Abstract
Training and credentialing for robotic surgery in otolaryngology - head and neck surgery is currently not standardized, but rather relies heavily on industry guidance. This manuscript represents a comprehensive review of this increasingly important topic and outlines clear recommendations to better standardize the practice. The recommendations provided can be used as a reference by individuals and institutions alike, and are expected to evolve over time.
Keywords: robotics, transoral, surgery, training, credentialing
INTRODUCTION
Robotic technology has afforded improvements in head and neck surgical techniques by providing enhanced visualization, increased manual dexterity, and the ability to perform surgery using a virtual environment. In 2009, the United States Food and Drug Administration (FDA) cleared the use of the da Vinci Surgical System (Intuitive Surgical, Sunnyvale, CA) for “transoral otolaryngology” procedures for T1 to T2 tumors based on a multicenter study demonstrating the safety and efficacy of transoral robotic surgery (TORS).1 Since then, the use of TORS in head and surgery has increased dramatically.1 TORS has been shown to be safe and oncologically effective when performed by experienced head and neck surgeons.2,3 In 2014, the FDA cleared the da Vinci Surgical System for use in benign base of tongue resection procedures.4,5 The da Vinci Surgical System remains under active investigation for obstructive sleep apnea. Although the system is not currently cleared for other procedures (eg, thyroidectomy, neck dissection), the use of robotics in head and neck surgery is likely to expand as indications for surgery evolve and technologies advance.
Currently, no governing-body mandated credentialing guidelines exist for robotic surgery. Certification of robotic skill proficiency remains at the institutional level, may be widely variable, and often relies on industry guidance. This process lacks standardization and is not competency based, which leaves tremendous room for improvement. The American Head and Neck Society (AHNS) and American Academy of Otolaryngology – Head and Neck Surgery (AAO-HNS) have yet to adopt guidelines for the training and credentialing of robotics in Otolaryngology and Head and Neck Surgery (OTO-HNS). Leadership in this area from the appropriate governing societies is critical for ensuring patient safety and public trust.6
In 2012, the Robotic Task Force drafted recommendations and guidelines for training and credentialing for the AAO-HNS. Since then, a subcommittee of the 2014 AHNS Education Committee was tasked with further exploring the current status of training and credentialing of robotics in head and neck surgery and developing more detailed recommendations to improve the quality and consistency of training. The guidelines were approved by both committees along with the AAO-HNS Sleep Disorders Committee. In the current integrated review, TORS is used as a prototypical robotic procedure. However, the concepts discussed should be relevant to all applications of robotics in OTO-HNS as well as to current and future technologies.
This document proposes standardized guidelines, or “best practices,” for training and credentialing of robotic surgery in OTO-HNS. The purpose of such standardized guidelines is to ensure to the public a good-faith effort to maximize the competency of practicing surgeons performing robotic surgery in our field. The recommendations provided can be used as a reference by individuals and institutions alike, and are expected to evolve over time. Importantly, for now, they can serve as a framework for institutions to use during the process of credentialing for privileges to perform robotic surgery in OTO-HNS. Recommendations for granting privileges for individual surgical procedures are not included. Appendix 1 includes definitions used in this review.
CURRENT STATUS OF TRAINING AND CREDENTIALING IN ROBOTIC SURGERY
In order to define the current status of training and credentialing criteria used for the granting of robotic surgery privileges, we examined the written requirements from a representative sample of academic and community hospitals across the United States. We requested robotic privileges credentialing criteria and application forms from 18 representative institutions and received complete documentation from 14 centers. At most hospitals, training and credentialing criteria applied for all robotic procedures, with only 3 institutions (21%) having additional criteria specific for head and neck procedures. Although case number requirements varied widely across institutions, credentialing criteria generally included 3 major components: (1) preclinical didactic training; (2) proctored cases; and (3) requirements for maintenance of privileges.
A preclinical didactic training course was required at all institutions. Only 4 (29%) institutions required completion of an industry-sponsored training course, whereas the majority (10; 71%) of institutions accepted an equivalent didactic course. In most cases where an equivalent course was acceptable, minimum requirements for the course were specified; typically 8 hours of instruction time, with at least 3 hours of personal hands-on time using the robot on either an animal or a cadaver. Four institutions (29%) specified additional requirements for preclinical training including: observation of at least 1 case, completion of the course within 2 months of the first robotic procedure, or completion of basic simulator training with a score >90%.
For the majority of respondent institutions (9; 64%), a surgeon was exempt from completing the preclinical didactic training course if an acceptable experience had been obtained during residency or fellowship. In general, a letter from the training program director stating that the trainee had received a “structured experience” in robotic surgery was all that was required. One institution (7%) waived only the didactic training requirement if the applicant surgeon had trained at that institution. Four institutions (29%) set minimum residency/fellowship case numbers, usually 10 cases.
After completion of the didactic training course (or in the event of exemption because of residency/fellowship training), all institutions specified that applicant robotic surgeons complete a minimum number of proctored cases (range, 2–10 cases). One institution (7%) required that applicant robotic surgeons serve as an assistant on 2 cases before performing proctored cases, and another institution (7%) required that applicant robotic surgeons have a credentialed robotic assistant for their first 7 cases. All institutions required that the proctor be credentialed for robotic surgery. Four institutions (29%) required that the proctor be from the same institution. The remaining institutions set a minimum experience level for the proctor between 20 and 40 cases.
