Abstract
Scope
Syzygium cumini (Jamun) is perhaps the only berry that has the diversity of anthocyanidins of blueberry and bilberry and the abundance of ellagitannins/ellagic acid of black raspberry. Here we report the potential of jamun against 17β-estrogen-mediated breast cancer and the role of miRNAs and other targets in disease inhibition.
Methods and results
Female ACI rats were given AIN-93M diet or diet supplemented with jamun. Two weeks later, animals received 17β-estradiol and were palpated weekly for the mammary tumors. At the end of 26 week, the jamun-diet significantly delayed the first tumor appearance by 21 days, and reduced the tumor incidence (65% vs 96%), tumor burden (313±95 vs 661±123 mm3) and tumor multiplicity (1.8±0.3 vs 4.2±0.4 tumors/rat) compared to control. The experimental diet significantly reduced the estrogen-associated growth of pituitary prolactinomas, circulating prolactin and estradiol levels and offset estrogen-associated increases in mammary cell-proliferation, ER-α and cyclinD1. MiRNAs that were either overexpressed (miR-182 and miR-375) or underexpressed (miR-127 and miR-206) following estrogen-treatment were significantly protected by jamun diet.
Conclusions
Together, our data show that jamun significantly offset estrogen-mediated alterations in mammary cell-proliferation, ER-α, cyclinD1, and candidate miRNAs, and that the modulation of these biomarkers correlated with a reduction in mammary carcinogenicity.
Keywords: Jamun pulp, Breast cancer, Estradiol, ACI rats, miRNAs
Graphical Abstract

Syzygium cumini (Jamun) has the diversity of anthocyanidins of blueberry and bilberry and the abundance of ellagitannins/ellagic acid of black raspberry. These chemopreventives from jamun effectively inhibit breast cancer tumorigenesis induced by 17β-estrogen in ACI rats modulating various genes and miRNAs.
1. Introduction
Breast cancer (BC) is the most frequently diagnosed cancer in American women after skin cancer and the second leading cause of cancer-related death. About 1 in 8 U.S. women (12%) will develop invasive BC over the course of lifetime. According to American Cancer Society, an estimated 231,840 new cases of invasive BC are expected to be diagnosed in the U.S., along with 60,290 new cases of non-invasive BC. About 40,290 women are expected die from BC [1]. In 2012, it represented about 12 percent of all new cancer cases and 25 percent of all cancers in women globally. Although deaths from breast cancer in the United States have been decreasing over the past few decades [2], worldwide BC incidence has increased by more than 20 percent, and mortality increased by 14 percent [3].
The development of BC has been associated with numerous risk factors including genetic, environmental, hormonal, and dietary influences [4]. Accumulating results from epidemiological studies increasingly suggest a role of both endogenous and exogenous reproductive hormones, particularly estrogens (E2) in the etiology and progression of BC. E2 is thought to bind with estrogen receptors in stromal cells to induce the release of growth factors, which then stimulate proliferation of epithelial cells in the mammary glands. Up-regulated ERα has been identified as an important factor during early stages of tumorigenesis in stimulating the proliferation of mammary cells leading to tumor development [5].
Besides genetic mutations, epigenetic mechanisms also have an important role in BC tumorigenesis. Therefore, research focusing on detailing epigenetic contributions to the development and progression of BC such as DNA methylation, histone modifications, and miRNA expression has increased. In particular miRNAs have gained much attention lately with a large pool of data indicating dysregulation of miRNA expression in BC [6]. Genome-wide miRNA expression studies have shown differential expression between normal tissue and breast tumors and can distinguish between breast tumor subgroups [7–9]. Interestingly, the differential expression of several miRNAs has also been correlated with the status of estrogen receptors, ERα and ERβ, PR and HER2/neu status [10, 11]. This is of particular importance as ERα is a target for endocrine-based therapies in 70% to 75% of early BC [12, 13].
Some recent reports have shown a link between E2 and/or ERs and miRNA expression [14, 15]. Our own studies have indicated that E2 treatment significantly modulated at least 33 miRNAs during ACI rat mammary gland carcinogenesis [16]. Moreover, a plant polyphenolic, ellagic acid (EA), was shown to reverse the effects of E2 on oncogenic miRNAs and tumor suppressor miRNA expression resulting in protective effects observed as decreased tumor incidences and tumor volume [16]. Given the important role of miRNAs in cancer cell proliferation and regression of tumors, it would be an advantageous approach to target estrogen-associated dysregulated miRNAs by dietary interventions.
Epidemiological studies have consistently shown that consumption of fruits and vegetables is strongly associated with reduced risk of cancer and other diseases [17]. Berries have recently received great attention because of their potential to prevent chemically-induced colon [18] and esophageal [19] cancers in animal models. Studies from Stoner and colleagues have demonstrated that black raspberry (BRB) shows high anticarcinogenic potential [20–22]. These protective effects were largely attributed to high content of EA in BRB. [23]
Anthocyanins are most prominent among the flavonoids, a large class of phenolic compounds that is abundant in dark-colored fruits, including berries. Anthocyanins present in berries are known to impart anticancer effects by induction of metabolizing enzymes, regulation of gene expression and their downstream signaling pathways, modulation of cell proliferation and apoptosis [24, 25]. The chemical profile of anthocyanidins present in berries is shown in Figure 1. Typically, BRB, which is a good source of EA contains almost exclusively cyanidin as the primary anthocyanin component, whereas BB, contains glycosides of five different anthocyanidins but is essentially devoid of EA [21, 25, 26]. Studies from our laboratory have shown that BB and BRB show comparable protection against mammary tumorigenesis suggesting role of polyphenolics other than EA behind BB activity [21]. Additionally, we reported 55% reduction in tumor burden in ACI rats treated with 5% BB powder in the diet [23]. Whole BB powder and extracts were also reported to modulate the growth and metastasis of triple-negative breast cancer MDA-MB-231 cells and tumors through modulation of the PI3K/AKT/NFκB pathway [27]. Thus, we hypothesized that berries containing diverse anthocyanidins and EA together could provide much enhanced protection against hormone-induced breast tumors.
Figure 1.
Photographs of blueberry (BB), black raspberry (BRB) and jamun berries shown along with the types and amounts of their anthocyanidins. Anthocyanidin structure is shown on the right side. In addition to ellagic acid (EA), jamun pulp powder contains anthocyanidins similarly as found in BB (delphinidin, cyanidin, peonidin, petunidin, and malvidin), however, their relative ratios are different. BRB almost exclusively contains cyanidin and EA.
Syzygium Cumini L., commonly known as ‘jamun’ in India and other Asian countries, is a popularly consumed berry and is used in treating certain ailments such as diabetes mellitus [28]. Jamun pulp contains anthocyanins including glycosides of delphinidin (Dp), malvidin (Mv), cyanidin (Cy), petunidin (Pt), and peonidin (Pe), and a significant amount of EA. Perhaps, this is the only berry that contains these five different anthocyanidins and EA [29]. Jamun pulp extracts was shown to exhibit antiproliferative effects and induce apoptosis in BC cell lines [30]. We have previously demonstrated the in vitro antioxidant and antiproliferative potential of jamun pulp [29]. In this study, we report on the chemopreventive potential of jamun berry in the estrogen- induced mammary carcinogenesis model and elucidate the possible mechanisms of action by analysis of various cellular and molecular markers.
2. Material and methods
17β-estradiol was purchased from Steraloids, Inc. (Newport, RI). The silastic tube (2.0 mm i.d.) was purchased from Allied Biomedical, Inc. (Ventura, CA) and medical-grade silicone adhesive was purchased from Factor II, Inc. (Lakeside, AZ).
