Abstract
One of the major challenges in biology is to explain how complex tissues and organs arise from the collective action of individual polarized cells. The best-studied model of this process is the cross talk between individual epithelial cells during their polarization to form the multicellular epithelial lumen during tissue morphogenesis. Multiple mechanisms of apical lumen formation have been proposed. Some epithelial lumens form from preexisting polarized epithelial structures. However, de novo lumen formation from nonpolarized cells has recently emerged as an important driver of epithelial tissue morphogenesis, especially during the formation of small epithelial tubule networks. In this review, we discuss the latest findings regarding the mechanisms and regulation of de novo lumen formation in vitro and in vivo.
Keywords: lumenogenesis, epithelial, coalescence
INTRODUCTION
Epithelial tissues are composed of polarized cells, which function as selectively permeable barriers. The plasma membrane (PM) of individual epithelial cells is divided into apical and basolateral domains; specialized junctional complexes between adjacent cells, such as the tight junctions (TJs), maintain separation of the apical and basolateral PM (Rodriguez-Boulan et al. 2004). Additionally, epithelial cells coordinate their polarization with neighboring cells to form an apical lumen, a key step in the establishment of renal and gut architecture and thus function (Bryant & Mostov 2008). Indeed, malfunctions in epithelial cell polarization and apical lumen formation are responsible for a variety of renal and intestinal disorders, such as polycystic kidney disease, renal tubular acidosis, microvilli inclusion disease (MVID), and diabetes insipidus. Additionally, tissue morphogenesis requires the coordinated action of groups of epithelial cells within three-dimensional (3D) space to establish spatial order and long-range luminal organization. How the formation of the apical lumen is regulated and coordinated has been a major question in the field of epithelial cell biology and development.
Recent work has shown that apical lumen formation is a complex process that involves dynamic restructuring of the cytoskeleton, endocytic membrane transport, and the formation of specialized polarity complexes. Despite these advances, many questions remain unanswered. How are apical endosomes targeted and regulated during apical lumen formation? How do cells establish a single apical lumen site? Do the mechanisms of lumen formation established in vitro also apply to epithelial morphogenesis in vivo? Improvement in imaging and the establishment of 3D tissue culture systems to study lumen formation now permit these questions to be addressed in greater detail. Indeed, during the past few years, multiple new models explaining the mechanisms of lumen formation have been proposed. Here, we discuss the latest findings regarding the mechanisms and regulation of de novo lumen formation in vitro and in vivo.
POLARIZATION OF INDIVIDUAL EPITHELIAL CELLS
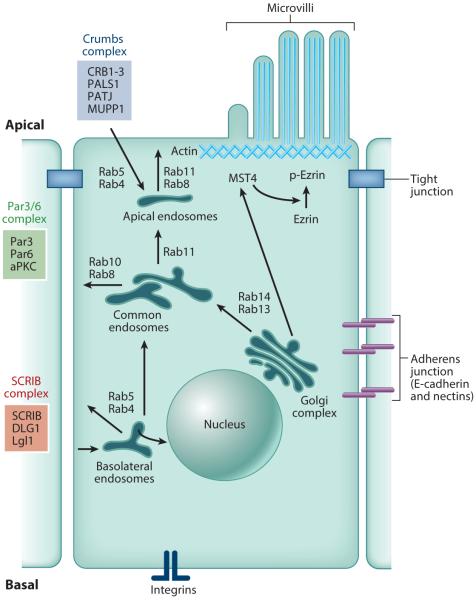
Epithelial cells are structurally and functionally polarized to directionally transport specific molecules while maintaining a transepithelial barrier. This selective transport is achieved by partitioning the PM into distinct apical and basolateral domains, which have distinct lipid compositions and specific sets of proteins. The apical PM is enriched in cholesterol, sphingolipids, and glycolipids and also contains various specific highly glycosylated proteins. By contrast, the basolateral membrane is enriched in E-cadherin, integrin molecules, and growth factor receptors (Mostov et al. 2003, Nelson 2003, Rodriguez-Boulan et al. 2004). TJs separate the apical and basolateral domains and provide a diffusion barrier that prevents the mixing of domain-specific membrane components (Figure 1). As the fidelity of apical versus basolateral protein and lipid distribution is crucial to a variety of epithelial functions, cells have developed mechanisms to selectively retain or deliver proteins to the basolateral and apical regions of the PM (Mostov et al. 2003, Nelson 2003, Rodriguez-Boulan et al. 2004).
Figure 1.
The proteins that establish and maintain epithelial cell polarity.
Polarity Complexes and the Actin Cytoskeleton
Polarity complexes are the key protein complexes that regulate the polarization of epithelial cells. Three major polarity complexes have been identified; each localizes to a different PM domain, and together they establish the identity and boundaries of the apical and basolateral domains (Bryant & Mostov 2008, Nelson 2003). The SCRIB and Crumbs complexes localize to and regulate the formation of the lateral and apical PM domains, respectively (Figure 1) (Nelson 2003, Schluter & Margolis 2012). By contrast, the Par3/6 complex localizes predominately to TJs and appears to play a key role in defining apical and lateral PM boundaries (Schluter & Margolis 2012). The key to Par3/6 complex function is the recruitment and activation of atypical PKC (aPKC) to TJs. Phosphorylation of Crumbs by aPKC promotes apical PM formation and expansion (Schluter & Margolis 2012), whereas phosphorylation of Lgl (a component of the SCRIB complex) leads to its degradation and the suppression of lateral PM expansion (Bryant & Mostov 2008, Schluter & Margolis 2012). The Par3/6 complex also interacts with Cdc42, a small monomeric GTPase that plays multiple roles during polarization. One of the main functions of Cdc42 is to regulate the recruitment of Par3/6 to TJs, thus promoting apical PM formation (Bryant & Mostov 2008, Schluter & Margolis 2012). Additionally, Cdc42 regulates apical actin polymerization, mitotic spindle orientation, and exocytosis/endocytosis of apical endosomes, the processes required for epithelial cell polarization. A detailed description of polarity complexes during epithelial cell polarization is outside the scope of this review, but several good reviews on this topic are available (Bryant & Mostov 2008, Nelson 2003, Schluter & Margolis 2012).
The actin cytoskeleton also plays a major role in the initiation and maintenance of epithelial cell polarity. It is well established that the actin cytoskeleton accumulates just beneath the apical PM (Figure 1) and that actin polymers form a dense network that also includes tropomyosin and cytokeratin-based intermediate filaments (Apodaca et al. 2012). This network, referred to as the terminal web, plays a major role in mediating apical localization of membrane proteins by anchoring them to the actin cytoskeleton via various actin binding proteins, such as EBP-50 (Bretscher et al. 2000). In addition, actin forms a junctional belt required for the formation and stability of TJs. At least to some extent, formation of the junctional belt is mediated by Cdc42 via recruitment of WASP and activation of Arp2/3 (Mellman & Nelson 2008).
