Abstract
Previous research has shown that physiological arousal and attentional responses to eye contact are modulated by one’s knowledge of whether they are seen by another person. Recently it was shown that this ‘eye contact effect’ can be elicited without seeing another person’s eyes at all. We aimed to investigate whether the eye contact effect is actually triggered by the mere knowledge of being seen by another individual, i.e. even in a condition when the perceiver does not see the other person at all. We measured experienced self-awareness and both autonomic and brain activity responses while participants were facing another person (a model) sitting behind a window. We manipulated the visibility of the model and the participants’ belief of whether or not the model could see them. When participants did not see the model but believed they were seen by the model, physiological responses were attenuated in comparison to when both parties saw each other. However, self-assessed public self-awareness was not attenuated in this condition. Thus, two requirements must be met for physiological responses to occur in response to eye contact: an experience of being seen by another individual and an experience of seeing the other individual.
Keywords: eye contact, skin conductance, heart rate, EEG, interaction
Introduction
Eye contact is a powerful signal which modulates social cognition as well as autonomic responses and brain activity in many ways. For example, faces with direct gaze are memorized more readily than faces with averted gaze (Mason et al., 2004), and faces with direct gaze can better hold attention, detracting from performance in concurrent cognitive tasks (Senju and Hasegawa, 2005; Conty et al., 2010). Seeing a face with direct vs averted gaze results in stronger autonomic responses as measured by skin conductance (Nichols and Champness, 1971; Helminen et al., 2011, 2015) and heart rate deceleration (Akechi et al., 2013). Neuroimaging studies have revealed stronger activation in response to faces with direct vs averted gaze in several brain areas, including the fusiform gyrus (George et al., 2001; Calder et al., 2002; Pageler et al., 2003;), superior temporal sulcus (Calder et al., 2002; Wicker et al., 2003), medial prefrontal cortex (Schilbach et al., 2006), orbitofrontal cortex (Wicker et al., 2003; Conty et al., 2007) and amygdala (Kawashima et al., 1999; Sato et al., 2004). Interestingly, it has been shown that the amygdala activates more strongly in response to direct than averted gaze even if the individual lacks conscious visual experience due to destruction of the primary visual cortex (i.e. cortical blindness; Burra et al., 2013).
Recently, several studies have shown that the effects of seeing a direct vs averted gaze may depend on whether participants are presented a real person or a picture or a video of a face. Compared with averted gaze, looking at direct gaze of a ‘live’ person has been shown to elicit larger skin conductance responses (SCR) (Hietanen et al., 2008; Pönkänen et al., 2011b), larger visual brain responses (Pönkänen et al., 2011a) and more pronounced relative left-side frontal electroencephalographic (EEG) alpha activity associated with approach motivation (Hietanen et al., 2008). Additionally, self-assessed public self-awareness (a tendency to attend to the aspects of the self that are on public display) has also been shown to be greater for direct than averted gaze when looking at a real person (Hietanen et al., 2008; Pönkänen et al., 2011b). Importantly, all these differences in physiological and self-assessed measures in response to direct vs averted gaze were observed only for real ‘live’ stimuli, and not for pictures of faces. It was suggested that one’s knowledge of being looked at by another person may be the pivotal factor modulating these responses to social stimuli (Hietanen et al., 2008; Pönkänen et al., 2011b). Supporting this suggestion, it has been shown that attention orienting by gaze cues (Teufel et al., 2010) and sensory visual adaptation to gaze direction stimuli (Teufel et al., 2009) are modulated by the belief of whether the stimulus-person can or cannot see the observer.
One plausible factor for the differential effects of gaze direction between a live gaze and a picture may be whether participants experience being observed by the stimulus person. In our recent study, we isolated and manipulated this factor while keeping other stimulus properties unchanged (Myllyneva and Hietanen, 2015, Experiment 1). We used a live person as the stimulus (model) and used a deception procedure to manipulate participants’ belief of whether they could be seen by the model. Importantly, the visual conditions were identical to the participants. Only when participants thought that they could be seen by the models did direct gaze elicit greater skin conductance and heart rate deceleration responses, pronounced EEG frontal P3 responses and higher public self-awareness. These results provided strong evidence that knowledge of being looked at by another person is an important factor underlying the enhanced responses to direct gaze.
If knowledge of being looked at is a key factor underlying enhanced physiological responses to eye contact, one can ask if seeing one’s eyes is even necessary to elicit the ‘eye contact effect’. We addressed this question in a second experiment (Myllyneva and Hietanen, 2015, Experiment 2) wherein we not only manipulated participants’ beliefs of being seen by the model but also the visibility of the model’s eyes by using different sunglasses. The results showed enhanced SCRs to direct gaze/head orientation independent of whether the model’s eyes were visible as long as participants knew that the model was able to see. However, when the model’s eyes were not visible and the participant was told that the model was not able to see through the sunglasses, the SCRs to direct head orientation were attenuated. These results provided further evidence that the critical factor behind the enhanced physiological responses is not eye contact per se, but rather the awareness that one is being observed by another person.
