Abstract
Childhood vaccination is one of the most effective public health strategies to control and prevent disease, but some parents decline or delay vaccinating their children. A variety of measures have been suggested to overcome vaccine noncompliance.
This is the first in a series of two articles about childhood and adult immunization in the United States. Part 2 will discuss adult vaccination, the role of pharmacists, and considerations for P&T committees.
INTRODUCTION
Childhood vaccination has proven to be one of the most effective public health strategies to control and prevent disease.1–7 In an effort to reduce childhood morbidity and mortality, the Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices (ACIP) issues annual recommendations and guidelines for childhood and adolescent immunizations.3,8–10 However, some parents decline or delay vaccinating their children or follow alternative immunization schedules because of medical, religious, philosophical, or socioeconomic reasons.3,4,6,7,9,11–16 Health care provider-based interventions have been suggested to overcome such vaccine noncompliance, including patient counseling; improving access to vaccinations; maximizing patient office visits; offering combination vaccines; and using electronic medical records (EMRs) and practice alerts.3,4,6,9,12,14 Community- and government-based interventions to improve parent and patient adherence include public education and reminder/recall strategies, financial incentives, and providing alternative venues for vaccination.3,9
THE IMPACT OF VACCINES ON PUBLIC HEALTH
The incidence, prevalence, morbidity, and mortality of many communicable diseases have significantly decreased in Western countries largely because of national immunization strategies aimed at infants and children.5–7 It has been estimated that for each U.S. birth cohort receiving recommended childhood immunizations, around 20 million illnesses and more than 40,000 deaths are prevented, resulting in $70 billion in savings.3,7 Vaccinations are effective primarily due to two factors.6 First, once a person is immunized against a specific pathogen, the rate of that disease, as well as its associated asymptomatic carrier state, is decreased.6 Second, when a large population is immunized, unvaccinated individuals benefit from “herd immunity,” which is a reduced risk of exposure to pathogens.6 Consequently, children’s health has improved, and the quality and length of their lives have increased.5
As a result of the availability of vaccinations, most vaccine-preventable diseases that had been health threats for centuries have experienced a dramatic decline in mortality and morbidity (Table 1).6 In addition, the decrease in mortality from tetanus and pertussis that has been directly attributed to vaccination has been estimated to be 99.2% and 99.3%, respectively.6
Table 1.
Estimates of the Decline in the Morbidity of Diseases due to Vaccination6
| Disease | Reduction |
|---|---|
| Diphtheria | 100% |
| Measles | 99.9% |
| Paralytic poliomyelitis | 100% |
| Rubella | 99.9% |
| Congenital rubella syndrome | 99.3% |
| Smallpox | 100% |
| Mumps | 95.9% |
| Tetanus | 92.9% |
| Pertussis | 92.2% |
Although vaccines were first introduced in the late 18th century, important advances in reducing childhood disease have continued throughout recent decades.6 In the 1980s, Haemophilus influenzae type b (Hib) was the leading cause of meningitis and other bacterial infections in children younger than 5 years old.6 However, in the early 1990s, the introduction of the Hib conjugate vaccine caused these infections to decline by 99%.6 Streptococcus pneumoniae then became the most common cause of meningitis and other bacterial infections in young children.6 Subsequently, in February 2000, the introduction of the sevenvalent pneumococcal conjugate vaccine (PCV7) caused a rapid decline in these illnesses by as early as 2001.6,7 The overall incidence of invasive pneumococcal disease decreased by 45% in children younger than 5 years old, and overall incidence decreased from around 99 cases per 100,000 during 1998–1999 to 21 cases per 100,000 in 2008.7 In 2010, a 13-valent vaccine (PCV13) became available, which offered protection against six additional serotypes of S. pneumoniae.7 By the end of 2011, the rates of pneumococcal disease due to these six additional serotypes had decreased by nearly 90% in children under 5 years of age, compared with the period prior to the introduction of PCV13.7
Since 2000, new vaccines against rotavirus (RV), meningococcal disease, and human papillomavirus (HPV) have also been introduced.7 The RV vaccine has been recommended for U.S. infants since 2006 and has had a substantial effect in reducing hospital admissions of young children because of diarrhea.7 After the vaccine was introduced, an estimated 77,000 fewer admissions for diarrhea occurred in U.S. children younger than 5 years during 2008–2009, reducing hospital costs by around $242 million.7 Substantial and sustained decreases in RV activity have occurred every year since; for example, positive RV tests decreased by 74% to 90% for the 2010–2012 RV seasons, compared with prevaccine years.7
After receiving Food and Drug Administration (FDA) approval in June 2006, the HPV vaccine demonstrated early evidence of efficacy in the United States.4 One study found a 56% decrease in the prevalence of vaccine-specific HPV among sexually active 14- to 19-year-old females during 2007–2010, compared with the prevaccine era of 2003–2006.4 Similarly, after the FDA approved the quadrivalent meningococcal vaccine (MCV4) in 2005, meningococcal meningitis declined markedly in children and other age groups.1
RECOMMENDED VACCINES FOR CHILDREN AND ADOLESCENTS
In an effort to reduce childhood morbidity and mortality, the ACIP issues annual recommendations and guidelines for childhood and adolescent immunizations.8–10 This committee consists of experts in vaccines, public health, infectious disease, and related disciplines.8,9 The official recommendations are also approved by the American Academy of Pediatrics (AAP), the American Academy of Family Physicians, and the American College of Obstetricians and Gynecologists.10
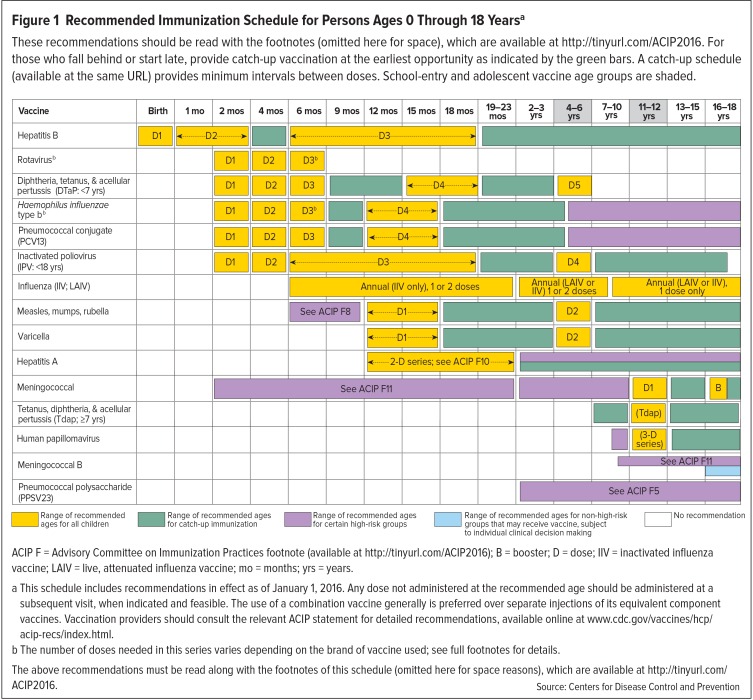
These annual immunization guidelines provide an evidence-based schedule of routine immunizations that are safe and effective, based on age and concurrent medical conditions.8,10 They describe each vaccine, indications and contraindications, background data, and other information, such as catch-up immunizations and recommendations for high-risk individuals or those planning to travel.8–10 Figure 1 presents the current vaccination schedule recommended by the ACIP for children and adolescents up to 18 years of age, as of January 1, 2016.8–10
Figure 1.
