Abstract
The accessibility of regulatory molecules to specific DNA sequences and chromatin regions in the nucleus is crucial to gene expression. In this study, we examined the chromatin structure in tomato leaf cells and in exponentially growing tomato cell suspension cultures. The structure of ribosomal chromatin was investigated by micrococcal nuclease and psoralen photocrosslinking. We showed that ribosomal genes in tomato are folded into two distinct types of chromatin: an open chromatin conformation and a closed nucleosomecontaining chromatin. In contrast to previous findings in Friend cells, where half of the ribosomal genes were found to be complexed within an inactive chromatin structure, we demonstrated that the canonical nucleosome-containing chromatin is present in the majority (approximately 80%) of the tomato rRNA-encoding DNA clusters. The minor open chromatin population (approximately 20% of the ribosomal genes) could be detected only after analysis following psoralen crosslinking. The relative amounts of the two ribosomal chromatin structures are similar in stationary and exponentially growing cells. This suggests that the proportions of open and closed chromatin structures present in either stationary or exponentially growing tomato cells are not dependent on the transcriptional process.
Full text
PDF

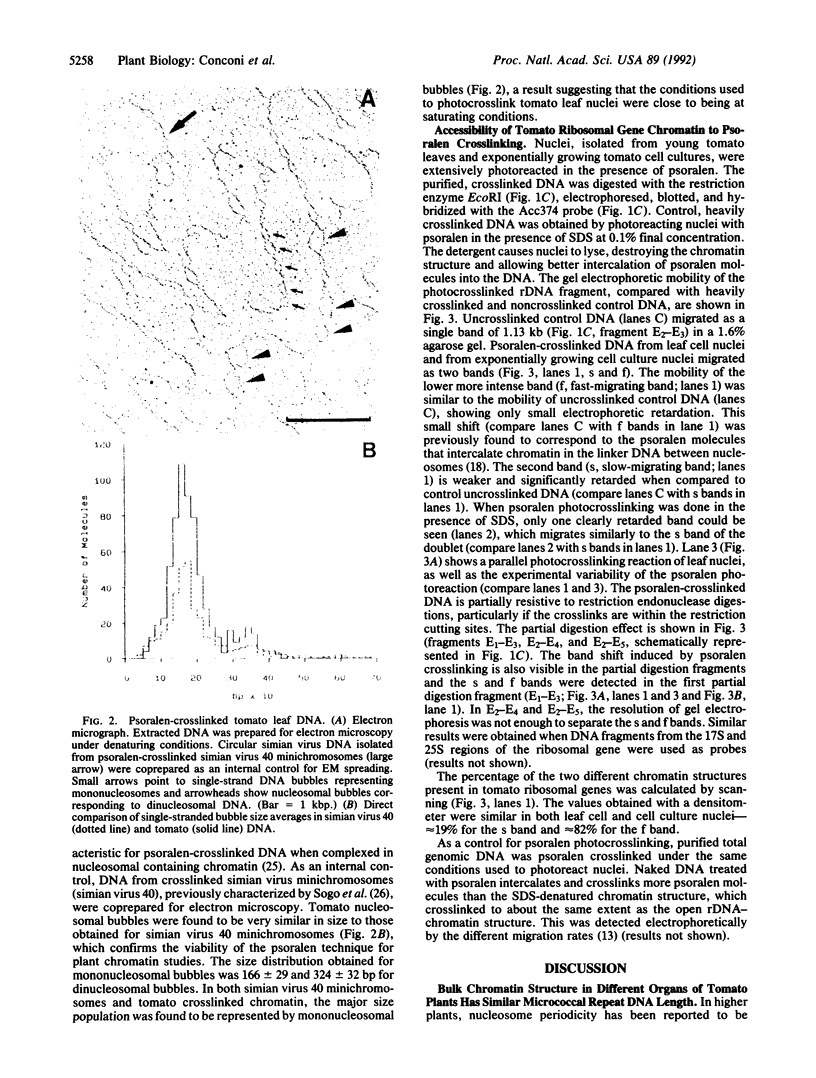
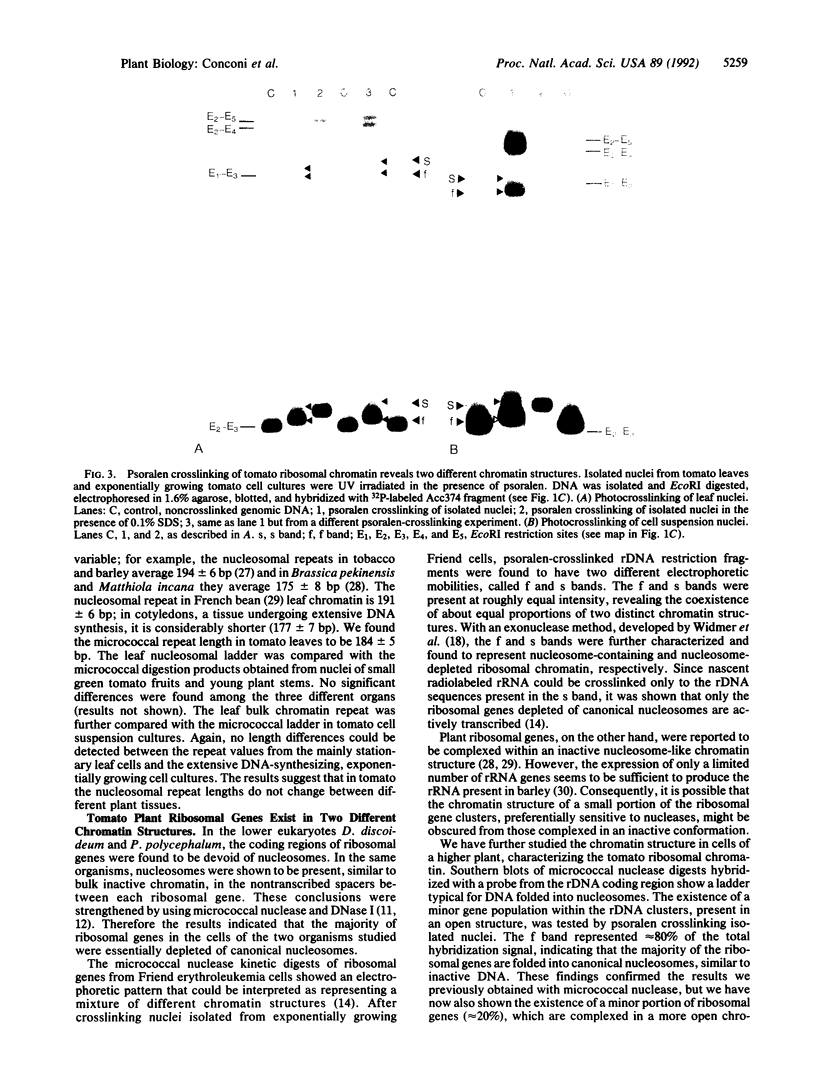

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butler P. J. The folding of chromatin. CRC Crit Rev Biochem. 1983;15(1):57–91. doi: 10.3109/10409238309102801. [DOI] [PubMed] [Google Scholar]
- Buttgereit D., Pflugfelder G., Grummt I. Growth-dependent regulation of rRNA synthesis is mediated by a transcription initiation factor (TIF-IA). Nucleic Acids Res. 1985 Nov 25;13(22):8165–8180. doi: 10.1093/nar/13.22.8165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conconi A., Losa R., Koller T., Sogo J. M. Psoralen-crosslinking of soluble and of H1-depleted soluble rat liver chromatin. J Mol Biol. 1984 Oct 5;178(4):920–928. doi: 10.1016/0022-2836(84)90319-x. [DOI] [PubMed] [Google Scholar]
- Conconi A., Widmer R. M., Koller T., Sogo J. M. Two different chromatin structures coexist in ribosomal RNA genes throughout the cell cycle. Cell. 1989 Jun 2;57(5):753–761. doi: 10.1016/0092-8674(89)90790-3. [DOI] [PubMed] [Google Scholar]
- Culotta V., Sollner-Webb B. Sites of topoisomerase I action on X. laevis ribosomal chromatin: transcriptionally active rDNA has an approximately 200 bp repeating structure. Cell. 1988 Feb 26;52(4):585–597. doi: 10.1016/0092-8674(88)90471-0. [DOI] [PubMed] [Google Scholar]
