Abstract
Background
The purpose of this study was for us to present our evaluation of the effectiveness of positron emission tomography (PET)/CT imaging in postoperative patients with oral cavity squamous cell carcinoma (SCC) before initiating adjuvant radiation therapy.
Methods
Treatment planning PET/CT scans were obtained in 44 patients with oral cavity SCC receiving adjuvant radiation. We identified target areas harboring macroscopic disease requiring higher radiation doses or additional surgery.
Results
Fourteen PET/CT scans were abnormal. Thirteen patients underwent surgery and/or biopsy, increased radiation dose, and/or addition of chemotherapy. Eleven patients received higher radiation doses. Patients undergoing imaging >8 weeks were more likely to have abnormal results (p = .01). One-year distant metastases-free survival was significantly worse in patients with positive PET/CT scans (61.5% vs 92.7%; p = .01). The estimated positive predictive value (PPV) was 38% for postoperative PET/CT scanning.
Conclusion
We demonstrated that 32% of patients have abnormal PET/CT scans resulting in management changes. Patients may benefit from postoperative PET/CT imaging to optimize adjuvant radiation treatment planning.
Keywords: oral cavity, squamous cell carcinoma, adjuvant radiation, surgery, postoperative
INTRODUCTION
Locally advanced oral cavity squamous cell carcinomas (SCCs) are aggressive malignancies of the head and neck. Treatment consists of surgery and is often followed with adjuvant radiation with or without chemotherapy.1,2 Unfortunately, local and regional recurrence rates remain high with limited salvage options.3–5 Identification of patients with recurrent or residual disease in the early postoperative setting and before initiation of adjuvant therapy may allow alteration in management of these high-risk patients with additional surgery, addition of systemic therapy, treating to higher radiation doses, or switching to palliative care in appropriate cases. With dose intensification in selected patients with worrisome postoperative radiological findings, we may be able to improve local control rates.
Positron emission tomography (PET) or PET/CT imaging has emerged as an essential tool to assess extent of disease and more recently to guide radiation treatment planning in head and neck, lung, and esophageal cancers.6–8 Specifically in the head and neck, PET/CT has been shown to have improved anatomic localization and accurate assessment of extent of disease.9–11 However, in the postoperative setting, PET or PET/CT scans are limited by their lack of specificity in distinguishing postoperative inflammatory changes from hypermetabolic tumor cells. Although a consensus on timing for follow-up PET or PET/CT scans has yet to be established, some authors have suggested that PET imaging should be performed no sooner than 2 to 3 months after surgery ± chemoradiation because of the potential high rates of false-positive results in this time period.12 However, given the importance of package time in adjuvant treatment for head and neck cancer, the use of PET and PET/CT may be considered earlier than later.13
The utility of PET or PET/CT scans in the adjuvant setting for head and neck cancers after surgery but before initiation of adjuvant treatment has been investigated in 2 recent studies. In both studies, PET/CT imaging demonstrated the presence of locoregional or metastatic disease that required the alteration of management in 15% to 24% of cases.14,15 Such early imaging in the postoperative setting is contrary to the traditional thinking that one must wait 2 to 3 months after surgery to obtain a meaningful PET to avoid false-positive results from postsurgical inflammation. The purpose of this retrospective study was to report our experience of postoperative PET/CT scans for patients with oral cavity SCC who underwent surgical resection of the primary tumor and were referred for postoperative radiation and received a postoperative PET/CT scan as part of restaging and radiation treatment planning. We determined the frequency of finding residual disease and altering management in these patients. Correlation with pathological findings and outcomes with the PET/CT scan results were made.
MATERIALS AND METHODS
Patient and disease characteristics
This retrospective study was reviewed and approved by the Memorial Sloan Kettering Cancer Center (MSKCC) Institutional Review Board. From January 2007 to October 2012, the records of 598 patients referred to the Department of Radiation Oncology with head and neck cancers were reviewed. All of these patients underwent PET/CT simulation for radiation treatment planning purposes. We identified 44 patients with locally advanced oral cavity SCC who had undergone surgical resection and recommended to receive adjuvant radiotherapy at MSKCC in Manhattan. Patients included in this study had very complex cases and were felt to require a referral for adjuvant radiation therapy. Patients who underwent biopsy only or primary surgery were excluded. Patients who had recurrent disease and had received previous definitive or adjuvant radiotherapy to the head and neck were also excluded. Patients who had biopsies performed at an outside institution but had definitive surgery at MSKCC were included. Six patients had prior early-stage oral cavity tumors treated with surgery alone with no adjuvant radiation treatment. One patient underwent robotic surgery for a posterior oral cavity SCC. Three patients received neoadjuvant chemotherapy before surgery.