Recommended Immunization Schedule for Persons Ages 0 Through 18 Yearsa
These recommendations should be read with the footnotes (omitted here for space), which are available at http://tinyurl.com/ACIP2016. For those who fall behind or start late, provide catch-up vaccination at the earliest opportunity as indicated by the green bars. A catch-up schedule (available at the same URL) provides minimum intervals between doses. School-entry and adolescent vaccine age groups are shaded.
ACIP F = Advisory Committee on Immunization Practices footnote (available at http://tinyurl.com/ACIP2016); B = booster; D = dose; IIV = inactivated influenza vaccine; LAIV = live, attenuated influenza vaccine; mo = months; yrs = years.
a This schedule includes recommendations in effect as of January 1, 2016. Any dose not administered at the recommended age should be administered at a subsequent visit, when indicated and feasible. The use of a combination vaccine generally is preferred over separate injections of its equivalent component vaccines. Vaccination providers should consult the relevant ACIP statement for detailed recommendations, available online at www.cdc.gov/vaccines/hcp/acip-recs/index.html.
b The number of doses needed in this series varies depending on the brand of vaccine used; see full footnotes for details.
The above recommendations must be read along with the footnotes of this schedule (omitted here for space reasons), which are available at http://tinyurl.com/ACIP2016.
Source: Centers for Disease Control and Prevention
Currently, 10 vaccines are included in the standard recommendations for children at specific ages between birth and 10 years: hepatitis A (HepA); hepatitis B (HepB); RV; diphtheria, tetanus, and acellular pertussis (DTaP); Hib; PCV13; inactivated poliovirus (IPV); inactivated influenza (IIV) or live-attenuated influenza (LAIV); measles, mumps, and rubella (MMR); and varicella (VAR).10 Currently, four vaccines are routinely recommended for adolescents: tetanus, diphtheria, and acellular pertussis (Tdap); MCV; HPV; and an annual IIV or LAIV vaccine.3,10 These vaccines are primarily recommended for 11- to 12-year-olds (except for the annual influenza vaccination), but can be administered throughout adolescence if not received previously.3,10 Additional details regarding some of these vaccines follow.
Haemophilus Influenzae Type B
Before vaccines, nearly all illnesses caused by H. influenzae type b infected children younger than 5 years old and more than two-thirds infected children younger than 15 months old.14 This age dependence is purportedly caused by the development of increased natural immunity with age.14 The ACIP recommends that, for routine vaccination, two or three doses of Hib vaccine should be administered during the primary series, and a booster dose (the third or fourth dose, depending on the vaccine used in the primary series) of any Hib vaccine (except Hiberix, GlaxoSmithKline) should be administered to children at 12 to 15 months of age.10 Unimmunized children at least 5 years old who are otherwise healthy do not require Hib immunization.10,14 However, children older than 5 years who have anatomic or functional asplenia or human immunodeficiency virus infection should receive this vaccination.10
Human Papillomavirus
Vaccination against HPV, the most common sexually transmitted infection in the U.S., has been recommended for preteens and young adults since 2006 for girls and since 2011 for boys.4,7 Infection from HPV has been linked to cervical, vaginal, and vulvar cancer in women. In men, the infection can cause penile cancer, while in both genders, it can cause anal and oropharyngeal cancer as well as genital warts.4,7 The HPV vaccines available in the U.S. include: Gardasil (Merck), a quadrivalent vaccine (4vHPV) that protects against HPV subtypes 6, 11, 16, and 18; and Cervarix (GlaxoSmithKline), a bivalent product (2vHPV) that protects against subtypes 16 and 18.1,2,4 In February 2015, Merck also introduced Gardisil 9 (9vHPV), a nine-valent HPV vaccine that contains antigens for HPV subtypes 6, 11, 16, 18, 31, 33, 45, 52, and 58.1,2,14 All of these vaccines target antigens against the HPV subtypes 16 and 18, which cause 70% of cervical cancers.1,2,4 However, the quadrivalent and nine-valent vaccines also protect against HPV 6 and 11, which are the main cause of more than 90% of genital warts.1,2,4 The nine-valent vaccine also protects against five additional subtypes of HPV, thereby expanding the provided protection against cervical cancers and precancerous lesions to approximately 90%.1,2,14
Since 2011, the ACIP has recommended routine vaccination for both boys (with 4vHPV or 9vHPV) and girls (with either 2vHPV, 4vHPV, or 9vHPV) at age 11 or 12 years, but the series can be initiated at 9 years of age in children with any history of sexual abuse or assault who have not initiated or completed the three-dose series.4,10,14 The ACIP also recommends catch-up immunization of girls and boys 13 to 18 years old, if not previously vaccinated.10 (Catch-up immunizations may continue to age 26 for females and age 21 for males, which will be discussed further in Part 2 of this series next month.)
