Abstract
Proliferative GN with monoclonal IgG deposits is an increasingly recognized form of GN, but its relation to hematologic malignancy remains poorly understood. Filgrastim, an analog for granulocyte colony–stimulating factor produced by recombinant DNA technology, is frequently used to stimulate bone marrow release of hematopoietic progenitor cells in preparation for stem cell transplant. We report an exceptional case of proliferative GN with monoclonal IgG2λ deposits in a young man whose disease progressed slowly to CKD, which was followed by a preemptive kidney transplant. The patient developed recurrent GN in the allograft and clinically detectable plasma cell neoplasm 9 years after the first renal manifestations. Contemporaneous with filgrastim administration for stem cell mobilization, the patient’s slowly progressive GN underwent severe crescentic transformation, leading to rapidly progressive and irreversible allograft failure. This report explores the spectrum of GN with monoclonal IgG deposits and the pathophysiologic role of granulocyte colony–stimulating factor in exacerbation of preexisting GN.
Keywords: glomerulonephritis, kidney biopsy, paraprotein
Proliferative GN with monoclonal Ig deposits is characterized by glomerular deposition of a monoclonal Ig (IgG, IgM, or IgA), complement activation, and variable patterns of proliferation. The diagnosis relies mainly on immunofluorescence, which is typically performed on frozen samples. However, when monoclonal Igs are masked, it may be necessary to uncover antigenic sites by repeating the immunofluorescence studies on paraffin sections after pronase digestion.
Proliferative GN with monoclonal IgG deposits (PGNMID) is the most common subtype. PGNMID is also the best characterized of these disorders because of the commercial availability of antibodies to the IgG subclasses, which are useful to confirm the monoclonal nature of the deposits.1 Our understanding of the association of PGNMID with dysproteinemia and hematologic neoplasms is evolving.
Filgrastim is a recombinant human granulocyte colony–stimulating factor (rHuG-CSF) commonly used to stimulate bone marrow production in patients who are neutropenic and collect progenitor cells for hematopoietic stem cell transplantation (HSCT).2 Although its renal safety profile is favorable, there are rare reports of GN flare in patients who received filgrastim.3–6 We describe the clinical and pathologic findings in a kidney transplant recipient who developed dramatic crescentic transformation of recurrent IgG2λ PGNMID during filgrastim treatment.
Case Report
A 31-year-old white man presented to an outside hospital with nephrotic syndrome and low serum C3. Serologic workup was negative for anti-nuclear antibody, antistreptococcal antibodies, hepatitis B and C, ANCA, and antiglomerular basement membrane antibodies. A renal biopsy sent to our institution for second opinion showed membranoproliferative GN with segmental membranous features (Figure 1A). Congo red stain was negative for amyloid material. The immunofluorescence revealed granular to smudgy mesangial and capillary wall staining for IgG2 (3+), C3 (3+), and λ (3+), with negative staining for IgG1, IgG3, IgG4, and κ. Electron microscopy displayed granular and focally organized deposits forming random fibrils as well as lattice–like parallel arrays (mean diameter of approximately 17 nm) in mesangial, subendothelial, and segmental subepithelial locations. Together, the findings favored IgG2λ PGNMID. Serum protein immunofixation showed a monoclonal IgGλ spike, whereas a bone marrow sample showed rare apparently polyclonal B cells without evidence for plasma cell dyscrasia. Three years later, serum creatinine increased from 1.1 to 2.6 mg/dl. The patient was transferred to our institution, where he received sequential therapy with rituximab, cyclosporine, mycophenolate mofetil, tacrolimus, cyclophosphamide, and adrenocorticotropic hormone. Despite aggressive treatment, he had progressive deterioration of renal function and persistence of serum IgGλ spike.