In some epithelial cells, the establishment of the apical terminal web is followed by the formation of microvilli, apical structures that are specialized for efficient nutrient uptake. Disruption of microvillus formation leads to MVID and microvillus atrophy (Ameen & Salas 2000, Erickson et al. 2008). The mechanisms mediating microvillus formation are only beginning to emerge, but recent work demonstrates that Rap2a delivery to the apical PM initiates a signaling cascade that leads to the apical recruitment of MST4 kinase from the trans-Golgi network (TGN) and the phosphorylation of Ezrin, resulting in the formation of actin-rich microvilli (Figure 1) (Gloerich et al. 2012). The terminal web is also required for microvilli stability, as microvilli terminate in actin filament rootlets anchored to the actin substructure of the terminal web by binding to Myo2 and Fodrin (Bretscher et al. 2002). What remains to be understood is how Rap2a is targeted to the apical PM. Recent work has shown that Rap2a localizes to Rab11 endosomes (Bruurs & Bos 2014). This raises the intriguing possibility that Rab11 endosomes deliver Rap2a to the apical PM to initiate microvilli formation. Consistent with this hypothesis, the Rab11-binding protein myosin-Vb is required for microvilli formation and is mutated in MVID patients (Muller et al. 2008, Szperl et al. 2011). Collectively, these new data reveal that cross talk between the polarity complexes, endocytic transport pathways, and actin cytoskeleton underlies the establishment of epithelial cell polarity.
Polarized Membrane Traffic
Epithelial polarity is tightly regulated by polarized membrane transport. Polarized epithelial cells have domain-specific early endosomes: apical and basolateral endosomes, as well as common recycling endosomes (Figure 1). In addition, most polarized epithelial cells have apical recycling endosomes (AREs), which are involved in regulated recycling of specialized apical proteins. For example, regulated insertion of H+/K+-ATPase into the apical PM of gastric parietal cells is responsible for the selective secretion of hydrochloric acid into the stomach lumen and is mediated by AREs (Calhoun et al. 1998, Duman et al. 1999). Most apically or basolaterally internalized proteins are eventually transferred to common recycling endosomes, where they are selectively segregated and sorted for transport to their final destinations (Figure 1). For instance, basolateral proteins such as E-cadherin, the transferrin receptor, or the low density lipoprotein receptor are targeted from common recycling endosomes to the basolateral PM via a pathway that involves the AP1B coat protein as well as the Exocyst protein complex (Lock & Stow 2005, Lock et al. 2005). In addition, newly synthesized proteins exiting the TGN are sorted and delivered to either the apical or basolateral PM. Some data suggest that many proteins are directed from the TGN to recycling endosomes before they are sorted to the apical or basolateral surfaces.
It is now well established that polarized epithelial cells rely on domain-specific endosomes to act as intermediaries for polarized transport of endocytic cargo to their appropriate PM domains (Figure 1) (Apodaca et al. 2012). Traffic through these domain-specific endosomes is mediated by Rabs, small monomeric GTPases that act as master regulators of membrane transport (Figure 1). Rabs function by recruiting various membrane transport regulators, known as effectors, to their respective membranes and then activating them. These Rab/effector complexes are responsible for very specific and selective regulation of distinct membrane transport steps. To date, several Rabs have been implicated in regulating epithelial transport, namely Rab8, Rab10, Rab11, Rab14, Rab17, and Rab25 (Figure 1) (Apodaca et al. 2012).
Rab11-family GTPases (Rab11a, Rab11b, and Rab25 isoforms) have emerged as key regulators of polarized transport in epithelial cells. Whereas Rab11a and Rab11b are ubiquitously expressed, Rab25 is present only in epithelial cells (Goldenring et al. 1993, 2001). Multiple studies have implicated Rab11-family proteins in regulating apical PM trafficking via AREs (Casanova et al. 1999). For example, expression of Rab11a/b dominant-negative mutants inhibits apical recycling and transcytosis of IgA (Casanova et al. 1999, Wang et al. 2000). Rab11 has also been implicated in regulating H+/K+-ATPase-dependent secretion of hydrochloric acid into the stomach lumen. In resting parietal cells, H+/K+-ATPase is sequestered within subapical tubulovesicles. Upon stimulation, the H+/K+-ATPase-containing tubulovesicles, whose function is probably equivalent to that of AREs, fuse with the apical PM, which results in the delivery of H+/K+-ATPase and the subsequent secretion of hydrochloric acid. This stimulated delivery of H+/K+-ATPase depends on the Rab11a/b GTPase, as well as its binding proteins (Calhoun et al. 1998). In addition to regulating apical recycling, Rab11 has been suggested to regulate transcytosis of proteins endocytosed at the basolateral PM for secretion at apical PM domains (Apodaca et al. 1994). Finally, recent studies have implicated Rab11 in regulating biosynthetic delivery of E-cadherin from the TGN to the basolateral PM (Lock et al. 2005) and Endolyn delivery from the TGN to the apical PM (Potter et al. 2006).
Because Rab GTPases often bind to multiple effector proteins, much effort has been dedicated to the identification of Rab11a/b effector proteins. Rab11a/b binds to myosin-Vb and Sec15 (a subunit of the Exocyst complex) (Hales et al. 2002, Zhang et al. 2004). This presumably allows specific targeting of Rab11 endosomes to the apical PM, as the Exocyst complex functions as a PM tether and myosin-Vb likely mediates the binding of Rab11 vesicles to the actin-rich terminal web. Rab11a also binds to and recruits Rabin8. Rabin8 is a GTP exchange factor (GEF) that activates Rab8, a GTPase also implicated in polarized endocytic transport (Figure 1) (Bryant et al. 2010). Indeed, the Rab11-Rabin8-Rab8 cascade regulates several polarized transport steps, including membrane transport to form primary cilia (Westlake et al. 2011). Rab11a/b GTPases also bind to Rab11 family–interacting proteins (known as FIPs) (Hales et al. 2001; Prekeris et al. 2000, 2001). FIPs act as scaffolds for the recruitment of additional endocytic transport factors (Prekeris 2003), with FIP2 and FIP5 involved in apically directed endosomal transport (Prekeris et al. 2000, Willenborg et al. 2011). FIP2 binds to myosin-Vb, thus mediating Rab11/FIP2/myosin-Vb complex formation (Hales et al. 2002, Lapierre et al. 2001). Similarly, FIP5 binds to Kinesin-2 and sorting nexin 18 (SNX18), both regulators of apical protein transport (Li et al. 2014a, Willenborg et al. 2011). Thus, a complex network of endosomal transport pathways and various Rab/effector complexes are required for epithelial cell polarization.
APICAL LUMEN FORMATION
A major challenge in biology is to explain how the interactions of individual polarized cells result in the formation of complex tissues. Although the mechanisms that govern individual epithelial cell polarization are now well understood, little is known about how these cells cross talk to organize and form polarized structures, such as the apical lumens within epithelial ducts and terminal endbuds. One way to form new lumens is from preexisting polarized epithelia, by either folding of the epithelial sheet or budding/sprouting from epithelial tubes. This type of lumen formation has been actively studied in the past couple of decades and is well described in a recent review (Andrew & Ewald 2010). More enigmatic is the de novo formation of apical lumens from nonpolarized cells. One key feature of de novo lumen formation is the simultaneous polarization of epithelial cells and establishment of a new lumen. The molecular machinery mediating the coordination between polarization of individual epithelial cells and apical lumen formation in vitro and in vivo is only beginning to be defined and will be the focus of the rest of this review.
