Abstract
Externalizing proneness, or trait disinhibition, is a concept relevant to multiple high-impact disorders involving impulsive-aggressive behavior. Its mechanisms remain disputed: major models posit hyperresponsive reward circuitry or heightened threat-system reactivity as sources of disinhibitory tendencies. This study evaluated alternative possibilities by examining relations between trait disinhibition and brain reactivity during preparation for and processing of visual affective stimuli. Forty females participated in a functional neuroimaging procedure with stimuli presented in blocks containing either pleasurable or aversive pictures interspersed with neutral, with each picture preceded by a preparation signal. Preparing to view elicited activation in regions including nucleus accumbens, whereas visual regions and bilateral amygdala were activated during viewing of emotional pictures. High disinhibition predicted reduced nucleus accumbens activation during preparation within pleasant/neutral picture blocks, along with enhanced amygdala reactivity during viewing of pleasant and aversive pictures. Follow-up analyses revealed that the augmented amygdala response was related to reduced preparatory activation. Findings indicate that participants high in disinhibition are less able to process implicit cues and mentally prepare for upcoming stimuli, leading to limbic hyperreactivity during processing of actual stimuli. This outcome is helpful for integrating findings from studies suggesting reward-system hyperreactivity and others suggesting threat-system hyperreactivity as mechanisms for externalizing proneness.
Keywords: amygdala, nucleus accumbens, fMRI, externalizing, disinhibition
Introduction
Externalizing proneness, or trait disinhibition1 (Sher and Trull, 1994; Patrick et al., 2013), represents a prominently heritable liability for a number of dysfunctional behaviors of high societal and personal relevance, including conduct problems in childhood, impulsive-aggressive behavior in adulthood, and substance-related addictions (Krueger et al., 2002, 2007). This dispositional liability can be assessed in a number of ways, including through diagnostic interview, self-report questionnaire, lab task performance, and psychophysiological reactivity (Young et al., 2009; Patrick et al., 2012, 2013). Indeed, recent research has demonstrated that general proneness to externalizing problems as operationalized by scores on a brief scale measure of trait disinhibitory tendencies indexes heritable variance in common with interview-assessed externalizing problems that in turn predicts aberrant brain response (Yancey et al., 2013).
In spite of its behavioral relevance, the neural mechanisms that underlie externalizing proneness remain unclear. On one hand, it has been proposed that this liability reflects increased sensitivity to immediate rewards at the expense of more distal goals, associated with dysfunction in the mesolimbic reward system of the brain (Beauchaine and McNulty, 2013). Other researchers, in contrast, propose that impulsive-aggressive tendencies arise from heightened sensitivity to threat cues associated with overreactivity of the brain’s amygdala-based defensive circuit, in conjunction with problems in reinforcement learning (Blair et al., 2014).
Results from differing studies have provided support for each of these hypotheses. For example, evidence for the deficient reward-system sensitivity hypothesis was provided by Buckholtz et al. (2010), who used positron emission tomography (PET) along with functional magnetic resonance imaging (fMRI) to examine brain responses in a monetary incentive delay task as a function of varying levels of impulsive-antisocial tendencies [closely akin to trait disinhibition (Blonigen et al., 2005)]. Specifically, on each trial of a simple button-pressing task, participants were informed beforehand whether they would win money or lose money. During the processing of these explicit cues, the authors found increased activation of the nucleus accumbens (nAcc) among subjects high in impulsive-antisocial tendencies. Their conclusion was that limbic hyperactivation during reward anticipation in high impulsive-antisocial (i.e. disinhibited) participants reflects increased motivational readiness for receipt of reward. These findings dovetail with work by Lawrence and Brooks (2014) reporting a high positive correlation between disinhibitory personality traits and the capacity of the ventral striatum (in which the nAcc is located) to synthesize dopamine, which they interpreted as a possible mechanism for genetic influences on externalizing behavior. It is noteworthy that both PET and fMRI index neural activation indirectly—using blood flow and oxygen depletion, respectively. It has been shown that results from the two methods can be successfully combined to characterize neural behavior in nAcc and the ventral striatum (Salimpoor et al., 2011; Heinz et al., 2014).
However, other research has emphasized overresponsiveness of the brain’s defensive system to threat stimuli as a key mechanism for disinhibitory-aggressive tendencies (Viding 2012; Blair et al. 2014; see also Davidson et al. 2000). For example, Viding et al. (2012) reported augmented amygdala reactivity to fearful face stimuli relative to neutral faces in young males with conduct problems but lacking core psychopathic symptoms, corresponding to high disinhibited (externalizing-prone) individuals (Patrick, 2008). The perspective here is that heightened reactivity of this system is associated with difficulties in processing of emotional cues in dynamic situational contexts, leading to affective dysregulation expressed as distress, anger and reactive aggression (Blair, 2013; Blair et al., 2014). Elsewhere, Iacono et al. (1999) demonstrated heightened physiological (electrodermal) reactivity to aversive noise blasts among high-disinhibited participants in a condition in which the blasts were signaled and thus predictable.2 Whereas low-disinhibited participants were able to use the predictive signal to down-regulate their responses to the succeeding noise blasts, high-disinhibited subjects were not—reacting at increased levels to the blasts. Notably, high-disinhibited subjects did not differ in responses to noise blasts that occurred without warning, indicating that impaired preparatory processing was the basis for reactivity differences. Findings from these studies appear consistent with clinical descriptions of high-disinhibited-externalizing individuals as hostile in their perceptions of others, deficient in anticipatory coping, low in frustration tolerance, and prone to react aggressively to threats (Dodge et al., 1995; Davidson et al., 2000; Frick and Marsee, 2006; Patrick, 2008).