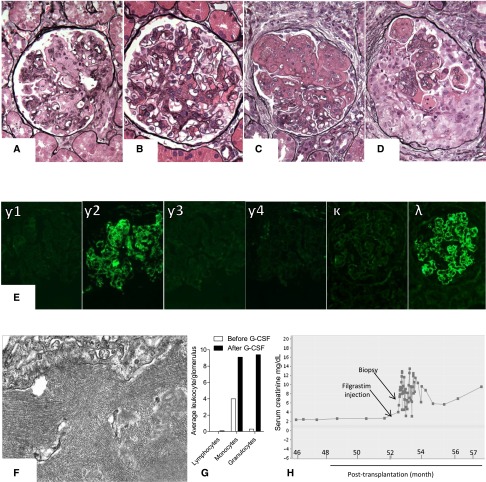
Figure 1.
Kidney biopsy findings in the native as well as the serial allograft biopsies. (A) Native kidney biopsy showing a glomerulus with expansion of the mesangial matrix and membranoproliferative features. (B) Second kidney allograft biopsy showing a glomerulus with largely mesangial proliferative features. (C) Third kidney allograft biopsy showing a glomerulus with expansion of the mesangial matrix and membranoproliferative features reminiscent of the pattern of injury observed in native kidney. (D) Fourth kidney allograft biopsy showing a glomerulus with circumferential cellular crescent (Jones methenamine silver). Original magnification, ×400. Notably, all allograft biopsies showed negative C4d staining along peritubular capillaries. Furthermore, circulating donor–specific antibodies, which were assessed only at the time of the third and fifth allograft biopsies using single–bead Luminex assay, were undetectable. (E) All native and allograft biopsies showed intense staining for γ2-heavy chain and λ-light chain with negative staining for γ1-, γ3-, γ4-heavy chains, and κ-light chain (fourth allograft biopsy; immunofluorescence). Original magnification, ×400. (F) Electron-dense deposits with 17-nm lattice–like parallel arrays (fourth allograft biopsy; electron microscopy). Original magnification, ×40,000. (G) Average number of intraglomerular lymphocytes, monocytes, and granulocytes assessed in the third and fourth allograft biopsies using immunohistochemical staining for CD3, CD68, and myeloperoxidase, respectively. (H) Change of serum creatinine in relation to filgrastim injection. G-CSF, granulocyte colony–stimulating factor.
Five years later, the patient received a preemptive, crossmatch–negative, four–antigen mismatch, living unrelated kidney allograft. After pretransplant plasmapheresis and thymoglobulin induction, he was maintained on tacrolimus and mycophenolic acid. One week post-transplantation, serum creatinine increased from 2.1 to 3.1 mg/dl in the setting of a hypotensive episode. The first allograft biopsy revealed focal cortical necrosis without rejection. Tacrolimus dose was decreased, and the patient maintained a stable serum creatinine of 1.6–2.2 mg/dl, despite the continuous presence of IgGλ serum spike. Two years post-transplantation, the patient developed low-grade proteinuria (400 mg/d). A second allograft biopsy showed mesangial proliferative GN (Figure 1B) with largely mesangial staining for monoclonal IgG2λ and 17-nm randomly oriented fibrils and lattice–like parallel arrays, consistent with recurrent PGNMID. Despite two 1000-mg doses of rituximab, proteinuria increased to 2.6 g/d. Over the next 2 years, the patient received two additional courses of rituximab (each of two 1000-mg doses), with partial stabilization of proteinuria (1.9–3.1 g/d) and serum creatinine (2.0–2.2 mg/dl). Four years post-transplantation, the patient was rebiopsied for increased serum creatinine (2.4 mg/dl), increased proteinuria (5.8 g/d), and new development of hypoalbuminemia (2.9 g/dl). This third allograft biopsy showed progression of the IgG2λ PGNMID manifested by membranoproliferative features without crescents (Figure 1C). The biopsy showed 20% tubulointerstitial scarring, mild to moderate vascular sclerosis, and mild patchy tubulointerstitial inflammation, which was attributed to the GN. A bone marrow biopsy, which was performed 4 months later (approximately 9 years after the first native kidney biopsy), disclosed a small plasma cell neoplasm comprising 5% of marrow cellularity with excess expression of λ. In preparation for autologous HSCT, the patient received 480 μg subcutaneous filgrastim injection twice daily for 5 days followed by stem cell collection. During treatment, absolute neutrophilic counts increased from 3000/mm2 to 37,000/mm2. Serum creatinine increased from 2.7 mg/dl before filgrastim administration to 7.4 mg/dl 1 day after completing treatment. The patient developed anuria requiring hemodialysis, and a fourth allograft biopsy was performed.