In Vitro Models of De Novo Apical Lumen Formation
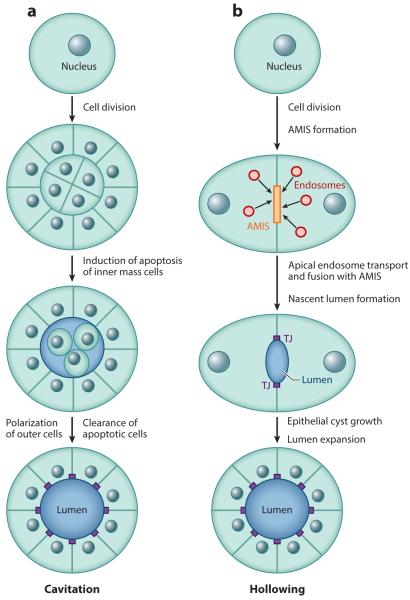
The key to advancing our understanding of lumenogenesis was the development of 3D tissue culture systems. These 3D assays rely on the suspension and growth of individual epithelial cells in the extracellular matrix (ECM). Typically, Matrigel or collagen matrices are used to mimic the in vivo extracellular environment. These matrices provide the specific structural and/or molecular signaling interactions required for epithelial cells to form spherical cysts with a single central hollow lumen. These cysts, or acini, are an excellent model system for studying the mechanisms of apical lumen formation during tissue morphogenesis. As the result of these studies two different, but not mutually exclusive, models of lumen formation have been proposed: cavitation and hollowing.
Cavitation model
One of the first models describing the mechanisms of de novo lumen formation in vitro was proposed by Joan Brugge and colleagues (Debnath & Brugge 2005) based on their work using the breast epithelial cell line MCF10A, and it is currently known as the cavitation model. Cavitation (also called canalization or lumenization) is the creation of a luminal space within a preexisting mass of cells that involves apoptosis of cells within the developing lumen. In an in vitro 3D model system, cells that are in contact with the ECM can differentiate and establish apical/basal polarity (Figure 2a) (Debnath & Brugge 2005). By contrast, cells located inside the spheroid (sometimes referred to as the mamosphere) are not able to contact the ECM. Consequently, they do not receive the necessary survival signals, triggering apoptosis (Debnath & Brugge 2005). The apoptotic cells are then cleared, resulting in the formation of hollow acini with a single apical lumen (Figure 2a).
Figure 2.
The (a) cavitation and (b) hollowing models of apical lumen formation in vitro. Red circles represent apical endosomes. Abbreviations: AMIS, apical membrane initiation site; TJ, tight junction.
Tissues that undergo cavitation include mammary and salivary glands, which rely on highly proliferative states to produce an interconnecting network of ducts with terminal endbuds (Coucouvanis & Martin 1995, Jaskoll & Melnick 1999, Mailleux et al. 2008). In 3D cultures of the nontumorigenic human mammary cell line MCF10A, targeted knockdown of the proapoptotic BCL-2 family proteins Bim and Bmf decreases apoptosis and impairs lumen formation (Mailleux et al. 2007, Reginato et al. 2005, Schmelzle et al. 2007). However, impaired lumen formation is only temporary; the acini do form hollow apical lumens after a few more days in culture (Mailleux et al. 2007). Interestingly, activation of ErbB2 (EGF receptor/Her2), the receptor overexpressed in some breast cancers, increases proliferation and filling of the apical lumen (Muthuswamy et al. 2001). Thus, it is clear that multiple, partially redundant molecular mechanisms are involved in cavitation, and further studies are needed to identify all the components of this lumen formation pathway.
Hollowing model
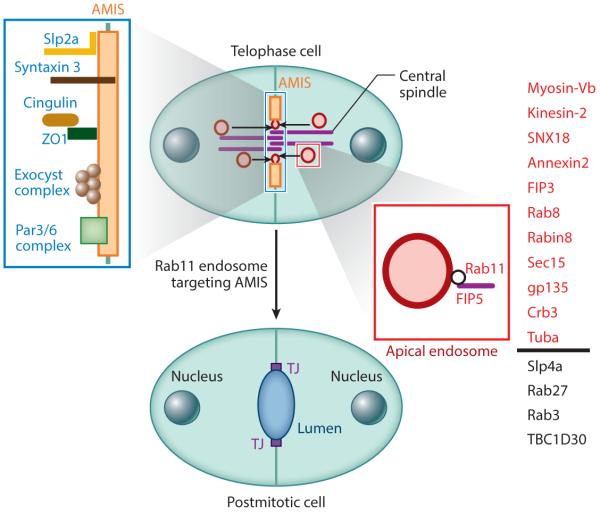
Although the cavitation model explains lumen formation in some tissues, in many cases apoptosis is not required to form an apical lumen. An apical lumen can form de novo between two cells as the result of coordinated cell division and polarization (Figure 2b) (Bryant et al. 2010, Li et al. 2014b). This mechanism of lumen formation, known as hollowing, was originally proposed by Keith Mostov and colleagues based on their work using Madin-Darby canine kidney (MDCK) cells (Bryant et al. 2010, Datta et al. 2011). In this model, the apical lumen forms between as few as two cells and is dependent on targeted trafficking of apical cargo to a specific location known as the apical membrane initiation site (AMIS) (Figure 2b). AMIS formation appears to be the key step in establishing the location of the nascent apical lumen and provides the targeting site for apical endosomes (Bryant et al. 2010, Li et al. 2014b, Willenborg et al. 2011). The AMIS is a transient structure that contains the Par3/Par6 polarity complex, the Exocyst targeting complex, and the canonical TJ proteins Cingulin and ZO1 (Bryant et al. 2010, Li et al. 2014b). As an apical lumen forms, the AMIS matures into the TJs that define the boundary between the apical and basolateral PM (Bryant et al. 2010, Ferrari et al. 2008, Li et al. 2014b) (Figure 2b).
Targeted transport and fusion of apical endosomes to the AMIS underlies the formation of nascent apical lumens (Figure 2b). Embedded in the ECM, each cyst begins as a nonpolarized single cell. In these cells, apical cargo such as Crumbs3a and glycoprotein-135 (gp135, also called podocalyxin) are either dispersed within the PM or located within the subpopulation of recycling endosomes (Bryant et al. 2010, Ferrari et al. 2008). During apical lumen initiation, gp135 and Crumbs3a are concentrated in Rab11a-containing transport vesicles. Formation and targeting of these vesicles is dependent on the interactions between Rab11 and its effector protein FIP5 (Figure 3). FIP5 depletion in MDCK cells induces the formation of multiluminal cysts (Willenborg et al. 2011). Furthermore, FIP5 directly interacts with SNX18, the protein shown to increase apical transport vesicle formation (Figure 3) (Willenborg et al. 2011).
Figure 3.
The role of the midbody during apical membrane initiation site (AMIS) formation and apical endosome recruitment. Red indicates apical endosomes and all proteins known to be associated with them. Blue indicates AMIS-associated proteins. Proteins listed in black are known to be required for lumen formation but have unclear subcellular localization.
