Abstract
Background
Entecavir (ETV) has been shown to be safe and efficacious in randomized controlled trials in highly selected patients with hepatitis B virus (HBV) infection.
Aim
To determine the safety and effectiveness of ETV in “real-world” HBV patients in the United States (US).
Methods
Treatment-naïve HBV patients ≥ 18 years old who received ETV for ≥12 months between 2005 and 2013 were included in a retrospective, cohort study. Rates of ALT normalization, undetectable HBV DNA, HBeAg and HBsAg loss/seroconversion, adverse events (AE) and clinical outcomes were evaluated.
Results
Of 841 patients, 658 [65% male, 83% Asian; median age 47 years] met the inclusion criteria. 36% were HBeAg+ and 9.3% cirrhotic. 89% had abnormal ALT. Baseline median HBV DNA was 5.8 log 10 IU/mL. Median duration of ETV treatment was 4 years.
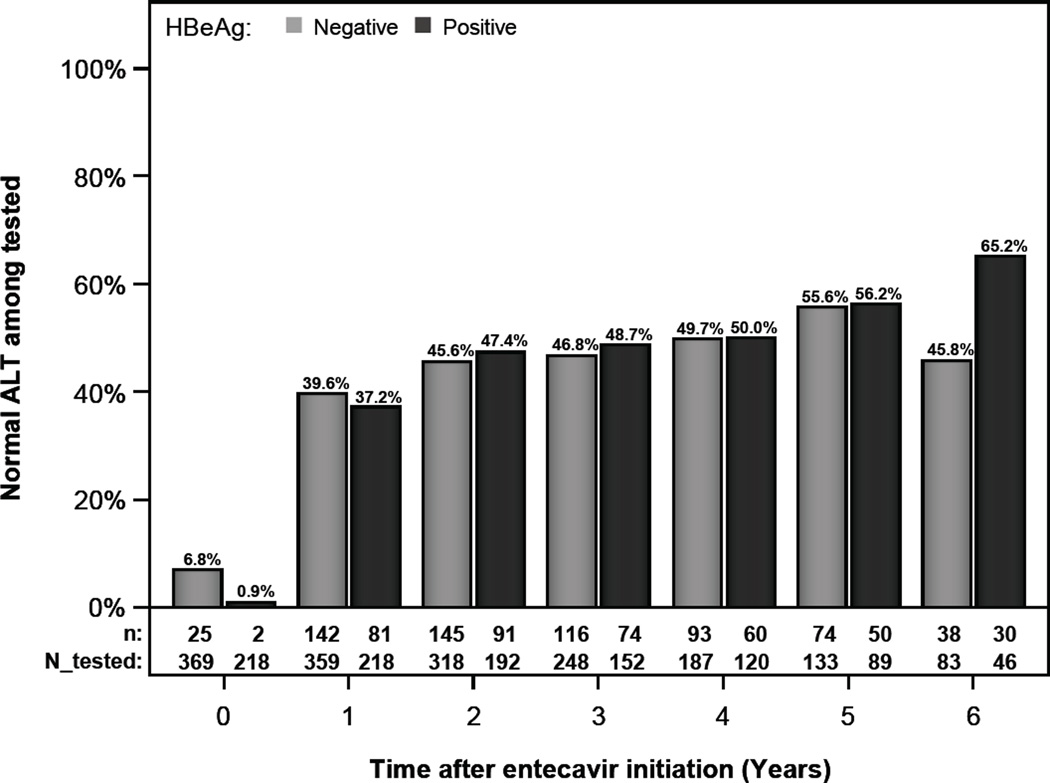
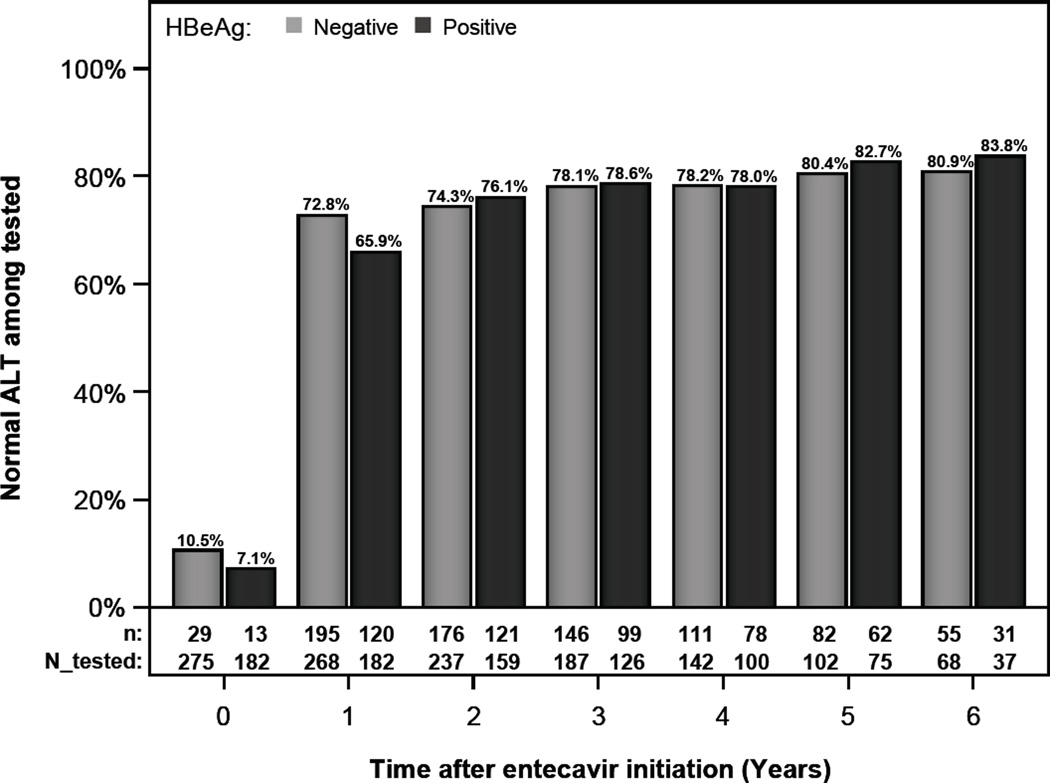
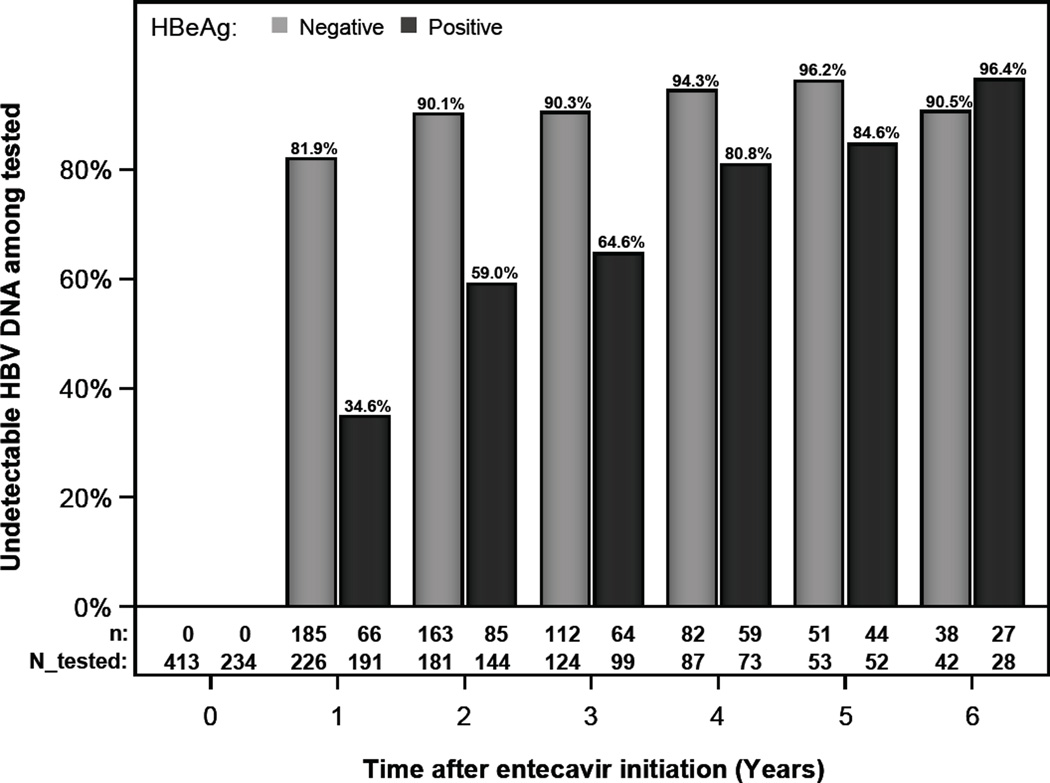
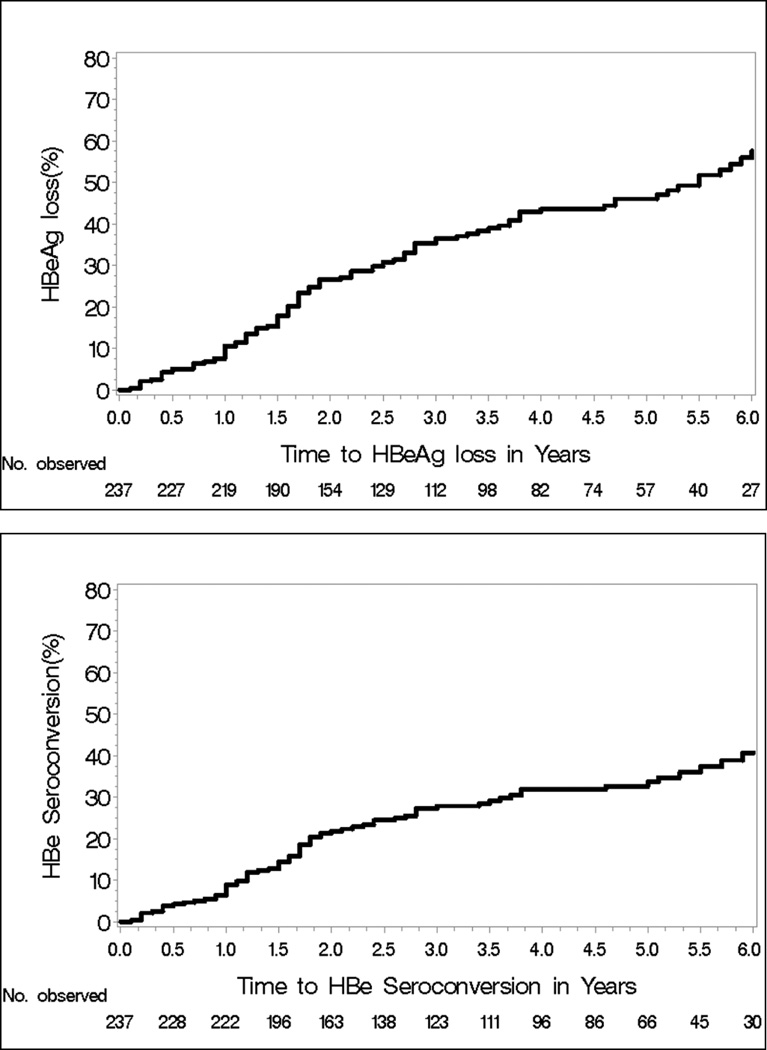
Rates of ALT normalization at 1, 3, and 5 years were 37.2%, 48.7%, and 56.2% in HBeAg+ and 39.6%, 46.8 %, and 55.6% in HBeAg- patients. HBV DNA was undetectable at 1, 3, and 5 years in 34.6%, 64.7%, and 84.6% in HBeAg+ patients, and 81.9%, 90.3%, and 96.2% in HBeAg- patients. 5 year cumulative probability of HBeAg loss and seroconversion was 46% and 33.7% and HBsAg loss was 4.6%. ETV was discontinued due to adverse events in 1.2% of patients. Hepatic decompensation occurred in 0.8%, liver cancer in 2.7%, and death in 0.6%.
Conclusion
Entecavir treatment was safe in a large cohort of US patients, but ALT normalization and HBV DNA suppression rates were lower than previously reported in clinical trials.
Keywords: hepatitis B, entecavir, real-world, HBeAg seroconversion, HBV DNA suppression
Introduction
