Abstract
Background
Whether elective lymph neck dissection (ELND) is associated with improved survival in oral squamous cell carcinomas (SCC) of the maxillary alveolus/hard palate is not known.
Methods
One hundred ninety-nine patients presenting de novo and receiving treatment for clinically node negative SCC of the maxillary alveolus/hard palate at 2 cancer centers between 1985 and 2011 were analyzed.
Results
Forty-two patients (21%) received ELND. Occult nodal metastases were present in 29% of the dissected necks. The ELND group had more T3 to T4 status tumors (62% vs 34%; p < .001) and positive-margin resections (59% vs 38%; p = .019). Patients undergoing ELND experienced lower rates of neck recurrence (6% vs 21%; p = .031), superior 5-year recurrence-free survival (68% vs 45%; p = .026), and overall survival (86% vs 62%; p = .043). ELND was associated with a 2-fold decrease in risk of recurrence in multivariable analysis.
Conclusion
ELND was associated with lower rates of recurrence and improved survival in SCC of the maxillary alveolus/hard palate.
Keywords: squamous cell carcinoma of head and neck, maxillary neoplasms, hard palate, lymphatic metastasis, neck dissection, disease free survival, treatment outcome
INTRODUCTION
Squamous cell carcinoma (SCC) arises less frequently in the maxillary (upper) alveolus and hard palate than in any other oral cavity subsite. These tumors tend to occur in older patients and in a higher proportion of women compared with other oral cancer subsites.1–4 These relatively uncommon tumors are often grouped together given their anatomic contiguity and the belief that their biologic behavior is similar.5–7 Available outcomes’ evidence to guide the management of this group of oral cavity cancers is limited. Most reports consist of small case series analyzed retrospectively.
The general consensus in the field of head and neck oncology states that appropriate initial management of these tumors is surgical extirpation with adjuvant treatment, as indicated. There is controversy in the field regarding the appropriateness of elective lymph node dissection (ELND). Data support the routine performance of ELND for oral SCCs arising in the lower half of the oral cavity or buccal mucosa for tumors with advanced T classification (generally T2 or greater) or tongue SCCs with depth of invasion of more than a threshold distance, generally 4 mm.8,9 In contrast, for maxillary alveolus and hard palate tumors, it has traditionally been believed that the risk of nodal metastases is not high enough to warrant ELND.10–14 Therefore, in the majority of cases, in the absence of clinical evidence of cervical nodal metastases, neck dissection is generally not recommended, independent of the size or depth.15–18 Several single-institution analyses have recently reported higher than expected rates of nodal failure among patients undergoing surgery, without neck dissection, for SCCs of the maxillary alveolus and hard palate.2–4,6,19 These hypothesis-generating analyses imply that ELND would be therapeutically beneficial in patients with clinically N0 SCC of the maxillary alveolus and hard palate, by potentially detecting occult nodal metastases, upstaging the N0 neck, facilitating appropriate adjuvant therapy, and thereby improving locoregional control and survival. To our knowledge, there are no reported data comparing the outcomes of patients with clinically node-negative SCC of the maxillary alveolus and hard palate who did or did not undergo ELND.
The purpose of this study was to investigate the impact of ELND on locoregional control and survival in patients with maxillary alveolus and hard palate SCC. Our multi-institutional analysis utilized patients treated at 2 oral cancer referral centers, Memorial Sloan Kettering Cancer Center (New York, NY) and the Princess Margaret Cancer Centre (Toronto, Ontario, Canada). Our secondary purposes were to ascertain the prevalence of occult nodal metastases in cN0 necks and to identify factors associated with occult nodal metastases, in order to potentially identify patients who may benefit most from ELND.
MATERIALS AND METHODS
This study was reviewed and approved by the institutional review board at each institution before the assembly of data. All patients treated for oral cancers by the head and neck surgery services at Memorial Sloan Kettering Cancer Center (MSKCC, New York, NY) and The Princess Margaret Cancer Centre (Toronto, Ontario, Canada) have been prospectively entered into institutional oral cancer registries (1985–2011 at MSKCC and 1995–2011 at Princess Margaret). We retrospectively assessed all patients with a diagnosis of SCC of the maxillary alveolus and hard palate for eligibility. We included all patients presenting untreated to either institution who were clinically node-negative (cN0), treated with curative intent using primary surgery with or without adjuvant therapy, as indicated, and followed for a minimum of 12 months if surviving. Patients were excluded if they had a history of head and neck cancer, were treated with primary radiotherapy rather than surgery, were treated with palliative intent, had inadequate follow-up (<12 months if surviving), or presented with clinical evidence of cervical nodal metastases (cN+) on clinical or radiological examination before definitive surgical treatment.