FIP5 directly interacts with the motor protein Kinesin-2 (Li et al. 2014a). This interaction allows Rab11/FIP5-containing vesicles to be transported along microtubules toward the forming apical domain (Figure 3) (Li et al. 2014a). Kinesin-2 competes with SNX18 for FIP5 binding, further supporting a stepwise flow from vesicle budding to vesicle transport (Li et al. 2014a). Although these vesicles are known to fuse at the AMIS, a specific targeting or tethering mechanism has yet to be determined; it is likely the result of a combination of factors. The Exocyst complex is involved in vesicle fusion and could serve as a tether for Rab11/FIP5 vesicles at the AMIS (Datta et al. 2011). The Rab11/FIP5 apical vesicles also contain Rab8 and Cdc42, which could regulate targeting to the aPKC/Par3 complex located at the AMIS (Figure 3) (Bryant et al. 2010). Apical endosome–associated Rab8 is activated by Rabin8, a GEF for Rab8 that is recruited to the apical vesicles through direct binding to Rab11a (Bryant et al. 2010). Although it is clear that Cdc42 and Rab8 are important for apical lumen formation, the molecular basis for their role during lumenogenesis remains to be fully defined.
Recently, several other molecules have been implicated in the regulation of vesicular transport and targeting to the AMIS. The synaptotagmin-like proteins Slp2a and Slp4a are required for single lumen formation (Figure 3) (Galvez-Santisteban et al. 2012). It has been proposed that Slp2a is concentrated at the AMIS through its interactions with PIP2, thus mediating the apical targeting of Rab27-containing transport vesicles. Slp4a targets Rab3 to the same Rab27-containing vesicles and may participate in targeting these vesicles to the AMIS (Galvez-Santisteban et al. 2012). It remains unclear whether Rab11 and FIP5 are also present in Rab27-containing organelles. One possibility is that Rab27/Rab3- and Rab11-dependent targeting pathways function as a coincidence detection system, ensuring the fidelity of apical protein targeting to the AMIS. Alternatively, Rab27/Rab3 and Rab11 vesicles may transport different apical cargo to the AMIS during lumen formation.
One question that still remains is how cells create a single AMIS. Formation of the midbody during the first cell division is the first symmetry-breaking event that initiates the polarization of the two daughter cells and induces lumen formation (Figure 3) (Li et al. 2014b, Schluter et al. 2009). The AMIS forms around the midbody during late telophase, thus marking the apical domain, which is retained and expanded with subsequent cell divisions (Li et al. 2014b). During metaphase and anaphase of the first cell division, Rab11/FIP5 endosomes are localized at the centrosomes and are not transported to the PM until after midbody formation in late telophase (Figure 3) (Li et al. 2014a,b). The machinery that mediates the timing of Rab11/FIP5-endosome transport remains to be fully defined. However, FIP5-T276 phosphorylation by GSK-3 during metaphase and anaphase inhibits FIP5 interaction with SNX18, thus preventing apical vesicle budding until late telophase (Li et al. 2014b).
Both the cavitation and hollowing models provide viable mechanisms of de novo lumen formation in vitro. Why two different means of lumen formation are needed in vivo remains to be understood. It is possible that distinct lumenogenesis mechanisms function in different tissues to accommodate tissue-specific dynamics and regulation of morphogenesis. It has been proposed that cavitation is the predominant mechanism of lumen formation during mammary duct morphogenesis and remodeling (Debnath & Brugge 2005). It is also possible that, at least in some tissues, the formation and maintenance of a single lumen is a result of both lumenogenesis methods. For example, an apical lumen may be initially formed by hollowing; apoptosis may only be used during lumen expansion and maintenance to remove any cells that begin to invade the luminal space.
Mechanisms of De Novo Apical Lumen Formation During Epithelial Tissue Morphogenesis
Our understanding of apical lumen formation has been derived primarily through in vitro 3D lumen formation assays, as described above. Although in vitro cell culture is a powerful experimental approach for identifying candidate molecular mechanisms of lumen formation, it lacks the inherent complexity of in vivo morphogenesis and organogenesis, which draw on dynamic polarity cues and positional information. Despite the wealth of information on lumen formation derived from in vitro studies, our understanding of how lumens form in vivo remains limited. Consequently, experimentally manipulatable animal models are needed to understand how the lumen forms in the context of in vivo development. In the next section, we highlight recent advances in understanding epithelial de novo lumen formation in vivo, using fly, worm, and fish models.
Drosophila melanogaster tracheal cells
The Drosophila tracheal system is a powerful model system for identifying and investigating molecular mechanisms of lumen formation in vivo. The fly tracheal system is composed of a network of epithelial tubes that transport oxygen to tissues. During embryonic development, the tracheal system forms by the invagination of epidermal placodes. Cells migrate from sites of placode invagination to form primary branches. These primary branches connect with cognate branches from adjacent primordia, building an interconnected network with a continuous lumen (Samakovlis et al. 1996).
De novo lumen formation occurs throughout the developing Drosophila tracheal system. Specialized fusion cells mediate lumen formation and elongation within primary branches. The site at which fusion cells contact each other acquires apical characteristics that depend on a localized increase in nucleation of the actin and microtubule cytoskeleton. Actin and microtubules aid in the targeted transport of apical cargo and establishment of cell structure (Lee et al. 2003, Lee & Kolodziej 2002). Vesicles and apical proteins, including the polarity proteins aPKC, Bazooka, and Crumbs are then targeted to the contact region to aid in lumen formation (Gervais et al. 2012). The small GTPase Arf-like 3 (Arl3) functions in the exocytic transport of cargo to the fusion site (Kakihara et al. 2008).
The fly tracheal system also contains terminal cells that connect to the tubular network via an invagination around a circular adherens junction. Previously, the terminal cell lumen was thought to form by the coalescence of intracellular vesicles. However, recent data suggest that the lumen is formed by the addition of apical membrane at the trunk cell junction site (Gervais & Casanova 2010). The initial site of lumen growth into terminal cells is defined by the accumulation of microtubules (Gervais & Casanova 2010). Microtubules extend from the intercellular junction to the cell boundary before the terminal cell elongates and any subcellular lumen is formed. Tyrosinated tubulin is specifically enriched at the front of the growing lumen and may act as a guide for lumenogenesis (Gervais & Casanova 2010), reminiscent of vesicle delivery in the formation of the lumen along central spindle microtubules during hollowing in vitro (see Figure 3).
Vesicle transport is also a key step during the formation of the lumen in terminal cells. Mutations in NSF2, the protein required for SNARE complex disassembly, disrupt apical membrane expansion (Song et al. 2013). Further, Germinal center kinase III is required for regulating the traffic of material to the apical domain (Song et al. 2013). The Exocyst complex, a known component of AMIS, is also required for PM morphogenesis in terminal cells; presumably it mediates the targeting and tethering of apical transport vesicles. Another AMIS component, the Par3/6 polarity complex, provides membrane localization cues for the Exocyst (Jones & Metzstein 2011). Rab35 has also been implicated in lumen formation in vivo (Schottenfeld-Roames & Ghabrial 2012), although its role in lumenogenesis remains to be defined.