Once privileges were granted, nearly all institutions (12; 86%) required either ongoing monitoring or minimum levels of robotic surgery volume. In most cases, both ongoing monitoring and minimum case volume standards were required. Most institutions (9; 64%) specified that robotic surgeons undergo a program of ongoing monitoring or evaluation to maintain credentialed status, sometimes referred to as Focused Professional Practice Evaluation. In some cases, this was limited to outcome review by the division chief or chair. Most institutions specified focused evaluations of the outcomes from the first 5 to 10 robotic cases. At 1 institution (7%), the newly credentialed robotic surgeon was required to perform 15 “basic” TORS cases before being granted privileges for more complex TORS surgery of the base of tongue, larynx, hypopharynx, or thyroid. One institution (7%) required that newly credentialed robotic surgeons inform patients if their total case volume was fewer than 5 cases. Six institutions (43%) also required specified minimum levels of surgical volume in order to maintain certification. In most cases, minimum requirement was 2 to 5 cases per year, with 1 institution (7%) requiring 10 cases per year, and 1 institution (7%) requiring 25 cases every 2 years. One institution (7%) additionally required that credentialed TORS surgeons maintain an ongoing volume of at least 10 open head and neck cases annually.
Currently, there is no reliable information available to the public regarding the training and credentialing of individual surgeons in robotics in OTO-HNS. Patients who seek a qualified TORS surgeon are left to rely on individual claims of expertise and or an arbitrary industry benchmark for case volume (n = 20; www.davincisurgeonlocator.com).
APPROACHES BY OTHER SPECIALTIES
In developing guidelines for training and credentialing of robotics in OTO-HNS, it is imperative to be familiar with what has already been established in other surgical subspecialties who have adopted the technology. The use of robotics in surgery first surged in urology with the description by Menon et al7 of the robotic radical prostatectomy over 12 years ago. In 2006, approximately 42% of all radical prostatectomies were performed robotically, and that number increased to 63% in 2007, and was estimated at 85% in 2008.8 The second subspecialty to incorporate robotic surgery was gynecology for both benign and malignant pathology. In this section, the current recommendations for credentialing and proctoring by the Society of Urological Robotic Surgeons (SURS) will be summarized and compared to those for the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) and the Minimally Invasive Robotic Association (MIRA).9
The SURS recommendations for credentialing of robotic-assisted radical prostatectomy are the most well-established and accepted guidelines to date.8 The primary objective of the SURS recommendations was to create a certification body and internationally recognized sets of standards and guidelines for the safe application of robotic-assisted radical prostatectomy free from industry. Individual institutions could then be responsible for credentialing surgeons in robotic-assisted radical prostatectomy based on these guidelines. The certification body, instead of industry, identifies proctors for robotic-assisted radical prostatectomy based on peer-reference, submitted case logs, and videos supporting their experience with the procedure. Furthermore, the certification body creates a standardized checklist for proctors to evaluate surgeons seeking credentialing. According to SURS guidelines, a novice surgeon requires proctoring for at least the first 3 to 5 cases before unrestricted privileges are granted. SURS guidelines include a requirement that the role of the proctor be included in the informed consent process. Individual institutions are expected to define the expectations of a proctor in case of emergency (ie, whether or not they are allowed to intervene). SURS recommendations also include regular surgical performance reviews by individual institutions for surgeons performing robotic-assisted radical prostatectomy to evaluate the safety and efficacy of the procedure, including a remediation process for substandard performance.
In 2006, a multidisciplinary consensus statement was also developed at the SAGES–MIRA Consensus Conference addressing credentialing and training in robotic surgery. The SAGES-MIRA credentialing guidelines were intended to help guide health care institutions in this process. According to the consensus recommendations, surgeons seeking robotic privileges should have accredited residency specialty training in the field. For surgeons who had formal robotic surgery training during residency or fellowship, a log of cases should be provided as well as a letter from the program director outlining the candidate’s robotic surgery experience. For candidate surgeons without prior formal robotic surgery training, a structured training program curriculum is required, which is established by the individual health care institution. Such a training program should consist of didactic training on the use and safety of the robotic device, review of robotic surgery videos, hands-on training in a dry laboratory environment, robotic surgery simulation, cadaver, and/or animal laboratory. In addition, it is strongly recommended to observe live surgical cases. The candidate surgeon’s competency in the procedure also needs to be verified before privileges are granted, as measured by knowledge of the procedure, indications, and decision-making. A certificate of competence can be granted to the surgeon seeking robotic privileges, and temporary privileges can be granted. During this time period, the surgeon is able to perform robotic surgery while being mentored or proctored for a certain number of cases with tracking of outcomes. The duration of the temporary privileges and number of preceptored or proctored cases is at the discretion of the chief of the service or the credentialing body. Once permanent privileges are granted, the institution may require outcomes reporting to track quality and safety of the procedure in the hands of the surgeon. In addition, appropriate continuing medical education, renewal, and denial of privilege policies are recommended.