For all eligible patients, demographic data, clinical tumor characteristics, risk factors (smoking and alcohol intake), and preoperative imaging results, if performed, were recorded. Neck management was categorized as either ELND or nodal observation. Recurrences were classified as local, regional, or distant. Isolated regional recurrence was defined as regional recurrence without antecedent or synchronous local recurrence. Salvage treatments were defined as successful if the patient remained free of disease after completion of salvage treatments until the end of follow-up. All patients were censored at last follow-up or death.
Patient data, disease, and treatment-related factors were summarized using descriptive statistics. All time-to-event statistics were calculated from the date of index treatment (ie, surgery) to the event of interest. Recurrence-free survival (RFS) was calculated from the time of surgery to the date of recurrence or death (whichever happened earlier). Patients without events were censored at the time of their last follow-up visit. All survival statistics were calculated by the Kaplan–Meier method.
Univariable analysis was performed using the log-rank test. Factors with a p value of < .2 were then entered into a multivariable Cox proportional hazard model using a forward selection algorithm. Disease stage, neck dissection, and adjuvant treatments were entered into the model based on a priori hypotheses. Hazard ratios (HRs) and 95% confidence intervals (CIs) were provided. Statistical significance was defined as a 2-sided p value < .05. All statistical analyses were conducted using SAS version 9.3 (SAS, Cary, NC).
RESULTS
Between 1985 and 2011, 199 patients met inclusion criteria (130 patients at MSKCC and 69 patients at the Princess Margaret). Patient and presenting disease characteristics are detailed in Table 1. The median age at diagnosis was 71 years. Half the patients were men (54%), and most were current or past tobacco or alcohol users. The majority of tumors was located on the maxillary alveolus (76%) and was early stage (ie, T1 and T2; 61%). Maxillectomy defect was repaired using microvascular free tissue transfer in 33 patients (16.6%). Forty-two patients (21%) underwent ELND. Comparing patients who underwent ELND to those who underwent nodal observation, ELND was significantly more common in patients who underwent free flap repair (82% vs 9%; p < .000) and patients with advanced T classification tumors (33% ELND in T3–T4 tumors vs 13% in T1–T2 tumors; p = .001; Table 1). There were no significant associations with ELND performance and median age, sex, and tobacco or alcohol use. Most neck dissections (71%; 30 of 42) were selective neck dissections of levels I to III.
TABLE 1.
Patient and tumor characteristics at presentation.
| Characteristics | All patients (%) | Elective neck dissection (%) | Nodal observation (%) | p value |
|---|---|---|---|---|
| No. of patients | 199 (100) | 42 (21.1) | 157 (78.9) | |
| Study site | ||||
| MSKCC | 130 (65.3) | 4 (3) | 126 (97) | |
| Princess Margaret | 69 (34.7) | 38 (55) | 31 (45) | |
| Sex | 1.00 | |||
| Female | 108 (54.3) | 23 (21) | 85 (79) | |
| Male | 91 (45.7) | 19 (21) | 72 (79) | |
| Median age, y (range) | 71 (24–95) | 67 (39–90) | 73 (24–95) | |
| Tobacco use | .06 | |||
| Never smoker | 85 (42.7) | 12 (14) | 73 (86) | |
| Past or current | 110 (55.3) | 28 (25) | 82 (75) | |
| Unknown | 4 (2) | 2 (50) | 2 (50) | |
| Alcohol use | .41 | |||
| Never drinker | 75 (37.7) | 16 (21) | 59 (79) | |
| Past heavy drinker >6/wk | 9 (4.5) | 4 (44) | 5 (56) | |
| Current drinker <6/wk | 82 (41.2) | 15 (18) | 67 (82) | |
| Current drinker >6/wk | 27 (13.6) | 4 (15) | 22 (85) | |
| Unknown | 6 (3) | 2 (33) | 4 (67) | |
| Tumor subsite | .08 | |||
| Maxillary alveolus | 153 (76.9) | 37 (24) | 116 (76) | |
| Hard palate | 46 (23.1) | 5 (11) | 41 (89) | |
| Clinical stage | .001 | |||
| T1 | 55 (27.6) | 3 (5) | 52 (95) | |
| T2 | 65 (32.7) | 13 (20) | 52 (80) | |
| T3 | 17 (8.5) | 4 (24) | 13 (76) | |
| T4 | 62 (31.2) | 22 (35) | 40 (65) | |
Abbreviation: MSKCC, Memorial Sloan Kettering Cancer Center.
