Abstract
Cancer represents the second cause of death in prepubertal children and adolescents, although it is currently associated with an overall survival rate of 80%–85%. The annual incidence rate is 186.6 per 1 million children and adolescents aged up to 19 years. Both disease and treatment options are associated with life-altering, long-term effects that require monitoring. Infertility is a common issue, and as such, fertility preservation represents an essential part in the management of young patients with cancer who are at risk of premature gonadal failure. This review deals with the up-to-date available data on fertility risk assessment and preservation strategies that should be addressed prior to antineoplastic therapy in this vulnerable subgroup of cancer patients.
Keywords: fertility, risk assessment, preservation, adolescents, cancer, radiotherapy, chemotherapy, surgery
Introduction
As a result of advances in cancer treatment, the five-year overall survival rate of adolescents and young adults currently stands at 80%–87% for both Europe1 and the United States of America.2 The American Cancer Society estimated that 10,380 new cases of cancer and 1,250 deaths from cancer would occur in 2016 among males and females aged 0–14 years.2 The most common cancers that occur in this age group include leukemias and lymphomas, brain and central nervous system tumors, embryonal tumors, sarcomas of bone and soft tissue, and gonadal germ cell tumors.2 Despite recent advances in the treatments of malignancies that may cure these young cancer patients,3–5 infertility is an important long-term toxicity in both females6 and males.7,8 Infertility is associated with significant psychological distress, with levels of depression twice that of the normal population in both young female9 and male cancer survivors.10 Even for patients who may have not planned to have children, most commonly due to their very young age, the threat of infertility can result in a deep sense of loss and anger.11
Since post-therapy recovery of gonadal function remains unpredictable, it is important to inform patients facing infertility of this possible side effect of their treatment and all the options available to prevent it.12 As survival worries may deviate from important life dreams, it is advisable to anticipate and facilitate the long-term perspectives that may not be readily apparent to young patients in this sensitive situation.13 Not surprisingly, fertility preservation concerns in many instances may influence patients’ treatment decisions, as for example in cases of breast cancer,14,15 although the general tendency of both patients and their parents is opposite.16,17
Herein, we present a comprehensive review of fertility risk assessment strategies including medical and surgical strategies that can preserve fertility in prepubertal and pubertal cancer patients.
Fertility Risk Assessment and Strategic Planning
Recent advances in cancer therapies have led to increased cure rates of male and female prepubertal and adolescent patients with malignant disease. As the likelihood of being long-term survivors is very high, it has become of utmost importance to assess the risk of infertility caused by treatment and to communicate this with the young patients and their parents, right at the beginning of their cancer journey (Fig. 1). Risk assessment for fertility preservation of a young patient includes the evaluation of both extrinsic and intrinsic factors that when combined assign patients into a group category of high, medium, and low risk (Table 1). Nevertheless, the exact assessment of individual factors is confounded by the constantly evolving treatment schedules and the difficulty to assess the gonadal reserve of each patient. The effect of chemotherapy on male and female gonads varies significantly among different drug combinations (Tables 2 and 3).
Figure 1.
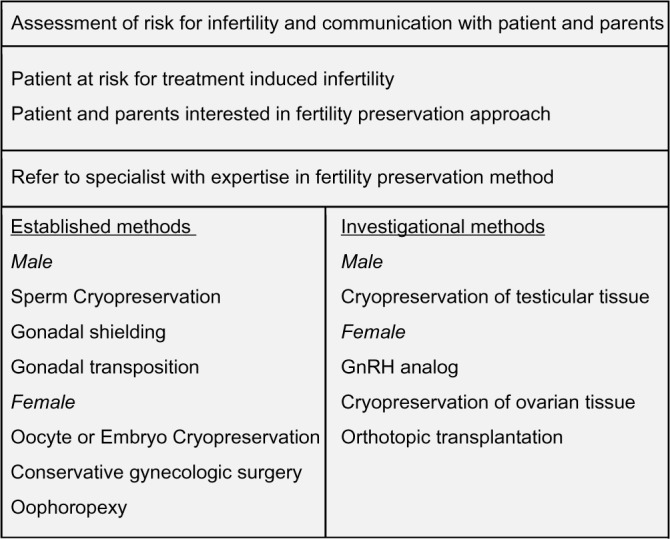
Flow diagram of fertility preservation strategy.
Table 1.
Extrinsic and intrinsic risk factors associated with infertility in adolescent cancer patients. Reprinted with permission, from: Wallace WH et al. J Clin Oncol. 30(1);2012:3–5. © 2012 American Society of Clinical Oncology. All rights reserved.122
| INTRINSIC FACTORS |
|---|
| Health status of patient |
| • Consent (patient/parent) |
| • Assessment of pubertal stage in young males (including testicular volume) |
| • Assessment of ovarian reserve in young females |
| • Tumor type, stage and location |
| • Performance status |
| • Ability to undergo fertility-sparingg procedures |
| EXTRINSIC FACTORS |
| • Treatment options |
| • Radiotherapy |
| • Surgery |
| • Chemotherapy (high/medium/low/uncertain risk for game) |
| • Dose and topology |
| • Time available for the procedure |
| • Access to Fertility Centers with specific expertise |
Table 2.
Classification of infertility risk induced by chemotherapy in females.
| CHEMOTHERAPY TREATMENT | DEGREE OF RISK |
|---|---|
| Hematopoietic stem cell transplantation and total body irradiation Radiotherapy to a field including the ovaries | High risk >80% |
| CAF, CMF, CEF x6 30–39 years of age ACx4 >40 years of age | Intermediate risk |
| ABVD,CHOP,CVP,AML, ALL CAF, CMF, CEF x6 <30 years of age ACx4 <40 years of age | Lower Risk (<20%) |
| Vincristine Methotrexate Fluorouracil |
Very Low or No Risk |
| Taxanes Irinotecan Oxaliplatin Monoclonal antibodies Tyrosine kinase inhibitors |
Unknown Risk |
Abbreviations: C, cyclophosphamide 600–1200 mg/m2; A, adriamycin 25–60 mg/m2; F, fluorouracil 600 mg/m2; E, epirubicin 60 mg/m2; M, methotrexate 40 mg/m2; B, bleomycin 10 U/m2; V, vinblastine 6 mg/m2; D, dacarbazine 375 mg/m2; V (O), vincristine 1.2 mg/m2–2 mg; P, prednisolone 40 mg/m2; H, hydroxydaunorubicin 50 mg/m2.
Table 3.
Classification of infertility risk induced by chemotherapy in males.
| CHEMOTHERAPY TREATMENT | EFFECT ON SPERM COUNT |
|---|---|
| Chlorambucil (1.4 g/m2) Cyclophosphamide (19 g/m2) Procarbazine (4 g/m2) Melphalan (140 mg/m2) Cisplatin (500 mg/m2) |
Prolonged or permanent azoospermia |
| BCNU (1 g/m2) CCNU (500 mg/m2) |
Azoospermia in adulthood if treated before puberty |
| Busulfan (600 mg/m2) Ifosfamide (42 g/m2) BCNU (300 mg/m2) Nitrogen mustard |
Azoospermia likely, and are often given with other highly sterilizing agents, adding to the effect |
| Doxorubicin (770 mg/m2) Thiotepa (400 mg/m2) Cytarabine (1 g/m2) Vinblastine (50 g/m2) Vincristine (8 g/m2) |
When used alone, cause only temporary reductions in sperm count. In conjunction with above agents, may be additive in causing azoospermia |
| Amsacrine Bleomycin Dacarbazine Daunorubicin Epirubicin Etoposide Fludarabine Fluorouracil 6-mercaptopurine Methotrexate Mitoxantrone Thioguanine |
When used in conventional regimens, cause only temporary reductions in sperm count. In conjunction with above agents, may be additive in causing azoospermia |
A special consideration includes the role of anti-Mullerian hormone (AMH) in predicting poor outcome in assisted reproduction, as shown by data from studies conducted more than 10 years ago.18 Of note, AMH values physiologically peak around the age of 26 years, so that its use is limited in females ≤25 years of age. Although there are limited data to reach robust conclusions on the relationship between AMH and the ovarian reserve in children and adolescents, there is growing evidence of its value as a potential marker of chemotherapy-induced ovarian follicular depletion. Recent studies indicate that AMH serves as an early plasma marker of chemotherapy-induced gonadal damage and is closely related to the ovarian reserve of patients before and after cancer treatment.19,20 In a prospective study including 22 females (17 prepubertal) of median age 4.4 years (range 0.3–15 years), it was shown that AMH was detectable prior to treatment in girls of all ages but fell rapidly during cancer treatment in both prepubertal and pubertal girls.21 Both the fall during treatment and recovery thereafter were linked with the risk of gonadotoxicity qualifying AMH as a clinically useful marker of damage to the ovarian reserve.21
For prepubertal patients, the American Society Clinical Oncology guidelines on fertility preservation recommend to use established methods of fertility preservation (gonadal tissue cryopreservation, radiation shielding, or ovarian transposition), with patient assent, if appropriate, and parent or guardian consent.22 Additionally, for adolescent patients, the American Society Clinical Oncology recommends to present information on additional methods that are investigational and refer for experimental protocols when available.22 The National Comprehensive Cancer Network (NCCN) guidelines also suggest the referral of all patients who choose it to fertility preservation clinics within 24 hours and to a mental health professional to assist with complex decision-making, if needed (NCCN guidelines version 1.2016; Adolescent and young adult oncology; Fertility and endocrine considerations; http://www.nccn.org).
