Abstract
Neurocognitive studies of psychopathy have predominantly focused on male samples. Studies have shown that female psychopaths exhibit similar affective deficits as their male counterparts, but results are less consistent across cognitive domains including response modulation. As such, there may be potential gender differences in error-related processing in psychopathic personality. Here we investigate response-locked event-related potential (ERP) components [the error-related negativity (ERN/Ne) related to early error-detection processes and the error-related positivity (Pe) involved in later post-error processing] in a sample of incarcerated adult female offenders (n = 121) who performed a response inhibition Go/NoGo task. Psychopathy was assessed using the Hare Psychopathy Checklist-Revised (PCL-R). The ERN/Ne and Pe were analyzed with classic windowed ERP components and principal component analysis (PCA). Consistent with previous research performed in psychopathic males, female psychopaths exhibited specific deficiencies in the neural correlates of post-error processing (as indexed by reduced Pe amplitude) but not in error monitoring (as indexed by intact ERN/Ne amplitude). Specifically, psychopathic traits reflecting interpersonal and affective dysfunction remained significant predictors of both time-domain and PCA measures reflecting reduced Pe mean amplitude. This is the first evidence to suggest that incarcerated female psychopaths exhibit similar dysfunctional post-error processing as male psychopaths.
Keywords: female psychopathy, event-related potentials, principal component analysis, error-related negativity, error-related positivity
Dysfunctional error-related processing in female psychopathy
Psychopathy is a serious personality disorder characterized by dysfunctional affective and behavioral symptoms. Psychopaths are defined by their overall absence of moral emotions and an impulsive lifestyle (Hare, 2003). Less than 1% of the general population is estimated to meet the established criteria for psychopathy, though the base rate increases to 15–25% in incarcerated settings (Hare, 2003). Psychopaths are also at least four times more likely to violently recidivate in the 12 months following institutional release compared to non-psychopathic criminal offenders (Rice and Harris, 1997). Understanding psychopathy is vital to the management of institutional populations. Considerable effort has been made in attempting to elucidate the construct of psychopathy in male samples; however, relatively little work has been specifically devoted to this construct in female counterparts.
Male psychopaths have regularly exhibited both affective and cognitive deficits through a variety of experimental paradigms. For example, male psychopaths have been characterized by reduced startle responses to affective stimuli including physiological reactions to unpleasant stimuli (Patrick et al., 1993) and identification of facial expressions of emotion (Kosson et al., 2002). Cognitive deficits in male psychopathy mainly center on response modulation deficits captured in passive avoidance learning (Newman and Kosson, 1986) and probabilistic learning paradigms (Budhani et al., 2006; von Borries et al., 2010). In these latter tasks, male psychopaths continually perseverate, exhibiting an inability to adjust their performance to meet the demands established by external sources (Baskin-Sommers et al., 2013; Newman, 1987).
The extensive affective and cognitive dysfunction associated with psychopathy suggests that a network of brain regions contribute to the disorder. Early theories concentrated on ventromedial prefrontal cortex and amygdala dysfunction (Blair, 2006). Another theory suggests that additional limbic and surrounding paralimbic regions help contribute to these deficits, including the anterior and posterior cingulate cortex, insula, parahippocampal gyrus and anterior superior temporal gyrus (Kiehl, 2006). Paralimbic dysfunction could give rise to a host of cognitive deficits associated with male psychopathy, including error-monitoring and post-error processing.
Event-related potentials (ERPs) are commonly used to examine different components of cognitive control including error-related processing. The two most-frequently investigated error-related ERPs are the error-related negativity (ERN/Ne) and the error-related positivity (Pe). Though closely related temporally, the ERN/Ne and Pe reflect distinct stages of error-processing. The ERN/Ne reflects initial automatic error-detection and action-monitoring processes (Falkenstein et al., 2000; Gehring et al., 1993). However, the Pe is involved in later, more elaborate error-processing stages, including the motivational (Ullsperger et al., 2010) and emotional appraisal (Overbeek et al., 2005) of error-related information. Additionally, the Pe may reflect an error-related subcomponent of the P300 ERP, as both components show similar topography and latency in response to errors (Leuthold and Sommer, 1999). Both the ERN/Ne and Pe are dependent on the participant’s conscious awareness of error occurrence, but this relationship is modulated by uncertainty and task parameters for the ERN/Ne (Shalgi and Deouell, 2012, 2013). Source localization attempts (Dehaene et al., 1994; Herrmann et al., 2004) and functional magnetic resonance imaging (fMRI) (van Veen and Carter, 2002; Edwards et al., 2012;) studies converge on the anterior cingulate cortex (ACC) as a generator for both the ERN/Ne and Pe, albeit separate sub regions. The ERN/Ne is said to arise within the cognitive, caudal division of the ACC, whereas the affective, rostral portion of the ACC is believed to contribute to the generation of the Pe (van Veen and Carter, 2002; Edwards et al., 2012).
Impulsive populations, such as those with externalizing disorders, show deficits in endogenously based performance monitoring as indexed by the ERN/Ne (Hall et al., 2007; Olvet and Hajcak, 2008), but intact monitoring of exogenous cues as indexed by the feedback-related negativity (Bernat et al., 2011). Other studies have found comparable ERN/Ne amplitudes between male psychopaths and matched controls when using affectively neutral stimuli (Munro et al., 2007; Brazil et al., 2009, 2011; von Borries et al., 2010). However, reduced ERN/Ne amplitude has also been observed in male psychopaths when incorporating evocative facial stimuli (Munro et al., 2007). An initial report suggests that post-error processing, as indexed by the Pe, appears to be implicated in male psychopathy (Brazil et al., 2009). Reduced Pe amplitude in psychopathy suggests a potential failure to incorporate information received from errors to improve subsequent behavior (Brazil et al., 2009).
In order to better conceptualize the construct of female psychopathy, we sought to expand upon the nascent understanding of cognitive processes implicated in this population. While female psychopaths are characterized by similar affective dysfunction as male psychopaths (Sutton et al., 2002; Eisenbarth et al., 2013; Verona et al., 2013), results are less consistent across cognitive domains. For example, female psychopathy is not associated with the same response perseveration deficits characteristic of male psychopathy (Vitale and Newman, 2001a; Vitale et al., 2011). Understanding of error-related processing in female psychopathy has been limited to predominantly female community samples, where females with elevated psychopathic traits exhibit reduced ERN/Ne amplitude (Dikman and Allen, 2000; Heritage and Benning, 2013). These reports did not investigate the Pe. However, it is important to note that these studies may be better measures of broader externalizing traits, which may not necessarily translate to incarcerated psychopathic populations (Patrick et al., 2009). Additionally, reduced ERN/Ne has been observed in females with Borderline Personality Disorder (BPD) (de Bruijn et al., 2006; Ruchsow et al., 2006). Some have argued that female psychopathy may be a phenotypic expression of BPD, as the two personality disorders exhibit a similar impulsive and aggressive nature (Sprague et al., 2012). Previous studies (Dikman and Allen, 2000; Heritage and Benning, 2013) did not examine the potential moderating influence of BPD in their results, which may potentially explain their reduced ERN/Ne finding.
Here, we investigate the relationship between psychopathy, BPD, and error-related electrophysiological indices using ERPs and a response inhibition Go/NoGo paradigm in a sample of incarcerated female offenders. Based on previous studies performed with male psychopaths (Munro et al., 2007; Brazil et al., 2009, 2011; von Borries et al., 2010), we hypothesized that female psychopaths would exhibit intact amplitude of the ERN/Ne and decreased amplitude of the Pe, suggesting dysfunctional post-error processing. Furthermore, as there may be a possible overlap between the Pe and P300 ERP components (Leuthold and Sommer, 1999), we investigated stimulus-locked ERPs (see Supplemental Materials) to test out the potential overlap in the Pe signal. In addition to classic time-domain measures of ERP components, we incorporated an approach based on principal component analysis (PCA) to allow for the examination of subcomponents encompassing the ERN/Ne and Pe (Steele et al., 2014).
