Abstract
Aims
The subcutaneous implantable cardioverter defibrillator (S-ICD) was introduced to overcome complications related to transvenous leads. Adoption of the S-ICD requires implanters to learn a new implantation technique. The aim of this study was to assess the learning curve for S-ICD implanters with respect to implant-related complications, procedure time, and inappropriate shocks (IASs).
Methods and results
In a pooled cohort from two clinical S-ICD databases, the IDE Trial and the EFFORTLESS Registry, complications, IASs at 180 days follow-up and implant procedure duration were assessed. Patients were grouped in quartiles based on experience of the implanter and Kaplan–Meier estimates of complication and IAS rates were calculated. A total of 882 patients implanted in 61 centres by 107 implanters with a median of 4 implants (IQR 1,8) were analysed. There were a total of 59 patients with complications and 48 patients with IAS. The complication rate decreased significantly from 9.8% in Quartile 1 (least experience) to 5.4% in Quartile 4 (most experience) (P = 0.02) and non-significantly for IAS from 7.9 to 4.8% (P = 0.10). Multivariable analysis demonstrated a hazard ratio of 0.78 (P = 0.045) for complications and 1.01 (P = 0.958) for IAS. Dual-zone programming increased with experience of the individual implanter (P < 0.001), which reduced IAS significantly in the multivariable model (HR 0.44, P = 0.01). Procedure time decreased from 75 to 65 min (P < 0.001). The complication rate and procedure time stabilized after Quartile 2 (>13 implants).
Conclusion
There is a short and significant learning curve associated with physicians adopting the S-ICD. Performance stabilizes after 13 implants.
Keywords: Implantable cardioverter-defibrillator, Subcutaneous implantable cardioverter-defibrillator, S-ICD, Learning curve, Complications, Inappropriate shock
What's new.
There is a learning curve for physicians adopting the S-ICD.
The complications rate decreased from 9.8 to 5.4%.
Performance stabilizes after 13 implants.
Inappropriate shocks (IASs) decreased during the study due to higher rates of dual-zone programming but were not related to physician experience.
The use of dual-zone programming will help new implanters achieve low IAS rates.
Procedure time stabilized at 65 min after 13 implants, which is similar to dual-chamber transvenous ICD implant durations.
Introduction
The subcutaneous implantable cardioverter defibrillator (S-ICD) was introduced to overcome complications related to transvenous leads. The available evidence from S-ICD studies shows a complication rate similar to transvenous implantable cardioverter defibrillator (ICD) therapy but includes the learning curve of Physicians becoming familiar with this new technology.1–3
It is widely accepted that increasing experience improves performance, particularly when adopting a new procedure. The introduction of the S-ICD required implanting physicians to adopt a new implant technique and different modes of ICD programming.4,5 The pulse generator of the S-ICD is positioned in the left lateral axillary line on the chest wall and uses a subcutaneous sensing and defibrillation lead, whereas conventional transvenous ICDs use endocardial leads. The S-ICD requires more energy for defibrillation, which increases the size of the pulse generator. Tunnelling the lead in this new position and larger size of the pulse generator and pocket require a different surgical approach than for transvenous implants.
The association between operator experience and the impact on adverse events is well documented.6 For cardiovascular procedures, operator training and volume have been associated with patient outcomes, including mortality in percutaneous coronary intervention procedures and infection and mechanical complications in transvenous ICD implants and replacements.7–9 Olde Nordkamp et al. observed more inappropriate shocks (IASs) and complications in initial experience per implanting centre compared with later experience in a Dutch S-ICD cohort.10
The aim of this study was to assess the learning curve for individual S-ICD implanters with respect to implant-related complications, procedure time, and IASs. We hypothesized the presence of a learning curve with the introduction of the S-ICD. Therefore, complications, implant procedure duration, and IASs were expected to decrease with increasing experience of the implanter, with stabilization after a given learning interval.
