Abstract
Human prosociality is often assumed to emerge from exerting reflective control over initial, selfish impulses. However, recent findings suggest that prosocial actions can also stem from processes that are fast, automatic and intuitive. Here, we attempt to clarify when prosocial behavior may be intuitive by examining prosociality as a form of reward seeking. Using event-related potentials (ERPs), we explored whether a neural signature that rapidly encodes the motivational salience of an event—the P300—can predict intuitive prosocial motivation. Participants allocated varying amounts of money between themselves and charities they initially labelled as high- or low-empathy targets under conditions that promoted intuitive or reflective decision making. Consistent with our predictions, P300 amplitude over centroparietal regions was greater when giving involved high-empathy targets than low-empathy targets, but only when deciding under intuitive conditions. Reflective conditions, alternatively, elicited an earlier frontocentral positivity related to response inhibition, regardless of target. Our findings suggest that during prosocial decision making, larger P300 amplitude could (i) signal intuitive prosocial motivation and (ii) predict subsequent engagement in prosocial behavior. This work offers novel insight into when prosociality may be driven by intuitive processes and the roots of such behaviors.
Keywords: prosociality, intuition, motivation, reward, P300, altruism
Introduction
Humans are a remarkably prosocial species. We routinely and voluntarily share, cooperate and help others, even to our own detriment (Warneken and Tomasello, 2006). This tendency is often credited to our ability to exert reflective control over our behavior (Stevens and Hauser, 2004; DeWall et al., 2008); however, mounting evidence suggests that prosocial actions may also stem from processes that are fast, automatic and intuitive (Rand et al., 2012; Righetti et al., 2013; Zaki and Mitchell, 2013; Keltner et al., 2014). If intuitive prosociality does exist, when might prosocial behaviors be motivated by intuitive processes, and what underlying neural processes could drive such behaviors? Here, we address these questions by drawing upon two markers proposed by Zaki and Mitchell (2013) to support an intuitive model of prosociality: (i) behavioral signs of automaticity associated with prosocial behaviors and (ii) neural signatures of value encoded during prosocial decision making.
Decision speed and giving
Recent behavioral evidence contradicts the assumption that prosociality requires reflection. First, individuals make prosocial decisions faster than selfish decisions (Rand et al., 2012). Second, prosocial behavior can increase when individuals make decisions under conditions that promote intuitive responding, such as time pressure (Rand et al., 2012, 2014) or distraction (Cornelissen et al., 2011; Schulz et al., 2012). Notably, Rand et al. (2012) found that participants who were forced to make quick, intuitive decisions contributed more money to others than those required to make slow, reflective decisions (Rand et al., 2012). However, this is not always the case. Some work finds prosocial behavior to be no different under intuitive or reflective conditions (Kinnunen and Windmann, 2013; Tinghög et al., 2013; Verkoeijen and Bouwmeester, 2014). While the importance of individual variability and context dependency in this phenomenon has been recognized (Zaki and Mitchell, 2013; Rand et al., 2014), research exploring such factors is sparse. If intuitive prosociality indeed depends on the situation and person, then it is important to clarify when and for whom it is most likely to occur. Here, we turn to evidence-linking prosocial behavior and reward-seeking neural activity—which could offer insight into when prosociality is intuitive.
Prosociality as reward-seeking
Humans often pursue rewarding goals intuitively (Custers and Aarts, 2010), and recent work supports the view that prosociality may represent a form of reward seeking in itself (Ruff and Fehr, 2014; Zaki and Mitchell, in press). Behavioral evidence suggests that prosocial actions are often experienced as more rewarding than selfish actions (Dunn et al., 2008). Moreover, functional MRI (fMRI) data suggest that people intrinsically value prosocial outcomes in an analogous manner to personal rewards (e.g. money; Zaki and Mitchel, 2011). Indeed, numerous studies reveal engagement of targets in the mesolimbic reward system both when we observe others experience positive outcomes (Morelli et al., 2015), and when we perform actions intended to benefit others (i.e. donating/sharing money; Moll et al., 2006; Harbaugh et al., 2007; Zaki et al., 2014). Specifically, the ventral striatum and ventromedial prefrontal cortex are thought to monitor the subjective value of behavioral outcomes, and implicitly guide decision making by increasing the motivational salience of potentially rewarding actions. In terms of prosociality, this reward-seeking circuitry could enhance the salience of cues that signal a prosocial opportunity—evoking an intuitive motive for action, prior to reflection.
This view helps illuminate why time pressure manipulations might increase prosociality. Imposing cognitive constraints on decision-making often increases dependence on reward-seeking circuitry for action selection—which enhances the salience of positive, over negative decision outcomes (Jones et al., 2011; Mather and Lighthall, 2012). Thus, in some situations, deciding intuitively might bias us towards actions that enhance others’ well-being (i.e. prosocial actions); whereas deciding reflectively could suppress this bias in favor of weighing the potential costs and benefits of acting prosocially.
To elucidate when prosociality is intuitive, a reward-seeking view would suggest a key role of the ventral striatum, which guides action by signaling events of motivational salience. Neuroimaging studies reveal that activation of the ventral striatum is particularly enhanced when engaging in prosocial actions towards targets we value or feel close to (Moll et al., 2006; Fareri et al., 2012). Such findings conform to the enduring view that humans are foremost concerned with the welfare of targets with whom we can readily empathize, such as people that we are socially connected with (e.g. a friend; de Waal, 2008) or people who we perceive to be in urgent need of help (e.g. a distressed stranger; Batson, 2011). Indeed, recent evidence suggests that the emotional rewards of prosocial behavior are greater when giving to those we feel close to (Aknin et al., 2011). Thus, together these findings suggest that examining prosocial decision making towards an empathized target could offer a useful starting point for examining the contextual emergence of intuitive prosociality.