COMPONENTS OF AN IDEAL MODEL FOR TRAINING
General
It has been nearly a decade since the SAGES-MIRA Consensus Conference met to develop recommendations for robotic training and credentialing.9 Yet, a unified model for training in robotic surgery remains elusive. This section aims to highlight the components of an ideal model for robotic surgery training for both OTO-HNS graduates (residents and fellows) and postgraduates.
The design of validated, standardized robot training initiatives is an area of active investigation. The Fundamental Skills of Robotic Surgery (FSRS) curriculum was developed to allow the novice surgeon to master the basic functions of the surgical console and psychomotor skills necessary to perform robotic surgery.10 Although this provides a solid fundamental platform from which to begin robotic training, it is not specialty-specific and procedure-based training is not incorporated. There have been nascent efforts to develop specific training curricula within the specialty of OTO-HNS, including a structured curricular training with TORS procedure-specific tasks.11,12 This curriculum verifies the utility of such training in improving human-machine operation skills and TORS-specific surgical skills with objective assessment.
There are general principles that can be applied to robotic surgery training in OTO-HNS. The design of any curriculum relies on setting expert determined goals and objectives, developing interventions to target these goals, and establishing assessment tools to certify competency of the desired skill sets. The curriculum must be structured and objective or competency-based and ideally should be free of industry influence. The development of robotic curricula should include both preclinical and clinical components.
There are important differences to consider between graduate and postgraduate surgeons training for robotic surgery in OTO-HNS. To a great extent, surgeons in training may be afforded time and graduated responsibility while gaining proficiency through a structured robotic surgery curriculum. These constructs unfortunately do not translate well to the training of practicing surgeons. Limitations, such as time, personal/practice finances, hospital resources, and lack of mentorship, have resulted in inconsistent TORS training of postgraduate surgeons. This problem is not unique to otolaryngology – head and neck surgery. Postgraduate robotic training in urology has faced similar obstacles and attempts to address these issues are ongoing.13–15
In an ideal model, any surgeon seeking robotic training should become familiar with the organizational structure of their institutional robotic surgery program. It is important to contact key robotic nursing and technical support staff, to determine the status of robot availability and needed instrumentation, and review credentialing requirements. Importantly, the candidate robotic surgeon will need to identify any robotic training needed for support staff. Concurrently, the candidate robotic surgeon would ideally review a head and neck specific robotic surgery video library (currently in development by the AHNS). Review of this material would be a prerequisite for enrolling in additional AAO-HNS/AHNS sponsored robotic surgery training courses (as proposed below).
Finally, any surgeon seeking robotic training in OTO-HNS should be strongly encouraged to attend a hands-on training course. This would ideally be offered independent of industry twice yearly at the AAO-HNS and AHNS annual meetings as breakout sessions similar to the American College of Surgeons ultrasound courses. These structured courses would have a curriculum developed and approved by a joint committee of the AAO-HNS and AHNS and staffed by approved proctors based on interest and level of experience. An ideal training program would include didactic sessions on patient selection, perioperative patient management, transoral anatomy, intraoperative troubleshooting, and potential complications, as well as hands-on robotic experience.
Independent credentialing committee
It would be ideal to have a centralized, independent credentialing committee for TORS formed jointly from the AAO-HNS and AHNS. Such a committee could be composed of experienced OTO-HNS robotic surgeons on a rotating basis. An independent credentialing committee would be best-suited to act in a transparent, comprehensive, and iterative manner to maintain a uniform standard for credentialing. Recommendations from the committee could be used by individual institutions as a minimum requirement for privileges. A surgeon seeking credentialing for robotic surgery in OTO-HNS would ideally submit a detailed description of preclinical and clinical curricula, including an assessment of knowledge and or simulation training, and letters supporting competency from instructors and or proctors. The goal of the committee would be to establish and maintain the public trust by setting a clear standard that prioritizes the safe application of new robotic technology. To this end, the committee could also serve as a centralized repository for outcomes data. A centralized registry, independent of industry, could document the safety and efficacy of robotic surgery in OTO-HNS for individuals, institutions, and society as a whole.