- Davis A. H., Reudelhuber T. L., Garrard W. T. Varigated chromatin structures of mouse ribosomal RNA genes. J Mol Biol. 1983 Jun 15;167(1):133–155. doi: 10.1016/s0022-2836(83)80038-2. [DOI] [PubMed] [Google Scholar]
- Hanson C. V., Shen C. K., Hearst J. E. Cross-linking of DNA in situ as a probe for chromatin structure. Science. 1976 Jul 2;193(4247):62–64. doi: 10.1126/science.935855. [DOI] [PubMed] [Google Scholar]
- Johnson E. M., Sterner R., Allfrey V. G. Altered nucleosomes of active nucleolar chromatin contain accessible histone H3 in its hyperacetylated forms. J Biol Chem. 1987 May 25;262(15):6943–6946. [PubMed] [Google Scholar]
- Kiss T., Kis M., Abel S., Solymosy F. Nucleotide sequence of the 17S-25S spacer region from tomato rDNA. Nucleic Acids Res. 1988 Jul 25;16(14B):7179–7179. doi: 10.1093/nar/16.14.7179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss T., Szkukálek A., Solymosy F. Nucleotide sequence of a 17S (18S) rRNA gene from tomato. Nucleic Acids Res. 1989 Mar 11;17(5):2127–2127. doi: 10.1093/nar/17.5.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leber B., Hemleben V. Structure of plant nuclear and ribosomal DNA containing chromatin. Nucleic Acids Res. 1979 Nov 10;7(5):1263–1281. doi: 10.1093/nar/7.5.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchini R., Pauli U., Braun R., Koller T., Sogo J. M. Structure of the extrachromosomal ribosomal RNA chromatin of Physarum polycephalum. J Mol Biol. 1987 Aug 20;196(4):829–843. doi: 10.1016/0022-2836(87)90408-6. [DOI] [PubMed] [Google Scholar]
- Luthe D. S., Quatrano R. S. Transcription in Isolated Wheat Nuclei: I. ISOLATION OF NUCLEI AND ELIMINATION OF ENDOGENOUS RIBONUCLEASE ACTIVITY. Plant Physiol. 1980 Feb;65(2):305–308. doi: 10.1104/pp.65.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui S. I., Weinfeld H., Sandberg A. A. Quantitative conservation of chromatin-bound RNA polymerases I and II in mitosis. Implications for chromosome structure. J Cell Biol. 1979 Feb;80(2):451–464. doi: 10.1083/jcb.80.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M. G., Thompson W. F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980 Oct 10;8(19):4321–4325. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ness P. J., Labhart P., Banz E., Koller T., Parish R. W. Chromatin structure along the ribosomal DNA of Dictyostelium. Regional differences and changes accompanying cell differentiation. J Mol Biol. 1983 May 25;166(3):361–381. doi: 10.1016/s0022-2836(83)80090-4. [DOI] [PubMed] [Google Scholar]
- Paule M. R., Iida C. T., Perna P. J., Harris G. H., Knoll D. A., D'Alessio J. M. In vitro evidence that eukaryotic ribosomal RNA transcription is regulated by modification of RNA polymerase I. Nucleic Acids Res. 1984 Nov 12;12(21):8161–8180. doi: 10.1093/nar/12.21.8161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pederson D. S., Thoma F., Simpson R. T. Core particle, fiber, and transcriptionally active chromatin structure. Annu Rev Cell Biol. 1986;2:117–147. doi: 10.1146/annurev.cb.02.110186.001001. [DOI] [PubMed] [Google Scholar]
