Abstract
Background and Aims
Cirrhosis from hepatitis C virus (HCV) infection is a major cause of end-stage liver disease and hepatocellular carcinoma worldwide. We determine the prevalence of cirrhosis among HCV-infected American adults including those unaware of their infection.
Methods
Using the National Health and Nutrition Examination Survey (NHANES) data, we identified participants aged≥20 years with detectable serum HCV RNA. The prevalence of advanced fibrosis and cirrhosis was determined for Eras 1 (1988-94), 2 (1999-2006) and 3 (2007-12) by using FIB-4 > 3.25 and APRI > 2.0, respectively.
Results
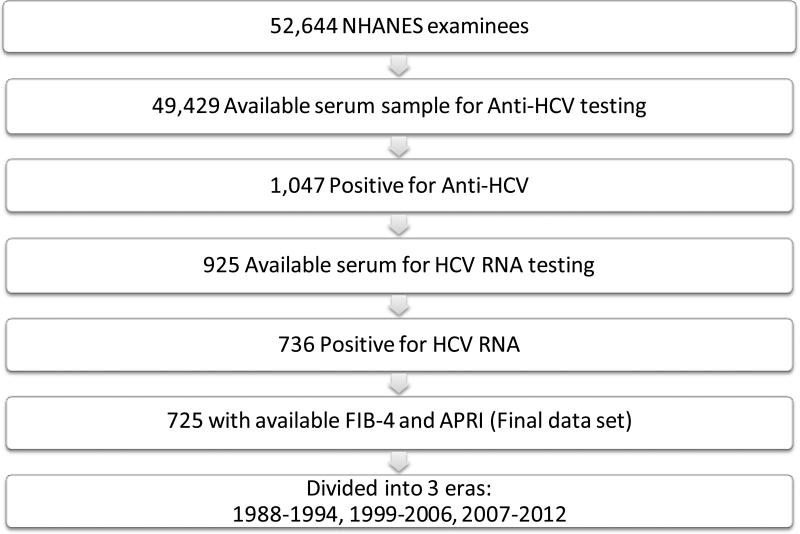
Out of 52,644 NHANES examinees, 49,429 were tested for HCV, of whom 725 met the inclusion criteria (positive HCV RNA with available data for FIB-4 and APRI). Based on APRI, 6.6% (95% confidence interval [CI]:2.2-11.0) of HCV-infected adults in Era 1, 7.6% (95%CI:3.4-11.8) in Era 2 and 17.0% (95%CI:8.0-26.0) in Era 3 had cirrhosis. In the multivariable regression analysis, this era effect was attributable to increasing age (odds ratio [OR]:1.04, 95%CI:1.02-1.07), diabetes (OR:2.33, 95%CI:1.01-5.40) and obesity (OR:2.96, 95%CI:1.15-7.57). Cirrhosis was as common among respondents who were unaware of their infection as those who were aware (both 11%). Results were identical when FIB-4 was used.
Conclusions
Among HCV-infected American adults, the proportion with cirrhosis has increased rapidly. Cirrhosis prevalence remains high in individuals unaware of their HCV infection. These data highlight the urgency for HCV screening regardless of symptoms, systematic assessment for liver fibrosis in those with HCV infection and institution of antivirals to prevent advanced liver disease.
Keywords: Hepatitis C Virus, Liver Fibrosis, Cirrhosis
Introduction
Chronic hepatitis C virus (HCV) infection, the most common chronic blood-borne infection in the United States, affects at least 3 million Americans.[1] As the leading cause of end-stage liver disease and hepatocellular carcinoma (HCC), it claims more lives annually than HIV infection.[2] Until its late sequelae develop, however, most patients with HCV infection remain asymptomatic, making its timely diagnosis difficult without purposeful screening. Approximately one half of US adults with HCV infection are yet to be diagnosed.[3]
Cirrhosis, the end result of progressive fibrosis, underlies most of the disease burden associated with HCV infection including hepatic decompensation and HCC. Evaluation of liver fibrosis is an essential element in the care of patients with chronic HCV infection, as the severity of liver fibrosis informs prognosis and treatment decisions. For example, responses to therapy available today are reduced in patients with decompensated cirrhosis, although they gain the largest benefit from successful antiviral therapy, which may halt the progression of liver fibrosis.[4] Many healthcare systems direct antiviral therapy to patients with advanced fibrosis and cirrhosis, as they attempt to prioritize utilization of the highly costly medications.
On the public health level, despite the importance of liver fibrosis in determining the current and future burden of HCV infection, reliable and generalizable data about the prevalence of HCV cirrhosis in the US are unavailable.[5] The prevalence of cirrhosis among people whose HCV infection is yet to be diagnosed remains even more uncertain. We address these questions by determining the prevalence of cirrhosis and advanced fibrosis in US residents with HCV infection and comparing the prevalence between individuals who are aware and unaware of their HCV infection based on population-based data generalizable to the entire US households.