The characteristics of these patients are shown in Table 1. Of the 44 analyzed patients, 28 were men and 16 were women. The median age was 58 years (range, 36–92 years). Margin status was determined by pathological review and was defined as negative, positive, or close as defined by <5 mm. Tumors were staged as per the American Joint Committee on Cancer classification16 based on pathological review of the resected specimen. One patient who received neoadjuvant chemotherapy was staged based on preoperative radiological imaging. Twenty-seven patients had preoperative PET/CT scans. Thirty-nine patients underwent ipsilateral neck dissection of at least levels 1 to 3; and 6 patients underwent contralateral neck lymph node dissections of levels 1 to 3. The resected specimens were evaluated for margin status and presence of perineural invasion (PNI) and extracapsular extension (ECE). Five patients were treated on clinical protocols.
TABLE 1.
General characteristics of patients included in this study.
| Characteristics | No. of patients (%) |
|---|---|
| Sex | |
| Male | 28 (64) |
| Female | 16 (36) |
| Median age, y | 58 (range, 36–92 y) |
| T classification | |
| T1 | 13 (30) |
| T2 | 13 (30) |
| T3 | 6 (14) |
| T4 | 9 (20) |
| N classification | |
| Node negative | 15 (34) |
| Node positive | 27 (61) |
| N1 | 12 (27) |
| N2a | 1 (2) |
| N2b | 13 (30) |
| N2c | 1 (2) |
| Presence of ECE | 10 (23) |
| Presence of PNI | 24 (55) |
| Margins | |
| Positive | 4 (9) |
| Close (<5 mm) | 17 (39) |
| Negative | 21 (48) |
Abbreviations: ECE, extracapsular extension; PNI, perineural invasion.
Positron emission tomography/CT imaging
Patients were evaluated by the department of radiation oncology and underwent a PET/CT simulation with intravenous contrast when feasible as part of radiation treatment planning and restaging. PET/CT scans were obtained at a median of 6 weeks (range, 4–14 weeks) postoperatively and were used for the purposes of radiation treatment planning. Two patients underwent PET/CT scans >10 weeks from surgery. Patients undergoing PET/ CT radiation treatment planning simulations were performed on a General Electric Discovery LS PET (Advance NXi)/CT (LightSpeed 4-slice) scanner (GE HealthCare, Waukesha, WI) with an intrinsic resolution of 4.2-mm full-width at half maximum, using standardized image acquisition protocols. Images were reviewed by both an attending nuclear medicine radiologist and attending radiation oncologist.
Patients who demonstrated new sites of distant disease or locoregionally residual/recurrent disease were identified. Biopsies and/or additional surgeries were performed in patients to confirm the PET findings when feasible. In patients who did not go undergo biopsy, the cases were discussed with the attending surgeon and treating radiation oncologist. Patients with positive PET/CT findings who were not able to be biopsied were discussed with the multidisciplinary team and, if suspected to have active disease, were treated with higher doses of radiation with possible modification of target volumes and received chemotherapy with concurrent radiation, when medically feasible.
Adjuvant therapy
All patients were treated with intensity-modulated radiotherapy using dose painting techniques. Details regarding delineation of target structures, total doses, and dose per fraction specifications used at MSKCC have been previously described in detail.11 The standard radiation dose delivered to the postoperative bed was 60 to 66 Gray (Gy). If margins were positive or close, the dose was increased to 66 Gy. In areas that were felt to harbor gross disease based on PET/CT imaging, the dose was further escalated to 70 Gy or more. In patients for whom biopsy for confirmation of suspicious PET finding was not feasible, the PET avid regions were boosted to 70 Gy after a thorough discussion between the radiation oncologist and the head and neck surgeon. The contralateral uninvolved neck was treated to 54 Gy.
Patients with gross disease, as determined by biopsy or PET/CT findings, were evaluated by the department of medical oncology regarding the need for systemic therapy. High-risk patients received cisplatin either given every 3 weeks at 100 mg/m2 or weekly at 30 to 40 mg/m2. Alternatively, patients were treated with cetuximab given as a loading dose of 400 mg/m2 and then weekly at 250 mg/m2. Patients who were not well enough to receive concurrent chemoradiation were treated with radiation alone.
Statistical methods
Patient charts were retrospectively reviewed to obtain prognostic information and time to events. Actuarial curves were generated using the Kaplan–Meier method and univariate analysis was performed with the log-rank test. Freedom from local failure and distant metastasis were analyzed similarly, using death or end of follow-up as censoring events. The relationship between pathologic variables and postoperative PET results was analyzed using Fisher’s exact tests. All statistical analysis was performed with the R language and environment for statistical computing, version 2.15.3 (http://www.R-project.prg).