- Philipps G., Gigot C. DNA associated with nucleosomes in plants. Nucleic Acids Res. 1977 Oct;4(10):3617–3626. doi: 10.1093/nar/4.10.3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior C. P., Cantor C. R., Johnson E. M., Littau V. C., Allfrey V. G. Reversible changes in nucleosome structure and histone H3 accessibility in transcriptionally active and inactive states of rDNA chromatin. Cell. 1983 Oct;34(3):1033–1042. doi: 10.1016/0092-8674(83)90561-5. [DOI] [PubMed] [Google Scholar]
- Scheer U., Rose K. M. Localization of RNA polymerase I in interphase cells and mitotic chromosomes by light and electron microscopic immunocytochemistry. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1431–1435. doi: 10.1073/pnas.81.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogo J. M., Ness P. J., Widmer R. M., Parish R. W., Koller T. Psoralen-crosslinking of DNA as a probe for the structure of active nucleolar chromatin. J Mol Biol. 1984 Oct 5;178(4):897–919. doi: 10.1016/0022-2836(84)90318-8. [DOI] [PubMed] [Google Scholar]
- Sogo J. M., Rodeño P., Koller T., Viñuela E., Salas M. Comparison of the A-T rich regions and the Bacillus subtilis RNA polymerase binding sites in phage phi 29 DNA. Nucleic Acids Res. 1979 Sep 11;7(1):107–120. doi: 10.1093/nar/7.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogo J. M., Stahl H., Koller T., Knippers R. Structure of replicating simian virus 40 minichromosomes. The replication fork, core histone segregation and terminal structures. J Mol Biol. 1986 May 5;189(1):189–204. doi: 10.1016/0022-2836(86)90390-6. [DOI] [PubMed] [Google Scholar]
- Sollner-Webb B., Mougey E. B. News from the nucleolus: rRNA gene expression. Trends Biochem Sci. 1991 Feb;16(2):58–62. doi: 10.1016/0968-0004(91)90025-q. [DOI] [PubMed] [Google Scholar]
- Sollner-Webb B., Tower J. Transcription of cloned eukaryotic ribosomal RNA genes. Annu Rev Biochem. 1986;55:801–830. doi: 10.1146/annurev.bi.55.070186.004101. [DOI] [PubMed] [Google Scholar]
- Thompson W. F., Flavell R. B. DNase I sensitivity of ribosomal RNA genes in chromatin and nucleolar dominance in wheat. J Mol Biol. 1988 Dec 5;204(3):535–548. doi: 10.1016/0022-2836(88)90353-1. [DOI] [PubMed] [Google Scholar]
- Widmer R. M., Koller T., Sogo J. M. Analysis of the psoralen-crosslinking pattern in chromatin DNA by exonuclease digestion. Nucleic Acids Res. 1988 Jul 25;16(14B):7013–7024. doi: 10.1093/nar/16.14.7013. [DOI] [PMC free article] [PubMed] [Google Scholar]