GRADUATES
Preclinical training
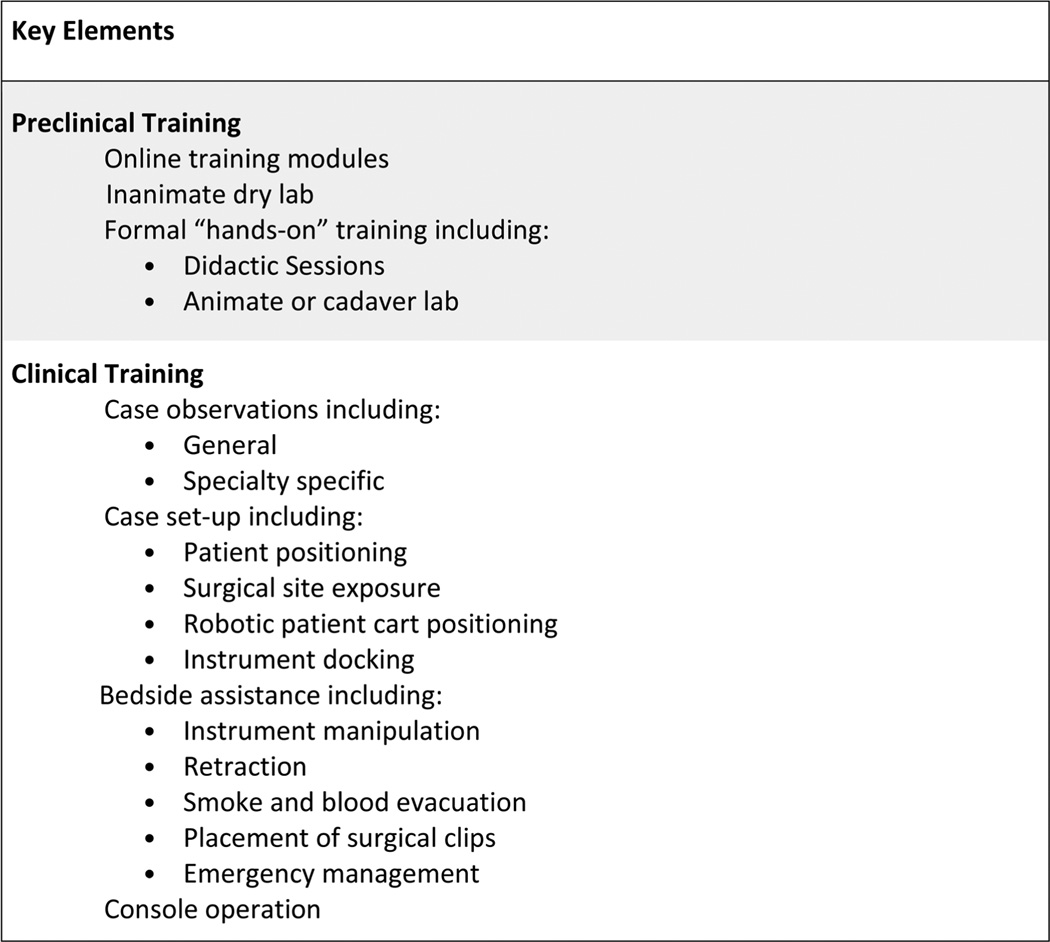
Appropriate didactic instruction in the functioning of the robotic surgical system should be mandatory at the onset of training (Figure 1). This can usually be achieved with completion of basic training modules (eg, on-line) specific for the robotic manufacturer’s surgical system. Such training should include a technical overview of the robot, functional aspects of the system, and troubleshooting tips. Robotic skill development can then begin with inanimate dry laboratory practice, which may include surgical simulation task boards or virtual reality simulation. Currently, the FSRS curriculum has been developed and validated for this purpose.16 After basic robotic aptitude is acquired, surgical skills should be honed on animate or cadaveric models. Such robotic training should be mandatory and should include TORS-specific tasks, including positioning and docking of the robotic system and performance of TORS-related procedures. Proficiency, as determined by expert mentorship or objective assessment of robotic skills, should be demonstrated before moving to the clinical stages rather than advancement based on a specific volume of tasks completed or static length of time spent training.
FIGURE 1.
Components of an ideal model: graduate.
Clinical training
Residents and fellows who train in a program with an active head and neck robotic practice have the opportunity to gain graduated experience and expertise in robotic surgery, similar to any other complex procedure (Figure 1). This ideally begins with observation of both general robotic and specialty specific cases, preferably both prerecorded video and live cases. A video library demonstrating commonly performed TORS procedures, as well as various techniques to address specific (currently in development by the AHNS), should be made available on-line for review.
The trainee should then assist at the bedside to develop a critical understanding and familiarity with the robot and common robotic procedures while retaining a subordinate role to the console surgeon. The bedside assistant develops knowledge of the functionality and limitations of the robot, as well as strategies utilized by the console surgeon for particular robotic procedures. A proficient bedside assistant (ie, able to anticipate the moves of the console surgeon, properly retract tissue to expose the area of interest, clutch and move the robotic arms to avoid collisions, cauterize and grasp tissue, and provide real-time feedback to the console surgeon) develops critical skills that make for a more competent console surgeon.
Finally, in an ideal model, a graduated, step-wise progression of defined tasks are executed by the trainee as console surgeon, based on degree of difficulty, under the supervision of the expert robotic surgeon. A rationale progression of operative experience could be the following: (1) benign tonsillar pathology; (2) lingual tonsillectomy; (3) lateral oropharyngectomy17 (often called “radical tonsillectomy”); (4) resection of the hemi-tongue base; and (5) supraglottic laryngectomy.
Until formal validated methods of assessment are developed to measure surgeon technical proficiency, it is recommended that an expert head and neck robotic surgeon proctor the trainee for each of the above procedures and document competence.
POSTGRADUATES
General
A structured training curriculum for postgraduate surgeons is also essential, ideally with a level of robotic competency commensurate to that achieved through formal graduate training. Postgraduate surgeons seeking to introduce robotic surgery into practice should have completed an accredited residency or fellowship training program and be board-eligible or board-certified in a surgical specialty. Moreover, the candidate robotic surgeon should have privileges to perform the equivalent open or endoscopic procedures, such that these procedures are within an existing scope of practice. Thus, the introduction of robotic technology should not be a driving force behind practice patterns. Surgery represents one component of patient care and the availability of a new instrument cannot supplant clinical expertise, experience, and judgment in a given subject area.