Methods
Data Source
The National Health and Nutrition Examination Survey (NHANES), conducted by the National Center for Health Statistics, is a program to assess the health and nutritional status of adults and children in the US over time. Hepatitis C testing began in the NHANES sample collected between 1988 and 1994. Subsequent NHANES data sets encompassing years 1999-2012 included hepatitis C testing as well. In this analysis, we divided the data sets into three periods: Era 1 (1988-94), Era 2 (1999-2006), and Era 3 (2007-12).
Details on the survey design for the NHANES is available online (http://www.cdc.gov/nchs/data/series/sr_02/sr02_155.pdf). From the wide array of information included in the NHANES data file, demographic (age, sex, race/ethnicity) and laboratory data (anti-HCV, HCV RNA, aspartate aminotransferase (AST), alanine aminotransferase (ALT) and platelet count) were extracted. Detailed description of laboratory methods used in the NHANES is publicly available.[6-8]
Since 2001, an additional survey was included in patients with positive anti-HCV in order to assess what proportion of the participants already knew of their infection status, what they know about HCV, and what actions were taken after their infection status was discovered. This survey was conducted by phone approximately 6 months after the original examination. The HCV Follow-up Questionnaire is available online (http://www.cdc.gov/nchs/data/nhanes/pf_hcq_03_08.pdf).
Study participants
Of the NHANES participants, we selected subjects aged 20 years or older, with detectable HCV RNA in the serum and available laboratory values consisting of AST, ALT and platelet count. In the process, survey respondents who did not undergo laboratory testing or did not have available a serum sample for HCV testing were excluded.
For the comparison of the prevalence of advanced fibrosis and cirrhosis between those who were aware and unaware of their infection, only those participants who responded to the question HCQ 030 “Was the test result in our letter the first time you were told you has hepatitis C?” in Hepatitis C Follow-Up Questionnaire were included.
The way in which race and ethnicity were defined in the NHANES data changed over time. To compare information for the three eras, race/ethnicity data were formatted following the classification used in Era 1, which included non-Hispanic white, non-Hispanic black, Mexican American and other. Body mass indices were used to group subjects into three categories: obese (BMI>30), overweight (BMI: 25-30) and lean (BMI<25). Of several variables on alcohol use, the most complete one that includes categories of current or previous drinker or non-drinker was included.
Main Study Outcomes
Assessment of liver fibrosis is essential for chronic HCV management, namely to determine the prognosis and to make therapeutic decisions. Thus, our main outcome measures were the proportions of HCV-infected individuals with advanced fibrosis and cirrhosis.
Severity of liver fibrosis has traditionally been gauged by liver histology, based on the location and amount of collagenous deposits in the hepatic parenchyma. More recently, non-invasive markers of liver fibrosis have been developed.[9] In this study, we employed APRI[10] and FIB-4[11] scores to evaluate the severity of liver fibrosis. These markers were developed among patients with chronic HCV infection and have been validated in patients with HCV and other liver disease.[12, 13]. The scores were calculated for participants with available AST, ALT and platelet count data based on the following formulas:
Both APRI and FIB-4 scores incorporate two cut-off values to correlate with Ishak fibrosis stages. For detection of cirrhosis, an APRI score > 2.0 is indicative of a high probability of cirrhosis (Ishak stages 5-6), whereas a score < 1.0 correlates with a low probability. A FIB-4 score > 3.25 correlates with a high probability of advanced fibrosis (Ishak stages 4–6) and a score < 1.45 with a low probability.[14] Of the two scores, APRI was used for the primary analysis and FIB-4 for a confirmatory analysis, since the latter includes age as a variable, which could potentially confound the trend in the prevalence of advanced fibrosis over time. Hence, advanced fibrosis/cirrhosis was defined by FIB-4 score > 3.25 and cirrhosis was defined by APRI > 2.0.
Data Analysis
NHANES uses a complex stratified survey design to ensure accurate projections for non-institutionalized, civilian households in the US and to account for oversampling of certain population and for non-response to the interview and physical examination. We incorporate the survey sample parameters (clusters, strata, and corresponding weights) in the analysis wherever appropriate, taking into account the fact that not all participants were tested for anti-HCV and not all participants with RIBA-positive or -indeterminate had available specimens for the HCV RNA test.[15, 16] All statistical analyses were performed using the SAS package (Version 9.4: SAS Institute Inc., Cary, NC).
The proportion of participants with cirrhosis was calculated in each era. The multiple logistic regression analyses were used to identify factors associated with cirrhosis with the main predictor variable being the era of survey. Covariates that may affect liver fibrosis such as age and alcohol consumption were considered in the analysis.
Based upon the results of the Hepatitis C Follow-up Survey, we determined characteristics of study participants with HCV infection who had been unaware of the infection before they were tested for HCV in the NHANES examination versus those who had already been diagnosed. Specifically, we compared the proportion with cirrhosis between those two groups, with a hypothesis that cirrhosis would be more common in subjects in whom HCV had been diagnosed than in those whose HCV was only detected in the NHANES survey. This was because subjects with diagnosed HCV infection might have been symptomatic possibly from advanced liver disease, leading to higher likelihood of medical evaluation and diagnosis of HCV.