Entecavir (ETV) is a cyclopentyl guanosine analogue, with potent activity against the hepatitis B virus (HBV) DNA polymerase. In 2005, the United States (US) Food and Drug Administration (FDA) approved ETV for the treatment of HBV based on randomized controlled trials demonstrating efficacy and safety in hepatitis B e antigen (HBeAg)-positive and HBeAg-negative patients that met entry criteria for those trials. These trials showed HBV DNA undetectable rates of 67%, alanine aminotransferase (ALT) normalization rates of 68%, and HBeAg seroconversion rates of 21% after 1 year of treatment in HBeAg-positive patients. (1) For HBeAg-negative patients, HBV DNA undetectable rates were 90% and ALT normalization rates were 78% at the end of 1 year of ETV treatment. (2) Subsequent rollover studies provided further follow-up data with undetectable HBV DNA in 94%, ALT normalization in 80%, an additional HBeAg seroconversion in 23%, and hepatitis B surface antigen (HBsAg) loss in 1.4% of HBeAg-positive patients by year 5. (3) Follow up data beyond year 1 for HBeAg-negative patients are less clear, as many of these patients had gaps in ETV treatment after year 1. (4) Overall, these studies confirmed long-term safety and a low rate of genotypic antiviral resistance among nucleoside naïve patients of 1.2% at 6 years. (3, 5, 6)
Due to trial design, the majority of subjects in these Phase III trials had discontinuation or interruption of ETV after the first year. (1–3) Thus, the trial design does not allow assessment of the outcomes of continuous treatment with ETV at the approved dose of 0.5 mg daily. The need for confirmation of the efficacy and safety of continuous ETV treatment at the standard dose, along with growing awareness regarding the distinction between clinical trial efficacy and “real-world” effectiveness led to studies of ETV treatment outcomes in clinical practice. (7–9) There have been several reports, mainly from Asia, showing variable effectiveness among patients treated with ETV in clinical practice attributed to enrollment of a more heterogeneous population as well as the challenges of supporting patients’ compliance to long-term treatment in the “real-world”. (10–17) However, there are limited data regarding the effectiveness and safety of ETV in the US. (18–19) We aimed to determine the safety and effectiveness of ETV in “real-world” practice settings in the US.
