In a retrospective review, the prevalence of delirium did not differ among intensive care unit patients who received either dexmedetomidine or propofol alone for 24 hours or more. Propofol was linked with more coma and shorter hospital stays.
Keywords: delirium, coma, mechanical ventilation, sedation, dexmedetomidine, propofol, intensive care
Abstract
Objective:
To assess the prevalence of delirium and coma in mechanically ventilated patients sedated with dexmedetomidine or propofol alone; to evaluate the hospital length of stay for both treatment groups; and to evaluate the level of sedation, adverse effects, and hospital outcomes.
Methods:
Medical records were reviewed retrospectively for patients who were admitted to the medical or surgical intensive care units (ICUs) in a 591-bed teaching hospital and who received either dexmedetomidine or propofol alone for 24 hours or more for sedation.
Results:
A total of 111 patients were included in the study, with 56 patients in the dexmedetomidine group and 55 patients in the propofol group. Results of the analysis showed that the propofol group had a higher prevalence of coma (43.6% versus 12.5%; P < 0.001). Dexmedetomidine patients had a longer median hospital length of stay of 23.5 days (interquartile range [IQR], 11.5–39.5 days) versus 15.0 days (IQR, 7.0–24.0 days; P = 0.01). The rates of delirium were similar in both groups, with 16% in dexmedetomidine-treated patients versus 20% in propofol-treated patients (P = 0.63).
Conclusion:
No difference in the prevalence of delirium was found when comparing the dexmedetomidine- and propofol-treated groups. Propofol was associated with more coma and oversedation; dexmedetomidine was associated with longer time to extubation, longer length of stay in the ICU, and longer hospital length of stay.
INTRODUCTION
Delirium is defined as a syndrome that is presented as an acute onset of cerebral dysfunction with a change or fluctuation in baseline mental status, inattention, and either disorganized thinking or an altered level of consciousness. In the intensive care unit (ICU), delirium is associated with increased duration of cognitive impairment, increased hospital length of stay, increased ICU length of stay, higher mortality, and higher hospital expenditures.1–4 Benzodiazepines such as lorazepam and midazolam are associated with delirium in the ICU.2
Dexmedetomidine is an alpha-2 adrenergic agonist with anesthetic and sedative properties thought to be due to activation of G-proteins by alpha 2A in the brainstem, resulting in inhibition of norepinephrine release. Dexmedetomidine is approved by the Food and Drug Administration for use of less than 48 hours sedation of intubated patients and for procedural sedation of nonintubated patients.5 Its mechanism of action is unique in comparison with other available sedatives that target gamma-aminobutyric acid (GABA) receptors.
In 2013, the Society of Critical Care Medicine (SCCM) published the Clinical Practice Guidelines for the Management of Pain, Agitation, and Delirium in Adult Patients in the Intensive Care Unit (PAD), which recommended nonbenzodiazepine-based sedation, specifically propofol or dexmedetomidine, in mechanically ventilated adult ICU patients to improve clinical outcomes.1 Recent literature comparing dexmedetomidine with lorazepam and midazolam has shown that dexmedetomidine as a sedative in the ICU may result in a lower prevalence of delirium and better hospital outcomes, such as decreased rate of mortality, shorter ICU length of stay, shorter hospital length of stay, and lower hospital expenditures.6,7 The PAD guidelines noted that there are insufficient data to determine the relationship between propofol use and the development of delirium in adult ICU patients.
METHODS
This was an institutional review board–approved single-center retrospective study that evaluated mechanically ventilated, adult, critically ill, medical or surgical intensive care unit (MICU or SICU) patients over a period of 21 months (January 2013 to September 2014). Inclusion criteria were: 18 years of age or older; admitted to the SICU or MICU; mechanically ventilated for more than 24 hours; received either dexmedetomidine or propofol continuous infusion as the only sedative for more than 24 hours; and record contained at least once-daily documentation of Confusion Assessment Method in the Intensive Care Unit (CAM-ICU)—a validated delirium screening tool— while on either sedative.8 Exclusion criteria were: allergy to any components of either sedative medication; not mechanically ventilated for more than 24 hours; inability to speak and understand English; pregnant or breastfeeding women; acute severe intracranial hemorrhage, trauma, spinal or neurological disorder; serious central nervous system disorders (e.g., stroke, seizures, and dementia); admitted to the neuro-ICU (NICU); admitted to the ICU for less than 24 hours; received prolonged neuromuscular blockade or other sedatives; and lack of CAM-ICU documentation.