Caenorhabditis elegans excretory cells
The C. elegans excretory system also provides significant insights into lumen formation in vivo. It consists of five epithelial cells that form fluid-filled tubules. The excretory cell is polarized, with an apical PM along the luminal surface, and contributes to most of the luminal structure of the system. During development, the excretory cell grows in an H shape, with four processes extending anteriorly and posteriorly along the body of the animal, and these processes continue to grow throughout development. Similar to MDCK cells in 3D tissue culture and fly terminal cells, the worm apical membrane grows distally from the cell body through the targeting and fusion of intracellular vesicles (Khan et al. 2013, Kolotuev et al. 2013). The cytoplasm surrounding the tube contains cyst-like membrane structures called canaliculi. In response to osmotic stress, canaliculi fuse to the luminal membrane to rapidly increase the size of the apical membrane (Khan et al. 2013, Kolotuev et al. 2013). The small GTPase RAL-1 and the polarity protein Par3 are both necessary for delivery of canaliculi to the lumen and excretory lumen outgrowth (Armenti et al. 2014).
Danio rerio intestinal cells
Zebrafish are increasingly used as a vertebrate model to understand lumen formation, owing in part to the ease of in vivo imaging and their amenability to genetic manipulation. Recent work on de novo lumen formation during development of the zebrafish vasculature system has provided valuable insights into the plasticity of lumen formation in vivo (Blum et al. 2008, Lenard et al. 2013).
The zebrafish intestine has emerged as an equally useful model for investigating epithelial de novo lumen formation. It originates as a solid rod of endodermal cells that differentiate into polarized epithelial cells prior to lumen formation. The gut lumen forms through the progressive coalescence of minilumens, and not through cavitation, as significant apoptosis is not seen (Ng et al. 2005). Initially, actin-rich foci localize to the site of nascent lumens (Horne-Badovinac et al. 2001). Formation of these actin foci resembles AMIS formation in vitro, as they contain many of the same proteins, including TJ components and the Par3/6 complex. Furthermore, the Par3/6 complex component aPKC is required to establish epithelial cell polarity; defects in aPKC function result in a gut with multiple lumens (Horne-Badovinac et al. 2001), resembling the phenotypes observed in 3D tissue culture systems. After the site of lumenogenesis is established, subsequent lumen expansion is driven by fluid accumulation generated by paracellular and transcellular ion transport. Ion transport in the zebrafish gut epithelium is regulated, at least in part, by the junctional protein Claudin 15 and Na+/K+-ATPase. Disruption of their function results in a multiple lumen defect (Bagnat et al. 2007).
The Mechanisms of Lumen Extension and Coalescence
Although in vitro models allow study of the mechanisms governing lumen initiation, they are primarily derived from studies of cysts forming from a single cell. In animal systems, by contrast, epithelial lumens often form from simultaneous polarization of multiple cells. Thus, the coalescence of multiple minilumens is a key step during the formation of apical tubules and networks. Lumen expansion and coalescence can be mediated through fluid accumulation and the generation of a turgor force. Turgor is generated by hydrostatic pressure, which builds through the accumulation of ion channels and pumps at the apical PM. For example, MDCK cysts use the cystic fibrosis transmembrane conductance regulator for lumen expansion (Bagnat et al. 2007). In the C. elegans excretory system, the water channel AQP8 mediates fluid accumulation and regulates tube size (Khan et al. 2013, Kolotuev et al. 2013).
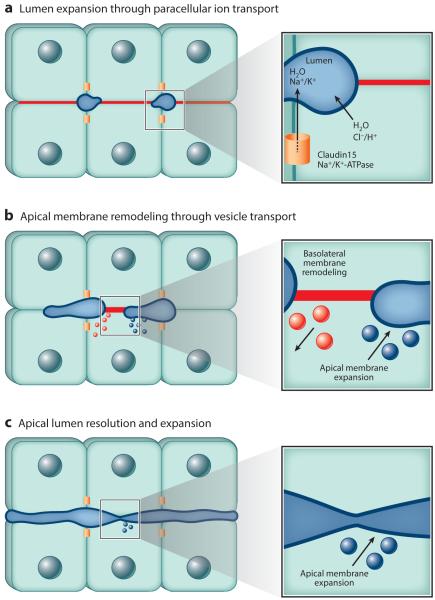
Most new insights into minilumen coalescence have come from work on lumen formation in the zebrafish gut. Lumenogenesis in the developing zebrafish gut does not require apoptosis (Ng et al. 2005), suggesting that hollowing-like processes drive the formation and remodeling of intestinal epithelia. Zebrafish gut lumenogenesis is initiated by de novo formation of multiple minilumens. Upon maturation, these minilumens fuse, providing a perfect system to study the machinery mediating lumen coalescence (Ng et al. 2005). Paracellular and transcellular ion transport, regulated by Claudin 15 and the Na+/K+-ATPase, drives fluid accumulation and is a key determinant of gut lumen expansion (Bagnat et al. 2007). However, lumen expansion resulting from fluid accumulation is not the sole mechanism driving lumen coalescence. Prior to lumen coalescence, enlarged lumens are present along the length of the zebrafish gut, separated by basolateral cell junctions that lack the apical junction and TJ markers podocalyxin and (Figure 4) (Alvers et al. 2014). The expansion of the zebrafish gut lumen is mediated, in part, through the Hedgehog pathway protein Smoothened and occurs through both the breaking of basolateral cell contacts and the expansion of the apical membrane (Alvers et al. 2014) (Figure 4). This process presumably requires the simultaneous targeted delivery and fusion of new apical endosomes and removal of basolateral PM proteins by endocytosis (Figure 4), although the machinery mediating and regulating this process remains unclear.
Figure 4.
Model depicting apical lumen coalescence during the formation of the zebrafish intestinal lumen.
(a) Definition of the apical lumen initiation site and initial formation of the zebrafish gut lumen require localization of apical and tight junction proteins. Both Claudin 15 and Na+/K+-ATPase are required for the paracellular ion transport necessary for lumen fluid accumulation and initial gut lumen expansion. (b) Gut lumen expansion occurs along adjacent basolateral membranes (red line) through Rab11-mediated apical trafficking (blue) and concurrent with basolateral membrane recycling (red ). (c) The mechanisms regulating the coalescence of multiple expanding apical lumens into a single continuous lumen remain unresolved. The Hedgehog pathway protein Smoothened is required for lumen resolution, but not lumen expansion, suggesting lumen expansion and resolution are two distinct processes.
SUMMARY AND FUTURE OBJECTIVES
Polarization of epithelial cells is a seminal step in epithelial tissue morphogenesis during development and epithelial tissue reorganization in adults. The emergence of in vitro 3D tissue culture models and novel imaging techniques has led to significant progress in identifying the factors that establish epithelial polarity at a single-cell level, as well as during formation of multicellular epithelial tissues. We now know that the targeted transport and fusion of apical endosomes play pivotal roles in delivering essential polarity factors and in the formation of the apical lumen (Bryant & Mostov 2008). The role of the actin and microtubule cytoskeleton during lumenogenesis is also well established. However, many questions remain unanswered. How are apical endosomes targeted during apical lumen formation? How do cells establish the site of a single apical lumen? How do multiple minilumens resolve to form intricate networks of tubes and terminal endbuds? Further study is required to answer these pivotal questions.