Results
In the NHANES data set, there were 52,644 adults 20 years or older who participated in the examination component and underwent laboratory testing (Fig. 1.). Of those, 49,429 (93.9%) had available serum samples for HCV testing, of whom 1,047 (2.1%) were positive for anti-HCV. Among anti-HCV-positive subjects, there were 736 (70.3%) who were positive for HCV RNA, whereas in 122 (11.6%), their HCV RNA status could not be ascertained, mostly for lack of available samples. Out of the 736 HCV-RNA-positive patients, a vast majority (n=725, 98.5%) had complete laboratory values for calculating APRI and FIB-4 scores and they constituted the final data set for this analysis.
Figure 1.
Flow diagram of subject selection
Table 1. depicts the characteristics of participants with regard to HCV results. Overall, there was a trend for decreasing prevalence of HCV infection. The prevalence of US adults with chronic HCV infection decreased from 1.5% (95% confidence interval [CI]: 1.0-2.0) in Era 1, to 1.2%, (95%CI: 1.0-1.4) in Era 2, and then to 1.0% (95%CI: 0.8-1.3) in Era 3, which project to 2.7 million (95%CI: 1.8-3.5 million), 2.5 million (95%CI, 2.1-2.9 million, and 2.2 million (95%CI, 1.7-2.8 million) Americans with HCV infection for Eras 1, 2, and 3, respectively.
Table 1.
Characteristics of N HAN ES participants, aged ≥ 20 (Weighted)
| Era 1 (1988-1994) | Era 2 (1999-2006) | Era 3 (2007-2012) | |
|---|---|---|---|
| Study Subjects | |||
| Number of US residents ≥ 20 years, in millions (95% confidence interval) | 177.2 (164.9-189.5) | 203.1 (191.8-214.3) | 219.4 (204.9-233.8) |
| Proportion of HCV RNA+ (95% confidence interval) | 1.5% (1.0-2.0) | 1.2% (1.0-1.4) | 1.0% (0.8-1.3) |
| HCV RNA+, in millions (95% confidence interval) | 2.7 (1.8-3.5) | 2.5 (2.1-2.9) | 2.2 (1.7-2.8) |
| Characteristics of HCV-RNA+ subjects, age ≥ 20 with available APRI and FIB-4 results | |||
| Number, in millions (95% confidence interval) | 2.6 (1.7-3.4) | 2.5 (2.1-2.9) | 2.2 (1.6-2.7) |
| Age* | 40.3 ± 0.9 | 47.5 ± 0.8 | 50.5 ± 0.8 |
| Sex (% female, (95%CI)) | 32.2 (23.8-40.6) | 33.2 (25.2-41.2) | 33.1 (24.2-42.0) |
| Race/Ethnicity (%, (95%CI)) | |||
| Non-Hispanic White | 55.9 (42.3-69.4) | 64.1 (56.3-72.0) | 63.2 (53.3-73.1) |
| Non-Hispanic Black | 22.3 (15.7-28.9) | 23.0 (17.0-29.0) | 23.4 (15.1-31.7) |
| Mexican American | 6.7 (3.7-9.7) | 6.0 (2.3-9.7) | 5.6 (2.5-8.8) |
| Other | 15.1 (2.4-27.8) | 6.9 (2.6-11.1) | 7.8 (3.3-12.3) |
| Body mass index(%, (95%CI)) | |||
| ≥30 (Obese) | 22.0 (12.3-31.8) | 19.5 (12.9-26.1) | 28.8 (19.8-37.9) |
| 25-29.9 (Overweight) | 23.3 (16.6-30.1) | 37.0 (31.3-42.7) | 35.2 (25.7-44.7) |
| <25 (Lean) | 54.6 (44.6-64.6) | 43.6 (35.3-51.9) | 36.0 (26.4-45.5) |
| Alcohol consumption (%, (95%CI)) | |||
| Nondrinker | 7.3 (0.2-14.4) | 7.0 (1.9-12.2) | 2.2 (0.1-4.4) |
| Current drinker | 68.9 (60.0-77.7) | 86.1 (80.1-92.1) | 83.8 (75.8-91.8) |
| Ex-drinker | 23.8 (14.8-32.9) | 6.8 (3.5-10.2) | 14.0 (6.3-21.6) |
| Diabetes Mellitus (%, (95%CI)) | 5.0 (2.1-7.9) | 7.5 (2.7-12.4) | 11.0 (5.8-16.3) |
| AST (U/L)*♯ | 48.5 ± 3.1 | 51.3 ± 3.0 | 63.4 ± 5.1 |
| ALT (U/L)* | 46.4 ± 3.1 | 57.8 ± 3.8 | 70.0 ± 7.2 |
| Platelets (×103/μL)* | 250.9 ± 7.7 | 251.1 ± 6.5 | 221.3 ± 6.9 |
| APRI score* | 0.73 ± 0.12 | 0.76 ± 0.06 | 1.14 ± 0.12 |
| FIB-4 score* | 1.87 ± 0.32 | 1.63 ± 0.13 | 2.21 ± 0.17 |
Mean ± Standard Deviation
Upper limits of normal1, 2: 40 U/L prior to 2000, 33 U/L thereafter
SI conversion factors: To convert AST and ALT to μkat/L, multiply values by 0.0167
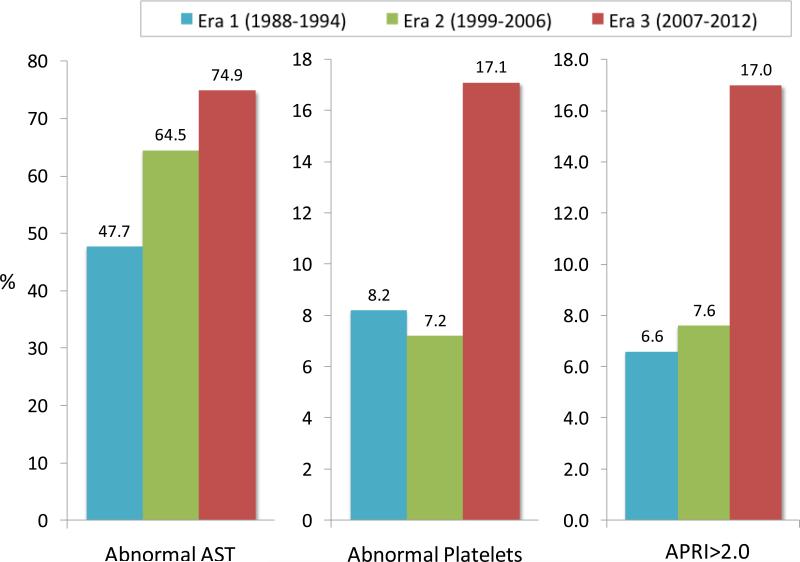