The primary endpoints of this study were the prevalence of delirium and coma. The prevalence of delirium was defined as a positive CAM-ICU. The prevalence of coma was defined as a Richmond Agitation Sedation Scale (RASS) score of −4 to −5.6 The RASS score was assessed during continuous sedation with dexmedetomidine or propofol. Secondary endpoints were level of sedation and adverse reactions, such as bradycardia and hypotension. Hospital outcomes were defined as hospital length of stay, ICU length of stay, time to extubation in days, and hospital mortality. Sedation at goal was the percentage of time in hours spent within a RASS score of 0 to −2, undersedation was the percentage of time in hours spent within a RASS score of +1 to +4, and oversedation was the percentage of time in hours spent within a RASS score of −3 to −5. Bradycardia was defined as heart rate less than 60 beats per minute and hypotension as systolic blood pressure less than 90 mm Hg or diastolic blood pressure less than 60 mm Hg. CAM-ICU documentation compliance ratio was defined as the number of days CAM-ICU was documented over the number of days patients received either dexmedetomidine or propofol continuous infusion. Our ICU sedation goal was defined as a RASS score of −2 to 0.1,4–6
Baseline characteristics and secondary outcomes were compared using the two-sample t-test, exact chi-square test, and Wilcoxon rank sum test. Multivariable logistic regression was used to find predictors of presence of delirium and presence of coma. All multivariable models were adjusted by age, gender, Acute Physiologic Assessment and Chronic Health Evaluation (APACHE) II scores, and creatinine clearance. All analyses were completed using SAS 9.4 (SAS Institute Inc., Cary, North Carolina).
RESULTS
Enrollment
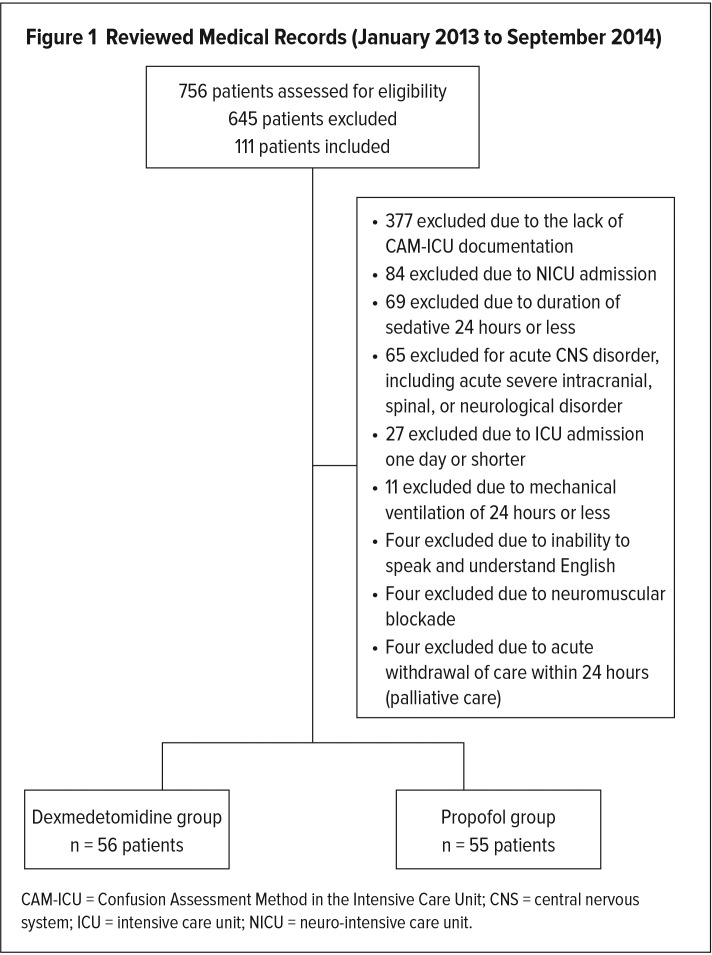
A total of 756 patient medical records were reviewed for eligibility. In this single-center retrospective study, 645 patients were excluded and 111 patients were enrolled based on the inclusion and exclusion criteria. Patients were further divided into two groups, a dexmedetomidine-treated group and a propofol-treated group. The most common reasons for exclusion were the following: lack of CAM-ICU documentation while on either sedative; NICU admissions with a neurological disorder; serious central nervous system pathology or acute severe intracranial, spinal, or neurological disorder; and being on a sedative for less than 24 hours. Of the 111 patients included in the study, 56 (50.5%) were enrolled in the dexmedetomidine group, and 55 (49.5%) were enrolled in the propofol group (Figure 1).