RESULTS
The characteristics for this patient group are shown in Table 1. The majority of patients were men and the median age was 58 years with a range of 36 to 92 years of age. Approximately 60% of patients had T1 or T2 classification of disease. Twenty-seven patients (61%) had pathologically involved lymph nodes, and 10 patients had ECE on pathologic review. PNI was detected in 24 patients (55%). Twenty-one patients (48%) had close (<5 mm) or positive margins.
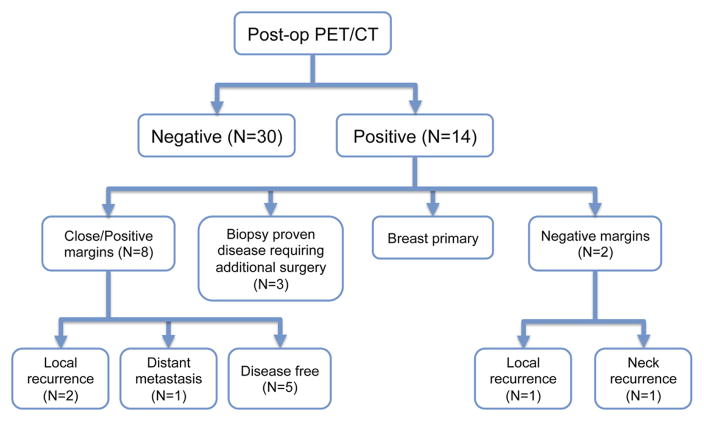
PET scans were obtained at a median of 6 weeks (range, 4–14 weeks) after definitive surgery. Scans of patients taken 8 weeks or later after surgery were significantly more likely to be abnormal (p = .01). Fourteen patients had abnormal PET/CT scans (see Figure 1 for additional characteristics of patients included in this review). One patient was found to have a new primary breast cancer and was not scored as requiring a change in management of her head and neck cancer treatment. Overall, 30% of patients (13/44) had a change in management of the adjuvant treatment of the head and neck after surgery. Ten of these 13 patients specifically had preoperative PET/CT imaging studies performed. One patient was found to have a lung primary cancer that had been previously detected and biopsied on preoperative imaging studies. However, this same patient was also noted to have abnormal nodular uptake in the left floor of the mouth postoperatively and was treated to a higher radiation dose. Two patients underwent additional biopsy of areas outside of the head and neck based on the PET/CT findings; biopsies were negative in both cases.
FIGURE 1.
Characteristics of patients included in this study who underwent postoperative planning positron emission tomography (PET)/CT simulations.
Three patients underwent additional biopsy in areas of the head and neck and subsequent surgery for pathologically confirmed disease. All 3 of these patients had preoperative PET/CT scans. An example of such a patient who was found to have locally advanced contralateral nodal disease and benefited from additional surgery before adjuvant radiation is shown in Figure 2. This patient underwent surgery for a pT2N2a of the left side of the oral tongue SCC. At 7 weeks, postoperative PET/CT imaging demonstrated contralateral neck nodal metastases. The patient underwent additional surgery demonstrating 2 metastatic right lymph nodes. Another patient underwent resection for an early-stage tongue cancer. Lymph nodal dissection demonstrated a single positive lymph node without ECE. The patient was recommended to receive radiation alone. However, the postoperative PET/CT demonstrated worrisome disease in a level 1b lymph node. The patient underwent additional surgery that demonstrated 2 additional lymph nodes with focal ECE. The patient went on to receive postoperative chemoradiation, but, given the patient’s high-risk features and multiple surgeries, received 70 Gy as a postoperative dose. The patient remains without evidence of disease at the last follow-up.
FIGURE 2.

Imaging studies of this patient with a pT2N2a of the left side of the oral tongue 7 weeks postoperatively demonstrated contralateral neck nodal metastases. The patient underwent additional surgery demonstrating 2 metastatic right lymph nodes. The patient is without evidence of disease at 16 months.
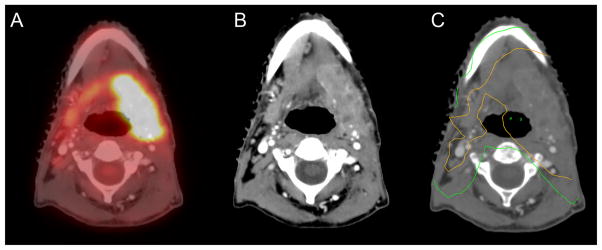
Eleven patients were treated to higher doses of radiation (70 Gy or higher) without additional biopsy confirmation, including the patient mentioned above. One patient underwent surgery for a pT3N2b oral tongue SCC; resection margins were positive and postoperative scan at 13 weeks suggested recurrent disease. The PET/CT images were used as a guide to increase the doses to specific areas of the head and neck that should receive a dose of 70 Gy (see Figure 3).
