Abstract
With congruent stimulation of one’s limb together with a fake counterpart, an illusory self-attribution of the fake limb can be induced. Such illusions have brought profound insights into the cognitive and neuronal mechanisms underlying temporary changes in body representation, but to put them in perspective, they need to be compared with ownership as experienced for one’s real body. We used functional magnetic resonance imaging (fMRI) to compare the neuronal correlates of touch under different degrees of body ownership. Participants’ left and right arms were stimulated either alone or together with a fake counterpart while this stimulation was synchronous, ambiguous or asynchronous. Synchronous stimulation induced illusory fake arm ownership, but the brain still differentiated between touch to one’s real arm and to an illusory ‘owned’ arm: the degree of arm ownership was encoded positively by activity in the ventromedial prefrontal cortex and lateral occipitotemporal cortex and negatively in the temporoparietal cortex. Conversely, the ventral premotor cortex responded more strongly to synchronous stimulation compared with asynchronous stimulation and with real arm only stimulation. These results offer new insights into the differential representation of the real body vs a body that is temporarily self-attributed following the resolution of multisensory conflict.
Keywords: body representation, ownership, rubber hand illusion, self-other distinction, touch
Introduction
The sense of having a body is one of the most basic conditions for self-awareness (Gallagher, 2000; Blanke and Metzinger, 2009; Tsakiris, 2010; Ehrsson, 2012). Although of such apparent importance, this sense of body ‘ownership’ can be easily manipulated in most individuals: synchronous touch applied to one’s hidden hand together with a visible fake counterpart is a reliable way to induce a self-attribution or feeling of ownership of the fake hand, known as the rubber hand illusion (RHI, Botvinick and Cohen, 1998). Initially, this stimulation produces a crossmodal conflict by vision of the touch occurring on the fake hand and simultaneous somatosensation on the real hand (Makin et al., 2008; Macaluso and Maravita, 2010). The RHI then results from the resolution of this conflict via integration (‘binding together’) of the simultaneously occurring seen and felt touches into one perceptual event—the touch ‘felt’ on the now self-attributed fake hand—which seems to involve a network comprising multisensory areas in the frontal, parietal and occipitotemporal cortex (Ehrsson et al., 2004; Tsakiris and Haggard, 2005; Costantini and Haggard, 2007; Tsakiris et al., 2007; Petkova et al., 2011; Blanke, 2012; Gentile et al., 2013; Limanowski and Blankenburg, 2015).
The interpretation of the RHI as a manipulation of body ownership implies that after induction, the body representation is updated so that the fake hand is represented as one’s own. However, the change in body representation induced by the RHI is only a temporary solution to an unusual crossmodal conflict, which requires ‘ignoring’ some bodily information such as proprioceptive information about one’s real hand's location (Botvinick and Cohen, 1998; Zeller et al., 2014). Correspondingly, the behavioral effects of the RHI are typically only partial, e.g. participants mislocalize their real hand merely toward the fake hand instead of at the fake hand’s location (Tsakiris and Haggard, 2005). This may suggest that the RHI is not a completely ‘convincing’ recalibration of one’s body representation. How then, if at all, does the brain’s representation of such a temporary illusory body differ from the representation of one’s lifelong owned body? Here, we addressed this question by comparing the neuronal correlates of touch to one’s real arm (RA) with touch to a fake arm (FA) that is perceived as one’s own due to an induced ownership illusion. We used an automated setup to stimulate our participants’ left and right arms inside an fMRI scanner, either alone or together with a FA of corresponding laterality. We hypothesized that experienced arm ownership would be strongest during stimulation of one’s RA. Further, we expected that synchronous, but not asynchronous, stimulation of RA and FA would induce illusory arm ownership (the RHI). We additionally introduced a mixed condition, in which touch to the RA and the FA was synchronous at one anatomical location and asynchronous at another one, speculating that this ambiguous input would be perceptually different from purely synchronous or asynchronous stimulation. In a first exploratory step, we looked for touch-related brain activity that would systematically co-vary with the degree of arm ownership, i.e. decrease or increase from stimulation of one’s RA through synchronous, ambiguous and asynchronous FA stimulation. Second, we looked for brain activity specific to multisensory integration processes thought to underlie the updating of body representation in the RHI. During the RHI, the synchronously occurring seen and felt touches are integrated, whereas during asynchronous stimulation, the seen and felt touches are clearly attributable to two different hands based on their temporal discrepancy (Botvinick and Cohen, 1998; Ehrsson, 2012; Hohwy, 2012). During touch to one’s real body, the seen and felt touch naturally correspond, and there is no need to resolve crossmodal conflicts. Therefore our second hypothesis was that brain areas involved in integrating multisensory body-related information during the RHI would show stronger responses during synchronous, compared with asynchronous FA stimulation and compared with RA stimulation alone. Finally, work in monkeys has demonstrated that the fronto-parietal brain areas implied in multisensory integration during the RHI contain neurons with body part-centered visuo-tactile receptive fields, which may help code the space around the body (Fogassi et al., 1996; Graziano, 1999; Graziano et al., 2000; Graziano and Cooke, 2006). Recent evidence from work in humans suggests that these areas may implement a general mechanism of updating one’s body representation during the RHI and similar paradigms, which generalizes across stimulation of different body parts (Tsakiris, 2008; Petkova et al., 2011; Blanke, 2012; Apps et al., 2013). Therefore we tested for consistent effects across both arm sides.
Methods
Participants
Thirteen healthy volunteers (10 females, age 23–35 years, all but one right-handed as measured with the Edinburgh Handedness Inventory, Oldfield, 1971) participated in the study. All participants signed an informed consent before and were paid after each of the scanning sessions. The study was approved by the local Ethical Committee of the Charité University Hospital Berlin and participants were treated in accordance with the Human Subject Guidelines of the Declaration of Helsinki.
Experimental design and procedure
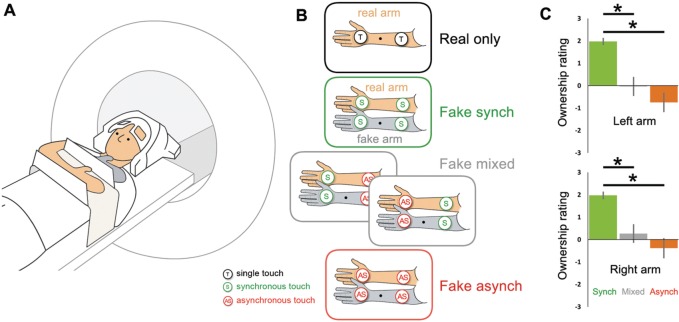
The study was conducted as a within-subject, repeated-measures design in four separate scanning sessions (Figure 1A). In two ‘RA’ sessions (conducted on the same day), participants viewed their RA being touched; in two ‘FA’ sessions (acquired at least 1 month from each other and from the RA sessions), participants viewed a FA being touched, while their RA was hidden from sight and also touched. In both RA and FA sessions, tactile stimulation occurred on the right arm in one session and on the left arm in the other session (using a mirrored version of the setup). In the FA sessions, a transparent console was set up atop the participants’ chest, with a realistic right or left FA attached to it (palm facing). The participants’ RA was placed 13 cm behind the FA in a congruent posture and was completely hidden from view, i.e. participants had direct vision on the FA with the corresponding brushes, but not of their RA. In the RA sessions, the participants' RA was mounted onto a similar console in a posture corresponding to that of the RA in the FA sessions; here, participants only saw their RA with corresponding brushes. Participants were instructed to fixate a black dot attached to the middle of the FA (FA sessions) or RA (RA sessions).
Fig. 1.