Materials & Methods
Study design and patient population
The ENtecavir Utilization, Management, and Efficacy in the United States: A MulTi-cEnter Study (ENUMERATE) is an observational, retrospective, multicenter cohort study of treatment-naïve, chronic HBV patients who received at least 12 months of ETV between April 2005 and April 2013 in 26 community and university clinical centers throughout the US. ENUMERATE was conducted in partnership with the advisors from the Asian Health Foundation (AHF), a non-profit organization dedicated to improving the health of Asian Americans & Pacific Islanders with a focus on viral hepatitis. The AHF advisors are comprised of hepatologists, gastroenterologists, and internists with clinical and research interest in HBV, practicing in 16 states. All authors had access to the study data and have reviewed and approved the final manuscript.
The study protocol and case report forms were approved by the Institutional Review Board (IRB) at each center. A central IRB was utilized for centers that did not have their own institutional IRB. Patients were identified at each center by searching internal databases and medical records. Demographic, clinical and laboratory data, HBV treatment history, and ETV start date, dosing and duration were recorded. All laboratory testing was obtained at the respective local laboratories for each center. A web based electronic case report form was designed for this study. Data collection was carried out between January 2013 and January 2014. An independent data monitor reviewed the submitted electronic case report forms for data quality, completeness, and accuracy.
Patients were included in the study if they were ≥ 18 years old, had chronic HBV infection, were HBV treatment naïve (no previous nucleos(t)ide analogues or interferon), and had been on ETV treatment for at least 12 months with a minimum of two sets of laboratory results that included HBV DNA and ALT after ETV initiation. Patients were excluded from the study if they did not have a baseline HBeAg result or a baseline ALT and HBV DNA (within 180 days prior to initiating ETV) but were not excluded based on a specific ALT or HBV DNA cutoff value criteria. Patients were also excluded if they were known to be co-infected with human immunodeficiency virus, hepatitis C virus, or hepatitis D virus, were pregnant or breastfeeding, had undergone solid organ transplantation, or were on chemotherapy for immunosuppressive therapy. Patients receiving combination therapy with another nucleot(s)ide analogue or interferon or were enrolled in other clinical trials for HBV were also excluded.
Baseline cirrhosis was determined based on liver histology, evidence of clinical decompensation (ascites, hepatic encephalopathy or variceal bleeding), and in the absence of histology or decompensation on radiological (nodular liver, intra-abdominal collaterals/varices of splenomegaly) or endoscopic (varices in the stomach or esophagus) findings.
Decision to initiate ETV treatment, starting dose, dose adjustment, and discontinuation of treatment were at the discretion of the investigators.
Assessment of treatment outcomes
Primary treatment endpoints included complete HBV DNA suppression rates (undetectable HBV DNA based on the lower limits of detection at each laboratory which varied between <10 IU/mL to <2000 copies/mL [~400 IU/mL]), ALT normalization, and HBeAg seroconversion. Two definitions of ALT normalization were utilized, the commonly used <40 U/L for both men and women, and the American Association for the Study of Liver Diseases (AASLD) HBV practice guideline threshold <30 U/L for men, <19 U/L for women, with ULN at each laboratory varying between 22–80 U/L. (20–21)
Secondary endpoints included adverse events (AE) leading to ETV dose reduction or discontinuation and clinical outcomes: cirrhosis (as defined for baseline cirrhosis), hepatic decompensation (defined as new development of ascites, hepatic encephalopathy, or variceal bleeding), liver transplantation, hepatocellular carcinoma (HCC) and death. HCC surveillance was conducted at each site at the discretion of the investigators. HBV resistance testing was not uniformly conducted in the study, but rates of virological breakthroughs (>1 log increase in HBV DNA levels from nadir) were noted. Renal function was assessed by estimating glomerular filtration rate (GFR) with the Modification of Diet in Renal Disease (MDRD) formula. Patients were followed from the initiation of ETV treatment to the end of data collection in January 2014.
Statistical analysis
Data analysis was performed using Version 9.4 of the SAS System (SAS Institute Inc., Cary, NC). For descriptive statistics of the baseline and follow-up data, mean with standard deviations or median (range) were calculated for continuous data and proportions (%) for categorical data. Between group comparisons were conducted by the student's t-test (or the Wilcoxon rank sum, if the distribution was not symmetric) for continuous variables and the chi-square test for categorical variables. Analyses were performed on subsets of patients with test results available at specific time intervals, and expressed as the number with response versus the number tested at each time point. Kaplan-Meier (KM) analysis was used to plot the cumulative probability of responses over time. Data analysis was performed for the entire cohort and also separately for the subgroup of patients recruited at community-based centers and those recruited at university-based centers. Statistical significance was assessed at the 0.05 level.
Results
Baseline clinical characteristics
Of 841 patients reviewed from 26 sites, a total of 658 met the inclusion criteria and were included in the study (Supplementary Figure 1). Ten study sites were community-based clinics and 16 were university-based. Table 1 shows the baseline patient characteristics: 64.9% were male, with a median age of 46.8 (18–83) years. The majority of the patients (83.3%) were Asian, 8.4% white, 4.6% black, and 3.8% were of other races. More than half of the patients (61.1%) were born outside the US, and 10.3% had a family history of liver cancer. At baseline, 237 (36%) were HBeAg-positive, 421 (64%) were HBeAg-negative. Baseline median ALT was 60 (6–3286) U/L. Baseline median HBV DNA was 7.4 log 10 IU/mL for HBeAg-positive and 5.2 log 10 IU/mL for HBeAg-negative patients. Cirrhosis was diagnosed in 61 (9.3%) patients: 15 based on liver biopsy and 46 based on radiological or endoscopic findings. At the time of ETV initiation, hepatic decompensation was present in 11 (1.7%) patients and 3 (0.5%) were listed for liver transplantation.
Table 1.
