Abstract
Some healthy older adults show departures from standard decision-making patterns exhibited by younger adults. We asked if such departures are uniform or if heterogeneous aging processes can designate which older adults show differing decision patterns. Thirty-three healthy older adults with varying decision-making patterns on a complex decision task (the Iowa Gambling Task) completed an intertemporal choice task while undergoing functional magnetic resonance imaging. We examined whether value representation in the canonical valuation network differed across older adults based on complex decision-making ability. Older adults with advantageous decision patterns showed increased activity in the valuation network, including the ventromedial prefrontal cortex (VMPFC) and striatum. In contrast, older adults with disadvantageous decision patterns showed reduced or absent activation in the VMPFC and striatum, and these older adults also showed greater blood oxygen level dependent signal temporal variability in the striatum. Our results suggest that a reduced representation of value in the brain, possibly driven by increased neural noise, relates to suboptimal decision-making in a subset of older adults, which could translate to poor decision-making in many aspects of life, including finance, health and long-term care. Understanding the connection between suboptimal decision-making and neural value signals is a step toward mitigating age-related decision-making impairments.
Keywords: aging, decision-making, ventromedial prefrontal cortex, Iowa Gambling Task, value
Introduction
Many healthy older adults show departures from the standard decision-making patterns exhibited by younger adults. For example, older adults show declines in reward learning (Eppinger and Kray, 2010; Samanez-Larkin et al., 2012), inconsistent decision patterns (Tymula et al., 2013), poorer decisional competency (Finucane and Gullion, 2010) and increased financial errors (Agarwal et al., 2009) relative to younger adults. Although age-related changes in decision-making are well described, individual differences in older adults’ decision patterns remain largely unexplored, and some decision-making capacities seem to remain fairly stable or even improve with age (e.g. Li et al., 2015). Therefore, we set out to address the following question: are these departures uniform, or do heterogeneous brain-based aging processes distinctively influence decision-making?
Decision-making involves integrating attributes of an outcome (e.g. magnitude, probability and delay to outcome) to create a common valuation so options can be compared (Levy and Glimcher, 2012). Most decisions involve subjective preferences that ultimately drive valuation and decisions (Kable and Glimcher, 2007). The ventromedial prefrontal cortex (VMPFC) and striatum represent value in the brain (Bartra et al., 2013; Clithero and Rangel, 2013), and dysfunction in these same regions contributes to decision-making debilities among healthy older adults (Mohr et al., 2010; Shohamy and Wimmer, 2013). For example, older adults show reduced striatal activation in anticipation of losses (Samanez-Larkin et al., 2007), increased variability in the striatum during decision-making (Samanez-Larkin et al., 2010) and reduced VMPFC activation during reward learning (Eppinger et al. 2013), compared with younger adults. Moreover, older adults with weaker correlations between mesolimbic activation and expected value make less optimal decisions (Samanez-Larkin et al., 2011b). This suggests that decision patterns are not uniform among older adults and that aberrant value representation may impede optimal decision-making.
However, individual differences contributing to aberrant value representation among older adults are not well understood. We predict that a subset of healthy older adults will have a diminished neural representation of subjective value (SV) and that this diminished representation can be identified by the Iowa Gambling Task (IGT), which involves integrating multiple decision attributes (e.g. ambiguity, risk, reward, loss) prior to making a selection (Bechara et al. 1994). Researchers designed the IGT to capture real-world decision-making deficits observed in patients with focal damage to the VMPFC; thus, the IGT was one of the first tasks to bring an emotional component into laboratory decision-making, and, indeed, patients with VMPFC damage showed marked deficits on the IGT (Bechara et al., 1994). This early lesion work was supported by neuroimaging findings, showing that activity in the VMPFC and striatum, among other areas, corresponds to IGT performance (Ernst et al., 2002; Li et al., 2010; Halfmann et al. 2014). These brain regions largely overlap with brain regions thought to represent SV, as described in neuroeconomics literature (Levy and Glimcher, 2012; Bartra et al., 2013; Clithero and Rangel, 2013). Of note, although most young adults show advantageous performance on the IGT, approximately one-third of seemingly healthy older adults show overtly disadvantageous performance, akin to individuals with focal damage to the VMPFC (Denburg et al., 2005). It seems plausible that poor performance on the IGT among older adults could stem from poor, absent or abnormal value representation in the brain.
Added support for this hypothesis comes from research that shows IGT deficits correlate with poor ecological decision-making abilities. For example, poor IGT performance relates to a greater intent to purchase products in misleading advertisements (Denburg et al., 2007) and poorer depth of reasoning in justification for financial decisions (Shivapour et al., 2012). In an effort to better understand why the IGT might affect these kinds of decisions, we examined whether IGT predicted a reduced or absent neural value signature among healthy older adults.
To test this prediction, we recruited healthy older adults with varying scores on the IGT and had them perform an intertemporal choice task while undergoing functional magnetic resonance imaging (fMRI). We used the intertemporal choice task during scanning because (i) it is easier to decompose than many other tasks (e.g. the IGT), (ii) we can test our main hypothesis related to value processing by using subjects’ preferences to compute SV on a trial-by-trial basis and use this as a regressor in our analyses, (iii) we can rule out alternative explanations by testing regressors such as the magnitude of the option and (iv) previous work shows blood oxygen level dependent (BOLD) activity in a canonical network, including the VMPFC and striatum, correlates with SV during this task (Smith and Huettel, 2010; Grabenhorst and Rolls, 2011; Peters and Buechel, 2011). Since age-related changes in activation during decision-making emerge in these same regions (Samanez-Larkin et al., 2007; Eppinger et al., 2013), we predicted SV-related activation in this network would correspond to individual differences in decision-making (measured by the IGT) among older adults. In other words, we predicted that older adults with the poorest complex decision-making performance would show reduced value processing during intertemporal choices in the value network.
Moreover, recent work suggests that intraindividual BOLD signal variability differs between young and old adults (Samanez-Larkin et al., 2010). It is thought that intraindividual variability in neuromodulatory systems, such as the dopaminergic system (Guitart-Masip et al., 2015), plays an important role in age-related changes in value-based decision-making (Li et al., 2010). We predicted intraindivdual variability in the canonical SV network would correspond to individual differences in decision-making (measured by the IGT) among older adults.
Materials and methods
Participants
Approximately 40 older adults (aged 60 years and above) from our laboratory’s participate database (described below) were invited to complete a behavioral pretest. Thirty-six (Mage = 77.0 years) chose to complete this pretest, of which 24 went on to participate in the fMRI research study reported here. The remaining 12 pretest participants were not eligible for participating in fMRI studies. In addition, 80 younger adults (Mage = 21 years) completed a similar pretest.
Twenty-four individuals from the pretest, and nine additional individuals (for a total of 33) were recruited from our laboratory’s database to participate in the fMRI study. These individuals were well-characterized, healthy older adults (59–88 years of age, Mage = 76 years). We omitted three individuals because the below described hyperbolic discount function did not fit their data well, with a cut-off of r2 < 0.30. We excluded one participant for only choosing the later option. Our final fMRI sample consisted of 29 participants (53% IGT-disadvantageous and 53% female).