These findings motivated the present experiment in which we took one further step and asked whether it is possible to observe the physiological responses to being seen by another person even when the other person is not visible at all. There is substantial evidence indicating that the autonomic nervous system can be activated without presenting sensory stimuli. For example, emotional and motor imagery tasks have been shown to result in similar autonomic activation compared with emotional sensory stimuli or actual motor performance, respectively (for reviews see: Lang, 1979; Guillot and Collet, 2005; Kreibig, 2010). It has also been shown that mental imagery can modulate cortical activity. For example, imaging faces has been shown to produce similar modulation of the face-sensitive N170 EEG response as seeing actual faces (Ganis and Schendan, 2008). In the present experiment, we compared physiological and self-evaluated responses in three different experimental conditions: (i) when the participant and the model could both see each other, (ii) when the participant could not see the model but was led to believe that the model could see him/her and (iii) when the participant could not see the model and was led to believe that the model also could not see him/her.
Following our previous study (Myllyneva and Hietanen, 2015), we measured SCR, heart rate deceleration responses, frontal P3 evoked-response potentials (ERP) and situational self-awareness. SCR index sympathetic affective arousal (Dawson et al., 2000), whereas the heart rate deceleration response is associated with orienting attention towards external stimuli (Graham and Clifton, 1966). The mid-latency frontal P3 ERPs analysed from the EEG signal are related to attention orientation caused by affectively arousing stimuli (Cuthbert et al., 2000; Keil et al., 2002). Self-assessed situational self-awareness consists of three components: private self-awareness, public self-awareness and awareness of one’s surroundings (Govern and Marsch, 2001). The component potentially sensitive to being observed by another person is public self-awareness because it is associated with the feeling of being evaluated by another person (Buss, 1980). Previous research has shown stronger responses to direct than averted gaze in SCR (Nichols and Champness, 1971; Hietanen et al., 2008; Helminen et al., 2011), heart rate deceleration response (Akechi et al., 2013), P3 ERP component (Conty et al., 2007) and public self-awareness (Hietanen et al., 2008; Pönkänen et al., 2011b). Importantly, these four measures were all analysed in our previous experiment (Myllyneva and Hietanen, 2015), where they were found to be modulated by participants’ beliefs of whether they were being observed by the model.
We made a straightforward hypothesis: we predicted greater skin conductance, HR deceleration responses and self-assessed public self-awareness when participants thought they were being observed compared with when they thought they were not being observed, regardless of whether the other person was visible. We expected P3 responses to be strongly modulated by the visual stimulus, but also expected to see a shift in the positive direction when participants believed that they were being observed, even when they did not see the other person at all.
Materials and methods
Participants
The participants were 25 right-handed undergraduate students (15 females, 10 males) with normal hearing and normal or corrected-to-normal vision. Seven female participants were excluded from the electrocardiogram (EKG) and SCR analyses due to an error in the script of the computer program causing data not to be recorded. Additionally, two male participants and three female participants were excluded from the P3 analysis due to excessive artefacts. Hence, the final data sample consisted of 25 participants for the questionnaire data, 18 participants (8 females, 10 males) for the SCR and EKG data and 20 participants (12 females, 8 males) for the ERP data. Ethical approval for this study was obtained from the Tampere Area Ethical Review Board. Participant consent was obtained according to the Declaration of Helsinki.
Stimuli
One-half of the female and male participants saw a male experimenter and the other half saw a female experimenter as the stimulus person (model). The model bore a neutral expression on his/her face and had a direct gaze. The model’s face was presented through a computer-operated voltage-sensitive liquid-crystal (LC) window (NSG UMU Products Co., Ltd.). The LC window was attached to a black panel positioned between the model and the participant. The size of the LC window was 30 × 40 cm. The participant was seated 60 cm from the window and the overall distance to the model sitting on the other side was 120 cm. Because the participants did not see the model in all conditions, a muffled buzzing sound was always presented to indicate the occurrence of the trial. The same buzzing sound was presented in all three blocks of trials (see below). The volume of the sound was set to be unobtrusive, but easily audible. The buzzing sound lasted until the offset of the trial.
Experimental procedure
The experiment was conducted in three separate blocks, one for each condition: (i) the participant (P) and the model (M) could see each other (P + /M+), (ii) the participant did not see the model, but believed that the model saw him/her (P−/M+) and (iii) neither the participant nor the model saw the other (P−/M−). Participants were instructed that their task was simply to watch forward, towards the panel behind which the model was sitting, independent of whether the participant could see the model or not. Participants were told that the model will be also watching the panel during all conditions. As described above, during the trials, a buzzing sound indicated when the LC-window was transparent.