Recently, the midbody was identified as a unique cellular structure intimately involved in regulating cell fate and polarization (Li et al. 2014b, Schluter et al. 2009, Wang et al. 2014). In vitro studies have shown that midbody formation is the first symmetry-breaking event that initiates epithelial polarization and determines the location of nascent apical lumen. Consistent with these concepts, a dramatic increase in cell division precedes the initiation of de novo lumen formation in vivo. Midbody effects are not limited to epithelial cells, as midbodies regulate mitotic spindle positioning during early C. elegans development (Singh & Pohl 2014). Similarly, midbodies mark the location of axon outgrowth in fly neurons (Pollarolo et al. 2011). Based on these and similar findings, midbodies are emerging as important regulators of epithelial polarization, although the machinery mediating these midbody functions remains unknown. Further research is needed to define midbody function during tissue morphogenesis.
Most work on apical lumen formation has been done using 3D tissue culture–based models. Although these are powerful experimental approaches, morphogenesis and organogenesis in vivo draws on complex polarity/positional information cues that cannot be fully replicated in cell culture. Thus, the extent to which proteins that regulate single lumen formation in 3D tissue culture systems contribute to lumen formation in vivo remains to be determined. Furthermore, whereas in vitro epithelial cysts start as single cells embedded in the ECM, most epithelial tissues form from hundreds of nonpolarized cells that simultaneously undergo polarization to generate apical lumens in vivo. How the formation and coalescence of these minilumens is organized and regulated remains unknown. The emergence of novel genomic editing approaches, as well as animal models such as zebrafish, will allow analysis of the machinery of epithelial morphogenesis in complex vertebrate organ systems.
ACKNOWLEDGMENTS
We apologize to our colleagues for not being able to cite all work related to cytokinesis, given the focused nature of this review and its requirement for brevity. We are grateful to James McManaman for critical reading of this manuscript. Research in R.P.’s laboratory is supported by the National Institutes of Health (R01 DK064380) and the Cancer League of Colorado Foundation. A.M. is supported by an NIH T32 training grant (T32 GM08730).
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Contributor Information
Alex J. Blasky, Department of Cell and Developmental Biology, University of Colorado Anschutz Medical Campus, Aurora, Colorado 80045
Anthony Mangan, Department of Cell and Developmental Biology, University of Colorado Anschutz Medical Campus, Aurora, Colorado 80045.
Rytis Prekeris, Department of Cell and Developmental Biology, University of Colorado Anschutz Medical Campus, Aurora, Colorado 80045; rytis.prekeris@ucdenver.edu.
LITERATURE CITED
- Alvers AL, Ryan S, Scherz PJ, Huisken J, Bagnat M. Single continuous lumen formation in the zebrafish gut is mediated by smoothened-dependent tissue remodeling. Development. 2014;141:1110–19. doi: 10.1242/dev.100313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameen NA, Salas PJ. Microvillus inclusion disease: a genetic defect affecting apical membrane protein traffic in intestinal epithelium. Traffic. 2000;1:76–83. doi: 10.1034/j.1600-0854.2000.010111.x. [DOI] [PubMed] [Google Scholar]
- Andrew DJ, Ewald AJ. Morphogenesis of epithelial tubes: insights into tube formation, elongation, and elaboration. Dev. Biol. 2010;341:34–55. doi: 10.1016/j.ydbio.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apodaca G, Gallo LI, Bryant DM. Role of membrane traffic in the generation of epithelial cell asymmetry. Nat. Cell Biol. 2012;14:1235–43. doi: 10.1038/ncb2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apodaca G, Katz LA, Mostov KE. Receptor-mediated transcytosis of IgA in MDCK cells is via apical recycling endosomes. J. Cell Biol. 1994;125:67–86. doi: 10.1083/jcb.125.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armenti ST, Chan E, Nance J. Polarized exocyst-mediated vesicle fusion directs intracellular lumenogenesis within the C. elegans excretory cell. Dev. Biol. 2014;394:110–21. doi: 10.1016/j.ydbio.2014.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnat M, Cheung ID, Mostov KE, Stainier DY. Genetic control of single lumen formation in the zebrafish gut. Nat. Cell Biol. 2007;9:954–60. doi: 10.1038/ncb1621. [DOI] [PubMed] [Google Scholar]
- Blum Y, Belting HG, Ellertsdottir E, Herwig L, Luders F, Affolter M. Complex cell rearrangements during intersegmental vessel sprouting and vessel fusion in the zebrafish embryo. Dev. Biol. 2008;316:312–22. doi: 10.1016/j.ydbio.2008.01.038. [DOI] [PubMed] [Google Scholar]
- Bretscher A, Chambers D, Nguyen R, Reczek D. ERM-Merlin and EBP50 protein families in plasma membrane organization and function. Annu. Rev. Cell Dev. Biol. 2000;16:113–43. doi: 10.1146/annurev.cellbio.16.1.113. [DOI] [PubMed] [Google Scholar]
- Bretscher A, Edwards K, Fehon RG. ERM proteins and merlin: integrators at the cell cortex. Nat. Rev. Mol. Cell Biol. 2002;3:586–99. doi: 10.1038/nrm882. [DOI] [PubMed] [Google Scholar]
- Bruurs LJ, Bos JL. Mechanisms of isoform specific Rap2 signaling during enterocytic brush border formation. PLOS ONE. 2014;9:e106687. doi: 10.1371/journal.pone.0106687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant DM, Datta A, Rodriguez-Fraticelli AE, Peranen J, Martin-Belmonte F, Mostov KE. A molecular network for de novo generation of the apical surface and lumen. Nat. Cell Biol. 2010;12:1035–45. doi: 10.1038/ncb2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant DM, Mostov KE. From cells to organs: building polarized tissue. Nat. Rev. Mol. Cell Biol. 2008;9:887–901. doi: 10.1038/nrm2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun BC, Lapierre LA, Chew CS, Goldenring JR. Rab11a redistributes to apical secretory canaliculus during stimulation of gastric parietal cells. Am. J. Physiol. 1998;275:C163–70. doi: 10.1152/ajpcell.1998.275.1.C163. [DOI] [PubMed] [Google Scholar]
- Casanova JE, Wang X, Kumar R, Bhartur SG, Navarre J, et al. Association of Rab25 and Rab11a with the apical recycling system of polarized Madin-Darby canine kidney cells. Mol. Biol. Cell. 1999;10:47–61. doi: 10.1091/mbc.10.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coucouvanis E, Martin GR. Signals for death and survival: a two-step mechanism for cavitation in the vertebrate embryo. Cell. 1995;83:279–87. doi: 10.1016/0092-8674(95)90169-8. [DOI] [PubMed] [Google Scholar]
