Palmisano A, Cobelli R, Buzio C, Vaglio A: Peri-renal collateral circles. Urology 74: 292–293, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Frech A, Gratl A, Fraedrich G, Glodny B, Klocker J: Periaortitis as a rare complication after endovascular aneurysm repair. Circulation 131: 1459–1461, 2015 [DOI] [PubMed] [Google Scholar]
- 23.Goldoni M, Bonini S, Urban ML, Palmisano A, De Palma G, Galletti E, Coggiola M, Buzio C, Mutti A, Vaglio A: Asbestos and smoking as risk factors for idiopathic retroperitoneal fibrosis: a case-control study. Ann Intern Med 161: 181–188, 2014 [DOI] [PubMed] [Google Scholar]
- 24.Salvarani C, Calamia KT, Matteson EL, Hunder GG, Pipitone N, Miller DV, Warrington KJ: Vasculitis of the gastrointestinal tract in chronic periaortitis. Medicine (Baltimore) 90: 28–39, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Magrey MN, Husni ME, Kushner I, Calabrese LH: Do acute-phase reactants predict response to glucocorticoid therapy in retroperitoneal fibrosis? Arthritis Rheum 61: 674–679, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Pelkmans LG, Aarnoudse AJ, Hendriksz TR, van Bommel EF: Value of acute-phase reactants in monitoring disease activity and treatment response in idiopathic retroperitoneal fibrosis. Nephrol Dial Transplant 27: 2819–2825, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Vaglio A, Versari A, Fraternali A, Ferrozzi F, Salvarani C, Buzio C: (18)F-fluorodeoxyglucose positron emission tomography in the diagnosis and followup of idiopathic retroperitoneal fibrosis. Arthritis Rheum 53: 122–125, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Vaglio A, Catanoso MG, Spaggiari L, Magnani L, Pipitone N, Macchioni P, Pulsatelli L, Nicastro M, Becchi G, Corradi D, Versari A, Boiardi L, Salvarani C: Interleukin-6 as an inflammatory mediator and target of therapy in chronic periaortitis. Arthritis Rheum 65: 2469–2475, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Kollert F, Binder M, Probst C, Uhl M, Zirlik A, Kayser G, Voll RE, Peter HH, Zissel G, Prasse A, Warnatz K: CCL18 -- potential biomarker of fibroinflammatory activity in chronic periaortitis. J Rheumatol 39: 1407–1412, 2012 [DOI] [PubMed] [Google Scholar]
- 30.Ceresini G, Urban ML, Corradi D, Lauretani F, Marina M, Usberti E, Palmisano A, Buzio C, Vaglio A: Association between idiopathic retroperitoneal fibrosis and autoimmune thyroiditis: a case-control study. Autoimmun Rev 14: 16–22, 2015 [DOI] [PubMed] [Google Scholar]
- 31.Fatourechi MM, Hay ID, McIver B, Sebo TJ, Fatourechi V: Invasive fibrous thyroiditis (Riedel thyroiditis): the Mayo Clinic experience, 1976-2008. Thyroid 21: 765–772, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Vaglio A, Palmisano A, Ferretti S, Alberici F, Casazza I, Salvarani C, Buzio C: Peripheral inflammatory arthritis in patients with chronic periaortitis: report of five cases and review of the literature. Rheumatology (Oxford) 47: 315–318, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Afeltra A, Gentilucci UV, Rabitti C, Amoroso A, Caricato M, Vadacca M, Valeri S, Zardi EM, Coppola R, Picardi A: Retroperitoneal fibrosis and ankylosing spondylitis: which links? Semin Arthritis Rheum 35: 43–48, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Vaglio A, Manenti L, Allegri L, Ferrozzi F, Corradi D, Buzio C: ANCA-positive periaortic vasculitis: does it fall within the spectrum of vasculitis? J Intern Med 251: 268–271, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Blockmans D, Baeyens H, Van Loon R, Lauwers G, Bobbaers H: Periaortitis and aortic dissection due to Wegener’s granulomatosis. Clin Rheumatol 19: 161–164, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Fujii K, Hidaka Y: Churg-Strauss syndrome complicated by chronic periaortitis: a case report and review of the literature. Intern Med 51: 109–112, 2012 [DOI] [PubMed] [Google Scholar]