All older adult participants (pretest and fMRI) were administered a full neuropsychological battery (Table 1) and health interview in a previous session. At the time of the initial neuropsychological testing and health interview, participants were excluded from the database if they had outstanding medical or psychiatric conditions and the following inclusion criteria were used: ability and willingness to provide consent, living independently, normal (age-related) hearing and vision, cognitive performance within the normal range (±1.5 s.d.) for age and educational attainment (Lezak et al., 2012) and no MRI contraindications.
Table 1.
Demographic and neuropsychological means and standard deviations for the IGT-disadvantageous and IGT-advantageous older adults
| Category | Measure | IGT-disadvantageous | IGT-advantageous | tdisadv–adv |
|---|---|---|---|---|
| Demographics | N | 15 | 14 | — |
| Age | 75.1 (8.1) | 76.4 (5.5) | −0.52 | |
| Sex | 53% female | 43% Female | — | |
| Education | 16.3 (3.0) | 16.6 (2.6) | −0.36 | |
| Handedness | 14 RH, 1 Ambi | 13 RH, 1 LH | — | |
| Intellect | WRAT reading | 108.9 (8.1) | 114.0 (5.6) | −1.97+ |
| Full scale IQ | 116.3 (12.9) | 124.6 (9.4) | −1.98+ | |
| Language | BNT | 19.3 (0.7) | 19.6 (0.6) | −1.22 |
| COWA | 43.4 (14.9) | 47.0 (12.3) | −0.71 | |
| Memory | ReyO delay | 14.1 (5.0) | 17.9 (6.4) | −1.75+ |
| AVLT delay | 8.9 (2.6) | 10.0 (3.6) | −0.92 | |
| Attention | WMI | 110.1 (13.5) | 112.2 (12.0) | −0.44 |
| BVRT errors | 7.2 (2.2) | 6.7 (1.6) | 0.20 | |
| Motor/construction | TM A (time) | 32.4 (6.9) | 33.3 (10.5) | −0.27 |
| ReyO copy | 31.3 (3.4) | 33.0 (3.3) | −1.33 | |
| Executive function | TM B (time) | 74.4 (22.2) | 75.1 (22.7) | −0.08 |
| WCST PE | 19.5 (17.11) | 10.8 (9.0) | 1.70+ | |
| IGT | −24.4 (15.9) | 36.5 (9.0) | −10.16*** | |
| Other | Numeracy | 8.5 (2.1) | 8.9 (2.1) | −0.50 |
| BDI | 4.2 (4.9) | 4.5 (4.0) | −0.18 |
WRAT, wide range achievement test; BNT, Boston naming test; COWA, Controlled Oral Word Association; ReyO, Rey Osterrieth complex figure; AVLT, auditory verbal learning test; WMI, working memory index from the Wechsler adult intelligence scale–IV; BVRT, Benton visual retention test; TM, trail making; WCST PE, Wisconsin card sorting task perseverative errors; IGT, Iowa Gambling Task; BDI, Beck depression inventory. +P < 0.10, ***P < 0.001.
All participants completed the computerized A′B′C′D′ version of the IGT an average of 5 years prior to this study (s.d. = 3.2 years). The IGT involves elements of ambiguity early in the task, and risk later in the task as the participant accumulates more knowledge of the reward/punishment contingencies of the card decks. Participants were not retested on the A′B′C′D′ version of the IGT because practice effects lead to improvements in performance for up to 5 years (Waters-Wood et al., 2012). Participants were categorized as IGT-advantageous (A′B′C′D′ scores > 16) or IGT-disadvantageous (A′B′C′D′ scores < 5; Table 1 for mean scores). We note that IGT-advantageous older adults trend toward having higher scores on measures of intellect, therefore, we conducted our analyses both with and without controlling for full scale IQ, as well as age.
Behavioral pretest
The behavioral pretest administered to the older adults (N = 36) consisted of 66 intertemporal choices, where participants indicated their preference for a sooner, smaller monetary gain or a later, larger monetary gain. The sooner, smaller amount was always $20.00 today. The later, larger amount varied between $20.00 and $50.00 at delays of 5, 10, 15, 30, 60 and 180 days. The resulting data were used to determine the range of magnitudes and delays used for this study. It should be noted that some participants who completed this behavioral pretest also went on to complete the intertemporal choice task in the fMRI portion of this study (N = 24); however, intertemporal choice preferences tend to be reliable across time (Simpson and Vuchnich, 2000), with test–retest correlations greater than r = 0.90 (Senecal et al., 2012), therefore test–retest is not a concern.
A sample of younger adults (N = 80) completed a similar behavioral pretest that included 36 questions with the sooner, smaller amount set at $20.00 today, and the later, larger amount varying between $20.00 and $50.00, with delays of 5, 15, 30, 60 and 180 days. Three younger adults were excluded because of a technical error saving the data. We included this younger adult sample to act as a comparison group with our older adults and replicate earlier results.
FMRI task and procedure
We tested each participant individually on a computerized version of an intertemporal choice task using E-Prime (version 2.0) while undergoing fMRI. We instructed participants, both verbally and in writing, that there were no right or wrong answers, and they should simply choose the option they most prefer. Choices were incentive compatible, meaning we paid participants based on one of their randomly selected choices via a Visa gift card. Participants completed 12 practice trials while on the scanner table, familiarizing them to the environment and the response pad. Two of these trials had a dominant response such that participants should choose the sooner option (e.g. $20.00 today or $19.00 in 30 days) to ensure that participants understood the task and button presses prior to the actual task. Participants then made 60 choices between an immediate monetary reward (always $20) and a larger, later monetary reward (between $20.25 and $40) with delays of 5, 15, 30, 60, 90 and 180 days. The range of delays and reward magnitudes for the fMRI study were optimized based on the pretest data to elicit ∼50% immediate reward and 50% later reward choices across participants.
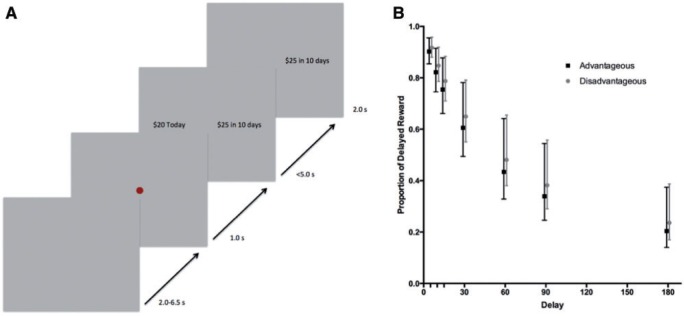
Participants chose by pressing a button on a fiber optic manipulandum, using their index finger to choose the Today option and their middle finger to choose the Later option. Participants had 5.0 s to make a choice and after their selection, the option they chose remained on the screen for an additional 2.0 s. This was followed by a 2.0- to 6.5-s jitter period and then a 1.0 s fixation, indicating that the next trial would begin (Figure 1A).
Fig. 1.