Methods
Participants
Participants included 121 incarcerated female offenders recruited from a medium security correctional facility. Participants were informed of their right to terminate participation at any point and were advised that their participation was not associated with institutional benefits or their facility or parole status. Participants received remuneration at the hourly labor wage of the facility. The work was approved by the University of New Mexico Health Science Center Human Research Review Committee and the Office of the Human Research Protections. All participants provided written informed consent prior to data collection (see Supplemental Material for more information).
Assessments
Psychopathy was assessed using the Hare Psychopathy Checklist-Revised (PCL-R) (Hare, 2003) which has been shown to reliably assess psychopathic traits in female samples (Vitale and Newman, 2001b). PCL-R Total Scores ranged from 3 to 35 (M = 18.75, SD = 6.37). For comparison to male samples, we examined a two-factor model of psychopathic traits, with Factor 1 reflecting interpersonal and affective traits, and Factor 2 consisting of lifestyle and antisocial traits (Harpur et al., 1989). Additionally, we examined the four-facet model of psychopathic traits with four latent dimensions encapsulating psychopathy: interpersonal, affective, behavioral and antisocial traits, respectively (Hare and Neumann, 2006). PCL-R Factor 1 and 2 scores were significantly correlated (r = 0.49, P < 0.001). See Table 1 for remainder of correlations between factors and facets of psychopathy.
Table 1.
Correlations among PCL-R variables and covariates
| Variable | PCL-R Total | PCL-R Factor1 | PCL-R Factor2 | PCL-R Facet 1 | PCL-R Facet 2 | PCL-R Facet 3 | PCL-R Facet 4 | Age | IQ | BPD | Sub. Dep. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PCL-R Total | |||||||||||
| PCL-R Factor 1 | 0.76** | ||||||||||
| PCL-R Factor 2 | 0.91** | 0.49** | |||||||||
| PCL-R Facet 1 | 0.64** | 0.74** | 0.45** | ||||||||
| PCL-R Facet 2 | 0.61** | 0.88** | 0.37** | 0.38** | |||||||
| PCL-R Facet 3 | 0.81** | 0.45** | 0.87** | 0.42** | 0.33** | ||||||
| PCL-R Facet 4 | 0.79** | 0.41** | 0.89** | 0.37** | 0.31** | 0.55** | |||||
| Age | −0.20** | −0.04 | −0.31** | −0.01 | −0.06 | −0.20* | −0.37** | ||||
| IQ | 0.01 | 0.01 | −0.04 | 0.12 | −0.08 | −0.07 | −0.01 | 0.06 | |||
| BPD | 0.57** | 0.32** | 0.61** | 0.22* | 0.29** | 0.42** | 0.67** | −0.26** | −0.05 | ||
| Sub. Dep. | 0.40** | 0.14 | 0.45** | 0.25** | −0.02 | 0.40** | 0.40** | −0.24* | −0.03 | 0.42* | |
| NoGo Errors | −0.09 | −0.06 | −0.14 | −0.10 | −0.02 | −0.08 | −0.15 | 0.22* | 0.14 | −0.04 | −0.08 |
Note. Assessments: PCL-R total is the total score derived from the Psychopathy Checklist-Revised (Hare, 2003); PCL-R Factor 1 and Factor 2 are Factor 1 and Factor 2 scores derived from the PCL-R; PCL-R Facet 1, Facet 2, Facet 3 and Facet 4 scores are Facet 1, 2, 3 and 4 scores derived from the PCL-R (Hare, 2003); IQ was calculated from the Wechsler Adult Intelligence Scale-Third Version (WAIS-III) (Wechsler, 1997); BPD is Borderline Personality Disorder symptomatology derived from the Structured Clinical Interview for DSM-IV Axis II Disorders (SCID-II) (First et al., 1997); Sub. Dep. is the number of substance dependencies calculated from the Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Version (SCID I/P) (First et al., 1995); NoGo errors are the mean number of FA’s each participant made.
*P < 0.05; **P < 0.01.
Additional assessments were administered to assess intelligence quotient (IQ), substance dependence, mental illness, BPD symptomatology and traumatic brain injury (TBI). Participants were excluded from analyses if they had a full-scale IQ < 70, reported a TBI accompanied with a significant loss of consciousness, or history of psychosis. Supplemental material includes further description and statistics of these assessments (see Supplemental Material for additional information).
Stimuli and task
EEG data were collected in a small room separate from the general population housing provided by the facility. After placement of electrodes, participants were seated in a comfortable chair 60 cm away from a computer monitor on which task stimuli were presented, and were instructed to refrain from excessive blinking and movement during data collection. Participants then performed a previously published response inhibition Go/NoGo task (Kiehl et al., 2000) containing a higher frequency of Go (84%, 412 trials, 206 on each run) than NoGo stimuli (16%, 78 trials, 39 on each run; see Supplemental Material for additional information).
EEG recordings
EEG data were collected on a 64-channel BioSemi ActiveTwo amplifier in accordance with the 10–20 International System (Jasper, 1958). Standard preprocessing steps were performed. Classic response-locked components, defined relative to a False Alarm (FA), were extracted (see Supplemental Material for additional EEG data collection and analysis information). An additional data reduction method based on PCA was also performed for response-locked data (Chapman and McCarry, 1995). A five-component response-locked solution was extracted for FA trials which accounted for 90.98% of the variance. Stimulus-locked ERP analyses are in included in the Supplemental Material.
Analytic strategy
We performed group-based analyses to directly compare to previous studies (Munro et al., 2007; Brazil et al., 2009; von Borries et al., 2010; Brazil et al., 2011). Additionally, linear, stepwise regressions were carried out to predict mean ERN/Ne and Pe amplitudes using psychopathy variables and five covariate measures (IQ, age, number of substance dependencies, BPD symptomatology and participant’s mean number of FAs) to take full advantage of our large sample size. Results are consistent with those reported below when the sample is reduced based on task performance (see Supplemental Analyses). As the ERN/Ne typically shows a maximal response on anterior electrodes and the Pe typically shows a more posterior distribution (Vocat et al., 2008), we selected a subset of nine electrodes representing maximal time-domain component activation (F3, Fz, F4, FC3, FCz, FC4, C3, Cz and C4 for the ERN/Ne and C3, Cz, C4, P3, Pz, P4, FO3, FOz and FO4 for the Pe) and used in subsequent time-domain and PCA analyses. Effects that did not reach statistical trend (P > 0.10) are not reported.
Results
Behavioral results
Response times (RTs) and frequency for Hits and FAs were analyzed. As expected, participants responded faster to NoGo (FA) stimuli (M = 332 ms, SD = 51 ms) compared with Go (Hit) stimuli (M = 669 ms, SD = 54 ms), t(120) = 41.60, P < 0.001. Participants made significantly more errors to NoGo stimuli (M = 16.85, SD = 11.06, range 1–66) compared with Go stimuli (M = 13.64, SD = 25.88, range 0–207), t(120) = 13.79, P < 0.001). There was a main effect for post-error slowing (PES) (Rabbit, 1981). Participants responded more slowly on post-error trials compared to post-correct trials, M = 33 ms, SD = 90 ms, t(120) = 4.03, P ≤ 0.001.This effect was not specific to psychopathy, t(42) = .003, P = 0.99, consistent with previous reports (Brazil et al., 2009, 2011; Munro et al., 2007). PES also did not correlate with PCL-R variables (Total, Factor or Facet scores) or time-domain or principal component analyses reflecting ERN/Ne and Pe mean amplitude (r’s < 0.15). Psychopathy scores and BPD symptomatology were not significantly correlated with RTs or error rates (r’s < 0.15).