It is imperative that any postgraduate surgeon seeking robotic training in OTO-HNS identify a mentor with experience and expertise in head and neck robotic surgery. Ideally, the mentor would serve in an assistive and advisory role to the candidate robotic surgeon throughout their robotic training and beyond. At a minimum, the mentor would provide case observation experience free-of-charge and be willing to serve as proctor. Ideally, the mentor would be the most qualified head and neck robotic surgeon in geographic proximity to the trainee and vetted by a joint committee of the AAO-HNS and AHNS based upon level of experience. Thus, training in robotic surgery for OTO-HNS would ideally be regionalized and nonindustry managed, minimizing travel time and expense.
Preclinical training
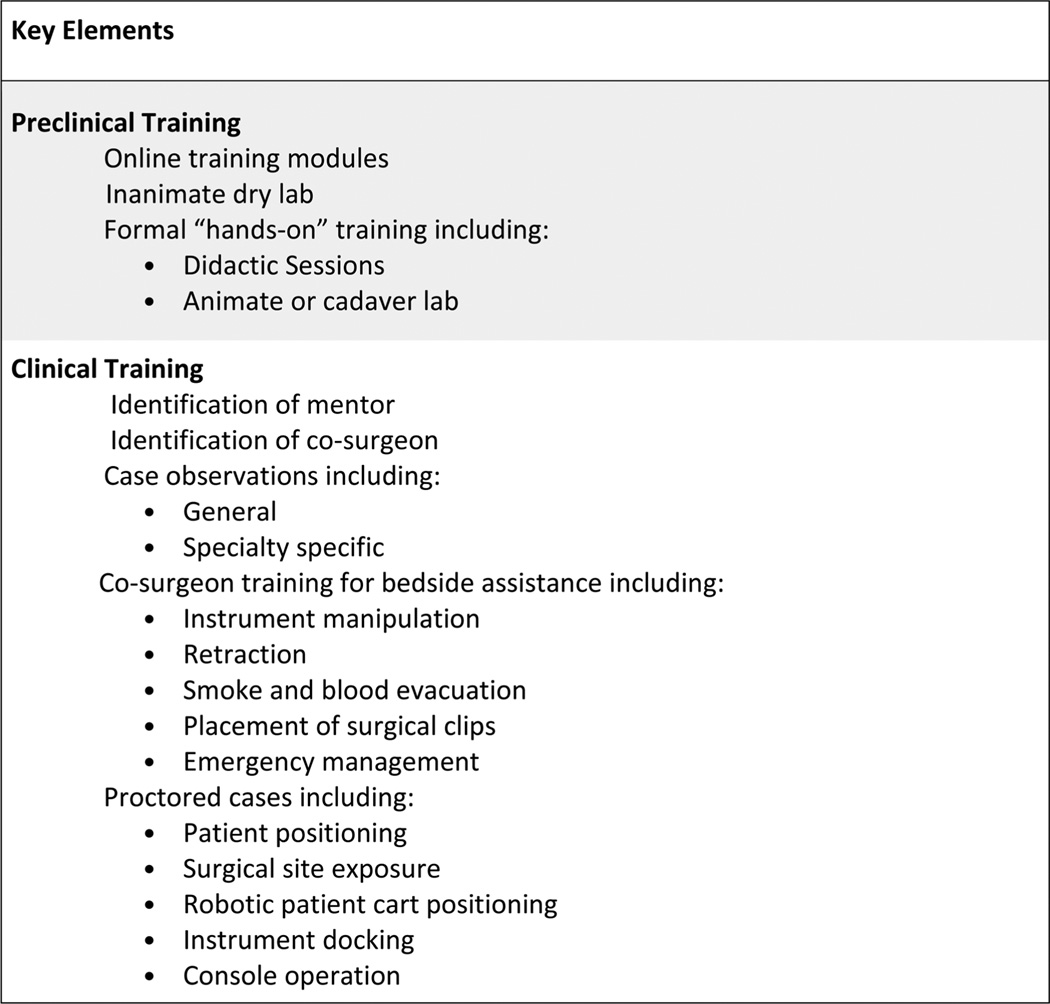
Preclinical robotic training for postgraduate surgeons is essentially the same as it is for graduates (Figure 2). The surgeon should receive detailed instruction on the operation and functionality of the robotic system to be used. This may be completed through on-line materials and/or during face-to-face encounters with manufacturer representatives and local robotic surgery support staff. Manufacturer recommendations regarding general technical specifications, setup, operation, handling, and troubleshooting should be encompassed. Basic exercises using either a virtual reality simulator or dry laboratories should then be undertaken to assist the candidate robotic surgeon in developing basic robotic dexterity and familiarity with the immersive environment. Simulator or dry laboratory exercises should include an assessment of technical proficiency via a validated scoring system, such as the FSRS16 or Global Evaluative Assessment of Robotic Skills.18 The candidate robotic surgeon would then be required to attend a handson training course, ideally offered independent of industry influence. The cadaver laboratory exercises should include demonstration of proficiency in patient positioning, exposure techniques, and bedside assistance. Ideally, each robotic surgeon trainee would perform a core set of TORS procedures on cadaveric specimens, including at least TORS lateral oropharyngectomy (radical tonsillectomy) and TORS hemi-tongue base resection.
FIGURE 2.
Components of an ideal model: postgraduate.