Baseline characteristics
| HBeAg Pos (n=237) | HBeAg Neg (n=421) | P value | |
|---|---|---|---|
| Age (yr) | 40.4 (18.2–80.2) | 50.1 (21.1–83.0) | <0.001 |
| Male, n(%) | 147 (62.0) | 280 (66.5) | 0.25 |
| Race, n(%) | 0.018 | ||
| Asian | 186 (78.5) | 362 (86.0) | |
| Black | 13 (5.5) | 17 (4.0) | |
| White | 30 (12.7) | 25 (5.9) | |
| Other | 8 (3.4) | 17 (4.0) | |
| Country of Birth, n(%) | 0.0001 | ||
| Outside US | 143 (60.3) | 259 (61.5) | |
| US | 38 (16.0) | 27 (6.4) | |
| Unknown | 56 (23.6) | 135 (32.1) | |
| Cirrhosis, n(%) | 23 (9.7) | 38 (9.0) | 0.25 |
| Hepatic Decompensation, n(%) | 3 (1.3) | 8 (1.9) | 0.75 |
| Listed for Liver Transplant, n(%) | 1 (0.4) | 2 (0.5) | 1 |
| Undetectable HBV DNA, n(%) | 3 (1.3) | 8 (1.9) | 0.75 |
| Normal ALT ( ALT < 30 for men and < 19 for women), n(%) | 19 (8.0) | 52 (12.4) | 0.085 |
| Normal ALT ( ALT < 40 for men and women), n(%) | 55 (23.2) | 146 (34.7) | 0.002 |
| HBV DNA (Log10 IU/ml) | 7.4 (2.0–10.2) | 5.2 (1.9–8.7) | <0.001 |
| ALT (U/L) | 71.0 (15.0–3286) | 55.0 (6.0–1783) | <0.001 |
| AST (U/L) | 46.0 (4.5–1334) | 39.0 (13.0–1385) | <0.01 |
| Albumin (g/dL) | 4.3 (2.7–5.1) | 4.4 (1.4–5.1) | <0.01 |
| Total Bilirubin (mg/dL) | 0.7 (0.0–13.4) | 0.7 (0.0–9.1) | 0.79 |
| Creatinine (mg/dL) | 0.9 (0.4–8.2) | 0.9 (0.5–13.4) | 0.67 |
| INR | 1.0 (0.9–2.5) | 1.1 (0.8–3.1) | 0.25 |
| Platelet Count (× 10^3/uL) | 203.0 (46.0–522.0) | 201.5 (43.0–1051) | 0.46 |
Starting dose of ETV was 0.5 mg/day in 584 (88.8%) patients, 1.0 mg/day in 69 (10.5%) patients, and <0.5 mg/day in 5 (0.8%) patients. Patients started at ETV 1.0 mg/day compared to those started at 0.5 mg/day had a statistically significantly higher rate of family history of HCC (18.8% vs. 9.4%), higher baseline HBV DNA (6.4 vs. 5.8 log 10 IU/mL), and lower baseline creatinine (0.8 vs. 0.9 mg/dL). There were no other statistically significant differences in baseline characteristics between these two ETV dose groups. Median duration of ETV treatment was 4 (1–8.3) years. The number of patients followed for 1, 2, 3, 4, and 5 years were 657 (99.9%), 579 (88%), 463 (70.4%), 370 (56.2%), and 265 (40.3%), respectively.
Approximately half the (52%) patients were enrolled at community-based sites. Differences in baseline characteristics of patients enrolled between university and community sites were notable for a higher proportion of patients with cirrhosis (14.2% vs. 4.7%), and hepatic decompensation (2.8% vs. 0.6%), and higher levels of ALT (65.5 vs. 51 U/L) at university sites as shown in Supplementary Table 1.
Primary outcomes
ALT normalization
For this analysis, only patients with elevated ALT at baseline were included. Among the patients tested at specific intervals, the proportions with normal ALT (defined as ALT<30 U/L in men and <19 U/L in women) at years 1, 3, and 5 were 37.2% (81/218), 48.7% (74/152), and 56.2% (50/89), respectively in HBeAg-positive patients and 39.6% (142/359), 46.8 % (116/248), and 55.6% (74/133), respectively in HBeAg-negative patients. (Figure 1a)
Figure 1.
a- ALT normalization by HBeAg status with normal ALT defined as < 30 U/L for men and < 19 U/L for women
b- ALT normalization by HBeAg status with normal ALT defined as < 40 U/L for both men and women
When normal ALT was defined as <40 U/L in both men and women, the proportions with normal ALT at years 1, 3, and 5 were 65.9% (120/182), 78.6% (99/126), and 82.7% (62/75), respectively in HBeAg-positive patients and 72.8% (195/268), 78.1% (146/187), and 80.4% (82/102), respectively in HBeAg-negative patients. (Figure 1b)
HBV DNA suppression
For this analysis, only patients with detectable HBV DNA at baseline were included. Among the patients tested at specific intervals, the proportion with undetectable DNA at years 1, 3, and 5 were 34.6% (66 /191), 64.6% (64/99) and 84.6% (44/52), respectively, in HBeAg-positive patients, and 81.9% (185/226), 90.3% (112/124) and 96.2% (51/53), respectively, in HBeAg-negative patients. (Figure 2) Comparisons of the 47 patients who failed to suppress HBV DNA by year 3 to those who suppressed HBV DNA showed that the former patients were significantly more likely to be HBeAg positive (74.5% vs. 36.4%) and to have higher HBV DNA levels (7.4 vs. 5.7 log 10 IU/mL) at baseline. There were no differences in HBV DNA suppression between patients receiving care in the community and university settings.
Figure 2.
HBV DNA undetectable rates by HBeAg status
HBeAg loss and seroconversion, and HBsAg loss
Among the 237 HBeAg-positive patients, 25 had HBeAg loss and 21 had HBeAg seroconversion by year 1 (8.8%); and 93 patients had HBeAg loss and 69 had HBeAg seroconversion by year 5 (29%). The cumulative probability of HBeAg loss at 1 and 5 years were 10.6% and 46%, respectively and of HBeAg seroconversion were 8.9% and 33.7%, respectively (Figure 3)
Figure 3.
Kaplan-Meier Cumulative Probability of HBeAg loss and HBeAg seroconversion
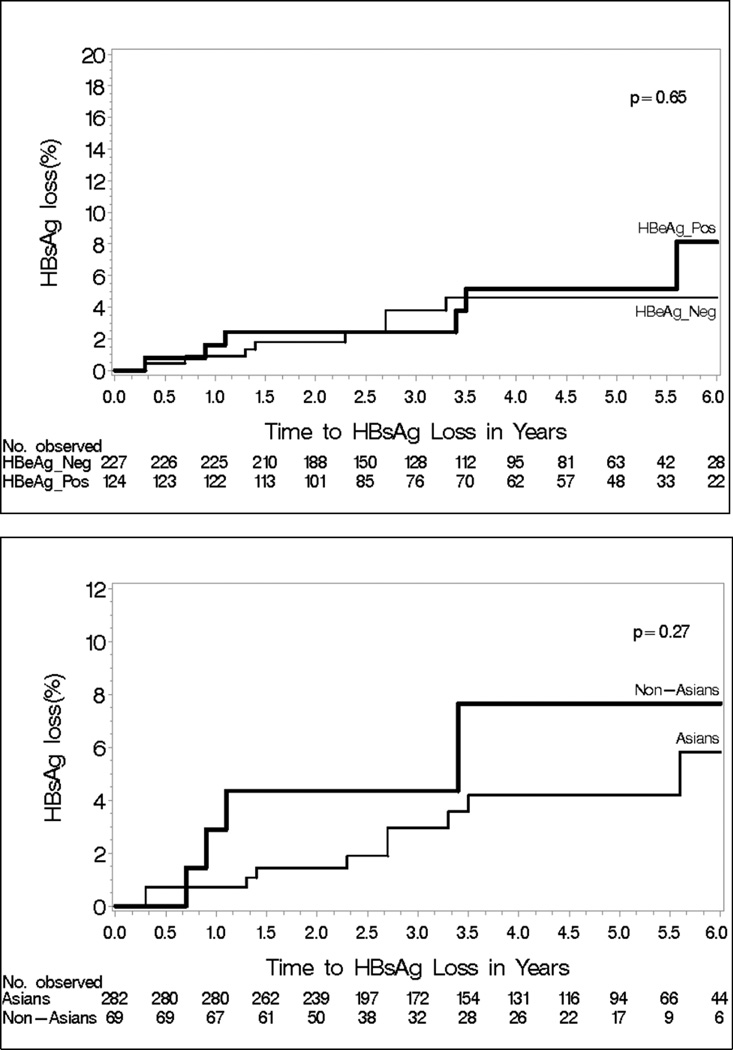
By year 5, 5 of the 237 HBeAg-positive patients and 8 of the 421 HBeAg-negative patients had lost HBsAg. The cumulative probability of HBsAg loss at year 5 was 5.2% in HBeAg-positive patients and 4.6% in HBeAg-negative patients. There were no statistically significant differences between Asian and non-Asian patients, although the overall number of patients with HBsAg loss was small. (Figure 4)
Figure 4.