(A) The trial structure is shown. Participants were instructed to choose whichever option they most preferred and that there were no right or wrong responses. During the task, a 2.0–6.5 s jittered intertrial interval preceded a red dot (1.0 s), signaling the start of a trial. Participants had up to 5.0 s to choose between an immediate option (always $20.00 today) and a delayed (5–180 days), larger ($20.25–$40.00) option (choice period). After making a selection, the option the participants chose remained on the screen for 2.0 s (feedback period). Participants responded using their index finger (immediate option) and middle finger (later option) on a fiber optic manipulandum. There were 60 trials total, following 12 practice trials. Participants were paid with a Visa gift card based on one of their randomly selected choices. (B) Discount functions for IGT indicating that IGT-disadvantageous and IGT-advantageous older adults do not differ in their mean discount rates. Error bars are the standard error of the mean discount rates.
Scan parameters
We collected MRI scans using a standard 12-channel head array on a Siemens 3T TIM Trio. Standard scan parameters were used (Supplementary Materials) and 31 interleaved slices were acquired in the oblique orientation, angled relative to AC-PC to minimize VMPFC signal dropout. Scan time fell between 8 min 33 s and 10 min 28 s and between 251 and 314 volumes were collected per subject. Differences in scan time were because trials were partially self-paced. The first two volumes were omitted to avoid saturation effects.
Behavioral analysis
We separately fit each participant’s data to a hyperbolic curve and calculated a single discounting parameter (k) using the following equation: , where SV is the subjective value, A is the objective value, D is the delay and k is the discount parameter (Kable and Glimcher, 2007). The model was fit using the function minimization tools in Matlab by fitting a logistic regression model, assuming hyperbolic discounting, to the binary choices by maximum likelihood. Next, we calculated the SV for each choice on a trial-by-trial basis, entering the trial-specific values along with the participant-specific k parameter into the above equation. We used SV as a parametric regressor in the following analyses to assess trial-by-trial BOLD signal fluctuations associated with SV during the choice and feedback periods. See Supplementary materials for additional detailed behavioral analyses.
Imaging analysis
We conducted our analyses using BrainVoyager QX 2.8 (Brain Innovation, Maastricht, Netherlands). We applied standard preprocessing steps (Supplementary Materials). We performed statistical analyses on a participant-averaged brain in Talairach space (Talairach and Tournoux, 1988).
We performed statistical analyses using a multistage approach. In the first stage, we estimated a general linear model with each part of the task (Choice Main, Feedback Main) convolved using a two-gamma hemodynamic response function corrected for temporal serial correlation using an AR(2) model. The intertrial interval was treated as the baseline. We also included parametric regressors for choice parametric and feedback parametric. The first two regressors fit the mean activity, collapsing across all trials, for each of their respective time points in the trial. The last two parametric regressors fit the deviations from the mean that were correlated with the SV of the delayed rewards at each time point across trials. SV was estimated based on each individual’s behaviorally derived hyperbolic preference curve (i.e. ). The model predicted 86% of the choices among the IGT-disadvantageous older adults and 88% of the choices among the IGT-advantageous older adults, t(27) = −0.83, P = 0.42, and the groups did not differ in the slope of the softmax, t(27) = 0.44, P = 0.67, suggesting that the model fit was similar across groups and differences in SV-correlated activation between groups is unlikely due to choice inconsistency. The parametric predictors were scaled to their standard deviation across trials. Higher values indicate that activation is more strongly correlated with the SV of the delayed reward. We also defined parametric predictors and ran parallel analyses replacing SV with the objective magnitude of the later reward or delay to the later reward or without a parametric regressor.
In the second stage, we used a multi-subject random-effects model with separate subject predictors. At this stage of the analysis, we applied a percent signal change transformation of each value with respect to the voxel time course mean value. We created separate statistical maps for each participant, using Brainvoyager’s volume map function, collapsing across the choice parametric and feedback parametric predictors to compute SV-associated activation (dependent measure). In other words, for each subject, we computed the percent signal change associated with SV during the choice and feedback portion of the trial.
For the whole-brain analysis, we ran a general linear model with IGT as a continuous predictor and SV-associated activation as the dependent measure. We set the threshold to a false discovery rate of q(FDR) = 0.05 (this corresponded to P = 0.002, 2-tailed) and submitted the thresholded maps to a whole-brain correction criterion based on the estimate of the map’s spatial smoothness (full width at half maximum = 2.829 mm). We then proceeded to run 1000 iterations of Monte Carlo simulations to estimate cluster-level false positive rates. By completing this iterative procedure, we obtained a minimum cluster size threshold for a cluster-level false positive rate of α < 0.05. Cluster threshold estimations are indicated in the results section. This iterative procedure was used in the following analyses to correct for multiple comparisons. To test our predictions, we assessed whether IGT scores correlated with BOLD signal change associated with SV. We next tested this effect controlling for age and IQ.
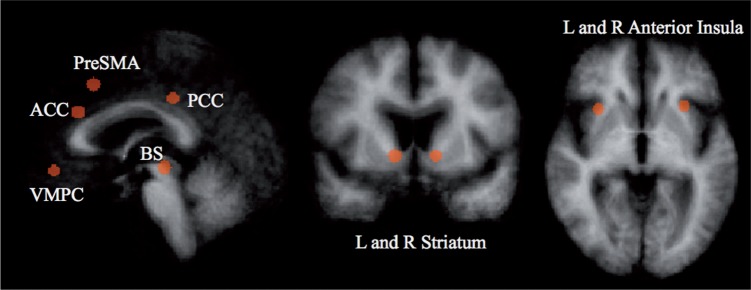
We also performed a Region of Interest (ROI) analysis using Pearson’s correlations with percent signal change as the dependent measure. Because commonly used nuisance factors (e.g. block or motion) were not an issue, as it is unlikely SV correlates with these factors, we did not include these in our analysis. This allowed us to test for effects in smaller brain regions, such as the striatum, that might not have been captured after cluster thresholding in the whole-brain analysis. We defined nine 8 mm sphere ROIs (Table 3 and Figure 3) based on results from a recent meta-analysis that showed activity in these regions positively correlate with SV across a large sample of empirical studies (Bartra et al., 2013). Reported SV-associated activity results represent the correlation between each individual’s trial-by-trial SV and the average (within subject) activity across all voxels in a given ROI. We further tested whether demographic or cognitive variables were able to predict SV representation in these ROIs.
Table 3.
Talairach coordinates of nine regions shown in a meta-analysis (Bartra et al., 2013) to be positively correlated with SV
| Region | X | Y | Z | IGT | IGT-Adv only |
IGT-Dis only |
||
|---|---|---|---|---|---|---|---|---|
| Ave r | % SΔ | Ave t | % SΔ | Ave t | ||||
| VMPFCa | 1 | 41 | −8 | 0.50* | 0.42 | 2.10 | −0.35 | −2.12 |
| L striatuma | −12 | 9 | −2 | 0.48* | 0.64 | 2.94* | −0.41 | −1.67 |
| R striatum | 11 | 7 | −2 | 0.38 | 0.66 | 2.80* | −0.14 | −0.68 |
| L ant insula | −29 | 18 | −2 | 0.31 | 0.90 | 3.75* | 0.17 | 0.98 |
| R ant insula | 30 | 16 | −1 | 0.21 | 0.63 | 2.62* | 0.20 | 0.68 |
| PCC | −3 | −28 | 34 | 0.36 | 0.98 | 3.48* | −0.12 | −0.39 |
| ACCa | −2 | 27 | 26 | 0.51* | 0.81 | 4.06* | −0.42 | −1.50 |
| Brainstem | −1 | −23 | −6 | 0.34 | 0.56 | 2.68* | −0.26 | −0.88 |
| Pre-SMA | −1 | 18 | 42 | 0.43* | 0.95 | 4.14* | −0.07 | −0.21 |
These regions served as regions of interest. The IGT column shows the Pearson’s correlation (r) between IGT scores and the average (across voxels within each participant’s ROI) SV-associated activity in each region of interest. The next columns (IGT-Adv only and IGT-Dis only) show the percent signal change correlated to SV (e.g. SV-associated activation) and the single sample t-values for IGT-advantageous older adults and IGT-disadvantageous older adults when they are split into two groups based on their scores. Ave, average; L, left; R, right; Ant, anterior; PCC, posterior cingulate cortex; ACC, anterior cingulate cortex; SMA, supplementary motor area; Adv, IGT-advantageous; Dis, IGT-disadvantageous; % SΔ, percent signal change correlated to SV. aIndicates ROI in subsequent analyses. *FDR(q) < 0.05.