Methods
Study cohort
The design of this pooled cohort has been described in detail elsewhere, but briefly: the pooled cohort consists of a total of 889 patients, 568 patients from the EFFORTLESS registry (NCT01085435), 308 from the IDE study (NCT01064076), and 13 patients who were in both studies.1,11,12 Data were collected until 12 November 2013 for the ongoing EFFORTLESS registry. The IDE study follow-up ended on 14 February 2012. Participation in both studies complied with the Helsinki declaration and required approval of local ethics or institutional review boards and informed consent. The device programming, including the use of the morphology-based discrimination algorithm, was at the physicians' discretion.
Endpoints
Endpoints included in the analysis were complications, procedure time, and IASs. Complications were defined as those related to the implant procedure and required surgical intervention. Complications unrelated to the implant procedure that did require a surgical intervention were excluded (inability to communicate with the device n = 1, IASs n = 12, and premature battery depletion n = 1). Procedure time was collected for patients enrolled in the EFFORTLESS registry and was defined as the time between incision and closure of the wound (skin-to-skin time). Inappropriate shocks were defined as shocks for any reason but ventricular tachyarrhythmias. Classification of complications and treated events were reported by the site, and appropriateness of therapy or detection was adjudicated by the sponsor (EFFORTLESS) or Clinical Events Committee (IDE). The complication rate and IAS rate were assessed by calculating Kaplan–Meier estimates at 180 days post-implantation.
Experience
Outcomes with S-ICD therapy were analysed for individual implanter experience: per implanter, all patients were chronologically ranked and subsequently distributed in quartiles. The first quartile of patients represents the initial experience of implanters, whereas the last quartile represents implant procedures when implanters had the most experience. During the EFFORTLESS study, the device was commercially available in Europe outside of the study. Therefore, the manufacturer's device tracking database was used to adjust the implant ranking for implants done outside of the EFFORTLESS study per individual implanters. The data presented in this study only represent patients who participated in the EFFORTLESS or IDE study.
Statistical analysis
The appropriateness of pooling the two studies was assessed by evaluation of the study as a predictor of each endpoint. Descriptive statistics are reported using the mean ± standard deviation for continuous variables and numbers with percentages for dichotomous variables unless otherwise indicated. Differences in baseline characteristics were tested using a Student's t-test or ANOVA for parametric numerical variables and Fisher's exact test for dichotomous variables unless otherwise indicated. Kaplan–Meier analyses and log-rank test were used to assess event rates across groups.
Univariable models were fit to assess which patient and programming characteristics were associated with each of the three endpoints. Cox proportional hazard models were used for the outcomes of complications and IASs. A general linear model was fit for the outcome procedure time. Each variable that was significant at the 0.10 level was included as a candidate for the final multivariable model for each outcome. Backward selection was used in a multivariable Cox proportional hazards model with a significance level for retention in the model of 0.10. All statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC). P values of <0.05 were deemed statistically significant.
Results
Study population
From August 2009 to November 2013, 889 patients were enrolled in both studies. There was no evidence that endpoints significantly differed by study (complications P = 0.47, IASs 0.12). Procedure time was only collected in the EFFORTLESS registry.
Of these 889 patients (73% men, mean age 50 ± 17 years), 70% received an S-ICD for primary prevention and 38% had ischaemic heart disease. A total of 882 implants were performed in 61 implanting centres by 107 implanters with a median of 4 implants (IQR 1,8). The remaining seven patients were not implanted. Most implanters in this cohort performed a total of 4 implants (Quartile 1) and 11 implanters performed more than 28 implants (Quartile 4). Additionally, there were 187 implants outside the EFFORTLESS study identified in the manufacturer's device tracking database that were used to adjust the implant order per individual implanter. The patient characteristics per quartile of increasing implant experience are summarized in Table 1.
Table 1.