In the P+/M+ condition, the model was presented through the LC window in such a way that both the participant and the model were able to see each other when the LC window became transparent. The P−/M+ condition was carried out by a deception procedure in which the participant was led to believe that a half-silvered ‘one-way’ mirror was attached to the LC window. While the participant was sitting in his/her seat and the LC window was transparent, the model slid an opaque, aluminum-coloured sheet onto the window and the participant was told that now the participant could not see the model but that model was still able to see them. To convince the participant, he/she was then taken to the other side of the LC window panel. While the participant was walking around the short partition, the model quickly replaced the opaque sheet with another sheet that was transparent. The participant then saw this transparent sheet from the model’s side and confirmed that, from this side, one could clearly see through it. When the participant returned to his/her own side of the table, the model cautiously and quickly replaced the transparent sheet again with the opaque sheet. The P−/M− condition was conducted by shutting the LC-window off and showing the participant that, in this condition, neither the participant nor the model was able to see through the window during a trial. The presentation order of the conditions was counterbalanced across the participants. Participants were informed about all these conditions before starting the experiment. To control for the suspicion of deceit, participants were asked after the experiment about possible differences in their experiences between the stimulus presentation conditions. During the final debriefing, the deceit was unveiled and participants were asked directly if they had any doubts about the model not seeing them during the P−/M+ block. A participant was excluded from analyses if he/she expressed doubts regarding the deceit.
Within each block, two series of measurements were conducted. The first series was intended for the measurements of the SCRs and HR deceleration responses and it consisted of 10 trials. The stimulus duration was 5 s with an inter-stimulus interval (ISI) varying between 20 and 45 s. A new trial was allowed after recovery from the previous SCR. After the first five trials, a short break (1‒2 min) was allowed.
The second series of measurements was to collect data for the ERPs and it consisted of 100 trials. The duration of stimulus presentation was 0.5 s with an ISI of 1.5 s. The stimuli were presented in 10-trial sequences. After each sequence, there was a 15-s break. After the first five sequences, participants were allowed a break of 1‒2 min. ISIs consisted of those moments, when the LC window was not transparent (there was no buzzing sound). After each block, participants completed a self-assessment questionnaire. The participants were asked to fill the nine-item Situational Self-Awareness Scale (SSAS) (Govern and Marsch, 2001). The SSAS measures three different forms of self-awareness: public self-awareness (e.g. ‘Right now, I am concerned about the way I present myself’), private self-awareness (e.g. ‘Right now, I am conscious about my inner feelings’) and awareness of one’s immediate surroundings (e.g. ‘Right now, I am keenly aware of everything in my environment’). The form used a 7-point scale. Participants were instructed to answer the questionnaire based on their feelings during the previous experimental block, not how they felt in general or at that point in their lives.
Acquisition of the physiological data
For the skin conductance measurements, two electrodes (Ag/AgCl) were attached to the palmar surface of the distal phalanxes of the index and middle fingers of the participant’s left hand. For the HR measures, two electrodes (Ag/AgCl) were placed on both arms. The sampling rate for the digitized signals was 1000 Hz.
Continuous EEG was recorded from 64 sites using actiCAP active electrodes, and the signal was amplified with a quickAmp amplifier (Brain products GmbH, Munich, Germany). An average reference was used. The sampling rate for the digitized signal was set to 1000 Hz. Additionally, vertical (VEOG) eye movements were recorded above and below the left eye. Skin abrasion and electrode paste were used to reduce electrode impedances below 25 kΩ. All physiological data collection was controlled with Brain Vision Professional Recorder (Brain products GmbH, Munich, Germany) running on a PC computer.
Data analysis
The SCR data were re-sampled offline to 100 Hz and filtered with a 10 Hz low-pass filter. No high-pass filtering was used. The SCR was defined as the maximum amplitude change from the baseline level (at the stimulus onset) during a 4-s time period starting after 1 s from the stimulus onset. In case there was more than 0.1 μs amplitude rise during the first second after stimulus onset, the trial was rejected. In this case, the response was too early to have been elicited by the stimulus. Of all trials, 6.0% were eliminated due to this criterion or because of technical errors. The data were averaged for each condition for each participant, including those trials with zero response. This method of calculation is referred to as the magnitude of galvanic skin response (Dawson et al., 2000).
Electrocardiogram (EKG) was analysed offline with an in-house (MATLAB-based) algorithm to measure the time intervals between two successive R-waves (interbeat interval, IBI). After computer-based detection of R-peaks, the data were manually checked and corrected in cases of falsely detected or missing peaks. Trials with excessive distortion in the signal were excluded from the analysis (1.9% of the trials). For a period between 5 s pre-stimulus and 5 s post-stimulus within each trial, the IBIs were quantified and assigned to 1-s intervals. This was done by averaging the IBIs in each interval weighted by the proportion of the interval occupied by that beat. Lastly, IBIs were converted to beats per minute (bpm) and averaged across trials within each condition. A baseline was defined as the average of the IBIs during the 5-s pre-stimulus period. The analyses were conducted on HR-change scores that were calculated by subtracting the bpm of each post-stimulus 1-s interval from baseline. Thus negative change-score values indicated HR deceleration and positive values HR acceleration.
The continuous EEG-signal was offline-filtered with 0.5‒30 band-pass filter with 24 dB/oct slope on both ends. The filtered signal was ocular-corrected using Gratton/Coles algorithm and manually checked for artefacts. Trials containing artefacts were rejected (4.0% of the trials). In order to study the ERP-responses, the signal was segmented into 600-ms long epochs starting 100 ms before stimulus onset and computed for each condition. The baseline was computed from the 100-ms pre-stimulus period. For the P3-component, we analysed the right and left anterior frontal and frontal pole regions [averaged over electrodes AF4, AF8 and Fp2 (right side), and AF3, AF7 and Fp1 (left side)], measuring the mean amplitude between 200 and 450 ms post-stimulus for each participant in each condition.