FIGURE 3.
(A–C) Imaging studies demonstrate the areas of gross disease in this patient with pT3N2b oral tongue squamous cell carcinoma (SCC). (A and B) Resections margins were positive and postoperative scan at 13 weeks demonstrated recurrent disease in both the fused positron emission tomography (PET)/CT component and the CT scan itself. (C) The area treated to 70 Gy is demonstrated by the orange line.
In some patients, radiographic areas of fluorodeoxyglucose (FDG) uptake correlated with areas of positive margins. The patient shown in Figure 4 had a pT4N0 gingival cancer with a positive anterior resection margin of the mandible. PET/CT imaging at 5.4 weeks postoperatively also demonstrated intense focal uptake at the anterior resection of the mandibular margin, which correlated with the area of the positive margin. This area was prescribed 70 Gy as a postoperative dose as it was felt to harbor residual or locally recurrent disease. A 3D margin of 5 to 10 mm around the area of FDG activity was generated and prescribed to a dose of 70 Gy. Radiographic morphological characteristics also suggested recurrent disease in a patient with a pT3N2b oral tongue SCC who was noted to have a focal FDG-avid rim-enhancing area on the PET/CT scan 7 week postoperatively. Although margins were negative in this patient, this well-circumscribed area was worrisome for residual disease. The area of FDG uptake was used to delineate the area prescribed to 70 Gy (see Figure 5).
FIGURE 4.
Imaging studies of this patient with a pT4 gingival cancer demonstrate intense fluorodeoxyglucose (FDG) uptake standardized uptake value (SUV) 7.2 in the anterior resection margin of the mandible, which was the same area as appositive margin was noted on pathological review. (A) The demonstrated hypermetabolic area on the fused positron emission tomography (PET)/CT scan. (B) The demonstrated area dosed to 70 Gy shown by the orange line.
FIGURE 5.
(A) At 7 weeks postoperatively, positron emission tomography (PET)/CT scan demonstrated a fluorodeoxyglucose (FDG) avid rim-enhancing 1.5 × 1.3 cm area with a standardized uptake value (SUV) of 8.2. Although no biopsy was performed, the patient was suspected to have recurrent disease and was treated to 70 Gy. (B) The area treated to 70 Gy is demonstrated by the orange line. (C) Demonstrates imaging studies 3 months after completion of chemoradiation showing resolution of the FDG avidity. The patient remains without evidence of disease of last follow-up at almost 2 years.
Ten of these identified patients also received concurrent chemotherapy; 2 of these patients would not have received systemic therapy based on pathology of the resected specimen. The 2 patients who had negative biopsies outside the head and neck were among these 11 patients treated to higher doses in the head and neck. See Table 2 for full patient characteristics of these 14 patients with positive postoperative PET/CT scans.
TABLE 2.
Characteristics of 14 patients with positive postoperative positron emission tomography/CT scans.