Kaplan-Meier Cumulative Probability of HBsAg loss by HBeAg status
There were no differences in HBeAg loss, HBeAg seroconversion, or HBsAg loss between patients receiving care in the community and university settings.
Secondary Outcomes
Median GFR for the entire cohort was 92.7 (4.1, 225.7) mL/min at baseline and 97.9 (6.5, 176.7) mL/min at 5 years. Two patients with normal GFR at baseline had deterioration of renal function during treatment necessitating dose reduction of ETV. No patients required hemodialysis or ETV discontinuation due to renal insufficiency.
Entecavir dose was increased from 0.5 mg daily to 1.0 mg daily in 53 (8.1%) patients by their providers. Forty had dose increase for persistently detectable HBV DNA 6 months to 7 years after initiation of treatment, 2 for “underlying cirrhosis”, and 9 for unspecified reasons. Two other patients had ETV dose increase for a breakthrough in HBV DNA level, confirmed on repeat testing but resistance mutation testing was not performed. One patient had subsequent suppression of HBV DNA to undetectable. The second patient had a decline in HBV DNA level from 22,100 IU/mL at breakthrough to 1910 IU/mL at the last follow up, 14 months after breakthrough.
Entecavir was discontinued in a total of 108 (16.4%) study patients as show in Table 2. Of these, 48 (44.4%) were due to self-discontinuation or loss to follow up and 25 (23.1%) per provider recommendation after achieving therapeutic endpoint. Other reasons included suboptimal HBV DNA response in 11 (10.2%) patients, AE in 8 (7.4%) patients, pregnancy or pregnancy planning in 6 (5.6%) patients, and nonspecific reasons in 6 (5.6%) patients. Entecavir was discontinued in 4 patients due to suspected drug resistance and Tenofovir 300 mg was substituted for these 4 patients. Of the 8 patients who discontinued ETV due to AE, 2 had nonfatal lactic acidosis which resolved with ETV discontinuation and 6 had rash, arthralgia, abdominal discomfort, fatigue, and/or alopecia.
Table 2.
Reasons for discontinuation of Entecavir
| Reason for discontinuation | Number | % |
|---|---|---|
| Self discontinuation | 48 | 44.4 |
| Provider recommendation- reached therapeutic endpoint | 25 | 23.1 |
| Provider recommendation- suboptimal clinical response | 11 | 10.2 |
| Adverse events: | 8 | 7.4 |
| Lactic acidosis | 2 | |
| GI, dermatologic, miscellaneous | 6 | |
| Pregnancy related | 6 | 5.6 |
| Miscellaneous- lost to follow up | 6 | 5.6 |
| Suspected ETV resistance | 4 | 3.7 |
| Total | 108 |
Among the 108 patients who discontinued ETV, 27 had substitution of another oral HBV antiviral with 24 being placed on tenofovir and three on emtricitabine + tenofovir combination. Twenty-five (3.8%) patients had tenofovir added to ETV, two for suspected drug resistance, 22 for incomplete HBV DNA suppression, and one for unspecified reasons. Two (0.3%) patients had adefovir added to ETV, one for incomplete HBV DNA suppression and one for suspected ETV resistance.
New hepatic decompensation occurred in 5 (0.8%) patients at a median of 1.55 (0.43–2.87) years after initiation of ETV. Three patients had ascites, one had variceal bleeding, and one had ascites and hepatic encephalopathy. Four of these patients had cirrhosis at ETV initiation, three had undetectable HBV DNA while the other two patients had HBV DNA of 387 IU/ml and 741 IU/ml at the time of hepatic decompensation.
HCC was diagnosed in 18 (2.7%) patients, 8 of whom had cirrhosis at baseline. Twelve of these patients achieved undetectable HBV DNA levels prior to the diagnosis of HCC but 2 of these 12 subsequently had detectable HBV DNA levels at the time of HCC diagnosis. Five patients were diagnosed to have HCC during the first 12 months of ETV therapy. Four (0.6%) patients died, two due to complications of cirrhosis or HCC and two from non-liver causes. Six patients, all of who had HCC, underwent liver transplantation.
There were no differences in these secondary outcomes between patients at university and community settings.
Discussion
ENUMERATE is the largest US “real-world” study of ETV treatment with 658 patients from 26 centers showing that ETV is safe and effective at HBV DNA suppression, ALT normalization, and HBeAg seroconversion. These patients were predominantly foreign-born (61%), Asians (83%), HBeAg-negative (64%), with a relatively low baseline ALT (60 U/L) and HBV DNA level (5.8 log 10 IU/mL). Although 61 (9.3%) patients had cirrhosis at baseline, only 5 patients developed new hepatic decompensation and only 2 died due to liver-related causes during a median follow-up of 4 years. Eighteen patients were diagnosed with HCC including 10 who did not have cirrhosis at baseline. Because liver biopsies or non-invasive assessments of cirrhosis were not routinely performed at the start of treatment, it is possible that some of these 10 patients may have had compensated cirrhosis that was not recognized. Given the small number of HCC cases, no clear relationship between HBV DNA suppression and the diagnosis of HCC can be concluded although it is of interest that undetectable HBV DNA was not protective against the subsequent development of HCC.
ALT normalization rates at 1 year were 65.9% for HBeAg-positive and 72.8% for HBeAg-negative patients, similar to that reported in Phase III study of 65.9% and 72.8%, respectively (1,2) when a traditional cutoff of 40 U/L was used to define “normal” ALT, but significantly lower, 37.2% for HBeAg-positive patients and 39.6% for HBeAg-negative patients, when cutoffs of 30 U/L for men and 19 U/L for women were used. The possibility that concomitant liver disease such as alcoholic or nonalcoholic fatty liver disease, hepatitis C or hepatitis D could have contributed to the lower ALT normalization rates cannot be excluded as the study design did not systematically record alcohol use or require baseline viral hepatitis C, hepatitis D, or HIV testing or protocol liver biopsies. Undetectable HBV DNA rates at 1 year were lower in our study, 34.6% for HBeAg-positive and 81.9% for HBeAg-negative patients compared to 67% and 90%, respectively in Phase III trials. HBeAg seroconversion rate at 1 year was also lower in our study, 8.9% compared to 21% in the Phase III trial. These lower rates of response may reflect the difference between clinical trials that enrolled selected patients who were provided free medications and monitored closely and effectiveness observations in “real-world” settings enrolling a more heterogeneous, potentially less-adherent patient pool with a wider possible range of comorbidities and demographics.