- 37.Demko TM, Diamond JR, Groff J: Obstructive nephropathy as a result of retroperitoneal fibrosis: a review of its pathogenesis and associations. J Am Soc Nephrol 8: 684–688, 1997 [PubMed] [Google Scholar]
- 38.Famularo G, Palmisano A, Afeltra A, Buzzulini F, Versari A, Minisola G, Vaglio A: Retroperitoneal fibrosis associated with psoriasis: a case series. Scand J Rheumatol 38: 68–69, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Palmisano A, Corradi D, Carnevali ML, Alberici F, Silini EM, Gatti R, Allegri L, Buzio C, Vaglio A: Chronic periaortitis associated with membranous nephropathy: clues to common pathogenetic mechanisms. Clin Nephrol 74: 485–490, 2010 [DOI] [PubMed] [Google Scholar]
- 40.Moroni G, Faricciotti A, Cappelletti M, Ponticelli C: Retroperitoneal fibrosis and membranous nephropathy. Improvement of both diseases after treatment with steroids and immunosuppressive agents. Nephrol Dial Transplant 14: 1303–1305, 1999 [DOI] [PubMed] [Google Scholar]
- 41.Prunotto M, Carnevali ML, Candiano G, Murtas C, Bruschi M, Corradini E, Trivelli A, Magnasco A, Petretto A, Santucci L, Mattei S, Gatti R, Scolari F, Kador P, Allegri L, Ghiggeri GM: Autoimmunity in membranous nephropathy targets aldose reductase and SOD2. J Am Soc Nephrol 21: 507–519, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buelli S, Perico L, Galbusera M, Abbate M, Morigi M, Novelli R, Gagliardini E, Tentori C, Rottoli D, Sabadini E, Saito T, Kawano M, Saeki T, Zoja C, Remuzzi G, Benigni A: Mitochondrial-dependent autoimmunity in membranous nephropathy of IgG4-related disease. EBioMedicine 2: 456–466, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cravedi P, Abbate M, Gagliardini E, Galbusera M, Buelli S, Sabadini E, Marasà M, Beck LH Jr, Salant DJ, Benigni A, D’Agati V, Remuzzi G: Membranous nephropathy associated with IgG4-related disease. Am J Kidney Dis 58: 272–275, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Alexander MP, Larsen CP, Gibson IW, Nasr SH, Sethi S, Fidler ME, Raissian Y, Takahashi N, Chari S, Smyrk TC, Cornell LD: Membranous glomerulonephritis is a manifestation of IgG4-related disease. Kidney Int 83: 455–462, 2013 [DOI] [PubMed] [Google Scholar]
- 45.Khosroshahi A, Ayalon R, Beck LH Jr, Salant DJ, Bloch DB, Stone JH: IgG4-related disease is not associated with antibody to the phospholipase A2 receptor. Int J Rheumatol 2012: 139409, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Szarf G, Bluemke DA: Case 83: multifocal fibrosclerosis with mediastinal-retroperitoneal involvement. Radiology 235: 829–832, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Stone JH, Zen Y, Deshpande V: IgG4-related disease. N Engl J Med 366: 539–551, 2012 [DOI] [PubMed] [Google Scholar]
- 48.Parums DV, Brown DL, Mitchinson MJ: Serum antibodies to oxidized low-density lipoprotein and ceroid in chronic periaortitis. Arch Pathol Lab Med 114: 383–387, 1990 [PubMed] [Google Scholar]
- 49.Uibu T, Vanhala E, Sajantila A, Lunetta P, Mäkelä-Bengs P, Goebeler S, Jäntti M, Tossavainen A: Asbestos fibers in para-aortic and mesenteric lymph nodes. Am J Ind Med 52: 464–470, 2009 [DOI] [PubMed] [Google Scholar]