All statistical analyses were conducted using repeated-measures analyses of variance (ANOVA). Planned comparisons were performed for analyses of simple main effects when interactions were observed. A Greenhouse-Geisser correction was applied when appropriate. When needed, data were normalized using natural-log transformations. All analyses were conducted on normally distributed variables.
Results
Situational self-awareness
A one-way ANOVA of experimental condition was performed on the ratings on each dimension of self-awareness (public, private and awareness of surroundings). A significant effect was found for public self-awareness (F2,48 = 5.98, P < 0.01, = 0.20) but not for the other dimensions (Ps > 0.4). Public self-awareness was greater both when the participant and the model could see each other (t24 = 4.38, P < 0.01, d = 0.88) and when the participant did not see the model but believed that the model could see him/her (t24 = 1.07, P = 0.049, d = 0.42) compared with when neither could see each other. Public self-awareness did not differ significantly between when the participant and the model could see each other and when the participant did not see the model but believed that the model could see him/her (t24 = 0.92, P = 0.37, d = 0.18). Thus, self-evaluated public self-awareness was enhanced independent of whether the participant saw the model as long as he/she believed themselves to be observed by the model. The self-awareness scores are presented in Table 1.
Table 1.
Scores on the SSAS for the three experimental conditions
| Self-awareness | P+/M+ |
P−/M+ |
P−/M− |
|||
|---|---|---|---|---|---|---|
| M | s.d. | M | s.d. | M | s.d. | |
| Private | 4.05 | 1.12 | 4.31 | 1.17 | 4.31 | 1.19 |
| Public | 3.04 | 1.61 | 2.81 | 1.58 | 2.21 | 1.37 |
| Surroundings | 4.27 | 1.37 | 4.10 | 1.54 | 4.49 | 1.16 |
SSAS scores include private self-awareness, public self-awareness, and awareness of one’s surroundings. The SSAS has a range of 1‒7. P+/M+ = the participant and the model could both see each other; P−/M+ = the participant could not see the model, but believes that the model could see him/her; P−/M− = neither the participant nor the model could see each other.
Skin conductance
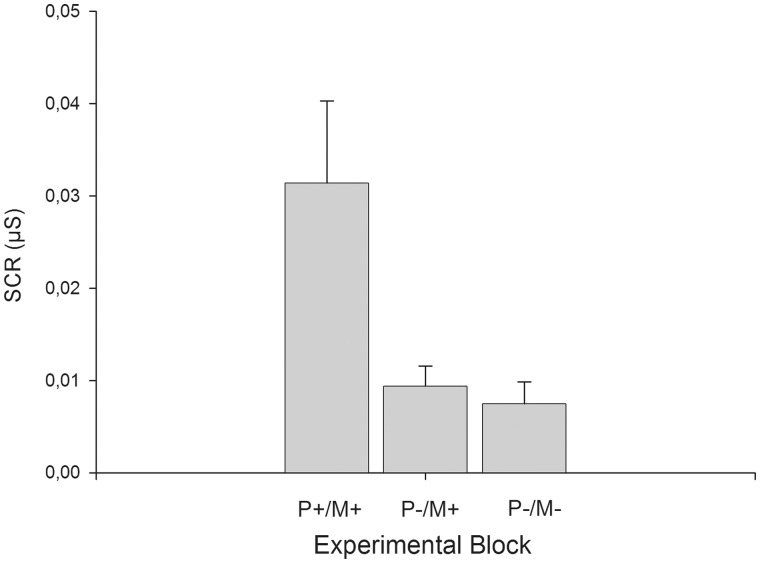
An ANOVA indicated a significant effect of experimental condition on SCR (F2,34 = 14.99, P < 0.01, = 0.47). T-tests revealed that when the model and the participant could see each other SCRs were greater compared with when the participant did not see the model but believed that the model could see him/her (t17 = 4.18, P < 0.01, d = 0.98) or when the model and participant could not see each other (t17 = 4.25, P < 0.01, d = 1.00). Importantly, there were no differences in SCR between the two conditions where the model was not visible (t17 = 0.51, P = 0.62, d = 0.12). Mean SCR are shown in Figure 1.
Fig. 1.
SCR (mean + s.e.m.) in the three different experimental conditions. P+/M+ = the participant and the model could both see each other; P−/M+ = the participant could not see the model, but believes that the model could see him/her; P−/M− = neither the participant nor the model could see each other.