Clinical training
Clinical robotic training for postgraduate surgeons is more structured than that for graduates given the relatively compressed timeframe for acquisition of knowledge and skills (Figure 2). Having obtained a core knowledge base and demonstrated proficiency in basic technical skills pertaining to TORS, the postgraduate candidate robotic surgeon would be well-served to observe general and specialty-specific robotic cases. Ideally, these cases would be performed by the candidate surgeon’s mentor. If the candidate robotic surgeon intends to perform robotic surgery for oncologic purposes, then the case observations should also be oncologic cases.
A postgraduate surgeon seeking robotic training in OTO-HNS should ideally serve as a co-surgeon to learn the critical role of bedside assistance. Although this may be difficult to do without a qualified mentor within the candidate’s institution, every attempt should be made to learn the key elements of bedside assistance, including instrument manipulation, retraction, smoke and blood evacuation, placement of surgical clips, and emergency management.
A postgraduate surgeon seeking robotic training in OTO-HNS should then have initial cases proctored by a qualified mentor, as described previously. The Society of Robotic Surgeons has set forth recommendations for robotic surgery proctoring that could easily be adopted by the AAO-HNS and AHNS (Appendix 2). The surgeon, proctor, and operative team should ideally debrief after each of the proctored cases to discuss performance. Preferably, the surgeon seeking robotic training should be assisted by the mentor until the proctor and trainee agree that the candidate robotic surgeon is safe and capable of operating independently. Documentation, using standardized evaluation forms provided online (ideally via the AAO-HNS and AHNS), could then be offered indicating that a core level of competency has been achieved (Appendix 3). Preferably, these would be reviewed and approved by a centralized, independent credentialing committee of the AAO-HNS and AHNS. A publicly available searchable database of qualified robotic surgeons in OTO-HNS who have met the standards for training and competency could then be generated.
RECOMMENDATIONS FOR TRAINING AND CREDENTIALING
Trainees and novice robotic surgeons are strongly encouraged to only perform those robotic procedures that have been cleared for use by the FDA. Currently, treatment of obstructive sleep apnea remains an off-label use of robotic technology.5 Any off-label use of complex medical technology places the surgeon and hospital at medicolegal risk and must be clearly communicated to the patient and included in the informed consent process.
METHODS
Recommendations for training and credentialing of robotic surgery in OTO-HNS were derived through a rigorous development process that began in 2012. The authors, as representatives of the AHNS Education Committee, the AAO-HNS Robotics Task, and the AAO-HNS Sleep Disorders Committee, combined expertise to construct the general framework of the recommendations. There was not consensus among authors regarding the number of cases recommended to be performed as bedside assistant and console surgeon. Rather, the final case number recommendations were determined by a majority vote of the authors. Supplemental Figures 1 and 2, online only, detail the voting results. Finally, draft recommendations were vetted through the respective committees and leadership of both the AHNS and AAO-HNS before submission for publication.
GRADUATES
Recommendations for training and credentialing of graduates from a residency program in otolaryngology – head and neck surgery or a fellowship program in head and neck surgery are as follows: (1) the applicant robotic surgeon must have or obtain privileges for performing the same or equivalent surgery via an open and/or endoscopic approach; (2) the residency, fellowship, and/or robotic program director must provide credentials to document satisfactory training and confirm competence of the applicant to independently perform robotic surgery; (3) the applicant robotic surgeon must provide evidence of appropriate didactic education during residency including: completion of the basic training modules (online or dry laboratory), specific for the robotic manufacturer’s surgical system; didactic education in the indications, contraindications, and perioperative management of patients undergoing robotic surgery in OTO-HNS; (4) the applicant robotic surgeon must provide evidence of a minimum of 10 robotic cases performed as the bedside assistant; and (5) the applicant robotic surgeon must provide evidence of a minimum of 10 robotic cases performed as the console surgeon.
POSTGRADUATES
Recommendations for training and credentialing of postgraduates from a residency program in otolaryngology – head and neck surgery are as follows: (1) the applicant robotic surgeon must have or obtain privileges for performing the same or equivalent surgery via an open and/or endoscopic approach; (2) the applicant robotic surgeon must provide evidence of appropriate didactic education including: completion of the basic training modules (online or dry laboratory) specific for the robotic manufacturer’s surgical system; didactic education in the indications, contraindications, and perioperative management of patients undergoing robotic surgery in OTO-HNS; formal instructor-led, “hands-on” training experience using the surgical robotic system. This training must include: (a) robotic surgical system setup and docking; (b) skills training using inanimate models or simulation; (c) animal laboratory experience; (d) cadaver laboratory experience (maximum 2 surgeons per cadaver); (3) the applicant robotic surgeon must provide evidence of case observations of robotic surgery in OTO-HNS (eg, TORS), performed by an experienced robotic surgeon; and (4) the applicant robotic surgeon must provide evidence of having been proctored for a minimum of 2 robotic cases with written confirmation by the proctor that the surgeon performed the robotic procedure(s) safely (Appendix 2 and Appendix 3).