Effect of anticancer therapy in prepubertal and pubertal ovarian function
The effects of cancer therapy on ovarian function in prepubertal girls are both underreported and heterogeneous, mainly due to the fact that it is difficult to be assessed prior and after the therapeutic strategies applied along with the low predictive value that hormonal tests have, and the various cancer types. Certain chemotherapy agents are thought to be more gonadotoxic than others, but young adolescent patients are less vulnerable compared with older females.23,24 Combination chemotherapies including alkylating agents are thought to be associated with a significant risk of premature ovarian failure. However, more than 50% of survivors of acute lymphoblastic leukemia who had received such treatment were shown to have little or no ovarian toxicity.25 Treatment with chemotherapy such as hydrazines and nitrosoureas for brain tumors or long-term anthracyclines and vinca alkaloids combination therapies for Hodgkin lymphoma may cause transient primary ovarian insufficiency, but eventually, an 80% of females enter and progress normally through puberty.26 Ovarian function appears to return to normal gradually over a period of years, while elevated gonadotropin levels decrease to baseline. For patients with brain tumors, cranial radiation has deleterious effects as it adds chronic endocrine disorders, related to hypothalamic–pituitary dysfunction, on the direct gonadal toxicity of chemotherapy.27 Pelvic surgery or radiotherapy including the ovaries may cause permanent ovarian failure.28 In a former study, it has been estimated that a total radiation exposure of 20 Gy fractionated over 6 weeks in younger women and children produces sterility with 95% confidence.29 Similarly, in a recent retrospective study including prepubertal and pubertal girls, all patients receiving >15 Gy radiotherapy to the ovaries developed ovarian failure.28
Histologic assessment of prepubertal ovaries in children treated with chemotherapy, such as single-agent cyclophosphamide, indicates a considerable damage, including follicular maturation arrest, stromal fibrosis, and a partially depleted oocyte population.30,31 Injury to blood vessels, focal ovarian cortical fibrosis, and direct apoptotic effect of chemotherapy on follicles have also been shown to occur.6,32 Despite this evidence for primary gonadal damage, ovarian recovery occurs and menarche may appear normally or even prematurely.30
Effect of anticancer therapy in prepubertal and pubertal testicular function
The testicular effects of cancer therapy in prepubertal boys are heterogeneous due to the various cancer diseases and therapeutic strategies, including surgery, chemotherapy, or radiotherapy. A recent systematic review indicated that testicular germ cell tumors are associated with semen abnormalities before orchiectomy and outside the treatment effects of orchiectomy, radiation, or chemotherapy.33
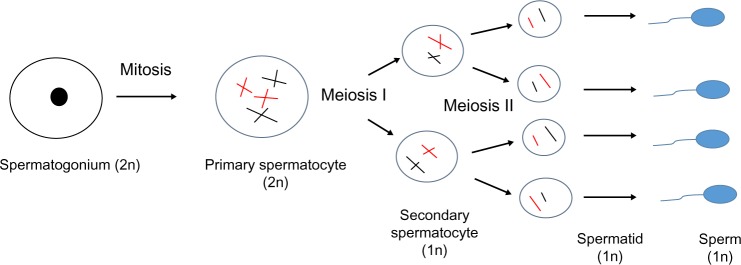
Prepubertal, adolescent, and adult male gonads exhibit similar sensitivity to chemotherapeutic agents (Table 3).34 Differentiating spermatogonia proliferate rapidly and are thus extremely vulnerable to cytotoxic agents, although the less active stem cell pool may also be depleted (Fig. 2).35 Modern adjuvant treatments for testicular germ cell tumor have drastic effects on spermatogenesis and sperm chromatin quality that decrease at 3–6 months and recover at 12 months following treatment with less than two cycles of bleomycin, etoposide, and cisplatin.36 However, the recovery period may become longer depending on treatment modalities, such as radiotherapy and more than two cycles of bleomycin, etoposide, and cisplatin, and patient’s characteristics such as pretreatment sperm production. Furthermore, despite lack of spermatogenesis completion in the prepubertal testis, cytotoxic treatment affects fertility by direct effect on early germ cells that undergo spontaneous degeneration before the haploid stage is reached.37
Figure 2.
The pathway of spermatogenesis. From Heller CG, Clermont Y. Spermatogenesis in man: an estimate of its duration. Science. 1963;140(3563):184–6. Reprinted with permission from AAAS.121
Recovery of sperm production after a cytotoxic therapy depends on the survival and ability of mitotically quiescent stem spermatogonia (type A dark) to transform into actively dividing stem and differentiating spermatogonia (type A pale).38 The somatic compartment of the testis may be more resistant to chemotherapeutic treatment, since these cells have a low or absent mitotic rate. Evidence of Sertoli cell functional impairment following chemotherapy, where germ cells have survived, has also been reported.39
Radiotherapy to the brain may damage the hypothalamic–pituitary axis if the dose is more than 30 Gy to the cranial region, resulting in endocrine complications involving the thyroid gland, the bone mass, and glucose homeostasis.40
The likelihood of infertility after radiation of the testes depends on the dose to the testes, shielding, and fractionation (single dose vs. multiple doses).41 Doses as small as 0.1 Gy can result in decreased sperm counts, and doses of 1.5–4 Gy can result in permanent sterility.41 The Leydig cells (responsible for testosterone production) are less sensitive to the effects of radiation, with damage occurring at 20 Gy in prepubescent males compared with 30 Gy in mature males.41
Female Fertility Preservation Approaches
Ovarian protection by GnRH analogs
One method of pharmacologic gonadal function preservation is based on the theory that germ cells are damaged by chemotherapy because they are rapidly dividing and reproducing; by administering a medical agent to stop the reproduction of these cells, the damage could be alleviated or even prevented. The temporary ovarian suppression with gonadotropin-releasing hormone analogs (GnRHa) targets for the prevention of chemotherapy-induced premature ovarian failure and for fertility preservation. A recent Cochrane systematic review concluded that the use of GnRHa should be considered in women of reproductive age receiving chemotherapy, as it seems to be effective in protecting ovaries during chemotherapy and should be given before or during treatment, although it was not associated with significant differences in pregnancy rates.42 Similarly, a 2014 meta-analysis of randomized trials showed that GnRHa significantly reduces the risk of chemotherapy-induced ovarian failure in young cancer patients.43 A new prospective phase III randomized study concluded that GnRHa administration with chemotherapy was associated with less premature ovarian failure and more pregnancies.44 Although two recently published randomized phase III studies indicated that the administration of GnRHa with chemotherapy may protect against ovarian failure,45,46 the trials did not include females under 18 years of age. Until today, there is no evidence supporting the role of GnRHa in prepubertal or pubertal male or female patients, and its use remains controversial.
Fertility-sparing surgery
Surgical techniques for preserving fertility in adolescents and young women include fertility-sparing surgery (FSS), ovarian transposition, modalities of ovarian transplantation, and ovarian tissue harvesting for cryopreservation.