Local-site reactions and syncope in teenage girls are the most common adverse events reported to the Vaccine Adverse Event Reporting System (which is cosponsored by the FDA and the CDC) with the HPV vaccine.4 Consequently, the ACIP recommends monitoring patients for 15 minutes after administering the HPV vaccine (or other immunizations) to adolescents because most episodes of syncope occur during this time period in this age group.4
Measles, Mumps, and Rubella
Measles, mumps, and rubella are viral diseases that occur in childhood and can be associated with serious complications or death.11 Measles, which is characterized by a rash over the entire body, is highly contagious and can cause encephalitis and pneumonia.11 Children who are exposed to measles and are not immune often get the disease.11 Mumps, characterized by swelling of the cheeks and jaw, is caused by inflammation of the salivary gland.11 Mumps can lead to orchitis, deafness, and aseptic meningitis.11 Rubella is usually a mild disease; however, it presents a danger to unborn babies when it is contracted in early pregnancy.11 Should this occur, there is an 80% chance that the baby will be born blind or deaf, with a damaged heart or small brain, or with mental retardation.11 Measles, mumps, and rubella are transmitted through breathing, coughing, or sneezing. Since immunization against these diseases began six decades ago, cases have declined more than 99%.11
For routine MMR vaccinations, the ACIP recommends administering a two-dose series to children—the first dose at 12 to 15 months old and the second at 4 to 6 years old.10 For catch-up vaccinations, all school-aged children and adolescents should have had two doses of MMR vaccine, with a minimal interval between doses of four weeks.10 The ACIP also provides further specific recommendations regarding the administration of MMR to infants and children during measles outbreaks or to those who will be traveling outside of the U.S.10
Pertussis
Pertussis, or whooping cough, continues to be a major cause of morbidity and mortality.8 Infants younger than 6 months of age are at the greatest risk for severe disease, and in the U.S., approximately 90% of pertussis-related deaths and complications occur in infants younger than 3 months old.3,8 Despite high pertussis vaccination coverage, a significant rise in reported cases in children has occurred in the U.S. in recent years.4 This is likely related to the decreased duration of protection provided by the acellular pertussis vaccine, which, to reduce side effects, replaced the whole-cell vaccine in the U.S. during the mid-1990s.4,7 In addition, improved diagnostic techniques and better recognition of this illness by health care providers are thought to have contributed to the increased reported incidence.4
Since the introduction of pertussis vaccinations, the epidemiology of pertussis infection has shifted.14 Previously, infection was primarily found in children younger than 10 years old; however, in the 1990s, the majority of cases were found in adolescents and adults.14 Infants who develop pertussis, therefore, do so most often due to transmission from adolescents or adults, rather than from other infants.14 Indeed, data indicate that transmission of pertussis to infants is most often from the mother or other household contacts.4 This presents an opportunity for pediatric providers to make a recommendation during prenatal or well-baby exams that pregnant women in their third trimester, new or expectant fathers, siblings, grandparents, or other household members receive the Tdap vaccine.7,14 Health care workers, day care employees, and other individuals exposed to infants should also be vaccinated.14 The practice of protecting infants from pertussis by vaccinating close contacts with Tdap is referred to as the “cocoon strategy.”4 This approach has not been easy or cost-effective; however, despite these difficulties, it is still recommended by the ACIP.4
With respect to childhood vaccinations, the ACIP recommends that one dose of Tdap be given to adolescents who are 11 to 12 years old, particularly those who will be exposed to infants.10,14 This can be done regardless of the timing of the patient’s previous tetanus and diphtheria toxoid-containing vaccinations.10,14 Tdap should also be administered to all pregnant adolescents during the 27th through 36th week of gestation.10 The ACIP provides additional age-specific recommendations regarding catch-up pertussis vaccination.10
Influenza
Each year, influenza viruses circulate in the U.S. from late fall to late spring.9 Although most infected children recover without complication, influenza can cause serious illness and death in this population.4 The 2012–2013 flu season demonstrated the unpredictability and severity of this disease.4 During this time, flu activity peaked early and was higher than the national baseline level for 15 weeks.4 During that season, 171 influenza-related pediatric deaths were reported.4 This was the highest number since data collection began in 2004, with the exception of the 2009 H1N1 pandemic.4
Annual vaccination is the most effective strategy for the prevention of influenza and its complications.4 Since 2010, the ACIP and AAP have recommended that all individuals ages 6 months to 18 years receive an annual flu vaccine.4,10 Various formulations of the flu vaccine are available in the U.S., some of which are indicated for use in children.4 IIVs, given by intramuscular injection, are approved for children 6 months of age and older.4,10 The LAIV vaccine is administered by intranasal spray and is approved for individuals 2 through 49 years old except: in children 2 through 4 years old with asthma; individuals who are immunosuppressed or pregnant; those who are on contraindicated medications; or people who are allergic to eggs or any component of the vaccine (see the ACIP schedule footnotes for additional details).4,10 As of August 2013, quadrivalent flu vaccines have been available in the U.S.4 These formulations are available as both a live-attenuated influenza vaccine (LAIV4) and an inactivated influenza vaccine (IIV4).4 The ACIP recommendations provide age-specific instruction regarding the number of flu vaccine doses that should be administered.1
Meningococcal Conjugate Vaccine
Despite decreasing incidence, meningococcal disease continues to cause severe and devastating illness in the U.S.4 Infants are at a higher risk of invasive meningococcal disease than any other age group.4 The highest incidence of invasive meningococcal disease occurs among infants less than 6 months old, most of whom are too young to have received the minimum two to three doses of vaccine needed for protection.4 Therefore, many recent studies and recommendations regarding MCVs have focused on protecting this age group.4 The second-highest incidence of meningococcal disease outside of infancy occurs in individuals 13 to 21 years old.3
The ACIP has updated its recommendations for the use of MCVs based on current epidemiology; the inclusion of younger age groups in the indications for a previously available vaccine; and the introduction of a new vaccine in 2012 for use in infants.4 Currently, the ACIP recommends MCV only for infants at high risk.4 High-risk conditions include persistent complement component deficiencies, community or institutional outbreaks, functional or anatomic asplenia, and travel to areas where meningococcal disease is prevalent.1,4 The indications, number of doses, and need for booster doses for these patients depend on the infant’s age and underlying risk factors.4 The ACIP has also noted that the epidemiology of meningococcal disease is dynamic and that routine immunization of all infants may become necessary if disease rates increase in the future.4 For adolescents, the ACIP recommends that a single dose of Menactra (Sanofi Pasteur) or Menveo (Novartis) be administered to individuals between the ages of 11 and 12 years, with a booster dose at age 16 years.10 The ACIP recommendation footnotes should be consulted for specific information regarding exceptions to the standard recommendation.10