Fig. 3.
Coordinates are reported in Table 4. Regions are based on a recent meta-analysis (Bartra et al., 2013). L, left; R, right; PCC, posterior cingulate cortex; ACC, anterior cingulate cortex; SMA, supplementary motor area.
Intraindividual variability analysis
As an exploratory analysis to better understand our results, we used mean square successive difference (MSSD) to estimate intraindividual variability of the BOLD signal. MSSD is typically used to assess heart rate variability; however, more recently MSSD has been applied to assess temporal variability, or within subject BOLD signal variability (Samanez-Larkin et al., 2010). We calculated MSSD values for each participant in the nine indicated ROIs across the full preprocessed, detrended and percent signal transformed activation time course (discarding the first five acquisitions). MSSD allows us to index BOLD signal variability by calculating the change in signal from one acquisition to the next. In other words, MSSD indexes temporal specificity, where greater MSSD values indicate less temporal specificity and more variability (Samanez-Larkin et al., 2010). The MSSD statistic was calculated using the following equation: . BOLD represents the percent signal change averaged across the specified ROI for acquisition j. The total number of acquisitions included in the analysis, N, equates to the participant’s volumes minus the first five.
Results
Behavioral results
Intertemporal choice pretest
Reported means in the following sections are geometric means and group comparisons were conducted on log(k). Using a t-test without assuming equal variance, we replicated earlier results (Green et al., 1994; Read and Read, 2004; Whelan and McHugh, 2009; Halfmann et al., 2013), showing that older adults (n = 36, μk = 0.003, SE = 0.005) discount the future at a lower rate than younger adults (n = 77, μk = 0.018, SE = 0.009), t(54.3) = 4.58, P < 0.001 (but see Read and Read, 2004 for a differing view). Results are shown in Supplementary Figure S1. This result held when using a nonparametric Mann–Whitney U test (P < 0.001) and when using area under the curve as the dependent measure (P < 0.001).
Intertemporal choice results acquired during fMRI
For this study, the mean discount rate was k = 0.006 (SE = 0.0075), with a minimum discount rate of k = 0.00095 and a maximum discount rate of k = 0.1751, demonstrating a large range of discount rates. The mean percent sooner choices was 62.6% (SE = 3.58%). On average, the hyperbolic discount function predicted 87% (SE = 1.41%) of the choices. IGT-advantageous and -disadvantageous older adults did not differ in discount rates, t27 = −0.24, P = 0.81, d = 0.09 (Figure 1B), percent sooner choices, t27 = −0.51, P = 0.61, d = 0.19 or model fit, t27 = −0.81, P = 0.42, d = 0.29. In other words, we observed meaningful variability (i.e. behavior does not appear to be at ceiling or floor) in discount rates and the individualized parametric regressors do not differ between groups, which makes it easier to interpret neural differences in SV signals.
Participants responded, on average, in 2097.4 ms (SE = 99.09 ms). As expected, the groups did not differ in their reaction time, t27 = 0.60, P = 0.55, d = 0.22. Reaction time did not correlate with age, education or discount rate (P > 0.16), which makes interpreting neural differences easier as the groups are making their selections within a similar time frame.
IGT and the neural representation of value
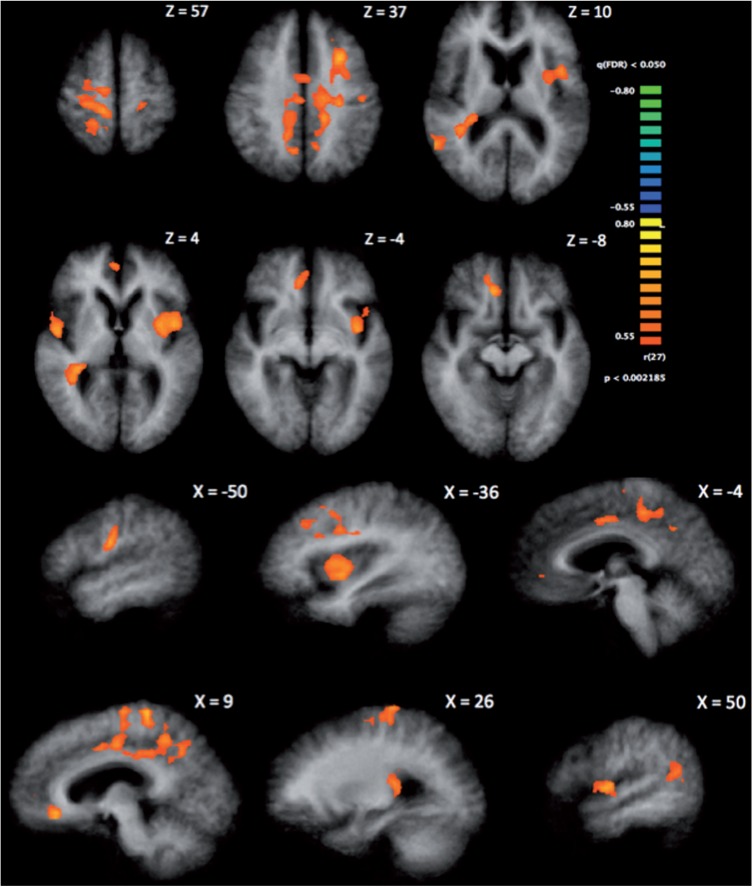
Higher scores on the IGT correlated with greater SV-associated activation in the right superior temporal gyrus, caudate, posterior cingulate and ventral anterior cingulate cortex (ACC)/VMPFC and in the left precentral gyrus and insula (α = 0.05, 2-tailed, corrected, minimum cluster size = 961 mm3; Table 2 and Figure 2). The posterior cingulate, ventral ACC/VMPFC and insula comprise a standard valuation network, indicating that better performance on the IGT relates to a greater BOLD signal change related to SV in the canonical value network. These results, coupled with prior research in younger adults (Kable and Glimcher, 2007), suggest that IGT-disadvantageous older adults have reduced representation of SV in the standard network. The results did not change when we controlled for age and intelligence quotient. Neither age nor IQ correlated with SV-associated activation in any regions, with the threshold set to the FDR, q = 0.05, or when set to a more liberal threshold of P < 0.006 (uncorrected, 2-tailed).