Patient characteristics per experience quartile
| Characteristic (number of implants) | Quartiles |
||||
|---|---|---|---|---|---|
| Q1 (1–4) | Q2 (5–12) | Q3 (13–28) | Q4 (>28) | P value | |
| Number of patients | 248 | 203 | 222 | 216 | |
| Implantersa | 95 | 48 | 25 | 11 | |
| Age (years) | 51.0 ± 17.0 | 47.3 ± 17.9 | 48.6 ± 16.4 | 53.9 ± 15.8 | <0.001 |
| Female (%) | 62 (25.1) | 54 (26.7) | 65 (30.2) | 60 (28.2) | 0.653 |
| Primary prevention (%) | 180 (72.9) | 135 (67.5) | 145 (67.4) | 150 (71.1) | 0.503 |
| QRS (ms) | 107 ± 27 | 103 ± 21 | 104 ± 22 | 108 ± 25 | 0.224 |
| LVEF (%) | 38 ± 17 | 40 ± 18 | 42 ± 18 | 37 ± 17 | 0.59 |
| BMI | 28.5 ± 6.7 | 28.5 ± 7.2 | 28.0 ± 6.9 | 27.9 ± 5.6 | 0.764 |
| Congestive heart failure (%) | 128 (52.0) | 80 (40.2) | 73 (34.0) | 88 (41.3) | 0.001 |
| AF (%) | 40 (16.3) | 23 (11.6) | 40 (18.6) | 40 (18.8) | 0.154 |
| NYHA Classes III and IV (%) | 33 (26.8) | 21 (28.8) | 26 (44.1) | 28 (38.9) | 0.070 |
| Diabetes (%) | 62 (25.2) | 25 (12.6) | 33 (15.3) | 36 (16.9) | 0.004 |
| COPD (%) | 18 (7.3) | 4 (2.0) | 21 (9.8) | 13 (6.1) | 0.007 |
| Hypertension (%) | 129 (52.4) | 70 (35.4) | 71 (33.0) | 61 (28.6) | <0.001 |
| Dual-zone programming (%) | 152 (64.1) | 154 (79.0) | 176 (83.4) | 207 (95.8) | <0.001 |
| Conditional zone rate | 195 ± 20 | 201 ± 20 | 201 ± 21 | 195 ± 14 | 0.79 |
LVEF, left ventricular ejection fraction; BMI, body mass index; CHF, congestive heart failure; HCM, hypertrophic cardiomyopathy; AF, atrial fibrillation; NYHA, New York Heart Association classification; COPD, chronic obstructive pulmonary disease.
aImplanters can contribute to more than one quartile.
Complications
There were 59 patients with 72 complications at 180 days follow-up. Of these complications, infections and suboptimal implantation were most common (Table 2). The median time from implant to complication was 18 days (mean 45 ± 71). The complication rate decreased almost by half from 9.8 to 5.4% (P = 0.019) over the experience quartiles (Figure 1). The absolute risk reduction, therefore, was 4.4% between Quartiles 1 and 4, and the relative risk reduction was 44.8%. The largest decrease was seen between Q1 and Q2. The complication rate stabilized in Quartile 3, which represents >13 procedures per implanter and indicates the end of the learning curve. In a Cox proportional hazard model for complications, increasing experience reduced the occurrence of complications per quartile (HR 0.78, P = 0.045, Table 3).
Table 2.
Complications in the first 6 months
| Description | Number of events |
|---|---|
| System infection | 16 |
| Electrode movement | 7 |
| Suboptimal electrode position | 7 |
| Erosion | 5 |
| Discomfort | 4 |
| Haematoma | 4 |
| Suboptimal pulse generator and electrode position | 4 |
| Adverse reaction to medication | 3 |
| Inadequate/prolonged healing of incision site | 3 |
| Incision/superficial infection | 3 |
| Pulse generator movement/revision | 3 |
| Suboptimal pulse generator position | 2 |
| Failed defibrillation threshold test | 2 |
| Acute hypoxic respiratory failure | 1 |
| Incomplete electrode connection to the device | 1 |
| Near syncope/dizziness/shortness of breath/confusion | 1 |
| Pleural effusion | 1 |
| Pneumothorax | 1 |
| Seroma | 1 |
| Suspected worsening of ischaemia | 1 |
| Suture discomfort | 1 |
| Undersensing | 1 |
| Grand total | 72 |
Figure 1.