Fortunately, the incidence of meningococcal disease in the U.S. remains low, and it is estimated that a routine vaccination program in infants would only prevent about 25% of cases in children younger than 5 years old.4 This is because about 60% of cases in this age group are attributable to serogroup B.4 In 2015, two meningococcal B vaccines were introduced in the United States: the three-dose series, Trumenba (Pfizer), and the two-dose series, Bexsero (GlaxoSmithKline).2 Both are only approved for individuals 10 to 25 years of age.1 However, although serotype B accounts for a larger proportion of all meningococcal disease than it did previously, it is still relatively rare.1
Pneumococcal Conjugate Vaccine
S. pneumoniae remains a leading cause of morbidity and mortality in children and adults worldwide.4 S. pneumoniae is a cause of meningitis, sepsis, pneumonia, sinusitis, and otitis media.4 Populations at an increased risk of being infected include infants, young children, the elderly, and individuals who have chronic diseases or are immunodeficient.4
Fortunately, the incidence of pneumonia, invasive pneumonia disease (IPD), and acute otitis media decreased dramatically after the introduction of PCV7.4 Primary care visits and hospitalizations for community-acquired pneumonia also decreased.4 These effects were observed in both vaccinated and unvaccinated individuals, which was likely due to a reduction in nasopharyngeal carriage of S. pneumoniae in vaccinated children and the herd immunity that resulted.4 However, an increase in IPD due to non-PCV7 serotypes, particularly serotype 19A, offset some of the decrease in disease caused by vaccine-covered serotypes.4 Data from the CDC’s Active Bacterial Core Surveillance showed that, although incidence of IPD due to the serotypes included in the PCV7 vaccine among children less than 5 years of age decreased by more than 95% within five years after the vaccine was introduced, the overall incidence had decreased by only 79% due to the emergence of nonvaccine serotypes.4
Since then, PCV13 and a 23-valent (PPSV23) vaccine have become available.10 The ACIP recommendations state that for routine vaccinations, PCV13 should be administered in a four-dose series to patients at ages 2, 4, and 6 months, and between 12 and 15 months.10 For children 14 to 59 months old who received a full series of PCV7, a single supplemental dose of PCV13 is sufficient.10 The ACIP has also issued age- and condition-specific recommendations for vaccination with PCV13 and PPSV23 in children at high risk.10
VACCINE COVERAGE IN CHILDREN AND ADOLESCENTS
Coverage for most vaccinations remains high in the U.S. in children 19 to 35 months old.17 In 2014, immunization series of three or more doses for DTap, IPV, HepB, and PCV were completed in 94.7%, 93.3%, 91.6%, and 92.6% of children in this age group, respectively.17 However, uptake of the HepA vaccine lagged behind, with just 57.5% of these children completing the two-dose recommendation that year.17 Less than 1% of children in this age group received no vaccinations at all in 2014.17
According to a 2015 CDC report, overall vaccination coverage in 49 reporting states and Washington, D.C., for children in kindergarten was high in 2014 (based on local vaccination requirements), with median coverage of 94.0% for MMR and 94.2% for DTap.18 In the states with a two-dose VAR vaccination requirement for school entry (including the District of Columbia), coverage was 93.6%.18 Median exemption levels vary by state but were low overall at 1.7%.18
In 2014, the immunization rates among adolescents 13 to 17 years of age were 86.0% for Tdap and 79.3% for meningococcal conjugate vaccine (MenACWY), demonstrating that high vaccine coverage among adolescents is possible.19 However, coverage for these vaccines varied widely by state, ranging from 70.8% in Mississippi to 94.8% in Connecticut for Tdap and from 46.0% in Mississippi to 95.2% in Pennsylvania for MenACWY.19
Despite the ACIP’s recommendations, coverage among adolescents for routine HPV vaccination, although improving slowly, is low.19 Only 60% of girls and 41.7% of boys ages 13 to 17 years old began the three-dose HPV series in 2014, and series completion was just 69.3% and 57.8% for girls and boys, respectively.19 These percentages, however, show a modest increase over the previous year.19
Adherence to influenza vaccination recommendations is also low, with only 59.3% of children ages 6 months through 17 years old receiving this vaccine in the 2014–2015 season.20 Vaccination coverage for the flu decreases with increasing age; whereas 74.6% of children 6 to 23 months old received at least one dose of influenza vaccine in the 2014–2015 season, only 46.6% of adolescents 13 to 17 years of age were vaccinated.20
PUBLIC HEALTH CONSEQUENCES OF NONCOMPLIANCE
Despite the widespread availability of vaccines, multiple resurgences of measles, rubella, mumps, and pertussis have occurred since the 1980s.12,14 These resurgences have been attributed to various causes, including refusal to vaccinate, incomplete vaccination series, waning immunity, and imported cases.7,12 Vaccine hesitancy and vaccine refusal have been implicated in outbreaks of invasive Hib, varicella, pneumococcus, measles, and pertussis. Notably, measles, which was declared eliminated in the United States in 2000, caused a record number of cases in 2014 (23 outbreaks and 644 cases in 27 states).7,14 Furthermore, in 2015 the United States experienced a large multistate measles outbreak that was thought to originate with an overseas traveler who visited Disneyland in California.13 The majority of people contract measles during such outbreaks because they are unvaccinated.13 These cases underscore the importance of maintaining high vaccination coverage in the U.S. population and advising travellers regarding immunization.7
In 2006, the largest U.S. outbreak of mumps in two decades occurred, with 6,584 cases reported.7 This outbreak took place mostly in eight Midwestern states and colleges.7 As is often true for mumps outbreaks in the U.S., cases mainly affected people living in close proximity (e.g., dormitories) and occurred despite high coverage rates with the two-dose MMR vaccine series.7 Administration of a third dose of MMR during mumps outbreaks and the need to develop an improved vaccine have been discussed; however, the benefit of these additional strategies is unclear.7
Unlike these episodic outbreaks of measles and mumps, the U.S. has had a sustained increase in pertussis cases.7 During the mid-2000s, despite high vaccination rates, illness in children and teens began to rise, prompting new recommendations for a pertussis booster in teenagers.7 Even with these new recommendations, rates of pertussis have continued to increase, evidenced by substantial outbreaks in California in 2010 and in Washington state in 2011–2012.7 A retrospective study in California showed that the cause of the disease resurgence was likely waning immunity with acellular pertussis vaccine.7
VACCINE HESITANCY
Some parents consciously choose to not have their children vaccinated, to delay vaccination, or to use alternative immunization schedules.6,9 This has caused a resurgence of many infectious diseases due to the loss of herd immunity, which puts many communities at risk.6,14