In comparison to fMRI, remarkably few studies have investigated prosocial behavior using event-related potentials (ERPs). However, given its high temporal precision, ERPs would be well-suited to uncover rapid neural mechanisms that support quick, intuitive prosocial decisions. In particular, a potentially valuable ERP component for such work is the centroparietal P300—a positive wave peaking roughly 300–400 ms after an event. Recent views of the P300 suggest that it rapidly encodes motivational salience (Lubman et al., 2008; Hajcak et al., 2010; Kleih et al., 2010)—or the capacity of a stimulus, usually by virtue of its relevance for reward prediction, to capture attention. For example, during incentive tasks P300 amplitude is enhanced to cues that signal rewarding opportunities (or outcomes), such as the prospect of gaining a monetary payoff (Broyd et al., 2012; Pfabigan et al., 2014) or positive social feedback (Cox et al., 2015; Flores et al., in press). Moreover, in such cases, P300 modulation has been proposed to reflect motivational signals that stem from mesolimbic reward regions (Cox et al., 2015; Flores et al., 2015; Knyazev, 2007; Pogarell et al., 2011). Specifically, combined ERP/fMRI work indicates that P300 activity positively co-varies with activation of the ventral striatum in response to rewarding cues (Pfabigan et al., 2014), suggesting that P300 modulation can capture early motivational processes that drive reward-seeking behaviors.
Crucially, initial evidence suggests that the P300 is also sensitive to prosocial opportunities and outcomes. For instance, greater P300 amplitudes occur when observing monetary gains for emotionally close others, such as friends, compared to strangers (Ma et al., 2011). Moreover, P300 amplitude increases with the perceived need of a target in a helping scenario, with situations involving a target with an urgent, unattended need (e.g. a serious injury) eliciting greater P300 responses than when help is not necessary (Chiu Loke et al., 2011). Additionally, P300 activity is positively associated with both self-reported prosocial habits (Chiu Loke et al., 2011) and implicit prosocial attitudes (Xiao et al., 2015).
Taken together, this work illustrates the possibility that the P300 may provide an early motivational signal that promotes intuitive prosocial behavior. Specifically, we propose that P300 modulation in this context represents the motivational salience of a perceived prosocial opportunity, with factors such as target empathy, perceived need, and one’s trait prosocial tendencies moderating the strength of this response. To our knowledge, no prior work has investigated the role of the P300 in a real prosocial choice scenario. As such, this work stands to offer novel insight into the temporal dynamics of prosociality.
Overview
In the present research, we tested whether the P300 can predict intuitive prosocial decisions. To do so we experimentally manipulated two factors while participants engaged with prosocial opportunities in a donation task. First, to discern the role of the P300 in intuitive as opposed to reflective decision-making, donation decisions were made under time pressure or time delay akin to Rand and colleagues (2012). Second, to assess the role of target empathy on P300 responses, donation decisions were made towards real charities that each participant had previously selected as their most and least empathized causes from a list of charities. Lastly, we measured two individual factors that could potentially moderate intuitive prosocial decisions, trait empathy and stable prosocial habits.
Behavioral predictions
First, we predicted that participants would both (a) donate more money and (b) accept more costly offers towards targets they reported empathizing with the most. These predictions follow from an abundant literature on the role of empathy in motivating prosocial actions (Batson, 2011; Decety et al., 2012; de Waal, 2012). Second, we predicted that participants would (c) make prosocial decisions quicker than selfish decisions, and (d) donate more under time pressure than time delay, but especially for their most empathized target. These predictions were based on the expectation that empathy would bolster the relationship between prosociality and intuitive decision making.
ERP predictions
At a neural level, we predicted that P300 amplitude would be (a) greater during intuitive, as opposed to reflective responding, and (b) specifically for an empathized target. This follows from the expectation that fast responding would increase reliance on reward salience processes (Mather and Lighthall, 2012), which would be enhanced for empathized targets—whom people reap greater emotional reward from helping. Slow responding, alternatively, would be expected to suppress this activity in favor of recruiting controlled, reflective processes. Finally, we predicted that (c) P300 amplitude would be greater when individuals subsequently engaged in prosocial behavior (i.e. accepted donation offers)—a prediction that follows from the intuitive model of prosociality (Zaki and Mitchell, 2013).
Materials and methods
Participants
Informed consent was obtained from 22 healthy undergraduates who participated in exchange for course credit. Two of these participants were excluded from analysis for making prosocial responses on every trial (thus providing insufficient data to model), leaving a total of 20 participants (Mage = 20.6, s.d. = 3.73, 14 female).
Trait measures
Participants reported their demographics including age, years of education, religious affiliation, and household income. Additionally, participants completed the Prosocial Tendencies Measure (PTM; Carlo and Randall, 2002)—a 23-item measure of stable prosocial habits, and a short form of the Empathy Quotient (EQ-Short; Wakabayashi et al., 2006)—a 22-item trait measure of cognitive and affective empathy.
Charity selection
Upon completing all questionnaires, participants read the mission statements of seven national charities supporting various groups (e.g. disaster victims). Each statement was accompanied by the charity’s logo, and outlined the needs of the individuals it supported and the positive outcomes of donating. Participants were instructed to ‘imagine the feelings’ of the individuals supported by each charity, and were then asked which charity they ‘empathize with the most and least’ (a discrete choice), ‘to what degree’ (a seven-point Likert scale rating), and ‘why’ (an open-ended question and manipulation check to ensure selections related to empathic concern). Unbeknownst to participants, their most empathized charity and least empathized charity were used as targets for the subsequent task.1
Donation task
Next, participants were seated in front of a CRT monitor (approximately 75 cm away) in a private booth for the anonymous donation task (adapted from Moll et al., 2006). Each participant was presented with a $20 bill (without prior notice), which they took ownership of by signing a lab receipt that acknowledged their payment. An instruction screen explained that the task required participants to ‘Accept’ or ‘Reject’ donation offers affecting their $20. Participants were also informed that all funds that they allocated to the targets—their most and least empathized charities—would be real donations.