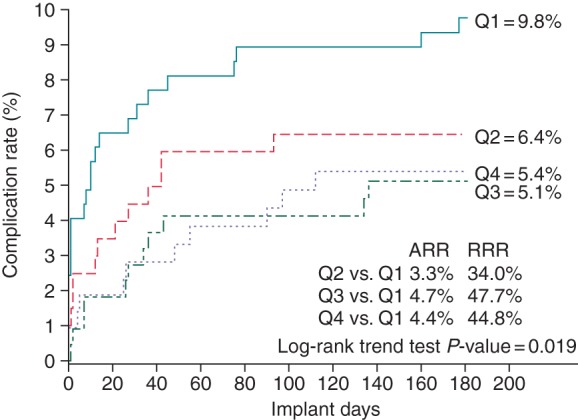
Kaplan–Meier analysis of experience quartiles and complications at 180 days. Q1: Experience Quartile 1 (implants 1–4); Q2: Experience Quartile 2 (implants 5–12); Q3: Experience Quartile 3 (implants 13–28); Q4: Experience Quartile 4 (implants >28); ARR, absolute risk reduction; RRR, relative risk reduction. P value is Kaplan–Meier trend test.
Table 3.
Multivariable Cox proportional hazard model for complications
| Characteristic | Hazard ratio (95% CI) | P value |
|---|---|---|
| Physician experience (per quartile) | 0.78 (0.61, 0.99) | 0.045 |
| BMI (per 5 units) | 1.15 (0.98, 1.34) | 0.090 |
| Age (per 5 years) | 0.89 (0.82, 0.96) | 0.005 |
| Atrial fibrillation (yes vs. no) | 1.91 (1.00, 3.67) | 0.051 |
In the univariable model, there was a non-significant trend towards fewer complication over the years 2009–2013 (P = 0.07). However, implant year was not retained in the multivariable model (Table 3). Implant year also did not interact with physician experience (Supplementary material online, Figure S1). No individual complication type was observed to drive the decrease in complications (Supplementary material online, Table S1).
The complication rate in implanter's first four S-ICD patients for those who ended as relatively high-volume implanters (>5 implants, 50% of implanters) was compared with lower-volume implanters in the study (1–5 implants, 50% of implanters). Higher-volume implanters had a similar complication rate (9.4%, 95% CI 5.6–15.7%) in the first four implants to lower-volume implanters (10.2%, 95% CI 5.8–17.6%, P = 0.429).
Procedure time
A sub-analysis among 434 EFFORTLESS patients for whom skin-to-skin procedure time was available showed a decrease from ∼75 ± 34 min in Quartile 1 to 65 ± 23 min in Quartile 4 (P < 0.001), which stabilized in Quartiles 3 and 4 (Figure 2). A generalized linear model for procedure time showed that the decrease in procedure time did not remain significant (P = 0.81) after adjustment for confounders: implant year, age, congenital heart disease, and prior ICD (Table 4).
Figure 2.