The mean age of survey participants with HCV infection increased from the early 40s to the 50s over time. The proportion of women did not change. The proportion of Non-Hispanic Whites increased, whereas those of Mexican Americans and of other race decreased. The proportion of overweight or obese subjects increased as well as those with diabetes, whereas the proportion of non-drinkers decreased. The mean serum activities of aminotransferases were higher than the upper limit of normal and tended to increase in each era. The mean platelet counts decrease from 251×103/μL in Eras 1 (95%CI: 235-266) and 2 (95%CI: 238-264) to 221×103/μL (95%CI: 207-235) in Era 3. The mean APRI and FIB-4 scores increased in Era 3 compared to previous eras, suggesting higher prevalence of advanced fibrosis in the last era.
In Fig. 2., the proportion of survey participants with abnormal AST and platelet results increased over time. Consequently, subjects with a high probability for cirrhosis (APRI>2.0) increased from 6.6% (95%CI: 2.2-11.0) in Era 1 and 7.6% (95%CI: 3.4-11.8) in Era 2 to 17.0% (95%CI: 8.0-26.0) in Era 3, corresponding to 170,100 (95%CI: 36,900-303,400) American adults with HCV cirrhosis in Era 1, 189,300 (95%CI: 82,900-295,700) in Era 2 and 370,500 (95%CI: 147,400-593,700) in Era 3. A similar trend was seen when FIB-4 was used to determine advanced fibrosis: 8.6% (95%CI: 4.0-13.1) in Era 1, 10.2% (95%CI: 5.4-14.9) in Era 2 and 16.0% (95%CI: 9.3-22.6) in Era 3. These estimates corresponded to 221,500 (95%CI: 77,000-366,000), 252,200 (95%CI: 124,900-379,500) and 347,800 (95%CI: 190,400-505,300) individuals with advanced fibrosis or cirrhosis for Eras 1, 2, and 3, respectively.
Figure 2.
Percentages of the individual with abnormal AST, platelet counts and APRI score > 2.0 in each era
Table 2. summarizes results of a series of logistic regression analyses for potential variables that may explain the higher prevalence of cirrhosis in the recent era. In univariate analyses, age, diabetes and BMI categories of overweight/obesity were associated with cirrhosis, in addition to the most recent era. These variables remained significant in bivariate models in which they were considered individually in conjunction with the eras. In the final model which included age (increased odds of cirrhosis by 4% (95%CI: 2-7%) per year), diabetes (increased odds by 2.33 fold, 95%CI: 1.01-5.40), and obesity (increased odds by 2.96 fold, 95%CI: 1.15-7.57), the effect of era became smaller and insignificant. When the analysis was repeated with FIB-4 score, the results were similar (Supplementary Table).
Table 2.
Factors associated with cirrhosis* in chronic HCV infection
| Variable | Odds Ratio (95% Confidence Interval) | ||
|---|---|---|---|
| Univariate Analysis | Bivariate Analysis** | Multivariable Analysis | |
| Era (reference=Era1) | |||
| Era3 | 2.91 (1.15-7.37) | N/A | 1.74 (0.65-4.69) |
| Era2 | 1.17 (0.47-2.90) | N/A | 0.84 (0.33-2.17) |
| Age | 1.05 (1.03-1.07) | 1.05 (1.02-1.07) | 1.04 (1.02-1.07) |
| Sex (reference=female) | 1.33 (0.54-3.24) | - | - |
| Race/Ethnicity (reference=NH white) | |||
| NH Black | 1.03 (0.50-2.11) | - | - |
| Mexican | 0.84 (0.34-2.08) | - | - |
| Other | 1.62 (0.63-4.21) | - | - |
| Diabetes | 4.33 (1.94-9.70) | 3.91 (1.80-8.49) | 2.33 (1.01-5.40) |
| BMI (reference=BMI < 25) | |||
| ≥30 | 3.42 (1.30-9.00) | 3.05 (1.17-7.96) | 2.96 (1.15-7.57) |
| 25-29.9 | 2.35 (1.05-5.23) | 2.14 (0.96-4.77) | 1.99 (0.91-4.36) |
| Drinking (reference=non-drinker) | |||
| Current drinker | 0.34 (0.06-1.87) | - | - |
| Previous drinker | 0.77 (0.12-4.79) | - | - |
Cirrhosis defined by APRI score > 2.0
Each variable was individually considered in conjunction with the era.