To further evaluate these alternative perspectives, this study examined effects of trait disinhibition on neural reactivity to both pleasurable and aversive stimuli (i.e. pictures) within the same task, under two conditions of processing: (i) preparatory cuing, and (ii) immediate viewing. As regards preparatory cuing, we were specifically interested in evaluating whether disinhibition involves selectively enhanced reactivity to cues for reward, or alternatively, a reduced capacity to develop representations for affective events more broadly—whether pleasant or aversive. To test this, we used an incidental pre-cuing manipulation in which participants were signaled in advance of each picture, but without explicit information about what type of picture would appear. Pictures were presented in blocks of either ‘pleasant + neutral’ or ‘unpleasant + neutral’—creating an opportunity for expectancies to develop regarding the nature of to-be-viewed pictures, based on emerging representations of the nature of stimuli occurring within blocks of each type. The study used female participants exclusively in order to avoid moderating effects of gender on emotional response (Cahill et al., 2001; Seo et al., 2011), and to allow for comparisons with other studies that have included women only (Ochsner et al., 2002; Kim and Hamann, 2007).
Within this experimental context, alternative hypotheses were proposed as follows, based on the two contrasting conceptions of disinhibitory as described above:
If trait disinhibition (externalizing proneness) primarily reflects hyperreactivity to cues for reward, then: (a) For the pre-cuing condition, high-disinhibited participants should show increased brain activation in reward-related regions during preparatory periods within pleasant + neutral blocks as compared to unpleasant + neutral blocks. Given consistent evidence for enhanced nAcc activation during anticipation of reward in impulsive-disinhibited individuals (Buckholtz et al., 2010; Lawrence and Brooks, 2014), we predicted enhanced activity for this region in particular. (b) For the viewing condition, participants high in disinhibition should show increased brain activation in reward-related regions for pleasant as compared to neutral pictures [particularly nAcc, as this region has been shown to be selectively reactive to pleasurable stimuli (Sabatinelli et al., 2007)]—perhaps along with increased activation of the amygdala and visual processing regions, since these brain areas are known to respond to the affective saliency of picture stimuli, irrespective of their valence (Bradley et al., 2003; Sabatinelli et al., 2005, 2007; Costa et al., 2010).
If trait disinhibition primarily reflects overresponsiveness to threat (Blair et al., 2014) as related to impaired preparatory processing (Iacono et al., 1999), then: (a) For the pre-cuing condition, high-disinhibited participants should show less evidence of differential brain activation during preparatory periods occurring within pleasant + neutral blocks as compared to unpleasant + neutral blocks [i.e. reduced recognition of the affective context at a neural-response level (cf. Iacono et al. 1999; see also Patrick 2008)]. (b) For the viewing condition, participants high in disinhibition should show increased activation of the amygdala in particular for unpleasant as compared to neutral pictures (Viding et al., 2012).
Materials and Methods
Participants
Participants were 45 female students recruited from undergraduate psychology classes and through university newspaper advertisements, screened to be free of hearing impairments, uncorrected visual impairments, and major psychiatric disorders. Due to missing or unusable fMRI data, five participants were excluded from analyses, leaving 40 in the final analysis sample (M age = 19.7 years, s.d. = 1.4). All were right-handed with the exception of one ambidextrous individual. Participants provided written informed consent prior to commencement of testing. Group-level, task-related findings for a subset of the current sample were reported previously by Seo et al. (2013).
Trait disinhibition measure
Trait disinhibition was operationalized by scores on a scale measure consisting of 30 items (Yancey et al., 2013) from the Externalizing Spectrum Inventory (Krueger et al., 2007). For details regarding scale content and properties, see Supplementary Methods.
Task stimuli
Participants viewed 150 photographic images from the International Affective Picture System [IAPS (Lang et al., 1999)] consisting of 60 pleasant, 60 unpleasant and 30 neutral scenes. Pleasant and unpleasant picture sets were selected to be matched for arousal (for details, see Supplementary Methods). Each picture was presented once during the test procedure. Order of picture presentation was counterbalanced across participants.
MRI scanning
The MRI testing session was conducted at the University of Minnesota’s Center for Magnetic Resonance Research, using a 3.0 Tesla Siemens Trio scanner. Participants viewed task stimuli (cues, pictures, fixation cross) using a mirror, attached to the head coil, that reflected images on a projection screen situated behind the scanner. Participants were in the scanner for ∼1.5 h (1 h for task procedures, and 30 min for setup and structural MRI scanning).
For each participant, structural brain information in the form of standard T1-weighted anatomical image volumes was first collected using a 3D Magnetization Prepared Rapid Gradient Echo (MPRAGE) sequence. Next, functional images were acquired using an 8-channel phased array coil as participants completed the picture processing task. These images were collected using an echo-planar imaging pulse sequence (36 slices, slice thickness = 2 mm with a 1.5-mm gap; field of view = 22.4 × 22.4 cm, matrix size = 6.4 × 64 for a nominal resolution of 3.5 mm; TE = 28 ms; TR = 2 s; flip angle = 80°; slice order = interleaved ascending). Slice orientation was oblique, roughly aligned with a line connecting the base of the cerebellum and the base of the frontal lobe, in order to maximize signal-to-noise ratio in the amygdala (Chen et al., 2003).
Procedure
At the beginning of each trial, participants were cued prior to picture onset either to simply view the picture when presented (cue = ‘V’) or decrease (suppress) their emotional reaction to it (cue = ‘D’). Current analyses focused on data from the View condition [for group results pertaining to the Decrease condition, see (Seo et al., 2013)]. For information regarding timing of picture stimuli and inter-stimulus intervals, see Supplementary Methods.
Participants completed six blocks of test trials, each containing 25 pictures, with a short rest period between blocks. Three blocks contained pleasant and neutral pictures, the other three contained unpleasant and neutral pictures. Participants were not explicitly advised prior to block onset whether pictures to be viewed would be pleasant and neutral, or unpleasant and neutral. Within blocks, participants were cued only as to processing instruction (‘view’ or ‘decrease’), not as to whether the upcoming picture would be emotional or neutral. At the end of the viewing period for each trial, participants rated the affective valence of the just-viewed picture. For further details regarding blocking of trials and picture valence ratings, see Supplementary Methods.