Experimental setup and stimulation conditions (right arm sessions; a mirrored version of this setup was used for the left arm). A: Participants lay inside the scanner with direct vision of their real arm (RA) with the corresponding brushes (‘RA’ sessions), or with direct vision of an equilateral fake arm (FA) with corresponding brushes, while their RA was hidden from view behind the FA (‘FA’ sessions). B: Brush strokes were automatically delivered at two stimulation locations (palm, forearm) in various combinations that allowed us to implement gradual differences in limb ownership, operationalized in terms of congruence of visuo-tactile information. In the Fake arm sessions, visuo-tactile stimulation of the corresponding locations of the RA and FA could be either synchronous (‘Fake synch’; RHI condition), mixed with respect to synchrony (‘Fake mixed’; palm stimulated synchronously and forearm stimulated asynchronously, or vice versa) or asynchronous (‘Fake asynch’; RHI control condition). C: Mean ratings of experienced ownership of the left and right FA during different types of visuo-tactile congruence. For both arm sides, synchronous stimulation produced greater illusory FA ownership than mixed or asynchronous stimulation. Asterisks denote significance at P < 0.0056 as obtained from Wilcoxon’s signed-rank test, applying Bonferroni adjusted significance levels, error bars are SEM.
Stimulation was delivered at two possible locations (palm and/or forearm) of the FA and/or the RA with sponge brushes moving back and forth at ∼1.3 Hz with random inter-stroking interval (0 or 250 ms) to intensify the illusion (Armel and Ramachandran, 2003). The brushes were driven by separate stepping motors controlled by a MATLAB (The Mathworks, Inc., Natick) script via parallel port output, which also received the scanner triggers to start and stop stimulation. The motors’ torque was transmitted into the scanner room via non-magnetic cables and gears. We used the same motors, stroking parameters and brushes for the stimulation in the FA and RA sessions, and ensured comparable positions of the arms and brushes and somatotopical representation of the stimulation locations (see Supplementary material). In the FA sessions, stimulation of the corresponding locations on the RA and the FA could occur synchronously (RHI condition), asynchronously (control condition) or in a mixed condition (one location stimulated synchronously, the other one asynchronously). Immediately after the scanning session, participants remained inside the scanner to rate the degree of experienced arm ownership in each condition (briefly presented again) by answering the question ‘During this stimulation, it felt as if the FA was my own arm’ (cf. Botvinick and Cohen, 1998) on a 7-point Likert-scale ranging from −3 (‘completely disagree’) to +3 (‘completely agree’). Subsequently, participants verbally indicated the onset of the illusion (any feeling of ownership of the FA as soon as first experienced, Ehrsson et al., 2004), which was measured by the experimenter with a stopwatch. Participants received stimulation in trials of 20.16 s duration and 12.6 s rest (10 trials total per condition) during five runs (two trials per run and condition) in each FA session (plus one visuo-tactile localizer run, see Supplementary material), and two runs (five trials per run and condition) in each RA session; the order of arms was randomized across participants and the order of stimulations was randomized for each run.
fMRI data acquisition, preprocessing and analysis
T2*-weighted functional images were acquired using a 3 Tesla Tim Trio scanner (Siemens, Germany, 32-channel head coil, high-resolution 3D-EPI sequence, Lutti et al., 2012). Parallel imaging (GRAPPA) was used along the phase and partition directions (acceleration factor 2), yielding a functional image resolution of 2.0 × 2.0 × 2.0 mm3 at an acquisition time of 2520 ms per volume (TR = 70 ms, matrix size [96, 96, 64], TE = 33 ms, nominal flip angle = 20°, BW = 1408 Hz). A total of 3596 functional volumes were recorded for each participant, plus GRE field maps (TE1 = 10.00 ms, TE2 = 12.46 ms) and a T1-weighted structural image (3D MPRAGE, voxel size = 1 × 1 × 1 mm, FOV = 256 × 256 mm, 176 slices, TR = 1900 ms, TE = 2.52 ms, TI = 900 ms, flip angle = 9°).
fMRI data were preprocessed and analyzed using SPM8 (Wellcome Department of Cognitive Neurology, London, UK: www.fil.ion.ucl.ac.uk/spm/). Physically based artifacts in individual slices were corrected via interpolation using the ArtRepair toolbox (Mazaika et al., 2009; default settings). Functional images were realigned and unwarped, coregistered to the respective structural image, spatially normalized to MNI space using DARTEL and spatially smoothed with a 6-mm full width at half maximum Gaussian kernel. Global blood oxygen level dependent (BOLD) signal effects were removed (Macey et al., 2004), and volumes with excessive head motion were interpolated using the ArtRepair toolbox (<10% of volumes corrected). To remove physiological noise from the gray matter BOLD signal, the first five principal components accounting for the most variance in the cerebrospinal fluid or white matter signal timecourse were added to the first-level general linear models (GLMs) as regressors of no interest (Behzadi et al., 2007).
On the first level, we fitted a GLM to each participant (microtime onset set to the middle slice, 300-s high-pass filter, the individual runs were concatenated into one run per arm and session). Each stimulation condition was modeled as a regressor (boxcar) and convoluted with the standard hemodynamic response function of SPM8. On the second level, the contrast images of each regressor vs baseline were entered into flexible factorial within-subject GLMs including a between-subjects factor modeling the subject constants.
For the first analysis, investigating linear effects of arm ownership, we calculated four Ownership regressors (Real only, Fake synch, Fake mixed, Fake asynch) per arm side on the first level. These regressors were modeling only those stimulations in which both locations (palm and forearm) were stimulated together, since the mixed condition could not be implemented by stimulating only one of the two locations. On the second level, to identify brain areas whose activity was parametrically modulated by the degree of experienced ownership of the touched arm, we calculated a balanced linear contrast with the contrast weights +1.5 (Real only), +0.5 (Fake synch), −0.5 (Fake mixed) and −1.5 (Fake asynch) for each arm side (or the respective negative weights for the reverse contrast). Moreover, to assess the effects of between-participant differences in experienced ownership, we calculated a parametric contrast on the first level, in which the contrast weight of each condition regressor was determined by the respective participant’s reported ownership ratings (values for Real only set to +3, the highest value of the rating scale, per default). These individually weighted contrast images were evaluated on the second level using a two-sample t-test.
For the second analysis, comparing the RHI condition with asynchronous and RA stimulation, we created separate first level GLMs, in which we randomly split each Fake synch condition into two separate regressors (Fake syncha, Fake synchb) to render the conjunction analyses across the Fake synch vs Real only and Fake synch vs Fake asynch contrasts independent. As this comparison did not involve the mixed condition, here we used regressors pooled across stimulation at the palm, forearm and both locations together in each condition. In addition, we compared the effects in the phase before and after the onset of the RHI by dividing each regressor into a pre-illusion onset and a post-illusion onset regressor on the first level, defined by each participant’s verbal onset report (Ehrsson et al., 2004). The second level GLMs used for this analysis thus comprised the regressors Real only, Fake syncha, Fake synchb and Fake asynch for each arm side, where these regressors modeled the entire stimulation period, or only the pre-illusion onset or post-illusion onset period, respectively.
Statistic images were assessed for significant activations in the whole brain using an initial voxel-wise threshold of P < 0.001, and a threshold of P < 0.05, family-wise error (FWE) corrected for false positives on the cluster level. For brain areas that we hypothesized to be involved in the RHI based on previous literature, we used small volume correction within 10 mm radius spheres centered upon the peak coordinates reported in Petkova et al. (2011). Since we were interested in effects that generalize across body parts (see Introduction), we performed global conjunction analyses (a test for voxels that show consistent effects across multiple contrasts, Friston et al., 2005) on the two respective contrasts of interest for each arm. Reported coordinates are in MNI space, neuroanatomical labels are derived from the SPM Anatomy toolbox (Eickhoff et al., 2005). Statistical parametric maps are projected onto the mean normalized structural image at P < 0.001, uncorrected, with a cluster extent threshold of 5 voxels.