MAINTENANCE AFTER INITIAL TRAINING AND CREDENTIALING
An ideal training model for robotic surgery in OTO-HNS should extend beyond the initial training period. The risk of complications after TORS has been shown to decrease with experience, suggesting that the learning curve for robotic surgery in OTO-HNS is steep.19,20 Increased experience also yields decreased operative time, length of intubation, and hospital stay.21 The results of a recent survey of TORS surgeons in the United States suggests that the risk of complications is significantly less for surgeons who have performed >50 TORS procedures.22 Therefore, it is recommended that newly credentialed robotic surgeons in OTO-HNS use a graduated approach to integrating robotic surgery into practice. The neophyte robotic surgeon should be comfortable operating independently and managing simple benign disease before attempting more advanced cases and malignancies. For example, it would be prudent for a novice TORS surgeon to perform several simple pharyngeal procedures (eg, excision of benign papilloma) before considering lateral oropharyngectomy (“radical tonsillectomy”) for squamous cell carcinoma (note that the da Vinci Surgical System is not cleared by the FDA for simple tonsillectomy). Adhering to these principles and maintaining ongoing communication with a robotic surgery mentor can help early robotic surgeons to establish and sustain success.
Requirements for maintenance after initial training and credentialing will vary across institutions. Options for maintenance include the following:
Provisional privileges
Provisional privileges may be appropriate for initial robotic surgical experience. The period of time and number of cases before unrestricted privileges may be granted should be determined at an institutional level by the medical staff committee, chief of service, or appropriate committee.
Monitoring of privileges
After the initial training and credentialing, clinical performance, surgical volume, and complications should be monitored via appropriate peer review, such as the independent credentialing committee, to ensure adequate robotic case volume and outcomes comparable to open and or endoscopic approaches.
Continuing medical education
Adequate evidence of continuing medical education activity in otolaryngology – head and neck surgery, including dedicated continuing medical education in robotic surgery should be obtained.
Supplementary Material
APPENDIX 1
DEFINITIONS
Best practices – A related set of generalizations derived from past experience arranged in a coherent structure to facilitate appropriate responses to specific situations. This set of standard operating practices has a broad base of acceptance among experts in the field.
Competency – Being adequately or well qualified to perform up to defined expectations.
Must/shall – Mandatory recommendation.
Should – Highly desirable recommendation.
May/could – Optional recommendation.
Credentials – Documented evidence of licensure, education, training, experience, or other qualifications.
APPENDIX 2
RECOMMENDED GUIDELINES FOR PROCTORS OF ROBOTIC SURGERY IN OTOLARYNOLOGY AND HEAD AND NECK SURGERY
The role of a proctor of robotic surgery in OTO-HNS should be defined at the institutional level. The following criteria, adapted from SURS recommendations, may be used as general guidance.
Proctors should be able to demonstrate substantial experience in transoral endoscopic head and neck surgery and robotics with a minimum of 20 cases similar to the one that is being proctored.
Informed consent must be obtained from the patient about the presence and responsibility of the proctor.
Granting temporary privileges to the proctor to assist during surgery may be considered.
The role and responsibility of the proctor should be clearly defined, including his/her responsibility in the event of a complication.
The proctor should be present in the operating room for the entire surgery.
The robotic proctor should evaluate whether or not additional malpractice insurance should be obtained.
APPENDIX 3
SAMPLE PROCTORING FORM
Name of the surgeon ______________________________
Name of proctor _________________________________
Date of proctored surgery __________________________
Procedure performed ______________________________
Was the surgery performed for an appropriate indication? Yes or No.
If no, discuss ____________________________________
Was the preoperative workup adequate? Yes or No.
If no, discuss ____________________________________
Please rate the surgeon’s knowledge of the surgical anatomy and the steps of the surgery. Poor, satisfactory, or excellent.
Comments, if any ________________________________
Please rate the surgical competence during this surgery, for his/her level of experience with robotics. Poor, satisfactory, or excellent.
Comments, if any ________________________________
Does the surgeon require proctoring for his/her cases in future? Yes or No.
If yes, for how many more cases would proctoring be required?
Comments, if any ________________________________
Signature: ______________________________________
Date: ________________
Name: _________________________________________
Address: ________________________________________
City: __________________________State: ____________
Zip: _________________________________________
Phone _________________________________
Footnotes
Additional Supporting Information may be found in the online version of this article.
REFERENCES
- 1.Chen MM, Roman SA, Kraus DH, Sosa JA, Judson BL. Transoral robotic surgery: a population-level analysis. Otolaryngol Head Neck Surg. 2014;150:968–975. doi: 10.1177/0194599814525747. [DOI] [PubMed] [Google Scholar]
- 2.Moore EJ, Olsen SM, Laborde RR, et al. Long-term functional and oncologic results of transoral robotic surgery for oropharyngeal squamous cell carcinoma. Mayo Clin Proc. 2012;87:219–225. doi: 10.1016/j.mayocp.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weinstein GS, O’Malley BW, Jr, Magnuson JS, et al. Transoral robotic surgery: a multicenter study to assess feasibility, safety, and surgical margins. Laryngoscope. 2012;122:1701–1707. doi: 10.1002/lary.23294. [DOI] [PubMed] [Google Scholar]
- 4.Hoff PT, D’Agostino MA, Thaler ER. Transoral robotic surgery in benign diseases including obstructive sleep apnea: safety and feasibility. Laryngoscope. 2015;125:1249–1253. doi: 10.1002/lary.25026. [DOI] [PubMed] [Google Scholar]
- 5.Department of Health & Human Services. Food and Drug Administration. [Accessed November 1, 2014];2014 Available at: http://www.accessdata.fda.gov/cdrh_docs/pdf12/K123329.pdf.