Kidney Allograft Biopsy (4)
Light microscopy sample contained 31 glomeruli, of which six were globally sclerotic. Predominantly cellular crescentic transformation was noted in 72% of the remaining 25 glomeruli (Figure 1D) with exuberant neutrophil infiltration. There was also diffuse mild to moderate interstitial inflammation with scattered but focally severe tubulitis. These changes were superimposed on 40% tubulointerstitial scarring and moderate vascular sclerosis without endothelialitis. Immunofluorescence revealed exclusively mesangial and glomerular capillary wall staining for IgG2 (2+), C3 (3+), and λ (2+) (Figure 1E). Electron microscopy showed mesangial, subendothelial, and segmentally subepithelial electron dense deposits focally assembled in 17- to 19-nm randomly oriented fibrils or lattice–like parallel arrays (Figure 1F). The above findings supported a crescentic transformation of the patient’s known IgG2λ GN. Comparative immunostains performed on the third and fourth allograft biopsies showed a 13-fold increase in intraglomerular neutrophils and a 2.3-fold increase in intraglomerular macrophages in the postfilgrastim biopsy without change in T cells (Figure 1G).
The patient was treated with two intravenous doses of 1000 mg methylprednisolone and four cycles of plasmapheresis. Melphalan conditioning (140 mg/m2) and autologous HSCT were performed 5 and 6 days postbiopsy, respectively. Fifteen days post-HSCT, a fifth allograft biopsy was obtained while the patient was still on hemodialysis, and it revealed minimal improvement in the GN. The 100-day post–HSCT bone marrow biopsy displayed a minute residual/persistent plasma cell neoplasm involving <0.1% of bone marrow cellularity. Eight months after the latest allograft biopsy, the patient remains on hemodialysis (Figure 1H).
Discussion
Knowledge of dysproteinemia–related renal disease is ever expanding. PGNMID is a recently described proliferative GN with monoclonal deposits restricted to a single IgG subclass and a single light–chain isotype.1 Although deposits of PGNMID usually have a primarily granular appearance by electron microscopy, they can show focal fibrils and/or lattice-like arrays in approximately 15% of patients.7 Our case was most consistent with PGNMID on the basis of the restricted glomerular staining for IgG2λ, exclusively glomerular distribution of the deposits, Congo red negativity, focal distribution of the fibrils, presence of codeposits of C3 with reduced serum C3, and lack of a demonstrable type 1 cryoglobulin.
Recent large studies have expanded our understanding of PGNMID7–9 and shown that this disease often occurs in older patients (mean age = 55 years old) and that only one third of patients with PGNMID have evidence of dysproteinemia, whereas even fewer (16%; range = 5%–35%) have detectable hematologic neoplasms.7–9 Crescentic transformation of PGNMID is rare but has been described after a second hit, such as reported in a patient after an upper respiratory infection.10 There are few reported patients with PGNMID recurrence in the allograft, and they often show the same histologic pattern as in the native kidney.11,12 Our case was unusual for the patient’s young age of onset, persistence of a monoclonal spike for years, and late detection of a plasma cell neoplasm (9 years after initial presentation and 2.5 years after the recurrence of PGNMID in the allograft). This case illustrates that PGNMID in young adults may precede clinical evidence of hematologic malignancy by up to a decade.