Results
Behavioral effects of visuo-tactile congruence
To verify that the RHI depended on visuo-tactile congruence, we analyzed the obtained ownership ratings, using the non-parametric Wilcoxon’s signed-rank test since they did not pass the Jarque-Bera test for normality, and applying Bonferroni adjusted significance levels of 0.05/9 = 0.0056 per test. For both arms, the synchronous condition produced significantly higher ownership ratings than the mixed and the asynchronous conditions (all Ps < 0.001, see Figure 1B). Mean ownership ratings showed a trend for being higher for the mixed than for the asynchronous conditions (left arm, P = 0.014; right arm, P = 0.016). No differences between arm sides were significant (all Ps > 0.45). The reported illusion onsets indicate an induction of the RHI early throughout stimulation (left arm: mean = 6.05 s, s.d. = 3.13; right arm: mean = 4.80 s, s.d. = 2.41; no significant differences between arm sides, two-tailed paired t-test, P = 0.14). A control experiment on a small independent sample similarly revealed significantly stronger mislocalization of the real hand following synchronous vs mixed or asynchronous stimulation and trends for mixed vs asynchronous stimulation (see Supplementary material).
Brain activity co-varying with subjective ownership of the touched arm
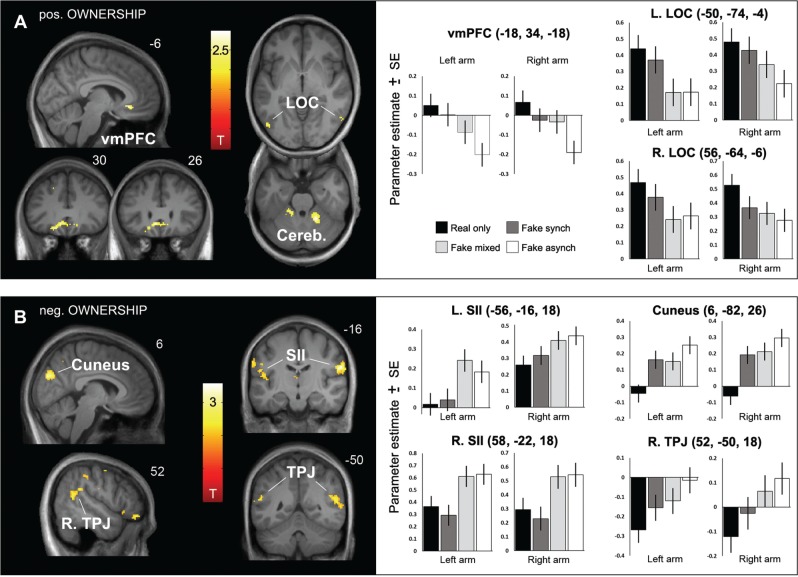
The conjunction across the linear ownership contrast (Real only > Fake synch > Fake mixed > Fake asynch) for the left and right arm revealed significant (P < 0.05, corrected) activity in the ventromedial prefrontal cortex (vmPFC), comprising bilateral rectal and mid orbital gyri and parts of the subgenual anterior cingulate cortex (sgACC), in the left (and a statistical trend for the right) lateral occipitotemporal cortex (LOC) and in the bilateral cerebellum and right parahippocampal gyrus (Figure 2A and Table 1). By weighting the subject-specific regressors with the individual ownership ratings, we found that between-participant differences related to arm ownership were also significantly (P < 0.05, corrected) reflected by activity in the vmPFC (x = −14, y = 36, z = −16, T = 4.86) and the bilateral LOC (L: x = −38, y = −66, z = 4, T = 3.25; R: x = 56, y = −64, z = 2, T = 4.03; P < 0.05, small volume corrected), and by a trend in the right cerebellum (x = 18, y = −48, z = −30, T = 3.64, P = 0.097, corrected). This analysis thus corroborated the results obtained using the linear ownership contrast, showing that the vmPFC, LOC and also the cerebellum responded more strongly, the higher the experienced ownership of the touched limb. Interestingly, the bilateral LOC activations obtained from both comparisons also fell within the body-selective extrastriate body area (EBA; defined by activations obtained from a functional localizer using the same scanner and pulse sequence in Limanowski and Blankenburg, 2015).
Fig. 2.
Activity differences related to the degree of arm ownership. A: Statistical parametric maps showing significant (P < 0.05, corrected) activity differences related positively to the degree of arm ownership in the vmPFC (comprising bilateral mOFC and sgACC), in the bilateral LOC (left, P < 0.05/right, P = 0.076, small volume corrected), and in the cerebellum (left, P = 0.05/right, P < 0.05), obtained from the conjunction across the positive linear ownership contrasts (Real only > Fake synch > Fake mixed > Fake asynch) for the left and right arm. See Table 1 for details. SPMs are thresholded at P < 0.001, bar plots depict the parameter estimates at the given coordinates for each arm side and condition with associated standard errors. B: Activity differences related inversely to the degree of arm ownership. SPMs show significant (P < 0.05, corrected) activity differences in the cuneus and in the SII (as defined by significant activations obtained from the tactile localizer runs for the respective contralateral arm side, see Supplementary material) and regions corresponding to the right TPJ (see Table 1 for details) obtained from the conjunction across the negative linear ownership contrasts (Real only < Fake synch < Fake mixed < Fake asynch) for each arm side.
Table 1.