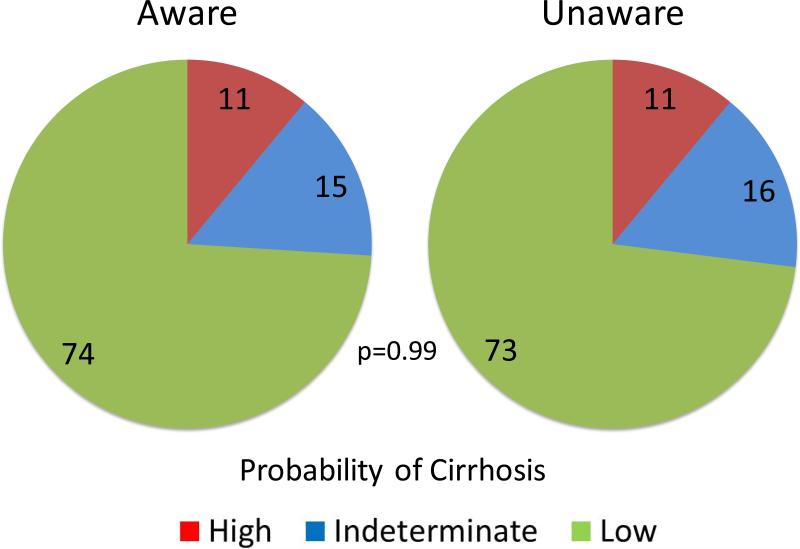
In NHANES 2001-2012, 163 participants who tested positive for HCV-RNA had available data for APRI and FIB-4 scores and responded to the Hepatitis C Follow-up Survey with regard to awareness of their HCV infection. In Table 3., those respondents were mostly male (67.5%, 110/163) and non-Hispanic White (44.8%, 73/163). The mean age was 52.5 ± 11.7, AST 55.5 ± 36.7 (U/L), ALT 57.6 ± 40.5 (U/L), and platelets 225.2 ± 83.3 (x103/μL). There was no difference in the mean APRI and FIB-4 scores between the two groups. Fig. 3. compares the proportions of respondents with a high, indeterminate, and low probability of cirrhosis, as determined by APRI. The proportion of cirrhosis was 11.2% among subjects unaware of their HCV infection, compared to 10.8% in those who were aware (p=0.99). A similar pattern was seen with the FIB-4: the proportion of a high, indeterminate and low probability for advanced fibrosis was 15%, 36% and 49% among respondents aware of their HCV infection, respectively, compared to 22%, 30% and 48% among those who were unaware, respectively (p=0.48).
Table 3.
Characteristics of positive HCV RNA participants who were and were not aware of their infection
| Aware | Not aware | p | |
|---|---|---|---|
| Survey Participants (n) | 80 | 83 | 0.81 |
| Sex (% female, (n)) | 37.5 (30) | 27.7 (23) | 0.18 |
| Race/Ethnicity (%, (n)) | |||
| Non-Hispanic White | 45.0 (36) | 44.6 (37) | |
| Non-Hispanic Black | 31.3 (25) | 37.4 (31) | 0.63 |
| Mexican American | 12.5 (10) | 12.1 (10) | |
| Other | 11.3 (9) | 6.0 (5) | |
| Age (years) | 52.8 ± 10.6 | 52.1 ± 12.7 | 0.70 |
| AST (U/L) | 54.9 ± 33.3 | 56.1 ± 39.9 | 0.83 |
| ALT (U/L) | 56.8 ± 37.7 | 58.3 ± 43.3 | 0.82 |
| Platelets(× 103/μL) | 219.8 ± 78.7 | 230.4 ± 87.7 | 0.42 |
| APRI score (n=163) | 1.02 ± 1.16 | 0.96 ± 1.05 | 0.76 |
| FIB-4 score (n=163) | 2.24 ± 1.96 | 2.17 ± 2.06 | 0.84 |
Figure 3.
Proportion of the respondents with high, indeterminate and low probability of cirrhosis based on APRI score
Discussion
With the advent of highly effective, yet highly costly antiviral agents against HCV, there has been much debate about how patients with HCV infection should be diagnosed, evaluated and treated.[17] The most contentious element in this debate is whether antiviral therapy should be offered to all with HCV infection or be prioritized to patients with evidence of significant liver fibrosis. There is little debate, however, that HCV patients with cirrhosis must be treated.[18] In this context, accurate data about the prevalence of cirrhosis are of critical importance in determining healthcare strategies regarding antiviral therapy and resource allocation for optimal management of HCV infection on the population level.