Conservative unilateral salpingo-oophorectomy
Up to date, the exact data from worldwide FSS modalities in adolescent cancer patients have not been reported, but an ongoing interest has been emerged, particularly for young patients with borderline ovarian tumors.47–50 One-half to two-thirds of ovarian malignancies in females up to 18 years derive from germ cells, most commonly dysgerminomas.51,52 Fertility-preserving surgery followed by chemotherapy, even in advanced-stage malignant germ cell tumors of the ovary, is effective in conserving the reproductive function of these women.53 It consists of a conservative unilateral salpingo-oophorectomy with preservation of the contralateral ovary and uterus.54 Removal of the ipsilateral fallopian tube is indicated because of the presence of lymphovascular connections between the tube and the ovary.54 Although the possibility of occult contralateral ovarian involvement is about 5%–10%, a wedge biopsy of a normal-appearing contralateral ovary is not indicated, as these tumors are sensitive to chemotherapy, and salvage rates are up to 94% even in the presence of advanced disease.54,55 In addition, a surgical intervention on a normal ovary, even with a biopsy, may lead to postoperative adhesions, and further fertility impairment.54 A retrospective study reported on 169 women (age range: 8–41 years) with various histological subtypes of malignant germ cell ovarian tumors (70 dysgerminomas, 28 endodermal sinus tumors, 24 mixed tumors, and 47 immature teratomas) and stages of the disease.56 In 138 patients (81.6%), a FSS was performed, and 81% of them received chemotherapy postoperatively. The survival rate was 94% for dysgerminoma, 89% for endodermal sinus tumors, 100% for mixed tumors, and 98% for immature teratomas. With regard to fertility, the authors reported 14 conceptions in 12 patients who did not receive chemotherapy postoperatively and 41 conceptions in 16 patients who received.
Another common subtype of ovarian tumors in adolescents is borderline ovarian tumors that have low-malignant potential and account for 30% of all ovarian tumors.52 A substantial number of these tumors are presented as stage I disease with a five-year survival rate of 95%–97%.52 In the past, hysterectomy with bilateral salpingo-oophorectomy, peritoneal washing, omentectomy, and peritoneal biopsies was the treatment of choice. However, many reports have demonstrated that conservative surgery, through either unilateral cystectomy or a unilateral oophorectomy, exerts no effect on the overall survival rate.48,52 A retrospective study indicated that women with borderline ovarian tumors undergoing minimal surgery with ovarian cyst excision had almost comparable recurrence rates to those treated with unilateral salpingo-oophorectomy.57 Concerning fertility, 25 (40.3%) of 62 patients attained 38 pregnancies, resulting in 35 deliveries. Another retrospective study indicated high disease relapse rates, but no difference in the mortality and an overall 63.6% pregnancy rate, in women undergoing FSS.58 A prospective randomized trial in patients with bilateral borderline ovarian tumors indicated that the ultraconservative fertility-sparing approach is more effective than the standard approach in terms of reproductive outcomes, but presents a higher oncological risk.59 In the case of borderline tumors, careful assessment of the ovaries and close follow-up are mandatory when conservative treatment is employed.
Ovarian transposition
Radiation therapy is a commonly applied treatment to adolescents with various tumors, such as sarcomas, medulloblastomas, and Hodgkin’s lymphomas, involving the genitourinary tract and the pelvis.60 However, there is a risk of ovarian damage after exposure of the gonadal tissue to radiation, especially when combined with alkylating chemotherapy drugs such as cyclophosphamide.61 The failure of the ovarian function has been related to the radiation dose, the age of the patient, the type of the chemotherapeutic drugs used, and the type of radiation.62 Wallace et al showed that a single dose of radiation of <4 Gy is able to destroy 50% of primordial follicles, while a single dose of 10 Gy for a total body irradiation may cause complete cessation of ovarian function in 55%–80% of patients before entering puberty.63
Ovarian transposition was first described in 1958 as a method of preserving ovarian function.64 In this context, the ovaries are transferred outside the field of radiation, through laparotomy, laparoscopy, or robotic surgery65 and are fixed in the paracolic gutters above the pelvic brim and the psoas muscle when central radiation is indicated, or medially behind the uterus, when lateral pelvic lymph node radiation is designed.66 Whatever is the reason of transposition, caution should be made in order to avoid any damage of the ovarian blood supply. After transposition, the ovarian vessels should be examined for the presence of any kinking, and hemoclips should be placed to secure their location during future abdominal X-rays.67 However, in 10%–14% of cases, the procedure can fail to protect the ovaries.60 Common complications of ovarian transposition include intestinal obstruction, dyspareunia, functional ovarian cysts, and tubal obstruction caused by adhesions.68 Spontaneous pregnancies are possible if tubal function is preserved as part of the oophoropexy; otherwise, in vitro fertilization is applied.
Orthotopic transplantation of cryopreserved ovarian tissue
Cryopreservation and orthotopic transplantation of ovarian tissue is a breakthrough option for fertility preservation in young female cancer patients facing sterilizing antineoplastic therapy.69,70 Based on small case series, there have been more than 30 pregnancies after ovarian tissue cryopreservation and transplantation.71,72 The success rate is unclear as the denominator that corresponds to the exact number of women who had frozen-thawed ovarian tissue reimplanted is unknown. With the given age-related decline from birth until menopause, in the number of non-growing follicles,73 and the difficulties associated with ovarian stimulation and oocyte collection, young adolescents facing gonadotoxic treatment are potentially ideal candidates for ovarian cortex harvesting before the initiation of therapy and reimplantation at a later time. The answer is probably hidden behind the medical axiom: “to do good or to do no harm”.
Reports on orthotopic reimplantation of cryopreserved ovarian tissue are encouraging, in terms of safety, feasibility, and efficacy, as it is performed through laparoscopy or laparotomy under general anesthesia and is the only method that may lead to spontaneous pregnancies.74–76 Consent for harvesting ovarian tissue is usually obtained from their parents, whereas informed consent for its reimplantation is obtained from the patients much later, when they are competent to assess the complex issues by themselves. Excision of the ovary is followed by freezing of the ovarian cortex with the prospect of reimplantation or in vitro maturation of oocytes at a later time.70,77 The frozen ovarian cortex reimplantation takes place either orthotopically (at the site of the remaining ovary) or heterotopically (in the subcutaneous tissue of the abdomen or forearm).70,78 In a series of 30 reimplanted, frozen, and thawed ovarian tissue specimens, the birth of six live newborns was reported.75 In another study of 45 young patients aged 4.4–17.8 years, in whom cryopreservation of the ovarian tissue was performed before chemotherapy, a high correlation between follicular density and age and a decrease in follicular quality after chemotherapy were reported.79 Authors suggested that ovarian tissue cryopreservation is the optimal method to preserve fertility in young patients with cancer, and the only option for prepubertal females.79 An important point of concern is the transplantation of cancer cells, requiring careful evaluation by preoperative imaging and histological/molecular investigation of fresh ovarian tissue for cancer cells.80 Another important question that has not been answered as yet is whether ovarian cortical strips are sufficient enough as an entire ovary.74,76 Although ovarian tissue cryopreservation is not a widely available procedure, it should be considered for fertility preservation in prepubertal girls and young patients who must urgently undergo aggressive chemotherapy and/or radiotherapy.81
Ovarian implantation and uterine transplantation
Given the largely avascular environment of ovarian follicles in the ovaries, the use of ovarian cortical grafts is associated with minimal oocyte loss from ischemia time; thus, there is no need for total ovarian removal.71 However, various malignant conditions in young adolescents such as sarcoma botryoides, cervical cancer, and adenocarcinoma of the vagina require more aggressive surgical approaches (total hysterectomy, subtotal hysterectomy with preservation of the ovaries, elective vaginal hysterectomy) and radiotherapy. Studies have proven that a whole ovary cryopreservation for future reimplantation is feasible and without signs of apoptosis or ultrastructural alterations in cells type.82–84 In cases where treatment modalities such as radiation may affect the capacity of the uterus to receive a fertilized egg, additional uterine transplantation could be the only possible option for this group of young women who wish to conceive.85 Although each year in the United States 5,000 hysterectomies are performed in women under the age of 24 years for various reasons, uterine transplantation remains under consideration for medical and ethical reasons and until now only one live birth has been reported.85,86
Male Fertility Preservation Approaches
Surgical methods preserving male fertility have been adopted including testis-sparing surgery, testicle transposition, and operative sperm retrieval strategies (testicular stem cell transplantation). On the other hand, no effective gonadal function preserving drugs are so far available for use in male patients.87 A recent animal study reported the protective effect of humanin analog on germ cells during chemotherapy in male mice,88 but no clinical studies are underway.