2.1 Jamun berries
Jamun fruit was handpicked in the garden of National Institute of Pharmaceutical Education and Research, S.A.S. Nagar, India. Jamun pulp was peeled, air dried and processed as described previously [31]. Briefly, jamun fruit was harvested, rinsed with deionized water, pulp was collected and dehydrated using commercial dehydrators (at 35°C–40°C), powdered, lyophilized to remove residual moisture, vacuum packed, and stored at −20 °C until use. Control AIN-93 M diet and diets supplemented with berries were prepared in pellet form by Harlan-Teklad, Inc. (Madison, WI). AIN-93 M diet was supplemented with 5% jamun pulp powder, by replacing the corn starch and fiber content, to adjust for the calorie content as described previously [31].
2.2 Animal study
Five to 6 week-old female ACI rats were purchased from Harlan Sprague-Dawley, Inc. (Indianapolis, IN). The rats were housed in cages and provided food and water ad libitum following an approved protocol (#13008) from the Institutional Animal Care and Use Committee. After acclimation, the rats were divided into 4 groups. Groups 1 and 3 received an AIN-93M diet, and groups 2 and 4 received a diet supplemented with (5%, w/w) Jamun powder. Two weeks later, rats from groups 3 and 4 (n=22–24) were implanted with 1.2 cm-silastic implant containing 9 ± 0.2 mg 17 β-estradiol (E2), as described previously [32]. Body weights and diet consumption were assessed weekly for all groups until termination. The animals challenged with E2 were palpated for mammary tumors every week to monitor the tumor appearance starting from week 12. Rats were euthanized at 26 weeks when the tumors reached 1.2 cm in size. When the palpable tumor incidence in the control group reached ~90%, all animals were euthanized by CO2 asphyxiation, blood was drawn, and tumors were measured. Mammary tumor, adjacent tissues, and pituitary gland were weighed, and a small piece of each tissue was transferred to 10% buffered formaldehyde for histopathological analysis and immunohistochemistry.
2.3 Plasma prolactin
Plasma prolactin levels were measured using the Rat Prolactin EIA kit (Alpco Diagnostics, Windham, NH).
2.4 Serum estradiol analysis
Circulating serum E2 was analyzed from blood samples by electrochemiluminescent detection using the Roche E170 immunoassay analyzer at the University Hospital’s Clinical Chemistry core facility as described [21]. The standard curve of E2 was linear in the range of 5–4300 pg/ml concentrations. An estradiol II reagent kit was purchased from Roche Diagnostics, Inc. (Indianapolis, IN) and conditions were followed as per the manufacturer’s instructions. The assay was calibrated using the standards provided by the vendor. The analysis was confirmed by using an enzyme immunoassay (EIA) kit for estradiol (Cayman Chem. Ann Arbor, MI). All samples were assayed in duplicate.
2.5 Immmuno histochemistry
Mammary tissue sections (5 μm) were stained for Proliferating Cell Nuclear Antigen (PCNA) using the Zymed Rat PCNA kit (Invitrogen Co., Carlsbad, CA) as described previously [28]. Briefly, sections cut from paraffin blocks of mammary tissues were dewaxed and rehydrated through graded ethanol to water for routine H&E staining and immunohistochemistry. Endogenous peroxidases were blocked with 3% H2O2 for 10 min. Antigen retrieval was carried out by boiling the sections in 0.01 M citrate buffer, pH 6.0 for 20 min in boiling water bath followed by staining with PCNA. Positive-stained cells were scored by 2 independent cytopathologists and average values were plotted.
2.6 Western blot analysis
Whole cell lysates (WCL) were prepared from mammary tissues of treated and untreated animals using RIPA buffer (Santa Cruz Biotechnology, Santa Cruz, CA) and analyzed by Western-blot analysis as described [33]. After transfer, the membrane was immunoblotted with Cyclin D1, ERα and PCNA antibodies (Cell-Signaling Technology, Danvers, MA). Appropriate secondary antibodies were used and detection carried out using an enhanced chemiluminescence reagent (Thermo Scientific, Waltham, MA). Equal loading of the proteins was confirmed using β-actin (Sigma-Aldrich, St. Louis, MO).
2.7 HPLC analysis
BRB, BB, and jamun extract samples (5 μL injection volume; 1 mg/mL concentrations) were analyzed on a Shim-Pack XR-ODS II column reverse phase column (Shimadzu; 150 × 3.0 mm i.d., 2.2μm). Anthocyanidins and other phenolics were separated in a gradient of 3.5% aqueous phosphoric acid and acetonitrile with flow rate 0.75 mL/min. Anthocyanidins were monitored at 520 nm as described [26].
2.8 qPCR analysis of selected miRNA
Small RNAs (<200 bp) were isolated from mammary tissues using the enrichment procedure of mirVana microRNA kit. qPCR analysis of miRNAs was performed using TaqMan microRNA Reverse Transcription Kit and TaqMan gene-specific MicroRNAassays (Applied Biosystems) according to the manufacturer’s instructions. All measurements were performed in triplicate. 5S RNA was used as housekeeping gene.
2.9 qRT-PCR for target gene expression
Primers for qRT-PCR were designed across the exon boundary using Primer Express 3.0 software (Applied Biosystems) and synthesized by Integrated DNA Technologies, Inc. as described [16]. The sequences of the forward and reverse primers for each gene tested are as follows: CyclinD1 forward, 5′-CCAGCCTTCTGACCTCTTTCC-3′; reverse, 5′-TCTTCGATTTGTTTTGCATCCA-3′, Cyclin D3 forward, 5′-TCTTGACCAGTACCAC ATTTTTGAG-3′; 5′-TCGAAAATATAGGTGTTGAGAGGTAGTG-3′, ERα forward 5′-GGCACATGAGTAACAAAGGCA-3′; reverse 5′-GGCATGAAGACGATGAGCAT-3′, PGR forward 5′-TCACAACGCTTCTATCAACTTACAAA-3′; reverse 5′-GGCAGCAATAA CTTCAGACA TCA-3′, 18S forward 5′-GCCAACCGTGAAAAGATG AC-3′; reverse 5′-ACCCTCATAGA TGGGCACAG-3′, BCl2 5′-GTATGATAACCGGGAG ATCG-3′; reverse 5′-AGCCAGGAG AAATCAAACAG-3′, Foxo forward 5′GATAAGGGC GACAGCAACAG-3′; reverse 5′-CTGTGCAGGGACAGGTTGT-3′ and Cdk4 forward 5′-GGATCTGATG CGCCAGTTTC-3′, reverse 5′-GGTCCCGGTGAACAATGC-3′.A One-Step SYBR green qRT-PCR Kit (Quanta Biosciences, Gaithersburg, MD) was used to perform cDNA synthesis and PCR amplification simultaneously from 100 ng of total RNA according to the manufacturer’s instructions. Reactions were run under the following conditions: hold at 50°C for 10 min, 95°C for 5 min, then 40 cycles at 95°C for 10 sec and 60°C for 30 sec. Relative gene expression was assessed using the differences in normalized Ct (ΔΔCt) method after normalization to 18s rRNA. Fold changes were calculated by 2−ΔΔCt.
2.10 Toxicity testing
Blood was collected separately at the time of euthanasia and hematological parameters were analyzed using whole blood by Cell Dyn 3500 hematology analyzer (Abbott laboratories, Santa Clara, CA). Serum was used to analyze the liver and kidney function enzymes. An ion selective electrode was used for analysis of various electrolytes and in-built spectrophotometric techniques were used for all other biochemical parameters analysis on automated AU640 Chemistry analyzed (Beckman Coulter, Inc., Brea, CA).