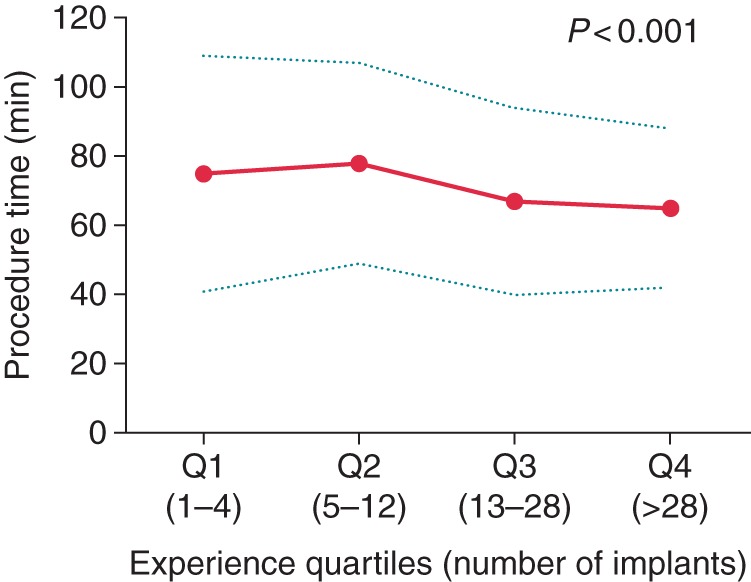
Skin-to-skin procedure time by experience quartiles. Q1: Experience Quartile 1 (implants 1–4); Q2: Experience Quartile 2 (implants 5–12); Q3: Experience Quartile 3 (implants 13–28); Q4: Experience Quartile 4 (implants >28). Solid line is mean, and dashed lines are ±1 SD. P value is trend test.
Table 4.
Generalized linear model for procedure time
| Characteristic | Effect (95% CI) | P value |
|---|---|---|
| Intercept | 101.2 (91.1, 111.3) | <0.001 |
| Physician quartile (per quartile) | 0.3 (−2.4, 3.1) | 0.807 |
| Implant year (per year starting in 2009) | −6.7 (−9.2, −4.1) | <0.001 |
| Age (per 5 years) | −1.7 (−2.4, −1.0) | <0.001 |
| Congenital disease (yes vs. no) | 26.1 (8.3, 43.9) | 0.004 |
| Prior ICD (yes vs. no) | 8.9 (2.0, 15.8) | 0.011 |
Inappropriate shocks
In this cohort, there were a total of 107 IASs in 48 patients during the first 180 days. Seventy-one per cent of the IASs were related to cardiac oversensing, 19% to supraventricular tachycardias, and 10% to non-cardiac oversensing. The median time between implant and the occurrence of the first IAS was 98 days (141 ± 105). There was a non-significant trend towards fewer IASs from 7.9 to 4.5% from Quartile 1 to Quartile 4 (P = 0.10) (Figure 3). Dual-zone programming increased significantly over the experience quartiles from 64 to 96% (P < 0.001). In a Cox proportional hazard model for IASs, increasing experience was not found to be a predictor for the decrease of IASs (HR 1.01, P = 0.96) (Table 5). However, dual-zone programming was significantly associated with fewer IASs (HR 0.44, P = 0.01).
Figure 3.
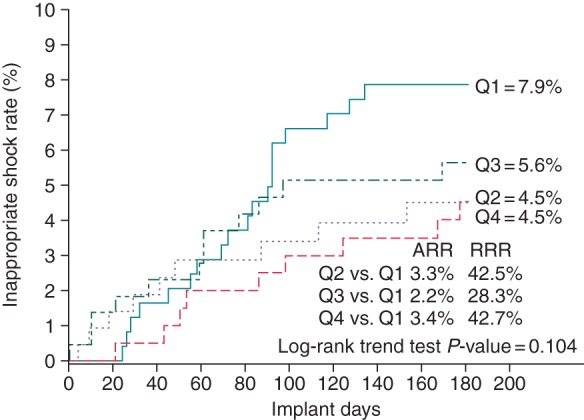
Kaplan–Meier analysis of experience quartiles and IASs at 180 days. Q1: Experience Quartile 1 (implants 1–4); Q2: Experience Quartile 2 (implants 5–12); Q3: Experience Quartile 3 (implants 13–28); Q4: Experience Quartile 4 (implants >28); ARR, absolute risk reduction; RRR, relative risk reduction. P value is Kaplan–Meier trend test.
Table 5.