Table 2.
Results from whole-brain analysis, indicating the regions in which IGT scores correlated SV-associated activity
| Region | BA | Peak x | Peak y | Peak z | mm3 | Mean x | Mean y | Mean z |
|---|---|---|---|---|---|---|---|---|
| STG | 22 | 56 | −50 | 9 | 1337 | 51 | −50 | 14 |
| STG | 22 | 50 | −2 | 3 | 1099 | 49 | 1 | 4 |
| Caudate | — | 32 | −32 | 5 | 2973 | 33 | −34 | 10 |
| MFG/PCC | 6/31 | 11 | −32 | 55 | 24 550 | −4 | −18 | 42 |
| vACC/VMPFC | 32 | 8 | 34 | −6 | 1334 | 7 | 40 | −3 |
| PCG | 4 | −13 | −26 | 67 | 1374 | −17 | −27 | 65 |
| PCG/insula | 44/13 | −43 | 4 | 6 | 4545 | −38 | 3 | 3 |
IGT positively correlated with SV-associated activation in each of the indicated regions. We used a cluster thresholding procedure to correct for multiple comparisons. One thousand iterations of Monte Carlo simulations were performed with an input threshold of q(FDR) = 0.05 (corresponding to P = 0.002, 2-tailed) and an estimated spatial smoothness of 2.829 mm. This resulted in a minimum cluster size of 961 mm3, corrected for a cluster-level false positive rate of α = 0.05. Coordinates are peak and mean Talairach coordinates. Results reported here correspond with Figure 2. BA, Brodmann area; STG, superior temporal gyrus; MFG, middle frontal gyrus; PCC, posterior cingulate cortex; vACC, ventral anterior cingulate cortex; PCG, precentral gyrus.
Fig. 2.
Correlation between IGT score and SV-associated activity at the corrected threshold of α = 0.05 (minimum cluster size = 961 mm3). Higher scores on the IGT correlate with greater SV-associated activity in the VMPFC/ventral anterior cingulate, caudate, posterior cingulate, insula, precentral gyrus and superior temporal gyrus. Results indicated here correspond with Table 2.
We found similar results when we examined each of the nine ROIs (Table 3 and Figure 3). First, we tested whether IGT correlated with SV-associated activity in a combined ROI that included each of the defined nine ROIs. IGT positively correlated with SV-associated activity in this combined ROI, r29 = 0.56, P = 0.002 (Table 3 for individual ROI correlations). In fact, we found SV significantly correlated with activity in eight out of the nine individual ROIs (FDR(q) = 0.05, d > 0.70; Table 3, Figure 4) when we examined the IGT-advantageous older adults alone. In contrast, a parallel analysis with the IGT-disadvantageous older adults revealed no significant correlations between activation and SV in the nine ROIs and a trend toward a negative correlation between activation and SV in the VMPFC, t14 = −2.1, P = 0.05, d = 0.55. Thus, the IGT-advantageous older adults seem to represent value in a similar way as healthy younger adults, whereas the IGT-disadvantageous older adults seem to be dissimilar to younger adults.
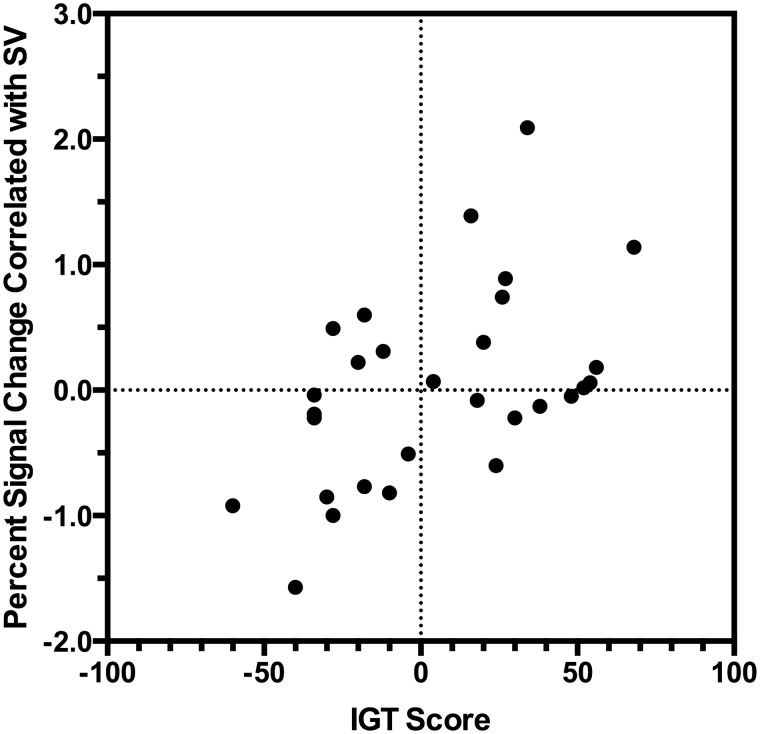
Fig. 4.
Relationship between IGT score and the percent signal change correlated with SV in the VMPFC. Higher scores on the IGT are correlated with a stronger SV-associated activity, r = 0.50, P (uncorrected) = 0.005, R2 = 0.25.
Demographics and cognitive factors did not show a significant relationship with SV-associated activation in any of the ROIs (r29 < |0.23|, P (uncorrected) > 0.10; Supplementary Table S3), with the exception of a correlation between education and SV-correlated activation in the right insula (r29 = −0.38, P (uncorrected) = 0.04). Model fit (R2), percent of choices predicted by the model, and noise (slope of the softmax) did not show significant correlations with SV representation in any of the ROIs (r < 0.33, P (uncorrected) > 0.08). These null findings, using a more liberal uncorrected threshold, are important as they suggest our results are specifically driven by emotional and cognitive capacities captured by the IGT and not demographic, cognitive or measurement-related confounds.
Using a whole-brain analysis, IGT did not predict magnitude- or delay-associated activation in any region at the FDR (but see the Supplementary Materials for sub-threshold and Supplementary Table S3 for ROI results). We did not observe significant differences in mean activation (e.g. when no parametric modulator was included). Neural activation involved in task-related demands does not differ as a function of IGT, suggesting our results are driven by SV-associated processing, not basic elements of the task.
Intraindividual variability
We next used MSSD to estimate intraindividual variability of the BOLD signal to test the theory that disadvantageous IGT performance is correlated with noise in the canonical value network. MSSD was recently used to measure temporal variability, or intraindividual BOLD signal variability, between young and old adults (Samanez-Larkin et al., 2010). This application of MSSD analyses is based on the idea that intraindividual variability in neuromodulatory systems, such as the dopaminergic system (Guitart-Masip et al., 2015), plays an important role in age-related changes in value-based decision-making (Li et al., 2010). Indeed, greater variability has been correlated with risk seeking mistakes in older adults (Samanez-Larkin et al., 2010). Samanez-Larkin et al. (2010) hypothesized that older adults may have a reduced ability to represent value. In an exploratory analysis, we sought to further test the theory that intraindividual variability correlates with decision-making and SV representation.