Data analysis
Functional analysis of the fMRI data was performed using the Statistical Parametric Mapping software (SPM8; Wellcome Institute of Imaging Neuroscience, London, UK) implemented in Matlab 7.11 (Mathworks Inc., Natick, MA). Data were re-aligned, corrected for slice timing effects, co-registered on a mean image of realigned and slice-timed images, normalized to a standard template (Montreal Neurological Institute), and smoothed with a Gaussian kernel of 5 mm (full-width at half-maximum). The fMRI design matrix used for parameter estimates included individual movement regressors.
The fMRI data were analyzed in two stages. Whole-brain level analyses were first performed across all participants, focusing on data from the preparation and viewing periods of each View trial. For the viewing period, activations occurring in response to emotional pictures were contrasted against activations for neutral pictures (i.e. contrast = View Emotional – View Neutral). The combination of pleasant and unpleasant View conditions into an emotional View condition was done after a preliminary analysis showed no difference between brain reactivity in the two emotional View conditions. For the preparation period, activations occurring between cue presentation (‘V’) and picture onset within pleasant + neutral trial blocks were contrasted against corresponding activations within unpleasant + neutral blocks. Distinct brain anatomic areas (see next paragraph) for which k = 10 or more voxels emerged as statistically significant for a given contrast were defined as regions of differential activation.
In the second analytic stage, neural activity in regions identified as differentially active in the whole-brain analyses was examined in relation to trait disinhibition scores, using correlational (Pearson’s r) and group (high vs low disinhibition) analyses. For details of how anatomic boundaries for regions of interest were defined and how mean regression-beta values (used as the units of measurement in figural depictions of results) were extracted from those regions, see Supplementary Methods. For each specified anatomic region, difference scores reflecting contrasts of interest (i.e. emotional−neutral for the viewing period; pleasant−unpleasant for the preparation period) were examined in relation to scores on trait disinhibition.
Results
Overall sample: whole-brain analysis
Preparation period
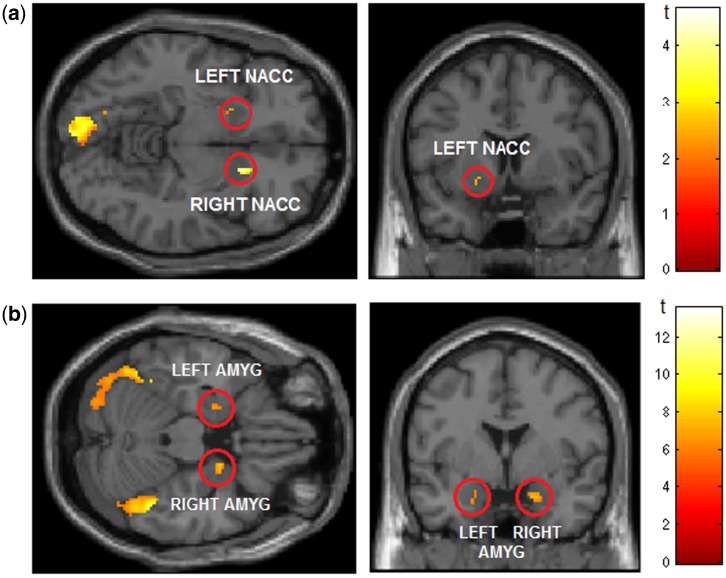
For the sample-wide contrast of pleasant vs unpleasant during the preparation period (Prep Pleasant−Prep Unpleasant), the only regions exhibiting significant activation after family-wise error (FWE) correction (α = 0.05) were left calcarine fissure, right nAcc, and left fusiform gyrus (Table 1 and Figure 1a).3
Table 1.
Region of interest (ROI) activations with a peak-level significance of P < 0.05 FWE (a) when preparing to view pleasant pictures or (b) viewing emotional pictures
|
Significant activation during preparation (contrast = Prep Pleasant − Prep Unpleasant) | |||
|---|---|---|---|
| Area | Coordinates (x, y, z) | T | Cluster size in voxels |
| Right nAcc | 16 14 − 8 | 4.65 | 21 |
| Left calcarine fissure | −14 − 84 0 | 4.68 | 179 |
| Left fusiform gyrus | −14 − 66 − 18 | 4.11 | 19 |
|
Significant activation during view (contrast = View Emotional − View Neutral) | |||
|---|---|---|---|
| Area | Coordinates (x, y, z) | T | Cluster size in voxels |
| Left amygdala | −22 − 2 − 26 | 6.82 | 80 |
| Right amygdala | 22 0 − 20 | 6.43 | 17 |
| Left calcarine fissure | −12 − 96 − 4 | 8.61 | 241 |
| Left fusiform gyrus | −22 − 78 − 20 | 6.78 | 70 |
| Right fusiform gyrus | 38 − 74 − 18 | 7.22 | 53 |
| Right inferior occipital gyrus | 22 − 98 2 | 8.86 | 230 |
Cluster threshold (k) =10. All coordinates are in Montreal Neurological Institute (MNI) space.
Fig. 1.
(a) Supra-threshold activation for the contrast of pleasant minus unpleasant pictures during the preparation period (contrast = Prep Pleasant − Prep Unpleasant) for a priori region nAcc (based on prior evidence) and other pre-specified regions of interest (see Methods); P = 0.05 uncorrected. Red circles indicate bilateral nAcc. Occipital activation peak lies in left calcarine fissure. View centered on: x = 16, y = 14, z = −8. (b) Supra-threshold activation for the contrast of emotional (pleasant/unpleasant)−neutral pictures during viewing period (whole brain, FWE corrected at P = 0.05; cluster threshold k = 20 voxels). Red circles indicate bilateral amygdala activation. View centered on: x = 20, y = 0, z = −22.
Viewing period
A sample-wide whole-brain analysis contrasting emotional and neutral pictures during the viewing period (View Emotional−View Neutral) revealed supra-threshold activation in areas corresponding to the amygdala and visual areas of the occipital cortex (Figure 1b). Analyses focusing on designated anatomic regions as described above confirmed that the only regions that exhibited significant activation after FWE correction for multiple comparisons (α = 0.05) were left and right amygdala, left and right fusiform gyrus, left calcarine fissure, and right inferior occipital cortex—with none of the other regions (OFC, ACC, DLPFC, nAcc) displaying a significant difference (Table 1).