To manipulate intuitive and deliberative thinking, each participant completed fast donation sessions that instructed them to make their decision as fast as possible, and slow donation sessions that instructed them to carefully consider their decision for at least 5 s2 (adapted from Rand et al., 2012). To enforce time pressure, offers in the fast sessions disappeared after 5 s, whereas to enforce time delay, responses to offers in the slow sessions could not be made until a signal indicated 5 s had elapsed. This manipulation allowed us to directly examine intuitive and reflective decisions while limiting the influence of individual tendencies towards intuitive or reflective thinking (see Stanovich and West, 1998). Response times were recorded to assess the processing speed of prosocial and selfish decisions towards each charity. This measure was of particular interest for fast sessions, as responses made under time pressure were expected to more directly reflect underlying intuitive preferences. The response window for analysis in the fast condition was 300–5000 ms; responses occurring in less than 300 ms—the minimum time deemed necessary for making above chance decisions (based on previous findings; Milosavljevic et al., 2011)—were excluded from analysis.3
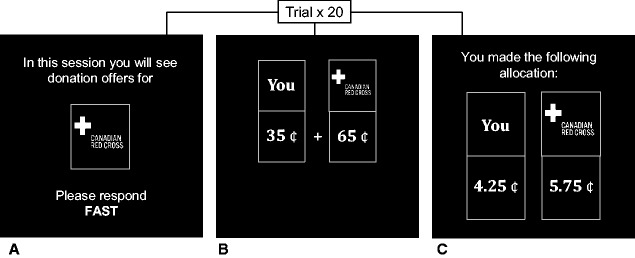
To ensure that participants were oriented to the keyboard, screen and task demands, they first completed a fast-response training round that involved responding to 10 trials of irrelevant stimuli (geometric shapes) within 2 s. Afterwards, participants completed 20 randomized donation blocks—each consisting of 20 trials. Each donation block had a specified recipient (high or low-empathy charity) and decision speed (fast or slow; see Figure 1A). Each trial randomly allocated $1 of the participants’ endowment between themselves and a charity in five cent increments varying between 5 and 95¢ (see Figure 1B). For instance, an offer could include a 30¢–70¢ split where 30¢ is kept by the participant and 70¢ is allocated to the charity. If accepted, 70¢ would be donated to the target charity, and if rejected, the entire dollar would be retained by the participant. After a response was made, the trial would terminate.
Fig. 1.
Sample block exhibiting the block introduction screen (A), a donation offer (B) and block feedback (C).
At the end of each donation block, participants received feedback on how much money they donated (see Figure 1C). A maximum of $10 per block could be donated to a charity, and participants were informed that one block for each charity would be selected at random as their actual donation to each organization to ensure each block was treated as a real allocation of their funds. Donation behavior was measured in two ways. First, we assessed the average amount participants donated to their most or least preferred charity under fast or slow conditions. To do so we computed the average block donation for each condition. Second, we assessed offer preferences by categorizing donation offers by their costliness to the participant. To do so we grouped offers as low (5–35¢), moderate/fair (40–60¢) and high (65–95¢) in costliness.4
ERP acquisition and analysis
Electroencephalography (EEG) was recorded using a 64-channel Ag/AgCl BioSemi electrode cap at standard 10–20 sites (BioSemi Active Two, Amsterdam). Ocular electrodes were placed at the external canthi of each eye and below each orbit, and two additional electrodes were positioned at each mastoid. All channels were digitized at a sampling rate of 512 Hz. Off-line processing was performed in BESA 5.3 (Brain Electrical Source Analysis, Gräfelfing, Germany), including high-pass filtering at 0.1 Hz (12 dB/oct), low-pass filtering at 30 Hz (12 dB/oct), re-referencing to the average of the mastoid electrodes and semi-automatic rejection of trials contaminated by blinks and eye movements.
ERPs were averaged for each target and decision speed condition, time-locked to the onset of donation offers and baseline corrected to the mean amplitude of the 200 ms pre-offer interval. A visual inspection of the grand average waveforms alongside their difference waves and scalp topographies revealed two potential effects. First, P300 amplitudes over centroparietal scalp regions appeared greater for fast decisions, but only for high empathy targets. A region of interest (ROI) including two centroparietal electrodes of maximal voltage was chosen (CPz, Pz), which aligned with previous research (Pfabigan et al., 2014). Second, an early positive deflection peaking at 230 ms over frontocentral scalp regions (henceforth P230) appeared greater for slow decisions than fast decisions, independent of target. An ROI including frontocentral electrodes (FCz, Cz) of maximal voltage was used. Mean amplitudes for the P230 (180–280 ms) and the P300 (300–400 ms) were then calculated for each condition.
Results
Behavioral data
We predicted that people would both (a) donate more and (b) accept more costly offers to a high-empathy target. Moreover, we predicted that people would (c) make prosocial decisions quicker than selfish decisions and (d) donate more under time pressure than time delay.
To begin we assessed whether giving was influenced by target empathy and decision speed by comparing average donations across all blocks in a 2 (target: high vs low-empathy) × 2 (decision: fast vs slow) ANOVA. As predicted, participants allocated significantly more money to a high-empathy target (M = 5.69, s.d. = 1.86) than a low-empathy target (M = 3.98, s.d. = 2.75), F(1, 19) = 14.12, P < 0.005 (see Figure 2). There was neither a main effect of decision speed, F(1, 19) = 1.33, P = 0.26, nor an interaction of target empathy and decision speed, F(1, 19) = 0.05, P = 0.83. However, since the repetitive nature of our task could attenuate such effects, we repeated this analysis for only donations made on participants’ first encounter with each condition (i.e. initial blocks). This analysis was expected to detect the strongest effect, as participants had no prior practice (Rand et al., 2012). Interestingly, during initial blocks participants donated significantly more when deciding intuitively (M = 5.46, s.d. = 2.51), than reflectively (M = 4.56, s.d. = 2.65, F(1, 19) = 5.00, P < 0.05), in addition to giving more to high-empathy (vs low-empathy) targets, F(1, 19) = 13.76, P < 0.005; however, no interaction occurred F(1, 19) = 0.36, P = 0.56). Together, these results suggest that while target empathy robustly elicits greater giving, the effect of decision speed on donating was too subtle to detect across all decision blocks.
Fig. 2.
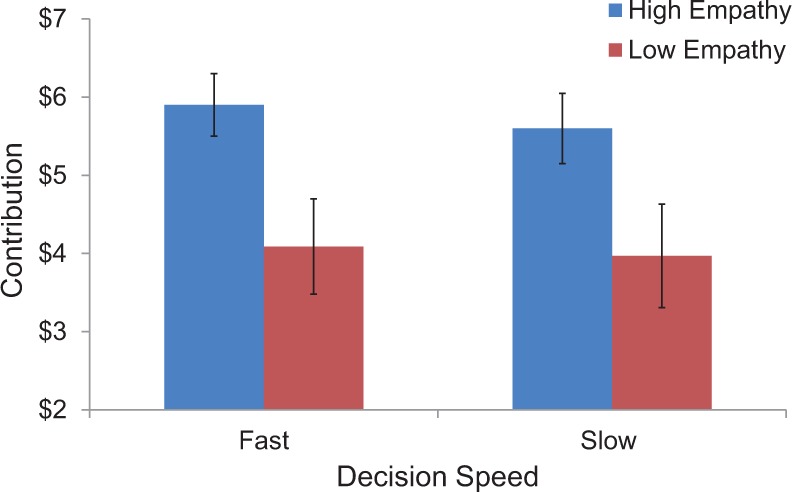
Average contributions (and standard error bars) made to high/low empathy targets with fast/slow decision speeds across all donation blocks.