- 50.Martorana D, Vaglio A, Greco P, Zanetti A, Moroni G, Salvarani C, Savi M, Buzio C, Neri TM: Chronic periaortitis and HLA-DRB1*03: another clue to an autoimmune origin. Arthritis Rheum 55: 126–130, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Boiardi L, Vaglio A, Nicoli D, Farnetti E, Palmisano A, Pipitone N, Maritati F, Casali B, Martorana D, Moroni G, Gallelli B, Buzio C, Salvarani C: CC chemokine receptor 5 polymorphism in chronic periaortitis. Rheumatology (Oxford) 50: 1025–1032, 2011 [DOI] [PubMed] [Google Scholar]
- 52.Zen Y, Fujii T, Harada K, Kawano M, Yamada K, Takahira M, Nakanuma Y: Th2 and regulatory immune reactions are increased in immunoglobin G4-related sclerosing pancreatitis and cholangitis. Hepatology 45: 1538–1546, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Maritati F, Corradi D, Versari A, Casali M, Urban ML, Buzio C, Vaglio A: Rituximab therapy for chronic periaortitis. Ann Rheum Dis 71: 1262–1264, 2012 [DOI] [PubMed] [Google Scholar]
- 54.Cronin CG, Lohan DG, Blake MA, Roche C, McCarthy P, Murphy JM: Retroperitoneal fibrosis: a review of clinical features and imaging findings. AJR Am J Roentgenol 191: 423–431, 2008 [DOI] [PubMed] [Google Scholar]
- 55.George V, Tammisetti VS, Surabhi VR, Shanbhogue AK: Chronic fibrosing conditions in abdominal imaging. Radiographics 33: 1053–1080, 2013 [DOI] [PubMed] [Google Scholar]
- 56.Bakir B, Yilmaz F, Turkay R, Ozel S, Bilgiç B, Velioglu A, Saka B, Salmaslioglu A: Role of diffusion-weighted MR imaging in the differentiation of benign retroperitoneal fibrosis from malignant neoplasm: preliminary study. Radiology 272: 438–445, 2014 [DOI] [PubMed] [Google Scholar]
- 57.Mirault T, Lambert M, Puech P, Argatu D, Renaud A, Duhamel A, Glovacki F, Villers A, Hachulla E, Biserte J, Hatron PY, Lemaitre L: Malignant retroperitoneal fibrosis: MRI characteristics in 50 patients. Medicine (Baltimore) 91: 242–250, 2012 [DOI] [PubMed] [Google Scholar]
- 58.Jansen I, Hendriksz TR, Han SH, Huiskes AW, van Bommel EF: (18)F-fluorodeoxyglucose position emission tomography (FDG-PET) for monitoring disease activity and treatment response in idiopathic retroperitoneal fibrosis. Eur J Intern Med 21: 216–221, 2010 [DOI] [PubMed] [Google Scholar]
- 59.Moroni G, Castellani M, Balzani A, Dore R, Bonelli N, Longhi S, Martinelli I, Messa P, Gerundini P: The value of (18)F‑FDG PET/CT in the assessment of active idiopathic retroperitoneal fibrosis. Eur J Nucl Med Mol Imaging 39: 1635–1642, 2012 [DOI] [PubMed] [Google Scholar]
- 60.Vaglio A, Greco P, Versari A, Filice A, Cobelli R, Manenti L, Salvarani C, Buzio C: Post-treatment residual tissue in idiopathic retroperitoneal fibrosis: active residual disease or silent “scar”? A study using 18F-fluorodeoxyglucose positron emission tomography. Clin Exp Rheumatol 23: 231–234, 2005 [PubMed] [Google Scholar]
- 61.Salvarani C, Pipitone N, Versari A, Vaglio A, Serafini D, Bajocchi G, Salvo D, Buzio C, Greco P, Boiardi L: Positron emission tomography (PET): evaluation of chronic periaortitis. Arthritis Rheum 53: 298–303, 2005 [DOI] [PubMed] [Google Scholar]
- 62.Abe H, Morikawa T, Araki A, Shima T, Nakatsu H, Fukayama M, Suzuki Y: IgG4-related periureteral fibrosis presenting as a unilateral ureteral mass. Pathol Res Pract 207: 712–714, 2011 [DOI] [PubMed] [Google Scholar]
- 63.Ellimoottil C, Hart S, Mehta V, Quek ML: Localized perirenal retroperitoneal fibrosis. Urology 81: e27–e28, 2013 [DOI] [PubMed] [Google Scholar]
- 64.van Bommel EF, Pelkmans LG, van Damme H, Hendriksz TR: Long-term safety and efficacy of a tamoxifen-based treatment strategy for idiopathic retroperitoneal fibrosis. Eur J Intern Med 24: 444–450, 2013 [DOI] [PubMed] [Google Scholar]