Group differences
Here, we report ANOVA analyses including the factors electrode (Fz, FCz, Cz and Pz) and extreme group (psychopath n = 23 and non-psychopath n = 21) to understand the specific error-related processes implicated in women with clinical levels of psychopathy. The initial categorization for male psychopathy was defined as one standard deviation above the mean (M = 22, SD = 8), resulting in a standard cut-off score of 30 or above for male psychopaths (Hare, 2003). Here, we report a mean PCL-R total score of 18.75 (SD = 6.37), resulting in a cut-off score of 25 for our sample, consistent with previous reports (Vitale and Newman, 2001a). n = 23 met the categorical classification for psychopathy in the current sample. Non-psychopaths consisted of n = 21 participants (PCL-R Total M = 8.80, SD = 2.84). Groups differed in respect to PCL-R Total scores (F1,42 = − 23.04, P < 0.001), number of substance dependencies (F1,38 = −4.068, P ≤ 0.001], and age (F1,42 = 2.18, P = 0.04). Groups did not differ in respect to IQ (F1,42 = 0.087, P = 0.93), BPD symptomatology (F1,37 = −1.751, P = 0.09) or mean number of FAs (F1,42 = 1.62, P = 0.11). Full descriptive statistics and t-tests to highlight group differences can be found in Supplementary Table S1.
Time-domain and PCA group differences
ERN/Ne
As expected, groups did not differ in respect to the mean ERN/Ne amplitude (F1,42 = 0.009, P = 0.93) or PC1 mean amplitude, reflecting the ERN/Ne mean amplitude (F1,42 = .067, p = .80].
Pe
The Pe was reduced for psychopaths compared to non-psychopaths (F1,42 = 3.960, P = 0.05) and for PC4 and PC5 mean amplitude, reflecting middle and late subcomponents underling the Pe (F1,42 = 4.259, P = 0.05) and (F1,42 = 4.453, P = 0.04), respectively. No group differences were observed in regards to PC3 mean amplitude, reflecting an early subcomponent of the Pe (F1,42 = 1.972, P = 0.17).
Regression analyses
Separate stepwise regressions using the average of the nine electrodes defined above were computed predicting the mean ERN/Ne and Pe amplitude with the mean measure of the five principal-component response-locked solution across the entire sample. The mean ERN/Ne was predicted by PC1 and PC3, and the mean Pe was predicted by PC2, PC3, PC4 and PC5 (P’s < 0.001). Although PC3 shared variance with both the ERN/Ne and Pe, PC3 reflects an early subcomponent of the Pe (see Fig. 1).
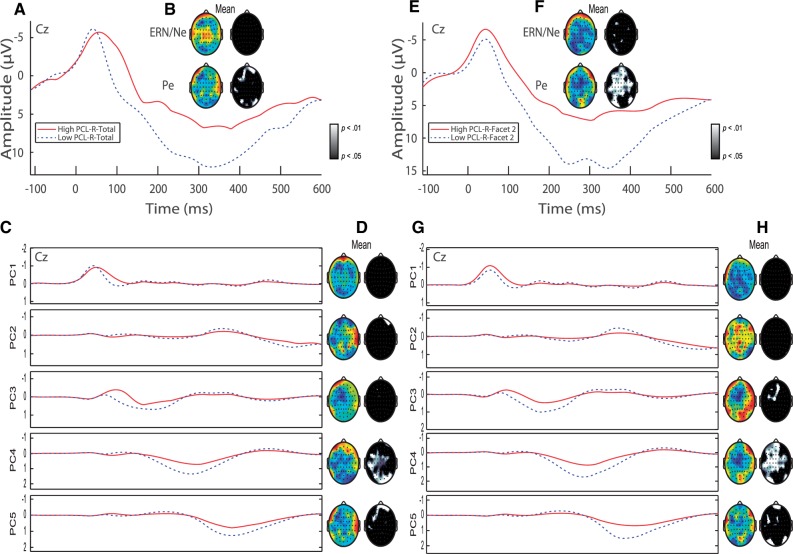
Fig. 1.
Response-locked ERP and PCA. (A) Representative ERP waveform plotted at Cz with negative voltage plotted up. ERP waveforms represent quartile splits of incarcerated adult females with elevated psychopathic traits (PCL-R Factor 1 score > 6, n = 41) (red) and low levels of psychopathic traits (PCL-R Factor 1 score < 3, n = 46) (dotted blue) are plotted. ERP components of interest (the ERN/Ne and Pe) are identified. (B) Topographic difference (color) and statistical (black and white) maps are plotted for each component window highlighting reduced Pe mean amplitude in adult females with elevated Factor 1 traits. Color maps were auto scaled to highlight topographical distribution of effects. Blue regions signify increased amplitudes for females with low levels of psychopathic traits compared to females with high levels of psychopathic traits. (C) Principal components extracted accounting for 90.98% of the variance. Principal components plotted for adult females with elevated Factor 1 traits (red line) and low levels of psychopathic traits (dotted blue line) are plotted at Cz. (D) Topographic difference (color) and statistical (black and white) maps are plotted for each principal component highlighting reduced Pe mean amplitude in adult females with elevated Factor 1 traits. (E) Representative ERP waveform plotted at Cz with negative voltage plotted up. ERP waveforms represent quartile splits of incarcerated adult females with elevated psychopathic traits (PCL-R Facet 2 score > 4, n = 43) (red) and low levels of psychopathic traits (PCL-R Facet 2 score < 1, n = 31) (dotted blue) are plotted. ERP components of interest (the ERN/Ne and Pe) are identified. (F) Topographic difference (color) and statistical (black and white) maps are plotted for each component window highlighting reduced Pe mean amplitude in adult females with elevated Facet 2 traits. (G) Principal components plotted for adult females with elevated Facet 2 traits (red line) and low levels of psychopathic traits (dotted blue line) are plotted at Cz. (H) Topographic difference (color) and statistical (black and white) maps are plotted for each principal component highlighting reduced Pe mean amplitude in adult females with elevated Facet 2 traits.
To sum, PC1 reflects the ERN/Ne mean amplitude, PC2 reflects post-Pe processing, and PC3, PC4 and PC5 reflect distinct subcomponents underlying the Pe (see Fig. 1) as detected in a previous report (Steele et al., 2014). The subcomponents underlying the Pe reflect unique patterns of hemodynamic activity (Edwards et al., 2012). In this study, the three Pe subcomponents show similar temporal distributions as the early, middle and late subcomponents defined previously (Edwards et al., 2012). As such, only PC2 was omitted from subsequent analyses, as it reflected post-Pe processing.
Time-domain ERP regression analyses
Separate linear regressions were performed to assess unique contributions to the mean ERN/Ne and Pe amplitude measured with classic windowed ERP components and PCA. Each regression included an ERP measure as the dependent measure, PCL-R measures (Regression 1: PCL-R Total Score; Regression 2: Factor Scores; Regression 3: Facet scores), and five covariate measures (IQ, age, number of substance dependencies, BPD symptomatology and participant’s mean number of FAs). The latter variables were therefore entered as simultaneous predictors alongside psychopathy scores in the regression analyses.
Regression 1 analyses showed that none of the variables included in analyses were significant predictors of ERN/Ne mean amplitude. Similarly, PCL-R Total Score did not emerge as a unique predictor of Pe mean amplitude. However, number of substance dependencies and age remained significant predictors of reduced Pe mean amplitude (Table 2).
Table 2.