Group-level activations obtained from the conjunction across the positive and negative linear ownership contrasts for the left and the right arm
| Peak (MNI) |
|||||||
|---|---|---|---|---|---|---|---|
| Anatomical description | Cluster size (voxels) | Peak T | Peak Z | P (corrected) | x | y | z |
| Positive ownership contrast: Real only > Fake synch > Fake mixed > Fake asynch | |||||||
| L./R. Middle orbital gyrus and | 180 | 2.98 | 4.48 | 0.005 | −18 | 32 | −18 |
| rectal gyrus (vmPFC)* | 14 | 34 | −14 | ||||
| R. Cerebellum (Lobule VI) | 155 | 2.96 | 4.46 | 0.011 | 20 | −50 | −26 |
| R. Parahippocampal gyrus | 111 | 2.74 | 4.18 | 0.048 | 30 | −32 | −16 |
| L. Cerebellum (Lobule V) | 110 | 2.69 | 4.12 | 0.050 | −20 | −40 | −24 |
| L. Inferior occipital gyrus (LOC) | 21 | 2.32 | 3.66 | 0.035a | −50 | −74 | −4 |
| R. Inferior temporal gyrus (LOC) | 7 | 1.99 | 3.23 | 0.076a | 56 | −64 | −6 |
| L. Fusiform gyrus | 12 | 2.63 | 4.05 | −40 | −80 | −14 | |
| L. Superior frontal gyrus | 7 | 2.12 | 3.39 | −20 | 32 | 42 | |
| L. Middle frontal gyrus | 6 | 2.05 | 3.31 | −26 | 14 | 50 | |
| Negative ownership contrast: Real only < Fake synch < Fake mixed < Fake asynch | |||||||
| R. Supramarginal gyrus, | 666 | 3.58 | 5.23 | <0.001 | 62 | −16 | 24 |
| parietal operculum (SII), and | 2.79 | 4.26 | 0.002b | 58 | −22 | 18 | |
| middle temporal gyrus (TPJ) | 2.62 | 4.04 | 0.006c | 52 | −50 | 18 | |
| R./L. Cuneus | 465 | 3.44 | 5.06 | <0.001 | 6 | −82 | 26 |
| L. Postcentral gyrus/parietal operculum (SII) | 54 | 2.50 | 3.88 | 0.016b | −56 | −16 | 18 |
| L. Precentral gyrus (PMd) | 117 | 2.90 | 4.30 | 0.039 | −54 | −6 | 50 |
| L. Postcentral gyrus/parietal operculum (SII) | 110 | 2.74 | 4.19 | 0.047b | −66 | −18 | 32 |
| L. Superior temporal gyrus | 58 | 2.59 | 4.00 | −40 | −30 | 0 | |
| L. Precentral gyrus | 41 | 2.58 | 3.99 | −60 | 6 | 30 | |
| L. Inferior frontal gyrus | 12 | 2.55 | 3.94 | −62 | 6 | 16 | |
| L. Precuneus | 15 | 2.49 | 3.88 | 2 | −62 | 46 | |
| L. Middle temporal gyrus (TPJ) | 69 | 2.44 | 3.81 | −56 | −52 | 16 | |
| R. Inferior frontal gyrus | 41 | 2.44 | 3.80 | 52 | 42 | −12 | |
| R. Middle frontal gyrus | 20 | 2.42 | 3.78 | 50 | −6 | 54 | |
| L. Thalamus | 26 | 2.36 | 3.70 | −8 | −10 | 12 | |
| R. Supramarginal gyrus | 20 | 2.27 | 3.60 | 50 | −30 | 44 | |
| R. Inferior frontal gyrus | 30 | 2.27 | 3.59 | 44 | 26 | 22 | |
| R. Inferior frontal gyrus | 21 | 2.27 | 3.59 | 60 | 18 | 32 | |
| L. SMA | 10 | 2.13 | 3.42 | 0 | −4 | 64 | |
| R. Inferior frontal gyrus | 16 | 2.07 | 3.33 | 50 | 22 | −6 | |
| L. Rolandic operculum | 5 | 2.05 | 3.30 | −44 | −28 | 22 | |
All significant activations at P < 0.001 (uncorrected, k > 5 voxels) are listed. vmPFC, ventromedial prefrontal cortex; LOC, lateral occipitotemporal cortex; PMd, dorsal premotor cortex; SII, secondary somatosensory cortex; SMA, supplementary motor area; TPJ, temporoparietal junction. FWE-corrected P values are based on cluster-wise correction or small volume correction as follows
a,bFWE-corrected peak P values based on small volume correction: awithin the left or right LOC as defined by the significant activations obtained from the visual localizer run for the left or right arm or bwithin the SII as defined by the significant activations obtained from the tactile localizer run for the contralateral arm side (see Supplementary material for details).
cFWE-corrected peak P values based on small volume correction using coordinates from Ionta et al. (2011).
*As this large cluster spanned to bilateral mid orbital gyrus, the peak coordinates of its local maximum in the right hemisphere are also listed.
The conjunction across the negative linear ownership contrasts (Real only < Fake synch < Fake mixed < Fake asynch) showed significant (P < 0.05, corrected) activity differences in large inferior parietal clusters, comprising the bilateral secondary somatosensory cortex (SII) and the right temporoparietal junction (TPJ), and further in the cuneus and left dorsal premotor cortex (Figure 2B and Table 1). When analyzing the individually weighted regressors, we found that between-participant differences related inversely to arm ownership were significantly (P < 0.05, corrected) reflected by activity in the bilateral TPJ and SII, cuneus, left PMd, right supplementary motor area (SMA) and right inferior frontal gyrus (IFG), at locations corresponding to those obtained from the linear contrast (Table 1). Notably, these individual differences were most strongly reflected by activity in the right TPJ (x = 62, y = −48, z = 12, T = 6.43, P < 0.05, corrected, cluster extent = 530 voxels).
Brain activity differences specific to the RHI
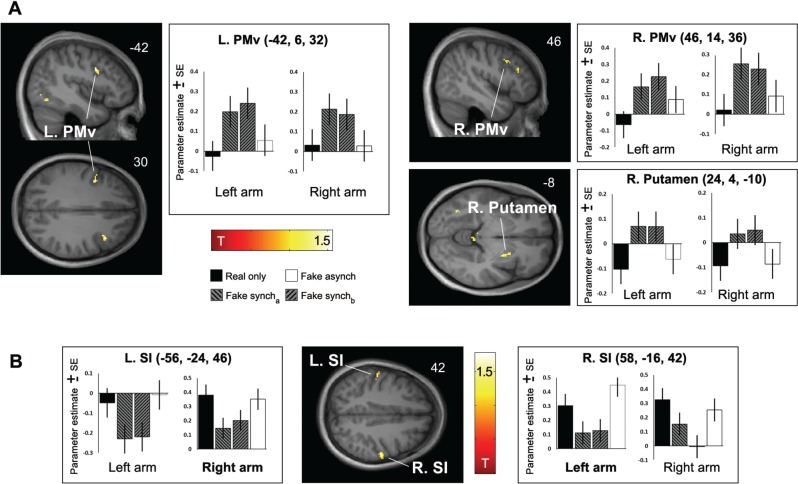
The conjunction across the Fake syncha vs Fake asynch and Fake synchb vs Real only contrasts for each arm side revealed significant (P < 0.05, small volume corrected) activity increases during synchronous stimulation in the bilateral ventral premotor cortex (PMv) and in the right putamen (Figure 3A and Table 2). Notably, activity differences at corresponding locations in the bilateral PMv (P < 0.05, small volume corrected) and a trend in the right putamen (P = 0.063, small volume corrected) emerged when only analyzing the post-illusion onset scans (no significant differences in the pre-illusion onset period); this comparison also revealed activity in the right posterior parietal cortex/intraparietal sulcus (IPS, x = 42, y = −56, z = 56, P < 0.001; at a lower threshold also in the left IPS: x = −38, y = −58, z = 56, P = 0.002).
Fig. 3.
Activity differences specific to the RHI. A: Results of the conjunction across the Fake syncha vs Fake asynch and Fake synchb vs Real only contrasts for each arm side (for this analysis, the Fake synch conditions were randomly split into Fake syncha, Fake synchb to obtain independent regressors for the conjunction analysis, see Methods). Significant (P < 0.05, small volume corrected) activity increases during synchronous FA stimulation compared with asynchronous FA stimulation and compared with RA stimulation were observed in the bilateral PMv and in the right putamen. See Table 2 for details. SPMs are thresholded at P < 0.001, bar plots depict the parameter estimates at the given coordinates for each arm side and condition with associated standard errors. B: The conjunction across the reverse contrasts (Real only vs Fake syncha and Fake asynch vs Fake synchb) revealed significant activity differences in the SI (P < 0.05, small volume corrected with significant activations obtained from the tactile localizer runs, see Table 2 and Supplementary material for details), which showed higher activity during stimulation of the RA and during asynchronous FA stimulation compared with synchronous FA stimulation (plots of the effects in the SI contralateral to the stimulated arm side are labeled in bold font).
Table 2.