We also assessed whether participants accepted more costly donations towards high-empathy charities. To do so we compared the proportion of high (95–65¢), medium (60–40¢), and low cost (35–5¢) offers accepted by condition using a 2 (donation target: high vs low-empathy charity) × 2 (decision speed: fast vs slow) × 3 (costliness: high vs fair vs low) repeated measures ANOVA. Analyses revealed a marginal main effect of target empathy, F(1,19) = 3.98, P = 0.06, and no main effect of decision speed, F(1, 19) = 0.07, P = 0.80. Interestingly, a significant interaction of target empathy with offer costliness emerged, F(2,38) = 9.89, P = 0.001. Follow-up contrasts revealed that participants accepted significantly more high offers to high-empathy (M% = 61.00, s.d. = 41.30) than low-empathy targets (M% = 33.75, s.d. = 41.95), t(19) = 3.43, P < 0.005, and more fair offers to high-empathy (M% = 56.11, s.d. = 20.92) than low-empathy targets (M% = 44.48, s.d. = 26.79), t(19) = 2.14, P < 0.05, but interestingly, accepted fewer low offers to high-empathy (M% = 38.66, s.d. = 42.50) than low-empathy targets (fair and low offer differences were marginally significant after a Bonferroni correction, α = 0.017). These results suggest that engaging in costly donating was not only heightened for high-empathy targets, but actually preferred, whereas the reverse was true of low-empathy targets (see Figure 3).
Fig. 3.
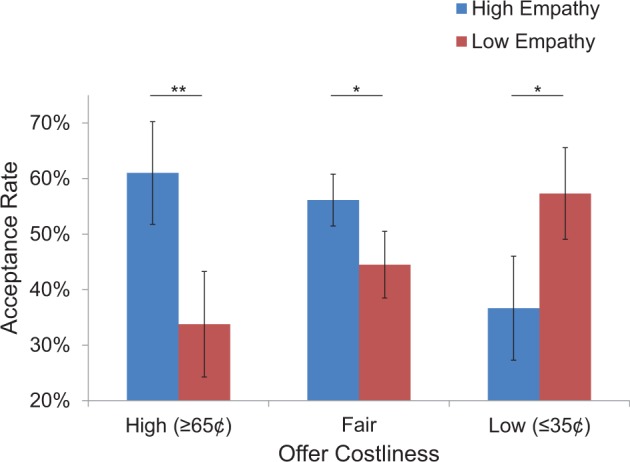
Average acceptance rates by target empathy and offer costliness (with standard error bars). Participants accepted more high and fair donation offers to high-empathy than low-empathy targets, but fewer low donation offers to high-empathy than low-empathy targets; *P < 0.05 (uncorrected), **P < 0.005.
Next we assessed whether prosocial decisions were made faster than selfish decisions. To test this we examined response times5 (ms) during fast blocks with a 2 (target empathy: high vs low) × 2 (offer outcome: accept vs reject) repeated measures ANOVA. We observed a significant effect of offer outcome on response times, F(1, 19) = 9.13, P < 0.01, such that decisions to accept donation offers (M = 1031.40, s.d. = 193) were faster than decisions to reject (M = 1127.46, s.d. = 256). However, there was no main effect of empathy, F(1, 19) = 1.08, P = 0.31, nor interaction between target empathy and offer outcome, F(1, 19) = 0.10, P = 0.76.
In summary, these results confirm that people donate more and accept more costly offers towards high empathy targets. Moreover, our results also indicate that while people make prosocial decisions quicker than selfish decisions, they donated similarly during fast and slow decisions across all blocks.
Electrophysiological data
Centroparietal P300 (300-400 ms)
We assessed whether P300 responses to donation offers were greater (a) for high-empathy targets, (b) when deciding quickly and (c) when making prosocial decisions.
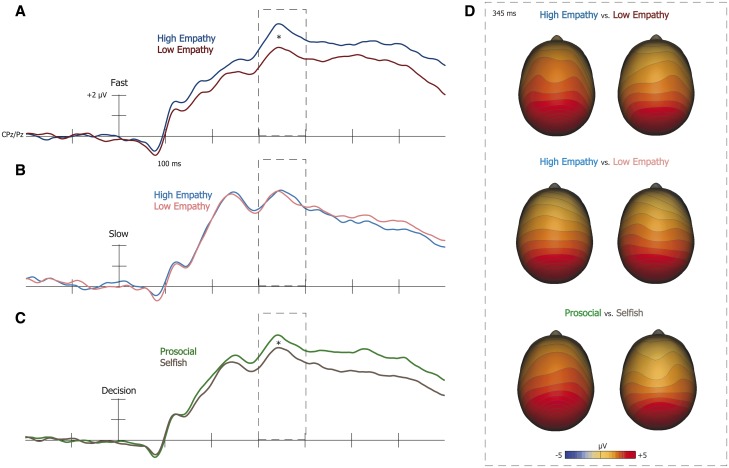
Mean P300 amplitude in response to donation offers was analyzed using a 2 (target empathy: high vs low) × 2 (decision speed: fast vs slow) × 2 (ROI: CPz, Pz) repeated measures ANOVA. We found a significant main effect of target empathy, F(1,19) = 4.83, P < 0.05, on P300 amplitudes. Moreover, while we found no main effect of decision speed, F(1,19) = 1.188, P = 0.29, critically, we observed a significant interaction between target empathy and decision speed, F(1,19) = 4.832, P < 0.05. Specifically, further contrasts revealed that P300 responses to donation offers were significantly greater for high-empathy targets (M = 10.11, s.d. = 3.61) than low-empathy targets (M = 7.92, s.d. = 4.66) during fast decisions, t(19) = 3.43, P < 0.005, but not slow decisions, t(19) = .04, P = 0.97 (see Figure 4A and B). Thus, P300 responses were sensitive to target empathy, but uniquely during intuitive decision making.