Multivariable Cox proportional hazard model for IASs
| Characteristic | Hazard ratio (95% CI) | P value |
|---|---|---|
| Physician quartile (per quartile) | 1.01 (0.77, 1.32) | 0.958 |
| Age (per 5 years) | 0.91 (0.83, 0.99) | 0.028 |
| Programming zones (dual vs. single) | 0.44 (0.24, 0.82) | 0.010 |
| NYHA class (II–IV vs. I) | 2.09 (1.13, 3.88) | 0.020 |
Discussion
Main findings
This study describes the learning curve for individual S-ICD implanters in the two largest cohorts to date regarding implant-related complications, procedure duration, and IASs. The main finding of this study is that implant-related complications significantly decrease from 9.8 to 5.4% with increasing experience of the individual implanter, and stabilized in Quartile 3, which represents >13 procedures per implanter. The unadjusted procedure duration decreased over the experience quartiles, but it did not remain significant after multivariable adjustment for baseline characteristics. There was a non-significant decrease of IASs over the experience quartiles, which was primarily explained by the increase in dual-zone programming of the S-ICD.
Complications
The complication rate decreased almost by half when the earliest experience (Quartile 1: first four procedures per implanter) was compared with the latest experience in this cohort. The largest reduction was seen between Q1 and Q2, indicating that most of the learning happens in the implanter's first four cases. It is possible that this result was driven by individual physicians who had a low complication rate in their early experience vs. implanters that may have experienced a higher complication rate early and stopped implanting. To explore whether this effect was driven by individual physicians, the complication rate in the first four implants per individual implanter was assessed.
High-volume implanters had a similar complication rate in the first four implants as those who implanted fewer S-ICDs. This indicates that the complication rate during the earliest experience is similar for all implanters, and individual physicians who became high-volume implanters did not have a lower complication rate in their early experience. If the low-volume implanter would have continued to increase their experience, they may reduce their complication rate to the level of high-volume implanters.
In the 2-year follow-up of the combined EFFORTLESS and IDE study, which included the earliest commercial and investigational experiences, Burke et al. found a complication rate of 7.7% at 180 days.1 That study showed that the complication rate reduced over time. In the current study with the same cohort, the difference in implant period did not remain significant in the multivariable model, whereas experience of the implanters did. Therefore, this analysis of the same cohort indicates that the complication rate of 7.7% was elevated by the early learning curve of individual implanters and that the rate for experienced implanters is lower.
Procedure time
The procedure time decreased significantly from 75 ± 34 to 65 ± 23 min over the experience quartiles in the unadjusted analysis (Figure 2). Interestingly, in the multivariable model, each increase in experience quartile did not significantly reduce procedure time, but each increment in implant year did. This indicates that the implant procedure has become more efficient over time, such as by the introduction of the two-incision technique that omits the superior parasternal incision.13 In the unadjusted analysis, the procedure time increased in the second quartile to 78 min compared with 75 the first quartile, which may be explained by the presence of a proctor during implants in Q1. In the presumed implants without a proctor (Q2–Q4), the trend of reduction in procedure time reduction is clearer. When procedure time in Quartile 4 is compared with unpublished data from MADIT-CRT, it is longer than in single-chamber ICDs, 65 ± 23 vs. 51 ± 25 min, respectively (P < 0.001), and similar to dual-chamber ICDs 63 ± 30 min (P = 0.414).14
Inappropriate shocks
There was a non-significant trend towards fewer IASs with increasing experience of the individual implanter. In the multivariable model, this trend was caused by increased dual-zone programming over the experience quartiles. The discrimination algorithm in the conditional zone between 170 and 250 beats per minutes has proved to be equally good at detecting ventricular arrhythmias and superior with respect to discrimination of supraventricular arrhythmia in a head-to-head comparison study published in 2012.5 Roughly half of the cohort of this study was implanted prior to this publication, which may explain the increase in dual-zone programming. The increased use of dual-zone programming can be considered as learning at a global level enabling knowledge to be transferred to new implanters. Potential other confounders such as discrimination algorithm improvements and reduction of myopotential oversensing after introduction of the suture sleeve may have contributed, but these variables were not collected in both studies. Improvement of the discrimination algorithm may continue to reduce the IAS shock rate but need to be proved in prospective studies.15 Future studies into programming of rate cut-off values for the conditional and unconditional zones are warranted in order to optimize sensitivity and specificity of the detection of ventricular arrhythmias.