We report in this work that while the overall prevalence of HCV infection has decreased over time[2, 5, 19], the proportion of HCV patients with cirrhosis more than doubled during the study period, reaching 17% (with the upper end of 95% confidence interval being 26%) in the latest era. Importantly, the prevalence of cirrhosis in patients who were unaware of their HCV infection was at least as high as that in those who had been diagnosed. Patients with HCV infection who have not been diagnosed should be urgently sought, so that appropriate hepatological care is provided, including modification of risk factors such as alcohol consumption and controlling body weight and disease monitoring, as well as appropriate antiviral therapy is chosen and administered.
Since only one half of HCV infection in the US is believed to have been diagnosed so far, our data suggest that at least 200,000 Americans with HCV cirrhosis remain undiagnosed. As NHANES excludes population with the highest HCV prevalence, such as homeless or incarcerated individuals, these figures likely underestimate the true number of those with HCV infection at risk of experiencing complications.[20, 21] To date, accurate population-based data about the prevalence of HCV cirrhosis in the US have been scarce. Several estimates, obtained with markovian models derived from presumed duration of infection and projected rates of fibrosis progression, varied widely from 261,000[22] to 600,000[23] and to nearly 800,000.[5] The only actual population-denominated, although not necessarily generalizable, data about advanced fibrosis are derived from the Veterans Administration system, which reported that the prevalence of HCV-related cirrhosis increased from 9% in 1996 to 18.5% in 2006.[24]
This rising trend is in part attributable to the aging of Americans with HCV infection, as shown in the demographic characteristics in Table 1. and the regression analysis in Table 2. It is well described that the majority of Americans with chronic HCV infection belong in the birth cohort between 1945 and 1965. As the generation with HCV infection becomes older, the duration of infection also increases, allowing liver fibrosis to progress.[25, 26] The significant increase in the proportion of participants with elevated AST level is consistent with increasing prevalence of advanced fibrosis and cirrhosis. On the other hand, ALT activities are commonly thought to decline as fibrosis progresses, The rising trend in ALT level in our data may be in part due to concomitant non-alcoholic fatty liver disease (NAFLD) which may accelerate fibrosis progression . As some of these patients have two different disease processes in the liver, The extent to which the presence of NAFLD may have influenced our assessment of fibrosis is uncertain, as for example, FIB-4 score which has ALT as a denominator in its formula still parallels the increasing trend of APRI. Nonetheless, these data highlight the need for the care of those patients to go beyond eradicating HCV and include comprehensive medical management.
Despite the recent recommendation for enhanced HCV screening,[27-29] a large proportion of Americans with HCV infection remains undiagnosed. Importantly, we found that cirrhosis was equally common in those unaware of their infection. This was contrary to our initial hypothesis that individuals who had their HCV infection diagnosed may have had a higher likelihood of having advanced liver disease. An alternate hypothesis that may associate HCV diagnosis with less severe fibrosis may be that patients aware of their HCV may be more likely to modify their life style (e.g., alcohol consumption) that could alter the trajectory of their disease progression. Unfortunately, information about whether HCV diagnosis led to behavioral changes is not available in the NHANES data. It is also possible that some of the diagnosed patients may have undergone antiviral therapy which might have temporized progression of fibrosis.
Thus, one of the limitations in this study is the lack of data on HCV treatment which might have affected fibrosis progression as well as overall HCV prevalence. The proportion of such patients is likely small as only a fraction of patients were treated in the prior antiviral era and even fewer patients responded successfully to therapy. Another limitation of this study is that while cirrhosis remains a histologic diagnosis, obtaining liver biopsies in a large epidemiological survey is impossible and, thus, we relied on surrogate indices to gauge fibrosis. Both APRI and FIB-4 scores have been developed and validated in patients with HCV and have been used to correlate with disease outcome and guide therapeutic decisions.[30] Recently, the World Health Organization adopted APRI as a tool to evaluate HCV patients in settings where more direct measures of liver fibrosis are not available. Both scores are limited by the fact that AST and ALT may be affected by factors other than fibrosis, such as liver inflammation from other causes (non-alcoholic fatty liver disease) as well as demographic factors (age, sex, and body mass index). However, when results produced with APRI and FIB-4 scores were taken together, the increasing trend and the approximate percentage of subjects with advanced fibrosis and cirrhosis were consistent with each other. Finally, our total sample size of 736 may appear relatively modest for a population-based study. However, a unique strength of this sample is that it is designed to be representative of the US general population.