Figure 1.
Reviewed Medical Records (January 2013 to September 2014)
CAM-ICU = Confusion Assessment Method in the Intensive Care Unit; CNS = central nervous system; ICU = intensive care unit; NICU = neuro-intensive care unit.
Baseline Characteristics
The mean ages for the two groups were 73 years and 68 years, respectively, for dexmedetomidine and propofol. Between the two groups, there were no statistically significant differences in mean age (P = 0.064), gender (P = 0.570), median creatinine clearance (P = 0.336), median APACHE II score (P = 0.423), and Child–Pugh score (P = 0.705). Baseline characteristics are displayed in Table 1.
Table 1.
Baseline Characteristics
| Group I (Dexmedetomidine) | Group II (Propofol) | P Value | |||||
|---|---|---|---|---|---|---|---|
| Age, years (range) | 73.2 (50–94) | 67.5 (22–93) | 0.064 | ||||
| Gender, n (%) | Male: 32 (57%) Female: 24 (43%) |
Male: 28 (51%) Female: 27 (49%) |
0.570 | ||||
| Creatinine clearance, mL/min, median (IQR) | 36.1 (20.5–66.8) | 48.8 (23.0–58.7) | 0.336 | ||||
| APACHE II score, median (IQR) | 18.0 (14.5–24.0) | 20.0 (16.0–25.0) | 0.423 | ||||
| Child–Pugh score, n (%) | A | B | C | A | B | C | 0.705 |
| 28 (50%) | 28 (50%) | 0 (0%) | 29 (53%) | 25 (45%) | 1 (2%) | ||
| CAM-ICU documentation compliance ratio, median (IQR) | 0.92 (0.50–1.00) | 0.67 (0.43–1.00) | 0.100 | ||||
APACHE = Acute Physiologic Assessment and Chronic Health Evaluation; CAM-ICU = Confusion Assessment Method in the Intensive Care Unit; IQR = interquartile range.
Primary Endpoints
Using the chi-square test, we compared the prevalence of delirium and coma. There was no significant difference in the prevalence of delirium in the two groups: 16.1% for the dexmedetomidine group versus 20% for the propofol group (P = 0.629). However, the prevalence of coma was statistically significantly higher in the propofol group compared with the dexmedetomidine group: 43.6% versus 12.5% (P < 0.001), respectively. Results for primary endpoints are displayed in Table 2.
Table 2.
Primary Endpoints
| Dexmedetomidine (n = 56) | Propofol (n = 55) | P Value | |
|---|---|---|---|
| Delirium positive | 9 (16.1%) | 11 (20.0%) | 0.629 |
| Coma positive | 7 (12.5%) | 24 (43.6%) | < 0.001 |
Secondary Endpoints
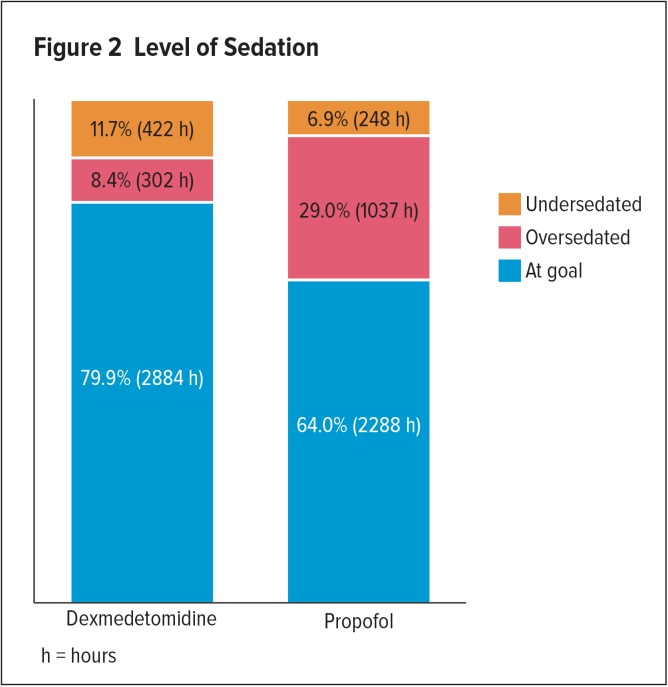
The results showed that dexmedetomidine patients spent a longer time in hours at sedation goal compared to propofol patients (79.9% versus 64%, respectively), a lower percentage of time in oversedation (8.4% versus 29.0%), and a higher percentage of time in undersedation (11.7% versus 6.9%). Level of sedation is listed in Figure 2. In regard to adverse effects, no difference in the prevalence of bradycardia or hypotension was found between the two groups (Table 3).