2.11 Statistical analyses
Differences between the means of the treatments were calculated for serum E2 levels, organ/ tissues weights, body weight and tumor multiplicity. A student unpaired t-test was used to compare treatment group averages for tumor volume. Generalized linear model (GLM) followed by post hoc Tukey’s multi-comparison test was used to compare treatment group averages for serum E2, prolactin and progesterone levels, organ/ tissues weights, body weight, and expression levels of proteins Cyclin D1, ERα and PCNA as well as biochemical and hematological parameters using SAS software. Tumor multiplicity was analyzed using the Negative-Binomial-regression model with logarithmic link. Latency was defined as the number of days of E2 treatment before the appearance of the first palpable mammary tumor based on weekly examination and determined using Kaplan-Meier survival estimates and plots. Relative fold change in miRNA and gene expression was analyzed using two-way ANOVA, followed by a Bonferroni’s multiple comparison posttest. In each assay a p value of <0.05 was considered to be statistically significant. The data are presented as mean ± SD.
3. Results
3.1 Effect of E2 Treatment on food consumption and growth
Control untreated rats fed on AIN-93M control diet consumed an average 9.6 ± 0.4 g of diet per day throughout the course of the experiment (Figure 2). The diet consumption was increased in E2-treated rats (10.7 ± 0.8 g). However, diet supplemented with jamun powder (5%, w/w) did not affect diet intake when compared with control irrespective of E2 treatment (Figure 2). At the end of the study (25 weeks), there was no significant difference between the animals fed with the control AIN-93M diet or jamun-supplemented diet suggesting no specific effect of jamun diet on animal weight gain. In addition, groups receiving E2-treatment also exhibited no difference in bodyweight over non-E2 treated groups. None of the groups lost weight until end of the study.
Figure 2.
Average weekly body weight gain and diet consumption. (A) Body weights of rats receiving either control diet or diet supplemented with 5% jamun pulp powder. Animals were weighed weekly and average body weight is presented. SD (≤7%) is not included for clarity. (B) Diet consumption by the ACI rats fed control diet, and diet supplemented with jamun, with and without estrogen treatment during the course of the study.
3.2 Effect of jamun diet on organs weight
Liver, lung, mammary and pituitary organ weights were correlated with bodyweight. Liver weight significantly (p=0.001) increased by 1 gm over the period of 180 days following E2 treatment. A significant decrease in lung weight with the jamun intervention compared to untreated control (Table I) was observed, while no difference was observed in the lung weight by E2 or E2/jamun diet. E2 treatment significantly enhanced the mammary tissue weight by almost 2-fold, which was partly offset by jamun intervention. The effect of jamun on decreasing E2-induced mammary weight was found to be significant (p= 0.005). Similarly, the weight of the pituitary was increased 6-fold with the E2 treatment. Although jamun treatment showed a modest decrease in pituitary weight, it was insignificant.
Table I.
Effect of Jamun intervention of body and organ weights.
| Group | Organ/ tissue weights at euthanasia
|
||||
|---|---|---|---|---|---|
| Body wt. (g) | Liver (g) | Lung (g) | Mammary (g) | Pituitary (mg) | |
| Control diet | 191.5 ± 11.6 | 5.9 ± 0.4 | 1.1 ± 0.2 | 4.4 ± 1.2 | 10.8 ± 0.9 |
| 5% Jamun | 193 ± 10 | 5.0 ± 0.5 | 0.8 ± 0.2* | 4.4 ± 0.6 | 9.1 ± 0.6 |
| E2/ Control diet | 202.4 ± 8.6 | 6.9 ± 0.6*** | 1.0 ± 0.1 | 7.0 ± 0.7*** | 52.0 ± 9.0*** |
| E2/ 5% Jamun | 198.9 ± 11 | 6.8 ± 0.7 * | 1.0 ± 0.1 | 6.1 ± 1.0***,## | 47.3 ± 11.7*** |
Data are reported as Mean ± SD. Statistical significance was analyzed by generalized linear model followed by post hoc Tukey’s multi-comparison test. Asterisks show significant difference at *p <0.05; **p <0.01; ***p <0.001 versus the control diet whereas # denotes comparison between E2/Jamun to E2 treated control animals at #p < 0.05; ##p <0.01.
3.3 Tumor indices
Administration of E2 to female ACI rats fed on the control diet resulted in the development of mammary tumors and the first palpable tumor was detected after 84 days of E2 treatment. Compared to E2-treated control, the jamun-supplemented diet delayed the first tumor appearance by 21 days. Consumption of the jamun diet markedly inhibited development of E2-induced mammary tumors over control group (65% vs 96%) by 25 weeks (Figure 3A). Both latency and final mammary tumor incidence differed significantly between animals fed with the control diet versus the jamun diet. Consumption of the jamun diet also reduced the tumor burden significantly in addition to reducing tumor incidence and increasing latency compared to the control diet. The average volume of mammary tumor tissues was 661 ± 123 mm3 in animals fed with the control diet compared to 313 ± 95 mm3 in the rats fed with the jamun diet (Figure 3B). A total of 101 mammary tumors were developed in the group of 24 animals fed with the control diet giving an average of 4.2 ± 0.4 tumors/rat. In contrast only 36 tumors developed in the group of 20 rats with the jamun diet intervention with an average of only 1.8 ± 0.3 tumors/rat (Figure 3C). The differences observed in tumor multiplicity and tumor volume in control vs jamun-treated animals were both statistically significant (p < 0.05).
Figure 3.
Effect of diet supplemented with 5% jamun pulp powder on tumor incidence (A), tumor volume (B), and tumor multiplicity (C). Female ACI rats were challenged with silastic implants of E2 and tumors were palpated after twelve weeks of E2 implantation. Tumor incidence was calculated from the weekly palpation report and analyzed using a nonparametric log-rank test. Asterisk indicates significant difference between animals fed with control diet versus experimental diet. Tumor volume (B) and tumor multiplicity (C) were calculated at the time of euthanasia and analyzed using unpaired two-tailed Student’s t-test. Asterisk indicate the significant differences of jamun diet in reducing both, the tumor volume and tumor multiplicity when compared with the E2-treated control. *p <0.05; **p <0.01; ***p <0.001.
3.4 Effect on mammary cell proliferations
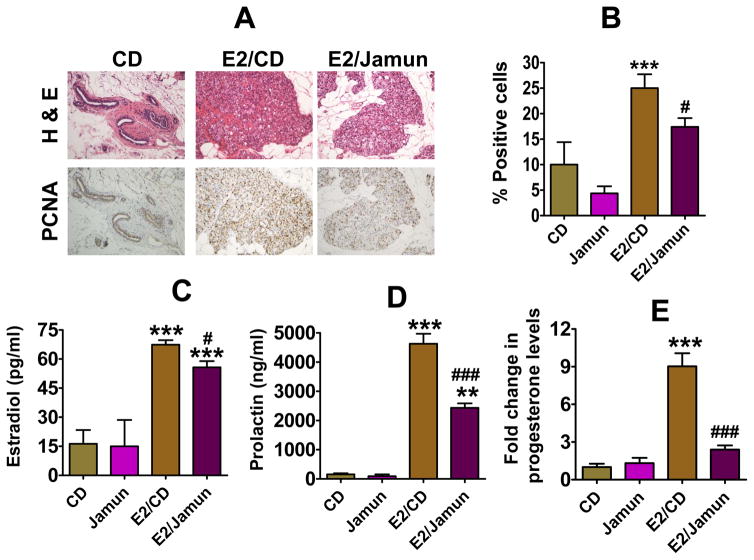
There was slight but insignificant decrease in the mammary cell proliferation index of control vs jamun-fed diet as shown by measurement of PCNA by immunohistochemistry. A marked stimulatory effect of E2 on mammary cell proliferation was evident; the fraction of cells staining positive for PCNA increased from 10% in untreated control to 25% in E2-treated rats. Dietary jamun significantly inhibited E2-associated mammary cell proliferation (Figure 4A and 4B). The jamun diet attenuated the ability of E2 to stimulate cell proliferation by 30%.
