Abstract
Narcissism is characterized by the search for affirmation and admiration from others. Might this motivation to find external sources of acclaim exist to compensate for neurostructural deficits that link the self with reward? Greater structural connectivity between brain areas that process self-relevant stimuli (i.e. the medial prefrontal cortex) and reward (i.e. the ventral striatum) is associated with fundamentally positive self-views. We predicted that narcissism would be associated with less integrity of this frontostriatal pathway. We used diffusion tensor imaging to assess the frontostriatal structural connectivity among 50 healthy undergraduates (32 females, 18 males) who also completed a measure of grandiose narcissism. White matter integrity in the frontostriatal pathway was negatively associated with narcissism. Our findings, while purely correlational, suggest that narcissism arises, in part, from a neural disconnect between the self and reward. The exhibitionism and immodesty of narcissists may then be a regulatory strategy to compensate for this neural deficit.
Keywords: narcissism, frontostriatal connectivity, diffusion tensor imaging, white matter, self-esteem
Introduction
Daily social interactions are festooned with the presence of egotistical and vain individuals. Yet what motivates the brazen swagger of these narcissists? In what follows, we argue that a structural deficit in the brain predicts narcissists’ blunted sense of reward in relation to the self. This lack of self-reward connectivity may then motivate their conceited attitudes and behavior to compensate for this deficiency.
Narcissism exists in two forms: grandiose and vulnerable (Morf and Rhodewalt, 2001; Miller et al., 2011). Grandiose narcissism is characterized by greater extraversion and lower agreeableness (Miller et al., 2011) and greater self-esteem (Miller et al., 2012). According to self-regulatory models of narcissism, grandiose narcissists use their interpersonal environment to obtain affirmation of their selves that they do not intrinsically generate (Campbell et al., 2006; Morf and Rhodewalt, 2001). To date, no study has examined whether neurostructural correlates of narcissism may help explain the source of this motivation. The current study fills this gap in the literature.
The underlying physiology of grandiose narcissists offers a clue regarding their drive for external admiration and affirmation. Although grandiose narcissism is unassociated with self-reports of rejection’s sting, it is associated with increased reactivity in regions of the brain that subserve the pain of rejection (Cascio et al., forthcoming), which goes on to predict whether they retaliate (Chester and DeWall, forthcoming). Narcissism is also associated with a greater stress responses in peripheral physiology during potential self-esteem threats (Edelstein et al., 2010). These findings suggest that grandiose narcissism is rooted in a physiological substrate that does not promote a stable, stoic and positive self. What remains unknown is whether grandiose narcissism and its associated motivation to seek extrinsic sources of affirmation arises in the structure of the nervous system.
The neural basis of positive self-regard has been a budding area of inquiry among neuroscientists. A key midline region, the medial prefrontal cortex (MPFC) shows robust sensitivity to the self-relevance of stimuli, particularly its rostral and ventral aspects (Denny et al., 2012). For example, MPFC activity corresponded to individuals’ judgments of whether personality traits related to themselves vs a close other (Heatherton et al., 2006). Further, the more ventral portions of the MPFC play a special role in self-valuation (D’Argembeau et al., 2012). If the MPFC is the neuroanatomical seat of self-relevant processing and the value of the self, then the extent to which this region acts in concert with other brain regions that subserve positive affect should predict self-esteem. The ventral striatum plays a critical role in the subjective experience of positive affect and hedonic reward (Berridge and Kringelbach, 2013). Chavez and Heatherton (forthcoming) demonstrated that dispositional self-esteem is associated with greater functional and structural connectivity between the ventral striatum and the MPFC (i.e. frontostriatal connectivity). These findings suggest that a neural link between self-relevant processing and pleasure subserves fundamentally positive self-views.
The pursuit of external self-affirmations among grandiose narcissists may reflect a compensatory strategy to counteract a deficit in this neural link. Much as sensation-seeking individuals turn to exciting behaviors (e.g. substance abuse) as a motivation to compensate for a tonic, biological state of reduced reward activity (Dawe et al., 2004), narcissists may self- aggrandize as a means to increase the chronically deficient reward that is experienced in relation to the self. We sought to test this possibility by assessing whether grandiose narcissism was associated with a neural disconnect between the self and reward. Specifically, we predicted that narcissism would be negatively associated with structural frontostriatal connectivity.