Brain reactivity differences associated with trait disinhibition
Preparation period
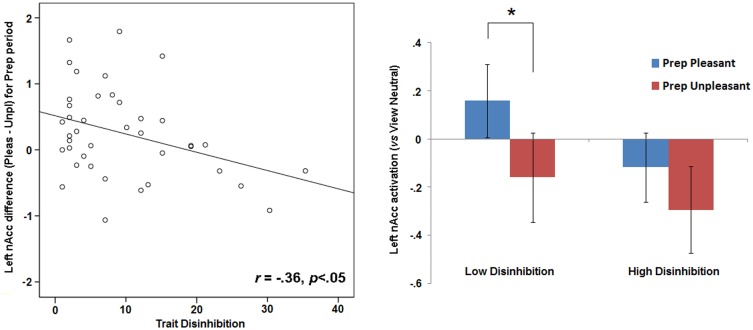
For the contrast of pleasant vs unpleasant pictures in the preparation period (Prep Pleasant−Prep Unpleasant), significant covariation was evident between disinhibition scores and differential activation of the left nAcc (r = −0.36, P < 0.05), with higher disinhibition associated with lesser pleasant−unpleasant differentiation during the preparation period (Figure 2). A significant relationship in the same direction was also found for the left calcarine fissure (r = −0.32, P < 0.05). However, when pleasant−unpleasant difference scores for both the left nAcc and left calcarine fissure were included as concurrent predictors of disinhibition scores in a regression model, only the relationship for the left nAcc emerged as significant (B = −0.31, P < 0.05; for calcarine fissure, B = −0.27, n.s.)—indicating that the effect for the calcarine fissure was secondary to (i.e. accounted for by) the effect for the nAcc.
Fig. 2.
Scatterplot on left depicts activation for left nAcc (mean beta values) during preparation to view pleasant pictures (contrast = Prep Pleasant − Prep Unpleasant). Bar plot on right depicts, for subjects scoring below and above the sample median on trait disinhibition, nAcc activation during prep periods within pleasant+neutral and unpleasant+neutral blocks−each contrasted against activation for the View Neutral condition. Error bars in right bar plot indicate standard error values. Asterisk indicates difference with P < 0.05.
Viewing period
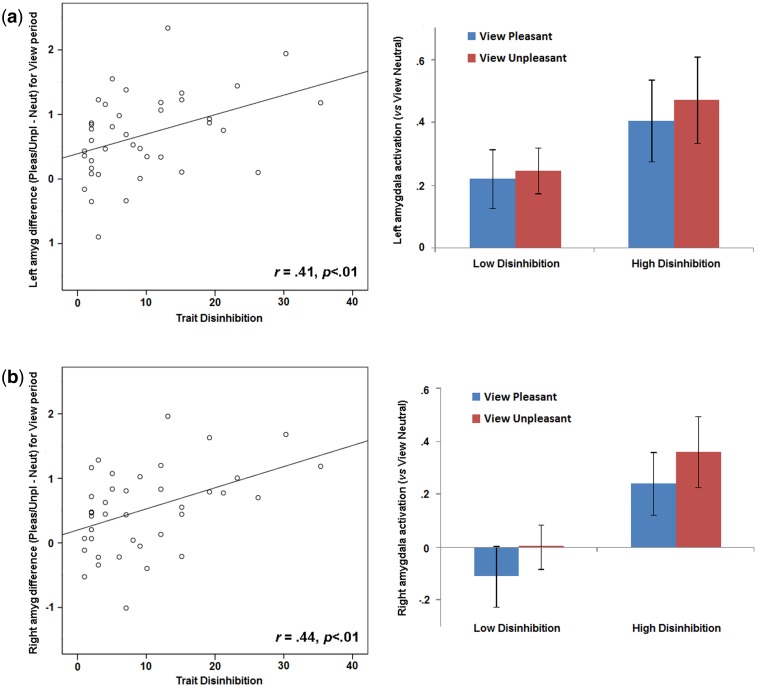
For the viewing period, difference scores (View Emotional—View Neutral) reflecting degree of enhanced activation in regions found to be significant in the whole-brain analysis (i.e. left and right amygdala, left and right fusiform gyrus, right inferior occipital gyrus, left calcarine fissure) were computed for individual participants and examined in relation to disinhibition scores. Increased differential activation was evident as function of higher disinhibition scores in the left and right amygdala regions, rs = 0.41 and 0.44, respectively, Ps < 0.01 (Figure 3). Effects for other brain regions exhibiting significant differential activation in the sample-wide analysis for the viewing period did not covary with disinhibition.
Fig. 3.
Scatterplots on left depict activation for left (a) and right (b) amygdala (mean beta values) during viewing of emotional pictures (contrast = View Emotional − View Neutral). Bar plots on right depict, for subjects scoring below and above the sample median on trait disinhibition, left (a) and right (b) amygdala activation during viewing of pleasant and unpleasant pictures, each contrasted against activation for the View Neutral condition. Error bars in right bar plots indicate standard error values. Within-subject effects (i.e., pleasant vs unpleasant) were not evident for either Disinhibition group in left or right amygdala; however, activation in both amygdala regions differed across groups as shown, ts (38) = −2.01/−2.12, respectively, ps = .04/.05.