We examined bivariate correlations between MSSD and IGT, age, discount rate (log of) and SV representation in the VMPFC, Striatum and ACC. We observed significant correlations between IGT and intraindividual variability in the left striatum, r29 = −0.41, P (uncorrected) = 0.03 and right striatum, r29 = −0.43, P (uncorrected) = 0.02 (Table 4). Greater intraindividual variability correlated with worse performance on the IGT. When we controlled for age and IQ, these correlations strengthened r26 > 0.53, P (uncorrected) < 0.004 and correlations in the VMPFC and left insula became significant (r26 = −0.42, P (uncorrected) = 0.03; r26 = −0.39, P (uncorrected) = 0.04, respectively).
Table 4.
Pearson correlations between mean squared successive difference (intraindividual variability) in nine regions of interests and 1) age, 2) IGT scores, 3) log of the discount rate (k), SV representation in the 4) VMPFC, 5) left striatum (LStri), 6) right striatum (RStri) and 7) anterior cingulate cortex (ACC)
| Region | Age | IGT | DR | VMPFCSV | LStriSV | RStriSV | ACCSV |
|---|---|---|---|---|---|---|---|
| VMPFCMSSD | 0.33+ | −0.32+ | −0.21 | −0.17 | −0.33+ | −0.05 | −0.17 |
| L striatumMSSD | 0.26 | −0.41* | −0.10 | −0.24 | −0.25 | 0.01 | −0.18 |
| R striatumMSSD | 0.27 | −0.43* | −0.19 | −0.40* | −0.34+ | 0.01 | −0.16 |
| L ant insulaMSSD | 0.22 | −0.30 | −0.20 | −0.28 | −0.24 | 0.07 | −0.03 |
| R ant insulaMSSD | 0.25 | −0.34+ | −0.02 | −0.19 | −0.16 | 0.08 | −0.22 |
| PCCMSSD | 0.27 | −0.31+ | 0.15 | −0.00 | 0.70 | 0.05 | −0.20 |
| ACCMSSD | 0.18 | −0.32+ | −0.04 | −0.25 | −0.14 | 0.04 | −0.31+ |
| BrainstemMSSD | −0.06 | −0.19 | −0.12 | −0.18 | −0.03 | 0.04 | −0.25 |
| Pre-SMAMSSD | 0.13 | −0.25 | −0.03 | −0.15 | −0.08 | 0.00 | −0.28 |
*P (uncorrected) < 0.05, +P (uncorrected) < 0.10.
Also, we found that intraindividual variability in the right striatum correlated with reduced SV representation in the VMPFC (r = −0.40, P < 0.05, uncorrected). This is consistent with previous aging work showing that BOLD variability influences value representation (e.g. Samanez-Larkin et al., 2010).
Discussion
We asked how heterogeneity in decision capacity contributes to functional neural correlates of value representation among healthy older adults. Previous work suggests that the IGT, a complex decision task that incorporates emotion and cognitive skills, predicts poor functioning on ecological decision tasks (Denburg et al., 2007; Shivapour et al., 2012). Here, we report that poor IGT performance also relates to reduced or absent neural correlates of SV. We have four main findings: (i) better performance on the IGT correlates with stronger SV-associated activation in the canonical subjective valuation network, including the VMPFC and striatum; (ii) individual differences in age, education, intellect and numeracy do not correlate with differences in the neural SV representation; (iii) better performance on the IGT did not significantly correlate with objective magnitude-, delay to reward- or task-associated activation and (iv) poorer performance on the IGT corresponds with increased variability in the striatum and, to a lesser extent, the VMPFC, during decision-making. Taken together, our results suggest that a reduced representation of value in the brain, possibly driven in part by increased neural noise, relates to suboptimal decision-making in a subset of older adults.
In our pretest, we replicated earlier research showing that younger adults discount at a steeper rate than older adults (Green et al., 1994; Loeckenhoff et al., 2011). Previous work has compared younger and older adults’ activation during intertemporal choice tasks and found reduced ventral striatal activation in response to immediate rewards among older adults (Eppinger et al., 2012). Another study found that striatal activation was relatively insensitive to delay in older compared with younger adults (Samanez-Larkin et al., 2011a). This work suggests that age is associated with changes in striatal activation patterns during intertemporal choice. We expand on this work by showing that individual differences in complex decision-making among older adults relate to differing SV representation, in brain regions such as the striatum and also in the VMPFC, during intertemporal choice. Importantly, we observed a large range of discount rates among the older adults, indicating that discounting was not approaching ceiling and we did indeed detect individual differences across the older adult sample. By focusing only on older adults, we were able to concurrently examine how these individual differences relate to neural differences and avoid common problems in aging research such as cohort effects and age-based differences in brain volume and hemodynamics (Samanez-Larkin and D'Esposito, 2008).
Given the reduced value signals and increased temporal variability we observed in the IGT-disadvantageous group, we might have expected this group to also exhibit noisier and less consistent choice patterns. We did not observe such differences. A likely explanation for this discrepancy is that the neural measures in this study (SV signal, MSSD) are simply more sensitive than the corresponding behavioral measures (R2, percent predicted, noise, discount rate). This may especially be the case as all participants faced identical choices (in order to facilitate individual difference analyses), and, therefore, many of the choices were not near indifference. A behavioral study that tailored choices to individual subjects such that most choices were near indifference might detect a reduction in choice consistency in the IGT-disadvantageous group that was not detected in this study.
The intertemporal choice task allowed us to determine trial-by-trial SV for each individual, as well as assess objective choice attributes (i.e. magnitude and delay), such that we could disentangle the contribution of each attribute to the BOLD signal. We show that IGT predicts greater differences in SV-associated activation than task-related activation or activation associated with magnitude and delay. These findings suggest that older adults’ heterogeneous decision-making patterns result specifically from differences in the ability to combine information about multiple attributes to form a value representation. This notion is consistent with the affective–integration–motivation framework, which proposes that affective, integration and motivational processes proceed sequentially to promote choice (Samanez-Larkin and Knutson, 2015). Specifically, the authors suggest that healthy aging may, in some cases, compromise value integration and optimal choices. Intertemporal choices require value integration; however, the task is simple enough that it may not reveal behavioral differences, consistent with previous work showing no differences between young and old on simple tasks, but individual differences in neural activation among those older adults able to match young adult performance on a complex task (Lighthall et al., 2014).
Thus, to compare individual differences in behavior, we turned to a more complex task: the IGT. Indeed, the value network represents SV using a common currency, on the same scale regardless of reward type or the attributes of the outcome. Decisions that require representing the common value of alternatives that differ on a number of attributes, such as in the IGT (e.g. ambiguity, risk, gains, losses), or intertemporal choice (e.g. magnitude of reward, delay to reward) require this kind of common currency to make comparisons. The IGT may be more sensitive to picking up behavioral effects of a reduced or absent neural value signal compared with the temporal discounting task. For example, the IGT may have more decisions where options are closer in value and require integrating more attributes, stressing the value network to a greater extent. We suggest reduced SV representation affects IGT performance, which requires ongoing value computation, and that this reduced representation can be observed during tasks that require the value network.