Filgrastim (rHuG-CSF) causes selective and transient increase in circulating neutrophils.13 In the transplant setting, filgrastim is regarded as a safe therapy that does not precipitate rejection.14 With regard to renal function, the effects of rHuG-CSF are usually restricted to transient elevation of serum creatinine and decreased urine output in a minority of patients, probably because of intrarenal leukostasis.13 In patients with severe congenital neutropenia, treatment with rHuG-CSF was rarely incriminated in triggering mesangial,3 membranoproliferative,4 and crescentic GN.5 During preparation for hematopoietic stem cell donation, one patient with asymptomatic microhematuria developed crescentic GN.6 In addition to increasing neutrophil counts, rHuG-CSF therapy enhances neutrophil activation,15 augments myeloid cell delivery to sites of inflammation,16 and induces endothelial cell activation15 (Figure 2). Taken together, the above findings suggest that filgrastim may trigger GN flare in a small subset of patients with potential genetic or acquired predisposition. This is supported by animal studies showing that granulocyte colony–stimulating factor is sufficient to trigger neutrophilic-induced GN in susceptible mice17 and increase the percentage of crescents in a murine model of passive anti-myeloperoxidase vasculitis.18
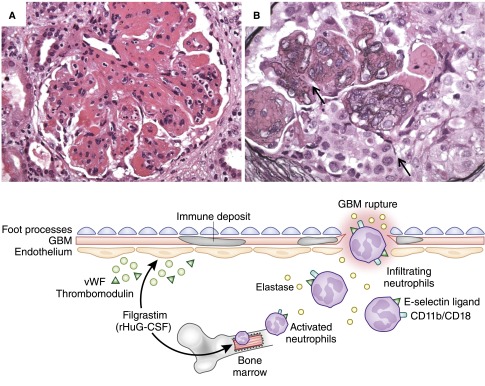
Figure 2.
The potential pathomechanisms of filgrastim–induced crescentic transformation. In addition to increasing neutrophil counts, filgrastim enhances neutrophil activation manifested by increasing CD11b/CD18 expression and elastase activity. It also induces endothelial activation via release of vWF and thrombomodulin and increases neutrophilic capability to bind to injured endothelium by enhancing neutrophil expression of E-selectin ligand. In the presence of another hit (e.g., preexisting immune complex GN), the localized deposition of Ig and complement in the glomeruli can attract the activated neutrophils to the glomerular microenvironment, leading to (A) glomerular neutrophilic infiltration (patient's fourth allograft biopsy; hematoxylin and eosin) and degranulation, which in turn, (B) promotes glomerular basement membrane (GBM) rupture and crescent formation (patient's fourth allograft biopsy; Jones methenamine silver). Original magnification, ×400 in A; ×600 in B.
Our patient had slowly progressive GN that probably made him prone to filgrastim–induced kidney injury via the sudden release of a large amount of activated neutrophils, which were attracted to the glomeruli because of preexisting deposits of IgG and complement (Figure 2). This scenario is supported by the strong temporal association between filgrastim treatment and the dramatic development of fulminant neutrophil–rich crescentic transformation of a longstanding slowly progressive GN that never exhibited cellular crescents before filgrastim treatment. In summary, this case strengthens the association of PGNMID with hematologic neoplasm and highlights the potential role of filgrastim in aggravating GN. We conclude that this agent should be used with caution in patients with preexisting GN.
Disclosures
None.
Acknowledgments
We thank the technicians of the Columbia Renal Pathology and Immunohistochemistry Laboratories for their valuable expertise.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Nasr SH, Markowitz GS, Stokes MB, Seshan SV, Valderrama E, Appel GB, Aucouturier P, D’Agati VD: Proliferative glomerulonephritis with monoclonal IgG deposits: A distinct entity mimicking immune-complex glomerulonephritis. Kidney Int 65: 85–96, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Deotare U, Al-Dawsari G, Couban S, Lipton JH: G-CSF-primed bone marrow as a source of stem cells for allografting: Revisiting the concept. Bone Marrow Transplant 50: 1150–1156, 2015 [DOI] [PubMed] [Google Scholar]
- 3.Bonilla MA, Dale D, Zeidler C, Last L, Reiter A, Ruggeiro M, Davis M, Koci B, Hammond W, Gillio A, Welte K: Long-term safety of treatment with recombinant human granulocyte colony-stimulating factor (r-metHuG-CSF) in patients with severe congenital neutropenias. Br J Haematol 88: 723–730, 1994 [DOI] [PubMed] [Google Scholar]