Figure 2.
Level of Sedation
Table 3.
Adverse Reactions
| Adverse Reactions | Dexmedetomidine (n = 56) | Propofol (n = 55) | P Value |
|---|---|---|---|
| Bradycardia | 17 (30.4%) | 12 (21.8%) | 0.389 |
| Hypotension | 53 (94.6%) | 52 (94.6%) | 0.999 |
There was no difference in hospital mortality between the two groups (35.7% versus 32.7%; P = 0.706). However, compared to propofol, the dexmedetomidine group had a longer median time to extubation (11 days versus seven days; P = 0.022), longer median hospital length of stay (23.5 days versus 15.0 days; P = 0.010), and longer ICU length of stay (13 days versus eight days; P = 0.001). Results for hospital outcomes are demonstrated in Table 4.
Table 4.
Hospital Outcomes
| Hospital Outcomes | Dexmedetomidine (n = 56) | Propofol (n = 55) | P Value |
|---|---|---|---|
| Hospital length of stay, days, median (IQR) | 23.5 (11.5–39.5) | 15.0 (7.0–24.0) | 0.010 |
| ICU length of stay, days, median (IQR) | 13.0 (7.0–27.5) | 8.0 (5.0–14.0) | 0.001 |
| Time to extubation, days, median (IQR) | 11 (4.0–25.5) | 7 (3.0–11.0) | 0.022 |
| Mortality, n (%) | 20 (35.7) | 18 (32.7) | 0.706 |
ICU = intensive care unit; IQR = interquartile range.
The multivariable model showed that patients sedated with propofol were 5.4 times more likely to have coma when compared to patients sedated with dexmedetomidine. In addition, older patients were less likely to have coma episodes, and men were more likely to have coma episodes (Table 5).
Table 5.
Adjusted Analysis of Delirium and Coma
| Delirium | Coma | |||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Propofol versus dexmedetomidine | 2.14 (0.69–6.61) | 0.188 | 5.43 (1.87–15.79) | 0.002 |
| Age | 0.98 (0.95–1.01) | 0.232 | 0.96 (0.93–0.99) | 0.021 |
| Male versus female | 1.09 (0.36–3.34) | 0.876 | 3.63 (1.23–10.74) | 0.020 |
| Creatinine clearance | 0.99 (0.98–1.01) | 0.321 | 1.00 (0.98–1.01) | 0.500 |
| APACHE II score | 1.03 (0.96–1.12) | 0.403 | 1.04 (0.97–1.13) | 0.283 |
| Nursing unit (SICU versus MICU) | 11.36 (2.67–48.42) | 0.001 | 0.89 (0.31–2.51) | 0.819 |
APACHE = Acute Physiologic Assessment and Chronic Health Evaluation; CI = confidence interval; MICU = medical intensive care unit; OR = odds ratio; SICU = surgical intensive care unit.
In regard to additional psychoactive medication use, five dexmedetomidine-treated patients received quetiapine and haloperidol for ICU delirium versus three patients in the propofol group.