Emerging data indicate that following eradication of HCV with antiviral therapy, cirrhotic patients may experience improvements in liver histology and long term clinical outcome.[4, 31] However, as antiviral therapy available to date has reduced efficacy in patients with cirrhosis, our data point to the urgency with which systematic screening should be implemented for HCV patients with cirrhosis who remain asymptomatic but at risk of developing hepatic decompensation and/or HCC in the foreseeable future. Moreover, cirrhosis may be even more common in people who were not captured in the NHANES study in whom the prevalence of co-existing conditions to accelerate fibrosis such as excessive alcohol use and HIV infection may be higher.[32]
In summary, data derived from the NHANES samples over a span of 14 years, generalizable to HCV-infected American adults, show that the number of adults with HCV cirrhosis is rising in an accelerated fashion. The prevalence of cirrhosis remains high even in subjects whose HCV infection has not been diagnosed. These data call for public health efforts to reduce the burden of HCV infection by ensuring adherence to the screening recommendations followed by systematic assessment for liver fibrosis and implementation of antiviral therapy in appropriate patients for primary and secondary prevention of cirrhosis and its complications.
Supplementary Material
Lay statement.
Chronic hepatitis C virus (HCV) infection is a major cause of cirrhosis, creating a large public health burden. Based on the US National Health and Nutrition Examination Survey sample, we found the proportion of patients with cirrhosis among Americans with HCV infection increased from 6.6% to 17.0% over the past two decades. Patients who were unaware of their infection was just as likely to have cirrhosis as those who knew about their infection, which highlights the need for screening and treatment for HCV at the population level.
Acknowledgments
Financial Support: The work was supported by grants from the National Institutes of Health (DK-34238 and DK-92336) attributable to WR Kim.
List of abbreviations in the order of appearance
- HCV
Hepatitis C Virus
- HCC
Hepatocellular Carcinoma
- APRI
AST to Platelet Ratio Index
- FIB-4
Fibrosis-4
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BMI
Body Mass Index
Footnotes
Conflict of Interest: Dr. Kim reports consulting and advisory board participation with Bristol-Myers-Squibb and Gilead Sciences. No other authors report potential conflicts.
Author's Contributions:
Prowpanga Udompap: acquisition of data; analysis and interpretation of data; drafting of the manuscript; statistical analysis
Ajitha Mannalithara: acquisition of data; analysis and interpretation of data
Naeyun Heo: critical revision of the manuscript for important intellectual content
Dong H. Kim: critical revision of the manuscript for important intellectual content
W. Ray Kim: study concept and design; obtained funding; administrative, technical, or material support; study supervision
References
- 1.Denniston MM, Jiles RB, Drobeniuc J, Klevens RM, Ward JW, McQuillan GM, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Annals of internal medicine. 2014;160:293–300. doi: 10.7326/M13-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ly KN, Xing J, Klevens RM, Jiles RB, Ward JW, Holmberg SD. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Annals of internal medicine. 2012;156:271–278. doi: 10.7326/0003-4819-156-4-201202210-00004. [DOI] [PubMed] [Google Scholar]
- 3.Denniston MM, Klevens RM, McQuillan GM, Jiles RB. Awareness of infection, knowledge of hepatitis C, and medical follow-up among individuals testing positive for hepatitis C: National Health and Nutrition Examination Survey 2001-2008. Hepatology. 2012;55:1652–1661. doi: 10.1002/hep.25556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Meer AJ, Veldt BJ, Feld JJ, Wedemeyer H, Dufour JF, Lammert F, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308:2584–2593. doi: 10.1001/jama.2012.144878. [DOI] [PubMed] [Google Scholar]
- 5.Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138:513–521. 521, e511–516. doi: 10.1053/j.gastro.2009.09.067. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention [April 1, 2015];2001-2002 Lab Methods. [cited; Available from: http://www.cdc.gov/nchs/nhanes/nhanes2001-2002/lab_methods_01_02.htm]
- 7.Centers for Disease Control and Prevention [April 1, 2015];NHANES III LABORATORY DATA FILE DOCUMENTATION. [cited; Available from: ftp://ftp.cdc.gov/pub/Health_Statistics/NCHS/nhanes/nhanes3/1A/lab-acc.pdf.]
- 8.Ioannou GN, Boyko EJ, Lee SP. The prevalence and predictors of elevated serum aminotransferase activity in the United States in 1999-2002. Am J Gastroenterol. 2006;101:76–82. doi: 10.1111/j.1572-0241.2005.00341.x. [DOI] [PubMed] [Google Scholar]
- 9.Martinez SM, Crespo G, Navasa M, Forns X. Noninvasive assessment of liver fibrosis. Hepatology. 2011;53:325–335. doi: 10.1002/hep.24013. [DOI] [PubMed] [Google Scholar]
- 10.Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 11.Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 12.Li J, Gordon SC, Rupp LB, Zhang T, Boscarino JA, Vijayadeva V, et al. The validity of serum markers for fibrosis staging in chronic hepatitis B and C. Journal of viral hepatitis. 2014;21:930–937. doi: 10.1111/jvh.12224. [DOI] [PubMed] [Google Scholar]
- 13.Teshale E, Lu M, Rupp LB, Holmberg SD, Moorman AC, Spradling P, et al. APRI and FIB-4 are good predictors of the stage of liver fibrosis in chronic hepatitis B: the Chronic Hepatitis Cohort Study (CHeCS). Journal of viral hepatitis. 2014;21:917–920. doi: 10.1111/jvh.12279. [DOI] [PubMed] [Google Scholar]
- 14.Schiavon Lde L, Narciso-Schiavon JL, de Carvalho-Filho RJ. Non-invasive diagnosis of liver fibrosis in chronic hepatitis C. World journal of gastroenterology. 2014;20:2854–2866. doi: 10.3748/wjg.v20.i11.2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention [April 1, 2015];ANALYTIC AND REPORTING GUIDELINES: The Third National Health and Nutrition Examination Survey, NHANES III (1988-94) [cited; Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes3/nh3gui.pdf]
- 16.Centers for Disease Control and Prevention [April 1, 2015];ANALYTIC AND REPORTING GUIDELINES. [cited; Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/nhanes_analytic_guidelines_dec_2005.pdf.]