Similar to the Phase III trials, serious adverse events were rare and only 8 (1.2%) patients required dose reduction or treatment discontinuation due to adverse events. However, overall changes in dose and treatment discontinuations were common. Of note, 8.1% of patients had increase in dose of ETV from 0.5 to 1.0 mg/day, mostly due to persistent viremia. Virologic breakthroughs were observed in only 4 (0.6%) patients though none were tested for genotypic resistance. Treatment was switched to tenofovir in 2 patients and ETV dose was increased in the other two patients.
ETV-related lactic acidosis was reported in 5 patients with decompensated cirrhosis but lactic acidosis was not observed in two prospective studies of ETV in patients with decompensated cirrhosis. (22–25) Two patients in our study who did not have baseline cirrhosis and did not progress to hepatic decompensation, developed non-fatal lactic acidosis. Both had ETV dose increase to 1.0 mg/day and were ambulatory patients with normal creatinine and bilirubin at the time of presentation with lactic acidosis. Neither required hospital admission. Both patients had no recurrence of symptoms after discontinuation of ETV and substitution with another antiviral agent.
Results from other “real-world” cohorts with chronic HBV who received ETV treatment are summarized in Table 3. Most of these were retrospective, single center studies with small sample size and short duration of follow up, and variable response rates. The largest study to date was a subgroup analysis of 1663 (29% HBeAg negative) Chinese patients showing a 1-year rate of undetectable HBV DNA of 60%, ALT normalization of 87%, and HBeAg seroconversion rate of 15% in HBV treatment-naïve patients; there were no significant adverse events. (12) Several multi-center studies from Europe reported similar results. (26–27) An Italian multicenter study of 418 patients (83% HBeAg-negative, 49% cirrhotic) found that by year 5, 100% of both HBeAg-positive and HBeAg-negative patients had undetectable HBV DNA, 52% had HBeAg seroconversion, and 33% had HBsAg loss. (28) The high rate of HBsAg loss in this study has not been observed in other studies. We observed lower rates of ALT normalization when using the more stringent normal ALT cutoff of 30 for men and 19 for women, and undetectable HBV DNA compared to other “real-world” studies. Our patients were predominantly Asian but our response rates remained lower even when compared to other studies from Asia.
Table 3.
“Real-world” studies of entecavir
| Buti | Baqai | Carey | Hou | Lampertico | Liu | Luo | Ono (2012)11 |
Yuen (2011)10 |
Wang | Zoutendijk | Ridruejo | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (2012)26 | (2009)19 | (2011)15 | (2013)12 | (2011)17 | (2013)18 | (201314 | (2012)13 | (2013)27 | (2013)16 | |||
| N | 190 | 153 | 154 | 1663 | 418 | 136 | 230 | 474 | 222 | 248 | 372 | 169 |
| Age (range) | 44 (35–54) | 51 (20–88) | 42 | 36 (16–70) | 58 | 39 | 42 | 47 (17–82) | 47 (21–77) | 39.4 | 43 (14) | 51 |
| Male sex | 73 | 71 | 79 | 79 | 76 | 61 | 85 | 68 | 71 | 69 | 73 | 77 |
| (%) | ||||||||||||
| Race | 84% white |
69% Asian |
ND | Asian | ND | ND | ND | ND | ND | ND | 48% white | 85% white |
| 9% Asian | 26% white |
27% Asian | 15% Asian |
|||||||||
| 3% black | 24% other | |||||||||||
| Country | Spain | US-Cali | UK | China | Italy | US- Cali | China | Japan | Hong Kong |
Taiwan | Europe | Argentina |
| Genotype | ND | ND | ND | ND | 90% D | ND | ND | 2.5% A | ND | ND | 9.4% A | ND |
| 85% B/C | 6.7% B | |||||||||||
| 10% C | ||||||||||||
| 36% D | ||||||||||||
| Cirrhosis | 34 | ND | 34 | 10 | 49 | ND | 74 | 22 | 0 | 16 | 24 | 23 |
| (%) | ||||||||||||
| HBeAg negative (%) |
70 | 55 | 69 | 29 (naïve) |
83 | 39 | 51 | 53 | 59 | 0 | 58 | 39 |
| ALT (IU/L) | 71.5 | 122 | NR | 122 (naïve) |
92 | 67 | 68 | 70 | 92 | 201 | NR | 139 |
| HBV DNA (log10 IU /ml) |
5.94 | >8 logs + in 10% |
4.6 | 6.78 (naïve) |
6 | 7.48 | 6.3 | 6.7 log 10 copies/ml |
7.1 | 7.6 | 6.2 | 6.88 |
| < 8 logs * in 90% | ||||||||||||
| Median follow up |
52 weeks |
28 months |
191 weeks |
5 years | 36 months |
27.5 months |
2.4 years | 3 years | 25 months |
20 months | 183 weeks |
|
| HBV DNA undetectable (%) |
83 | 86+ | 76 | 60 | 100 | 41 | 89.4 (year 1) |
88 (year 1) | 96 | 52 (year 1) | 68 (week 48) |
100 |
| (week 48) |
(36 months) |
(12 months) |
(week 48) |
(e antigen positive) |
(12 months) |
100 (year 5) |
83 (year 3) | 93 | (240 weeks in e antigen positive) |
|||
| 98* | 99 | 85 | (144 weeks) | (192 weeks in e antigen negative) |
||||||||
| (36 months) |
(e antigen negative) |
(36 months) |
||||||||||
| ALT normalization (%) |
82 | 82 | ND | 87 | 90 | ND | 73.9 (year 1) |
83 (year 1) | 90 | 83 (year 1) | 78 | ND |
| (week 48) |
(12 months) |
(week 48) |
(year 3) | 82.8 (year 3) |
93 (year 4) | 95 (year 3) | ||||||
| HBeAg seroconversion (%) |
2 | ND | 8 | 15 | 55 (cumulative rate) |
4.8 (12 months |
21 | 16 (year 1) | 53 | 27.7 | 17 | 68 |
| (week 48) |
(12 months) |
(week 48) |
30 (36 months) |
(year 2) | 38 (year 4) | |||||||
| HBSAg loss (%) |
1 | ND | 1 | ND | 34 (cumulative rate) |
ND | 0.4 | ND | 0.5 | ND | 1 | 14 |
| (12 months) |
The limitations of this study are inherent in its retrospective study design. The lack of a standardized monitoring protocol may have led to underreporting of adverse events or clinical outcomes. Laboratory tests were performed locally, which could have influenced our assessment of virologic response. Nevertheless, the lower limits of detection of HBV DNA assays were fairly similar throughout the sites as all sites used PCR assays with detection limits between 10–400 IU/mL. The types and frequency of other laboratory tests varied widely across sites, and contributed to the exclusion of 166 patients due to incomplete baseline laboratory data. Our study included both university and community sites, and therefore reflects inherent “real-world” variation in practice patterns which differ significantly from standardized clinical trial protocols.