Psychopathy variables and covariates predicting error-related positivity (Pe) mean amplitude
| Predictors | B | SE B | Wald | β | Sig | |
|---|---|---|---|---|---|---|
| Stepwise regression 1: PCL-R factor scores | ||||||
| Step 1 | PCL-R Factor 1 | −0.795 | 0.280 | −2.839 | −0.278 | .006 |
| PCL-R Factor 2 | 0.828 | .410 | ||||
| IQ | 0.632 | .529 | ||||
| Age | −2.480 | .015 | ||||
| Sub. Dep. | −1.402 | .164 | ||||
| BPD | 0.711 | .479 | ||||
| NoGo Errors | −1.146 | .255 | ||||
| Step 2 | PCL-R Factor 1 | −0.750 | 0.273 | −2.744 | −0.263 | .007 |
| PCL-R Factor 2 | 0.072 | .943 | ||||
| IQ | 0.553 | .582 | ||||
| Age | −0.193 | 0.078 | −2.480 | −0.237 | .015 | |
| Sub Dep. | −1.702 | .092 | ||||
| BPD | −0.046 | .963 | ||||
| NoGo Errors | −0.835 | .406 | ||||
| Stepwise regression 2: PCL-R facet scores | ||||||
| Step 1 | PCL-R Facet 1 | −1.657 | .101 | |||
| PCL-R Facet 2 | −2.677 | .009 | ||||
| PCL-R Facet 3 | −1.370 | .174 | ||||
| PCL-R Facet 4 | −0.718 | .475 | ||||
| IQ | 0.326 | .745 | ||||
| Age | −0.208 | 0.080 | −2.580 | −0.255 | .011 | |
| Sub. Dep. | −2.025 | .046 | ||||
| BPD | −0.997 | .322 | ||||
| NoGo Errors | −0.779 | .438 | ||||
| Step 2 | PCL-R Facet 1 | −0.905 | .368 | |||
| PCL-R Facet 2 | −1.026 | 0.383 | −2.677 | −0.256 | .009 | |
| PCL-R Facet 3 | −0.523 | .602 | ||||
| PCL-R Facet 4 | 0.024 | .981 | ||||
| IQ | 0.328 | .744 | ||||
| Age | −0.209 | 0.078 | −2.677 | −0.256 | .009 | |
| Sub. Dep. | −1.919 | .058 | ||||
| BPD | −0.191 | .849 | ||||
| NoGo Errors | −0.826 | .411 | ||||
Regression 1: Step 1: R2 = 0.077, R = 0.278, F(1,96) = 8.059; Step 2: R2 = 0.134, R = 0.365, F(1,95) = 6.149.
Regression 2: Step 1: R2 = 0.065, R = 0.255, F(1,96) = 6.656; Step 2: R2 = 0.130, R = 0.361, F(1,95) = 7.165.
Note. Assessments: PCL-R Factor 1 and Factor 2 are Factor 1 and Factor 2 scores derived from the Hare Psychopathy Checklist-Revised (PCL-R; Hare, 2003); PCL-R Facet 1, Facet 2, Facet 3 and Facet 4 scores are Facet 1, 2, 3 and 4 scores derived from the PCL-R (Hare, 2003); IQ was calculated from the WAIS-III (Wechsler, 1997); BPD is Borderline Personality Disorder symptomatology derived from the SCID-II (Firstet al., 1997); Sub. Dep. is the number of substance dependencies calculated from the Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Version (SCID I/P) (First et al., 1995). NoGo errors are the mean number of FA's each participant made.
Regression 2 analyses showed that neither PCL-R Factor scores nor covariate measures were significant predictors of ERN/Ne mean amplitude. However, PCL-R Factor 1 scores and age emerged as significant predictors of reduced Pe mean amplitude (Table 2 and Fig. 1).
Finally, Regression 3 showed that none of the variables entered emerged as significant predictors of ERN/Ne mean amplitude. PCL-R Facet 2 scores, age, and number of substance dependencies emerged as significant predictors of reduced Pe mean amplitude (Table 2 and Supplementary Figure S1).
PCA regression analyses
ERN/Ne
The same psychopathy variables (either PCL-R Total, Factor or Facet scores) and covariate measures used to predict time-domain ERP amplitudes (IQ, age, number of substance dependencies, BPD symptomatology and participant’s mean number of FAs) were used in subsequent PCA regression analyses. Psychopathy variables and the other five covariate measures did not emerge as significant predictors of PC1 mean amplitude, reflecting the ERN/Ne mean amplitude.
Pe
Psychopathy variables emerged as significant predictors of PC3, PC4 and PC5 mean amplitudes, reflecting early, middle, and late subcomponents of the Pe, respectively. Regression 1 analyses showed that none of the variables included in analyses emerged as significant predictors of PC3 mean amplitude. Similarly, PCL-R Total Score did not emerge as a significant predictor of PC4 mean amplitude, while number of substance dependencies, age and participant’s mean number of FAs (Table 3). Likewise, PCL-R Total Score was not a significant predictor of PC5 mean amplitude, but number of substance dependencies emerged as a unique predictor (Table 4).
Table 3.
Psychopathy variables and covariates predicting PC3 mean amplitude
| Predictors | B | SE B | Wald | β | Sig | ||
|---|---|---|---|---|---|---|---|
| Stepwise Regression 1: PCL-R Factor Scores | |||||||
| Step 1 | PCL-R Factor 1 | −0.025 | 0.012 | −2.050 | −0.205 | .003 | |
| PCL-R Factor 2 | 0.385 | .701 | |||||
| IQ | 0.527 | .599 | |||||
| Age | −0.773 | .441 | |||||
| Sub. Dep. | −0.198 | .843 | |||||
| BPD | −0.881 | .380 | |||||
| NoGo Errors | −0.390 | .697 | |||||
Regression 1: Step 1: R2 = .042, R = .205, F(1,96) = 4.204.
Note. Assessments: PCL-R Factor 1 and Factor 2 are Factor 1 and Factor 2 scores derived from the Psychopathy Checklist-Revised (Hare, 2003); IQ was calculated from the Wechsler Adult Intelligence Scale – Third Version (WAIS-III) (Wechsler, 1997); BPD is Borderline Personality Disorder symptomatology derived from the Structured Clinical Interview for DSM-IV Axis II Disorders (SCID-II) (First et al., 1997); Sub. Dep. is the number of substance dependencies calculated from the Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Version (SCID I/P) (First et al., 1995). NoGo errors are the mean number of FA's each participant made.
Table 4.
Psychopathy variables and covariates predicting PC4 mean amplitude
| Predictors | B | SE B | Wald | β | Sig | ||
|---|---|---|---|---|---|---|---|
| Stepwise regression 1: PCL-R factor scores | |||||||
| Step 1 | PCL-R Factor 1 | −2.538 | .013 | ||||
| PCL-R Factor 2 | −2.295 | .024 | |||||
| IQ | 0.249 | .804 | |||||
| Age | −0.014 | 0.005 | −3.060 | −0.298 | .003 | ||
| Sub. Dep. | −3.049 | .003 | |||||
| BPD | −2.014 | .047 | |||||
| NoGo Errors | −1.507 | .135 | |||||
| Step 2 | PCL-R Factor 1 | −2.205 | .030 | ||||
| PCL-R Factor 2 | −1.026 | .307 | |||||
| IQ | 0.025 | .980 | |||||
| Age | −0.016 | 0.005 | −3.447 | −0.323 | .001 | ||
| Sub Dep. | −0.091 | 0.030 | −3.049 | −0.286 | .003 | ||
| BPD | −0.873 | .385 | |||||
| NoGo Errors | −1.873 | .064 | |||||
| Step 3 | PCL-R Factor 1 | −0.035 | 0.016 | −2.205 | −0.205 | .030 | |
| PCL-R Factor 2 | 0.003 | .997 | |||||
| IQ | 0.224 | .823 | |||||
| Age | −0.015 | 0.004 | −3.332 | −0.307 | .001 | ||
| Sub Dep. | −0.082 | 0.030 | −2.764 | −0.257 | .007 | ||
| BPD | −0.165 | .869 | |||||
| NoGo Errors | −1.907 | .060 | |||||
| Stepwise regression 2: PCL-R facet scores | |||||||
| Step 1 | PCL-R Facet 1 | −1.630 | .106 | ||||
| PCL-R Facet 2 | −2.415 | .018 | |||||
| PCL-R Facet 3 | −2.119 | .037 | |||||
| PCL-R Facet 4 | −1.882 | .063 | |||||
| IQ | 0.249 | .804 | |||||
| Age | −0.014 | 0.005 | −3.060 | –0.298 | .003 | ||
| Sub. Dep. | −3.049 | .003 | |||||
| BPD | −2.014 | .047 | |||||
| NoGo Errors | −1.507 | .135 | |||||
| Step 2 | PCL-R Facet 1 | −1.116 | .267 | ||||
| PCL-R Facet 2 | −2.321 | .022 | |||||
| PCL-R Facet 3 | −1.033 | .304 | |||||
| PCL-R Facet 4 | −0.693 | .490 | |||||
| IQ | 0.025 | .980 | |||||
| Age | −0.016 | 0.005 | −3.447 | −0.323 | .001 | ||
| Sub. Dep. | −0.091 | 0.030 | −3.049 | −0.286 | .003 | ||
| BPD | −0.873 | .385 | |||||
| NoGo Errors | −1.873 | .064 | |||||
| Step 3 | PCL-R Facet 1 | −0.451 | .653 | ||||
| PCL-R Facet 2 | −0.050 | 0.022 | −2.321 | −0.213 | .022 | ||
| PCL-R Facet 3 | −0.269 | .789 | |||||
| PCL-R Facet 4 | −0.045 | .964 | |||||
| IQ | 0.328 | .744 | |||||
| Age | −0.016 | 0.004 | −3.527 | −0.324 | .001 | ||
| Sub. Dep. | −0.087 | 0.029 | −2.967 | −0.273 | .004 | ||
| BPD | −0.160 | .874 | |||||
| NoGo Errors | −1.924 | .057 | |||||
Regression 1: Step 1: R2 = 0.089, R = 0.298, F(1,96) = 9.362; Step 2: R2 = 0.170 R = 0.412, F(1,95) = 9.296; Step 3: R2 = 0.211, R = 0.459, F(1,94) = 4.860.