| Patient no. | Sex | Age, y | Site | Ipsilateral neck dissection | Margin status | Stage | ECS | PNI | PET result | Management change | Status at last follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 46 | Lateral tongue | Levels 1–4 | Close (<1 mm) | T2N2b | Yes | Yes | Breast primary | No change in head and neck treatment. Treated to 66 Gy. Patient went on for breast treatment. | Alive with disease |
| 2 | M | 49 | Lateral tongue | Levels 1–5 | Positive | T3N2b | Yes | Yes | Resection margin: Levels 3–5 ipsilateral. Level 2 contralateral. |
Treated to higher dose of radiation with cetuximab (70 Gy) | Alive with disease |
| 3 | M | 56 | Lower gum | Levels 1–3 | Positive | T4N0 | N/A | No | Resection margin | Treated to higher dose of radiation with cisplatin (70 Gy). | Distant metastasis |
| 4* | M | 57 | Ventral tongue | Levels 1–4 | Negative | T1N2b | Yes | No | Uptake in mediastinum | Biopsy of mediastinum. Treated to higher dose of radiation. On protocol 08-138 (70 Gy). | No evidence of disease |
| 5*,† | M | 50 | Lateral tongue | Levels 1–4 | Close (0.3 mm) | T3N2b | No | Yes | Uptake at hyoid bone/ resection margin. | Treated to higher dose of radiation on protocol 10-081 (70 Gy). | No evidence of disease |
| 6† | F | 62 | Hard palate | Levels 1–3 | Close (4 mm) | T3N0 | N/A | Yes | Uptake in mediastinum | Biopsy of mediastinum. Treated to higher dose of radiation (70 Gy). | No evidence of disease |
| 7 | M | 72 | Lateral tongue | Levels 1–5 | Negative | T2N2a | No | Yes | Contralateral level 3 neck | Additional biopsy and surgery of neck (level 3). Standard postoperative doses. | No evidence of disease |
| 8 | F | 56 | Lateral tongue | Levels 1–4 | Negative | T1N2b | Yes | No | Ipsilateral level 1 neck | Additional biopsy and surgery of neck (level 1). Treated to higher dose of radiation on protocol 08-138 (70 Gy). | No evidence of disease |
| 9 | M | 84 | Ventral tongue | Levels 1–4 | Close (1 mm) | T2N0 | N/A | No | Uptake in a nodular area in left floor of mouth. Uptake in lung primary. | Treated to higher dose of radiation with carboplatin (70 Gy). | No evidence of disease |
| 10 | M | 44 | Lateral tongue | Levels 1–5 | Negative | T4N1 | Yes | Yes | Ipsilateral level 1 neck | Treated to higher dose of radiation with cisplatin (70 Gy). | Alive with disease |
| 11*,‡ | F | 60 | Oral tongue | N/A | Close (<0.6 mm) | rT2N1 | No | No | Focal uptake with associated soft tissue fullness and focal enhancement. | Treated to higher dose of radiation with cisplatin (70 Gy). | No evidence of disease |
| 12 | M | 76 | Oral tongue | Levels 1–5 | Close (<0.5 mm) | TxN1 | Yes | No | Uptake in soft tissue mass in submandibular area and ipsilateral neck. | Additional biopsy and surgery in the head and neck. Standard RT (66 Gy) with cisplatin after additional surgery. | Distant metastasis |
| 13§ | F | 36 | Buccal mucosa | Levels 1–4 | Negative | T3N2b | No | Yes | Uptake in area lateral to mandibular condyle. | Treated to higher dose of radiation (74 Gy) with cetuximab. | Local recurrence |
| 14†,§ | F | 72 | Oral tongue | Levels 1–4 | Close (<0.6 mm) | T3N2b | Yes | Yes | Uptake adjacent to flap SUV and at ipsilateral neck. Additional <1 cm lesions. | Treated to higher dose of radiation with cisplatin (70 Gy). | Distant metastasis |
Abbreviations: ECS, extracapsular spread; PNI, perineural invasion; PET, positron emission tomography; N/A, not applicable; RT, radiotherapy; SUV, standardized uptake value.
No preoperative PET/CT.
Contralateral neck levels 1–3 dissected.
Recurrent setting.
Received neoadjuvant chemotherapy.
Patients with abnormal PET/CT scans felt to require changes in treatment.
Margin status, PNI, and presence of ECE were correlated with positive findings on the postoperative PET/CT scan. None of these pathological risk factors were significantly associated with positive PET scans in our patient population (see Table 3). However, we identified that a longer time to simulation PET-CT was significantly correlated with a positive scan result (p = .01).
TABLE 3.
Pathological risk factors.
| Pathological characteristics | Odds ratio | p value |
|---|---|---|
| T classification T3/T4 | 2.07 | .31 |
| N classification | 2.11 | .48 |
| ECE | 4.66 | .11 |
| PNI | 0.96 | 1 |
| Margin status (negative vs close/positive) | 1.93 | .51 |
Abbreviations: ECE, extracapsular extension; PNI, perineural invasion.
The median follow-up for this patient group was 22 months (range, 0–58.1 months). Locoregional control, distant metastases, and overall survival rates for patients with positive and negative PET/CT scans are shown in Figure 6. The local control rate for patients with positive PET/CT scans was 76.9% for both 1 and 2 years. In comparison, patients with negative PET/CT scans had local control rates of 89.7% and 86.1% at 1 and 2 years, respectively. Neither of these were statistically significant (p = .39). Overall survival was also not statistically significant between the 2 groups. For patients with positive PET/CT scans, 1-year and 2-year survival rates were 75.5% and 64.7%, respectively. In comparison, the 1-year and 2-year survival rates for patients with negative PET/CT scans were 89.6% and 85.5%, respectively (p = .16). However, distant metastasis-free survival in patients with positive PET/CT scans was statistically different. Distant metastasis-free survival at 1 and 2 years was 61.5% and 53.8%, respectively, in patients with positive PET/CT scans compared to 92.7% at both years for patients with negative PET/CT scans (p = .01).