Methods
Participants
Fifty healthy, right-handed undergraduate students (64% female; age: M = 18.78, s.d. = 1.04) were recruited to participate from the University of Kentucky Introductory Psychology Subject Pool. Participants were compensated with partial course credit and $45. Participants were excluded from the study if they reported any history of psychological or neurological pathology, claustrophobia, seizures, major medical issues, issues with substance abuse, current use of psychoactive medication, learning disorders, color blindness or a body-mass index indicating obesity (i.e. >30). For safety reasons, participants were also excluded if they reported any metallic objects or devices inside their body. All participants provided informed consent and all procedures were approved by the University of Kentucky Office of Research Integrity.
These data were collected as part of a larger study on the role of negative emotion in impulsivity. Because of this larger aim, participants were recruited to be relatively high or low in impulsivity and neuroticism, as determined by their scores being above the scale’s midpoint for both of these constructs. All reported effects in this manuscript remain significant after controlling for participants’ group assignment. We did not assess correlations with impulsivity or neuroticism with frontostriatal connectivity as our hypotheses did not pertain to these constructs.
Materials
Narcissistic personality index (NPI-16)
To measure dispositional individual differences in grandiose narcissism, participants completed the 16-item Narcissistic Personality Inventory (NPI-16; Ames et al., 2006; Miller et al., 2012). In this measure, participants repeatedly decide between a dichotomous narcissistic (e.g. I think I am a special person) or non-narcissistic (e.g. I am no better or worse than most people) response. These items focus more on the grandiose elements of narcissism (e.g. self-enhancement) and less on the vulnerable aspects (e.g. hostility; Miller et al., 2012). Scores across all 16 items were scored such that narcissistic responses were coded as 1 and non-narcissistic responses were coded as 0. Responses were then averaged across all 16 items to yield a trait narcissism score that could range from 0 to 1.
Procedure
Participants first completed an intake session in which they were screened for safety and comfort in the magnetic resonance imaging (MRI) environment and then they completed a battery of questionnaires that assessed demographic information and trait narcissism. Several days later, participants arrived at the University of Kentucky’s Magnetic Resonance Imaging and Spectroscopy Center where they were again screened for MRI safety and comfort. Participants then entered the MRI scanner and underwent diffusion tensor imaging for approximately 10 min.
Magnetic resonance imaging
Data acquisition
All MRI data were acquired on a 3.0-Tesla Siemens MAGNETOM Trio scanner using a 32-channel head coil. Diffusion tensor imaging (DTI) was acquired across the entire brain using an axial double-refocused spin-echo echo planar imaging sequence (TR = 8000 ms, TE = 96 ms, FOV = 224 mm, 52 slices, 2 mm isotropic resolution). DT images were acquired with 64 noncollinear encoding direction (b = 1000 s/mm2) and six images without diffusion weighting (b = 0 s/mm2, b0). Then, a high-resolution T1-weighted MP-RAGE sequence was acquired from each participant to allow for native space registration of the DTI data (parameters: 1mm3 isotropic voxel size, TR = 1.69 s, TE = 2.56 ms, flip angle = 12°).
Preprocessing and FA extraction
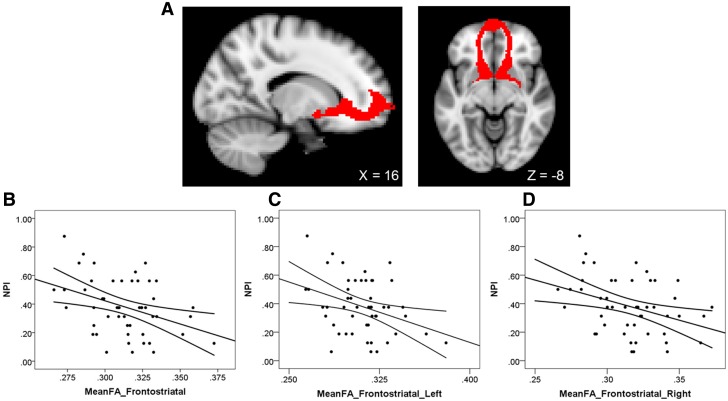
DTI data were analyzed using FMRIB’s Software Library (FSL v5.0; Smith et al., 2004; Jenkinson et al., 2012). Raw DT images were preprocessed to correct for head motion and residual eddy current distortion using a 12-parameter affine alignment to the corresponding b0 image via FMRIB’s Linear Image Registration Tool (FLIRT). Next, FMRIB’s Diffusion Toolbox (FDT v2.0) was used to fit the diffusion tensor and calculate fractional anisotropy (FA) eigenvalues. FA images were then registered into MNI152 space using FSL’s tract-based spatial statistics (TBSS v1.2) program. All participants’ FA images were aligned to a common target using a nonlinear registration approach and then affine registered and resampled to 2 mm3 MNI152 space. Frontostriatal tract masks for the left and right hemisphere were acquired from Chavez and Heatherton (forthcoming; Figure 1A). Mean FA values were extracted for each hemisphere of the frontostriatal tract for each participant. The FA values from each hemisphere of the frontostriatal tract were then averaged together to create a single frontostriatal FA value for each participant.