Vaccination is compulsory for school-age children in the U.S.; however, public health officials are increasingly fearful of the option for parents to claim exemptions from vaccination requirements.9 Because of outbreaks of vaccine-preventable disease, rising attention has been focused on vaccine hesitancy, causing some state legislatures to enact new vaccine exemption laws.15 Currently, exemptions are allowed due to medical reasons in all states; religious grounds in 48 states; and philosophical objections in 20 states.9,16 It has been estimated that 1% to 3% of children are excused from immunization because of these exemptions, but in some communities the exemption rate is as high as 20%.9 Even when a low percentage of children are excused from immunization, the risk of disease outbreaks in schools with exemption rates as low as 2% to 4% increases.9 Illustratively, in southern Pennsylvania, health care providers have frequently expressed frustration with morbidity and mortality from preventable infectious diseases that are traced to many Amish parents’ decisions not to have their children immunized.9
Paradoxically, one reason for vaccine hesitancy among parents may be the widespread success of immunization.6 Because of high vaccination rates in children under 5 years of age, most vaccine-preventable diseases have declined to historically low levels in the U.S.6 This success has masked the health dangers of once-prevalent communicable infections, causing young parents to be unaware of the threat that these diseases posed to previous generations.6 Consequently, the perceived risk of vaccine-preventable diseases is low because people have had little to no experience with them, making it a challenge for health care providers to communicate the need for vaccination.9
Some parents who decline or delay having their children vaccinated may also believe that vaccine-preventable diseases were disappearing before the use of vaccines, or they may prefer disease-induced immunity for their children because it is “more natural.”12 These parents may also believe they can control their children’s susceptibility to infection or that their child will be protected from childhood diseases by herd immunity, or they may question the accuracy of vaccine information.12 Other vaccine-hesitant parents may believe that the decline in vaccine-preventable diseases has been due to factors other than vaccination, such as improved health care, hygiene, and sanitation.6
REASONS FOR NONCOMPLIANCE
Concern About Side Effects
Many misconceptions about vaccines exist.12 In a survey of the parents of 13,000 children 8 to 35 months old, the most commonly cited barrier to vaccination was concern about side effects.12 Some parents question the safety of vaccines, think their children are more likely to acquire infectious diseases if vaccinated, and even consider vaccines to cause attention-deficit/hyperactivity disorder and/or autism.6 Some parents believe that vaccines will weaken their child’s immune system or cause chronic illnesses, such as asthma or multiple sclerosis.6 Others parents assert that infants and young children should not be vaccinated because their bodies are still immature and fragile.6 These parents are often concerned about the number of shots per session, pain from injections, the ingredients in the vaccines, and side effects.6
Thimerisol, a mercury-containing preservative used in some vaccines, has also been the focus of fear concerning neurological damage.9 In the late 1990s, despite a lack of scientific evidence linking thimerisol to neurological sequelae, an outcry by activists about possible adverse effects from exposure to environmental mercury led the CDC and the AAP to ask pharmaceutical companies to remove it from vaccines as quickly as possible.9 Consequently, as of 1999, the U.S. transitioned to using thimerisol-free vaccines with the exception of some flu immunizations.9
The perception of risk associated with vaccinations has also been fed by media attention on actual or potential side effects.9 The news media have reported cases of childhood disability after immunization, which typically generate a public response.9 The public also heavily uses the CDC’s National Immunization Information hotline to inquire about side effects.9 Health care providers also report frequent queries from parents and guardians who are concerned about vaccine safety.9
Fear of Autism
One prevalent fear about vaccine safety risks involves autism. Some parents have suggested that there is a causal link between autism and the MMR vaccine; however, numerous large-scale studies have failed to reveal any connection.9 The possibility of this linkage was initially raised through case reports and then in a 1998 Lancet study of children with autism-spectrum disorders who had received the MMR vaccine.9 However, the Lancet and most of the study co-authors have since retracted that research as flawed.9
Numerous studies have been undertaken to investigate the proposed relationship between vaccines and the onset of autism, yet despite the use of different methodological approaches, no causal relationship has been demonstrated.9 One potential explanation for the suspicion that vaccinations cause autism may be because diagnosis of autism often occurs between the ages of 18 months and 3 years, the same time frame in which children get the bulk of their vaccinations.9 Still, parents are wary about vaccine safety and autism advocacy groups continue to pursue this issue.9
Objection to the Large Number of Injections
Another concern that parents say deters them from complying with the immunization schedule is the large number of injections that are recommended.9 During the decade from 1990 to 2000, four diseases involving 10 to 12 injections were added to the ACIP schedule.9 According to the current ACIP schedule, it is possible for a 12-month-old child to get as many as eight different injections in a single visit.9 Some provider offices use anesthetic sprays or creams to reduce discomfort and distraction or therapeutic play to reduce the child’s distress; however, this is still a large number of injections.9
Evaluation of historical trends has suggested that the more complex vaccination regimens become, the more compliance declines.9 In a survey of parents of 13,000 children ages 8 to 35 months, two-thirds of the respondents indicated that they preferred their children to receive no more than two immunizations in one visit.12 Parents have also cited concern that the quantity of childhood immunizations could overwhelm the immune system and contribute to the increases in asthma and autoimmune diseases that have occurred in recent decades.9 These fears also promote wariness among parents regarding combination vaccinations.9 However, so far, combination vaccines have demonstrated immune responses comparable to individual vaccinations.9
Moral or Religious Grounds
Objection to vaccination on the basis of moral or religious grounds is particularly relevant to the HPV vaccine.9 For many families, the decision to vaccinate their children against HPV is associated with moral or religious decision-making and the separation of church and state.9 Therefore, the withholding of parental consent is a concern with respect to HPV vaccination of preadolescent girls.9 Other common reasons for the refusal of the HPV vaccine include: “not sexually active,” “not appropriate age,” and “safety concerns/side effects.”4 These results reflect misconceptions regarding the HPV vaccine, such as believing that it’s not safe or that it’s only necessary for sexually active teens.4