Fig. 4.
In response to fast offers (A), significantly larger P300 amplitude occurred when offers affected high-, than low-empathy targets (*P = 0.004). However, in response to slow offers (B), no differences in P300 amplitude occurred depending on target empathy (P = 0.53). Moreover (C), P300 amplitude was significantly larger prior to accepting, than rejecting offers (*P < 0.001), indicating that higher amplitude was associated prosocial decisions. These differences can also be observed from EEG scalp topography (D).
Next we analyzed whether P300 amplitude predicted subsequent donation decisions using a 2 (target empathy: high vs low) × 2 (offer outcome: accepted vs rejected) repeated measures ANOVA.6 We found both a significant main effect of target empathy, F(1, 19) = 4.993, P < 0.05, and offer outcome, F(1, 19) = 4.99, P < 0.001, on P300 amplitude, however no significant interaction emerged between target and offer outcome, F(1, 19) = 0.88, P = 0.36. An a priori planned contrast revealed P300 amplitude at our ROI was significantly greater when participants eventually accepted (M = 9.41, s.d. = 3.43), than rejected (M = 8.26, s.d. = 3.39) offers towards targets, F(1, 19) = 4.99, P < 0.001 (Figure 4C). Thus, P300 activity was not only sensitive to target empathy, but also predicted subsequent donation behavior.
Supporting the above findings, we found a positive correlation in the fast condition between P300 amplitude responses and overall donations, r(18) = .21, P < 0.05 (Figure 5), suggesting that greater P300 responses were associated with greater prosocial behavior. However, P300 amplitude was unrelated to EQ scores, r(18) = 0.08, P = 0.73 and PTM scores r(18) = 0.11, P = 0.65.
Fig. 5.
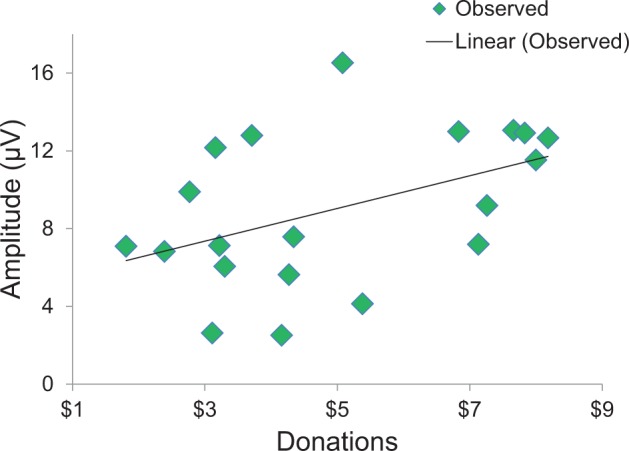
P300 amplitude (μV) in response to donation offers was positively correlated with overall donations to targets in the fast condition.
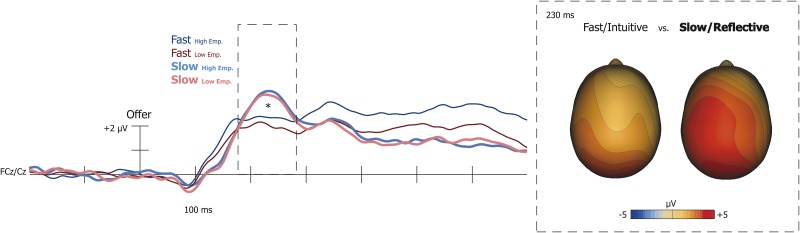
Frontocentral P230 (180–280 ms)
Lastly, we examined whether frontocentral P230 responses to donation offers were greater when making slow decisions, than fast decisions. A 2 (decision speed: fast vs slow) × 2 (target: high empathy vs low empathy) repeated measures ANOVA revealed that frontocentral P230 amplitude was significantly larger in the slow condition than the fast condition, F(1, 19) = 8.98, P < 0.01 (Figure 6). However, no differences occurred based on target empathy (P = 0.11).
Fig. 6.
Significantly larger frontal P230 responses occurred during slow offers, than fast offers (*P = 0.007).
In sum, our electrophysiological data confirmed that P300 amplitudes were greater when making fast donation decisions for high-empathy (vs low empathy) targets, and when subsequently making prosocial decisions. Moreover, we found that greater frontocentral P230 responses occurred in our task when participants were required to make slow, reflective decisions, regardless of target.
Discussion
The present research investigated the temporal dynamics of intuitive prosocial behavior. Particularly, we examined whether P300 amplitude could signal the motivational salience of a donation opportunity, and predict subsequent donating. Consistent with our predictions, the P300 was sensitive to target empathy, displaying increased amplitude centroparietally when donation offers involved a highly empathized target. Importantly, as expected this distinction was only observed when participants made fast, intuitive decisions, as opposed to slow, reflective decisions. In addition, we found that greater P300 responses predicted subsequent decisions to donate, offering initial evidence that P300 modulation may constitute an important predictor of our motivation to engage in prosociality. In further support of this view, during fast offers we found a positive correlation between participants’ P300 responses and their overall average donations, suggesting that greater P300 amplitude was related to greater giving.
Conversely, while engaging in reflective decision-making donation offers elicited an early frontocentral positivity (P230). The timing and spatial distribution of this component suggest that it represents a cognitive control process, as similar components are observed in well-known tasks requiring response inhibition (e.g. Go-NoGo and Stop Signal tasks, Schmajuk et al., 2006; Nash et al., 2013; Wessel and Aron, 2015). Additionally, both source modelling (Albert et al., 2013) and fMRI work (Liddle et al., 2001; Garavan et al., 2002) indicate that such components have neural generators in regions supporting controlled behavior, such as the dorsolateral prefrontal cortex. Thus, the observed P230 likely represents a top-down, reflective process that suppresses motivationally salient events (e.g. a donation opportunity) when individuals choose to, or are experimentally required to, reflect and delay their decision.