Several possible explanations for the absence of the SV signal and the increased variability in the valuation network stand out. First, anatomical differences may exist between the groups. For example, striatal volume declines with age (Walhovd et al., 2011), along with global thinning of gray matter in the PFC, and specifically in the VMPFC (Raz et al., 1997). Moreover, dopamine transporter availability (van Dyck et al., 2002), receptor density (Volkow et al., 2000) and overall concentration (Eppinger et al., 2011) deplete with age, and declines in dopamine or serotonin are theorized to increase neural noise (Mohr and Nagel, 2010). It could be that some older adults, in this case the IGT-disadvantageous, are undergoing speedier structural and neurochemical decline. Put one way, the common neural currency (Levy and Glimcher, 2012) used to compute value is running out.
Second, the groups may have functional differences. In other words, the two groups may have a similar anatomy, but the IGT-disadvantageous older adults less effectively use or recruit the appropriate systems. For example, changes in neurovascular coupling could cause neural dedifferentiation. Dedifferentiation mirrors a broader tuning curve in the neural signal (Park and Reuter-Lorenz, 2009). This physiological deficit then breeds a failure to selectively and effectively recruit task-relevant regions, possibly by reducing neural specificity (Li et al., 2010). In this case, the IGT-disadvantageous older adults were not effectively and/or selectively recruiting the value network during an intertemporal choice task.
Functional deficits such as dedifferentiation also raise the possibility that, as suggested in previous work (Denburg et al., 2006), IGT-disadvantageous older adults have diminished ‘somatic markers’. Somatic markers indicate value based on previously learned information. Decision attributes, such as ambiguity, risk and reward magnitude and their associated outcomes contribute to the development of somatic markers. For example, high levels of risk may be associated with a negative somatic/affective signal, leading an individual to avoid that option. It seems plausible that reduced functioning in the VMPFC and related structures might lead to diminished somatic markers and therefore poor affect-based decision-making. This is consistent with patient work showing that VMPFC damage leads to impairments on the IGT (Bechara et al., 1994) and the notion of SV signals in the VMPFC as affect or emotion signals (Phelps et al., 2014). Unlike previous work that tested whether good or bad performance on the IGT related to differences in BOLD signal during the IGT (Halfmann et al., 2013, 2014), we were able to show here that the group differences were related to SV signals in the VMPFC. Since the intertemporal choice task used here does not involve learning, we were able to more precisely measure SV signals and distinctive components of each choice (e.g. magnitude) that may contribute to neural differences.
An important goal of neurocognitive aging research entails identifying individuals most at-risk for cognitive decline or real-world problems and understanding what triggers that risk. IGT predicts individual differences in real-world decision-making, and, here, we have identified a potential neural mechanism for these differences: reduced neural representation of SV. A reduced representation of SV could translate to poor decision-making in many aspects of life, including finance, health and long-term care. For example, choosing how to invest and spend money or what treatment plan meets one’s needs requires integrating the attributes of each alternative and comparing them on a common scale to make a sound decision. Future work should explore the relationship between IGT and other complex laboratory measures of decision-making and how they relate to real-world financial performance.
Future work should investigate lifestyle factors that might contribute to both neurobiological and behavioral deficits related to decision-making among older adults. By establishing a link between suboptimal decision-making and the neural instantiation of SV, we offer a step toward mitigating age-related decision-making impairments. Future work should investigate whether interventions could enhance SV signals in the VMPFC and striatum among at-risk older adults. For example, recent work has demonstrated that attention can modulate SV (Schonberg et al., 2014). Therefore, it is plausible that interventions could help mitigate SV abnormalities among at-risk older adults.
Supplementary data
Supplementary data are available at SCAN online.
Acknowledgements
We thank Andrew Jones and Thomas Goetz for assistance with data preprocessing.
Funding
Preparation for the article was supported by a Dana Foundation Program in Brain and Immuno Imaging Grant to N.L.D.
Conflict of interest. None declared.
References
- Agarwal S., Driscoll J.C., Gabaix X., Laibson D. (2009). The age of reason: financial decisions over the life-cycle with implications for regulation. Brookings Papers on Economic Activity , 2, 51–117 [Google Scholar]
- Bartra O., McGuire J.T., Kable J.W. (2013). The valuation system: a coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. NeuroImage , 76, 412–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A., Damasio A.R., Damasio H., Anderson S. (1994). Insensitivity to future consequences following damage to human prefrontal cortex. Cognition , 50, 7–15. [DOI] [PubMed] [Google Scholar]
- Clithero J.A., Rangel R. (2013). Informatic parcellation of the network involved in the computation of subjective value. Social, Cognitive, and Affective Neuroscience , 9, 1289–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denburg N.L., Cole C.A., Hernandex M., et al. (2007). The orbitofrontal cortex, real-world decision-making, and normal aging. Annals of the New York Acadamy of Sciences , 1121, 480–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denburg N.L., Recknor E.C., Bechara A., Tranel D. (2006). Psychophysiological anticipation of positive outcomes promotes advantageous decision-making in normal older persons. International Journal of Psychophysiology , 61, 19–25. [DOI] [PubMed] [Google Scholar]
- Denburg N.L., Tranel D., Bechara A. (2005). The ability to decide advantageously declines prematurely in some normal older persons. Neuropsychologia , 43, 1099–106. [DOI] [PubMed] [Google Scholar]
- Eppinger B., Hämmerer D., Li S.-C. (2011). Neuromodulation of reward-based learning and decision making in human aging. Annals of the New York Acadamy of Sciences , 1235, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppinger B., Kray J. (2010). To choose or to avoid: age differences in learning from positive and negative feedback. Journal of Cognitive Neuroscience , 23, 41–52. [DOI] [PubMed] [Google Scholar]
- Eppinger B., Nystrom L.E., Cohen J.D. (2012). Reduced sensitivity to immediate reward during decision-making in older than younger adults. PLoS One , 7, e36953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppinger B., Schuck N.W., Nystrom L.E., Cohen J.D. (2013). Reduced striatal responses to reward prediction errors in older compared with younger adults. The Journal of Neuroscience , 33,9905–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M., Bolla K., Mouratidis M., et al. (2002). Decision making in a risk-taking task: a PET study. Neuropsychopharmacology , 26,682–91. [DOI] [PubMed] [Google Scholar]
- Finucane M.L., Gullion C.M. (2010). Developing a tool for measuring the decision-making competence of older adults. Psychology and Aging , 25, 271–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabenhorst F., Rolls E.T. (2011). Value, pleasure and choice in the ventral prefrontal cortex. Trends in Cognitive Sciences , 15, 56–67. [DOI] [PubMed] [Google Scholar]
- Green L., Fry A.F., Myerson J. (1994). Discounting of delayed rewards: a life-span comparison. Psychological Science , 5, 33–6. [Google Scholar]