All patients were classified as cN0 at presentation.
The details of surgical treatment and pathologic characteristics are summarized in Table 2. The majority of patients (114; 57.3%) underwent negative margin resection of primary tumors. Perineural and lymphovascular invasion was observed in a minority of patients (14.6% and 8%, respectively). There was no statistical difference between the ELND and nodal observation group with respect to lymphovascular or perineural invasion. However, more patients in the ELND group had positive or close margins (58.5% vs 38.2%; p = .02).
TABLE 2.
Details of treatment and pathology.
| Characteristics | All patients (n = 199) (%) | Elective neck dissection (n = 42) (%) | Nodal observation (n = 157) (%) | p value |
|---|---|---|---|---|
| T classification | .002 | |||
| T1 | 76 (38.2) | 8 (10) | 68 (90) | |
| T2 | 53 (26.6) | 19 (36) | 34 (64) | |
| T3 | 6 (3) | 3 (50) | 3 (50) | |
| T4 | 64 (32.2) | 12 (19) | 52 (71) | |
| N classification | ||||
| N0 | 30 (71.4) | 30 (71.4) | ||
| N1 | 6 (14.3) | 6 (14.3) | ||
| N2a | 0 (0) | 0 (0) | ||
| N2b | 5 (11.9) | 5 (11.9) | ||
| N2c | 1 (2.4) | 1 (2.4) | ||
| Stage | ||||
| I | 74 (37.2) | 6 (8) | 68 (92) | |
| II | 49 (24.6) | 15 (31) | 34 (69) | |
| III | 8 (4) | 5 (63) | 3 (37) | |
| IV | 68 (34.2) | 16 (24) | 52 (76) | |
| Free flap reconstruction | < .000 | |||
| Yes | 33 | 27 (82) | 6 (18) | |
| No | 166 | 15 (9) | 151 (91) | |
| Surgical margins | .003 | |||
| Negative | 114 (57.3) | 17 (15) | 97 (85) | |
| Close (<5 mm) | 42 (21.1) | 16 (38) | 26 (62) | |
| Positive | 42 (21.1) | 8 (19) | 34 (81) | |
| Unknown | 1 (0.5) | 1 (100) | 0 (0) | |
| Perineural invasion | .5 | |||
| No | 170 (85.4) | 34 (20) | 136 (80) | |
| Yes | 29 (14.6) | 8 (28) | 21 (72) | |
| Lymphovascular invasion | .94 | |||
| No | 183 (92) | 38 (21) | 145 (79) | |
| Yes | 16 (8) | 4 (25) | 12 (75) | |
| Extracapsular extension | ||||
| No | 36 (86) | 36 | N/A | |
| Yes | 6 (14) | 6 | N/A | |
| Adjuvant treatments | .14 | |||
| None | 155 (77.9) | 28 (18) | 127 (82) | |
| Radiotherapy | 44 (22.1) | 14 (32) | 30 (68) | |
| Primary site only | 12 (6) | 4 (33) | 8 (66) | |
| Primary site and neck | 25 (12.6) | 9 (36) | 16 (64) | |
| Radiation site unknown | 7 (3.5) | 1 (22) | 6 (78) | |
Abbreviation: N/A, not applicable.
The figures in bold indicate statistical significance.
Of the 42 ELNDs, 12 specimens (29%) harbored occult nodal metastases. The median number of positive nodes was 1.5. Patients with tumors pT2 to pT4 had >20% incidence of occult nodal metastases (Table 3). Fifty percent of positive specimens showed evidence of extracapsular extension (6 of 12). Of the entire cohort, 44 patients (22%) received adjuvant radiation. Of the 12 patients with positive neck dissection specimens, 7 (58.3%) received adjuvant radiation. Of the 5 patients who did not receive radiation, 4 had pN1 disease and 1 patient with pN2 disease was 90 years old at the time of diagnosis. The majority of patients who did not undergo ELND did not receive elective nodal irradiation to the neck (127; 80.9%).