Figure 4.
Antiproliferative effects of the control diet (CD) and diet supplemented with jamun pulp powder with and without estrogen (E2) treatment evaluated by immunohistochemical staining for Proliferating Cell Nuclear Antigen (PCNA) at euthanasia. Photomicrographs are 20× magnification of normal and hyperplastic mammary tissues (A); corresponding graph representing the quantitation of deeply stained cells for PCNA (B). Circulating estradiol (A), prolactin (B), and progesterone (C) levels measured at euthanasia in rats fed control diet or diet supplemented with jamun. Values represent average ± SD. Statistical significance was analyzed by generalized linear model (GLM) followed by post hoc Tukey’s multi-comparison test to compare treatment groups using SAS software. Asterisk shows significant difference at *p <0.05; **p <0.01; ***p <0.001 versus the control diet whereas # denotes comparison between E2/Jamun to E2 treated control animals at #p < 0.05; ##p <0.01; ###p <0.001.
3.5 Circulating estradiol and prolactin
The serum levels of E2 did not differ between the control diet and the diet supplemented with jamun at the time of euthanasia. A significant increase in E2 levels was observed (p < 0.0001) in the animals that received exogenous E2 via implants. E2 levels were 16.3 ± 7.1 pg/ml in the untreated animals, which increased to 67.4 ± 6.7 pg/ml with the E2 treatment. The jamun diet in E2-treated group significantly reduced the elevated level of circulating E2 to 55.7 ± 7.2 pg/ml (Figure 4C).
Similar to circulating E2 levels, no significant differences were found in the plasma prolactin levels between untreated and rats fed the 5% jamun diet. However, in animals receiving exogenous E2, the serum prolactin levels increased by nearly 30-fold, from 155 ± 35 ng/ml to 4,629 ± 839 ng/ml (p < 0.0001). The circulating prolactin levels in the E2-treated animals were abrogated by jamun diet by one-half to 2,434 ± 343 ng/ml (p=0.0001) (Figure 4D).
3.6 Analysis of progesterone
The difference in progesterone levels in animals fed with the control or the jamun diet with or without E2 was determined by RT-PCR analysis and expressed as fold change. While no difference was observed in control vs jamun diet, the progesterone levels were significantly increased to 9-fold with the E2 treatment. The effect of the latter was almost completely diminished by dietary jamun intervention (p=0.0003) (Figure 4E).
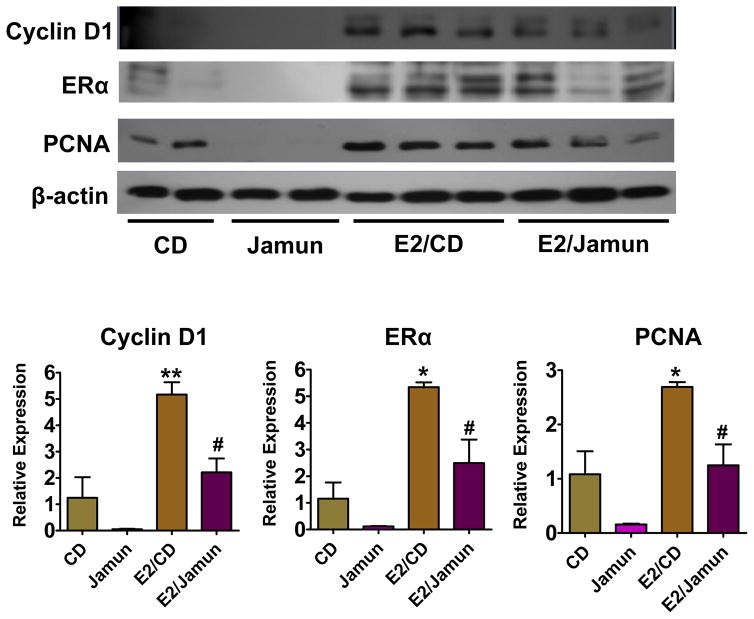
3.7 Cyclin D1, ERα and PCNA protein expression
Cyclin D1, ERα and PCNA protein expression in mammary gland tissues of ACI rats treated with or without E2 and fed either control or jamun diet for 7 months was determined by western blot analysis (Figure 5). Compared with age-matched control, a significant rise in cyclin D1, ERα and PCNA was detected in animals treated with E2/control diet but not in E2/Jamun diet group. Suggesting that jamun counteracted the E2-associated increases in mammary cell proliferation (PCNA) (p=0.06), ERα (p=0.03) and cyclin D1 (p=0.013). The data also indicated that dietary jamun reduced the basal levels of cyclin D1, ERα and PCNA protein expression observed in untreated animals.
Figure 5.
Effect of jamun on protein expression levels of selected markers. Western blot analysis was performed on the mammary protein lysates isolated using RIPA buffer. 30 μg protein was separated by gel electrophoresis and probed for the indicated proteins. Blots were stripped and reprobed with β-actin to confirm the equal loading. Densitometry was performed using ImageJ software and the bar graph is given representing average ± SD of 4–6 samples. Statistical significance was analyzed by generalized linear model (GLM) followed by post hoc Tukey’s multi-comparison test to compare treatment groups using SAS software. Asterisks show significant difference at *p <0.05; **p <0.01 versus the control diet whereas # denotes comparison between E2/Jamun to E2 treated control animals at #p < 0.05.
3.8 Modulation of select miRNA expression and their targets by jamun diet
The association of several miRNAs is well established in development and progression of breast cancers [34]. We earlier demonstrated a ‘signature’ of 33 significantly modulated miRNAs during the process of mammary tumorigenesis in E2-treated ACI rats [16]. In this study, we analyzed effect of jamun diet on four E2-specific miRNAs (miR-127, -206, -182, and -375). No difference was found in the expression of any of the analyzed miRNAs following the treatment with jamun over untreated control. In agreement with our previous study, miR-127 and miR-206 were significantly down-regulated by 17- and 38-fold with the E2 treatment, respectively, and miR-375 and miR-182 were upregulated by 6.9 and 4.2-fold, respectively (Figure 6). Expression levels of miRNAs miR-127, miR-206 and miR-375 were significantly modulated by jamun diet compared to control diet receiving E2 treatment.
Figure 6.
Effect of diet supplemented with jamun pulp powder (5% w/w) on indicted miRNAs on E2-mediated mammary tumorigenesis. The small RNAs were isolated by mirVana miRNA kit and quantified by Bioanalyzer. qPCR analysis was performed using a TaqMan miRNA kit using manufacturer’s guidelines. Reverse Transcription Kit and TaqMan gene-specific miRNA assays. Select miRNAs were analyzed by qRT-PCR in tissues of E2-alone and jamun-treated ACI rats. Data represent mean ± SD of fold change in mRNA expression relative to the untreated control of five animals. Statistical significance was analyzed by two-way ANOVA after applying the Bonferroni post hoc test for multiple comparisons at *p <0.05; **p <0.01; ***p <0.001 versus the control diet. Student’s t test was done to compare E2/Jamun to E2-treated control animals where #p < 0.05; ##p <0.01; ###p <0.001.