Consistent with our ERP findings, we found that people donate more to highly empathized targets, and make prosocial decisions faster than selfish decisions. We also initially observed increased donations during fast offers; however this effect was negligible across all blocks. We suspect that the difference in donations during fast and slow blocks was somewhat attenuated by practice effects (see Rand and Nowak, 2013), though including donations for low-empathy targets in our main analysis—for whom donating is rather unintuitive—may have also curbed this distinction. In either case, our ERP findings offer clear and compelling evidence that functionally distinct neural processes were at play when participants engaged in fast and slow decision making. Thus, we remain optimistic about the insights our work offers on the role of the P300 in the temporal unfolding prosocial motivation, and subsequent prosocial behavior.
The roots of prosociality
In line with a reward-seeking model of prosociality, our neural data suggest that people may engage with prosocial opportunities in a manner akin to opportunities to obtain personal rewards, such as money or food. Indeed, by encoding the motivational salience of cues that are relevant to benefitting others, P300 modulation could offer insight into when prosociality arises intuitively. For instance, our work suggests that the intuitive appeal of such opportunities likely depends in part on our social and emotional connection to the recipient.
This observation is especially apt with consideration to the roots of prosociality. The personal value we imbue in the well-being of others was probably largely shaped by evolutionarily ancient mechanisms for parent-offspring bonding (de Waal, 2008), which could today make helping those we ‘feel close to’ especially intuitive. However, the value we attach to prosocial outcomes is also undoubtedly a product of human culture and personal experience. For instance, prosocial preferences for fairness have a demonstrated capacity to influence valuation processes in the ventral striatum and ventromedial prefrontal cortex, which may have supported the cultivation of prosocial principles that helped shape modern societies (Tabibnia et al., 2008; Ruff and Fehr, 2014). Moreover, prosocial intuitions could be reinforced when we act prosocially or observe others do so in daily life, as such actions typically afford the actor reputational benefits and reciprocal social ties (Rand et al., 2012). Furthermore, a growing literature now supports the idea that behaving prosocially makes actors feel happier (Aknin et al., 2013), and the emotional rewards we derive from these experiences could endorse the formation of prosocial habits (Aknin et al., 2012). Together, these motivational forces may largely shape our capacity for prosocial intuitions.
Yet the extent to which such intuitions dictate our behavior remains deeply context-dependent. Intuitive prosociality would be likely to emerge in situations where we perceive the need for immediate action (e.g. holding an elevator door open for a stranger), and among people that (a) are prone to intuitive thinking and (b) have had positive experiences with prosociality (Rand et al., 2012). Reflective prosociality, on the other hand, may be a more common among situations where the most intuitively appealing option is self-interested, and must be suppressed in the strategic pursuit of a higher order goal (e.g. agreeing to work late to gain favor with your boss). Drawing such distinctions could perhaps clarify conflicting studies that link prosocial decisions to brain regions involved in controlled, reflective behavior (e.g. dorsolateral prefrontal cortex, Baumgartner et al., 2011; Ruff et al., 2013).
Caveats and future directions
Of course, despite its utility, a reward-seeking model cannot fully account for a phenomenon as multifaceted as prosocial behavior. Prosociality can be supported by a complex array of neural and bodily processes involved in emotion and motivation (Morelli et al., 2014), social cognition (Bartz et al., 2011) and the perception of mental states in others (Waytz et al., 2012). For instance, autonomic changes (e.g. skin conductance responses) can be an equally useful predictor of our willingness to help when observing an individual in pain (Hein et al., 2011). Such work highlights the need for further research clarifying which physiological mechanisms facilitate prosociality for different people and situations.
Similarly, since the P300 has many neural inputs and has been associated with numerous affective and cognitive processes across different tasks, further work is needed to investigate the modulation of the P300 in different prosocial contexts, especially beyond monetary-based choice scenarios. Similarly, given that our work examined responses to charitable organizations, future work could benefit by examining P300 responses during prosocial decisions made towards specific, identifiable individuals under intuitive demands (see Genevsky et al., 2013), as such work may reveal more nuanced neural dynamics at play.
These considerations aside, our work offers initial evidence for a rapid neural signature (the P300) that could predict our motivational engagement with prosocial cues analogously to personally rewarding prospects. This work dovetails with a growing view that in the same intuitive manner with which we humans pursue opportunities to improve our own welfare, we may also pursue opportunities to enhance the welfare of others.
Funding
This research was supported by grants from the Natural Sciences and Engineering Research Council of Canada (NSERC) and the Canada Foundation for Innovation (CFI) to M.L., and by grants from the Social Sciences and Humanities Research Council of Canada (SSHRC) and the Canadian Institute for Advanced Research (CIFAR) to L.B.A.
Conflict of interest. None declared.
Footnotes
1 Here we refer to ‘empathy’ as a pre-existing motivational factor concerning a specific target, as opposed to the active process of taking on another’s perspective during decision making.
2 A 5 s, opposed to the 10-s constraint/delay in Rand et al., (2012) was used due to the length of the task required for ERP averaging.
3 Less than 1% of the overall trials (N = 73) in the donation task were missed or excluded from analyses. Proportional corrections were applied to behavioral data for the affected blocks.
4 The 20 donation trials per block were divided into 7 high offers (95–65¢), 6 fair/moderate offers (60–40¢) and 7 low offers (35–5¢). Fifty-cent offers occurred twice per block, which was mathematically necessary for blocks to correctly allocate the cash endowment.
5 On average, decisions were made in 1030.51 ms during fast rounds (SD = 218.27) and 5772.06 ms during slow rounds (SD = 289.14).
6 Fast and slow decision blocks were not analyzed separately here due to individual variability in accept/reject decisions, which left insufficient trial counts for such tests. However, this effect is likely driven by fast blocks due to the lack of P300-response differentiation during slow blocks.