TABLE 3.
Pathologic nodal classification in patients with neck dissection stratified by pathologic tumor classification.
| pN classification | pT1 (%) | pT2 (%) | pT3 (%) | pT4 (%) | Total |
|---|---|---|---|---|---|
| pN0 | 6 (75) | 15 (79) | 1 (34) | 8 (66) | 30 |
| pN1 | 2 (25) | 1 (5) | 1 (33) | 2 (17) | 6 |
| pN2 | 0 | 3 (16) | 1 (33) | 2 (17) | 6 |
| Total | 8 | 19 | 3 | 12 | 42 |
Median follow-up time for the entire cohort was 52.5 months (range, 0.6–261 months). Recurrence of disease was observed in 80 patients (40.2%). Details of the recurrent cases are presented in Table 4. The most common site of recurrence was local (46.2%) followed by regional recurrence in the undissected neck (40%). Only 5% of patients experienced distant failures. There were no differences in the rates of local (5-year failure rate: 18% vs 25%; p = .21) or distant failures (5-year failure rate: 5% vs 1%; p = .14) between the ELND and nodal observation groups (Table 4).
TABLE 4.
Patterns of failure in 80 patients with evidence of recurrent disease.
| Site of failure | All patients (%) | Elective neck dissection (%) | Nodal observation (%) | p value (log-rank test) |
|---|---|---|---|---|
| Local | 37 (46) | 4 (11) | 33 (89) | .21 |
| Regional | 32 (40) | 2 (6) | 30 (94) | .031 |
| Local + regional | 7 (9) | 2 (29) | 5 (71) | .64 |
| Distant | 4 (5) | 2 (50) | 2 (50) | .15 |
| Total | 80 (100) | 10 (13) | 70 (87) | .021 |
The figures in bold indicate statistical significance.
Of the 32 patients experiencing isolated regional recurrences, 26 (81%) underwent salvage treatments. The most common method of salvage was surgery plus radiotherapy (18; 69.2%), although some patients underwent surgery alone (3; 11.5%) or surgery and chemoradiation in 2 patients (7.7%). The details of salvage treatment were unavailable for 3 patients (11.5%). Seventeen patients (53%) were successfully salvaged and 15 (47%) died because of recurrent disease.
At the end of follow-up, 100 patients were alive with no disease (50.3%), 9 patients were alive with disease (4.5%), 42 had died of disease (21.1%), and 48 had died of other causes (24.1%). Median RFS was 62 months (95% CI = 34–101). The 5-year probability of RFS was 50% (95% CI = 44–58). Median overall survival was 124 months (95% CI = 106–164) and 5-year overall survival was 68% (95% CI = 61–75).
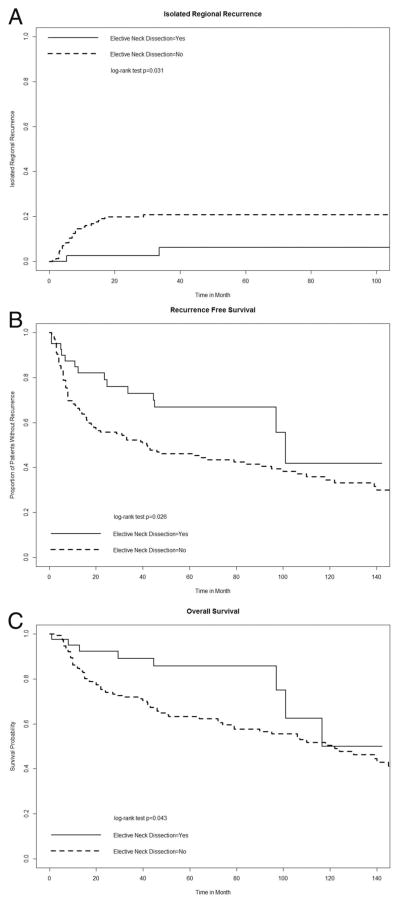
Isolated regional recurrence was observed in 32 patients (16%). The 5-year probability of isolated regional failure was 18% (95% CI = 12–23). Only 2 patients (5%) in the ELND group experienced isolated regional failure. The first patient was classified as pN0 and the second was pN1 after the neck dissection. Neither had received adjuvant radiotherapy. Patients undergoing nodal observation had a higher rate of regional recurrence (21% vs 6%; p = .031; Figure 1A) and a lower probability of RFS (5-year RFS 45% vs 68%; p = .026; Figure 1B). Overall survival was also shorter for patients in the nodal observation group (5-year survival probability: 62% vs 86%; p = .043; Figure 1C).