Heart rate
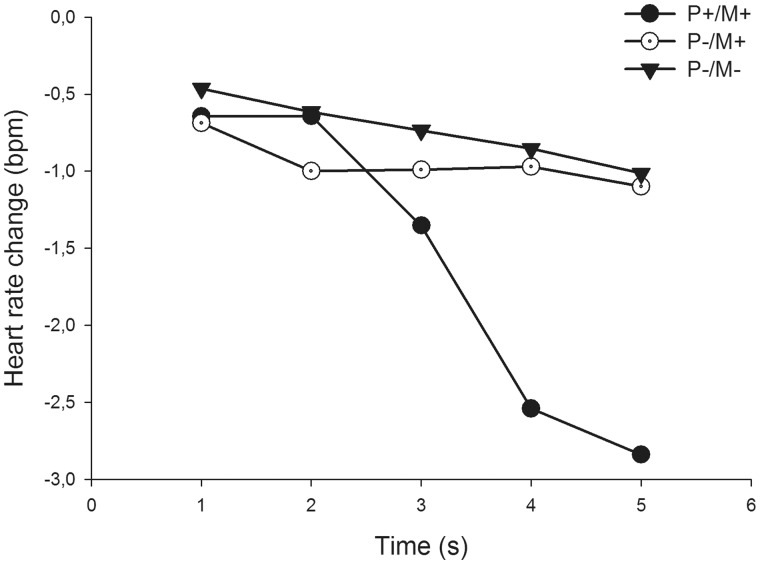
A 5 × 3 ANOVA (time × experimental condition) on heart rate response revealed a main effect of time (F4,72 = 5.23, P < 0.01, = 0.23) and a significant interaction between time and experimental condition (F8,144 = 3.97, P < 0.01, = 0.18). We used t-tests to compare the maximal HR-decelerations between experimental conditions. The HR-deceleration was stronger when the participant and the model could see each other compared with when the participant did not see the model but believed that the model could see him/her (t18 = 2.81, P = 0.01, d = 0.64), and when the model or participant could not see each other (t18 = 2.64, P = 0.01, d = 0.61). Again, there were no differences in responses between the two conditions where the model was not visible (t18 = 0.21, P = 0.83, d = 0.04). Heart rate deceleration responses are shown in Figure 2.
Fig. 2.
Heart rate changes in the three different experimental conditions. P+/M+ = the participant and the model could both see each other; P−/M+ = the participant could not see the model, but believes that the model could see him/her; P−/M− = neither the participant nor the model could see each other.
P3 response
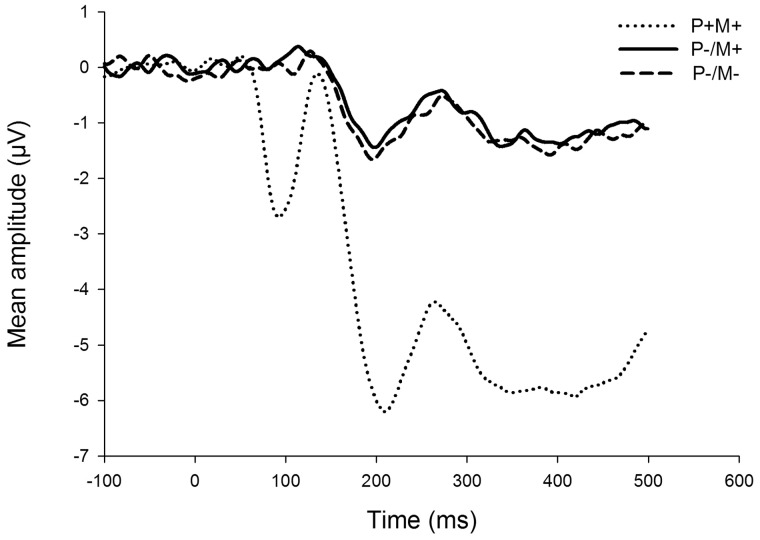
There was an expected modulation of the ERP responses whereby the presence of the visual stimulus induced a prominent negative shift in the waveform for the condition where the participant was able to see the model. For the P3 response, a 3 × 2 ANOVA (experimental condition × hemisphere) showed a main effect of experimental condition (F2,36 = 61.91, P < 0.01, = 0.78) reflecting this negative shift. Importantly, however, there were no differences in the P3 responses between the two conditions where the model was not visible (t18 = 0.03, P = 0.97, d < 0.01), averaged across both left and right hemispheres. Thus, compatible with the skin conductance and heart rate deceleration responses, the P3 response was not larger when the participant did not see the model but still believed that the model could see him/her compared with when neither could see each other. The ERP responses are shown in Figure 3.
Fig. 3.
Mean waveforms from frontal electrode sites in the three different experimental conditions. The presented waveforms are averaged over the left and right hemispheres. The timeframe of the P3 response is 200‒450 ms. P+/M+ = the participant and the model could both see each other; P−/M+ = the participant could not see the model, but believes that the model could see him/her; P−/M− = neither the participant nor the model could see each other.