- Datta A, Bryant DM, Mostov KE. Molecular regulation of lumen morphogenesis. Curr. Biol. 2011;21:R126–36. doi: 10.1016/j.cub.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnath J, Brugge JS. Modelling glandular epithelial cancers in three-dimensional cultures. Nat. Rev. Cancer. 2005;5:675–88. doi: 10.1038/nrc1695. [DOI] [PubMed] [Google Scholar]
- Duman JG, Tyagarajan K, Kolsi MS, Moore HP, Forte JG. Expression of rab11a N124I in gastric parietal cells inhibits stimulatory recruitment of the H+-K+-ATPase. Am. J. Physiol. 1999;277:C361–72. doi: 10.1152/ajpcell.1999.277.3.C361. [DOI] [PubMed] [Google Scholar]
- Erickson RP, Larson-Thome K, Valenzuela RK, Whitaker SE, Shub MD. Navajo microvillous inclusion disease is due to a mutation in MYO5B. Am. J. Med. Genet. A. 2008;146A:3117–19. doi: 10.1002/ajmg.a.32605. [DOI] [PubMed] [Google Scholar]
- Ferrari A, Veligodskiy A, Berge U, Lucas MS, Kroschewski R. ROCK-mediated contractility, tight junctions and channels contribute to the conversion of a preapical patch into apical surface during isochoric lumen initiation. J. Cell Sci. 2008;121:3649–63. doi: 10.1242/jcs.018648. [DOI] [PubMed] [Google Scholar]
- Galvez-Santisteban M, Rodriguez-Fraticelli AE, Bryant DM, Vergarajauregui S, Yasuda T, et al. Synaptotagmin-like proteins control the formation of a single apical membrane domain in epithelial cells. Nat. Cell Biol. 2012;14:838–49. doi: 10.1038/ncb2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervais L, Casanova J. In vivo coupling of cell elongation and lumen formation in a single cell. Curr. Biol. 2010;20:359–66. doi: 10.1016/j.cub.2009.12.043. [DOI] [PubMed] [Google Scholar]
- Gervais L, Lebreton G, Casanova J. The making of a fusion branch in the Drosophila trachea. Dev. Biol. 2012;362:187–93. doi: 10.1016/j.ydbio.2011.11.018. [DOI] [PubMed] [Google Scholar]
- Gloerich M, ten Klooster JP, Vliem MJ, Koorman T, Zwartkruis FJ, et al. Rap2A links intestinal cell polarity to brush border formation. Nat. Cell Biol. 2012;14:793–801. doi: 10.1038/ncb2537. [DOI] [PubMed] [Google Scholar]
- Goldenring JR, Aron LM, Lapierre LA, Navarre J, Casanova JE. Expression and properties of Rab25 in polarized Madin-Darby canine kidney cells. Methods Enzymol. 2001;329:225–34. doi: 10.1016/s0076-6879(01)29082-x. [DOI] [PubMed] [Google Scholar]
- Goldenring JR, Shen KR, Vaughan HD, Modlin IM. Identification of a small GTP-binding protein, Rab25, expressed in the gastrointestinal mucosa, kidney, and lung. J. Biol. Chem. 1993;268:18419–22. [PubMed] [Google Scholar]
- Hales CM, Griner R, Hobdy-Henderson KC, Dorn MC, Hardy D, et al. Identification and characterization of a family of Rab11-interacting proteins. J. Biol. Chem. 2001;276:39067–75. doi: 10.1074/jbc.M104831200. [DOI] [PubMed] [Google Scholar]
- Hales CM, Vaerman JP, Goldenring JR. Rab11 family interacting protein 2 associates with Myosin Vb and regulates plasma membrane recycling. J. Biol. Chem. 2002;277:50415–21. doi: 10.1074/jbc.M209270200. [DOI] [PubMed] [Google Scholar]
- Horne-Badovinac S, Lin D, Waldron S, Schwarz M, Mbamalu G, et al. Positional cloning of heart and soul reveals multiple roles for PKCλ in zebrafish organogenesis. Curr. Biol. 2001;11:1492–502. doi: 10.1016/s0960-9822(01)00458-4. [DOI] [PubMed] [Google Scholar]
- Jaskoll T, Melnick M. Submandibular gland morphogenesis: stage-specific expression of TGF-α/EGF, IGF, TGF-β, TNF, and IL-6 signal transduction in normal embryonic mice and the phenotypic effects of TGF-β2, TGF-β3, and EGF-r null mutations. Anat. Rec. 1999;256:252–68. doi: 10.1002/(SICI)1097-0185(19991101)256:3<252::AID-AR5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Jones TA, Metzstein MM. A novel function for the PAR complex in subcellular morphogenesis of tracheal terminal cells in Drosophila melanogaster. Genetics. 2011;189:153–64. doi: 10.1534/genetics.111.130351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakihara K, Shinmyozu K, Kato K, Wada H, Hayashi S. Conversion of plasma membrane topology during epithelial tube connection requires Arf-like 3 small GTPase in Drosophila. Mech. Dev. 2008;125:325–36. doi: 10.1016/j.mod.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Khan LA, Zhang H, Abraham N, Sun L, Fleming JT, et al. Intracellular lumen extension requires ERM-1-dependent apical membrane expansion and AQP-8-mediated flux. Nat. Cell Biol. 2013;15:143–56. doi: 10.1038/ncb2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolotuev I, Hyenne V, Schwab Y, Rodriguez D, Labouesse M. A pathway for unicellular tube extension depending on the lymphatic vessel determinant Prox1 and on osmoregulation. Nat. Cell Biol. 2013;15:157–68. doi: 10.1038/ncb2662. [DOI] [PubMed] [Google Scholar]
- Lapierre LA, Kumar R, Hales CM, Navarre J, Bhartur SG, et al. Myosin vb is associated with plasma membrane recycling systems. Mol. Biol. Cell. 2001;12:1843–57. doi: 10.1091/mbc.12.6.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Lee S, Zadeh AD, Kolodziej PA. Distinct sites in E-cadherin regulate different steps in Drosophila tracheal tube fusion. Development. 2003;130:5989–99. doi: 10.1242/dev.00806. [DOI] [PubMed] [Google Scholar]
- Lee S, Kolodziej PA. Short Stop provides an essential link between F-actin and microtubules during axon extension. Development. 2002;129:1195–204. doi: 10.1242/dev.129.5.1195. [DOI] [PubMed] [Google Scholar]
- Lenard A, Ellertsdottir E, Herwig L, Krudewig A, Sauteur L, et al. In vivo analysis reveals a highly stereotypic morphogenetic pathway of vascular anastomosis. Dev. Cell. 2013;25:492–506. doi: 10.1016/j.devcel.2013.05.010. [DOI] [PubMed] [Google Scholar]
- Li D, Kuehn EW, Prekeris R. Kinesin-2 mediates apical endosome transport during epithelial lumen formation. Cell Logist. 2014a;4:e28928. doi: 10.4161/cl.28928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Mangan A, Cicchini L, Margolis B, Prekeris R. FIP5 phosphorylation during mitosis regulates apical trafficking and lumenogenesis. EMBO Rep. 2014b;15:428–37. doi: 10.1002/embr.201338128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock JG, Hammond LA, Houghton F, Gleeson PA, Stow JL. E-cadherin transport from the trans-Golgi network in tubulovesicular carriers is selectively regulated by golgin-97. Traffic. 2005;6:1142–56. doi: 10.1111/j.1600-0854.2005.00349.x. [DOI] [PubMed] [Google Scholar]
- Lock JG, Stow JL. Rab11 in recycling endosomes regulates the sorting and basolateral transport of E-cadherin. Mol. Biol. Cell. 2005;16:1744–55. doi: 10.1091/mbc.E04-10-0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailleux AA, Overholtzer M, Brugge JS. Lumen formation during mammary epithelial morphogenesis: insights from in vitro and in vivo models. Cell Cycle. 2008;7:57–62. doi: 10.4161/cc.7.1.5150. [DOI] [PubMed] [Google Scholar]