Overlap in effects for preparation and viewing periods
Follow-up analyses were conducted to assess for interdependency between disinhibition-related effects for the nAcc during preparation and for the amygdala during viewing. As noted above, disinhibition scores predicted both left nAcc differentiation during preparation (pleasant−unpleasant trial blocks) and left/right amygdala reactivity during picture viewing (emotional−neutral pictures): rs = −0.36 and 0.41/0.44, respectively. In addition, reduced left nAcc activation during preparation significantly predicted enhanced left and right amygdala reactivity during viewing: rs = −0.43 and −0.41, respectively, Ps < 0.01. To directly evaluate whether reduced left nAcc differentiation prior to picture onset contributed to enhanced amygdala reactivity for high-disinhibited participants during viewing, we performed an estimation of indirect effects analysis (Preacher and Hayes, 2008) to formally test for mediation. This analysis confirmed a significant indirect effect (i.e. mediating role) of preparatory left nAcc activation in the relationship between disinhibition scores and reactivity to emotional pictures for both left and right amygdala [95% confidence intervals = (0.04, 1.61) and (0.06, 1.48), respectively].
Discussion
This study investigated differences in degree of activation of relevant brain regions in participants scoring higher or lower in trait disinhibition when preparing for or viewing emotional picture stimuli. The results were expected to support either the view that disinhibition is related to heightened reward sensitivity, or that it reflects enhanced reactivity to negative emotional stimuli.
For the overall sample, we found increased limbic activation in the nAcc during preparation to view pleasant pictures as compared with unpleasant pictures. This is in accordance with prior literature describing this area as a reward anticipation region (Knutson et al., 2001; Knutson and Cooper, 2005). During actual viewing, emotional pictures elicited increased bilateral amygdala activation relative to neutral, as has been reported previously (Costa et al., 2010; Sabatinelli et al., 2005), along with increased activity in visual processing regions [calcarine fissure, fusiform gyrus, occipital gyrus (Bradley et al., 2003; Keil et al., 2009)].
As regards trait disinhibition, we found that high disinhibition scores were associated with reduced activation of the left nAcc during preparatory periods within pleasant/neutral trial blocks compared with unpleasant/neutral blocks, together with enhanced bilateral amygdala reactivity to both pleasant and unpleasant pictures (relative to neutral) during subsequent viewing periods. A formal mediation analysis showed these effects to be interrelated, revealing that reduced pleasant/unpleasant differentiation in the nAcc during preparation contributed to enhanced emotional/neutral differentiation in the amygdala during viewing. The implication is that the relationship between trait disinhibition and augmented amygdala reactivity during the viewing of emotional pictures within pleasant vs unpleasant blocks was at least partially attributable to reduced anticipation of the possible affective quality of upcoming pictures.
These findings both replicate and extend prior research findings. Evidence was found for augmented brain reactivity to both positive (Buckholtz et al., 2010; Lawrence and Brooks, 2014) and negative (Iacono et al., 1999; Viding et al., 2012) emotional stimuli. In addition, we found this increased reactivity to be related to reduced preparatory responding—indicating a failure on the part of high-disinhibited individuals to develop expectancies regarding the nature of to-be-viewed pictures over the course of presentations occurring within blocks of each type. This aspect of the current findings coincides with results from Iacono et al. (1999), who reported reduced ability to down-regulate physiological-affective reactions to aversive noise blasts when the noises were made predictable. The implication is that high disinhibition (externalizing proneness) entails a deficit in the natural tendency to formulate contextual representations or schemas for ongoing stimulus events having motivational significance. As a function of reduced anticipatory response, high-disinhibited participants showed enhanced amygdala reactivity to picture stimuli at the time of their occurrence—interpretable as increased motive-driven attention to these stimuli (Gallagher and Holland, 1992; Lang et al., 1997). The fact that amygdala reactivity was enhanced during viewing of both pleasurable and aversive pictures suggests a global impairment in the development of schemas for affective events, as opposed to a selective anomaly in the processing of either reward or threat stimuli.
Notably, the current study used an incidental pre-cuing manipulation in which participants were signaled in advance of each picture, without explicit information as to what type of picture would appear. However, pictures were presented in blocks of either ‘pleasant + neutral’ or ‘unpleasant + neutral’—creating an opportunity for expectancies to develop regarding the nature of to-be-viewed pictures. This differs from explicit-cuing procedures used in other studies that have reported increased rather than decreased nAcc activation in high-disinhibited individuals during anticipation of pleasurable events. For example, Buckholtz et al. (2010) reported enhanced nAcc activation for high impulsive-antisocial participants in a monetary incentive delay task in which participants were cued explicitly prior to the receipt of financial rewards. The implication is that findings for high-disinhibited individuals can differ dramatically depending on experimental task parameters [cf. (Steinberg, 2008)]. Implicit vs explicit cuing appears to be one such parameter: whereas Buckholtz et al.’s findings indicate overresponsiveness of high-disinhibited individuals to explicit cues for reward, current findings indicate that these individuals are less sensitive to possibilities for pleasurable outcomes in implicit cuing contexts (e.g. where prospects for reward need to be inferred). Additionally, our results suggest that impairments in the capacity to process implicit motivational information in such individuals may extend to aversive outcomes. Taken together, these contrasting findings point to a need for systematic research comparing reactions of high- vs low-disinhibited individuals to both pleasurable and aversive cues in implicit as well as explicit cuing contexts.
Some ostensible limitations of the current work need to be considered in interpreting our results and evaluating their implications for understanding of serious impulse-control problems. One potential concern is that participants were not patients diagnosed with externalizing disorders but rather college students varying in levels of disinhibitory tendencies as indexed by a self-report scale. However, this concern is mitigated by prior work demonstrating that externalizing psychopathology is continuous rather than discrete (Krueger et al., 2002, 2005) and that electrocortical response differences evident in clinical samples with severe externalizing problems (Venables and Patrick, 2014) are also observed in individuals from college or community samples who score high in disinhibitory tendencies (Nelson et al., 2011; Patrick et al., 2013). Further, the effect of reduced responsiveness to reward is consistent with data from patient populations (Barkley et al., 2001; Demurie et al., 2012; White et al., 2014). Regarding the scale measure of disinhibition we used, this measure was carefully designed to index liability toward externalizing problems (Krueger et al., 2007; Patrick et al., 2013), and prior research has shown that scores on this scale covary substantially with interview-assessed externalizing symptoms—largely as a function of shared genetic influences that covary in turn with brain response (Yancey et al., 2013). As such, this scale measure provides an effective vehicle for bridging between brain response measures and clinical problems (Patrick et al., 2013).