Testis-sparing surgery
The most common type of cancer of the testis in young men is germ cell tumors.89 Synchronous and metachronous bilateral germ cell tumors occur in 2%–5% of patients and bilateral radical orchiectomy will lead not only to infertility but also to increased cumulative risk of metabolic syndrome and cardiovascular events,90 everlasting dependency on exogenous testosterone replacement, and severe psychological disorders due to bilateral castration at such a young age. Organ-sparing surgery with tumor enucleation has resulted in fertility preservation of 10 patients who achieved spontaneous pregnancies and 5 who used the in vitro fertilization technique.91 Two major conditions that allow for testis-sparing surgery are the small size (20 mm or less) of the tumor and its confinement to the testis.91 As adjacent foci of testicular intraepithelial neoplasia may occur in up to 85% of the entire population,92,93 and local recurrence of invasive malignancy in 5% of patients treated with enucleation, close monitoring during follow-up is required.91 According to the European Society of Medical Oncology guidelines for testicular intraepithelial neoplasia patients who are willing to father children, definitive treatment by radiotherapy could be deferred until resolution, although substituted by close surveillance.94,95 The same guidelines also emphasize the need of semen sample before surgery and that a postponement of radiotherapy should be discussed only with patients with confirmed normal semen. Alternative options of sperm donation or testicular sperm extraction should also be discussed at the same consultation. In the less frequent case of bilateral germ cell testicular cancer, fertility preservation strategies should be similar as the survival rate matches that of unilateral disease. Similar to germ cell tumors, Leydig cell tumors that account for 0.8%–3% of all testicular neoplasms are traditionally treated by radical orchiectomy.96 However, in two studies including a total of 61 patients treated with testicular conservative surgery, no recurrence was noted.97,98 Authors’ criteria for a sparing surgery included no symptoms at presentation, laboratory data, the size of the tumor, and frozen-section analysis during surgery.97
Of note, testicular or paratesticular neoplasms are rare in children and adolescent males,99 but if proven, careful surveillance is mandatory.100 In all cases, the written informed consent of the patient is of paramount significance, concerning the right treatment option, including orchiectomy, and alternative strategies of organ preservation, including chemotherapy and radiotherapy.101
Testicular transposition
Radiation may damage the testicular function in a dose-dependent mode; if the dose is between 20 and 200 cGy, the damage may be reversible, but total irreversible azoospermia will be developed at a dose over 400 cGy.102 Testicular transposition was first described in a young male with a paratesticular rhabdomyosarcoma of the left testis. After left orchiectomy and radical retroperitoneal lymphadenectomy, a course of radiation was suggested. To avoid damage from radiation of the right normal testis, transposition of the testis to the right thigh was performed.103 Following radiotherapy completion, the testis was replaced in the scrotum. This approach could also enable fatherhood in young males with rhabdomyosarcoma of the bladder or prostate where radiotherapy is necessary.103 Furthermore, Acosta et al.104 modified the abovementioned technique by wrapping the testis in a Silastic® sheath to prevent adhesions between the spermatic structures/testis and the surrounding anatomical tissues. In addition, they proposed to relocate the testes in the upper medial thighs in the case of the whole abdominal region that needs to be exposed to irradiation.104 However, the clinical role of these methods warrants further investigation.105
Sperm extraction and banking
Sperm banking from adolescents scheduled for cancer therapy may be produced by masturbation.106,107 In a study of 238 adolescents patients (aged 12–19 years) with various types of cancer (Hodgkin’s and non-Hodgkin’s lymphoma, osteosarcoma, Ewing’s sarcoma, acute myeloid leukemia, acute lymphoblastic leukemia, testicular cancer, leukemia, and lymphoma), the majority of patients (86.1%) were able to offer adequate semen sample for future fertilization with modern assisted reproductive technologies.108 However, in the cases of failure to deliver a semen sample because of masturbation problems, other alternative methods such as vibrostimulation or electroejaculation could be performed under general anesthesia.109,110 Novel techniques for sperm extraction are currently available.111 These include: (a) removal of seminiferous tubules with their sperm included within them, after an incision in the scrotum and testis, testicular sperm aspiration by using a needle sized 16–22 G from seminiferous tubules, and (b) extraction of sperm from the epididymis, which represents the primary site of sperm maturation and motility gaining. Percutaneous epididymal sperm aspiration can be performed either by using a small-caliber needle (23–25 G) or with the use of an operating microscope.
All in all, prior to orchiectomy, in order to avoid fertility impairment, semen cryopreservation remains the appropriate option. A recent study from France indicated a large inter-center variation in practices involving young patients seeking to preserve their fertility before cancer therapy pointing an urgent need for decisive changes in public health policy to facilitate the access to reproductive health care for all young cancer patients.112
Female and Male Fertility Preservation by in vitro Procedures
Gametes and embryo cryopreservation
Gametes and embryo cryopreservation are considered as standard practice in young individuals with cancer and are widely accessible.22
For young males, sperm cryopreservation is an easy and effective, although underused, method before starting treatment. For young females, oocyte cryopreservation is a currently offered method, through either controlled ovarian stimulation or no stimulation at all, via in vitro maturation.22,113,114 This method is of particular importance for women who do not have a male partner or do not want to use donor sperm. Embryo cryopreservation is the most used method of fertility preservation, further enhancing its capabilities through the freezing method of vitrification.115 The latter is a standardized, simple, reproducible, and efficient option, easily applied in the storage of all the mentioned specimens.116 In addition, newer strategies in the form of mild stimulation regimens, such as letrozole, constitute an important advance in the field of reproductive endocrinology.117
Notably, even though results from both the cryopreservation of ovarian cortical strips and in vitro maturation, mentioned above, are encouraging, both approaches are not routinely performed and thus cannot be considered as standard of care at the moment.118,119
Prepubertal boys cannot benefit from sperm banking; a potential alternative strategy for preserving their fertility involves storage of immature gametes and gonadal stem cells after testicular tissue sampling in the hope that future technologies will allow its safe utilization.87 A recent review indicates that the generation of male gametes from stem cells is a promising option for the future.120
Conclusions
Based on recent improvements on the survival of adolescent patients with cancer and the progress of reproductive techniques, oncologists can assess the risk of infertility and discuss the options of fertility preservation with both young patients and their parents. Patients should have active counseling about fertility preservation strategies, their risks, and success rates before the initiation of antineoplastic treatment, so that fertility preservation can be incorporated into their designated treatment plan. It is therefore of utmost importance that an effective collaboration between oncologists and gynecologists specialized in reproductive medicine is implemented to improve adolescent cancer patients’ access to assisted reproductive technologies. Nevertheless, more effort is required to improve the efficacy and safety of the available strategies and advance the field of fertility preservation in cancer patients.
Footnotes
ACADEMIC EDITOR: William Chi-shing Cho, Editor in Chief
PEER REVIEW: Three peer reviewers contributed to the peer review report. Reviewers’ reports totaled 739 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no external funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE). Provenance: the authors were invited to submit this paper.
Author Contributions
Conceived and designed the review: NZ, AK. Analyzed the data: CS, AK. Wrote the first draft of the manuscript: NZ, AS. Contributed to the writing of the manuscript: CS, AK. Agree with manuscript results and conclusions: NZ, AS, CS, AK. Jointly developed the structure and arguments for the paper: NZ, CS. Made critical revisions and approved the final version of the manuscript: NZ, AS, CS, AK. All authors have contributed to the preparation of the manuscript and approved its final version.