FIGURE 1.
Regional failure and survival of patients who underwent neck dissection in comparison to nodal observation group. (A) Isolated regional recurrence: patients who underwent elective neck dissection, experienced significantly less isolated regional recurrences (5-year regional recurrence: 6% vs 21%; p = .031). (B) Disease-free survival: patients who underwent elective neck dissection had longer recurrence-free survival (67% vs 45% 5-year survival; p = .026). (C) Overall survival: patients who underwent elective neck dissection had improved overall survival (86% vs 62% 5-year survival; p = .043).
Univariable analysis of factors associated with isolated regional recurrence demonstrated that tumor site and ELND were both significant (Table 5). No factors were significant on multivariable analysis (Table 6), although performance of ELND trended toward an association with improved regional control (p = .09).
TABLE 5.
Univariable analysis of isolated regional failure and recurrence-free survival using a Cox proportional hazards model.
| Variables | Isolated regional failure
|
RFS
|
||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Age, y | 1.01 (0.98–1.04) | .48 | 1.03 (1.01–1.05) | .0012 |
| Sex | .43 | .21 | ||
| Female | Reference | Reference | ||
| Male | 1.32 (0.66–2.65) | 0.79 (0.54–1.14) | ||
| Tobacco use | .93 | .21 | ||
| Never smoker | Reference | Reference | ||
| Past or current user | 0.97 (0.48–1.95) | 0.79 (0.54–1.15) | ||
| Alcohol use | .89 | .014 | ||
| Never drinker | Reference | Reference | ||
| Past or current drinker | 0.95 (0.46–1.94) | 0.62 (0.43–0.91) | ||
| Primary site | .02 | .0095 | ||
| Maxillary alveolus | Reference | Reference | ||
| Hard palate | 2.35 (1.15–4.81) | 1.70 (1.14–2.53) | ||
| Elective neck dissection | .048 | .028 | ||
| Yes | Reference | Reference | ||
| No | 4.24 (1.01–17.74) | 1.88 (1.07–3.30) | ||
| Clinical T classification | .22 | .26 | ||
| Early T1–T2 | Reference | Reference | ||
| Advanced T3–T4 | 0.61 (0.28–1.33) | 1.23 (0.85–1.79) | ||
| Margins | .24 | .077 | ||
| Negative | Reference | Reference | ||
| Positive | 1.58(0.73–3.42) | 1.47 (0.96–2.27) | ||
| Pathologic T classification | .83 | < .001 | ||
| Early T1–T2 | Reference | Reference | ||
| Advanced T3–T4 | 1.08 (0.52–2.25) | 1.95 (1.34–2.82) | ||
| Perineural invasion | .34 | .69 | ||
| No | Reference | Reference | ||
| Yes | 1.54 (0.63–3.74) | 1.12 (0.65–1.93) | ||
| Lymphovascular invasion | .77 | .25 | ||
| No | Reference | Reference | ||
| Yes | 0.81 (0.19–3.38) | 1.47 (0.76–2.82) | ||
| Pathologic N classification | .15 | .072 | ||
| No neck dissection | Reference | .071 | Reference | .024 |
| N0 | 0.16 (0.02–1.17) | .44 | 0.45(0.23–0.90) | .58 |
| N+ | 0.46 (0.06–3.34) | 0.77 (0.31–1.90) | ||
| Adjuvant radiotherapy | .37 | .89 | ||
| No | Reference | Reference | ||
| Yes | 0.62 (0.22–1.76) | 1.04 (0.64–1.69) | ||
Abbreviations: RFS, recurrence-free survival; HR, hazard ratio; 95% CI, 95% confidence interval.
The figures in bold indicate statistical significance.
TABLE 6.