Currently in the U.S., parents of children and adolescents under the age of 18 years must give consent for medical procedures.9 However, it has been suggested that the age of informed consent for vaccinations recommended by the CDC be lowered, on the grounds that children have the right to be protected from disease even when their parents withhold consent.9 Some states are also seeking to mandate the HPV vaccine, despite opposition on moral grounds by various religious organizations.9
Lack of Access Due to Cost and Other Reasons
Another major contributor to vaccination noncompliance is the lack of access to health care due to socioeconomic and other factors.6 Many parents go through hard times because of job loss, divorce, home foreclosure, or other financial hardship.6 Some parents are single, overwhelmed, and overworked, and not able to keep up with their children’s vaccinations and well-child visits.6 If they lose their jobs and health insurance, some parents don’t know that they could qualify for Medicaid to maintain their health care.6 Families may also have inadequate access to health care because of lack of transportation or inconvenient clinic hours.9,12 Additional problems that hinder access to vaccinations include child care for children not being vaccinated, lack of knowledge, and difficulty in reserving an appointment.9
It is well known that vaccination rates are influenced by poverty level.6 There is no difference between children living under and above the poverty level for MMR, IPV, and Hep B vaccinations, which are provided under the Vaccines For Children program.6 However, the vaccination rate for children living below the poverty level lags for newer vaccines and those that require four doses to complete the series.6 Black children have a lower vaccination rate for DTaP, Hib, PCV, and RV than white children.6 However, this difference disappears after adjustment for socioeconomic status, which suggests that a greater prevalence of poverty for black children could explain the decrease in vaccine coverage.6
Lack of Information
Language barriers and insufficient knowledge about immunizations contribute to reduced immunization adherence.9 Parents may not be aware of the threat of vaccine-preventable illness or know that effective and safe vaccinations are available against these diseases.9,12 In a national survey of 1,600 parents conducted by the National Network for Immunization Information, many parents indicated that they need more information about how vaccines work, possible side effects, and changes made to the guidelines.9 Common reasons cited for the refusal of HPV and other adolescent vaccines are: “not recommended (by provider),” “not needed or necessary,” ’‘lack of knowledge,” and “don’t know.”4
MEASURES TO IMPROVE COMPLIANCE
The CDC’s Task Force on Community Prevention Services has identified three categories for interventions to overcome vaccine noncompliance: increasing community demand for vaccination, enhancing access to vaccination services, and provider-based interventions.9 This section describes health provider-based and government or community interventions that may increase vaccination compliance.
Health Provider-Based Interventions
Studies have consistently shown that absent or weak recommendations from health care providers are primary drivers of poor vaccine uptake.3 Consequently, it is important to develop interventions that target health care providers and their practices (Table 2), including patient counseling and automated EMR-based reminder systems.3 A description of these, as well as other provider-based interventions, follows.
Table 2.
| Provide Parent and Patient Counseling |
|
| Maximize Opportunities for Vaccination |
|
| Offer Combination Vaccines |
|
| Improve Accessibility to Vaccinations |
|
| Use Electronic Medical Records |
|
Patient Counseling
Studies have found that the most important factor influencing parental decisions about vaccinations is communication with the health care provider.4 Parental and patient education provided by primary care physicians can be particularly important in influencing higher vaccine uptake.4 Most parents are not familiar with the specific vaccines that their children receive and the infections that they prevent.6
In numerous studies, a strong provider recommendation was shown to be a key factor in encouraging adolescent vaccination.3 One potential intervention is to improve provider communication strategies regarding adolescent vaccines (particularly HPV and influenza) by providing training and materials to providers to use when they encounter vaccine-hesitant parents.3 The risks and benefits of the HPV vaccine and its efficacy in preventing cervical cancer are among the most common topics of discussion pursued by patients.4 A health care provider recommendation has also been reported as the strongest factor linked to the initiation of the HPV vaccine series in boys.4 Research has focused on training health care providers to use proven communication strategies, such as motivational interviewing, when vaccine hesitancy is encountered.3 Several of these methods have shown promise, and others have had minimal effect or mixed results.3
Health care providers should also discuss research concerning unsubstantiated safety concerns about immunizations with parents.9 During office visits, parents should be allowed time to discuss the concerns they have about immunizations, which the health care provider should respectfully address.9,12 It is also helpful to describe the diseases that the vaccines prevent.6 For example, many vaccine-hesitant parents agree to vaccinate their child when they hear that Hib and streptococcus pneumonia are the two leading causes of meningitis or that a rotavirus infection will likely cause their child to be admitted to the hospital.6 Defining the health risks, as well as the potential for large medical bills, often provides parents with a clearer picture with which to better assess their decision.6
The health care provider’s office should also provide parents with information about upcoming immunizations before a child’s scheduled visit, so that they can gain an understanding of any recommended vaccinations.9 The CDC website provides numerous handouts that can be downloaded and distributed to parents and patients.9 Parents should also be provided with a vaccination record that summarizes all of their child’s past immunizations and the recommended dates for future vaccinations.4,6
Another evidence-based intervention is the patient reminder.14 This can be a letter or a call to families to remind them that a recommended date for vaccinations is upcoming.14 Parents who delay or refuse vaccination might also be asked to sign an exemption form.6 Requesting this can help the parent make a more informed decision because it provides them with an opportunity to read about the benefits of vaccines and the risks of not being vaccinated.6
Parents should also be told that there is no maximum interval between doses of any of the routine vaccinations that would cause the series to be restarted.3 Therefore, vaccination records should be reviewed at all visits to see if a series may have begun years before and never been completed.3 This is of particular importance for the HPV vaccination because adolescents often don’t have regularly scheduled well-child checks.3
Maximize Opportunities During Patient Visits
In the U.S., up to two-thirds of undervaccination in children younger than 2 years has been attributed to missed opportunities.14 Missed visits and failure to provide needed immunizations at every opportunity contribute to incomplete immunization requirements.12 After 2 years of age, most children are only brought to the doctor for sick visits.6 This is because parents often assume that after reaching age 2, healthy children don’t need to be seen by a health care provider.6 This is particularly true for patients who are disadvantaged socioeconomically and go to the doctor only when they need to, that is, when they are sick or need a prescription.6