- Guitart-Masip M., Salami A., Garrett D., Rieckmann A., Lindenberger U., Backman L. (2015). BOLD variability is related to dopaminergic neurotransmission and cognitive aging. Cerebral Cortex, doi: 10.1093/cercor/bhv029. [DOI] [PubMed] [Google Scholar]
- Halfmann K., Hedgcock W., Bechara A., Denburg N.L. (2014). Functional neuroimaging of the Iowa Gambling Task in older adults. Neuropsychology , 6, 870–80. [DOI] [PubMed] [Google Scholar]
- Halfmann K., Hedgcock W., Denburg N.L. (2013). Age-related differences in discounting future gains and losses. Journal of Neuroscience, Psychology, and Economics , 6, 42–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable J.W., Glimcher P.W. (2007). The neural correlates of subjective value during intertemporal choice. Nature Neuroscience , 10, 1625–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D.J., Glimcher P.W. (2012). The root of all value: a neural common currency for choice. Current Opinion in Neurobiology , 22, 1027–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak M.D., Howieson D.B., Bigler E.D., Tranel D. (2012). Neuropsychological Assessment, 5th edn New York: Oxford University Press. [Google Scholar]
- Li S.C., Gao J., Enkavi A.Z., Zaval L., Weber E.U., Johnson E.J. (2015). Sound credit scores and financial decisions despite cognitive aging. Proceedings of the National Academy of Sciences of the United States of America , 112, 65–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S.-C., Lindenberger U., Backman L. (2010). Dopaminergic modulation of cognition across the life span. Neuroscience and Biobehavioral Reviews , 34, 625–30. [DOI] [PubMed] [Google Scholar]
- Li X., Lu Z.-L., D'Argembeau A., Ng M., Bechara A. (2010). The Iowa Gambling Task in fMRI images. Human Brain Mapping , 31, 410–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lighthall N.R., Huettel S.A., Cabeza R. (2014). Functional compensation in the ventromedial prefrontal cortex improves memory-dependent decisions in older adults. The Journal of Neuroscience , 34, 15648–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeckenhoff C.E., O'Donoghue T., Dunning D. (2011). Age differences in temporal discounting: the role of dispositional affect and anticipated emotions. Psychology and Aging , 26, 274–84. [DOI] [PubMed] [Google Scholar]
- Mohr P.N.C., Li S.-C., Heekeren H.R. (2010). Neuroeconomics and aging: neuromodulation of economic decision making in old age. Neuroscience and Biobehavioral Reviews , 34, 678–88. [DOI] [PubMed] [Google Scholar]
- Mohr P.N.C., Nagel I.E. (2010). Variability in brain activity as an individual difference measure in neuroscience? The Journal of Neuroscience , 30, 7755–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D.C., Reuter-Lorenz P. (2009). The adaptive brain: aging and neurocognitive scaffolding. The Annual Review of Psychology , 60, 173–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J., Buechel C. (2011). The neural mechanisms of inter-temporal decision-making: understanding variability. Trends in Cognitive Sciences , 15, 227–39. [DOI] [PubMed] [Google Scholar]
- Phelps E.A., Lempert K.M., Sokol-Hessner P. (2014). Emotion and decision making: multiple modulatory neural circuits. The Annual Review of Neuroscience , 37, 263–87. [DOI] [PubMed] [Google Scholar]
- Raz N., Guning F.M., Head D., et al. (1997). Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cerebral Cortex , 7, 268–82. [DOI] [PubMed] [Google Scholar]
- Read D., Read N.L. (2004). Time discounting over the lifespan. Organizational Behavior and Human Decision Processes , 94, 22–32. [Google Scholar]
- Samanez-Larkin G.R., D'Esposito M. (2008). Group comparisons: imaging the aging brain. Social, Cognitive, and Affective Neuroscience , 3, 290–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanez-Larkin G.R., Gibbs S.E.B., Khanna K., Nielsen L., Carstensen L.L., Knutson B. (2007). Anticipation of monetary gain but not loss in healthy older adults. Nature Neuroscience , 10, 787–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanez-Larkin G.R., Knutson B. (2015). Decision making in the ageing brain: changes in affective and motivational circuits. Nature Reviews Neuroscience, doi:10.1038/nrn3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanez-Larkin G.R., Kuhnen C.M., Yoo D.J., Knutson B. (2010). Variability in nucleus accumbens activity mediates age-related suboptimal financial risk taking. The Journal of Neuroscience , 30, 1426–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanez-Larkin G.R., Levens S.M., Perry L.M., Dougherty R.F., Knutson B. (2012). Frontostriatal white matter integrity mediates adult age differences in probabilistic reward learning. The Journal of Neuroscience , 32, 5333–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanez-Larkin G.R., Mata R., Radu P.T., Ballard I.C., Carstensen L.L., McClure S.M. (2011a). Age differences in striatal delay sensitivity during intertemporal choice in healthy adults. Frontiers in Neuroscience , 5, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanez-Larkin G.R., Wagner A.D., Knutson B. (2011b). Expected value information improves financial risk taking across the adult lifespan. Social, Cognitive, and Affective Neuroscience , 6, 207–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonberg T., Bakkour A., Hover A.M., et al. (2014). Changing value through cued approach: an automatic mechanism of behavior change. Nature Neuroscience , 17, 625–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senecal N., Wang T., Thompson E., Kable J.W. (2012). Normative arguments from experts and peers reduce delay discounting. Judgment and Decision Making , 7, 566–89. [PMC free article] [PubMed] [Google Scholar]
- Shivapour S.K., Nguyen C.M., Cole C.A., Denburg N.L. (2012). Effects of age, sex, and neuropsychological performance on financial decision-making. Frontiers in Neuroscience , 6, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohamy D., Wimmer G.E. (2013). Dopamine and the cost of aging. Nature Neuroscience , 16, 519–21. [DOI] [PubMed] [Google Scholar]
- Simpson C.A., Vuchnich R.E. (2000). Reliability of a measure of temporal discounting. The Psychological Record , 50, 3–16. [Google Scholar]
- Smith D.V., Huettel S.A. (2010). Decision neuroscience: neuroeconomics. WIREs Cognitive Science , 1, 854–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J., Tournoux P. (1988). Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical Publishers, Inc. [Google Scholar]
- Tymula A., Belmaker L.A.R., Ruderman L., Glimcher P.W., Levy I. (2013). Like cognitive function, decision making across the lifespand shows profound age-related changes. Proceedings of the National Academy of Sciences of the United States of America , 110, 17143–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dyck C.H., Seibyl J.P., Malison R.T., et al. (2002). Age-related decline in dopamine transporter: analysis of striatal subregions, nonlinear effects and hemispheric asymmetries. The American Journal of Geriatric Psychiatry , 10, 36–43. [PubMed] [Google Scholar]
- Volkow N.D., Logan J., Fowler J.S., et al. (2000). Association between age-related decline in brain dopamine activity and impairment in frontal and cingulate metabolism. The American Journal of Psychiatry , 157, 75–80. [DOI] [PubMed] [Google Scholar]
- Walhovd K.B., Westlye L.T., Amlien I., et al. (2011). Consistent neuroanatomical age-related volume differences across multiple samples. Neurobiology of Aging , 32, 916–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters-Wood S.M., Xiao L., Denburg N.L., Hernandex M., Bechara A. (2012). Failure to learn from repeated mistakes: persistent decision-making impairments as measured by the Iowa Gambling Task in patients with ventromedial prefrontal cortex lesions. Journal of the International Neuropsychological Society , 18, 927–30. [DOI] [PubMed] [Google Scholar]
- Whelan R., McHugh L.A. (2009). Temporal discounting of hypothetical monetary rewards by adolescents, adults, and older adults. Psychological Record , 59, 247–58. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.