Another limitation of the study is that participants consisted of college-aged women, raising questions about the generalizability of findings to older female samples as well as to younger and older men. Notably, other published work has demonstrated associations between disinhibition and electrocortical response in older mixed-gender samples [e.g. (Patrick et al., 2013; Yancey et al., 2013; Brislin et al., 2014)]. Additionally, studies utilizing mixed-gender samples that have tested for moderating effects of gender on relations between disinhibition and electrocortical response have not found such effects (Bernat et al., 2011; Nelson et al., 2011). Beyond this, twin modeling studies that have tested for gender differences in externalizing tendencies have found mean-level differences between men and women in broad disinhibitory liability, but no differences in the phenotypic structure of such tendencies or in etiologic influences contributing to them (Krueger et al., 2002; Hicks et al., 2004). While these lines of evidence suggest that current findings for high-disinhibited women would likely extend to men, follow-up studies examining disinhibition-related differences in fMRI brain response in mixed-gender samples of differing ages are clearly needed before firm conclusions can be drawn. While the sample size of this study is above-average for fMRI studies (David et al., 2013) and of sufficient size to detect relevant effects (Desmond and Glover, 2002), it bears noting that due to the correlational nature of this study, a larger-scale study could have found stronger or additional effects.
Notwithstanding these limitations, findings from this study provide important new insights into the nature of brain-based processing deviations in individuals prone to impulse-control problems. In a task that included aversive as well as pleasurable visual stimuli and an incidental pre-cuing manipulation, high-disinhibited participants showed heightened amygdala response to stimulus presentations of both types, in the context of reduced anticipatory nAcc differentiation. That disinhibition-related effects emerged for these subcortical brain regions in particular points to differences in basic motive-driven processing. Specifically, our results indicate that high-disinhibited participants failed to develop motivational recognition of to-be-viewed stimuli within pleasurable vs aversive blocks, and as a function of this, responded with more intense amygdala activation to affective stimulus presentations. This pattern is indicative of a stimulus-driven orientation in which affective cues are processed in an immediate, local manner without being assimilated into a broader representation of the ongoing task context [Bechara et al. 1994; Iacono et al., 1999; see also Miller and Cohen 2001]. This stimulus-driven processing style could be expected to give rise to behavioral patterns characterized by pursuit of immediate tangible rewards and disregard for potential adverse consequences (Davidson et al., 2000; Patrick et al., 2012).
Importantly, our findings in conjunction with prior work also suggest that the direction of relationship between externalizing proneness and reward-system (nAcc) response is likely to depend on the cuing parameters of a task. In tasks where cues for an upcoming reward are salient and explicit, high-disinhibited individuals can be expected to show increased reward-system response [cf. (Buckholtz et al., 2010)]. In contrast, in tasks where prospects for reward are implicit rather than explicit, as in the current study, high-disinhibited individuals are likely to show weaker reward-system response. The ability to recognize possibilities for reward under implicit, contextual cuing is likely dependent on regions such as the hippocampus and prefrontal cortex (Miller and Cohen, 2001) that may be dysfunctional in high-disinhibited individuals. Further research will be needed to evaluate this possibility as related to reward processing across differing contexts. Additionally, vis-à-vis alternative hypotheses stated at the outset, our findings suggest a broader implicit processing/context-representation deficit in externalizing individuals—insofar as enhanced amygdala reactivity was evident for aversive as well as pleasant stimulus presentations, suggesting a failure to anticipate affective events of either type within blocks in which they occurred. This finding coincides with prior work demonstrating reduced ability to down-regulate emotional reactivity to aversive events under conditions of enhanced predictability (Iacono et al., 1999). We encourage further systematic research directed at evaluating whether a common stimulus-driven orientation accounts for disinhibition-related differences in reactivity to aversive as well as pleasurable events within particular contexts of cuing. We also encourage research aimed at further clarifying the roles of enhanced subcortical-circuit reactivity and reinforcement learning deficits in disinhibitory problems (Blair et al., 2014).
Supplementary Material
Acknowledgements
Data collection for this work was supported by NIH BTRC grant P41 RR008079 (NMR Imaging and Spectroscopy). Preparation of this article was supported in part by US Army grant W911NF-14‐1‐0027 and NIMH grant MH089727. The views, opinions, and/or findings contained in this report are those of the authors and shall not be construed as an official Department of the Army position, policy, or decision, unless so designated by other documents.
Footnotes
1 Externalizing proneness is conceptualized as a latent liability factor (Krueger et al., 2002), for which trait disinhibition serves as a report-based operationalization (Patrick et al., 2013).
2 While electrodermal response is considered to arise from a different branch of the nervous system than fMRI response (i.e. autonomic/sympathetic vs central/brain), the two are clearly interrelated. Electrodermal reactivity to task stimuli is influenced by central brain structures, including frontal regions implicated in externalizing problems (Bechara et al., 2000; Davidson et al., 2000), and research has shown that electrodermal and fMRI responses covary (Critchley et al., 2000) and that this covariation occurs in differing task contexts, including resting state measurement (Patterson et al., 2002).
3 Using a less conservative designated-ROI criterion of 0.01 (uncorrected) for regions predicted a priori to show effects, differential activation was also evident for the left nAcc.