- 6.Pradarelli JC, Campbell DA, Jr, Dimick JB. Hospital credentialing and privileging of surgeons: a potential safety blind spot. JAMA. 2015;313:1313–1314. doi: 10.1001/jama.2015.1943. [DOI] [PubMed] [Google Scholar]
- 7.Menon M, Shrivastava A, Tewari A, et al. Laparoscopic and robot assisted radical prostatectomy: establishment of a structured program and preliminary analysis of outcomes. J Urol. 2002;168:945–949. doi: 10.1016/S0022-5347(05)64548-X. [DOI] [PubMed] [Google Scholar]
- 8.Zorn KC, Gautam G, Shalhav AL, et al. Training, credentialing, proctoring and medicolegal risks of robotic urological surgery: recommendations of the Society of Urologic Robotic Surgeons. J Urol. 2009;182:1126–1132. doi: 10.1016/j.juro.2009.05.042. [DOI] [PubMed] [Google Scholar]
- 9.Herron DM, Marohn M SAGES-MIRA Robotic Surgery Consensus Group. A consensus document on robotic surgery. Surg Endosc. 2008;22:313–325. doi: 10.1007/s00464-007-9727-5. discussion 326–327. [DOI] [PubMed] [Google Scholar]
- 10.Attalla K, Raza SJ, Rehman S, et al. Effectiveness of a dedicated robot-assisted surgery training program. Can J Urol. 2013;20:7084–7090. [PubMed] [Google Scholar]
- 11.Moles JJ, Connelly PE, Sarti EE, Baredes S. Establishing a training program for residents in robotic surgery. Laryngoscope. 2009;119:1927–1931. doi: 10.1002/lary.20508. [DOI] [PubMed] [Google Scholar]
- 12.Zhang N, Sumer BD. Transoral robotic surgery: simulation-based standardized training. JAMA Otolaryngol Head Neck Surg. 2013;139:1111–1117. doi: 10.1001/jamaoto.2013.4720. [DOI] [PubMed] [Google Scholar]
- 13.Gamboa AJ, Santos RT, Sargent ER, et al. Long-term impact of a robot assisted laparoscopic prostatectomy mini fellowship training program on postgraduate urological practice patterns. J Urol. 2009;181:778–782. doi: 10.1016/j.juro.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 14.Lee JY, Mucksavage P, Sundaram CP, McDougall EM. Best practices for robotic surgery training and credentialing. J Urol. 2011;185:1191–1197. doi: 10.1016/j.juro.2010.11.067. [DOI] [PubMed] [Google Scholar]
- 15.Mirheydar H, Jones M, Koeneman KS, Sweet RM. Robotic surgical education: a collaborative approach to training postgraduate urologists and endourology fellows. JSLS. 2009;13:287–292. [PMC free article] [PubMed] [Google Scholar]
- 16.Stegemann AP, Ahmed K, Syed JR, et al. Fundamental skills of robotic surgery: a multi-institutional randomized controlled trial for validation of a simulation-based curriculum. Urology. 2013;81:767–774. doi: 10.1016/j.urology.2012.12.033. [DOI] [PubMed] [Google Scholar]
- 17.Holsinger FC, McWhorter AJ, Ménard M, Garcia D, Laccourreye O. Transoral lateral oropharyngectomy for squamous cell carcinoma of the tonsillar region: I. Technique, complications, and functional results. Arch Otolaryngol Head Neck Surg. 2005;131:583–591. doi: 10.1001/archotol.131.7.583. [DOI] [PubMed] [Google Scholar]
- 18.Goh AC, Goldfarb DW, Sander JC, Miles BJ, Dunkin BJ. Global evaluative assessment of robotic skills: validation of a clinical assessment tool to measure robotic surgical skills. J Urol. 2012;187:247–252. doi: 10.1016/j.juro.2011.09.032. [DOI] [PubMed] [Google Scholar]
- 19.Genden EM, Desai S, Sung CK. Transoral robotic surgery for the management of head and neck cancer: a preliminary experience. Head Neck. 2009;31:283–289. doi: 10.1002/hed.20972. [DOI] [PubMed] [Google Scholar]
- 20.Lawson G, Matar N, Remacle M, Jamart J, Bachy V. Transoral robotic surgery for the management of head and neck tumors: learning curve. Eur Arch Otorhinolaryngol. 2011;268:1795–1801. doi: 10.1007/s00405-011-1537-7. [DOI] [PubMed] [Google Scholar]
- 21.White HN, Frederick J, Zimmerman T, Carroll WR, Magnuson JS. Learning curve for transoral robotic surgery: a 4-year analysis. JAMA Otolaryngol Head Neck Surg. 2013;139:564–567. doi: 10.1001/jamaoto.2013.3007. [DOI] [PubMed] [Google Scholar]
- 22.Chia SH, Gross ND, Richmon JD. Surgeon experience and complications with transoral robotic surgery (TORS) Otolaryngol Head Neck Surg. 2013;149:885–892. doi: 10.1177/0194599813503446. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.