Multivariable analysis of predictive factors in isolated regional recurrence and recurrence-free survival.
| Characteristics | Isolated regional failure
|
RFS
|
||
|---|---|---|---|---|
| RR (95% CI) | p value | RR (95% CI) | p value | |
| Tobacco use | .74 | |||
| Never smoker | Reference | |||
| Past or current smoker | 1.07 (0.7–1.65) | |||
| Alcohol use | .067 | |||
| Never drinker | Reference | |||
| Past or current drinker | 0.67 (0.43–1.03) | |||
| Pathologic T classification | .8 | .0016 | ||
| T1–T2 | Reference | Reference | ||
| T3–T4 | 0.9 (0.4–2.03) | 1.98 (1.3–3.03) | ||
| Primary tumor site | .05 | |||
| Maxillary alveolus | Reference | |||
| Hard palate | 2.14 (1–4.56) | |||
| Elective neck dissection | .09 | .025 | ||
| Yes | Reference | Reference | ||
| No | 3.43 (0.81–14.52) | 2.05 (1.09–3.82) | ||
| Surgical margins | .27 | .61 | ||
| Negative | Reference | Reference | ||
| Positive | 1.75 (0.83–3.69 | 1.16 (0.66–2.04) | ||
| Adjuvant radiotherapy | .37 | .36 | ||
| No | Reference | Reference | ||
| Yes | 0.57 (0.16–1.97) | 0.75 (0.41–1.38) | ||
Abbreviations: RFS, recurrence-free survival; RR, risk ratio; 95% CI, 95% confidence interval.
The figures in bold indicate statistical significance.
Univariable analysis of RFS demonstrated several factors as significantly associated with RFS: age, alcohol use, site, ELND, and pathologic T classification (Table 5). On multivariable analysis, ELND and pathologic T classification remained significant (p value: stage = .0016; neck dissection = .025; Table 6). Nodal observation was associated with a 2-fold increased risk of disease recurrence even after controlling for other factors. These analyses indicate that the performance of ELND is associated with longer overall survival and RFS, and improved regional control for patients with SCC of the maxillary alveolus and hard palate.
DISCUSSION
Elective neck dissection is routinely performed in the SCC of the lower oral cavity when the risk of metastasis is considered higher than 20%.20 A recent randomized trial by D’Cruz et al9 provided proof of improved disease-free and overall survival after ELND in patients with early-stage oral cancers for the first time. In that study, however, maxillary alveolus and hard palate tumors were excluded. Traditionally, ELND has not been recommended for SCC arising from the maxillary alveolus or hard palate. However, we and others have recently reported a higher than expected rate of occult metastases in patients with these tumors.2–4,19 At MSKCC and Princess Margaret, we have previously published the 2 largest single center analyses of patients with SCC of the maxillary alveolus and hard palate. We found high rates of occult nodal metastases in the cN0 neck: 26% at Princess Margaret and 29.5% at MSKCC. We also found significant rates of regional recurrence among patients not undergoing ELND.2,3 Other smaller case series have also consistently showed high rates of occult cervical metastases from these tumors.4,21,22 Therefore, we hypothesized that ELND may be associated with an improvement in survival or locoregional control in patients with SCC of the maxillary alveolus and hard palate. None of the prior studies had samples sizes or ELND rates large enough to address this question.
In this study, we analyzed the outcomes of patients treated at 2 high-volume head and neck oncology centers for SCC of the maxillary alveolus and hard palate. Although neither center used a policy of routine ELND for these tumors, the wider use of microvascular reconstruction for high T classification tumors led to an increasing integration of nodal dissection into surgical management. This cohort of patients provided an opportunity to examine outcomes of patients treated with ELND for SCC of the maxillary alveolus and hard palate.
Of the 199 patients, 42 (21%) underwent ELND. Of the nodal dissections in cN0 necks, 29% harbored occult metastases. Because ELND was often performed in cases undergoing microvascular reconstruction, the ELND group comprised more advanced T classification tumors, which were more likely to be resected with positive margins. Nevertheless, the higher-risk patients in the ELND group experienced superior regional control, RFS, and overall survival. The performance of ELND, by upstaging disease and facilitating appropriate adjuvant therapy, was associated with a lower regional recurrence rate (6% vs 21%; p = .03), higher 5-year RFS (67% vs 45%; p = .026), and higher 5-year overall survival (86% vs 62%; p = .043).
Neck dissection remains a significant factor in improved RFS even after controlling for stage and adjuvant treatments. Patients who did not undergo ELND had a 2-fold increased risk of recurrence.