Group-level activations obtained from the conjunction across the contrasts Fake syncha vs Fake asynch and Fake synchb vs Real only (and the conjunction across the respective reverse contrasts) for the left and right arm
| Peak (MNI) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Anatomical description | Cluster size (voxels) | Peak T | Peak Z | P (corrected) | x | y | z | |
| Fake synchavs Fake asynch and Fake synchbvs Real only | ||||||||
| L. Precentral gyrus (PMv) | 38 | 1.55 | 4.18 | 0.006a | −42 | 6 | 32 | |
| R. Inferior frontal gyrus (PMv) | 26 | 1.38 | 3.87 | 0.016a | 38 | 20 | 30 | |
| R. Putamen | 51 | 1.45 | 3.99 | 0.028a | 24 | 4 | −10 | |
| L./R. Superior medial gyrus | 59 | 1.54 | 4.15 | 2 | 36 | 36 | ||
| L. Inferior occipital gyrus | 28 | 1.49 | 4.06 | −38 | −64 | −10 | ||
| R. Inferior frontal gyrus | 45 | 1.45 | 4.00 | 54 | 30 | 16 | ||
| Cerebellar vermis | 32 | 1.39 | 3.89 | 0 | −36 | −6 | ||
| L. Inferior occipital gyrus (LOC) | 7 | 1.22 | 3.59 | −46 | −70 | −4 | ||
| L. Anterior insula | 6 | 1.18 | 3.52 | −30 | 18 | −4 | ||
| R. Anterior insula | 11 | 1.11 | 3.40 | 28 | 26 | 0 | ||
| L. Superior medial gyrus | 8 | 1.10 | 3.39 | −6 | 36 | 36 | ||
| Real only vs Fake syncha and Fake asynch vs Fake synchb | ||||||||
| R. Postcentral gyrus (SI) | 45 | 1.60 | 4.25 | 0.006b | 58 | −16 | 42 | |
| L. Postcentral gyrus/inferior parietal lobule (SI) | 31 | 1.55 | 4.17 | 0.008c | −56 | −24 | 46 | |
| L. Precentral gyrus | 60 | 1.77 | 4.56 | −38 | −14 | 66 | ||
| L. Postcentral gyrus/superior parietal lobule | 34 | 1.61 | 4.27 | −20 | −42 | 68 | ||
| L. Superior temporal gyrus | 24 | 1.28 | 3.70 | −62 | −2 | 0 | ||
| R. SMA | 16 | 1.24 | 3.63 | 4 | −12 | 70 | ||
| R. SMA | 7 | 1.20 | 3.56 | 14 | −4 | 68 | ||
| R. Precentral gyrus | 5 | 1.15 | 3.47 | 42 | −14 | 58 | ||
| R. Superior temporal gyrus | 7 | 1.00 | 3.22 | 66 | −10 | 10 | ||
All significant activations at P < 0.001 (uncorrected, k > 5 voxels) are listed. PMv, ventral premotor cortex; LOC, lateral occipitotemporal cortex; SI, primary somatosensory cortex, SMA = supplementary motor area.
aFWE-corrected peak P values based on small volume correction using coordinates from Petkova et al. (2011).
b,cFWE-corrected peak P values based on small volume correction within SI as defined by the significant activations obtained from the tactile localizer run for the left armb or right armc (see Supplementary material for details).
The conjunction across the reverse contrasts (Real only vs Fake syncha and Fake asynch vs Fake synchb) revealed significant activity differences within the bilateral primary somatosensory cortex (SI; P < 0.05, small volume corrected within masks created from the tactile localizer, see Table 2, Figure 3B and Supplementary material).
Discussion
In this study, brain activity during touch to one’s real arm vs to a (self-attributed) fake arm was compared in two different ways: we first identified brain areas whose activity co-varied with the degree of ownership of the touched arm. Second, we looked for brain activity increases specific to the RHI, which may indicate an update of the body representation.
Brain areas encoding the degree of ownership of the touched arm
Activity in the vmPFC and the bilateral LOC, and also in the cerebellum co-varied with the degree of ownership of the touched arm, gradually decreasing from stimulation of one’s RA alone, through synchronous, ambiguous and asynchronous stimulation of a FA together with one’s RA. These activation patterns were consistent for the left and right arm.
The vmPFC cluster encompassed bilateral medial orbitofrontal cortex (mOFC) and parts of the sgACC. The vmPFC/mOFC (often used synonymously, Zald and Rauch, 2006) receives information from all sensory modalities (Gusnard et al., 2001; Kringelbach, 2005) and hence may qualify as a ‘hub’ region (Northoff et al., 2006). VmPFC activity increases are generally observed during self-referential reflective tasks (Kjaer et al., 2002; D’Argembeau et al., 2005; Northoff et al., 2006; Sui et al., 2013) and during rest (in the absence of attention-demanding tasks, Raichle et al., 2001; Gusnard et al., 2001; D’Argembeau et al., 2005). It has hence been argued that the vmPFC might instantiate basic self-referential processes that could modulate other cognitive and sensory processes (Northoff and Bermpohl, 2004; Schmitz and Johnson, 2007; Liao et al., 2010; Bai et al., 2012; however, perhaps the observed vmPFC activations may also be explained by general inferential or memory processes that are not exclusively self-specific, cf. Gillihan and Farah, 2005; Legrand and Ruby, 2009). Along similar lines, the vmPFC/mOFC contributes to stimulus valuation and stimulus-reinforcement learning (Rolls, 2004; Kringelbach, 2005; Nicolle et al., 2012), in particular to the evaluation of self-associated vs other-associated objects (Kim and Johnson, 2015) and to forming novel associations between neutral stimuli and oneself vs others (Sui et al., 2013). The vmPFC is also involved in social cognition, for example, in adjusting social interactions to knowledge about the other (Stolk et al., 2015) or processing others’ predictions (Apps et al., 2015) and value representations (Garvert et al., 2015). Interestingly, in some cases this leads to updating one’s own beliefs and preferences (Garvert et al., 2015). Correspondingly, Murray et al. (2014) have proposed that the vmPFC could contribute to self-updating via the integration of self-relevant social information with one’s self-representation.
Taken together, the increased activation of the vmPFC by conditions with higher arm ownership could reflect a response to the self-relevance of the seen touches, determined by the subjective ownership of the touched arm. Perhaps the (arguably) pleasant touch of the sponge brushes could have increased this effect further, as the vmPFC/mOFC reliably responds to pleasant touch to the hand (Rolls et al., 2003), and this response may be subject to cognitive modulations (McCabe et al., 2008). Likewise, the vmPFC could have contributed to ‘higher-level’ (cognitive) self-vs-other processing and self-updating. However, this part of our analysis was exploratory and the existing literature at present does not suggest a role of the vmPFC in body ownership. Future work should therefore try to follow up on this finding, and clarify the potential function of the vmPFC in body ownership as related to self-vs-other representation and/or self-referential information processing (e.g. as rather an affective one, a cognitive one or both, Spangler and Allen, 2012).
The linear ownership contrast also revealed activity in the bilateral LOC. These activations fell within regions activated by the moving brushes in the visual localizer (touch to the FA only), but did not respond to tactile stimulation per se (no activation by the tactile localizer, see Supplementary material). Activity increases in the LOC are reliably evoked by spatially and temporally congruent vs incongruent stimulation of the real and fake body part during the RHI, and the extent of these activity increases correlates positively with the reported intensity of the illusion (Gentile et al., 2013; Limanowski and Blankenburg, 2015). Here, we observed a similar pattern, however, with even stronger responses to touch seen on one’s RA. Makin et al. (2007) have shown that the LOC preferentially responds to moving stimuli in perihand space, which aligns nicely with previous findings that vision of body parts alone is sufficient to influence the processing of stimuli in peripersonal space (Graziano et al., 2000; Làdavas, 2002; Graziano and Cooke, 2006). A similar importance of vision of the body is implied by crossmodal interactions between vision and somatosensation. For example, touch applied to the same location as a visual stimulus enhances visual cortex activity (Macaluso et al., 2000). Similarly, non-informative vision of a body part improves tactile acuity on that body part (Kennett et al., 2001; Haggard et al., 2007). There is evidence that these effects are further enhanced when viewing a body part that is self-attributed (Haggard et al., 2007; Longo et al., 2008). It has therefore been proposed that these crossmodal effects may depend on body ownership processes and the attribution of these stimuli to one’s body (rather than mere attentional effects, Whiteley et al., 2004; Macaluso and Maravita, 2010). Notably, the LOC activations we observed also fell within body part-selective regions of the extrastriate visual cortex known as the EBA (Downing et al., 2001; Limanowski and Blankenburg, 2015). Interestingly, there is evidence that the EBA also processes non-visual (e.g. proprioceptive) body-related information (Astafiev et al., 2004), and several authors have proposed that the EBA may implement self-other distinction mechanisms (Jeannerod, 2004; Arzy et al., 2006; David et al., 2007; Haggard et al., 2007). Taken together, a compelling interpretation of our results is that the LOC/EBA represents the body and the space around it differently than it does for a fake body (even if currently self-attributed) and may thus contribute to self-other distinction.