The effect of dietary jamun intervention in E2 treated was analyzed for mRNA expression levels on the predicted targets (Cyclin D1, Cyclin D3, Foxo1, Bcl2, and Cdk4) of the selected miRNAs (Figure 6B). Of these, four mRNA targets were downregulated with the E2/jamun treatment compared to animals receiving control diet and E2. These molecular targets suggest E2-associated increase in the expressions levels were favorably counteracted by the jamun intervention.
3.9 Effect of jamun diet on liver and kidney functions and hematological parameters
Data in Table II and III demonstrate the effect of E2 and jamun diet for potential toxicity. There was no significant effect of 5% jamun supplemented diet on the body weights of the animals following administration for almost 7 months suggesting no signs of gross toxicity. No differences were observed in liver function enzymes (like aspartate transaminase, alanine aminotransferase, alkaline phosphatase, gamma-glutamyl transpeptidase, amylase, or lipase), or biochemical parameters of kidney function (like creatinine, Ca2+, Na+, and Cl− levels) except blood urea nitrogen (BUN) and BUN/creatinine ratio with dietary jamun compared with the animals fed with control diet. The BUN levels were significantly reduced (p= 0.01) from 28.5 ± 2.4 in control animals to 20.7 ± 2.1 in jamun diet group. A similar trend was also evident in the E2/jamun treated animals (Table II). When compared to untreated age-matched control animals, E2/jamun treatment showed significant (p= 0.02) decrease in blood glucose levels, while E2/control diet group exhibited slight but insignificant decrease in blood glucose levels. Furthermore, jamun diet resulted in significant decrease in total cholesterol levels compared to untreated control irrespective of absence or presence of E2. Triglyceride levels on other hand were significantly decreased in all groups (jamun, E2/CD and E2/jamun) compared to control diet receiving animals (Table II).
Table II.
Effect on biochemical profile (systemic toxicity) following 6 months exposure to 5% Jamun diet in female ACI rats.
| Biochemical profile | 6 Month Exposure
|
|||
|---|---|---|---|---|
| Control | Jamun | E2/Control | E2/Jamun | |
| Liver profile | ||||
| AST (SGOT) | 154 ± 49.3 | 152.0 ± 7.9 | 188.4 ± 84.5 | 131.0 ± 25.5 |
| ALT (SGPT) | 41.3 ± 12.8 | 37.0 ± 4.4 | 37.3 ± 6.7 | 38.8 ± 7.5 |
| Alk Phosphatase | 29.8 ± 12.5 | 40.0 ± 10.0 | 27.3 ± 6.1 | 36.3 ± 8.1 |
| GGT | 1.5 ± 0.6 | 2.7 ± 1.2 | 2.3 ± 0.8 | 2.3 ± 0.5 |
| Amylase | 654.5 ± 57.2 | 536.3 ± 36.0 | 560.5 ± 47.1 | 618.8 ± 105.9 |
| CPK | 798.3 ± 295.3 | 781.7 ± 112.0 | 1010.3 ± 549.3 | 550.0 ± 131.1 |
| Kidney profile | ||||
| BUN | 28.5 ± 2.4 | 20.7 ± 2.1** | 25.7 ± 2.3 | 20.0 ± 2.4***,# |
| BUN/Creatinine Ratio | 71.5 ± 6.0 | 41.3 ± 4.2*** | 55.7 ± 8.8* | 38.3 ± 6.0***,## |
| Phosphorus | 15.2 ± 0.9 | 17.0 ± 0.6 | 12.5 ± 1.4* | 14.7 ± 1.8# |
| Calcium | 10.9 ± 0.9 | 11.1 ± 0.5 | 11.5 ± 0.3 | 11.8 ± 0.3 |
| Total Protein | 8.1 ± 0.4 | 7.4 ± 0.3* | 8.0 ± 0.3 | 8.0 ± 0.3 |
| Albumin | 4.9 ± 0.3 | 4.1 ± 0.6 | 4.7 ± 0.3 | 4.4 ± 0.4 |
| Globulin | 3.3 ± 0.1 | 3.3 ± 0.5 | 3.3 ± 0.4 | 3.6 ± 0.4 |
| A/G Ratio | 1.5 ± 0.1 | 1.5 ± 0.1 | 1.4 ± 0.2 | 1.3 ± 0.2 |
| Glucose | 154.7 ± 40.5 | 158.0 ± 46.5 | 107.8 ± 7.1 | 87.8 ± 10.8* |
| Cholesterol | 142.3 ± 29.7 | 82.7 ± 11.2** | 120.7 ± 8.8 | 109.0 ± 6.6* |
| Triglyceride | 203.7 ± 28.1 | 110.0 ± 29.6* | 107.0 ± 44.7** | 91.8 ± 19.4** |
Female ACI rats (6–7) weeks old were provided either control diet (AIN 93M) or diet supplemented with 5% (w/w) Jamun powder and water ad libitum. At euthanasia blood was collected and analyzed using an automated AU640® Chemistry Analyzer by Antech diagnostics. Data represent average and standard deviation of four animals. Statistical significance was analyzed by generalized linear model followed by post hoc Tukey’s multi-comparison test. Asterisk shows significant difference at *p <0.05; **p <0.01; ***p <0.001 versus the control diet whereas # denotes comparison between E2/Jamun to E2 treated control animals at #P < 0.05; ##p <0.01.
Table III.
Effect on the hematological parameters (systemic toxicity) following 6 months exposure to 5% (w/w) Jamun diet in ACI rats.
| Hematological profile | 6 Months exposure of Jamun diet
|
|||
|---|---|---|---|---|
| Control | Jamun | E2/Control | E2/Jamun | |
| WBC | 5.1 ± 1.1 | 4.3 ± 0.9 | 4.4 ± 1.5 | 5.0 ± 1.6 |
| HGB | 13.3 ± 0.6 | 12.4 ± 0.4 | 10.5 ± 0.9** | 10.5 ± 1.1** |
| HCT | 46.7 ± 1.2 | 40.3 ± 0.6 | 34.8 ± 4.1** | 34.3 ± 3.8** |
| MCV | 54.5 ± 1.5 | 51.7 ± 1.5 | 52.8 ± 2.5 | 52.8 ± 2.6 |
| MCHC | 28.7 ± 0.6 | 30.7 ± 1.2 | 30.2 ± 1.6 | 30.5 ± 1.9 |
| Platelet Count | 939.3 ± 61.1 | 907.7 ± 93.1 | 745.3 ±208.0 | 824.3 ± 34.4 |
| Neutrophils | 17.8 ± 7.5 | 16.7 ± 3.2 | 28.0 ± 11.5 | 25.3 ± 13.5 |
| Lymphocytes | 76.8 ± 7.8 | 80.3 ± 2.5 | 64.8 ± 12.6 | 69.0 ± 14.8 |
Female ACI rats (6–7) weeks old were provided either control diet (AIN 93M) or diet supplemented with 5% (w/w) Jamun powder and water ad libitum. At euthanasia blood was collected and analyzed using an automated AU640® Chemistry Analyzer by Antech diagnostics. Data represent average and standard deviation of four animals. Statistical significance was analyzed by generalized linear model followed by post hoc Tukey’s multi-comparison test. Asterisk shows significant difference at *p <0.05; **p <0.01 versus the control diet.