Clinical implications
This study has several implications for training and instruction of new S-ICD implanters. First, we observed rapid improved performance by implanters resulting in a stable complication rate after 13 implants. The complication rate decreased for all implanters after four implants. Complications occurring during the early experience should encourage the implanter to analyse and adjust the workflow where needed and continue the learning process. Interruption of implants in response to complications stops the learning process and diminishes the impact of skills acquired up to that point.
There was no individual implanters learning curve with respect to IASs and procedure time. Strategies to reduce IASs can be transferred to new implanters.16,17 Therefore, the IAS rate in Quartile 4 in this analysis is achievable for new implanters from their first procedure.
Study limitations
This study has several limitations. First, this is a retrospective cohort analysis that can only establish associations and not determine causation. Implant experience may, therefore, be a surrogate marker for an unidentified confounder. Other confounders that may influence the learning curve for individual implanters such as differences in transvenous ICD implant experience/volume or the number of implants that were performed in the presence of a proctor were not part of the current analysis.
Conclusions
There is a short and significant learning curve associated with physicians adopting the S-ICD. The complication rate of 9.8% decreased with increasing experience of individual implanters and stabilized after 13 implants per implanter at 5.4%. The reduction of IASs and procedure time likely reflects a global learning effect.
Supplementary material
Funding
The S-ICD IDE study and the EFFORTLESS S-ICD registry are sponsored in their entirety by Cameron Health, Inc., a subsidiary of Boston Scientific Corporation.
Supplementary Material
Acknowledgements
The authors acknowledge the large contribution of investigators and institutions participating in the EFFORTLESS registry and IDE study as listed in Supplementary material online, Appendix S1, and the MADIT-CRT investigators for sharing procedure time data of transvenous ICDs.
Conflict of interest: R.E.K. has received grant support and consulting fees from Boston Scientific. D.A.T. has received institutional grant support and consulting fees from Boston Scientific. L.B. has received consulting fees from Medtronic and Boston Scientific; and speaking fees from Medtronic, Boston Scientific, and Biotronik. R.W. has received consulting fees, speaking fees, and research grant support from Biosense Webster, Biotronik, Boston Scientific, Medtronic, and St Jude Medical. P.N. has received research grant support from Boston Scientific, Medtronic, and St Jude Medical. P.D.L. has received speaker fees and research support from Boston Scientific, Medtronic, St Jude Medical, and UCLH Biomedicine NIHR. A.R.L. has received research grant support from Boston Scientific. P.J. and N.W. are employees of Boston Scientific. A.A.G. has received consulting fees from Boston Scientific. M.C.B. has received research grant support, speaking fees, and consulting fees from Boston Scientific, Medtronic, and St Jude Medical.