We also observed some activity modulations by arm ownership in the cerebellum, which has also been consistently reported in RHI studies (Ehrsson et al., 2004; Petkova et al., 2011; Gentile et al., 2013). The cerebellum plays a role in the estimation of the sensory state of the body (Herzfeld and Shadmehr, 2013), in the prediction of sensory events (Roth et al., 2013), and in the detection of intersensory congruence (Miall et al., 1993), which could explain its activation by the increased predictability of touch sensation from the visual stimulus (highest for RA stimulation, lowest for asynchronous stimulation).
Strong activity increases with decreasing arm ownership were observed in the right (and to a lesser degree also in the left) TPJ. The right TPJ has been associated with abnormal bodily states like out-of-body experiences (Blanke et al., 2005; De Ridder et al., 2007) and disembodied self-location (Arzy et al., 2006). In a recent fMRI study, TPJ activity reflected changes in perceived self-location induced by multisensory conflicts (Ionta et al., 2011). Farrer et al. (2003, 2004) have shown that right inferior parietal/TPJ activity increases were related to decreasing feeling of control of a virtual fake hand. Tsakiris et al. (2008) found that interfering with right TPJ activity increased the RHI induced with a non-hand object, and speculated that the TPJ may ‘test for fit’ of a fake object with one’s body representation. Interpreted with some caution, the increasing activation of the TPJ by conditions with increasing visuo-tactile incongruence (i.e. decreasing arm ownership) we observed could similarly imply a response to violations of bodily predictions. We observed further activity differences in neighboring inferior parietal regions including bilateral SII, which could reflect sensory mirroring (Keysers et al., 2010) or the anticipation of a tactile stimulus on one’s real limb (Carlsson et al., 2000; Tsakiris et al., 2007), since in the mixed and asynchronous conditions the FA was always touched before the RA.
Brain responses specific to touch to a temporarily self-attributed arm
We expected to observe activity increases during the RHI in brain areas related to multisensory integration, i.e. the binding of seen touches on the ‘owned’ FA and felt touches on the RA, which is not occurring during asynchronous or RA stimulation. We identified such responses in the bilateral PMv and the right putamen. This corroborates the results of previous RHI studies implying the PMv (Ehrsson et al., 2004; Brozzoli et al., 2012; Gentile et al., 2013; Zeller et al., 2014) and also the putamen (Petkova et al., 2011) in the integration of multisensory input and the updating of one’s body representation during the RHI. Correspondingly, the response differences in the PMv were particularly pronounced in the period after onset of the RHI (at a lower threshold, this analysis revealed similar responses in the bilateral IPS, which has been implied in similar multisensory processes, Ehrsson et al., 2004; Gentile et al., 2013).
The reverse comparison revealed that touch-related activity in the SI contralateral to the stimulated arm was lower during the RHI than during both RA stimulation and asynchronous stimulation (similar patterns emerged in the ipsilateral SI). This result replicates a recent electroencephalography study reporting reduced early touch-evoked potentials in SI during the RHI (Zeller et al., 2014), which the authors interpret as a suppression of somatosensory signals from the real hand that could be required to enable a recalibration of hand representation from one’s real hand onto the fake hand.
Conclusion
In sum, our results suggest that whereas integrating visuo-tactile information and updating the body representation under crossmodal conflict involves known multisensory integration areas, the self-relevance of touch to the body may be encoded in brain areas thought to implement self-referential processes and self-other distinction. These areas could perhaps in this way differentiate between oneself, i.e. one’s real body parts and those temporarily self-attributed as a result of an illusion. Both processes—updating the body representation and self-other distinction—may be considered crucial for a basic self-representation. Recently, it has been proposed that hierarchical prediction error minimization in the brain could underlie the sense of body ownership and even the experience of selfhood (Friston, 2011; Seth et al., 2011; Hohwy, 2012; Limanowski and Blankenburg, 2013; Apps and Tsakiris, 2014). These accounts are based on the assumption that the brain implements a hierarchical generative model of the world to predict its sensory input, and that this predictive model also instantiates the representation of one’s body and self. Some anatomical candidate regions for the underlying architecture are the PMv, LOC/EBA, IPS and also the TPJ, which have been proposed repeatedly as parts of a network implementing one’s body representation (Tsakiris et al., 2007; Makin et al., 2008; Tsakiris, 2010; Blanke, 2012; Ehrsson, 2012; Gentile et al., 2013; Apps and Tsakiris, 2014; Limanowski and Blankenburg, 2015). To identify the specific contribution of individual brain areas and larger networks in predictive body- and self-modeling is an important and promising task for future research. An understanding of such a predictive model’s processing hierarchy could shed more light onto the mechanisms underlying a flexible self-representation and self-other distinction; specifically, it could help clarify which parts of one’s body representation can in principle be changed or updated ‘completely’, and whether a fake body (and touch to it) can ever be represented just like the real body.
Supplementary Material
Acknowledgements
We thank Evgeniya Kirilina and Bernhard Spitzer for helpful comments and technical advice, and Simon Ludwig for assistance during the behavioral experiment.