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
References
- Barkley R.A., Edwards G., Laneri M., Fletcher K., Metevia L. (2001). Executive functioning, temporal discounting, and sense of time in adolescents with attention deficit hyperactivity disorder (ADHD) and oppositional defiant disorder (ODD). Journal of Abnormal Child Psychology, 29(6), 541–56. [DOI] [PubMed] [Google Scholar]
- Beauchaine T.P., McNulty T. (2013). Comorbidities and continuities as ontogenic processes: toward a developmental spectrum model of externalizing psychopathology. Development and Psychopathology, 25(4 Pt 2), 1505–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A., Damasio A.R., Damasio H., Anderson S.W. (1994). Insensitivity to future consequences following damage to human prefrontal cortex. Cognition, 50(1‐3), 7–15. [DOI] [PubMed] [Google Scholar]
- Bechara A., Damasio H., Damasio A.R. (2000). Emotion, decision making, and the orbitofrontal cortex. Cerebral Cortex, 10(3), 295–307. [DOI] [PubMed] [Google Scholar]
- Bernat E.M., Nelson L.D., Steele V.R., Gehring W.J., Patrick C.J. (2011). Externalizing psychopathology and gain/loss feedback in a simulated gambling task: dissociable components of brain response revealed by time-frequency analysis. Journal of Abnormal Psychology, 120, 352–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair R.J.R. (2013). The neurobiology of psychopathic traits in youths. Nature Neuroscience, 14, 786–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair R.J.R., Leibenluft E., Pine D.S. (2014). Conduct disorder and callous-unemotional traits in youth. New England Journal of Medicine, 371, 2207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blonigen D.M., Hicks B.M., Krueger R.F., Patrick C.J., Iacono W.G. (2005). Psychopathic personality traits: heritability and genetic overlap with internalizing and externalizing psychopathology. Psychological Medicine, 35(5), 637–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley M.M., Sabatinelli D., Lang P.J., Fitzsimmons J.R., King W., Desai P. (2003). Activation of the visual cortex in motivated attention. Behavioral Neuroscience, 117(2), 369–80. [DOI] [PubMed] [Google Scholar]
- Brislin S.J., Yancey J.R., Drislane L.E., Bowyer C.B., Roche N., Patrick C.J. (2014). P3 amplitude shows differential relations with triarchic psychopathy facets. Psychophysiology, 51, S33–4. [Google Scholar]
- Buckholtz J.W., Treadway M.T., Cowan R.L., et al. (2010). Mesolimbic dopamine reward system hypersensitivity in individuals with psychopathic traits. Nature Neuroscience, 13(4), 419–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L., Haier R.J., White N.S., et al. (2001). Sex-related difference in amygdala activity during emotionally influenced memory storage. Neurobiology of Learning and Memory, 75(1), 1–9. [DOI] [PubMed] [Google Scholar]
- Chen N.K., Dickey C.C., Yoo S.S., Guttmann C.R., Panych L.P. (2003). Selection of voxel size and slice orientation for fMRI in the presence of susceptibility field gradients: application to imaging of the amygdala. Neuroimage, 19(3), 817–25. [DOI] [PubMed] [Google Scholar]
- Costa V.D., Lang P.J., Sabatinelli D., Versace F., Bradley M.M. (2010). Emotional imagery: assessing pleasure and arousal in the brain's reward circuitry. Human Brain Mapping, 31(9), 1446–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley H.D., Elliott R., Mathias C.J., Dolan R.J. (2000). Neural activity relating to generation and representation of galvanic skin conductance responses: a functional magnetic resonance imaging study. The Journal of Neuroscience, 20, 3033–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David S.P., Ware J.J., Chu I.M., et al. (2013). Potential reporting bias in fMRI studies of the brain. PLoS One, 8(7), e70104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson R.J., Putnam K.M., Larson C.L. (2000). Dysfunction in the neural circuitry of emotion regulation—a possible prelude to violence. Science, 289(5479), 591–4. [DOI] [PubMed] [Google Scholar]
- Demurie E., Roeyers H., Baeyens D., Sonuga-Barke E. (2012). Temporal discounting of monetary rewards in children and adolescents with ADHD and autism spectrum disorders. Developmental Science, 15(6), 791–800. [DOI] [PubMed] [Google Scholar]
- Desmond J.R., Glover G.H. (2002). Estimating sample size in functional MRI (fMRI) neuroimaging studies: statistical power analyses. Journal of Neuroscience Methods, 118, 115–28. [DOI] [PubMed] [Google Scholar]
- Dodge K.A., Pettit G.S., Bates J.E., Valente E. (1995). Social information-processing patterns partially mediate the effect of early physical abuse on later conduct problems. Journal of Abnormal Psychology, 104, 632–43. [DOI] [PubMed] [Google Scholar]
- Frick P.J., Marsee M.A. (2006). Psychopathy and developmental pathways to antisocial behavior in youth. In: Patrick C.J.editor. Handbook of Psychopathy. New York: Guilford Press, 353–74. [Google Scholar]
- Gallagher M., Holland P.C. (1992). The amygdala complex: multiple roles in associative learning and attention. Proceedings of the National Academy of Sciences of the United States of America, 91, 11771–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A., Siessmeier T., Wrase J., et al. (2014). Correlation between dopamine D2 receptors in the ventral striatum and central processing of alcohol cues and craving. The American Journal of Psychiatry, 161, 1783–9. [DOI] [PubMed] [Google Scholar]
- Hicks B.M., Krueger R.F., Iacono W.G., McGue M., Patrick C.J. (2004). Family transmission and heritability of externalizing disorders: a twin-family study. Archives of General Psychiatry, 61(9), 922–8. [DOI] [PubMed] [Google Scholar]
- Iacono W.G., Carlson S.R., Taylor J., Elkins I.J., McGue M. (1999). Behavioral disinhibition and the development of substance-use disorders: findings from the Minnesota Twin Family Study. Development and Psychopathology, 11(4), 869–900. [DOI] [PubMed] [Google Scholar]
- Keil A., Sabatinelli D., Ding M., Lang P.J., Ihssen N., Heim S. (2009). Re-entrant projections modulate visual cortex in affective perception: evidence from Granger causality analysis. Human Brain Mapping, 30(2), 532–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.H., Hamann S. (2007). Neural correlates of positive and negative emotion regulation. Journal of Cognitive Neuroscience, 9(5), 776–98. [DOI] [PubMed] [Google Scholar]