Fig. 1.
(A) Sagittal and axial views of the frontostriatal masks, displayed in red, overlaid atop an MNI152 normalized template brain. Coordinates are in MNI space. (B–D) Scatterplots depicting negative associations between average frontostriatal fractional anisotropy and scores on the narcissistic personality inventory for (B) bilateral, (C) left and (D) right hemispheres of the pathway. Curved lines represent 95% confidence intervals around the regression line.
Quality check
Reconstructed FA volumes were visually inspected prior to eddy current correction for excessive distortions and signs of excessive motion during the scan (e.g. striations, displacement), and then again after the eddy current correction's affine registration phase for misalignments between the original and target volumes. After normalization to MNI152 standard-space, FA volumes were displayed in a vertical slice directory for visual inspection against one another to detect misalignments or other deviations. None of the participants’ DTI volumes were excluded as no serious abnormalities were detected.
Results
We predicted that narcissism would relate to lower FA values in the frontostriatal pathway. Because narcissism tends to be higher for males than for females (Twenge et al., 2008), we controlled for participant sex in our analysis. Frontostriatal FA did not differ by participant sex, β = 0.19, t(47) = 1.42, P = 0.164.
As predicted, narcissism related to lower structural integrity between the MPFC and ventral striatum. Frontostriatal FA was negatively associated with grandiose narcissism, β = −0.34, t(47) = −2.46, P = 0.017 [95% bias-corrected and accelerated confidence interval: β = −0.63 to −0.06 (Figure 1B)]. This association was observed in both the left, β = −0.30, t(47) = −2.13, P = 0.039, and right, β = −0.32, t(47) = −2.40, P = 0.020, hemispheres of the frontostriatal tract. Thus, narcissistic motivation to secure external admiration and affirmation may arise from a deficit in neural pathways that connect self-relevant processing with reward.
Discussion
Grandiose narcissists display bloated self-esteem that they seek to bolster from external sources of self-affirmation (Morf and Rhodewalt, 2001). Yet whether this motivation is associated with a neural deficit in intrinsically positive self-views is unknown. Using DTI, we found that grandiose narcissism predicted reduced white matter integrity between brain regions that, in concert, subserve self-esteem (Chavez and Heatherton, forthcoming). This observed tendency of individuals higher in narcissism to have less frontostriatal connectivity mirrors other work showing that the biology of narcissists reveals a far different view than merely self-reports would allow for (Cascio et al., 2015).
Our results fit well with regulatory models of narcissism (Morf and Rhodewalt, 2001; Campbell et al., 2006). The results paint a picture of narcissists as seeking positive self-related experiences in a similar fashion to sensation-seekers who crave excitement as a compensation for their internally dull mental states (Dawe et al., 2004). These findings also support the notion of the frontostriatal pathway as a crucial neural correlate of truly positive self-views (Chavez and Heatherton, forthcoming). Future research should examine this tract’s role in other phenomena characterized by vulnerable self-esteem (e.g. depression, disorder eating).
According to our compensatory model of narcissism, in which narcissists seek external self-affirmation to compensate for their internal deficit in self-reward connectivity, narcissists have a larger disparity between their baseline and desired levels of self-reward connectivity than non-narcissists. However, an alternative explanation could be that narcissists possess a similar baseline of self-reward connectivity to others, but the amount or magnitude of stimuli necessary to reach their desired levels of self-esteem may be larger. Much like the need of substance-dependent individuals to require greater and greater doses to achieve their desired high, narcissists may require substantially more external affirmation than their less-vain counterparts because each instance is associated with less reward. Indeed, if narcissists do not possess an intrinsically robust frontostriatal connection, external affirmations are unlikely to hold a strong hedonic magnitude. Our findings are unable to determine which of these accounts is more probable, or if they both have some basis in fact. Future research might benefit from experimentally pitting these models against one another, perhaps through functional MRI tasks that test neural BOLD response changes during external self-affirmations.