- 17.Lutchman G, Kim WR. A glass half full: Implications of screening for hepatitis C virus in the era of highly effective antiviral therapy. Hepatology. 2015;61:1455–1458. doi: 10.1002/hep.27718. [DOI] [PubMed] [Google Scholar]
- 18.Koretz RL, Lin KW, Ioannidis JP, Lenzer J. Is widespread screening for hepatitis C justified? BMJ. 2015;350:g7809. doi: 10.1136/bmj.g7809. [DOI] [PubMed] [Google Scholar]
- 19.Ditah I, Ditah F, Devaki P, Ewelukwa O, Ditah C, Njei B, et al. The changing epidemiology of hepatitis C virus infection in the United States: National Health and Nutrition Examination Survey 2001 through 2010. Journal of hepatology. 2014;60:691–698. doi: 10.1016/j.jhep.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 20.Chak E, Talal AH, Sherman KE, Schiff ER, Saab S. Hepatitis C virus infection in USA: an estimate of true prevalence. Liver international. 2011;31:1090–1101. doi: 10.1111/j.1478-3231.2011.02494.x. [DOI] [PubMed] [Google Scholar]
- 21.Moorman AC, Xing J, Ko S, Rupp LB, Xu F, Gordon SC, et al. Late diagnosis of hepatitis C virus infection in the Chronic Hepatitis Cohort Study (CHeCS): Missed opportunities for intervention. Hepatology. 2015;61:1479–1484. doi: 10.1002/hep.27365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGarry LJ, Pawar VS, Panchmatia HR, Rubin JL, Davis GL, Younossi ZM, et al. Economic model of a birth cohort screening program for hepatitis C virus. Hepatology. 2012;55:1344–1355. doi: 10.1002/hep.25510. [DOI] [PubMed] [Google Scholar]
- 23.Razavi H, Elkhoury AC, Elbasha E, Estes C, Pasini K, Poynard T, et al. Chronic hepatitis C virus (HCV) disease burden and cost in the United States. Hepatology. 2013;57:2164–2170. doi: 10.1002/hep.26218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanwal F, Hoang T, Kramer JR, Asch SM, Goetz MB, Zeringue A, et al. Increasing prevalence of HCC and cirrhosis in patients with chronic hepatitis C virus infection. Gastroenterology. 2011;140:1182–1188. e1181. doi: 10.1053/j.gastro.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thein HH, Yi Q, Dore GJ, Krahn MD. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology. 2008;48:418–431. doi: 10.1002/hep.22375. [DOI] [PubMed] [Google Scholar]
- 26.Rein DB, Wittenborn JS, Weinbaum CM, Sabin M, Smith BD, Lesesne SB. Forecasting the morbidity and mortality associated with prevalent cases of pre-cirrhotic chronic hepatitis C in the United States. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2011;43:66–72. doi: 10.1016/j.dld.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 27.Moyer VA, Force USPST Screening for hepatitis C virus infection in adults: U.S. Preventive Services Task Force recommendation statement. Annals of internal medicine. 2013;159:349–357. doi: 10.7326/0003-4819-159-5-201309030-00672. [DOI] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recommendations and reports : Morbidity and mortality weekly report Recommendations and reports / Centers for Disease Control. 1998;47:1–39. [PubMed] [Google Scholar]
- 29.Chou R, Cottrell EB, Wasson N, Rahman B, Guise JM. Screening for hepatitis C virus infection in adults: a systematic review for the U.S. Preventive Services Task Force. Annals of internal medicine. 2013;158:101–108. doi: 10.7326/0003-4819-158-2-201301150-00574. [DOI] [PubMed] [Google Scholar]
- 30.Holmberg SD, Lu M, Rupp LB, Lamerato LE, Moorman AC, Vijayadeva V, et al. Noninvasive serum fibrosis markers for screening and staging chronic hepatitis C virus patients in a large US cohort. Clinical infectious diseases. 2013;57:240–246. doi: 10.1093/cid/cit245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poynard T, McHutchison J, Manns M, Trepo C, Lindsay K, Goodman Z, et al. Impact of pegylated interferon alfa-2b and ribavirin on liver fibrosis in patients with chronic hepatitis C. Gastroenterology. 2002;122:1303–1313. doi: 10.1053/gast.2002.33023. [DOI] [PubMed] [Google Scholar]
- 32.Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. Lancet. 1997;349:825–832. doi: 10.1016/s0140-6736(96)07642-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.