Regression 2: Step 1: R2 = 0.089, R = 0.298, F(1,96) = 9.362; Step 2: R2 = 0.170, R = 0.412, F(1,95) = 9.296; Step 3: R2 = .215, R = .464, F(1,94) = 5.389.
Note. Assessments: PCL-R Factor 1 and Factor 2 are Factor 1 and Factor 2 scores derived from the Psychopathy Checklist-Revised; PCL-R Facet 1, Facet 2, Facet 3 and Facet 4 scores are Facet 1, 2, 3 and 4 scores derived from the PCL-R (Hare, 2003); IQ was calculated from the WAIS-III (Wechsler, 1997); BPD is Borderline Personality Disorder symptomatology derived from the Structured Clinical Interview for DSM-IV Axis II Disorders (SCID-II) (First et al., 1997); Sub. Dep. is the number of substance dependencies calculated from the Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Version (SCID I/P) (First et al., 1995). NoGo errors are the mean number of FA's each participant made.
Regression 2 analyses including PCL-R Factor scores along with covariate measures showed that none of the variables entered were significant predictors of PC3 mean amplitude. Covariates number of substance dependencies, age, and participant’s mean number of FAs emerged as significant predictors of PC4 mean amplitude (Table 3). Additionally, number of substance dependencies emerged as a significant predictor of PC5 mean amplitude (Table 4).
Finally, Regression 3 analyses showed that none of the variables included in analyses were significant predictors of PC3 mean amplitude. However, number of substance dependencies emerged as a significant predictor of PC4 mean amplitude (Table 3). Furthermore, PCL-R Facet 2 along with the covariate measure number of substance dependencies emerged as significant predictors of PC5 mean amplitude (Tables 4 and 5).
Table 5.
Psychopathy variables and covariates predicting PC5 mean amplitude
| Predictors | B | SE B | Wald | β | Sig | |
|---|---|---|---|---|---|---|
| Stepwise regression 1: PCL-R factor scores | ||||||
| Step 1 | PCL-R Factor 1 | −0.045 | 0.019 | −2.351 | −0.233 | .021 |
| PCL-R Factor 2 | −0.727 | .469 | ||||
| IQ | 1.115 | .268 | ||||
| Age | −1.408 | .162 | ||||
| Sub. Dep. | −1.936 | .056 | ||||
| BPD | −0.489 | .626 | ||||
| NoGo Errors | −0.540 | .591 | ||||
| Stepwise regression 2: PCL-R facet scores | ||||||
| Step 1 | PCL-R Facet 1 | -0.677 | .500 | |||
| PCL-R Facet 2 | −0.063 | 0.027 | -2.359 | −0.234 | .020 | |
| PCL-R Facet 3 | −0.805 | .423 | ||||
| PCL-R Facet 4 | −0.791 | .431 | ||||
| IQ | 0.925 | .357 | ||||
| Age | −1.579 | .118 | ||||
| Sub. Dep. | −2.104 | .038 | ||||
| BPD | −0.536 | .593 | ||||
| NoGo Errors | −0.558 | .578 | ||||
| Step 2 | PCL-R Facet 1 | −0.339 | .735 | |||
| PCL-R Facet 2 | −0.059 | 0.026 | −2.263 | −0.221 | .026 | |
| PCL-R Facet 3 | 0.052 | .959 | ||||
| PCL-R Facet 4 | 0.119 | .905 | ||||
| IQ | 0.787 | .433 | ||||
| Age | −1.809 | 0.074 | ||||
| Sub. Dep. | −0.074 | 0.035 | −2.104 | −0.206 | .038 | |
| BPD | 0.383 | .702 | ||||
| NoGo Errors | 0.432 | .432 | ||||
Regression 1: Step 1: R2 = 0.054, R = 0.233, F(1,96) = 5.525.
Regression 2: Step 1: R2 = 0.055, R = 0.234, F(1,96) = 5.563; Step 2: R2 = 0.097, R = 0.311, F(1,95) = 4.425.
Note. Assessments: PCL-R Factor 1 and Factor 2 are Factor 1 and Factor 2 scores derived from the Psychopathy Checklist-Revised (Hare, 2003); PCL-R Facet 1, Facet 2, Facet 3 and Facet 4 scores are Facet 1, 2, 3 and 4 scores derived from the PCL-R (Hare, 2003); IQ was calculated from the WAIS-III (Wechsler, 1997); BPD is Borderline Personality Disorder symptomatology derived from the Structured Clinical Interview for DSM-IV Axis II Disorders (SCID-II) (First et al., 1997); Sub. Dep. is the number of substance dependencies calculated from the Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Version (SCID I/P) (First et al., 1995). NoGo errors are the mean number of FAapos;s each participant made.
Discussion
This study examined whether dysfunctional error-related processing characteristic of male psychopathy (Munro et al., 2007; Brazil et al., 2009, 2011; von Borries et al., 2010) was also present in incarcerated female psychopaths. Consistent with hypotheses, based upon group-based and dimensional analyses, females with elevated psychopathic traits displayed intact early error-detection and action-monitoring processes as indexed by ERN/Ne mean amplitude (Gehring et al., 1993; Falkenstein et al., 2000). Female psychopathy was associated with reduced amplitude of later stages of error-processing, as indexed by both time-domain and PCA analyses reflecting Pe mean amplitude. Stepwise linear regressions associated reduced Pe mean amplitude specifically with PCL-R subscales related to interpersonal and affective dysfunction.
Our results lie contrary to previous research associating reduced ERN/Ne mean amplitudes in predominantly female community samples with psychopathic tendencies (Dikman and Allen, 2000; Heritage and Benning, 2013). However, these studies may be better measures of broader externalizing traits as psychopathy was assessed via self-report. Externalizing populations often show decreased ERN/Ne amplitude (Hall et al., 2007; Olvet and Hajcak, 2008). Additionally, these studies did not examine the potential moderating influence of BPD in their results, an important consideration as BPD has been previously associated with reduced ERN/Ne amplitude (de Bruijn et al., 2006; Ruchsow et al., 2006). In the present study, neither psychopathy scores nor covariate measures emerged as significant predictors of ERN/Ne mean amplitude. This suggests that female psychopathy is not associated with impairments in error detection or action-monitoring processes (Gehring et al., 1993; Falkenstein et al., 2000).
Female psychopathy was associated with dysfunction in later stages of error-processes as indexed by reduced Pe mean amplitude. This is consistent with an initial report showing reduced Pe amplitude in male psychopathy (Brazil et al., 2009). The Pe is believed to be implicated in later, more elaborate error-processing stages, including the motivational (Ullsperger et al., 2010) and emotional appraisal (Overbeek et al., 2005) of error-related information.