In addition to liver and kidney function tests, the effect of dietary jamun was examined on the hematological profile as depicted in Table III. Modest but significant decrease was observed in hemoglobulin (HGB) and hematocrit (HCT) with jamun and/or E2 treatment. No significant differences in white blood cells, hemoglobin and other molecules like MCV, and MCHC were observed with dietary jamun; neutrophils, lymphocytes, monocytes, eosinophils, and basophils were also unaffected.
4. Discussion
Data presented herein suggest that a 5% (w/w) jamun pulp powder diet was well tolerated and prominently inhibited E2-mediated mammary tumorigenesis in ACI rats. Although other berries, namely BB and BRB, have been shown to inhibit mammary carcinogenesis [21], but this is the first report to show tumor growth inhibition by jamun that shares bioactive profile of BB viz all five anthos, Cy, Dp, Pe, Pt, and Mv and of BRB that contains almost exclusively Cy and abundant EA. To the best of our knowledge, jamun is the only known fruit that provides this atypical profile of phytochemicals.
We have previously reported breast cancer chemopreventive and therapeutic activity of BB diet [21, 23]. In this study, jamun provides activity similar to BB. Noteworthy is that jamun contains di-glucosides of five distinct anthocyanidins whereas BB has been shown to contain mono-glucosides of same anthocyanidins [21, 29]. The similar activity could be due to possible in vivo conversion of anthocyanins to anthocyanidins. Anthocyanins are known to lose their glucose moiety upon acid hydrolysis [29, 35]. In this study, jamun not only increased tumor latency by 21 days but also showed marked reduction in both tumor volume and tumor multiplicity.
The mechanism of E2-induced mammary tumorigenesis in ACI rats is complex. However, two principle pathways are proposed by which E2 exerts its carcinogenic effects on the breast that include hormone receptor-mediated cell-proliferation and metabolism in which hydroxylated metabolites (4E2 or 4E1) that are genotoxic, induce mutations leading to carcinogenesis. Low serum E2 levels (approximately 60–120 pg/ml) have been shown to be sufficient to induce mammary tumors that remarkably resemble human ductal BC histopathologically [36]. In our study, the serum E2 levels were 67.4 ± 6.7 pg/ml in animals receiving exogenous E2 via implants and correlated well with the above study. The four-fold higher levels of circulating estrogen over control clearly suggest an important role of E2 in tumorigenesis. Reports indicate dietary interventions to inhibit mammary tumorigenesis by reducing circulatory E2 or prolactin [37]. The serum E2 levels were significantly reduced by the jamun diet compared to animals fed with the control diet, suggesting that the jamun diet possibly, in part, altered the circulating E2 levels. In contrast to our previous studies where the circulatory E2 levels were >300 pg/ml from the implant containing 27 mg E2, and dietary intervention of berry did not affect E2 levels [22], in this study a significant reduction of E2 levels observed could be due to a lower level of E2 in circulation (67 pg/ml) from the 9 mg E2 implant. On the other hand, BB and BRB provide evidence of protective effects against estrogen metabolism by berry diet [31, 32]. Our previous study indicated that a BB diet significant reduces levels of CYP1A1 [23] that catalyzes the conversion of E2 into its metabolites (2E2 and 4E2). Since jamun contains the same anthocyanidins as BB with additional EA, it could also possibly reduce the CYPs expressions leading to the inhibition of redox cycle and reactive oxygen species formation [38]. However, we have not analyzed CYPs in this study.
Prolactin is considered to play a major role in the development of mammary tumors in the ACI rat model [39]. Remarkable correlation between the ratios of serum E2 and plasma prolactin levels has been shown in our earlier study confirming the direct effect of E2 on prolactin levels [32]. Therefore, reduction in pituitary weight and the corresponding prolactin levels noted in this study suggest the effectiveness of the jamun diet against mammary tumorigenesis. It is not clear, however, whether the prolactin levels are directly modulated by the berries or whether the production of prolactin is reduced due to decrease in circulating estrogen levels facilitated by the berry diets. Tamoxifen, an established selective estrogen receptor modulation (SERM), is known to reduce mammary tumors in female ACI rats [40]. Significant reduction in plasma prolactin by jamun diet suggests that phytocompounds in jamun have SERM-like activity. Anthocyanidins and ellagic acid both are shown to have potent anti-estrogenic activities [22].
It has been demonstrated by Shull and colleagues that ovariectomy inhibits E2-induced mammary tumors development suggesting requirement of one or more ovarian factors, like progesterone for tumor development [41]. Various studies suggest the role of progesterone in the etiology, promotion, and progression of human BC [42]. In this study, the jamun diet significantly abrogated the estrogen-associated increase in progesterone.
The cell proliferative effect of estrogen leading to cancers is mediated by the estrogen receptors (ER), ERα and ERβ. Most cells in the human body possess ERs and the gene transcription function of estrogen was thought to be mediated via its interaction with this receptor. Notably, in the present study, the jamun diet significantly inhibited E2-induced mammary epithelial cell proliferation even in the presence of elevated E2 and prolactin. This reduction in biological effect of E2 by ERα further suggests its role against mammary tumorigenesis in animals treated with E2. Cell cycle deregulation, particularly overexpressed cyclin D1, has been implicated in various human cancers including BC [32]. Cyclin D1 protein is overexpressed in >50% of human BCs and regulation of its expression has been associated with changes in the proliferation rate of BC cells [43]. Jamun bioactives target various pathways and modulate E2-associated molecules including estrogen-associated mammary cell proliferation, ER-α and cyclin D1 indicating its protective effects against mammary tumorigenesis in animals treated with E2.
Numerous studies have identified miRNAs modulated by E2 and dysregulated in BC and other cancers [9, 11, 34, 44]. miRNAs are small, non-coding RNA molecules that play important roles in controlling cell growth, differentiation, apoptosis, and tumorigenesis through the translational repression of their cognate target genes. Targeting specific miRNAs for inhibiting specific stages of a cancer is being explored [44]. Observations from our recent study have incited us to hypothesize that specific miRNAs can mediate the anti-cancer activity. Our previous study examining mammary tissues of E2-treated ACI rats indicated modulation of at least 33 miRNAs at a 2-fold cutoff [16]. In this study we tested a jamun diet on four select miRNAs that were highly modulated following E2 treatment. Interestingly, dietary jamun favorably modulated E2- associated changes in the select miRNAs (miR-127, -206, and -375) and several of their mRNA targets (cyclin D1, cyclin D3, Foxo1, Bcl2, and Cdk4). The most remarkable changes, confirmed by RT-PCR, were specific to the modulation of miR-127 and miR-375 expression, which modulated early and remained altered throughout the carcinogenic process in our previous study [30]. Modulation of these miRNAs has been reported in various cancers including BC [45–47]. We have observed a similar effect in our previous studies with EA [16] and BB that contains similar anthocyanidins as jamun on other miRNAs [23]. However, to the best of our knowledge, this is the first report to show effect of jamun that contains both EA and anthocyanidins on the E2-associated changes in miRNAs. In summary, jamun modulates estrogen-associated changes in selected miRNAs and their associated targets suggesting the involvement of these miRNAs in BC and a possible target for dietary interventions.