A lingering question remains from our findings: if narcissism is associated with high self-esteem (Miller et al., 2012), and high self-esteem is associated with greater frontostriatal connectivity (Chavez and Heatherton, forthcoming), how could narcissism be associated with lesser frontostriatal connectivity? To question our findings on these grounds is to fall victim to a syllogistic logical fallacy in which the observed, positive associations between several constructs (narcissism and self-esteem, self-esteem and frontostriatal connectivity) fictitiously implies a third, positive association (narcissism and frontostriatal connectivity). At a theoretical level, there are two reasons for the observed disparity between our findings and what might ‘logically’ be expected. First, narcissists’ higher reports of self-esteem might suggest that they are successful in obtaining the external sources of self-affirmation that they require. It is even possible that external self-reports of high self-esteem may serve this affirming purpose. Second, a contentious body of literature has suggested that there is a disconnect between narcissists’ explicit and implicit sense of self-esteem (Zeigler-Hill, 2006). Therefore, our connectivity findings may reflect the impaired and threatened implicit self-esteem of narcissists, that does not appear in explicit self-reports. Such discrepant self-esteem may arise early in human life from the internalization of high explicit self-esteem from overly affirming parents (Brummelman et al., 2015), while leaving the frontostriatal connection unchanged.
As a potential limitation, our narcissism measure, the NPI-16, tends to capture the more grandiose facets of narcissism and its unitary nature fails to assess the multidimensionality of dispositional narcissism (e.g. authority, exhibitionism; Ackerman et al., 2011). Thus, it is uncertain what the associations between frontostriatal integrity would be with vulnerable and other subtypes of narcissism. We also assessed subclinical levels of narcissism, it is then unclear if our findings could extend to individuals with narcissistic personality disorder. Given the many divergences between clinical and subclinical narcissism (Miler and Campbell, 2008), we would not necessarily predict that individuals with narcissistic personality disorder would show a weakened frontostriatal pathway. Further, it must be stressed that these findings are purely correlational and obtained from a sample population of 50 undergraduate students. This relatively small sample size makes our results an optimum target for future replication efforts.
Our findings do not suggest that narcissists are a ‘lost cause’ because of any perceived immutability of brain structure. Indeed, clinical interventions can readily alter white matter integrity (Voss et al., 2013). Therefore, our findings may help inform interventions targeting reductions in narcissistic tendencies by suggesting that they should foster intrinsic (and perhaps biological) connections between the self and reward, perhaps through repeated administrations of intrinsic self- affirmations (Schimel et al., 2004). Such approaches underscore the value of structural and neural investigations of maladaptive dispositions and will hopefully lead to their reduced prevalence in daily life.
Funding
This experiment was funded by a grant from the University of Kentucky’s Center for Drug Abuse Research Translation (CDART; Sponsor: National Institute on Drug Abuse, Grant number: DA005312) to C. N. DeWall and D. R. Lynam.
Conflict of interest. None declared.
Acknowledgements
The authors are grateful to Robert Chavez for providing his generous assistance in acquiring the frontostriatal masks and other analytic advice regarding this project. We thank Richard Milich and Donald Lynam for their assistance in running and guiding this project.