- 4.Magen D, Mandel H, Berant M, Ben-Izhak O, Zelikovic I: MPGN type I induced by granulocyte colony stimulating factor. Pediatr Nephrol 17: 370–372, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Sotomatsu M, Kanazawa T, Ogawa C, Watanabe T, Morikawa A: Complication of rapidly progressive glomerulonephritis in severe congenital neutropenia treated with long-term granulocyte colony-stimulating factor (filgrastim). Br J Haematol 110: 234–235, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Nasilowska-Adamska B, Perkowska-Ptasinska A, Tomaszewska A, Serwacka A, Marianska B: Acute glomerulonephritis in a donor as a side effect of allogeneic peripheral blood stem cell mobilization with granulocyte colony-stimulating factor. Int J Hematol 92: 765–768, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Nasr SH, Satoskar A, Markowitz GS, Valeri AM, Appel GB, Stokes MB, Nadasdy T, D’Agati VD: Proliferative glomerulonephritis with monoclonal IgG deposits. J Am Soc Nephrol 20: 2055–2064, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guiard E, Karras A, Plaisier E, Duong Van Huyen JP, Fakhouri F, Rougier JP, Noel LH, Callard P, Delahousse M, Ronco P: Patterns of noncryoglobulinemic glomerulonephritis with monoclonal Ig deposits: Correlation with IgG subclass and response to rituximab. Clin J Am Soc Nephrol 6: 1609–1616, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Bhutani G, Nasr SH, Said SM, Sethi S, Fervenza FC, Morice WG, Kurtin PJ, Buadi FK, Dingli D, Dispenzieri A, Gertz MA, Lacy MQ, Kapoor P, Kumar S, Kyle RA, Rajkumar SV, Leung N: Hematologic characteristics of proliferative glomerulonephritides with nonorganized monoclonal immunoglobulin deposits. Mayo Clin Proc 90: 587–596, 2015 [DOI] [PubMed] [Google Scholar]
- 10.Oshio M, Fujii T, Kusaura T, Nagahama K: Relapsing proliferative glomerulonephritis with monoclonal IgG deposits showing circumferential crescentic glomerulonephritis. Clin Kidney J 6: 635–638, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nasr SH, Sethi S, Cornell LD, Fidler ME, Boelkins M, Fervenza FC, Cosio FG, D’Agati VD: Proliferative glomerulonephritis with monoclonal IgG deposits recurs in the allograft. Clin J Am Soc Nephrol 6: 122–132, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Batal I, Bijol V, Schlossman RL, Rennke HG: Proliferative glomerulonephritis with monoclonal immunoglobulin deposits in a kidney allograft. Am J Kidney Dis 63: 318–323, 2014 [DOI] [PubMed] [Google Scholar]
- 13.D’Souza A, Jaiyesimi I, Trainor L, Venuturumili P: Granulocyte colony-stimulating factor administration: Adverse events. Transfus Med Rev 22: 280–290, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Peddi VR, Hariharan S, Schroeder TJ, First MR: Role of granulocyte colony stimulating factor (G-CSF) in reversing neutropenia in renal allograft recipients. Clin Transplant 10: 20–23, 1996 [PubMed] [Google Scholar]
- 15.Falanga A, Marchetti M, Evangelista V, Manarini S, Oldani E, Giovanelli S, Galbusera M, Cerletti C, Barbui T: Neutrophil activation and hemostatic changes in healthy donors receiving granulocyte colony-stimulating factor. Blood 93: 2506–2514, 1999 [PubMed] [Google Scholar]
- 16.Dagia NM, Gadhoum SZ, Knoblauch CA, Spencer JA, Zamiri P, Lin CP, Sackstein R: G-CSF induces E-selectin ligand expression on human myeloid cells. Nat Med 12: 1185–1190, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Kitching AR, Ru Huang X, Turner AL, Tipping PG, Dunn AR, Holdsworth SR: The requirement for granulocyte-macrophage colony-stimulating factor and granulocyte colony-stimulating factor in leukocyte-mediated immune glomerular injury. J Am Soc Nephrol 13: 350–358, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Freeley SJ, Coughlan AM, Popat RJ, Dunn-Walters DK, Robson MG: Granulocyte colony stimulating factor exacerbates antineutrophil cytoplasmic antibody vasculitis. Ann Rheum Dis 72: 1053–1058, 2013 [DOI] [PubMed] [Google Scholar]