All clinical encounters, including visits for injuries or mild illness, should be considered an opportunity to administer needed vaccines.9,12 Studies have demonstrated that immunizing children at sick visits may reduce their subsequent need for care.14 However, the decision to provide vaccinations during sick visits should involve weighing the risks versus the benefits as they apply to the specific patient and family.14 Postsurgical, posthospitalization, and follow-up visits also present an opportunity to administer needed vaccinations.6
A standing order for the vaccination of patients should be issued to allow nurses to do so independently of physicians.6 One situation where this is particularly important is when patients are scheduled only for a weight check or a flu vaccine administered by a nurse.6 During such visits, nurses should routinely verify patients’ vaccination status and offer to administer any other needed vaccines.6
Administer Combination Vaccines
The desire to simplify the immunization regimen to promote patient adherence has prompted a global trend toward developing combination vaccines.9 The simultaneous administration of childhood vaccines has been deemed both safe and effective, and will avoid the need for a return visit.14 In addition, it has been observed that when the advantages of combination vaccines are explained to parents, adherence can be improved.9 It is true, however, that combination vaccines require scrupulous record-keeping to ensure that children get all the immunizations they need and not more (or less) than those recommended.9 Combination vaccines also make adverse reactions more difficult to track.9
In some instances, giving a child an unneeded immunization (e.g., an extra HepB vaccination in a DTaP-IPV-HepB combination) may be preferable to administering multiple individual vaccines.14 However, it should be noted that this isn’t always the case, such as when administering a combined measles, mumps, rubella, and varicella (MMR-V) vaccine, which has been associated with an increased risk for febrile seizures following the first dose, when compared to giving the vaccinations individually.14 Therefore, unless a parent expresses a preference for the MMR-V vaccine, separate MMR and varicella vaccines should be administered.14 However, the combination vaccine should still be offered if separate vaccines are unavailable.14
Improve Access to Vaccinations
Parents of patients from disadvantaged socioeconomic groups encounter many obstacles that interfere with vaccine compliance, such as job responsibilities; not being able to keep up with appointments; unreliable transportation; relocating frequently; or having difficult family circumstances.6 For parents and patients belonging to this group, the most effective intervention to increase vaccination rates is to make access to vaccinations easier.6 This can be done by allowing patients to be seen on the same day they call to make an appointment.6 Such walk-in visits are usually scheduled for a minor illness, but should also include a check of vaccination status.6 If a patient has only a minor illness, vaccinations can be given during this encounter.6 Supportive staff, convenient office times, and limited wait time for immunizations also contribute to vaccine compliance.12
Use EMRs and Practice Alerts
Computerized tracking of patient records across health care venues has the potential to improve communication, reduce immunization errors, and reduce missed opportunities for vaccination.9 Health care systems should therefore utilize consolidated electronic immunization records to conduct system-wide and cross-system checks.9
Data have shown that practices with electronic reminder systems in place can increase immunization rates.12 In one study, establishing a practice alert for HPV vaccination in the EMRs of 11 pediatric practices in Philadelphia resulted in a higher proportion of parents discussing the vaccine with their child’s provider, compared to practices that had not instituted the alert (84% versus 70%).3 In a larger randomized controlled trial, clinician-focused practice alerts resulted in initial HPV vaccination levels 8 percentage points higher than offices without the intervention (24% versus 16%) and 6 percentage points higher than offices that had used an educational intervention instead (18%).3
EMRs also improve efficiency and accuracy by standardizing record-keeping regarding immunizations and missed visits.12 Office practice staff can use the EMR reminder system to identify patients who are not up to date with their immunizations.6 A notice can then be sent or a call can be made to these patients to schedule an appointment for a well-child visit and vaccinations.6
Community- and Government-Based Interventions
Community- and government-based approaches to enhance vaccination rates include increasing outreach and educational programs; using recall and reminder strategies; providing financial incentives; and offering vaccination at nontraditional sites (Table 3).4 A more detailed discussion of these interventions follows.
Table 3.
| Public Education |
|
| Public Reminder and Recall Strategies |
|
| Free Vaccines and Other Financial Incentives |
|
| Alternative Public and Private Venues for Vaccination |
|
Public Education
It has not yet been definitively proven that parent-driven or patient-based education can improve immunization rates without additional interventions.3 However, it has been shown that the efficacy of these efforts does improve when combined with community- or government-based measures.3 Rather than relying solely on direct parent or patient education, using newer educational modalities that incorporate community input and Web-based tools for information dissemination can be particularly effective.3 For example, an educational brochure for parents about adolescent vaccines was created in close collaboration with a focus group.3 Pilot testing of the feedback from the parents of middle and high school students where the intervention was scheduled was conducted.3 Overall, 67% of these parents recalled receiving the brochure, 90% read it, and more than half discussed it with family or friends.3
Brief public messaging interventions directed at parents and adolescents also show promise, particularly around increasing the intention to vaccinate.3 However, the positive effect of brief messages may only have a short-lived impact on behavior.3 Therefore, it is important to evaluate the effect of different messaging strategies on intention and vaccine receipt when the messages are delivered in a setting where vaccinations can be administered.3 Messaging can also be useful to inform parents that the HPV vaccine is routinely recommended for boys as well as girls.3 Without such awareness, many of these parents would not know to have their sons vaccinated.3
Public Reminder and Recall Strategies
Parent and clinician “reminders” regarding upcoming vaccines and “recall” for vaccines past due are another evidence-based approach for improving vaccination rates.3 Typically, these interventions use mail- or phone-based approaches and are instituted at the practice level.3 However, with advances in EMR and other immunization information systems, a novel development in reminder/recall is to “centralize” the process so that a coordinating agency (such as a health department) can implement it.3 Centralized reminder/recall at the payer level for adolescent vaccination is also being examined.3 A centralized reminder/recall approach conducted by a managed care organization found that both the telephone and postal mail arms of the study achieved immunization levels for each of the four vaccines recommended for adolescents that were four to nine percentage points greater than the control group for each vaccine.3 Another study reported a completion rate for a three-dose vaccine that was nearly 10% higher for a group that had received a reminder, compared with a control group.3