PCL-R Facet 2 emerged as a significant predictor of reduced Pe mean amplitude. This facet directly taps into affective dysfunction associated with female psychopathy, including shallow affect, callousness, and a lack of remorse and empathy. Our current results appear to support the affective-processing hypothesis of the Pe (Overbeek et al., 2005). As the Pe is believed to arise from the rostral, affective subdivision of the ACC (van Veen and Carter, 2002; Edwards et al., 2012), reduced Pe amplitude suggests female psychopathy is associated with decreased emotional appraisal of error-related information.
In addition to psychopathy variables, age, number of substance dependencies and participant’s mean number of FAs emerged as significant predictors of reduced Pe mean amplitude. The Pe may reflect an error-related subcomponent of the P300 ERP component which has been shown to decrease in amplitude with increasing age (Juckel et al., 2012). Both components show a similar topography and latency in response to errors (Leuthold and Sommer, 1999; Nieuwenhuis et al., 2001). The Pe may reflect a P300 elicited by rare error trials, but also, involved in post-error response adjustment strategies, including the updating of environmental contexts (Leuthold and Sommer, 1999).
Age emerged as a significant predictor of reduced Pe mean amplitude. It’s relevant to note that psychopathic traits, particularly lifestyle and antisocial traits, have been shown to decrease with increasing age (Harpur and Hare, 1994). Thus, age-related dysfunctional post-error processing may augment baseline deficits associated with Factor 1 and Facet 2 psychopathic traits. As such, early intervention to potentially curb error-related dysfunction in female psychopathy may prove essential as the problem may increase with age. Age may have emerged as a significant predictor of reduced Pe amplitude as P300 amplitude has been shown to reduce in amplitude with increasing age (Juckel et al., 2012). To address this issue, we conducted group-based and dimensional analyses investigating the P300 ERP component (see Supplemental Materials). Our supplementary results support the interpretation of the Pe as a separate entity compared to the P300, as female psychopathy was associated with a deficit in Pe amplitude, not the P300.
Additionally, number of substance dependencies emerged as a significant predictor of reduced Pe mean amplitude. Psychopathic traits, particularly Factor 2 traits, and substance use disorders (SUDs) are highly comorbid (Smith and Newman, 1990; Walsh et al., 2007). Both disorders are characterized by dysfunction of broader externalizing dimensions, including impulsivity, poor decision-making and dysfunctional error-related processing (Franken et al., 2007; Goldstein et al., 2007). Furthermore, SUDs appear to be specifically related to a form of impulsivity captured by the Barratt Impulsivity Scale subscale Non-Planning Impulsiveness (Patton et al., 1995). This measure is directly related to the quantity and severity of substance use (Stanford et al., 2009). The inclusion of substance dependence as a covariate measure may be capturing a more specific form of impulsivity directly related to the poor planning of future behavior (Hare, 2003). It is possible that predictor criterion overlap between the number of substance dependencies and Factor 2 psychopathic traits led to the former being consistent in regression analyses and the latter non-significant. Future studies might consider identifying a sample of participants scoring high in psychopathy and low substance abuse to better disentangle these effects.
BPD symptomatology was not a significant predictor of either ERN/Ne or Pe mean amplitude. Researchers have investigated the putative role of BPD in female psychopathy, as the two disorders exhibit a similar impulsive and aggressive nature (Sprague et al., 2012). Sprague et al. (2012) suggest the possibility of a BPD-variant of female psychopathy closely resembling the construct of secondary psychopathy in male samples. In this model, manipulative and callous traits arise as a means of coping with environmental stressors and are often accompanied by emotional dysregulation (Karpman, 1941; Sprague et al., 2012). The present sample scored in the low range of BPD symptomatology (only 7 of 121 participants met the SCID-II classification for BPD). Thus, our results appear to be investigating the primary variant of female psychopathy, rather than the BPD-variant of secondary psychopathy found in previous reports (Hicks et al., 2010; Sprague et al., 2012). Future research may benefit from investigating error-related processing in this secondary variant of female psychopathy, as primary and secondary variants of female psychopathy may exhibit dissimilar error-related processing, which could potential impact treatment interventions aimed towards these disorders.
PCL-R Total Score and Factor 2 scores were not significant predictors of reduced Pe mean amplitude through regression analyses; rather, Pe amplitude reduction was associated with interpersonal and affective dysfunction characterized by PCL-R Factor 1 and Facet 2 scores. The variance associated with Factor 2 tends to dominate the PCL-R Total Score in female samples (Kennealy et al., 2007). This may explain why PCL-R Total Score was not a significant predictor of either time-domain or PCA measures reflecting Pe mean amplitude.
We observed significant PES after error trials compared to correct trials. PES did not correlate with time-domain or PCA measures reflecting Pe mean amplitude (r’s < 0.15). Also, we did not observe an association between PES and psychopathy, consistent with previous incarcerated adult male samples (Munro et al., 2007; Brazil et al., 2009; 2011). Recently, PES has been associated with self-reported psychopathic traits in community samples comprised of males and females (Bresin et al., 2014). This raises the possibility that methodological differences may account for the discrepant findings, including differences in measurement of psychopathy (self-report vs expert-rater devices) and clinical levels of psychopathy (community samples vs forensic samples). Also, the present task did not measure error awareness, so we cannot directly relate behavioral data to the reduced Pe amplitude in female psychopathy as being an inability to use error-related information to subsequently improve future behavior.
Limitations
Female psychopathy is less prevalent than male psychopathy with 9–16% of incarcerated females meeting the criteria for psychopathy compared with 15–25% of males (Vitale et al., 2002). In the present study, only 5% of the sample scored above the typical threshold used for male psychopathy (i.e. a PCL-R Total Score ≥ 30), with n = 23 participants scoring one standard deviation above the mean. Therefore, it is not known whether our results with female offenders with elevated psychopathic traits will extend to women scoring in the more extreme range of PCL-R Total Scores.
For comparison to male samples, we used the standard PCL-R Factor and Facet items algorithms used in male samples (Hare, 2003; Hare and Neumann, 2006). It is possible that female psychopathy may have a different factor and facet structure than male psychopathy. For example, PCL-R items such as promiscuous sexual behavior do not load onto either Factor 1 or Factor 2 for male psychopathy, whereas one study found this item loads onto to Factor 2 for female psychopathy (Salekin et al., 1997). While the PCL-R has been validated for its use in incarcerated female samples (Vitale and Newman, 2001b), future analyses may benefit by investigating which PCL-R items load onto respective Factors and Facets appropriately to better conceptualize the phenomenon of female psychopathy.
Our study included participants with low error rates to NoGo stimuli. Some have suggested excluding such participants, as the ERN/Ne and Pe can be quantified in as little as six to eight errors (Olvet and Hajcak, 2009; Pontifex et al., 2010; Rietdijk et al., 2014). However, we included participants who committed fewer than six FA’s (n = 16). Reliability analyses were performed on the current data set, showing a reliable ERN/Ne signal with as few as four error trials and Pe signal in as few as two error trials. Effects are consistent with those reported earlier when excluding participants based on the necessary number of trials for a reliable signal (see Supplemental Analyses). Still, replication is necessary to evaluate our findings in similar populations with low error rates.
Conclusions
Female psychopathy was associated with reduced Pe mean amplitude and intact ERN/Ne amplitude. Results suggest that female psychopaths exhibit intact, automatic error-detection and action-monitoring processes as indexed by the ERN/Ne. However, this population exhibits specific deficits in post-error processing, as indexed by reduced Pe amplitude. Results specifically associated reduced Pe mean amplitude with interpersonal and affective traits considered central to female psychopathy. This is the first evidence to suggest that female psychopaths exhibit similar post-error processing dysfunction as male psychopaths, which may partly explain this population’s increased propensity towards prolific substance use, with violence, recidivism and incarceration.