REFERENCES
- 1.Gatta G, Zigon G, Capocaccia R, et al. Survival of European children and young adults with cancer diagnosed 1995–2002. Eur J Cancer. 2009;45(6):992–1005. doi: 10.1016/j.ejca.2008.11.042. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.Hodgson DC, Pintilie M, Gitterman L, et al. Fertility among female Hodgkin lymphoma survivors attempting pregnancy following ABVD chemotherapy. Hematol Oncol. 2007;25(1):11–5. doi: 10.1002/hon.802. [DOI] [PubMed] [Google Scholar]
- 4.Koumarianou AA, Xiros N, Papageorgiou E, Pectasides D, Economopoulos T. Survival improvement of young patients, aged 16–23, with Hodgkin lymphoma (HL) during the last three decades. Anticancer Res. 2007;27(2):1191–7. [PubMed] [Google Scholar]
- 5.Pectasides D, Pectasides E, Papaxoinis G, et al. Testicular function in poor-risk nonseminomatous germ cell tumors treated with methotrexate, paclitaxel, ifosfamide, and cisplatin combination chemotherapy. J Androl. 2009;30(3):280–6. doi: 10.2164/jandrol.108.006437. [DOI] [PubMed] [Google Scholar]
- 6.Meirow D, Nugent D. The effects of radiotherapy and chemotherapy on female reproduction. Hum Reprod Update. 2001;7(6):535–43. doi: 10.1093/humupd/7.6.535. [DOI] [PubMed] [Google Scholar]
- 7.Howell SJ, Shalet SM. Testicular function following chemotherapy. Hum Reprod Update. 2001;7(4):363–9. doi: 10.1093/humupd/7.4.363. [DOI] [PubMed] [Google Scholar]
- 8.Byrne J, Mulvihill JJ, Myers MH, et al. Effects of treatment on fertility in long-term survivors of childhood or adolescent cancer. N Engl J Med. 1987;317(21):1315–21. doi: 10.1056/NEJM198711193172104. [DOI] [PubMed] [Google Scholar]
- 9.Carter J, Rowland K, Chi D, et al. Gynecologic cancer treatment and the impact of cancer-related infertility. Gynecol Oncol. 2005;97(1):90–5. doi: 10.1016/j.ygyno.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 10.Green D, Galvin H, Horne B. The psycho-social impact of infertility on young male cancer survivors: a qualitative investigation. Psychooncology. 2003;12(2):141–52. doi: 10.1002/pon.622. [DOI] [PubMed] [Google Scholar]
- 11.Duffy C, Allen S. Medical and psychosocial aspects of fertility after cancer. Cancer J. 2009;15(1):27–33. doi: 10.1097/PPO.0b013e3181976602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wallace WH, Anderson RA, Irvine DS. Fertility preservation for young patients with cancer: who is at risk and what can be offered? Lancet Oncol. 2005;6(4):209–18. doi: 10.1016/S1470-2045(05)70092-9. [DOI] [PubMed] [Google Scholar]
- 13.Loscalzo MJ, Clark KL. The psychosocial context of cancer-related infertility. Cancer Treat Res. 2007;138:180–90. doi: 10.1007/978-0-387-72293-1_13. [DOI] [PubMed] [Google Scholar]
- 14.Partridge AH, Gelber S, Peppercorn J, et al. Web-based survey of fertility issues in young women with breast cancer. J Clin Oncol. 2004;22(20):4174–83. doi: 10.1200/JCO.2004.01.159. [DOI] [PubMed] [Google Scholar]
- 15.Ruddy KJ, Gelber SI, Tamimi RM, et al. Prospective study of fertility concerns and preservation strategies in young women with breast cancer. J Clin Oncol. 2014;32(11):1151–6. doi: 10.1200/JCO.2013.52.8877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burns KC, Boudreau C, Panepinto JA. Attitudes regarding fertility preservation in female adolescent cancer patients. J Pediatr Hematol Oncol. 2006;28(6):350–4. doi: 10.1097/00043426-200606000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Loi K, Lau M, Loh SF, et al. Attitudes toward fertility preservation in female cancer patients. J Reprod Med. 2010;55(9–10):411–6. [PubMed] [Google Scholar]
- 18.Lutchman Singh K, Davies M, Chatterjee R. Fertility in female cancer survivors: pathophysiology, preservation and the role of ovarian reserve testing. Hum Reprod Update. 2005;11(1):69–89. doi: 10.1093/humupd/dmh052. [DOI] [PubMed] [Google Scholar]
- 19.Bozza C, Puglisi F, Lambertini M, Osa EO, Manno M, Del Mastro L. Anti-Mullerian hormone: determination of ovarian reserve in early breast cancer patients. Endocr Relat Cancer. 2014;21(1):R51–65. doi: 10.1530/ERC-13-0335. [DOI] [PubMed] [Google Scholar]
- 20.Dunlop CE, Anderson RA. Uses of anti-Mullerian hormone (AMH) measurement before and after cancer treatment in women. Maturitas. 2015;80(3):245–50. doi: 10.1016/j.maturitas.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Brougham MF, Crofton PM, Johnson EJ, Evans N, Anderson RA, Wallace WH. Anti-Mullerian hormone is a marker of gonadotoxicity in pre- and postpubertal girls treated for cancer: a prospective study. J Clin Endocrinol Metab. 2012;97(6):2059–67. doi: 10.1210/jc.2011-3180. [DOI] [PubMed] [Google Scholar]
- 22.Loren AW, Mangu PB, Beck LN, et al. American Society of Clinical O: fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31(19):2500–10. doi: 10.1200/JCO.2013.49.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mackie EJ, Radford M, Shalet SM. Gonadal function following chemotherapy for childhood Hodgkin’s disease. Med Pediatr Oncol. 1996;27(2):74–8. doi: 10.1002/(SICI)1096-911X(199608)27:2<74::AID-MPO2>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 24.Whitehead E, Shalet SM, Blackledge G, Todd I, Crowther D, Beardwell CG. The effect of combination chemotherapy on ovarian function in women treated for Hodgkin’s disease. Cancer. 1983;52(6):988–93. doi: 10.1002/1097-0142(19830915)52:6<988::aid-cncr2820520610>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 25.Wallace WH, Shalet SM, Tetlow LJ, Morris-Jones PH. Ovarian function following the treatment of childhood acute lymphoblastic leukaemia. Med Pediatr Oncol. 1993;21(5):333–9. doi: 10.1002/mpo.2950210505. [DOI] [PubMed] [Google Scholar]
- 26.Schilsky RL, Sherins RJ, Hubbard SM, Wesley MN, Young RC, DeVita VT. Long-term follow up of ovarian function in women treated with MOPP chemotherapy for Hodgkin’s disease. Am J Med. 1981;71(4):552–6. doi: 10.1016/0002-9343(81)90205-9. [DOI] [PubMed] [Google Scholar]
- 27.Constine LS, Woolf PD, Cann D, et al. Hypothalamic-pituitary dysfunction after radiation for brain tumors. N Engl J Med. 1993;328(2):87–94. doi: 10.1056/NEJM199301143280203. [DOI] [PubMed] [Google Scholar]
- 28.Schuck A, Hamelmann V, Bramswig JH, et al. Ovarian function following pelvic irradiation in prepubertal and pubertal girls and young adult women. Strahlenther Onkol. 2005;181(8):534–9. doi: 10.1007/s00066-005-9500-4. [DOI] [PubMed] [Google Scholar]
- 29.Lushbaugh CC, Casarett GW. The effects of gonadal irradiation in clinical radiation therapy: a review. Cancer. 1976;37(2 suppl):1111–25. doi: 10.1002/1097-0142(197602)37:2+<1111::aid-cncr2820370821>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 30.Quigley C, Cowell C, Jimenez M, et al. Normal or early development of puberty despite gonadal damage in children treated for acute lymphoblastic leukemia. N Engl J Med. 1989;321(3):143–51. doi: 10.1056/NEJM198907203210303. [DOI] [PubMed] [Google Scholar]
- 31.Pennisi AJ, Grushkin CM, Lieberman E. Gonadal function in children with nephrosis treated with cyclophosphamide. Am J Dis Child. 1975;129(3):315–8. doi: 10.1001/archpedi.1975.02120400027006. [DOI] [PubMed] [Google Scholar]
- 32.Meirow D, Dor J, Kaufman B, et al. Cortical fibrosis and blood-vessels damage in human ovaries exposed to chemotherapy. Potential mechanisms of ovarian injury. Hum Reprod. 2007;22(6):1626–33. doi: 10.1093/humrep/dem027. [DOI] [PubMed] [Google Scholar]