- 65.Pipitone N, Versari A, Vaglio A, Salvarani C: Role of 18F-fluorodeoxyglucose positron emission tomography in the workup of retroperitoneal fibrosis. Clin Exp Rheumatol 29[Suppl 64]: S72–S78, 2011 [PubMed] [Google Scholar]
- 66.Greco P, Vaglio A, Corradi D, Cobelli R, Zompatori M, Buzio C: Tuberculosis as a trigger of retroperitoneal fibrosis. Clin Infect Dis 41: e72–e75, 2005 [DOI] [PubMed] [Google Scholar]
- 67.Akhan SE, Dogan Y, Akhan S, Iyibozkurt AC, Topuz S, Yalcin O: Pelvic actinomycosis mimicking ovarian malignancy: three cases. Eur J Gynaecol Oncol 29: 294–297, 2008 [PubMed] [Google Scholar]
- 68.Couderc M, Mathieu S, Dubost JJ, Soubrier M: Retroperitoneal fibrosis during etanercept therapy for rheumatoid arthritis. J Rheumatol 40: 1931–1933, 2013 [DOI] [PubMed] [Google Scholar]
- 69.Diamond EL, Dagna L, Hyman DM, Cavalli G, Janku F, Estrada-Veras J, Ferrarini M, Abdel-Wahab O, Heaney ML, Scheel PJ, Feeley NK, Ferrero E, McClain KL, Vaglio A, Colby T, Arnaud L, Haroche J: Consensus guidelines for the diagnosis and clinical management of Erdheim-Chester disease. Blood 124: 483–492, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Scheel PJ Jr, Feeley N: Retroperitoneal fibrosis. Rheum Dis Clin North Am 39: 365–381, 2013 [DOI] [PubMed] [Google Scholar]
- 71.Mertens S, Zeegers AG, Wertheimer PA, Hendriksz TR, van Bommel EF: Efficacy and complications of urinary drainage procedures in idiopathic retroperitoneal fibrosis complicated by extrinsic ureteral obstruction. Int J Urol 21: 283–288, 2014 [DOI] [PubMed] [Google Scholar]
- 72.van Bommel EF, Siemes C, Hak LE, van der Veer SJ, Hendriksz TR: Long-term renal and patient outcome in idiopathic retroperitoneal fibrosis treated with prednisone. Am J Kidney Dis 49: 615–625, 2007 [DOI] [PubMed] [Google Scholar]
- 73.van Bommel EF, Hendriksz TR, Huiskes AW, Zeegers AG: Brief communication: tamoxifen therapy for nonmalignant retroperitoneal fibrosis. Ann Intern Med 144: 101–106, 2006 [DOI] [PubMed] [Google Scholar]
- 74.Brandt AS, Kamper L, Kukuk S, Haage P, Roth S: Tamoxifen monotherapy in the treatment of retroperitoneal fibrosis. Urol Int 93: 320–325, 2014 [DOI] [PubMed] [Google Scholar]
- 75.Jois RN, Kerrigan N, Scott DG: Mycophenolate mofetil for maintenance of remission in idiopathic retroperitoneal fibrosis. Rheumatology (Oxford) 46: 717–718, 2007 [DOI] [PubMed] [Google Scholar]
- 76.Scheel PJ Jr, Feeley N, Sozio SM: Combined prednisone and mycophenolate mofetil treatment for retroperitoneal fibrosis: a case series. Ann Intern Med 154: 31–36, 2011 [DOI] [PubMed] [Google Scholar]
- 77.Binder M, Uhl M, Wiech T, Kollert F, Thiel J, Sass JO, Walker UA, Peter HH, Warnatz K: Cyclophosphamide is a highly effective and safe induction therapy in chronic periaortitis: a long-term follow-up of 35 patients with chronic periaortitis. Ann Rheum Dis 71: 311–312, 2012 [DOI] [PubMed] [Google Scholar]
- 78.Marcolongo R, Tavolini IM, Laveder F, Busa M, Noventa F, Bassi P, Semenzato G: Immunosuppressive therapy for idiopathic retroperitoneal fibrosis: a retrospective analysis of 26 cases. Am J Med 116: 194–197, 2004 [DOI] [PubMed] [Google Scholar]
- 79.Alberici F, Palmisano A, Urban ML, Maritati F, Oliva E, Manenti L, Ferretti S, Cobelli R, Buzio C, Vaglio A: Methotrexate plus prednisone in patients with relapsing idiopathic retroperitoneal fibrosis. Ann Rheum Dis 72: 1584–1586, 2013 [DOI] [PubMed] [Google Scholar]
- 80.Carruthers MN, Topazian MD, Khosroshahi A, Witzig TE, Wallace ZS, Hart PA, Deshpande V, Smyrk TC, Chari S, Stone JH: Rituximab for IgG4-related disease: a prospective, open-label trial. Ann Rheum Dis 74: 1171–1177, 2015 [DOI] [PubMed] [Google Scholar]