Advances in electronic communications have been essential in enabling the rapid sharing of health and safety information.7 These communication capacities allow real-time health updates and the broad sharing of information that enhance public health partnerships.7 Most reminder and recall strategies have focused on paper- or telephone-based reminder systems.3 However, with the increased use of mobile phones for health-related activities, the impact of a reminder or recall sent by text message or social media is now being examined.3
Free Vaccines and Other Financial Incentives
Issuing financial incentives to parents or patients, such as an entry into a lottery for a gift certificate or providing vaccines for free to the uninsured, are other strategies that may improve immunization rates.3,9 However, studies published regarding adolescent HPV vaccination suggest that providing free vaccines has a limited effect.3 With respect to incentivized vaccination, one study determined that providing a shopping voucher to girls 16 to 18 years old at each of the three visits required for the HPV vaccine led to significant and substantial increases in series completion, with double to quadruple the rates found in the control group.3 However, the low overall completion rates (12.4%–22.4%) indicate that additional approaches may be required to achieve higher vaccination rates.3 A literature review regarding the influence of financial incentives on parents in increasing preschool vaccination also found insufficient evidence to conclude that this strategy was effective, suggesting that more research may be necessary.3
Alternative Public and Private Venues for Vaccination
Many studies have provided evidence supporting school-and day care-based immunization programs.3,9 Improvement in childhood and adolescent immunization rates has also been achieved by opening a walk-in vaccination clinic run by a nurse practitioner on evenings and weekends.9 Pharmacies, which provide convenient access to vaccinations during off hours, such as evenings and weekends, have also been successful.3 Adults have been the greatest users of pharmacy vaccination services, but some groups have begun to explore the potential for pharmacies to improve adolescent vaccination coverage, particularly for HPV.3 Given the proven success with adults, a similar approach to adolescent vaccination may also be successful.3 Other possible alternative immunization venues include emergency departments, Women, Infants, and Children (WIC) program offices, inpatient settings, and home visits specifically for vaccine administration.9
CONCLUSION
The incidence and associated risks of many communicable diseases have significantly decreased in Western countries due to immunization strategies aimed at infants and children.5–7 The potential for vaccines to prevent morbidity and to save lives has never been greater, but this potential can only manifest if parents and patients comply with the recommendations for childhood and adolescent immunization.14 Efforts by health care providers, as well as community- and government-based interventions to increase vaccine coverage, must continue in order to reduce morbidity and mortality in children due to vaccine-preventable diseases.4
REFERENCES
- 1.Campos-Outcalt D. Immunization update: what’s changed, what’s on the way. J Fam Pract. 2015;64(3):177–180. [PubMed] [Google Scholar]
- 2.Half of all flu shots in the U.S. are provided by pharmacists. Human Vaccin Immunother. 2014;10(11):3103–3106. [PubMed] [Google Scholar]
- 3.Dempsey AF, Zimet GD. Interventions to improve adolescent vaccination: what may work and what still needs to be tested. Vaccine. 2015;33(suppl 4):D106–D113. doi: 10.1016/j.vaccine.2015.09.032. [DOI] [PubMed] [Google Scholar]
- 4.Kao CM, Schneyer RJ, Bocchini JA. Child and adolescent immunizations: selected review of recent U.S. recommendations and literature. Curr Opin Pediatr. 2014;26(3):383–395. doi: 10.1097/MOP.0000000000000093. [DOI] [PubMed] [Google Scholar]
- 5.Esposito S, Durando P, Bosis S, et al. Vaccine-preventable diseases: from paediatric to adult targets. Eur J Intern Med. 2014;25(3):203–212. doi: 10.1016/j.ejim.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Temoka E. Becoming a vaccine champion: evidence-based interventions to address the challenges of vaccination. SD Med. 2013:68–72. spec no: [PubMed] [Google Scholar]
- 7.Khabbaz RF, Moseley RR, Steiner RJ, et al. Challenges of infectious diseases in the U.S.A. Lancet. 2014;384(9937):53–63. doi: 10.1016/S0140-6736(14)60890-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poland GA, Schaffner W, Hopkins RH, Jr, U.S. Department of Health and Human Services Immunization guidelines in the United States: new vaccines and new recommendations for children, adolescents, and adults. Vaccine. 2013;31(42):4689–4693. doi: 10.1016/j.vaccine.2013.03.031. [DOI] [PubMed] [Google Scholar]
- 9.Sharts-Hopko NC. Issues in pediatric immunization. MCN Am J Matern Child Nurs. 2009;34(2):80–88. doi: 10.1097/01.NMC.0000347300.39714.19. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention Recommended immunization schedule for persons aged 0 through 18 years. Jan 1, 2016. Available at: www.cdc.gov/vaccines/schedules/downloads/child/0-18yrs-child-combined-schedule.pdf. Accessed February 29, 2016.
- 11.Jones CM, Harshbarger JS. Pediatric and adolescent vaccines update. US Pharm. 2008;33(3):28–47. [Google Scholar]
- 12.Anderson E. Recommended solutions to the barriers to immunization in children and adults. Mo Med. 2014;111(4):344–348. [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention Measles cases and outbreaks. May 2, 2016. Available at: www.cdc.gov/measles/cases-outbreaks.html. Accessed February 29, 2016.
- 14.Oldfield BJ, Stewart RW. Common misconceptions, advancements, and updates in pediatric vaccine administration. South Med J. 2016;109(1):38–41. doi: 10.14423/SMJ.0000000000000399. [DOI] [PubMed] [Google Scholar]
- 15.Wang E, Clymer J, Davis-Hayes C, Buttenheim A. Non-medical exemptions from school immunization requirements: a systematic review. Am J Public Health. 2014;104:e62–e84. doi: 10.2105/AJPH.2014.302190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Conference of State Legislatures States with religious and philosophical exemptions from school immunization requirements. Jan 21, 2016. Available at: www.ncsl.org/research/health/school-immunization-exemption-state-laws.aspx. Accessed February 29, 2016.
- 17.Hill HA, Elam-Evans LD, Yankey D, et al. National, state, and selected local area vaccination coverage among children aged 19–35 months—United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64(33):889–896. doi: 10.15585/mmwr.mm6433a1. [DOI] [PubMed] [Google Scholar]
- 18.Seither R, Calhoun K, Knighton CL, et al. Vaccination coverage among children in kindergarten—United States, 2014–15 school year. MMWR Morb Mortal Wkly Rep. 2015;64(33):897–904. doi: 10.15585/mmwr.mm6433a2. [DOI] [PubMed] [Google Scholar]
- 19.Reagan-Steiner S, Yankey D, Jeyarajah J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64(29):784–792. doi: 10.15585/mmwr.mm6429a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention Flu vaccination coverage, United States, 2014–15 influenza season. Jan 28, 2016. Available at: www.cdc.gov/flu/fluvaxview/coverage-1415estimates.htm. Accessed May 25, 2016.