DISCUSSION
The prevalence of ICU delirium was 16% to 20% in our study, significantly less than other study findings. The Safety and Efficacy of Dexmedetomidine Compared with Midazolam (SEDCOM) and Maximizing Efficacy of Targeted Sedation and Reducing Neurological Dysfunction (MENDS) trials cited the prevalence of delirium in 54% to 77% of patients.6,7
In our study, patients in the propofol group had more than three times higher prevalence of coma than the dexmedetomidine group. This result was expected due to propofol’s ability to provide deep sedation and dexmedetomidine’s unique GABA-sparing mechanism of action.6,7,9 Our results showed a much lower prevalence of coma in dexmedetomidine-treated patients versus the MENDS trial. The MENDS trial reported a 63% prevalence of coma in the dexmedetomidine-treated group versus 12.5% in our study.6
In regard to level of sedation, the dexmedetomidine group spent a longer time within sedation goal and undersedation. Conversely, the propofol group spent more time within over-sedation. These results were expected and also have been demonstrated in previous studies.6,7
In comparison with previous trials, we used different endpoints in regard to level of sedation. While we used a blanket target for sedation goal (RASS −2 to 0), other studies used percentage of days spent within 1 RASS point of individualized sedation goals, −2 to +1, and −3 to 0 for the MENDS, SEDCOM, and PRODEX (dexmedetomidine versus propofol)/MIDEX (dexmedetomidine versus midazolam) studies, respectively.6,7,9
Similar to previous study findings, our study showed hypotension was present in both groups, and bradycardia was more prevalent in the dexmedetomidine group.6,7 In the PRODEX trial, hypotension and bradycardia appeared with both propofol and dexmedetomidine.9
Unlike the PRODEX trial findings, time to extubation and ICU length of stay were longer with dexmedetomidine in our study. These results were statistically significant and were not as we expected. The PRODEX trial found that there were no differences in ICU length of stay and time to extubation when comparing dexmedetomidine to propofol (97 hours versus 118 hours; P = 0.24).9
STUDY LIMITATIONS
The limitations of this study include single institution data; small sample size; lack of documentation of CAM-ICU in many patients, which resulted in study exclusion; retrospective study design; and no dose analysis. Additionally, lack of data collection on vasopressor requirements and sedation regimen before and after the study period could be potential confounding factors that affect our study results in regard to longer ICU and hospital length of stay. Lastly, selection bias in the choice of sedative agents could be another reason why we are seeing overall less delirium and longer length of stay in this study. Our prescribers preferred to use dexmedetomidine in ICU patients who had a high risk of delirium or were already delirious.
CONCLUSION
No statistically significant difference in the prevalence of delirium was observed between dexmedetomidine- and propofol-treated, mechanically ventilated, critically ill adult patients. A higher prevalence of coma and longer time spent in over sedation were associated with propofol. In addition, dexmedetomidine use in mechanically ventilated SICU and MICU patients was associated with longer time to extubation, longer ICU length of stay, and longer hospital length of stay without a mortality benefit.
Footnotes
Disclosure: The authors report no commercial or financial interests in regard to this article.
REFERENCES
- 1.Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41(1):263–306. doi: 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]
- 2.Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753–1762. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 3.Milbrandt EB, Deppen S, Harrison PL, et al. Costs associated with delirium in mechanically ventilated patients. Crit Care Med. 2004;32(4):955–962. doi: 10.1097/01.ccm.0000119429.16055.92. [DOI] [PubMed] [Google Scholar]
- 4.Reardon DP, Anger KE, Adams CD, Szumita PM. Role of dexmedetomidine in adults in the intensive care unit: an update. Am J Health-Syst Pharm. 2013;70(9):767–777. doi: 10.2146/ajhp120211. [DOI] [PubMed] [Google Scholar]
- 5.Precedex (dexmedetomidine hydrochloride) prescribing information. Lake Forest, Illinois: Hospira, Inc.; 2012. [Google Scholar]
- 6.Pandharipande PP, Pun BT, Herr DL, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298(22):2644–2653. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]
- 7.Riker RR, Shehabi Y, Bokesch PM, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301(5):489–499. doi: 10.1001/jama.2009.56. [DOI] [PubMed] [Google Scholar]
- 8.Shi Q, Warren L, Saposnik G, Macdermid JC. Confusion assessment method: a systematic review and meta-analysis of diagnostic accuracy. Neuropsychiatr Dis Treat. 2013;9:1359–1370. doi: 10.2147/NDT.S49520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruokonen E, Parviainen I, Jakob SM, et al. Dexmedetomidine versus propofol/midazolam for long-term sedation during mechanical ventilation. Intensive Care Med. 2009;35(2):282–290. doi: 10.1007/s00134-008-1296-0. [DOI] [PubMed] [Google Scholar]