Supplementary Material
Acknowledgements
We are grateful to the staff and clients at the New Mexico Women’s Correctional Facility for their support and making this research possible.
Funding
This research was supported by grants from the National 100 Institute of Mental Health (R01 MH085010-01A1, MH070539-01) (PI: K.A.K.) and (K08 MH080239) (PI: E.M.B.) and National Institute on Drug Abuse (R01 DA026964-01A1, DA026505-01A1) (PI: K.A.K).
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
References
- Baskin-Sommers A.R., Curtin J.J., Newman J.P. (2013). Emotion-modulation startle in psychopathy: Clarifying familiar effects. Journal of Abnormal Psychology , 122(2), 458–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernat E.M., Nelson L.D., Steele V.R., Gehring W.J., Patrick C.J. (2011). Externalizing psychopathology and gain-loss feedback in a simulated gambling task: dissociable components of brain response revealed by time-frequency analysis. Journal of Abnormal Psychology , 120(2), 352–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair R.J. (2006). The emergence of psychopathy: Implications for the neuropsychological approach to developmental disorders. Cognition , 101(2), 414–42. [DOI] [PubMed] [Google Scholar]
- Brazil I. A., de Bruijn E.R., Bulten B.H., von Borries A.K., van Lankveld J.J., Buitelaar J.K., Verkes R.J. (2009). Early and late components of error monitoring in violent offenders with psychopathy. Biological Psychiatry , 65(2), 137–43. [DOI] [PubMed] [Google Scholar]
- Brazil I.A., Mars R.B., Bulten B.H., Buitelaar J.K., Verkes R.J., De Bruijn E.R. (2011). A neurophysiological dissociation between monitoring one’s own and others’ actions in psychopathy. Biological Psychiatry , 69(7), 693–99. [DOI] [PubMed] [Google Scholar]
- Bresin K., Finy S., Sprague J., Verona E. (2014). Response monitoring and adjustment: Differential relations with psychopathic traits. Journal of Abnormal Child Psychology , 123(3), 634–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budhani S., Richell R.A., Blair R.J. (2006). Impaired reversal but intact acquisition: probabilistic response reversal deficits in adult individuals with psychopathy. Journal of Abnormal Psychology , 115(3), 552–8. [DOI] [PubMed] [Google Scholar]
- Chapman R.M., McCarry J.W. (1995). EP component identification and measurement by principal component analysis. Brain and Cognition , 27, 288–310. [DOI] [PubMed] [Google Scholar]
- de Bruijn E.R., Grootens K.P., Verkes R.J., Buchholz V., Hummelen J.W., Hulstijn W. (2006). Neural correlates of impulsive responding in borderline personality disorder: ERP evidence for reduced action monitoring. Journal of Psychiatric Research , 40(5), 428–37. [DOI] [PubMed] [Google Scholar]
- Dehaene S., Posner M.I., Tucker D.M. (1994). Localization of a neural system for error detection and compensation. Psychological Science , 5(5), 303–90. [Google Scholar]
- Dikman Z.V., Allen J.J.B. (2000). Error monitoring during reward and avoidance learning in high- and low-socialized individuals. Psychophysiology , 37(1), 43–54. [PubMed] [Google Scholar]
- Edwards B.G., Calhoun V.D., Kiehl K.A. (2012). Joint ICA of ERP and fMRI during error-monitoring. NeuroImage , 59(2), 1896–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenbarth H., Angrilli A., Calogero A., Harper J., Olson L.A., Bernat E. (2013). Reduced negative affect response in female psychopaths. Biological Psychology , 94(2), 310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M.B., Gibbon M., Spitzer R.L., Williams J.B.W., Benjamin L.S. (1997). Structured Clinical Interview for DSM-IV Axis II Personality Disorder (SCID-II). Washington DC: American Psychiatric Press. [Google Scholar]
- First M.B., Spitzer M., Gibbon M., Williams J.B.W. (1995). tructured Clinical Interview for DSM-IV Axis I Disorders - Patient Version. New York, NY: New York State Psychiatric Institute, Biometrics Research Department. [Google Scholar]
- Falkenstein M., Hoormann J., Christ S., Hohnsbein J. (2000). ERP components on reaction errors and their functional significance: a tutorial. Biological Psychology , 51, 87–107. [DOI] [PubMed] [Google Scholar]
- Franken I.H., van Strien J.W., Franzek E.J., van de Wetering B.J. (2007). Error-processing deficits in patients with cocaine dependence. Biological Psychiatry , 75(1), 45–51. [DOI] [PubMed] [Google Scholar]
- Gehring W.J., Goss B., Coles M.G.H., Meyer D.E., Donchin E. (1993). A neural system for error detection and compensation. Psychological Science , 4(6), 385–390. [Google Scholar]
- Goldstein R.Z., Tomasi D., Rajaram S., Cottone L.A., Zhang L., Maloney T., et al. (2007). Role of the anterior cingulate and medial orbitofrontal cortex in processing drug cues in cocaine addiction. Neuroscience , 144(4), 1153–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J., Bernat E.M., Patrick C.J. (2007). Externalizing psychopathology and the error-related negativity. Psychological Science , 18(4), 326–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare R.D. (2003). Manual for the Hare Psychopathy Checklist - Revised (2nd Ed). Toronto, Canada: Multi-Health Systems. [Google Scholar]
- Hare R.D., Neumann C.S. (2006). The PCL-R assessment of psychopathy: development, structural properties, and new directions. In: Patrick C. editor. Handbook of Psychopathy, pp. 58–88. New York: Guilford Press. [Google Scholar]
- Harpur T.J., Hare R.D. (1994). Assessment of psychopathy as a function of age. Journal of Abnormal Psychology , 103(4), 604–9. [DOI] [PubMed] [Google Scholar]
- Harpur T.J., Hare R.D., Hakstian R. (1989). Two-factor conceptualization of psychopathy: Construct validity and assessment implications. Psychological Assessment , 1(1), 6–17. [Google Scholar]
- Heritage A.J., Benning S. (2013). Impulsivity and response modulation deficits in psychopathy: evidence from the ERN and N1. Journal of Abnormal Psychology , 122(1), 215–22. [DOI] [PubMed] [Google Scholar]
- Herrmann M.J., Rommler J., Ehlis A.C., Heidrich A., Fallgatter A.J. (2004). Source localization (LORETA) of the error-related-negativity (ERN/Ne) and positivity (Pe). Cognitive Brain Research , 20(2), 294–9. [DOI] [PubMed] [Google Scholar]
- Hicks B.M., Vaidyanathan U., Patrick C.J. (2010). Validating female psychopathy subtypes: Differences in personality, antisocial and violent behavior, substance abuse, trauma, and mental health. Personality Disorders: Theory, Research, and Treatment , 1(1), 38–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasper H.H. (1958). The ten-twenty electrode system of the International Federation. Electroencephalography and Clinical Neurophysiology , 10, 371–5. [PubMed] [Google Scholar]
- Juckel G., Karch S., Kawohl W., Kirsch V., Jager L., Leicht G., et al. (2012). Age effects on the P300 potential and the corresponding fMRI BOLD-signal. NeuroImage , 60(4), 2027–34. [DOI] [PubMed] [Google Scholar]
- Karpman B. (1941). On the need of separating psychopathy into two distinct clinical types: the symptomatic and the idiopathic. Journal of Criminal Psychopathology , 3, 112–37. [Google Scholar]
- Kennealy P.J., Hicks B.M., Patrick C.J. (2007). Validity of factors of the Psychopathy Checklist–Revised in female prisoners: discriminant relations with antisocial behavior, substance abuse, and personality. Assessment , 14(4), 323–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehl K.A. (2006). A cognitive neuroscience perspective on psychopathy: evidence for paralimbic system dysfunction. Psychiatry Research , 142(2-3), 107–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehl K.A., Liddle P.F., Hopfinger J.B. (2000). Error processing and the rostral anterior cingulate: an event-related fMRI study. Psychophysiology , 37(2), 216–23. [PubMed] [Google Scholar]