Animals fed with a diet supplemented with jamun were observed for any toxicity by analyzing systemic toxicological effects as determined by various hematological and serum biochemical parameters of liver and kidney functions. No difference in body weight gain or changes in terms of in hematological parameters and liver/kidney function tests suggest that jamun diet was well tolerated at the given dose. Gross examination of different organs at the time of necropsy did not reveal any differences between untreated control and jamun fed animals. Although minor reductions in BUN and BUN/Creatinine levels were observed, their levels were well within the normal physiological range of age-matched control animals. Significant reductions in glucose levels were also observed, but this effect was due to the E2 and consistent with literature reports [48]. A significant decrease in cholesterol was observed in jamun- treated groups while triglyceride was decreased in both jamun treated groups with or without E2 as well as E2-treated animals receiving control diet. However, these intriguing findings need to be confirmed. HGB and HCT were also significantly decreased in E2-treated groups irrespective of diet they received indicating plausible influence of E2 on these factors.
In summary, we have provided several pieces of evidence suggesting that Syzygium cumini (jamun) contains phytochemicals such as anthocyanins and ellagic acid capable of reducing E2-induced mammary tumorigenesis in ACI rats and provides benefits of both BB and BRB. Jamun exerts a chemopreventive/chemotherapeutic response by interfering in several E2-associated pathways that include mammary cell proliferation, ERα and E2-induced cyclin D1 in mammary tumorigenesis. Further studies are required to confirm the SERM effects of this dietary agent in the various estrogen-responsive organs of the ACI rat such as the pituitary, ovary, uterus, adrenal and mammary. Jamun also reduced E2-associated elevated levels of circulating estradiol, prolactin and progesterone. Finally, jamun modulates E2-associated changes in selected miRNAs and their associated molecules suggesting a possible target for dietary interventions. The data presented here re-emphasize jamun to be non-toxic and promising in the prevention and treatment of BC.
Acknowledgments
This work was supported from Agnes Brown Duggan Endowment, Helmsley grant and the NIH grants CA-118114 and CA-125152. Drs. Srivani Ravoori and Swarupa Gadre are thankfully acknowledged for tissue histology data analysis and Manicka Vadhanam for discussion. We also thank Director of NIPER for supporting this work. Mr. Stephen Cohen, Assistant director for graduate studies writing, of University writing center is thankfully acknowledged for editorial corrections.
Abbreviations
- ACI rat
August-Copenhagen Irish rats
- BB
Blueberry
- BC
Breast cancer
- BRB
Black raspberry
- Cy
Cyanidin
- Dp
Delphinidin
- EA
Ellagic acid
- E2
17β-estradiol
- 4E2
4-Hydroxy-17β-estradiol
- ER-α
Estrogen receptor-alpha
- ER
Estrogen receptor
- Mv
Malvidin
- Pt
Petunidin
- Pe
Peonidin
- PCNA
Proliferating cell nuclear antigen
- PR
progesterone receptor
Footnotes
Author contributions
FA and RCG designed the project, analyzed the data and wrote the manuscript. FA, JJ, and RM conducted the study, collected the data, and RM also contributed to writing of the manuscript. IPS contributed in providing plant materials and writing of the manuscript.
Conflict of interests
The authors have declared no conflict of interest.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: a cancer journal for clinicians. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA: a cancer journal for clinicians. 2014;64:52–62. doi: 10.3322/caac.21203. [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, et al. GLOBOCAN 2012. International Agency for Research on Cancer; Lyon, France: 2013. [Google Scholar]
- 4.Weiderpass E, Meo M, Vainio H. Risk factors for breast cancer, including occupational exposures. Saf Health Work. 2011;2:1–8. doi: 10.5491/SHAW.2011.2.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354:270–282. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- 6.Taylor MA. Circulating MicroRNAs as Biomarkers and Mediators of Cell–Cell Communication in Cancer. Biomedicines. 2015;3:270–281. doi: 10.3390/biomedicines3040270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 8.Iorio MV, Ferracin M, Liu CG, Veronese A, et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005;65:7065–7070. doi: 10.1158/0008-5472.CAN-05-1783. [DOI] [PubMed] [Google Scholar]
- 9.Blenkiron C, Goldstein LD, Thorne NP, Spiteri I, et al. MicroRNA expression profiling of human breast cancer identifies new markers of tumor subtype. Genome Biol. 2007;8:R214. doi: 10.1186/gb-2007-8-10-r214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mattie MD, Benz CC, Bowers J, Sensinger K, et al. Optimized high-throughput microRNA expression profiling provides novel biomarker assessment of clinical prostate and breast cancer biopsies. Molecular Cancer. 2006;5 doi: 10.1186/1476-4598-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iorio MV, Ferracin M, Liu CG, Veronese A, et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Research. 2005;65:7065–7070. doi: 10.1158/0008-5472.CAN-05-1783. [DOI] [PubMed] [Google Scholar]
- 12.Herynk MH, Fuque SAW. Estrogen receptors in resistance to hormone therapy. Breast Cancer Chemosensitivity. 2007;608:130–143. doi: 10.1007/978-0-387-74039-3_10. [DOI] [PubMed] [Google Scholar]
- 13.Speirs V, Walker RA. New perspectives into the biological and clinical relevance of oestrogen receptors in the human breast. Journal of Pathology. 2007;211:499–506. doi: 10.1002/path.2130. [DOI] [PubMed] [Google Scholar]
- 14.Adams BD, Furneaux H, White BA. The micro-ribonucleic acid (miRNA) miR-206 targets the human estrogen receptor-alpha (ERalpha) and represses ERalpha messenger RNA and protein expression in breast cancer cell lines. Mol Endocrinol. 2007;21:1132–1147. doi: 10.1210/me.2007-0022. [DOI] [PubMed] [Google Scholar]
- 15.Wickramasinghe NS, Manavalan TT, Dougherty SM, Riggs KA, et al. Estradiol downregulates miR-21 expression and increases miR-21 target gene expression in MCF-7 breast cancer cells. Nucleic Acids Res. 2009;37:2584–2595. doi: 10.1093/nar/gkp117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munagala R, Aqil F, Vadhanam MV, Gupta RC. MicroRNA ‘signature’ during estrogen-mediated mammary carcinogenesis and its reversal by ellagic acid intervention. Cancer Letters. 2013;339:175–184. doi: 10.1016/j.canlet.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu RH. Potential synergy of phytochemicals in cancer prevention: Mechanism of action. J Nutr. 2004;134:3479s–3485s. doi: 10.1093/jn/134.12.3479S. [DOI] [PubMed] [Google Scholar]
- 18.Harris GK, Gupta A, Nines RG, Kresty LA, et al. Effects of lyophilized black raspberries on azoxymethane-induced colon cancer and 8-hydroxy-2′-deoxyguanosine levels in the Fischer 344 rat. Nutr Cancer. 2001;40:125–133. doi: 10.1207/S15327914NC402_8. [DOI] [PubMed] [Google Scholar]