- 33.Djaladat H, Burner E, Parikh PM, Beroukhim Kay D, Hays K. The association between testis cancer and semen abnormalities before orchiectomy: a systematic review. J Adolesc Young Adult Oncol. 2014;3(4):153–9. doi: 10.1089/jayao.2014.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rivkees SA, Crawford JD. The relationship of gonadal activity and chemotherapy-induced gonadal damage. JAMA. 1988;259(14):2123–5. [PubMed] [Google Scholar]
- 35.Bucci LR, Meistrich ML. Effects of busulfan on murine spermatogenesis: cytotoxicity, sterility, sperm abnormalities, and dominant lethal mutations. Mutat Res. 1987;176(2):259–68. doi: 10.1016/0027-5107(87)90057-1. [DOI] [PubMed] [Google Scholar]
- 36.Bujan L, Walschaerts M, Moinard N, et al. Impact of chemotherapy and radiotherapy for testicular germ cell tumors on spermatogenesis and sperm DNA: a multicenter prospective study from the CECOS network. Fertil Steril. 2013;100(3):673–80. doi: 10.1016/j.fertnstert.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 37.Kelnar CJ, McKinnell C, Walker M, et al. Testicular changes during infantile ‘quiescence’ in the marmoset and their gonadotrophin dependence: a model for investigating susceptibility of the prepubertal human testis to cancer therapy? Hum Reprod. 2002;17(5):1367–78. doi: 10.1093/humrep/17.5.1367. [DOI] [PubMed] [Google Scholar]
- 38.van Alphen MM, van de Kant HJ, de Rooij DG. Depletion of the spermatogonia from the seminiferous epithelium of the rhesus monkey after X irradiation. Radiat Res. 1988;113(3):473–86. [PubMed] [Google Scholar]
- 39.Bar-Shira Maymon B, Yogev L, Marks A, Hauser R, Botchan A, Yavetz H. Sertoli cell inactivation by cytotoxic damage to the human testis after cancer chemotherapy. Fertil Steril. 2004;81(5):1391–4. doi: 10.1016/j.fertnstert.2003.09.078. [DOI] [PubMed] [Google Scholar]
- 40.Chemaitilly W, Sklar CA. Endocrine complications in long-term survivors of childhood cancers. Endocr Relat Cancer. 2010;17(3):R141–59. doi: 10.1677/ERC-10-0002. [DOI] [PubMed] [Google Scholar]
- 41.Shalet SM. Effect of irradiation treatment on gonadal function in men treated for germ cell cancer. Eur Urol. 1993;23(1):148–51. doi: 10.1159/000474584. discussion 152. [DOI] [PubMed] [Google Scholar]
- 42.Chen H, Li J, Cui T, Hu L. Adjuvant gonadotropin-releasing hormone analogues for the prevention of chemotherapy induced premature ovarian failure in premenopausal women. Cochrane Database Syst Rev. 2011;9(11):CD008018. doi: 10.1002/14651858.CD008018.pub2. [DOI] [PubMed] [Google Scholar]
- 43.Del Mastro L, Ceppi M, Poggio F, et al. Gonadotropin-releasing hormone analogues for the prevention of chemotherapy-induced premature ovarian failure in cancer women: systematic review and meta-analysis of randomized trials. Cancer Treat Rev. 2014;40(5):675–83. doi: 10.1016/j.ctrv.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 44.Moore H, Unger J, Phillips K, et al. American Society for Clinical Oncology: 2014. Chicago, USA: 2014. Phase III trial (Prevention of Early Menopause Study [POEMS]-SWOG S0230) of LHRH analog during chemotherapy (CT) to reduce ovarian failure in early-stage, hormone receptor-negative breast cancer: an International Intergroup trial of SWOG, IBCSG, ECOG, and CALGB (Alliance) [Google Scholar]
- 45.Del Mastro L, Boni L, Michelotti A, et al. Effect of the gonadotropin-releasing hormone analogue triptorelin on the occurrence of chemotherapy-induced early menopause in premenopausal women with breast cancer: a randomized trial. JAMA. 2011;306(3):269–76. doi: 10.1001/jama.2011.991. [DOI] [PubMed] [Google Scholar]
- 46.Moore HC, Unger JM, Phillips KA, et al. Goserelin for ovarian protection during breast-cancer adjuvant chemotherapy. N Engl J Med. 2015;372(10):923–32. doi: 10.1056/NEJMoa1413204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gershenson DM. Fertility-sparing surgery for malignancies in women. J Natl Cancer Inst Monogr. 2005;(34):43–7. doi: 10.1093/jncimonographs/lgi011. [DOI] [PubMed] [Google Scholar]
- 48.Gotlieb WH, Flikker S, Davidson B, Korach Y, Kopolovic J, Ben-Baruch G. Borderline tumors of the ovary: fertility treatment, conservative management, and pregnancy outcome. Cancer. 1998;82(1):141–6. [PubMed] [Google Scholar]
- 49.Jeruss JS, Woodruff TK. Preservation of fertility in patients with cancer. N Engl J Med. 2009;360(9):902–11. doi: 10.1056/NEJMra0801454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schilder JM, Thompson AM, DePriest PD, et al. Outcome of reproductive age women with stage IA or IC invasive epithelial ovarian cancer treated with fertility-sparing therapy. Gynecol Oncol. 2002;87(1):1–7. doi: 10.1006/gyno.2002.6805. [DOI] [PubMed] [Google Scholar]
- 51.Ehren IM, Mahour GH, Isaacs H., Jr Benign and malignant ovarian tumors in children and adolescents. A review of 63 cases. Am J Surg. 1984;147(3):339–44. doi: 10.1016/0002-9610(84)90163-6. [DOI] [PubMed] [Google Scholar]
- 52.Fotiou SK. Ovarian malignancies in adolescence. Ann N Y Acad Sci. 1997;816:338–46. doi: 10.1111/j.1749-6632.1997.tb52159.x. [DOI] [PubMed] [Google Scholar]
- 53.Tangir J, Zelterman D, Ma W, Schwartz PE. Reproductive function after conservative surgery and chemotherapy for malignant germ cell tumors of the ovary. Obstet Gynecol. 2003;101(2):251–7. doi: 10.1016/s0029-7844(02)02508-5. [DOI] [PubMed] [Google Scholar]
- 54.Low JJ, Perrin LC, Crandon AJ, Hacker NF. Conservative surgery to preserve ovarian function in patients with malignant ovarian germ cell tumors. A review of 74 cases. Cancer. 2000;89(2):391–8. [PubMed] [Google Scholar]
- 55.Gordon A, Lipton D, Woodruff JD. Dysgerminoma: a review of 158 cases from the Emil Novak Ovarian Tumor Registry. Obstet Gynecol. 1981;58(4):497–504. [PubMed] [Google Scholar]
- 56.Zanetta G, Bonazzi C, Cantu M, et al. Survival and reproductive function after treatment of malignant germ cell ovarian tumors. J Clin Oncol. 2001;19(4):1015–20. doi: 10.1200/JCO.2001.19.4.1015. [DOI] [PubMed] [Google Scholar]
- 57.Yinon Y, Beiner ME, Gotlieb WH, Korach Y, Perri T, Ben-Baruch G. Clinical outcome of cystectomy compared with unilateral salpingo-oophorectomy as fertility-sparing treatment of borderline ovarian tumors. Fertil Steril. 2007;88(2):479–84. doi: 10.1016/j.fertnstert.2006.11.128. [DOI] [PubMed] [Google Scholar]
- 58.Donnez J, Munschke A, Berliere M, et al. Safety of conservative management and fertility outcome in women with borderline tumors of the ovary. Fertil Steril. 2003;79(5):1216–21. doi: 10.1016/s0015-0282(03)00160-2. [DOI] [PubMed] [Google Scholar]
- 59.Palomba S, Falbo A, Del Negro S, et al. Ultra-conservative fertility-sparing strategy for bilateral borderline ovarian tumours: an 11-year follow-up. Hum Reprod. 2010;25(8):1966–72. doi: 10.1093/humrep/deq159. [DOI] [PubMed] [Google Scholar]
- 60.Irtan S, Orbach D, Helfre S, Sarnacki S. Ovarian transposition in prepubescent and adolescent girls with cancer. Lancet Oncol. 2013;14(13):e601–8. doi: 10.1016/S1470-2045(13)70288-2. [DOI] [PubMed] [Google Scholar]