References
- Ackerman R.A., Witt E.A., Donnellan M.B., Trzesniewski K.H., Robins R.W., Kashy D.A. (2011). What does the narcissistic personality inventory really measure? Assessment, 18, 67–87. [DOI] [PubMed] [Google Scholar]
- Ames D.R., Rose P., Anderson C.P. (2006). The NPI-16 as a short measure of narcissism. Journal of Research in Personality, 40, 440–50. [Google Scholar]
- Berridge K.C., Kringelbach M.L. (2013). Neuroscience of affect: brain mechanisms of pleasure and displeasure. Current Opinion in Neurobiology, 23, 294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummelman E., Thomaes S., Nelemans S.A., Castro B.O. de, Overbeek G., Bushman B.J. (2015). Origins of narcissism in children. Proceedings of the National Academy of Sciences, 112, 3659–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell W.K., Brunell A.B., Finkel E.J. (2006). Narcissism, interpersonal self regulation, and romantic relationships: An agency model approach. In Finkel E.J., Vohs K.D., editors. Self and Relationships: Connecting Intrapersonal and Interpersonal Processes. New York: Guilford. [Google Scholar]
- Cascio C.N., Konrath S.H., Falk E.B. (2015). Narcissists’ social pain seen only in the brain. Social Cognitive and Affective Neuroscience , 10, 335–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez R.S., Heatherton T. F. (forthcoming). Multimodal frontostriatal connectivity underlies individual differences in self-esteem. Social Cognitive and Affective Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester D.S., DeWall C.N. (forthcoming). Sound the alarm: The effect of narcissism on retaliatory aggression is moderated by dACC reactivity to rejection. Journal of Personality. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Argembeau A., Jedidi H., Balteau E., Bahri M., Phillips C., Salmon E. (2012). Valuing one’s self: medial prefrontal involvement in epistemic and emotive investments in self-views. Cerebral Cortex, 22, 659–67. [DOI] [PubMed] [Google Scholar]
- Dawe S., Gullo M.J., Loxton N.J. (2004). Reward drive and rash impulsiveness as dimensions of impulsivity: Implications for substance misuse. Addictive Behaviors, 29, 1389–405. [DOI] [PubMed] [Google Scholar]
- Denny B. T., Kober H., Wager T.D., Ochsner K.N. (2012). A meta-analysis of functional neuroimaging studies of self and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. Journal of Cognitive Neuroscience, 24, 1742–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelstein R.S., Yim I.S., Quas J.A. (2010). Narcissism predicts heightened cortisol reactivity to a psychosocial stressor in men. Journal of Research in Personality, 44, 565–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton T.F., Wyland C.L., Macrae C.N., Demos K.E., Denny B.T., Kelley W.M. (2006). Medial prefrontal activity differentiates self from close others. Social Cognitive and Affective Neuroscience, 1, 18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Beckmann C.F., Behrens T.E.J., Woolrich M.W., Smith S.M. (2012). FSL. NeuroImage, 62, 782–90. [DOI] [PubMed] [Google Scholar]
- Miller J.D., Campbell W.K. (2008). Comparing clinical and social-personality conceptualizations of narcissism. Journal of Personality, 76, 449–76. [DOI] [PubMed] [Google Scholar]
- Miller J.D., Hoffman B.J., Gaughan E.T., Gentile B., Maples J., Campbell W.K. (2011). Grandiose and vulnerable narcissism: a nomological network analysis. Journal of Personality, 79, 1013–42. [DOI] [PubMed] [Google Scholar]
- Miller J.D., Price J., Campbell W.K. (2012). Is the narcissistic personality inventory still relevant? A test of independent grandiosity and entitlement scales in the assessment of narcissism. Assessment, 19, 8–13. [DOI] [PubMed] [Google Scholar]
- Morf C.C., Rhodewalt F. (2001). Unraveling the paradoxes of narcissism: a dynamic self-regulatory processing model. Psychological Inquiry, 12, 177–96. [Google Scholar]
- Schimel J., Arndt J., Banko K.M., Cook A. (2004). Not all self-affirmations were created equal: the cognitive and social benefits of affirming the intrinsic (vs. Extrinsic) self. Social Cognition, 22, 75–99. [Google Scholar]
- Smith S. M., Jenkinson M., Woolrich M.W., et al. (2004). Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage, 23, Supplement 1(0), S208–19. [DOI] [PubMed] [Google Scholar]
- Twenge J.M., Konrath S., Foster J.D., Campbell W.K., Bushman B.J. (2008). Egos inflating over time: A cross-temporal meta-analysis of the narcissistic personality inventory. Journal of Personality , 76, 875–901. [DOI] [PubMed] [Google Scholar]
- Voss M.W., Heo S., Prakash R.S., et al. (2013). The influence of aerobic fitness on cerebral white matter integrity and cognitive function in older adults: Results of a one-year exercise intervention. Human Brain Mapping, 34, 2972–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeigler-Hill V. (2006). Discrepancies between implicit and explicit self-esteem: implications for narcissism and self-esteem instability. Journal of Personality, 74, 119–44. [DOI] [PubMed] [Google Scholar]