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
References
- Apps M.A., Lesage E., Ramnani N. (2015). Vicarious reinforcement learning signals when instructing others. The Journal of Neuroscience , 35, 2904–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apps M.A., Tajadura-Jiménez A., Sereno M., Blanke O., Tsakiris M. (2013). Plasticity in unimodal and multimodal brain areas reflects multisensory changes in self-face identification. Cerebral Cortex , 25, 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apps M.A., Tsakiris M. (2014). The free-energy self: a predictive coding account of self-recognition. Neuroscience and Biobehavioral Reviews, 41, 85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armel K.C., Ramachandran V.S. (2003). Projecting sensations to external objects: evidence from skin conductance response. Proceedings of the Royal Society of London B , 270, 1499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arzy S., Thut G., Mohr C., Michel C.M., Blanke O. (2006). Neural basis of embodiment: distinct contributions of temporoparietal junction and extrastriate body area. The Journal of Neuroscience , 26, 8074–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astafiev S.V., Stanley C.M., Shulman G.L., Corbetta M. (2004). Extrastriate body area in human occipital cortex responds to the performance of motor actions. Nature Neuroscience, 7,542–8. [DOI] [PubMed] [Google Scholar]
- Bai F., Shi Y., Yuan Y., Wang Y., Yue C., Teng Y., et al. (2012). Altered self-referential network in resting-state amnestic type mild cognitive impairment. Cortex , 48, 604–13. [DOI] [PubMed] [Google Scholar]
- Behzadi Y., Restom K., Liau J., Liu T.T. (2007). A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage, 37, 90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanke O. (2012). Multisensory brain mechanisms of bodily self-consciousness. Nature Reviews. Neuroscience , 13, 556–71. [DOI] [PubMed] [Google Scholar]
- Blanke O., Metzinger T. (2009). Full-body illusions and minimal phenomenal selfhood. Trends in Cognitive Sciences, 13, 7–13. [DOI] [PubMed] [Google Scholar]
- Blanke O., Mohr C., Michel C.M., Pascual-Leone A., Brugger P., Seeck M., et al. (2005). Linking out-of-body experience and self processing to mental own-body imagery at the temporoparietal junction . The Journal of Neuroscience , 25, 550–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick M., Cohen J. (1998). Rubber hands ‘feel’ touch that eyes see. Nature, 391, 756. [DOI] [PubMed] [Google Scholar]
- Brozzoli C., Gentile G., Ehrsson H.H. (2012). That’s near my hand! Parietal and premotor coding of hand-centered space contributes to localization and self-attribution of the hand. The Journal of Neuroscience, 32, 14573–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson K., Petrovic P., Skare S., Peterson K.M., Ingvar M. (2000). Tickling expectations: neural processing in anticipation of a sensory stimulus. Journal of Cognitive Neuroscience, 12, 691–703. [DOI] [PubMed] [Google Scholar]
- Costantini M., Haggard P. (2007). The rubber hand illusion: sensitivity and reference frame for body ownership. Consciousness and Cognition, 16, 229–40. [DOI] [PubMed] [Google Scholar]
- D’Argembeau A., Collette F., Van der Linden M., Laureys S., Del Fiore G., Degueldre C., et al. (2005). Self-referential reflective activity and its relationship with rest: a PET study. NeuroImage ,25, 616–24. [DOI] [PubMed] [Google Scholar]
- David N., Cohen M.X., Newen A., Bewernick B.H., Shah N.J., Fink G.R., et al. (2007). The extrastriate cortex distinguishes between the consequences of one’s own and others’ behavior. NeuroImage , 36, 1004–14. [DOI] [PubMed] [Google Scholar]
- De Ridder D., Van Laere K., Dupont P., Menovsky T., Van de Heyning P. (2007). Visualizing out-of-body experience in the brain. New England Journal of Medicine , 357, 1829–33. [DOI] [PubMed] [Google Scholar]
- Downing P.E., Jiang Y., Shuman M., Kanwisher N. (2001). A cortical area selective for visual processing of the human body. Science , 293, 2470–3. [DOI] [PubMed] [Google Scholar]
- Ehrsson H.H. (2012). The concept of body ownership and its relation to multisensory integration. In: Stein B.E., editors. The New Handbook of Multisensory Processes. Cambridge, MA: MIT Press. [Google Scholar]
- Ehrsson H.H., Spence C., Passingham R.E. (2004). That’s my hand! Activity in premotor cortex reflects feeling of ownership of a limb. Science, 305, 875–7. [DOI] [PubMed] [Google Scholar]
- Eickhoff S.B., Stephan K.E., Mohlberg H., Grefkes C., Fink G.R., Amunts K., et al. (2005). A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage, 25, 1325–35. [DOI] [PubMed] [Google Scholar]
- Farrer C., Franck N., Frith C.D., Decety J., Georgieff N., d’Amato T., et al. (2004). Neural correlates of action attribution in schizophrenia. Psychiatry Research , 131, 31–44. [DOI] [PubMed] [Google Scholar]
- Farrer C., Franck N., Georgieff N., Frith C.D., Decety J., Jeannerod M. (2003). Modulating the experience of agency: a positron emission tomography study. NeuroImage , 18, 324–33. [DOI] [PubMed] [Google Scholar]
- Fogassi L., Gallese V., Fadiga L., Luppino G., Matelli M., Rizzolatti G. (1996). Coding of peripersonal space in inferior premotor cortex (area F4). Journal of Neurophysiology, 76, 141–57. [DOI] [PubMed] [Google Scholar]
- Friston K. (2011). Embodied inference: or “I think therefore I am, if I am what I think”. In: Tschacher W., Bergomi C., editors. The Implications of Embodiment: Cognition and Communication. UK: Imprint Academic, pp. 89–125. [Google Scholar]
- Friston K.J., Penny W.D., Glaser D.E. (2005). Conjunction revisited. NeuroImage, 25, 661–7. [DOI] [PubMed] [Google Scholar]
- Gallagher S. (2000). Philosophical conceptions of the self: implications for cognitive science. Trends in Cognitive Sciences , 4, 14–21. [DOI] [PubMed] [Google Scholar]
- Garvert M.M., Moutoussis M., Kurth-Nelson Z., Behrens T.E., Dolan R.J. (2015). Learning-induced plasticity in medial prefrontal cortex predicts preference malleability. Neuron , 85, 418–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile G., Guterstam A., Brozzoli C., Ehrsson H.H. (2013). Disintegration of multisensory signals from the real hand reduces default limb self-attribution: an fMRI study. The Journal of Neuroscience, 33, 13350–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillihan S.J., Farah M.J. (2005). Is self special? A critical review of evidence from experimental psychology and cognitive neuroscience. Psychological Bulletin , 131, 76–97. [DOI] [PubMed] [Google Scholar]
- Graziano M.S. (1999). Where is my arm? The relative role of vision and proprioception in the neuronal representation of limb position. Proceedings of the National Academy of Sciences , 96, 10418–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano M.S., Cooke D.F. (2006). Parieto-frontal interactions, personal space, and defensive behavior. Neuropsychologia , 44, 845–59. [DOI] [PubMed] [Google Scholar]
- Graziano M.S., Cooke D.F., Taylor C.S. (2000). Coding the location of the arm by sight. Science, 290, 1782–6. [DOI] [PubMed] [Google Scholar]
- Gusnard D. A., Akbudak E., Shulman G.L., Raichle M.E. (2001). Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proceedings of the National Academy of Sciences , 98, 4259–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggard P., Christakou A., Serino A. (2007). Viewing the body modulates tactile receptive fields. Experimental Brain Research,180, 187–93. [DOI] [PubMed] [Google Scholar]
- Herzfeld D.J., Shadmehr R. (2013). Cerebellum estimates the sensory state of the body. Trends in Cognitive Scences, 18,66–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohwy J. (2012). Attention and conscious perception in the hypothesis testing brain. Frontiers in Psychology, 3, 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionta S., Heydrich L., Lenggenhager B., Mouthon M., Fornari E., Chapuis D., et al. (2011). Multisensory mechanisms in temporo-parietal cortex support self-location and first-person perspective. Neuron , 70, 363–74. [DOI] [PubMed] [Google Scholar]
- Jeannerod M. (2004). Visual and action cues contribute to the self–other distinction. Nature Neuroscience, 7, 422–3. [DOI] [PubMed] [Google Scholar]