FIGURE 6.

Shown here are: (A) locoregional control rates; (B) distant failures; and (C) overall survival graphs.
DISCUSSION
We have previously demonstrated the utility of PET and MRI scans in delineation of target volumes for patients undergoing definitive chemoradiation for oropharyngeal cancers.17 With newer advances with PET/CT fusion technologies and using the same immobilization devices, PET-CT fusion with the CT-simulation has been shown to have significant impact on staging and planning of radiation.18 It is now standard in our department to perform PET/CT simulations for patients requiring definitive radiation treatments for locally advanced head and neck cancer malignancies.
However, the role of PET/CT imaging in the postoperative setting and in treatment planning has not been clearly established. Patients with oral cavity primary cancers are inherently at an increased risk of locoregional disease and may also harbor distant metastases. Many of these patients have almost immediately recurrent disease reflecting bad biology of the tumor that is at risk of further progression before the initiation of adjuvant treatment. Proper recognition of either of these scenarios is critical in treatment planning in the postoperative setting to deliver the appropriate radiation dose.
In our study, we identified 13 patients (30%) who were felt to have abnormal PET/CT scans in the head and neck area that warranted a change in management. This percentage of patients is similar to the results reported by Shintani et al14 and Liao et al15 in a similar patient population. Among these 13 patients, we identified 5 patients who underwent additional biopsy or surgery based on the PET/CT findings; 2 patients had negative biopsies in the mediastinum and 3 patients were found to have gross disease and required subsequent surgery in the head and neck.
Two patients had negative biopsies alluding to the relevant false-positive rates of postoperative PET/CT imaging. However, in both these patients, biopsies were of areas in the mediastinum and not in the operative bed where there may be a question of postoperative inflammatory changes. Identifying areas of distant disease outside of the head and neck may result in abandonment of adjuvant treatment. In the study by Shintani et al,14 the investigators identified 4 of 91 patients with widely metastatic disease and adjuvant radiotherapy was abandoned. However, only 1 of these patients had SCC, suggesting that metastatic disease development shortly after surgery is relatively uncommon in this patient population, provided adequate preoperative staging is performed.
Eleven patients were treated with higher doses of radiation with or without chemotherapy. Biopsy of the head and neck area with tissue confirmation of positive disease is not always feasible in all postoperative patients. It has been our practice to discuss the postoperative findings when worrisome with the multidisciplinary team before proceeding with an alternation of patient management. Of the remaining patients who were treated with higher doses of radiation, the radiological imaging was worrisome enough to warrant a change in management. Two patients who would not have otherwise received chemotherapy went on to receive concurrent chemoradiation because macroscopic disease was highly suspected.
Despite aggressive treatment with dose escalation to 70 Gy or higher to areas of PET positivity and the addition of chemotherapy, some patients still have local recurrence of their disease within a short interval after completing adjuvant or presumably definitive therapy. Although there was no statistically significant difference between patients who had positive or negative postoperative PET/CT scans, patients with positive scans were more likely to have recurrence earlier. These outcomes suggest that these tumors can be aggressive and patients may benefit from additional surgery if residual or recurrent disease is suspected at the time of simulation.
We attempted to estimate the positive predictive value (PPV) for postoperative PET/CT scanning. However, without tissue confirmation of the PET positive area, accurate calculation is not feasible. Instead, we used surrogate markers to determine a range of the PPV for postoperative PET/CT scans. Among the 13 patients who had a change in management of adjuvant therapy, we identified 3 patients who had gross disease who required additional surgery. In addition, 2 patients with close or positive margins had local recurrence of their disease, suggesting that the residual microscopic disease at the margin was accurately detected on PET/CT imaging, but not amenable to treatment intensification to effect local disease control. If we consider these 5 patients to be positive, then the PPV is at minimum 38% for a postoperative PET/CT scan.
Because an additional 5 patients who had close/positive margins remained disease free at the time of the last follow-up, it is not clear if they benefited from the additional treatments or received unnecessary escalated therapy. Above, we discussed the patient in Figure 5 who underwent surgery for a pT4 gingival cancer. The anterior resection margin of the mandible was positive and postoperative imaging demonstrated intense FDG uptake at the same area. If these clinicopathological and radiographic correlations are considered, the PPV is likely to be much higher than 38%. However, because biopsies were not performed on all FDG-avid areas, this number still must be interpreted in the background of a post-surgical setting where areas of inflammation and wound healing are likely to be detected on PET/CT imaging.