Local and distant failure rates did not differ between the groups (Table 4), making the regional recurrence rate a possible explanation for the poorer outcome in the nodal observation group. These associations remained significant on multivariable analysis.
Together, these results allow us to conclude that a significant proportion of patients with SCC of the maxillary alveolus and hard palate harbor occult cervical metastases, and that identifying these occult metastases with elective nodal dissection facilitates appropriate risk stratification for adjuvant therapy. This was associated with improved regional control and survival.
Regional recurrences for oral cavity SCC are generally difficult to successfully salvage.4 Studies of oral cavity SCC failing regionally reveal that salvage neck dissection generally requires more extensive operations than selective supraomohyoid neck dissection.23 In this study, patients who experienced a regional recurrence had lower survival, and salvage was successful only 53% of the time.
Similar to the data reported by D’Cruz et al,9 our data demonstrate that ELND was associated with superior regional control and survival, despite it being performed in patients with generally more advanced disease than those undergoing nodal observation. Our findings extend the conclusions by D’Cruz et al9 to the maxillary alveolus and hard palate subsites. Therefore, the traditional belief that SCCs of the maxillary alveolus and hard palate do not require ELND is unlikely to be applicable in all situations. Although the rate of occult nodal metastases in SCCs arising from these areas may be incrementally lower than other oral cavity sites, our data suggest that ELND, by pathologically staging the neck and facilitating risk-adjusted adjuvant therapy, may improve disease control in properly selected patients. Unfortunately, these retrospective data cannot precisely define the criteria for patient selection, but we do recommend considering ELND for these tumors, akin to the calculus for other oral cavity SCCs.
We acknowledge the limitations of our study. Similar to all case series, these patients were treated and collected during a long period of time. The possibility for variations in diagnostic methods, treatment modality, and follow-up capabilities do exist. Although this was not a randomized clinical trial, these retrospective data are hypothesis-generating data that represent the first comparison of patients receiving ELND versus observation in the SCCs of the maxillary alveolus and hard palate.
In conclusion, we present the largest series of maxillary alveolus and hard palate tumors without evidence of cervical lymph node metastases at diagnosis. We identified a considerable rate of occult cervical metastases in this group (28.6%). Furthermore, our results suggest that ELND in this group of patients provides the most accurate staging method and could potentially reduce the risk of regional recurrence and improve disease-free and overall survival. Larger, prospective, controlled trials are necessary to test this hypothesis. Conducting such a study might be logistically challenging considering the low incidence of this disease. In the absence of prospective controlled trials, the data presented here and our previous reports2,3 provide high-quality retrospective data in support of routine ELND for patients with T2 and greater oral cavity SCC arising in the maxillary alveolus and hard palate.
Acknowledgments
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748 and NIH K08 DE024774 (to LGTM).
References
- 1.Lin HW, Bhattacharyya N. Survival impact of nodal disease in hard palate and maxillary alveolus cancer. Laryngoscope. 2009;119:312–315. doi: 10.1002/lary.20054. [DOI] [PubMed] [Google Scholar]
- 2.Morris LG, Patel SG, Shah JP, Ganly I. High rates of regional failure in squamous cell carcinoma of the hard palate and maxillary alveolus. Head Neck. 2011;33:824–830. doi: 10.1002/hed.21547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eskander A, Givi B, Gullane PJ, et al. Outcome predictors in squamous cell carcinoma of the maxillary alveolus and hard palate. Laryngoscope. 2013;123:2453–2458. doi: 10.1002/lary.24079. [DOI] [PubMed] [Google Scholar]