- Kennett S., Taylor-Clarke M., Haggard P. (2001). Noninformative vision improves the spatial resolution of touch in humans. Current Biology , 11, 1188–91. [DOI] [PubMed] [Google Scholar]
- Keysers C., Kaas J.H., Gazzola V. (2010). Somatosensation in social perception. Nature Reviews Neuroscience, 11, 417–28. [DOI] [PubMed] [Google Scholar]
- Kim K., Johnson M.K. (2015). Distinct neural networks support the mere ownership effect under different motivational contexts. Social Neuroscience, 10, 376–390. [DOI] [PubMed] [Google Scholar]
- Kjaer T.W., Nowak M., Lou H.C. (2002). Reflective self-awareness and conscious states: PET evidence for a common midline parietofrontal core. NeuroImage , 17, 1080–6. [PubMed] [Google Scholar]
- Kringelbach M.L. (2005). The human orbitofrontal cortex: linking reward to hedonic experience. Nature Reviews Neuroscience , 6, 691–702. [DOI] [PubMed] [Google Scholar]
- Làdavas E. (2002). Functional and dynamic properties of visual peripersonal space. Trends in Cognitive Sciences, 6, 17–22. [DOI] [PubMed] [Google Scholar]
- Legrand D., Ruby P. (2009). What is self-specific? Theoretical investigation and critical review of neuroimaging results. Psychological Review , 116, 252–82. [DOI] [PubMed] [Google Scholar]
- Liao W., Mantini D., Zhang Z., Pan Z., Ding J., Gong Q., et al. (2010). Evaluating the effective connectivity of resting state networks using conditional Granger causality. Biological Cybernetics , 102, 57–69. [DOI] [PubMed] [Google Scholar]
- Limanowski J., Blankenburg F. (2013). Minimal self-models and the free energy principle. Frontiers in Human Neuroscience, 7,547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limanowski J., Blankenburg F. (2015). Network activity underlying the illusory self-attribution of a dummy arm. Human Brain Mapping, 36, 2284–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo M.R., Cardozo S., Haggard P. (2008). Visual enhancement of touch and the bodily self. Consciousness and Cognition , 17,1181–91. [DOI] [PubMed] [Google Scholar]
- Lutti A., Thomas D.L., Hutton C., Weiskopf N. (2012). High-resolution functional MRI at 3T: 3D/2D echo-planar imaging with optimized physiological noise correction. Magnetic Resonance in Medicine, 69, 1657–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaluso E., Frith C.D., Driver J. (2000). Modulation of human visual cortex by crossmodal spatial attention. Science , 289,1206–8. [DOI] [PubMed] [Google Scholar]
- Macaluso E., Maravita A. (2010). The representation of space near the body through touch and vision. Neuropsychologia , 48,782–95. [DOI] [PubMed] [Google Scholar]
- Macey P.M., Macey K.E., Kumar R., Harper R.M. (2004). A method for removal of global effects from fMRI time series. NeuroImage, 22, 360–6. [DOI] [PubMed] [Google Scholar]
- Makin T.R., Holmes N.P., Ehrsson H.H. (2008). On the other hand: dummy hands and peripersonal space. Behavioural Brain Research , 191, 1–10. [DOI] [PubMed] [Google Scholar]
- Makin T.R., Holmes N.P., Zohary E. (2007). Is that near my hand? Multisensory representation of peripersonal space in human intraparietal sulcus. The Journal of Neuroscience , 27, 731–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazaika P., Hoeft F., Glover G.H., Reiss A.L. (2009). Methods and Software for fMRI Analysis for Clinical Subjects. San Francisco, CA: Annual Meeting of the Organization for Human Brain Mapping. [Google Scholar]
- McCabe C., Rolls E.T., Bilderbeck A., McGlone F. (2008). Cognitive influences on the affective representation of touch and the sight of touch in the human brain. Social Cognitive and Affective Neuroscience , 3, 97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miall R.C., Weir D.J., Wolpert D.M., Stein J.F. (1993). Is the cerebellum a smith predictor? Journal of Motor Behavior , 25,203–16. [DOI] [PubMed] [Google Scholar]
- Murray R.J., Debbane M., Fox P.T., Bzdok D., Eickhoff S.B. (2014). Functional connectivity mapping of regions associated with self- and other- processing. Human Brain Mapping , 36, 1304–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolle A., Klein-Flügge M.C., Hunt L.T., Vlaev I., Dolan R.J., Behrens T.E. (2012). An agent independent axis for executed and modeled choice in medial prefrontal cortex. Neuron , 75, 1114–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G., Bermpohl F. (2004). Cortical midline structures and the self. Trends in Cognitive Sciences, 8, 102–7. [DOI] [PubMed] [Google Scholar]
- Northoff G., Heinzel A., de Greck M., Bermpohl F., Dobrowolny H., Panksepp J. (2006). Self-referential processing in our brain—a meta-analysis of imaging studies on the self. NeuroImage , 31, 440–57. [DOI] [PubMed] [Google Scholar]
- Oldfield R.C. (1971). The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia, 9, 97–113. [DOI] [PubMed] [Google Scholar]
- Petkova V.I., Björnsdotter M., Gentile G., Jonsson T., Li T.Q., Ehrsson H.H. (2011). From part-to whole-body ownership in the multisensory brain. Current Biology, 21, 1118–22. [DOI] [PubMed] [Google Scholar]
- Raichle M.E., MacLeod A.M., Snyder A.Z., Powers W.J., Gusnard D.A., Shulman G.L. (2001). A default mode of brain function. Proceedings of the National Academy of Sciences , 98, 676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls E.T. (2004). The functions of the orbitofrontal cortex. Brain and cognition , 55, 11–29. [DOI] [PubMed] [Google Scholar]
- Rolls E.T., O’Doherty J., Kringelbach M.L., Francis S., Bowtell R., McGlone F. (2003). Representations of pleasant and painful touch in the human orbitofrontal and cingulate cortices. Cerebral Cortex , 13, 308–17. [DOI] [PubMed] [Google Scholar]
- Roth M.J., Synofzik M., Lindner A. (2013). The cerebellum optimizes perceptual predictions about external sensory events. Current Biology 23, 930–5. [DOI] [PubMed] [Google Scholar]
- Schmitz T.W., Johnson S.C. (2007). Relevance to self: a brief review and framework of neural systems underlying appraisal. Neuroscience and Biobehavioral Reviews , 31, 585–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth A.K., Suzuki K., Critchley H.D. (2011). An interoceptive predictive coding model of conscious presence. Frontiers in psychology , 2, 395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangler D.L., Allen M.D. (2012). An fMRI investigation of emotional processing of body shape in bulimia nervosa. International Journal of Eating Disorders , 45, 17–25. [DOI] [PubMed] [Google Scholar]
- Stolk A., D’Imperio D., di Pellegrino G., Toni I. (2015). Altered communicative decisions following ventromedial prefrontal lesions. Current Biology , 25, 1469–74. [DOI] [PubMed] [Google Scholar]
- Sui J., Rotshtein P., Humphreys G.W. (2013). Coupling social attention to the self forms a network for personal significance. Proceedings of the National Academy of Sciences , 110,7607–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsakiris M. (2008). Looking for myself: current multisensory input alters self-face recognition. PLoS One , 3(12), e4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsakiris M. (2010). My body in the brain: a neurocognitive model of body-ownership. Neuropsychologia, 48, 703–12. [DOI] [PubMed] [Google Scholar]
- Tsakiris M., Costantini M., Haggard P. (2008). The role of the right temporo-parietal junction in maintaining a coherent sense of one’s body. Neuropsychologia , 46,3014–8. [DOI] [PubMed] [Google Scholar]
- Tsakiris M., Haggard P. (2005). The rubber hand illusion revisited: visuotactile integration and self-attribution. Journal of Experimental Psychology. Human Perception and Performance, 31,80–91. [DOI] [PubMed] [Google Scholar]
- Tsakiris M., Hesse M.D., Boy C., Haggard P., Fink G.R. (2007). Neural signatures of body ownership: a sensory network for bodily self-consciousness. Cerebral Cortex, 17, 2235–44. [DOI] [PubMed] [Google Scholar]
- Whiteley L., Kennett S., Taylor-Clarke M., Haggard P. (2004). Facilitated processing of visual stimuli associated with the body. Perception , 33, 307–14. [DOI] [PubMed] [Google Scholar]
- Zald D.H., Rauch S.L. (2006). The Orbitofrontal Cortex. New York: Oxford University Press. [Google Scholar]
- Zeller D., Litvak V., Friston K.J., Classen J. (2014). Sensory processing and the rubber hand illusion—an evoked potentials study. Journal of Cognitive Neuroscience , 27, 573–82. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.