Discussion
In this study, we explored whether the mere belief of being seen by another person, without actually seeing the person, is enough to elicit similar self-awareness and physiological responses indexing arousal and attention allocation that typically follow making eye contact with another person. The self-assessed situational public self-awareness was higher when participants believed that they were being seen by the model compared with when not being seen, even when the model was not visible to the participant. When participants believed that they were being observed, they responded similarly regardless of whether they saw the observer or not. This result is consistent with our hypothesis, and not surprising given that Govern and Marsch (2001) described public awareness as a tendency to attend to the aspects of the self that are on public display. Public self-awareness is also associated with the feeling of being evaluated by another person (Buss, 1980). In our previous study (Myllyneva and Hietanen, 2015), we showed that public self-awareness decreased when participants were led to believe that they were not being observed by another person even when their own ability to see the other person was not affected. Thus, for public self-awareness, the crucial factor seems to be the knowledge of being seen by another person. The visibility of this observer does not have an effect on this subjective experience.
The results from our physiological measurements clearly differed from the results of the public self-awareness ratings. Against our hypothesis, one’s belief of being seen by a nonvisible model had no effect on any of the measured physiological responses: SCR, HR and the P3 ERP component. The findings of our previous study (Myllyneva and Hietanen, 2015) showed that when participants believed they were being seen, autonomic activation was enhanced independent of whether they saw the observer’s eyes or not. Instead, this study convincingly shows that the mental state of believing oneself to be observed by another person is not enough to elicit the enhanced physiological arousal and attention allocation. Together with our previous study, the present experiment strongly indicates that to elicit greater physiological arousal, the experience of being observed by another person must be accompanied by sensory evidence of this person.
One may question whether our manipulation really worked and whether the participants were convinced that they were being seen by the model even when they did not see the model themselves. Participants were interviewed after the experiment, and not a single participant expressed strong doubts regarding the deceit before being informed of the experiment. However, five participants did express doubts of deceit after the deception was unveiled. We re-analysed the data leaving out these participants, and still there was no sign of differential physiological responses to being seen vs not being seen when the model was not visible. Additionally, the results from the public self-awareness ratings strongly indicate that our experimental manipulation worked as intended. It is difficult to account for the stronger public self-awareness when the participants believe they are being observed by the hidden model if our manipulation did not work properly.
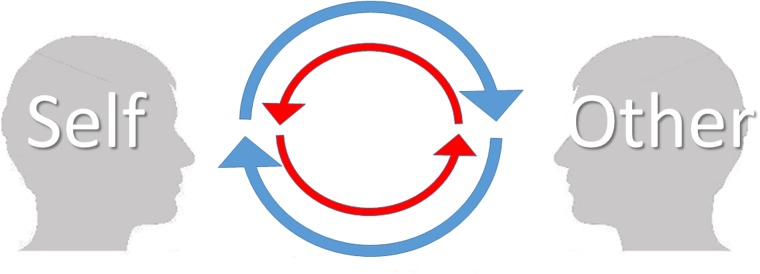
Many studies show that the autonomic nervous system can be activated without a sensory stimulus, e.g. with emotional or motor imagery (Lang, 1979; Guillot and Collet, 2005; Kreibig, 2010). However, our results show that the mere belief of being looked at by another individual is not enough to increase autonomic activation without any sensory (visual) information of this observer. This result calls for a revision of our previous suggestion that the belief that one is being watched is the pivotal factor behind the enhanced autonomic and brain responses during eye contact (Myllyneva and Hietanen, 2015). In light of the present evidence, it appears that there are actually two requirements which must be met: (i) having the experience of being looked at by another individual and (ii) having the experience of looking at the other individual. Only in this kind of condition is there a possibility for mutual social influence; without the experience of being looked at by another individual, one may merely be a passive observer, comparable to a television viewer, without the possibility to impact another individual. Without the simultaneous experience of looking at the other individual, one is essentially locked in an observation room with a one-way mirror without any chance to be impacted by the other individual’s behaviour (Figure 4). This model is the most parsimonious explanation of the results from our previous and the present study. The function of the sympathetic nervous system is to prepare the body for action. Our results show that the presence of another person activates this system only to direct sensory stimuli in a condition where mutual interaction is possible. In contrast, public self-awareness is an internal mental state and can be heightened just by the belief that one is being looked at by another individual.
Fig. 4.
An illustration describing mutual, visual interaction between two persons: the ‘self’ and the ‘other’. Blue arrows depict an individual’s experience of seeing the other person. Red arrows depict an individual’s simultaneous experience of being influenced by the other person’s behaviour. If one is not able to see the other (e.g. as in this study) or if the other is not able to see the self (e.g. Myllyneva and Hietanen, 2015) the upper or lower half, respectively, of this ‘circle of interaction’ breaks down, preventing mutual (visual) interaction. This model can also be applied to interactions involving other sensory modalities.
Our model is consistent with a recent account by De Jaegher et al. (2010) proposing that interaction includes at least two agents co-regulating and mutually affecting each other while preserving their individual autonomy. In fact, De Jaegher et al. (2010) explicitly suggest that the belief of another person’s presence is not enough to constitute genuine social interaction. In a recent seminal review, Schilbach et al. (2013) use a term ‘spectatorial gap’ to describe a situation where a person is merely observing his/her surroundings without any possibility to interact with it. They suggest that when a possibility for interaction and/or emotional engagement is prevented social cognition may be fundamentally different compared with a natural encounter with another person. Our previous and present results are well in line with these considerations and provide empirical evidence for them. Our results show that closing or not closing this spectatorial gap can, indeed, have a major effect on attention and affective arousal-related autonomic and brain responses.