References
- Aknin L.B., Barrington-Leigh C.P., Dunn E.W., et al. (2013). Prosocial spending and well-being: Cross-cultural evidence for a psychological universal. Journal of Personality and Social Psychology , 104, 635–52. [DOI] [PubMed] [Google Scholar]
- Aknin L.B., Dunn E.W., Norton M.I. (2012). Happiness runs in a circular motion: Evidence for a positive feedback loop between prosocial spending and happiness. Journal of Happiness Studies, 13, 347–55. [Google Scholar]
- Aknin L.B., Sandstrom G.M., Dunn E.W., Norton M.I. (2011). It's the recipient that counts: Spending money on strong social ties leads to greater happiness than spending on weak social ties. PloS One, 6, e17018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert J., López-Martín S., Hinojosa J.A., Carretié L. (2013). Spatiotemporal characterization of response inhibition. NeuroImage, 76, 272–81. [DOI] [PubMed] [Google Scholar]
- Bartz J.A., Zaki J., Bolger N., Ochsner K.N. (2011). Social effects of oxytocin in humans: context and person matter. Trends in Cognitive Sciences, 15, 301–9. [DOI] [PubMed] [Google Scholar]
- Batson C.D. (2011). Altruism in Humans. New York: Oxford University Press. [Google Scholar]
- Baumgartner T., Knoch D., Hotz P., Eisenegger C., Fehr E. (2011). Dorsolateral and ventromedial prefrontal cortex orchestrate normative choice. Nature Neuroscience, 14, 1468–74. [DOI] [PubMed] [Google Scholar]
- Broyd S.J., Richards H.J., Helps S.K., Chronaki G., Bamford S., Sonuga-Barke E.J. (2012). An electrophysiological monetary incentive delay (e-MID) task: a way to decompose the different components of neural response to positive and negative monetary reinforcement. Journal of Neuroscience Methods, 209, 40–9. [DOI] [PubMed] [Google Scholar]
- Carlo G., Randall B.A. (2002). The development of a measure of prosocial behaviors for late adolescents. Journal of Youth and Adolescence, 31, 31–44. [Google Scholar]
- Chiu Loke I., Evans A.D., Lee K. (2011). The neural correlates of reasoning about prosocial–helping decisions: An event-related brain potentials study. Brain Research, 1369, 140–8. [DOI] [PubMed] [Google Scholar]
- Cornelissen G., Dewitte S., Warlop L. (2011). Are social value orientations expressed automatically? Decision making in the dictator game. Personality and Social Psychology Bulletin , 37, 1080–90. [DOI] [PubMed] [Google Scholar]
- Cox A., Kohls G., Naples A.J., et al. (2015). Diminished social reward anticipation in the broad autism phenotype as revealed by event-related brain potentials. Social Cognitive and Affective Neuroscience, nsv024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Custers R., Aarts H. (2010). The unconscious will: How the pursuit of goals operates outside of conscious awareness. Science, 329, 47–50. [DOI] [PubMed] [Google Scholar]
- de Waal F.B. (2008). Putting the altruism back into altruism: The evolution of empathy. Annual Review of Psychology , 59, 279–300. [DOI] [PubMed] [Google Scholar]
- de Waal F.B. (2012). The antiquity of empathy. Science, 336, 874–76. [DOI] [PubMed] [Google Scholar]
- DeWall C.N., Baumeister R.F., Gailliot M.T., Maner J.K. (2008). Depletion makes the heart grow less helpful: Helping as a function of self-regulatory energy and genetic relatedness. Personality and Social Psychology Bulletin, 34, 1653–62. [DOI] [PubMed] [Google Scholar]
- Decety J., Norman G.J., Berntson G.G., Cacioppo J.T. (2012). A neurobehavioral evolutionary perspective on the mechanisms underlying empathy. Progress in Neurobiology, 98, 38–48. [DOI] [PubMed] [Google Scholar]
- Dunn E.W., Aknin L.B., Norton M.I. (2008). Spending money on others promotes happiness. Science, 319, 1687–88. [DOI] [PubMed] [Google Scholar]
- Fareri D.S., Niznikiewicz M.A., Lee V.K., Delgado M.R. (2012). Social network modulation of reward-related signals. The Journal of Neuroscience , 32, 9045–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores A., Münte T.F., Doñamayor N. (2015). Event-related EEG responses to anticipation and delivery of monetary and social reward. Biological Psychology, 109, 10–9. [DOI] [PubMed] [Google Scholar]
- Garavan H., Ross T.J., Murphy K., Roche R.A., Stein E.A. (2002). Dissociable executive functions in the dynamic control of behavior: inhibition, error detection, and correction. NeuroImage,17, 1820–29. [DOI] [PubMed] [Google Scholar]
- Genevsky A., Västfjäll D., Slovic P., Knutson B. (2013). Neural underpinnings of the identifiable victim effect: Affect shifts preferences for giving. The Journal of Neuroscience , 33, 17188–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G., MacNamara A., Olvet D.M. (2010). Event-related potentials, emotion, and emotion regulation: An integrative review. Developmental Neuropsychology, 35, 129–55. [DOI] [PubMed] [Google Scholar]
- Harbaugh W.T., Mayr U., Burghart D.R. (2007). Neural responses to taxation and voluntary giving reveal motives for charitable donations. Science, 316, 1622–5. [DOI] [PubMed] [Google Scholar]
- Hein G., Lamm C., Brodbeck C., Singer T. (2011). Skin conductance response to the pain of others predicts later costly helping. PloS One, 6, e22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C.L., Minati L., Harrison N.A., Ward J., Critchley H.D. (2011). Under pressure: response urgency modulates striatal and insula activity during decision-making under risk. PLoS One, 6, e20942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keltner D., Kogan A., Piff P.K., Saturn S.R. (2014). The sociocultural appraisals, values, and emotions (SAVE) framework of prosociality: Core processes from gene to meme. Annual Review of Psychology, 65, 425–60. [DOI] [PubMed] [Google Scholar]
- Kinnunen S.P., Windmann S. (2013). Dual-processing altruism. Frontiers in Psychology, 4, 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleih S.C., Nijboer F., Halder S., Kübler A. (2010). Motivation modulates the P300 amplitude during brain–computer interface use. Clinical Neurophysiology , 121, 1023–31. [DOI] [PubMed] [Google Scholar]
- Knyazev G.G. (2007). Motivation, emotion, and their inhibitory control mirrored in brain oscillations. Neuroscience and Biobehavioral Reviews, 31, 377–95. [DOI] [PubMed] [Google Scholar]