- 61.Sklar C. Reproductive physiology and treatment-related loss of sex hormone production. Med Pediatr Oncol. 1999;33(1):2–8. doi: 10.1002/(sici)1096-911x(199907)33:1<2::aid-mpo2>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 62.Bath LE, Wallace WH, Critchley HO. Late effects of the treatment of childhood cancer on the female reproductive system and the potential for fertility preservation. BJOG. 2002;109(2):107–14. doi: 10.1111/j.1471-0528.2002.t01-1-01007.x. [DOI] [PubMed] [Google Scholar]
- 63.Wallace WH, Thomson AB, Kelsey TW. The radiosensitivity of the human oocyte. Hum Reprod. 2003;18(1):117–21. doi: 10.1093/humrep/deg016. [DOI] [PubMed] [Google Scholar]
- 64.Mc CM, Keaty EC, Thompson JD. Conservation of ovarian tissue in the treatment of carcinoma of the cervix with radical surgery. Am J Obstet Gynecol. 1958;75(3):590–600. doi: 10.1016/0002-9378(58)90614-8. discussion 600–595. [DOI] [PubMed] [Google Scholar]
- 65.Molpus KL, Wedergren JS, Carlson MA. Robotically assisted endoscopic ovarian transposition. JSLS. 2003;7(1):59–62. [PMC free article] [PubMed] [Google Scholar]
- 66.Hadar H, Loven D, Herskovitz P, Bairey O, Yagoda A, Levavi H. An evaluation of lateral and medial transposition of the ovaries out of radiation fields. Cancer. 1994;74(2):774–9. doi: 10.1002/1097-0142(19940715)74:2<774::aid-cncr2820740234>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 67.Hwang JH, Yoo HJ, Park SH, et al. Association between the location of transposed ovary and ovarian function in patients with uterine cervical cancer treated with (postoperative or primary) pelvic radiotherapy. Fertil Steril. 2012;97(6):e1–2. doi: 10.1016/j.fertnstert.2012.02.052. [DOI] [PubMed] [Google Scholar]
- 68.Thibaud E, Ramirez M, Brauner R, et al. Preservation of ovarian function by ovarian transposition performed before pelvic irradiation during childhood. J Pediatr. 1992;121(6):880–4. doi: 10.1016/s0022-3476(05)80332-4. [DOI] [PubMed] [Google Scholar]
- 69.Meirow D, Levron J, Eldar-Geva T, et al. Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. N Engl J Med. 2005;353(3):318–21. doi: 10.1056/NEJMc055237. [DOI] [PubMed] [Google Scholar]
- 70.Donnez J, Dolmans MM, Demylle D, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364(9443):1405–10. doi: 10.1016/S0140-6736(04)17222-X. [DOI] [PubMed] [Google Scholar]
- 71.Meirow D, Ra’anani H, Biderman H. Ovarian tissue cryopreservation and transplantation: a realistic, effective technology for fertility preservation. Methods Mol Biol. 2014;1154:455–73. doi: 10.1007/978-1-4939-0659-8_21. [DOI] [PubMed] [Google Scholar]
- 72.Donnez J, Silber S, Andersen CY, et al. Children born after autotransplantation of cryopreserved ovarian tissue. a review of 13 live births. Ann Med. 2011;43(6):437–50. doi: 10.3109/07853890.2010.546807. [DOI] [PubMed] [Google Scholar]
- 73.Wallace WH, Kelsey TW. Human ovarian reserve from conception to the menopause. PLoS One. 2010;5(1):e8772. doi: 10.1371/journal.pone.0008772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jadoul P, Dolmans MM, Donnez J. Fertility preservation in girls during childhood: is it feasible, efficient and safe and to whom should it be proposed? Hum Reprod Update. 2010;16(6):617–30. doi: 10.1093/humupd/dmq010. [DOI] [PubMed] [Google Scholar]
- 75.Donnez J, Jadoul P, Squifflet J, et al. Ovarian tissue cryopreservation and transplantation in cancer patients. Best Pract Res Clin Obstet Gynaecol. 2010;24(1):87–100. doi: 10.1016/j.bpobgyn.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 76.Anderson RA, Wallace WH, Baird DT. Ovarian cryopreservation for fertility preservation: indications and outcomes. Reproduction. 2008;136(6):681–9. doi: 10.1530/REP-08-0097. [DOI] [PubMed] [Google Scholar]
- 77.Meirow D, Fasouliotis SJ, Nugent D, Schenker JG, Gosden RG, Rutherford AJ. A laparoscopic technique for obtaining ovarian cortical biopsy specimens for fertility conservation in patients with cancer. Fertil Steril. 1999;71(5):948–51. doi: 10.1016/s0015-0282(99)00067-9. [DOI] [PubMed] [Google Scholar]
- 78.Oktay K, Buyuk E, Veeck L, et al. Embryo development after heterotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;363(9412):837–40. doi: 10.1016/S0140-6736(04)15728-0. [DOI] [PubMed] [Google Scholar]
- 79.Fabbri R, Vicenti R, Macciocca M, et al. Cryopreservation of ovarian tissue in pediatric patients. Obstet Gynecol Int. 2012;2012:910698. doi: 10.1155/2012/910698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Meirow D, Hardan I, Dor J, et al. Searching for evidence of disease and malignant cell contamination in ovarian tissue stored from hematologic cancer patients. Hum Reprod. 2008;23(5):1007–13. doi: 10.1093/humrep/den055. [DOI] [PubMed] [Google Scholar]
- 81.Practice Committee of American Society for Reproductive M Ovarian tissue cryopreservation: a committee opinion. Fertil Steril. 2014;101(5):1237–43. doi: 10.1016/j.fertnstert.2014.02.052. [DOI] [PubMed] [Google Scholar]
- 82.Martinez-Madrid B, Camboni A, Dolmans MM, Nottola S, Van Langendonckt A, Donnez J. Apoptosis and ultrastructural assessment after cryopreservation of whole human ovaries with their vascular pedicle. Fertil Steril. 2007;87(5):1153–65. doi: 10.1016/j.fertnstert.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 83.Jadoul P, Donnez J, Dolmans MM, Squifflet J, Lengele B, Martinez-Madrid B. Laparoscopic ovariectomy for whole human ovary cryopreservation: technical aspects. Fertil Steril. 2007;87(4):971–5. doi: 10.1016/j.fertnstert.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 84.Martinez-Madrid B, Dolmans MM, Van Langendonckt A, Defrere S, Donnez J. Freeze-thawing intact human ovary with its vascular pedicle with a passive cooling device. Fertil Steril. 2004;82(5):1390–4. doi: 10.1016/j.fertnstert.2004.06.036. [DOI] [PubMed] [Google Scholar]
- 85.Nair A, Stega J, Smith JR, Del Priore G. Uterus transplant: evidence and ethics. Ann N Y Acad Sci. 2008;1127:83–91. doi: 10.1196/annals.1434.003. [DOI] [PubMed] [Google Scholar]
- 86.Tzakis AG. The first live birth subsequent to uterus transplantation. Transplantation. 2015;99(1):8–9. doi: 10.1097/TP.0000000000000564. [DOI] [PubMed] [Google Scholar]
- 87.Wyns C, Curaba M, Vanabelle B, Van Langendonckt A, Donnez J. Options for fertility preservation in prepubertal boys. Hum Reprod Update. 2010;16(3):312–28. doi: 10.1093/humupd/dmp054. [DOI] [PubMed] [Google Scholar]
- 88.Lue Y, Swerdloff R, Wan J, et al. The potent humanin analogue (HNG) protects germ cells and leucocytes while enhancing chemotherapy-induced suppression of cancer metastases in male mice. Endocrinology. 2015;156(12):4511–21. doi: 10.1210/en.2015-1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gobel U, Schneider DT, Calaminus G, Haas RJ, Schmidt P, Harms D. Germ-cell tumors in childhood and adolescence. GPOH MAKEI and the MAHO study groups. Ann Oncol. 2000;11(3):263–71. doi: 10.1023/a:1008360523160. [DOI] [PubMed] [Google Scholar]
- 90.Haugnes HS, Wethal T, Aass N, et al. Cardiovascular risk factors and morbidity in long-term survivors of testicular cancer: a 20-year follow-up study. J Clin Oncol. 2010;28(30):4649–57. doi: 10.1200/JCO.2010.29.9362. [DOI] [PubMed] [Google Scholar]
- 91.Heidenreich A, Weissbach L, Holtl W, et al. German Testicular Cancer Study G: organ sparing surgery for malignant germ cell tumor of the testis. J Urol. 2001;166(6):2161–5. doi: 10.1016/s0022-5347(05)65526-7. [DOI] [PubMed] [Google Scholar]