During the past decade, there has been active conversation about the importance of using real social stimuli and real social situations in social neuroscience and social cognition research (Hietanen et al., 2008; Hari and Kujala, 2009; Risko et al., 2012; Teufel et al., 2012; Schilbach et al., 2013). Many novel methods have been developed to overcome the difficulties of researching social phenomena during true interactions (Teufel et al., 2009; Wilms et al., 2010; Konvalinka and Roepstorff, 2012; Schilbach, 2014). Our method of using a liquid crystal window between two people and manipulating the participant’s mental state while recording physiological responses offers one functional solution for creating experiments with a second-person approach. The present results show that the potential for genuine, mutual interaction can be a pivotal factor modulating arousal and attention-related responses. In future studies it would be interesting to explore which types of visual information may be enough (e.g. parts of the body or a silhouette) to elicit physiological responses similar to those when seeing the other person completely, and whether information via sensory modalities other than vision could be used to establish a similar mutual social contact resulting in enhanced physiological responses. This knowledge would be interesting not only from the perspective of social perception, but could also be relevant for social media and video communication technology.
Acknowledgements
This work was supported by the Academy of Finland (project no. 266187 to J.K.H.).
Conflict of interest. None declared.
References
- Akechi H., Senju A., Uibo H., Kikuchi Y., Hasegawa T., Hietanen J.K. (2013). Attention to eye contact in the West and East: autonomic responses and evaluative ratings. PloS One, 8, e59312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burra N., Hervais-Adelman A., Kerzel D., Tamietto M., De Gelder B., Pegna A.J. (2013). Amygdala activation for eye contact despite complete cortical blindness. The Journal of Neuroscience, 33, 10483–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss A.H. (1980). Self-consciousness and Social Anxiety. San Francisco: Freeman. [Google Scholar]
- Calder A.J., Lawrence A.D., Keane J., Scott S.K., Owen A.M., Christoffels I., Young A.W. (2002). Reading the mind from eye gaze. Neuropsychologia, 40, 1129–38. [DOI] [PubMed] [Google Scholar]
- Conty L., Gimmig D., Belletier C., George N., Huguet P. (2010). The cost of being watched: Stroop interference increases under concomitant eye contact. Cognition, 115, 133–9. [DOI] [PubMed] [Google Scholar]
- Conty L., N’Diaye K., Tijus C., George N. (2007). When eye creates the contact! ERP evidence for early dissociation between direct and averted gaze motion processing. Neuropsychologia , 45, 3024–37. [DOI] [PubMed] [Google Scholar]
- Cuthbert B.N., Schupp H.T., Bradley M.M., Birbaumer N., Lang P.J. (2000). Brain potentials in affective picture processing: covariation with autonomic arousal and affective report. Biological Psychology , 52, 95–111. [DOI] [PubMed] [Google Scholar]
- Dawson M.E., Schell A.M., Filion D.L. (2000). The electrodermal system. In: Cacioppo J.T., Tassinary L.G., Berntson G.G., editors. Handbook of Psychophysiology, 2nd edn, Cambridge University Press, Cambridge, 200–33. [Google Scholar]
- De Jaegher H., Di Paolo E., Gallagher S. (2010). Can social interaction constitute social cognition?. Trends in Cognitive Sciences, 14, 441–7. [DOI] [PubMed] [Google Scholar]
- Ganis G., Schendan H.E. (2008). Visual mental imagery and perception produce opposite adaptation effects on early brain potentials. Neuroimage, 42, 1714–27. [DOI] [PubMed] [Google Scholar]
- George N., Driver J., Dolan R.J. (2001). Seen gaze-direction modulates fusiform activity and its coupling with other brain areas during face processing. Neuroimage, 13, 1102–12. [DOI] [PubMed] [Google Scholar]
- Govern J.M., Marsch L.A. (2001). Development and validation of the situational self-awareness scale. Consciousness and Cognition, 10, 366–78. [DOI] [PubMed] [Google Scholar]
- Graham F.K., Clifton R.K. (1966). Heart-rate change as a component of the orienting response. Psychological Bulletin, 65, 305–20. [DOI] [PubMed] [Google Scholar]
- Guillot A., Collet C. (2005). Duration of mentally simulated movement: a review. Journal of motor behavior, 37, 10–20. [DOI] [PubMed] [Google Scholar]
- Hari R., Kujala M.V. (2009). Brain basis of human social interaction: from concepts to brain imaging. Physiological Reviews, 89, 453–79. [DOI] [PubMed] [Google Scholar]
- Helminen T.M., Kaasinen S.M., Hietanen J.K. (2011). Eye contact and arousal: the effects of stimulus duration. Biological Psychology, 88, 124–30. [DOI] [PubMed] [Google Scholar]