References
- 1. Burke MC, Gold MR, Knight BP, Barr CS, Theuns DA, Boersma LV et al. Safety and efficacy of the totally subcutaneous implantable defibrillator. J Am Coll Cardiol 2015;65:1605–15. [DOI] [PubMed] [Google Scholar]
- 2. Kirkfeldt RE, Johansen JB, Nohr EA, Jorgensen OD, Nielsen JC. Complications after cardiac implantable electronic device implantations: an analysis of a complete, nationwide cohort in Denmark. Eur Heart J 2014;35:1186–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gadler F, Valzania C, Linde C. Current use of implantable electrical devices in Sweden: data from the Swedish pacemaker and implantable cardioverter-defibrillator registry. Europace 2014;17:69–77. [DOI] [PubMed] [Google Scholar]
- 4. Bardy G, Smith W, Hood M, Crozier I, Melton I, Jordaens L et al. An entirely subcutaneous implantable cardioverter–defibrillator. N Engl J Med 2010;363:36–44. [DOI] [PubMed] [Google Scholar]
- 5. Gold MR, Theuns DA, Knight BP, Sturdivant JL, Sanghera R, Ellenbogen KA et al. Head-to-head comparison of arrhythmia discrimination performance of subcutaneous and transvenous ICD arrhythmia detection algorithms: the START study. J Cardiovasc Electrophysiol 2012;23:359–66. [DOI] [PubMed] [Google Scholar]
- 6. Hopper A, Jamison M, Lewis W. Learning curves in surgical practice. Postgrad Med J 2007;83:777–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Al-Khatib SM, Lucas FL, Jollis JG, Malenka DJ, Wennberg DE. The relation between patients’ outcomes and the volume of cardioverter-defibrillator implantation procedures performed by physicians treating Medicare beneficiaries. J Am Coll Cardiol 2005;46:1536–40. [DOI] [PubMed] [Google Scholar]
- 8. Krahn AD, Lee DS, Birnie D, Healey JS, Crystal E, Dorian P et al. Predictors of short-term complications after implantable cardioverter-defibrillator replacement: results from the Ontario ICD database. Circ Arrhythm Electrophysiol 2011;4:136–42. [DOI] [PubMed] [Google Scholar]
- 9. Curtis JP, Luebbert JJ, Wang Y, Rathore SS, Heidenreich PA, Hammill SC et al. Association of physician certification and outcomes among patients receiving. JAMA 2009;301:1661–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Olde Nordkamp LR, Dabiri Abkenari L, Boersma LV, Maass AH, de Groot JR, van Oostrom AJ et al. The entirely subcutaneous implantable cardioverter-defibrillator: initial clinical experience in a large Dutch cohort. J Am Coll Cardiol 2012;60:1933–9. [DOI] [PubMed] [Google Scholar]
- 11. Weiss R, Knight BP, Gold MR, Leon AR, Herre JM, Hood M et al. Safety and efficacy of a totally subcutaneous implantable-cardioverter defibrillator. Circulation 2013;128:944–53. [DOI] [PubMed] [Google Scholar]
- 12. Lambiase PD, Barr C, Theuns DA, Knops RE, Neuzil P, Johansen JB et al. Worldwide experience with a totally subcutaneous implantable defibrillator: early results from the EFFORTLESS S-ICD registry. Eur Heart J 2014;35:1657–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Knops RE, Olde Nordkamp LR, de Groot JR, Wilde AA. Two-incision technique for implantation of the subcutaneous implantable cardioverter-defibrillator. Heart Rhythm 2013;10:1240–3. [DOI] [PubMed] [Google Scholar]
- 14. Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med 2009;361:1329–38. [DOI] [PubMed] [Google Scholar]
- 15. Brisben AJ, Burke MC, Knight BP, Hahn SJ, Herrmann KL, Allavatam V et al. A new algorithm to reduce inappropriate therapy in the S-ICD system. J Cardiovasc Electrophysiol 2015;26:417–23. [DOI] [PubMed] [Google Scholar]
- 16. Kooiman KM, Knops RE, Olde Nordkamp L, Wilde AA, de Groot JR. Inappropriate subcutaneous implantable cardioverter-defibrillator shocks due to T-wave oversensing can be prevented: implications for management. Heart Rhythm 2014;11:426–34. [DOI] [PubMed] [Google Scholar]
- 17. Olde Nordkamp LR, Brouwer TF, Barr C, Theuns DA, Boersma LV, Johansen JB et al. Inappropriate shocks in the subcutaneous ICD: incidence, predictors and management. Int J Cardiol 2015;195:126–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