- Mailleux AA, Overholtzer M, Schmelzle T, Bouillet P, Strasser A, Brugge JS. BIM regulates apoptosis during mammary ductal morphogenesis, and its absence reveals alternative cell death mechanisms. Dev. Cell. 2007;12:221–34. doi: 10.1016/j.devcel.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I, Nelson WJ. Coordinated protein sorting, targeting and distribution in polarized cells. Nat. Rev. Mol. Cell Biol. 2008;9:833–45. doi: 10.1038/nrm2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostov K, Su T, ter Beest M. Polarized epithelial membrane traffic: conservation and plasticity. Nat. Cell Biol. 2003;5:287–93. doi: 10.1038/ncb0403-287. [DOI] [PubMed] [Google Scholar]
- Muller T, Hess MW, Schiefermeier N, Pfaller K, Ebner HL, et al. MYO5B mutations cause microvillus inclusion disease and disrupt epithelial cell polarity. Nat. Genet. 2008;40:1163–65. doi: 10.1038/ng.225. [DOI] [PubMed] [Google Scholar]
- Muthuswamy SK, Li D, Lelievre S, Bissell MJ, Brugge JS. ErbB2, but not ErbB1, reinitiates proliferation and induces luminal repopulation in epithelial acini. Nat. Cell Biol. 2001;3:785–92. doi: 10.1038/ncb0901-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson WJ. Adaptation of core mechanisms to generate cell polarity. Nature. 2003;422:766–74. doi: 10.1038/nature01602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng AN, de Jong-Curtain TA, Mawdsley DJ, White SJ, Shin J, et al. Formation of the digestive system in zebrafish: III. Intestinal epithelium morphogenesis. Dev. Biol. 2005;286:114–35. doi: 10.1016/j.ydbio.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Pollarolo G, Schulz JG, Munck S, Dotti CG. Cytokinesis remnants define first neuronal asymmetry in vivo. Nat. Neurosci. 2011;14:1525–33. doi: 10.1038/nn.2976. [DOI] [PubMed] [Google Scholar]
- Potter BA, Weixel KM, Bruns JR, Ihrke G, Weisz OA. N-glycans mediate apical recycling of the sialomucin endolyn in polarized MDCK cells. Traffic. 2006;7:146–54. doi: 10.1111/j.1600-0854.2005.00371.x. [DOI] [PubMed] [Google Scholar]
- Prekeris R. Rabs, Rips, FIPs, and endocytic membrane traffic. Sci. World J. 2003;3:870–80. doi: 10.1100/tsw.2003.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prekeris R, Davies JM, Scheller RH. Identification of a novel Rab11/25 binding domain present in Eferin and Rip proteins. J. Biol. Chem. 2001;276:38966–70. doi: 10.1074/jbc.M106133200. [DOI] [PubMed] [Google Scholar]
- Prekeris R, Klumperman J, Scheller RH. A Rab11/Rip11 protein complex regulates apical membrane trafficking via recycling endosomes. Mol. Cell. 2000;6:1437–48. doi: 10.1016/s1097-2765(00)00140-4. [DOI] [PubMed] [Google Scholar]
- Reginato MJ, Mills KR, Becker EB, Lynch DK, Bonni A, et al. Bim regulation of lumen formation in cultured mammary epithelial acini is targeted by oncogenes. Mol. Cell. Biol. 2005;25:4591–601. doi: 10.1128/MCB.25.11.4591-4601.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Boulan E, Musch A, LeBivic A. Epithelial trafficking: new routes to familiar places. Curr. Opin. Cell Biol. 2004;16:436–42. doi: 10.1016/j.ceb.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Samakovlis C, Hacohen N, Manning G, Sutherland DC, Guillemin K, Krasnow MA. Development of the Drosophila tracheal system occurs by a series of morphologically distinct but genetically coupled branching events. Development. 1996;122:1395–407. doi: 10.1242/dev.122.5.1395. [DOI] [PubMed] [Google Scholar]
- Schluter MA, Margolis B. Apicobasal polarity in the kidney. Exp. Cell Res. 2012;318:1033–39. doi: 10.1016/j.yexcr.2012.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluter MA, Pfarr CS, Pieczynski J, Whiteman EL, Hurd TW, et al. Trafficking of Crumbs3 during cytokinesis is crucial for lumen formation. Mol. Biol. Cell. 2009;20:4652–63. doi: 10.1091/mbc.E09-02-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelzle T, Mailleux AA, Overholtzer M, Carroll JS, Solimini NL, et al. Functional role and oncogene-regulated expression of the BH3-only factor Bmf in mammary epithelial anoikis and morphogenesis. PNAS. 2007;104:3787–92. doi: 10.1073/pnas.0700115104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schottenfeld-Roames J, Ghabrial AS. Whacked and Rab35 polarize dynein-motor-complex-dependent seamless tube growth. Nat. Cell Biol. 2012;14:386–93. doi: 10.1038/ncb2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh D, Pohl C. Coupling of rotational cortical flow, asymmetric midbody positioning, and spindle rotation mediates dorsoventral axis formation in C. elegans. Dev. Cell. 2014;28:253–67. doi: 10.1016/j.devcel.2014.01.002. [DOI] [PubMed] [Google Scholar]
- Song Y, Eng M, Ghabrial AS. Focal defects in single-celled tubes mutant for cerebral cavernous malformation 3, GCKIII, or NSF2. Dev. Cell. 2013;25:507–19. doi: 10.1016/j.devcel.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szperl AM, Golachowska MR, Bruinenberg M, Prekeris R, Thunnissen AM, et al. Functional characterization of mutations in the myosin Vb gene associated with microvillus inclusion disease. J. Pediatr. Gastroenterol. Nutr. 2011;52:307–13. doi: 10.1097/MPG.0b013e3181eea177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Yanger K, Stanger BZ, Cassio D, Bi E. Cytokinesis defines a spatial landmark for hepatocyte polarization and apical lumen formation. J. Cell Sci. 2014;127:2483–92. doi: 10.1242/jcs.139923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Kumar R, Navarre J, Casanova JE, Goldenring JR. Regulation of vesicle trafficking in Madin-Darby canine kidney cells by Rab11a and Rab25. J. Biol. Chem. 2000;275:29138–46. doi: 10.1074/jbc.M004410200. [DOI] [PubMed] [Google Scholar]
- Westlake CJ, Baye LM, Nachury MV, Wright KJ, Ervin KE, et al. Primary cilia membrane assembly is initiated by Rab11 and transport protein particle II (TRAPPII) complex-dependent trafficking of Rabin8 to the centrosome. PNAS. 2011;108:2759–64. doi: 10.1073/pnas.1018823108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willenborg C, Jing J, Wu C, Matern H, Schaack J, et al. Interaction between FIP5 and SNX18 regulates epithelial lumen formation. J. Cell Biol. 2011;195:71–86. doi: 10.1083/jcb.201011112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XM, Ellis S, Sriratana A, Mitchell CA, Rowe T. Sec15 is an effector for the Rab11 GTPase in mammalian cells. J. Biol. Chem. 2004;279:43027–34. doi: 10.1074/jbc.M402264200. [DOI] [PubMed] [Google Scholar]