- Helminen T.M., Pasanen T.P., Hietanen J.K. (2015). Learning under your gaze: the mediating role of affective arousal between perceived direct gaze and memory performance. Psychological Research, 1–13. [DOI] [PubMed] [Google Scholar]
- Hietanen J.K., Leppänen J.M., Peltola M.J., Linna-aho K., Ruuhiala H.J. (2008). Seeing direct and averted gaze activates the approach-avoidance motivational brain systems. Neuropsychologia, 46, 2423–30. [DOI] [PubMed] [Google Scholar]
- Kawashima R., Sugiura M., Kato T., et al. (1999). The human amygdala plays an important role in gaze monitoring. A PET study. Brain, 122, 779–83. [DOI] [PubMed] [Google Scholar]
- Keil A., Bradley M.M., Hauk O., Rockstroh B., Elbert T., Lang P.J. (2002). Large-scale neural correlates of affective picture processing. Psychophysiology, 39, 641–9. [DOI] [PubMed] [Google Scholar]
- Konvalinka I., Roepstorff A. (2012). The two-brain approach: how can mutually interacting brains teach us something about social interaction?. Frontiers in Human Neuroscience, 6, 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreibig S.D. (2010). Autonomic nervous system activity in emotion: a review. Biological Psychology, 84, 394–421. [DOI] [PubMed] [Google Scholar]
- Lang P.J. (1979). A bio‐informational theory of emotional imagery. Psychophysiology, 16, 495–512. [DOI] [PubMed] [Google Scholar]
- Mason M., Hood B., Macrae C.N. (2004). Look into my eyes: gaze direction and person memory. Memory, 12, 637–43. [DOI] [PubMed] [Google Scholar]
- Myllyneva A., Hietanen J.K. (2015). There is more to eye contact than meets the eye. Cognition , 134, 100–9. [DOI] [PubMed] [Google Scholar]
- Nichols K.A., Champness B.G. (1971). Eye gaze and the GSR. Journal of Experimental Social Psychology, 7, 623–6. [Google Scholar]
- Pageler N.M., Menon V., Merin N.M., Eliez S., Brown W.E., Reiss A.L. (2003). Effect of head orientation on gaze processing in fusiform gyrus and superior temporal sulcus. Neuroimage, 20, 318–29. [DOI] [PubMed] [Google Scholar]
- Pönkänen L.M., Alhoniemi A., Leppänen J.M., Hietanen J.K. (2011a). Does it make a difference if I have an eye contact with you or with your picture? An ERP study. Social Cognitive and Affective Neuroscience , 6, 486–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pönkänen L.M., Peltola M.J., Hietanen J.K. (2011b). The observer observed: frontal EEG asymmetry and autonomic responses differentiate between another person’s direct and averted gaze when the face is seen live. International Journal of Psychophysiology, 82, 180–7. [DOI] [PubMed] [Google Scholar]
- Risko E.F., Laidlaw K.E.W., Freeth M., Foulsham T., Kingstone A. (2012). Social attention with real versus reel stimuli: toward an empirical approach to concerns about ecological validity. Frontiers in Human Neuroscience, 106, 1487–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato W., Yoshikawa S., Kochiyama T., Matsumura M. (2004). The amygdala processes the emotional significance of facial expressions: an fMRI investigation using the interaction between expression and face direction. Neuroimage, 22, 1006–13. [DOI] [PubMed] [Google Scholar]
- Schilbach L. (2014). On the relationship of online and offline social cognition. Frontiers in Human Neuroscience, 8, 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilbach L., Timmermans B., Reddy V., et al. (2013). Toward a second-person neuroscience. Behavioral and Brain Sciences, 36, 393–414. [DOI] [PubMed] [Google Scholar]
- Schilbach L., Wohlschlaeger A.M., Kraemer N.C., et al. (2006). Being with virtual others: neural correlates of social interaction. Neuropsychologia, 44, 718–30. [DOI] [PubMed] [Google Scholar]
- Senju A., Hasegawa T. (2005). Direct gaze captures visuospatial attention. Visual Cognition, 12, 127–44. [Google Scholar]
- Teufel C., Alexis D.M., Clayton N.S., Davis G. (2010). Mental-state attribution drives rapid, reflexive gaze following. Attention, Perception, & Psychophysics, 72, 695–705. [DOI] [PubMed] [Google Scholar]
- Teufel C., Alexis D.M., Todd H., Lawrance-Owen A.J., Clayton N.S., Davis G. (2009). Social cognition modulates the sensory coding of observed gaze direction. Current Biology, 19, 1274–7. [DOI] [PubMed] [Google Scholar]
- Teufel C., von dem Hagen E., Plaisted-Grant K.C., et al. (2012). What is social about social perception research?. Frontiers in Integrative Neuroscience, 6, 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicker B., Perrett D.I., Baron-Cohen S., Decety J. (2003). Being the target of another’s emotion: a PET study. Neuropsychologia, 41, 139–46. [DOI] [PubMed] [Google Scholar]
- Wilms M., Schilbach L., Pfeiffer U., Bente G., Fink G.R., Vogeley K. (2010). It’s in your eyes—using gaze-contingent stimuli to create truly interactive paradigms for social cognitive and affective neuroscience. Social Cognitive and Affective Neuroscience, 5, 98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]