- Liddle P.F., Kiehel K.A., Smith A.M. (2001). Event-related fMRI study of response inhibition. Human Brain Mapping, 12, 100–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubman D.I., Allen N.B., Peters L.A., Deakin J.W. (2008). Electrophysiological evidence that drug cues have greater salience than other affective stimuli in opiate addiction. Journal of Psychopharmacology, 22, 836–42. [DOI] [PubMed] [Google Scholar]
- Ma Q., Shen Q., Xu Q., Li D., Shu L., Weber B. (2011). Empathic responses to others’ gains and losses: An electrophysiological investigation. NeuroImage, 54, 2472–80. [DOI] [PubMed] [Google Scholar]
- Mather M., Lighthall N.R. (2012). Risk and reward are processed differently in decisions made under stress. Current Directions in Psychological Science, 21, 36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milosavljevic M., Koch C., Rangel A. (2011). Consumers can make decisions in as little as a third of a second. Judgment and Decision-making, 6, 45–52. [Google Scholar]
- Moll J., Krueger F., Zahn R., Pardini M., de Oliveira-Souza R., Grafman J. (2006). Human fronto–corticostriatal networks guide decisions about charitable donation. Proceedings of the National Academy of Sciences, 103, 15623–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli S.A., Rameson L.T., Lieberman M.D. (2014). The neural components of empathy: predicting daily prosocial behavior. Social Cognitive and Affective Neuroscience , 9, 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli S.A., Sacchet M.D., Zaki J. (2015). Common and distinct neural correlates of personal and vicarious reward: A quantitative meta-analysis. NeuroImage, 112, 244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash K., Schiller B., Gianotti L.R., Baumgartner T., Knoch D. (2013). Electrophysiological indices of response inhibition in a Go/NoGo task predict self-control in a social context. PloS One, 8, e79462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfabigan D.M., Seidel E.M., Sladky R., et al. (2014). P300 amplitude variation is related to ventral striatum BOLD response during gain and loss anticipation: An EEG and fMRI experiment. NeuroImage, 96, 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogarell O., Padberg F., Karch S., et al. (2011). Dopaminergic mechanisms of target detection—P300 event related potential and striatal dopamine. Psychiatry Research: Neuroimaging, 194, 212–8. [DOI] [PubMed] [Google Scholar]
- Rand D.G., Greene J.D., Nowak M.A. (2012). Spontaneous giving and calculated greed. Nature , 489, 427–30. [DOI] [PubMed] [Google Scholar]
- Rand D.G., Nowak M.A. (2013). Human cooperation. Trends in Cognitive Sciences, 17, 413. [DOI] [PubMed] [Google Scholar]
- Rand D.G., Peysakhovich A., Kraft-Todd G.T., et al. (2014). Social heuristics shape intuitive cooperation. Nature Communications, 5, 3677. [DOI] [PubMed] [Google Scholar]
- Righetti F., Finkenauer C., Finkel E.J. (2013). Low self-control promotes the willingness to sacrifice in close relationships. Psychological Science , 24, 1533–40. [DOI] [PubMed] [Google Scholar]
- Ruff C.C., Fehr E. (2014). The neurobiology of rewards and values in social decision-making. Nature Reviews Neuroscience, 15, 549–62. [DOI] [PubMed] [Google Scholar]
- Ruff C.C., Ugazio G., Fehr E. (2013). Changing social norm compliance with noninvasive brain stimulation. Science, 342, 482–4. [DOI] [PubMed] [Google Scholar]
- Schulz J.F., Fischbacher U., Thöni C., Utikal V. (2012). Affect and fairness: Dictator games under cognitive load. Journal of Economic Psychology , 41, 77–87. [Google Scholar]
- Schmajuk M., Liotti M., Busse L., Woldorff M.G. (2006). Electrophysiological activity underlying inhibitory control processes in normal adults. Neuropsychologia, 44, 384–95. [DOI] [PubMed] [Google Scholar]
- Stanovich K.E., West R.F. (1998). Individual differences in rational thought. Journal of Experimental Psychology, 127, 161. [Google Scholar]
- Stevens J.R., Hauser M.D. (2004). Why be nice? Psychological constraints on the evolution of cooperation. Trends in Cognitive Sciences, 8, 60–5. [DOI] [PubMed] [Google Scholar]
- Tabibnia G., Satpute A.B., Lieberman M.D. (2008). The sunny side of fairness preference for fairness activates reward circuitry (and disregarding unfairness activates self-control circuitry). Psychological Science, 19, 339–47. [DOI] [PubMed] [Google Scholar]
- Tinghög G., Andersson D., Bonn C., et al. (2013). Intuition and cooperation reconsidered. Nature, 498, E1–2. [DOI] [PubMed] [Google Scholar]
- Verkoeijen P.P., Bouwmeester S. (2014). Does intuition cause cooperation? PloS one, 9, e96654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi A., Baron-Cohen S., Wheelwright S., et al. (2006). Development of short forms of the Empathy Quotient (EQ-Short) and the Systemizing Quotient (SQ-Short). Personality and Individual Differences, 41, 929–40. [Google Scholar]
- Warneken F., Tomasello M. (2006). Altruistic helping in human infants and young chimpanzees. Science, 311, 1301–3. [DOI] [PubMed] [Google Scholar]
- Waytz A., Zaki J., Mitchell J.P. (2012). Response of dorsomedial prefrontal cortex predicts altruistic behavior. The Journal of Neuroscience, 32, 7646–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel J.R., Aron A.R. (2015). It's not too late: The onset of the frontocentral P3 indexes successful response inhibition in the stop‐signal paradigm. Psychophysiology, 52, 472–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F., Zheng Z., Wang Y., Cui J., Chen Y. (in press). Conflict monitoring and stimulus categorization processes involved in the prosocial attitude implicit association test: Evidence from event-related potentials. Social Neuroscience. [DOI] [PubMed] [Google Scholar]
- Zaki J., Mitchell J. (2011). Equitable decision-making is associated with neural markers of subjective value. Proceedings of the National Academy of Sciences , 108, 19761–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki J., Mitchell J. (2013). Intuitive prosociality. Current Directions in Psychological Science, 22, 466–70. [Google Scholar]
- Zaki J., Mitchell J. (in press). Prosociality as a form of reward-seeking. In: Greene J., Morrison I.editors. Positive Neuroscience. Oxford: Oxford University Press. [Google Scholar]
- Zaki J., López G., Mitchell J.P. (2014). Activity in ventromedial prefrontal cortex co-varies with revealed social preferences: Evidence for person-invariant value. Social Cognitive and Affective Neuroscience, 9, 464–9. [DOI] [PMC free article] [PubMed] [Google Scholar]