We correlated presence of PNI, ECE, nodal status, and presence of close or positive margins with PET positivity. We did not find significant correlation of the presence of PNI or ECE, nodal stage, or margin status with PET/CT scan results. However, this study was limited in its number of patients and is likely underpowered to detect significant correlations with these risk factors. Although we recommend all high-risk patients undergo postoperative imaging, patients with ECE may be at a higher risk and may benefit from PET/CT imaging based on a trend toward correlation and their inherent increased risk of disease recurrence. Although nearly 50% of patients in this cohort had positive or close margins, there may be a selection bias for this group because MSKCC’s role as a tertiary care referral center for challenging oral cavity cancer cases. In addition, patients who were felt to be at high risk for recurrence may have preferentially referred internally to our institution, possibly enhancing the close or positive margin rate in this group.
Timing of PET/CT scans was found to be statistically significant with patients undergoing scans at 8 weeks or more after surgery (p = .01). This finding corresponds with the well-known importance of package time in patients undergoing surgery for postoperative oral cavity cancers.1,2 Proper identification of these patients who have recurrent gross disease is critical to intensify treatment with higher radiation doses and add chemotherapy in an effort to achieve local control. Additional data are needed to determine if dose escalation can overcome delays in patients’ package time.
We also identified a patient who had a false negative PET/CT scan in this patient cohort. This patient with pT3N2b oral tongue SCC underwent PET/CT imaging 6.4 weeks after surgery. Subcentimeter lymph nodes with a standardized uptake value (SUV) of 2.7 were felt to be inflammatory. The patient was treated with radiation alone to a dose of 60 Gy. Chemotherapy was not recommended because of the patient’s medical comorbidities. Unfortunately, 3.5 months after completion of adjuvant radiation, follow-up imaging demonstrated progression of disease in the head and neck (see Figure 7). Although it is not known if the addition of chemotherapy or treating the patient to a higher dose would have prevented the locoregional recurrence, this patient illustrates the point that all patients with even borderline worrisome findings should be offered biopsy and possible dose intensification.
FIGURE 7.
This patient underwent resection for a pT3N2b oral tongue squamous cell carcinoma (SCC). Pathologically involved lymph nodes were noted in levels IIA and III of the left neck. (A and B) Postoperative positron emission tomography (PET)/CT scan at 6.4 weeks demonstrated mildly fluorodeoxyglucose (FDG)-avid left supraclavicular lymph nodes with a standardized uptake value (SUV) 2.7, measuring 0.8 × 0.6 cm. The patient was treated with radiation alone to a dose of 60 Gy. (C) Follow-up PET/CT at 3.5 months after completing radiotherapy that demonstrated a large FDG avid left supraclavicular lymph node consistent with disease progression.
In summary, this study suggests a benefit of PET/CT scanning in the postoperative setting before the initiation of adjuvant radiotherapy in patients with head and neck SCC of the oral cavity. We show that about 30% of patients had some type of alteration in management, including additional surgery, treating to higher radiation doses, and adding chemotherapy in selected patients. Future studies with longer follow up will be needed to assess both the oncologic and functional impact of this approach. We did not identify any pathological risk factors that were significantly associated with positive postoperative PET/CT scans; however, the lack of correlation is likely related to the limited patient numbers. Likewise, although patients with positive PET/CT scans had worse local control and overall survival, these outcome measurements were not found to be statistically significant. Nonetheless, we suggest that patients with oral cavity SCC may benefit from preoperative imaging with a PET/CT scan to ensure that the appropriate surgical management is performed. Patients with high-risk features who require adjuvant radiotherapy may benefit from PET/CT imaging after surgery and before radiation treatment planning, as adjuvant treatment in these patients may be altered depending on these findings. Biopsies of suspected areas of disease should be performed and managed with additional surgery or higher doses of radiation with chemotherapy when appropriate. Patients undergoing adjuvant treatment longer than 8 weeks from surgery are at increased risk of harboring recurrent disease. We recommend PET/CT imaging in these patients who were statistically more likely to have positive findings worrisome for recurrent disease and who therefore may benefit the most from repeat surgery or dose escalation above standard treatment.