- 19.Stoner GD, Wang LS, Seguin C, Rocha C, et al. Multiple berry types prevent N-nitrosomethylbenzylamine-induced esophageal cancer in rats. Pharm Res. 2010;27:1138–1145. doi: 10.1007/s11095-010-0102-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cai T, Huang C. In: Berries and Cancer Prevention. Stoner G, Seeram N, editors. Springer; New York: 2010. [Google Scholar]
- 21.Ravoori S, Vadhanam MV, Aqil F, Gupta RC. Inhibition of estrogen-mediated mammary tumorigenesis by blueberry and black raspberry. Journal of agricultural and food chemistry. 2012;60:5547–5555. doi: 10.1021/jf205325p. [DOI] [PubMed] [Google Scholar]
- 22.Aiyer HS, Gupta RC. Berries and ellagic acid prevent estrogen-induced mammary tumorigenesis by modulating enzymes of estrogen metabolism. Cancer prevention research. 2010;3:727–737. doi: 10.1158/1940-6207.CAPR-09-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeyabalan J, Aqil F, Munagala R, Annamalai L, et al. Chemopreventive and therapeutic activity of dietary blueberry against estrogen-mediated breast cancer. J Agric Food Chem. 2014;62:3963–3971. doi: 10.1021/jf403734j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aiyer HS, Warri AM, Woode DR, Hilakivi-Clarke L, Clarke R. Influence of berry polyphenols on receptor signaling and cell-death pathways: implications for breast cancer prevention. Journal of agricultural and food chemistry. 2012;60:5693–5708. doi: 10.1021/jf204084f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kausar H, Jeyabalan J, Aqil F, Chabba D, et al. Berry anthocyanidins synergistically suppress growth and invasive potential of human non-small-cell lung cancer cells. Cancer letters. 2012;325:54–62. doi: 10.1016/j.canlet.2012.05.029. [DOI] [PubMed] [Google Scholar]
- 26.Aqil F, Vadhanam MV, Jeyabalan J, Cai J, et al. Detection of Anthocyanins/Anthocyanidins in Animal Tissues. Journal of agricultural and food chemistry. 2014;62:3912–3918. doi: 10.1021/jf500467b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adams LS, Kanaya N, Phung S, Liu Z, Chen S. Whole blueberry powder modulates the growth and metastasis of MDA-MB-231 triple negative breast tumors in nude mice. J Nutr. 2011;141:1805–1812. doi: 10.3945/jn.111.140178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ayyanar M, Subash-Babu P, Ignacimuthu S. Syzygium cumini (L.) Skeels. a novel therapeutic agent for diabetes: folk medicinal and pharmacological evidences. Complement Ther Med. 2013;21:232–243. doi: 10.1016/j.ctim.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 29.Aqil F, Gupta A, Munagala R, Jeyabalan J, et al. Antioxidant and antiproliferative activities of anthocyanin/ellagitannin-enriched extracts from Syzygium cumini L. (Jamun, the Indian Blackberry) Nutrition and cancer. 2012;64:428–438. doi: 10.1080/01635581.2012.657766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li LY, Adams LS, Chen S, Killian C, et al. Eugenia Jambolana Lam. Berry Extract Inhibits Growth and Induces Apoptosis of Human Breast Cancer but Not Non-Tumorigenic Breast Cells. Journal of agricultural and food chemistry. 2009;57:826–831. doi: 10.1021/jf803407q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aiyer HS, Srinivasan C, Gupta RC. Dietary berries and ellagic acid diminish estrogen-mediated mammary tumorigenesis in ACI rats. Nutrition and Cancer-an International Journal. 2008;60:227–234. doi: 10.1080/01635580701624712. [DOI] [PubMed] [Google Scholar]
- 32.Ravoori S, Vadhanam MV, Sahoo S, Srinivasan C, Gupta RC. Mammary tumor induction in ACI rats exposed to low levels of 17 beta-estradiol. International Journal of Oncology. 2007;31:113–120. [PubMed] [Google Scholar]
- 33.Munagala R, Kausar H, Munjal C, Gupta RC. Withaferin A induces p53-dependent apoptosis by repression of HPV oncogenes and upregulation of tumor suppressor proteins in human cervical cancer cells. Carcinogenesis. 2011;32:1697–1705. doi: 10.1093/carcin/bgr192. [DOI] [PubMed] [Google Scholar]
- 34.O’day E, Lal A. MicroRNAs and their target gene networks in breast cancer. Breast Cancer Research. 2010;12 doi: 10.1186/bcr2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Truong VD, Deighton N, Thompson RT, McFeeters RF, et al. Characterization of anthocyanins and anthocyanidins in purple-fleshed sweetpotatoes by HPLC-DAD/ESI-MS/MS. Journal of agricultural and food chemistry. 2010;58:404–410. doi: 10.1021/jf902799a. [DOI] [PubMed] [Google Scholar]
- 36.Weroha SJ, Li SA, Tawfik O, Li JJ. Overexpression of cyclins D1 and D3 during estrogen-induced breast oncogenesis in female ACI rats. Carcinogenesis. 2006;27:491–498. doi: 10.1093/carcin/bgi278. [DOI] [PubMed] [Google Scholar]
- 37.Aiyer HS. University of kentucky. 2007. [Google Scholar]
- 38.Liehr JG. Is estradiol a genotoxic mutagenic carcinogen? Endocrine Reviews. 2000;21:40–54. doi: 10.1210/edrv.21.1.0386. [DOI] [PubMed] [Google Scholar]
- 39.Holtzman S, Stone JP, Shellabarger CJ. Influence of Diethylstilbestrol Treatment on Prolactin Cells of Female Aci and Sprague-Dawley Rats. Cancer Research. 1979;39:779–784. [PubMed] [Google Scholar]
- 40.Bentrem DJ, Gaiha P, Jordan VC. Oestrogens, oestrogen receptors and breast cancer. Ejc Supplements. 2003;1:1–12. [Google Scholar]
- 41.Shull JD, Spady TJ, Snyder MC, Johansson SL, Pennington KL. Ovary-intact, but not ovariectomized female ACI rats treated with 17 beta estradiol rapidly develop mammary carcinoma. Carcinogenesis. 1997;18:1595–1601. doi: 10.1093/carcin/18.8.1595. [DOI] [PubMed] [Google Scholar]
- 42.Blank EW, Wong PY, Lakshmanaswamy R, Guzman R, Nandi S. Both ovarian hormones estrogen and progesterone are necessary for hormonal mammary carcinogenesis in ovariectomized ACI rats. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:3527–3532. doi: 10.1073/pnas.0710535105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Castro-Rivera E, Samudio I, Safe S. Estrogen-regulation of cyclin D1 gene expression in ZR-75 breast cancer cells involves multiple enhancer elements. Journal of Biological Chemistry. 2001;276:30853–30861. doi: 10.1074/jbc.M103339200. [DOI] [PubMed] [Google Scholar]
- 44.Sassen S, Miska EA, Caldas C. MicroRNA - implications for cancer. Virchows Archiv. 2008;452:1–10. doi: 10.1007/s00428-007-0532-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simonini PDR, Breiling A, Gupta N, Malekpour M, et al. Epigenetically Deregulated microRNA-375 Is Involved in a Positive Feedback Loop with Estrogen Receptor alpha in Breast Cancer Cells. Cancer Research. 2010;70:9175–9184. doi: 10.1158/0008-5472.CAN-10-1318. [DOI] [PubMed] [Google Scholar]
- 46.Zhao HJ, Zhu L, Jin YJ, Ji HB, et al. miR-375 is highly expressed and possibly transactivated by achaete-scute complex homolog 1 in small-cell lung cancer cells. Acta Biochimica Et Biophysica Sinica. 2012;44:177–182. doi: 10.1093/abbs/gmr110. [DOI] [PubMed] [Google Scholar]
- 47.He XX, Chang Y, Meng FY, Wang MY, et al. MicroRNA-375 targets AEG-1 in hepatocellular carcinoma and suppresses liver cancer cell growth in vitro and in vivo. Oncogene. 2012;31:3357–3369. doi: 10.1038/onc.2011.500. [DOI] [PubMed] [Google Scholar]
- 48.Kojima T, Lindheim SR, Duffy DM, Vijod MA, et al. Insulin Sensitivity Is Decreased in Normal Women by Doses of Ethinyl Estradiol Used in Oral-Contraceptives. American Journal of Obstetrics and Gynecology. 1993;169:1540–1544. doi: 10.1016/0002-9378(93)90432-i. [DOI] [PubMed] [Google Scholar]