- 92.Dieckmann KP, Skakkebaek NE. Carcinoma in situ of the testis: review of biological and clinical features. Int J Cancer. 1999;83(6):815–22. doi: 10.1002/(sici)1097-0215(19991210)83:6<815::aid-ijc21>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 93.Holzbeierlein JM, Sogani PC, Sheinfeld J. Histology and clinical outcomes in patients with bilateral testicular germ cell tumors: the memorial sloan kettering cancer center experience 1950 to 2001. J Urol. 2003;169(6):2122–5. doi: 10.1097/01.ju.0000067462.24562.8b. [DOI] [PubMed] [Google Scholar]
- 94.Schmoll HJ, Jordan K, Huddart R, et al. Testicular non-seminoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(suppl 5):v147–54. doi: 10.1093/annonc/mdq177. [DOI] [PubMed] [Google Scholar]
- 95.Schmoll HJ, Jordan K, Huddart R, et al. Testicular seminoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(suppl 5):v140–6. doi: 10.1093/annonc/mdq176. [DOI] [PubMed] [Google Scholar]
- 96.Kim I, Young RH, Scully RE. Leydig cell tumors of the testis. A clinicopathological analysis of 40 cases and review of the literature. Am J Surg Pathol. 1985;9(3):177–92. doi: 10.1097/00000478-198503000-00002. [DOI] [PubMed] [Google Scholar]
- 97.Suardi N, Strada E, Colombo R, et al. Leydig cell tumour of the testis: presentation, therapy, long-term follow-up and the role of organ-sparing surgery in a single-institution experience. BJU Int. 2009;103(2):197–200. doi: 10.1111/j.1464-410X.2008.08016.x. [DOI] [PubMed] [Google Scholar]
- 98.Giannarini G, Menchini Fabris F, Mogorovich A, Morelli G. Re: long-term follow-up and clinical characteristics of testicular Leydig cell tumor: experience with 24 cases. J Urol. 2007;177(5):1955. doi: 10.1016/j.juro.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 99.Agarwal PK, Palmer JS. Testicular and paratesticular neoplasms in prepubertal males. J Urol. 2006;176(3):875–81. doi: 10.1016/j.juro.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 100.Forbes CM, Metcalfe C, Murray N, Black PC. Three primary testicular tumours: trials and tribulations of testicular preservation. Can Urol Assoc J. 2013;7(9–10):E630–3. doi: 10.5489/cuaj.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zuniga A, Lawrentschuk N, Jewett MA. Organ-sparing approaches for testicular masses. Nat Rev Urol. 2010;7(8):454–64. doi: 10.1038/nrurol.2010.100. [DOI] [PubMed] [Google Scholar]
- 102.Rowley MJ, Leach DR, Warner GA, Heller CG. Effect of graded doses of ionizing radiation on the human testis. Radiat Res. 1974;59(3):665–78. [PubMed] [Google Scholar]
- 103.Cheng BS, King LR, Kinney TR. Testicle transposition in children who undergo low-pelvic or scrotal irradiation. Urology. 1984;24(5):476–8. doi: 10.1016/0090-4295(84)90326-1. [DOI] [PubMed] [Google Scholar]
- 104.Acosta JM, Tiao G, Stein JE, Mahour GH. Temporary relocation of testes to the anterior abdominal wall before radiation therapy of the pelvis or perineum. J Pediatr Surg. 2002;37(8):1232–3. doi: 10.1053/jpsu.2002.34488. [DOI] [PubMed] [Google Scholar]
- 105.Hussein AA, Tran ND, Smith JF. Fertility preservation for boys and adolescents facing sterilizing medical therapy. Transl Androl Urol. 2014;3(4):382–90. doi: 10.3978/j.issn.2223-4683.2014.11.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schover LR, Brey K, Lichtin A, Lipshultz LI, Jeha S. Knowledge and experience regarding cancer, infertility, and sperm banking in younger male survivors. J Clin Oncol. 2002;20(7):1880–9. doi: 10.1200/JCO.2002.07.175. [DOI] [PubMed] [Google Scholar]
- 107.Kliesch S, Behre HM, Jurgens H, Nieschlag E. Cryopreservation of semen from adolescent patients with malignancies. Med Pediatr Oncol. 1996;26(1):20–7. doi: 10.1002/(SICI)1096-911X(199601)26:1<20::AID-MPO3>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 108.Bahadur G, Ling KL, Hart R, et al. Semen quality and cryopreservation in adolescent cancer patients. Hum Reprod. 2002;17(12):3157–61. doi: 10.1093/humrep/17.12.3157. [DOI] [PubMed] [Google Scholar]
- 109.Muller J, Sonksen J, Sommer P, et al. Cryopreservation of semen from pubertal boys with cancer. Med Pediatr Oncol. 2000;34(3):191–4. doi: 10.1002/(sici)1096-911x(200003)34:3<191::aid-mpo5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 110.Schmiegelow ML, Sommer P, Carlsen E, Sonksen JO, Schmiegelow K, Muller JR. Penile vibratory stimulation and electroejaculation before anticancer therapy in two pubertal boys. J Pediatr Hematol Oncol. 1998;20(5):429–30. doi: 10.1097/00043426-199809000-00004. [DOI] [PubMed] [Google Scholar]
- 111.Kort JD, Eisenberg ML, Millheiser LS, Westphal LM. Fertility issues in cancer survivorship. CA Cancer J Clin. 2014;64(2):118–34. doi: 10.3322/caac.21205. [DOI] [PubMed] [Google Scholar]
- 112.Daudin M, Rives N, Walschaerts M, et al. Sperm cryopreservation in adolescents and young adults with cancer: results of the French national sperm banking network (CECOS) Fertil Steril. 2015;103(2):478–86e471. doi: 10.1016/j.fertnstert.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 113.Grynberg M, El Hachem H, de Bantel A, Benard J, le Parco S, Fanchin R. In vitro maturation of oocytes: uncommon indications. Fertil Steril. 2013;99(5):1182–8. doi: 10.1016/j.fertnstert.2013.01.090. [DOI] [PubMed] [Google Scholar]
- 114.Chian RC, Uzelac PS, Nargund G. In vitro maturation of human immature oocytes for fertility preservation. Fertil Steril. 2013;99(5):1173–81. doi: 10.1016/j.fertnstert.2013.01.141. [DOI] [PubMed] [Google Scholar]
- 115.Donnez J, Dolmans MM. Fertility preservation in women. Nat Rev Endocrinol. 2013;9(12):735–49. doi: 10.1038/nrendo.2013.205. [DOI] [PubMed] [Google Scholar]
- 116.Cobo A, Garcia-Velasco JA, Domingo J, Remohi J, Pellicer A. Is vitrification of oocytes useful for fertility preservation for age-related fertility decline and in cancer patients? Fertil Steril. 2013;99(6):1485–95. doi: 10.1016/j.fertnstert.2013.02.050. [DOI] [PubMed] [Google Scholar]
- 117.Azim AA, Costantini-Ferrando M, Lostritto K, Oktay K. Relative potencies of anastrozole and letrozole to suppress estradiol in breast cancer patients undergoing ovarian stimulation before in vitro fertilization. J Clin Endocrinol Metab. 2007;92(6):2197–200. doi: 10.1210/jc.2007-0247. [DOI] [PubMed] [Google Scholar]
- 118.Berwanger AL, Finet A, El Hachem H, le Parco S, Hesters L, Grynberg M. New trends in female fertility preservation: in vitro maturation of oocytes. Future Oncol. 2012;8(12):1567–73. doi: 10.2217/fon.12.144. [DOI] [PubMed] [Google Scholar]
- 119.Lieberman B. Function of ovarian tissue after long-term storage. Reprod Biomed Online. 2012;25(2):96–7. doi: 10.1016/j.rbmo.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 120.Tournaye H, Dohle GR, Barratt CL. Fertility preservation in men with cancer. Lancet. 2014;384(9950):1295–301. doi: 10.1016/S0140-6736(14)60495-5. [DOI] [PubMed] [Google Scholar]
- 121.Heller CG, Clermont Y. Spermatogenesis in man: an estimate of its duration. Science. 1963;140(3563):184–6. doi: 10.1126/science.140.3563.184. [DOI] [PubMed] [Google Scholar]
- 122.Wallace WH, Critchley HO, Anderson RA. Optimizing reproductive outcome in children and young people with cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012;30(1):3–5. doi: 10.1200/JCO.2011.38.3877. [DOI] [PubMed] [Google Scholar]