The potential weaknesses of the study such as allowing inclusion of a more heterogeneous pool of patients who would not qualify for Phase III studies e.g. patients who had normal ALT or low HBV DNA at baseline or those who were initiated on a higher ETV dose of 1.0 mg daily can be its strength as it can better reflect the “real-world” practice of HBV treatment. The strengths of this study are its robust sample size, diverse mix of patients from university and community settings from different geographic regions of the United States, and long-term follow-up of the cohort. We observed a high rate of treatment discontinuation, which did not appear to be attributable to treatment failure or provider recommendation, and likely reflect “real-world” challenges in maintaining patients on long term HBV treatment.
In summary, our study of a large cohort of “real-world” patients who received ETV for chronic hepatitis B in the US demonstrated safety and long-term effectiveness despite a heterogeneous patient population and challenges in ensuring adherence to ETV and follow-up in clinical practice.
Supplementary Material
Acknowledgments
The authors would like to thank the following ENUMERATE investigators and AHF members for their contributions:
Jocelyn Woog- Asian Health Foundation
Ho Bae- Asian Pacific Liver Center
Steve-Huy Han- UCLA
Hie-Won L. Hann- Jefferson University Hospitals
Daryl Lau- Beth Israel Deaconess Medical Center
Loc Trong Le- Woodholme Gastroenterology Associates
Truong-Sinh Leduc- Leduc Medical Group
James Park- New York University
Anjana Pillai- Emory University
Myron Tong- UCLA
San Van Tran- Sang Van Tran PC
Clifford Wong- San Francisco, CA
Grant Support- This study was supported by an investigator-initiated grant to the Asian Health Foundation by Bristol Myers Squibb.
Abbreviations
- AE
adverse events
- AHF
Asian Health Foundation
- ALT
alanine aminotransferase
- Anti-HBe
hepatitis B e antibody
- AST
aspartate aminotransferase
- ETV
entecavir
- FDA
Food and Drug Administration
- GFR
glomerular filtration rate
- HBeAg
hepatitis B e antigen
- HBsAg
hepatitis B surface antigen
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- IRB
institutional review board
- KM
Kaplan-Meier
- MDRD
modification of diet in renal disease
- ULN
upper limit of normal
- US
United States
Footnotes
Authorship Statement
Joseph Ahn is acting as the submission’s guarantor and takes responsibility for the integrity of the work as a whole, from inception to published article. All authors approved the final version of the article, including the authorship list.
- Joseph Ahn, Hannah Lee, Joseph Lim, Calvin Pan, Mindie Nguyen, W. Ray Kim, Anna Lok- study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; obtained funding; study supervision
- Ajitha Mannalithara- statistical analysis
- Huy Trinh, Danny Chu, Tram Tran, Albert Min, Son Do, Helen Te, Raj Reddy- acquisition of data; critical revision of the manuscript for important intellectual content
Writing and Data Analyses Assistance- none
Statement of Interests
- Joseph Ahn- Research grant support from Bristol-Myers Squibb
- Calvin Pan- Speaker, consultant for Bristol-Myers Squibb
- Mindie Nguyen- Advisory board, research grant support from Bristol-Myers Squibb
- Danny Chu- Advisory board, speaker’s bureau for Gilead Sciences.
- Tram Tran- Research grant support from Bristol-Myers Squibb and Gilead Sciences. Advisory board for Bristol-Myers Squibb and Gilead Sciences.
- Albert Min- Research grant support from Bristol-Myers Squibb, Janssen and Gilead. Advisory board for Bristol-Myers Squibb, Gilead Sciences, Janssen. Speaker’s bureau for Bristol-Myers Squibb and Gilead Sciences.
- Helen Te- Research grant support from Abbvie, Conatus, Bristol-Myers Squibb. Advisory board for Bristol-Myers Squibb.
- K. Rajender Reddy- Research grant support from Bristol-Myers Squibb, Gilead Sciences, Abbvie, Merck, Jannsen. Advisory board for Merck, Bristol-Myers Squibb, Gilead Sciences, Abbvie, and Janssen.
- Anna S. Lok- Research grant support from Bristol-Myers Squibb and Gilead Sciences. Advisory panel for Gilead Sciences and safety panel for GlaxoSmithKline. Funded in part by NIH grant U01 DK082863.
- None declared- for all other authors
Contributor Information
Joseph Ahn, Division of Gastroenterology & Hepatology, Oregon Health & Science University, Portland, USA.
Hannah M. Lee, Gastroenterology/Hepatology Division, Tufts Medical Center, Boston, USA
Joseph K. Lim, Digestive Diseases, Yale University, New Haven, USA
Calvin Q. Pan, Department of Medicine, NYU Langone, NYC, USA
Mindie H. Nguyen, Division of Gastroenterology & Hepatology, Stanford University, Stanford, USA
W. Ray Kim, Division of Gastroenterology & Hepatology, Stanford University, Stanford, USA.
Ajitha Mannalithara, Division of Gastroenterology & Hepatology, Stanford University, Stanford, USA.
Huy Trinh, San Jose Gastroenterology, San Jose, USA.
Danny Chu, Albert Einstein College of Medicine, NYC, USA.
Tram Tran, Department of Medicine, Cedars Sinai Medical Center, Los Angeles, USA.
Albert Min, Division of Gastroenterology, Mount Sinai Beth Israel, NYC, USA.
Son Do, Digestive Health Associates of Texas, Plano, USA.
Helen Te, Digestive Disease Center, University of Chicago, Chicago, USA.