- 4.Montes DM, Carlson ER, Fernandes R, et al. Oral maxillary squamous carcinoma: an indication for neck dissection in the clinically negative neck. Head Neck. 2011;33:1581–1585. doi: 10.1002/hed.21631. [DOI] [PubMed] [Google Scholar]
- 5.Janeway HH. The treatment of tumors of the superior maxilla. Ann Surg. 1918;68:353–370. doi: 10.1097/00000658-191810000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simental AA, Jr, Johnson JT, Myers EN. Cervical metastasis from squamous cell carcinoma of the maxillary alveolus and hard palate. Laryngoscope. 2006;116:1682–1684. doi: 10.1097/01.mlg.0000233607.41540.28. [DOI] [PubMed] [Google Scholar]
- 7.Mourouzis C, Pratt C, Brennan PA. Squamous cell carcinoma of the maxillary gingiva, alveolus, and hard palate: is there a need for elective neck dissection? Br J Oral Maxillofac Surg. 2010;48:345–348. doi: 10.1016/j.bjoms.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 8.Huang SH, Hwang D, Lockwood G, Goldstein DP, O’Sullivan B. Predictive value of tumor thickness for cervical lymph-node involvement in squamous cell carcinoma of the oral cavity: a meta-analysis of reported studies. Cancer. 2009;115:1489–1497. doi: 10.1002/cncr.24161. [DOI] [PubMed] [Google Scholar]
- 9.D’Cruz AK, Vaish R, Kapre N, et al. Elective versus therapeutic neck dissection in node-negative oral cancer. N Engl J Med. 2015;373:521–529. doi: 10.1056/NEJMoa1506007. [DOI] [PubMed] [Google Scholar]
- 10.MacFee WF. Resection of the upper jaw for carcinoma. Am J Surg. 1935;30:21–35. [Google Scholar]
- 11.Martin H. Cancer of the gums (gingivae) Am J Surg. 1941;54:769–806. [Google Scholar]
- 12.Cady B, Catlin D. Epidermoid carcinoma of the gum. A 20-year survey. Cancer. 1969;23:551–569. doi: 10.1002/1097-0142(196903)23:3<551::aid-cncr2820230306>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 13.Evans JF, Shah JP. Epidermoid carcinoma of the palate. Am J Surg. 1981;142:451–455. doi: 10.1016/0002-9610(81)90373-1. [DOI] [PubMed] [Google Scholar]
- 14.Soo KC, Spiro RH, King W, Harvey W, Strong EW. Squamous carcinoma of the gums. Am J Surg. 1988;156:281–285. doi: 10.1016/s0002-9610(88)80292-7. [DOI] [PubMed] [Google Scholar]
- 15.Jones AS. Oral cancer. In: Shah JP, Johnson NW, Batsakis JG, editors. British Journal of Surgery. London, UK: Martin Dunitz; 2003. p. 496. [Google Scholar]
- 16.Myers EN, Suen MD, Myers J, editors. Cancer of the head and neck. 4. Philadelphia, PA: Saunders; 2003. p. 850. [Google Scholar]
- 17.Genden EM, Varvares MA. Head and neck cancer: an evidence-based team approach. New York: Thieme; 2008. [Google Scholar]
- 18.Montgomery PQ, Rhys Evans PH, Gullane PJ, editors. Principles and practice of head and neck surgery and oncology. 2. Boca Raton, FL: CRC Press; 2009. [Google Scholar]
- 19.Brown JS, Bekiroglu F, Shaw RJ, Woolgar JA, Rogers SN. Management of the neck and regional recurrence in squamous cell carcinoma of the maxillary alveolus and hard palate compared with other sites in the oral cavity. Head Neck. 2013;35:265–269. doi: 10.1002/hed.22957. [DOI] [PubMed] [Google Scholar]
- 20.Weiss MH, Harrison LB, Isaacs RS. Use of decision analysis in planning a management strategy for the stage N0 neck. Arch Otolaryngol Head Neck Surg. 1994;120:699–702. doi: 10.1001/archotol.1994.01880310005001. [DOI] [PubMed] [Google Scholar]
- 21.Binahmed A, Nason RW, Hussain A, Abdoh AA, Sándor GK. Treatment outcomes in squamous cell carcinoma of the maxillary alveolus and palate: a population-based study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:750–754. doi: 10.1016/j.tripleo.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Wang TC, Hua CH, Lin CC, Tsou YA, Tseng HC, Tsai MH. Risk factors affect the survival outcome of hard palatal and maxillary alveolus squamous cell carcinoma: 10-year review in a tertiary referral center. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110:11–17. doi: 10.1016/j.tripleo.2009.11.035. [DOI] [PubMed] [Google Scholar]
- 23.Yuen AP, Ho CM, Chow TL, et al. Prospective randomized study of selective neck dissection versus observation for N0 neck of early tongue carcinoma. Head Neck. 2009;31:765–772. doi: 10.1002/hed.21033. [DOI] [PubMed] [Google Scholar]