- Kosson D.S., Suchy Y., Mayer A.R., Libby J. (2002). Facial affect recognition in criminal psychopaths. Emotion , 2(4), 398–411. [DOI] [PubMed] [Google Scholar]
- Leuthold H., Sommer W. (1999). ERP correlates of error processing in spatial S-R compatibility tasks. Clinical Neurophysiology , 110(2), 342–57. [DOI] [PubMed] [Google Scholar]
- Munro G.E., Dywan J., Harris G.T., McKee S., Unsal A., Segalowitz S.J. (2007). ERN varies with degree of psychopathy in an emotion discrimination task. Biological Psychology , 76(1–2), 31–42. [DOI] [PubMed] [Google Scholar]
- Newman J.P. (1987). Reaction to punishment in extraverts and psychopaths: Implications for the impulsive behavior of disinhibited individuals. Journal of Research in Personality , 21(4), 464–80. [Google Scholar]
- Newman J.P., Kosson D. S. (1986). Passive avoidance learning in psychopathic and nonpsychopathic offenders. Journal of Abnormal Psychology , 95(3), 252–6. [PubMed] [Google Scholar]
- Nieuwenhuis S., Ridderinkhof R.R., Blom J., Band G.P., Kok A. (2001). Error-related brain potentials are differentially related to awareness of response errors: evidence from an antisaccade task. Psychophysiology , 38(5), 752–60. [PubMed] [Google Scholar]
- Olvet D.M., Hajcak G. (2008). The error-related negativity (ERN) and psychopathology: toward an endophenotype. Clinical Psychology Review , 28(8), 1343–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olvet D.M., Hajcak G. (2009). The stability of error-related brain activity with increasing trials. Psychophysiology , 46(5), 957–61. [DOI] [PubMed] [Google Scholar]
- Overbeek T.J.M., Nieuwenhuis S., Ridderinkhof K.R. (2005). Dissociable components of error processing. Journal of Psychophysiology , 19(4), 319–29. [Google Scholar]
- Patrick C.J., Bradley M.M., Lang P.J. (1993). Emotion in the criminal psychopath: startle reflex modulation. Journal of Abnormal Psychology , 102(1), 82–92. [DOI] [PubMed] [Google Scholar]
- Patrick C.J., Fowles D.C., Krueger R.F. (2009). Triarchic conceptualization of psychopathy: developmental origins of disinhibition, boldness, and meanness. Development and Psychopathology , 21(3), 913–39. [DOI] [PubMed] [Google Scholar]
- Patton J.H., Stanford M. S., Barratt E. S. (1995). Factor structure of the Barratt Impulsiveness Scale. Journal of Clinical Psychology , 6, 768–74. [DOI] [PubMed] [Google Scholar]
- Pontifex M.B., Scudder M.R., Brown M.L., O’Leary K.C., Wu C.T., Themanson J.R., et al. (2010). On the number of trials necessary for stabilization of error-related brain activity across the life span. Psychophysiology , 47(4), 767–73. [DOI] [PubMed] [Google Scholar]
- Rabbit P.M.A. (1981). Sequential reactions. In: Holding D. editor. Human Skills, pp. 153–75. New York: Wiley. [Google Scholar]
- Rice M.E., Harris G.T. (1997). Cross-validation and extension of the Violence Risk Appraisal Guide for child molestors and rapists. Law and Human Behavior , 21(2), 231–41. [DOI] [PubMed] [Google Scholar]
- Rietdijk W.J.R., Franken I.H.A., Thurik A.R. (2014). Internal consistency of event-related potentials associated with cognitive control: N2/P3 and ERN/Pe. PLoS One , 9(7), 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruchsow M., Walter H., Buchheim A., Martius P., Spitzer M., Kachele H., et al. (2006). Electrophysiological correlates of error processing in borderline personality disorder. Biological Psychology , 72(2), 133–40. [DOI] [PubMed] [Google Scholar]
- Salekin R.T., Rogers R., Sewell K.W. (1997). Construct validity of psychopathy in a female offender sample: a multitrait-multimethod evaluation. Journal of Abnormal Psychology , 106(4), 576–85. [DOI] [PubMed] [Google Scholar]
- Shalgi S., Deouell L.Y. (2012). Is any awareness necessary for an Ne? Frontiers in Human Neuroscience , 6(124), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalgi S., Deouell L.Y. (2013). Is there any electrophysiological evidence for subliminal error processing? Frontiers in Neuroscience , 7(150), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.S., Newman J.P. (1990). Alcohol and drug abuse-dependence disorders in psychopathic and nonpsychopathic criminal offenders. Journal of Abnormal Psychology , 99(4), 430–39. [DOI] [PubMed] [Google Scholar]
- Sprague J., Javdani S., Sadeh N., Newman J.P., Verona E. (2012). Borderline personality disorder as a female phenotypic expression of psychopathy? Personality Disorders: Theory, Research, and Treatment , 3(2), 127–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford M.S., Mathias C.W., Dougherty D.M., Lake S.L., Anderson N.E., Patton J.H. (2009). Fifty years of the Barratt Impulsiveness Scale: an update and review. Personality and Individual Differences , 47(5), 385–95. [Google Scholar]
- Steele V.R., Fink B.C., Maurer J.M., Arbabshirani M.R., Wilber C.H., Jaffe A.J., et al. (2014). Brain potentials measured during a go/nogo task predict completion of substance abuse treatment. Biological Psychiatry , 76(1), 75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton S.K., Vitale J.E., Newman J.P. (2002). Emotion among women with psychopathy during picture perception. Journal of Abnormal Psychology , 111(4), 610–9. [DOI] [PubMed] [Google Scholar]
- Ullsperger M., Harsay H.A., Wessel J.R., Ridderinkhof K.R. (2010). Conscious perception of errors and its relation to the anterior insula. Brain Structure and Function , 214(5–6), 629–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veen V., Carter C.S. (2002). The timing of action-monitoring processes in the anterior cingulate cortex. Journal of Cognitive Neuroscience , 14(4), 593–602. [DOI] [PubMed] [Google Scholar]
- Verona E., Bresin K., Patrick C.J. (2013). Revisiting psychopathy in women: Cleckley/Hare conceptions and affective response. Journal of Abnormal Psychology , 122(4), 1088–93. [DOI] [PubMed] [Google Scholar]
- Vitale J.E., Maccoon D.G., Newman J.P. (2011). Emotion facilitation and passive avoidance learning in psychopathic female offenders. Criminal Justice and Behavior , 38(7), 641–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale J.E., Newman J.P. (2001a). Response perseveration in psychopathic women. Journal of Abnormal Psychology , 110(4), 644–47. [DOI] [PubMed] [Google Scholar]
- Vitale J.E., Newman J.P. (2001b). Using the Psychopathy Checklist-Revised with female samples: reliability, validity, and implications for clinical utility. Clinical Psychology: Science and Practice , 8(1), 117–32. [Google Scholar]
- Vitale J.E., Smith S.S., Brinkley C.A., Newman J.P. (2002). The reliability and validity of the Psychopath Checklist—revised in a sample of female offenders. Criminal Justice and Behavior , 29(2), 202–231. [Google Scholar]
- Vocat R., Pourtois G., Vuilleumier P. (2008). Unavoidable errors: A spatio-temporal analysis of time-course and neural sources of evoked potentials associated with error processing in a speeded task. Neuropsychologia , 46(10), 2545–555. [DOI] [PubMed] [Google Scholar]
- von Borries A.K., Brazil I.A., Bulten B.H., Buitelaar J.K., Verkes R.J., de Bruijn E.R. (2010). Neural correlates of error-related learning deficits in individuals with psychopathy. Psychological Medicine , 40(9), 1559–68. [DOI] [PubMed] [Google Scholar]
- Walsh Z., Allen L.C., Kosson D.S. (2007). Beyond social deviance: substance use disorders and the dimensions of psychopathy. Journal of Personality Disorders , 21(3), 273–88. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.