References
- 1.Ang KK, Trotti A, Brown BW, et al. Randomized trial addressing risk features and time factors of surgery plus radiotherapy in advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;51:571–578. doi: 10.1016/s0360-3016(01)01690-x. [DOI] [PubMed] [Google Scholar]
- 2.Peters LJ, Goepfert H, Ang KK, et al. Evaluation of the dose for postoperative radiation therapy of head and neck cancer: first report of a prospective randomized trial. Int J Radiat Oncol Biol Phys. 1993;26:3–11. doi: 10.1016/0360-3016(93)90167-t. [DOI] [PubMed] [Google Scholar]
- 3.Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350:1945–1952. doi: 10.1056/NEJMoa032641. [DOI] [PubMed] [Google Scholar]
- 4.Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350:1937–1944. doi: 10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz GJ, Mehta RH, Wenig BL, Shaligram C, Portugal LG. Salvage treatment for recurrent squamous cell carcinoma of the oral cavity. Head Neck. 2000;22:34–41. doi: 10.1002/(sici)1097-0347(200001)22:1<34::aid-hed6>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 6.Mac Manus MP, Hicks RJ. The role of positron emission tomography/computed tomography in radiation therapy planning for patients with lung cancer. Semin Nucl Med. 2012;42:308–319. doi: 10.1053/j.semnuclmed.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Wang YC, Hsieh TC, Yu CY, et al. The clinical application of 4D 18F-FDG PET/CT on gross tumor volume delineation for radiotherapy planning in esophageal squamous cell cancer. J Radiat Res. 2012;53:594–600. doi: 10.1093/jrr/rrs009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zygogianni A, Kyrgias G, Kouvaris J, Pistevou–Gompaki K, Kouloulias V. A new role of PET/CT for target delineation for radiotherapy treatment planning for head and neck carcinomas. Hell J Nucl Med. 2012;15:139–143. [PubMed] [Google Scholar]
- 9.Schöder H, Yeung HW, Gonen M, Kraus D, Larson SM. Head and neck cancer: clinical usefulness and accuracy of PET/CT image fusion. Radiology. 2004;231:65–72. doi: 10.1148/radiol.2311030271. [DOI] [PubMed] [Google Scholar]
- 10.Branstetter BF, IV, Blodgett TM, Zimmer LA, et al. Head and neck malignancy: is PET/CT more accurate than PET or CT alone? Radiology. 2005;235:580–586. doi: 10.1148/radiol.2352040134. [DOI] [PubMed] [Google Scholar]
- 11.Gomez DR, Zhung JE, Gomez J, et al. Intensity-modulated radiotherapy in postoperative treatment of oral cavity cancers. Int J Radiat Oncol Biol Phys. 2009;73:1096–1103. doi: 10.1016/j.ijrobp.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 12.Zimmer LA, Branstetter BF, Nayak JV, Johnson JT. Current use of 18F-fluorodeoxyglucose positron emission tomography and combined positron emission tomography and computed tomography in squamous cell carcinoma of the head and neck. Laryngoscope. 2005;115:2029–2034. doi: 10.1097/01.MLG.0000181495.94611.A6. [DOI] [PubMed] [Google Scholar]
- 13.Rosenthal DI, Liu L, Lee JH, et al. Importance of the treatment package time in surgery and postoperative radiation therapy for squamous carcinoma of the head and neck. Head Neck. 2002;24:115–126. doi: 10.1002/hed.10038. [DOI] [PubMed] [Google Scholar]
- 14.Shintani SA, Foote RL, Lowe VJ, Brown PD, Garces YI, Kasperbauer JL. Utility of PET/CT imaging performed early after surgical resection in the adjuvant treatment planning for head and neck cancer. Int J Radiat Oncol Biol Phys. 2008;70:322–329. doi: 10.1016/j.ijrobp.2007.06.038. [DOI] [PubMed] [Google Scholar]
- 15.Liao CT, Fan KH, Lin CY, et al. Impact of a second FDG PET scan before adjuvant therapy for the early detection of residual/relapsing tumours in high-risk patients with oral cavity cancer and pathological extracapsular spread. Eur J Nucl Med Mol Imaging. 2012;39:944–955. doi: 10.1007/s00259-012-2103-2. [DOI] [PubMed] [Google Scholar]
- 16.Edge SB, Byrd DR, Compton C, Fritz AG, Greene FL, Trotti A III, editors. AJCC Cancer Staging Manual. 7. New York, NY: Springer; 2010. [Google Scholar]
- 17.Thiagarajan A, Caria N, Schöder H, et al. Target volume delineation in oropharyngeal cancer: impact of PET, MRI, and physical examination. Int J Radiat Oncol Biol Phys. 2012;83:220–227. doi: 10.1016/j.ijrobp.2011.05.060. [DOI] [PubMed] [Google Scholar]
- 18.Koshy M, Paulino AC, Howell R, Schuster D, Halkar R, Davis LW. F-18 FDG PET-CT fusion in radiotherapy treatment planning for head and neck cancer. Head Neck. 2005;27:494–502. doi: 10.1002/hed.20179. [DOI] [PubMed] [Google Scholar]