- Knutson B., Adams C.M., Fong G.W., Hommer D. (2001). Anticipation of increasing monetary reward selectively recruits nucleus accumbens. The Journal of Neuroscience, 21(16), RC159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B., Cooper J.C. (2005). Functional magnetic resonance imaging of reward prediction. Current Opinion in Neurology, 18(4), 411–7. [DOI] [PubMed] [Google Scholar]
- Krueger R.F., Hicks B.M., Patrick C.J., Carlson S.R., Iacono W.G., McGue M. (2002). Etiologic connections among substance dependence, antisocial behavior, and personality: modeling the externalizing spectrum. Journal of Abnormal Psychology, 111, 411–24. [PubMed] [Google Scholar]
- Krueger R.F., Markon K.E., Patrick C.J., Benning S.D., Kramer M.D. (2007). Linking antisocial behavior, substance use, and personality: an integrative quantitative model of the adult externalizing spectrum. Journal of Abnormal Psychology, 116, 645–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger R.F., Markon K.E., Patrick C.J., Iacono W.G. (2005). Externalizing psychopathology in adulthood: a dimensional-spectrum conceptualization and its implications for DSM-V. Journal of Abnormal Psychology, 114(4), 537–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang P.J., Bradley M.M., Cuthbert B.N. (1997). Motivated attention: affect, activation, and action. In: Lang P.J., Simmons R.F., Balaban M.T.editors. Attention and Orienting: Sensory and Motivational Processes. Hillsdale, NJ: Erlbaum, 97–135. [Google Scholar]
- Lang P.J., Bradley M.M., Cuthbert B.N. (1999). International Affective Picture System (IAPS): Technical Manual and Affective Ratings. Gainesville: University of Florida, Center for Research in Psychophysiology. [Google Scholar]
- Lawrence A.D., Brooks D.J. (2014). Ventral striatal dopamine synthesis capacity is associated with individual differences in behavioral disinhibition. Frontiers in Behavioral Neuroscience, 8, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E.K., Cohen J.D. (2001). Integrative theory of prefrontal cortex function. Annual Review of Neuroscience, 24, 167–202. [DOI] [PubMed] [Google Scholar]
- Nelson L.D., Patrick C.J., Bernat E.M. (2011). Operationalizing proneness to externalizing psychopathology as a multivariate psychophysiological phenotype. Psychophysiology , 48, 64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner K.N., Bunge S.A., Gross J.J., Gabrieli J.D. (2002). Rethinking feelings: an fMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience, 14(8), 1215–29. [DOI] [PubMed] [Google Scholar]
- Patrick C.J. (2008). Psychophysiological correlates of aggression and violence: an integrative review. Philosophical Transactions of the Royal Society B (Biological Sciences), 363, 2543–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick C.J., Durbin C.E., Moser J.S. (2012). Conceptualizing proneness to antisocial deviance in neurobehavioral terms. Development and Psychopathology, 24, 1047–71. [DOI] [PubMed] [Google Scholar]
- Patrick C.J., Venables N.C., Yancey J.R., Hicks B.M., Nelson L.D., Kramer M.D. (2013). A construct-network approach to bridging diagnostic and physiological domains: application to assessment of externalizing psychopathology. Journal of Abnormal Psychology, 122(3), 902–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson J.C., Ungerleider L.G., Bandettini P.A. (2002). Task-independent functional brain activity correlation with skin conductance changes: an fMRI study. Neuroimage, 17, 1797–806. [DOI] [PubMed] [Google Scholar]
- Preacher K.J., Hayes A.F. (2008). Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods, 40, 879–91. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D., Bradley M.M., Fitzsimmons J.R., Lang P.J. (2005). Parallel amygdala and inferotemporal activation reflect emotional intensity and fear relevance. Neuroimage, 24(4), 1265–70. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D., Bradley M.M., Lang P.J., Costa V.D., Versace F. (2007). Pleasure rather than salience activates human nucleus accumbens and medial prefrontal cortex. Journal of Neurophysiology, 98, 1374–9. [DOI] [PubMed] [Google Scholar]
- Salimpoor V.N., Benovoy M., Larcher K., Dagher A., Zatorre R.J. (2011). Anatomically distinct dopamine release during anticipation and experience of peak emotion to music. Nature Neuroscience, 14(2), 257–62. [DOI] [PubMed] [Google Scholar]
- Seo D., Jia Z., Lacadie C.M., Tsou K.A., Bergquist K., Sinha R. (2011). Sex differences in neural responses to stress and alcohol context cues. Human Brain Mapping, 32(11), 1998–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo D., Olman C.A., Haut K.M., Sinha R., MacDonald A.W., III, Patrick C.J. (2013). Neural correlates of preparatory and regulatory control over positive and negative emotion. Social Cognitive and Affective Neuroscience, 9(4), 494–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher K.J., Trull T.J. (1994). Personality and disinhibitory psychopathology: alcoholism and antisocial personality disorder. Journal of Abnormal Psychology, 103, 92–102. [DOI] [PubMed] [Google Scholar]
- Steinberg L. (2008). A social neuroscience perspective on adolescent risk-taking. Developmental Review, 28(1), 78–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venables N.C., Patrick C.J. (2014). P3 brain response amplitude in criminal psychopathy: distinct relations with impulsive-antisocial versus affective-interpersonal features. Psychophysiology, 51, 427–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viding E., Sebastian C.L., Dadds M.R., et al. (2012). Amygdala response to preattentive masked fear in children with conduct problems: the role of callous-unemotional traits. The American Journal of Psychiatry, 169, 109–16. [DOI] [PubMed] [Google Scholar]
- White S.F., Clanton R., Brislin S.J., et al. (2014). Reward: empirical contribution. Temporal discounting and conduct disorder in adolescents. Journal of Personality Disorders, 28(1), 5–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey J.R., Venables N.C., Hicks B.M., Patrick C.J. (2013). Evidence for a heritable brain basis to deviance-promoting deficits in self-control. Journal of Criminal Justice, 41(5), 309–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S.E., Friedman N.P., Miyake A., et al. (2009). Behavioral disinhibition: liability for externalizing spectrum disorders and its genetic and environmental relation to response inhibition across adolescence. Journal of Abnormal Psychology, 118, 117–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.