K. Rajender Reddy, Division of Gastroenterology and Hepatology, University of Pennsylvania, Philadelphia, USA.
Anna S. Lok, Division of Gastroenterology and Hepatology, University of Michigan, Ann Arbor, USA
Bibliography
- 1.Chang TT, Gish RG, de Man R, Gadano A, et al. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. NEJM. 2006;354(10):1001–1010. doi: 10.1056/NEJMoa051285. [DOI] [PubMed] [Google Scholar]
- 2.Lai CL, Shouval D, Lok AS, et al. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. NEJM. 2006;354(10):1011–1020. doi: 10.1056/NEJMoa051287. [DOI] [PubMed] [Google Scholar]
- 3.Chang TT, Lai CL, Kew Yoon S, et al. Entecavir treatment for up to 5 years in patients with hepatitis B e antigen-positive chronic hepatitis B. Hepatology. 2010;51(2):422–430. doi: 10.1002/hep.23327. [DOI] [PubMed] [Google Scholar]
- 4.Shouval D, Lai CL, Chang TT, et al. Relapse of hepatitis B in HBeAg-negative chronic hepatitis B patients who discontinued successful entecavir treatment: the case for continuous antiviral therapy. J Hepatol. 2009;50(2):289–295. doi: 10.1016/j.jhep.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 5.Manns MP, Akarca US, Chang TT, et al. Long-term safety and tolerability of entecavir in patients with chronic hepatitis B in the rollover study ETV-901. Expert Opin Drug Saf. 2012;11(3):361–368. doi: 10.1517/14740338.2012.653340. [DOI] [PubMed] [Google Scholar]
- 6.Tenney DJ, Rose RE, Baldick CJ, et al. Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naïve patients is rare through 5 years of therapy. Hepatology. 2009;49(5):1503–1514. doi: 10.1002/hep.22841. [DOI] [PubMed] [Google Scholar]
- 7.Scaglione SJ, Lok AS. Effectiveness of hepatitis B treatment in clinical practice. Gastroenterology. 2012;142(6):1360–1368. doi: 10.1053/j.gastro.2012.01.044. [DOI] [PubMed] [Google Scholar]
- 8.Pol S, Lampertico P. First-line treatment of chronic hepatitis B with entecavir or tenofovir in 'real-life' settings: from clinical trials to clinical practice. J Viral Hepat. 2012;19(6):377–386. doi: 10.1111/j.1365-2893.2012.01602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rothwell PM. External validity of randomised controlled trials: "to whom do the results of this trial apply?". Lancet. 2005;365(9453):82–93. doi: 10.1016/S0140-6736(04)17670-8. [DOI] [PubMed] [Google Scholar]
- 10.Yuen MF, Seto WK, Fung J, et al. Three years of continuous entecavir therapy in treatment-naïve chronic hepatitis B patients: VIRAL suppression, viral resistance, and clinical safety. Am J Gastroenterol. 2011;106(7):1264–1271. doi: 10.1038/ajg.2011.45. [DOI] [PubMed] [Google Scholar]
- 11.Ono A, Suzuki F, Kawamura Y, et al. Long-term continuous entecavir therapy in nucleos(t)ide-naïve chronic hepatitis B patients. J Hepatol. 2012;57(3):508–514. doi: 10.1016/j.jhep.2012.04.037. [DOI] [PubMed] [Google Scholar]
- 12.Hou JL, Jia JD, Wei L, et al. Efficacy and safety of entecavir treatment in a heterogeneous CHB population from a ‘real-world’ clinical practice setting in China. J Viral Hepat. 2013;20(11):811–820. doi: 10.1111/jvh.12115. [DOI] [PubMed] [Google Scholar]
- 13.Wang CC, Tseng KC, Peng CY, et al. Viral load and alanine aminotransferase correlate with serologic response in chronic hepatitis B patients treated with entecavir. J Gastroenterol Hepatol. 2013;28(1):46–50. doi: 10.1111/j.1440-1746.2012.07269.x. [DOI] [PubMed] [Google Scholar]
- 14.Luo J, Li X, Wu Y, et al. Efficacy of entecavir treatment for up to 5 years in nucleos(t)ide-naïve chronic hepatitis B patients in real life. Int J Med Sci. 2013;10(4):427–433. doi: 10.7150/ijms.5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carey I, Nguyen HL, Joe D. Denovo antiviral therapy with nucleos(t)ide analogues in ‘real-life’ patients with chronic hepatitis B infection: comparison of virological response between lamivudine + adefovir, entecavir vs. tenofovir therapy. Hepatology. 2011;54:A1396. [Google Scholar]
- 16.Ridruejo E, Adrover R, Cocozzella D, et al. Effectiveness of entecavir in chronic hepatitis B NUC-naive patients in routine clinical practice. Int J Clin Pract. 2011;65(8):866–870. doi: 10.1111/j.1742-1241.2011.02719.x. [DOI] [PubMed] [Google Scholar]
- 17.Lampertico P, Vigano M, Soffredini R. Entecavir monotherapy for nuc-naïve chronic hepatitis B patients from field practice: high efficacy and favorable safety profile over 3 year. Hepatology. 2011;54:A1436. [Google Scholar]
- 18.Liu A, Ha NB, Lin B, et al. Low hepatitis B envelope antigen seroconversion rate in chronic hepatitis B patients on long-term entecavir 0.5 mg daily in routine clinical practice. Eur J Gastroenterol Hepatol. 2013;25(3):338–343. doi: 10.1097/MEG.0b013e32835b3677. [DOI] [PubMed] [Google Scholar]
- 19.Baqai SF, Yi DH, Gish RG. Profound virologic response in chronic hepatitis B (CHB) patients treated with entecavir (ETV) in HBeAg positive and negative disease. Hepatology. 2009;50(S4):530A. [Google Scholar]
- 20.Prati D, Taioli E, Zanella A, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137(1):1–10. doi: 10.7326/0003-4819-137-1-200207020-00006. [DOI] [PubMed] [Google Scholar]
- 21.Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50(3):661–662. doi: 10.1002/hep.23190. [DOI] [PubMed] [Google Scholar]
- 22.Lange CM, Bojunga J, Hofmann WP, et al. Severe lactic acidosis during treatment of chronic hepatitis B with entecavir in patients with impaired liver function. Hepatology. 2009;50(6):2001–2006. doi: 10.1002/hep.23346. [DOI] [PubMed] [Google Scholar]
- 23.Liaw YF, Raptopoulou-Gigi M, Cheinquer H, et al. Efficacy and safety of entecavir versus adefovir in chronic hepatitis B patients with hepatic decompensation: a randomized, open-label study. Hepatology. 2011;54(1):91–100. doi: 10.1002/hep.24361. [DOI] [PubMed] [Google Scholar]
- 24.Liaw YF, Sheen IS, Lee CM, et al. Tenofovir disoproxil fumarate (TDF), emtricitabine/TDF, and entecavir in patients with decompensated chronic hepatitis B liver disease. Hepatology. 2011;53(1):62–72. doi: 10.1002/hep.23952. [DOI] [PubMed] [Google Scholar]
- 25.Köklü S, Tuna Y, Gülşen MT, et al. Long-term efficacy and safety of lamivudine, entecavir, and tenofovir for treatment of hepatitis B virus-related cirrhosis. Clin Gastroenterol Hepatol. 2013;11(1):88–94. doi: 10.1016/j.cgh.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 26.Buti M, Morillas RM, Prieto M, et al. Efficacy and safety of entecavir in clinical practice in treatment-naive Caucasian chronic hepatitis B patients. Eur J Gastroenterol Hepatol. 2012;24(5):535–542. doi: 10.1097/MEG.0b013e3283511287. [DOI] [PubMed] [Google Scholar]
- 27.Zoutendijk R, Reijinders JG, Zoulim F, et al. Virological response to entecavir is associated with a better clinical outcome in chronic hepatitis B patients with cirrhosis. Gut. 2013;62(5):760–765. doi: 10.1136/gutjnl-2012-302024. [DOI] [PubMed] [Google Scholar]
- 28.Lampertico P, Soffredini R, Vigano M, et al. 5-year entecavir treatment in NUC-naive, field-practice patients with chronic hepatitis B showed excellent viral suppression and safety profile but no prevention of HCC in cirrhotics. J Hepatol. 2013;58(S1):A755. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.