Abstract
Background
Platelet rich plasma (PRP) consists of platelet derived growth factor (PDGF) and Transforming growth factor-beta (TGF-β) that increase cell proliferation of mesenchymal stem cells (MSCs), whereas, bone morphogenic Protein-2 (BMP2) promotes osteogenic differentiation of MSCs. However, the high degradation rate of fibrin leads to the dissociation of cytokines even before the process of bone regeneration has begun. Hence, for the first time, we studied the combined effect of sustained released PRP from alginate beads on BMP2 modified MSCs osteogenic differentiation in vitro and of sustained PRP alone on a fracture defect model ex vivo as well as its effect on the calvarial suture closure.
Methods
After optimizing the concentration of alginate for the microspheres, the osteogenic and mineralization effect of PRP and BMP2 in combinations on MSCs was studied. A self-setting alginate hydrogel carrying PRP was tested on a femur defect model ex-vivo. The effect of PRP was studied on the closure of the embryonic (E15) mouse calvaria sutures ex vivo.
Results
Increase of PRP concentration promoted cellular proliferation of MSCs. 2.5%–10% of PRP displayed gradually increased ALP activity on the cells in a dose dependent manner. Sustained release PRP and BMP2 demonstrated a significantly higher ALP and mineralization activity (p<0.05). The radiographs of alginate hydrogel with PRP treated bone demonstrated a nearly complete healing of the fracture and the histological sections of the embryonic calvaria revealed that PRP leads to suture fusion.
Conclusions
Sustained release of PRP along with BMP2 gene modified MSCs can significantly promote bone regeneration.
Keywords: Platelet rich plasma, BMP-2, Mesenchymal stem cells, periodontal regeneration, bone regeneration, Alginate
Bone regeneration is a complicated and well-orchestrated physiological process of bone formation (1). Since periodontitis, trauma, tumor and other bone conditions involve the loss of alveolar bone, regeneration of the lost hard tissue is of primary importance (2). Presently, there is a surfeit of various strategies to repair the impaired bone-regeneration process, which includes the autologous bone graft, allograft implantation, and use of cytokines and other proteins (3–5). Most of the materials that are being presently employed in the field of bone engineering exhibit relatively satisfactory results (6). However, there are associated drawbacks and limitations including the donor site morbidity, the need for second surgery and limited availability in case of the autogenous bone grafts, as well as increased risk of immune rejection as far as autologous bone grafts are concerned and increased costs in terms of xenografts and synthetic bone grafts (hydroxyapatite, bioglass) (7). Therefore, there is a need to develop innovative treatments as alternatives or adjuncts to the standard methods used for bone regeneration (8).
Recently, Platelet rich plasma (PRP) has been extensively studied for its regenerative potential in Periodontics (9, 10). PRP is a blood derivative that is abundant in cytokines with more than 15 different factors including platelet derived growth factor (PDGF including PDGFBB,- AB und -AA isoforms), transforming growth factor-beta (TGF-beta), platelet factor 4 (PF4), interleukin 1 (IL-1), platelet-derived angiogenesis factor (PDAF), vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), platelet-derived endothelial growth factor (PDEGF), epithelial cell growth factor (ECGF), insulin-like growth factor (IGF), osteocalcin (Oc), osteonectin (On), fibrinogen (Fg), vitronectin (Vn), fibronectin (Fn) and thrombospontin-1(TSP-1), which are essential for bone regeneration and vascularization (11, 12) The reason that makes PRP a suitable candidate as an adjunct to bone formation in the treatment of Periodontitis is its ease of isolation, handling and storage properties, and its ability to reduce inflammation along with being cost effective(13, 14). PRP can also be derived from the same patient thus eliminating the risk of disease transmission(15). However, the disadvantage of the use of PRP is its early dissolution, thus leading to the exhaustion of the cytokines, even before the process of bone formation has occurred, which makes it necessary to deliver PRP via an agent that degrades slowly as well as releases PRP cytokines in a sustained manner(14, 16).
Alginate microspheres can readily form gels under mild conditions upon exposure to divalent cations, hence providing an aqueous environment for increased transportation to the localized site in a sustained dosage form (17). Additionally, they are semi-permeable which enables the adherence of cells to it thus resulting in good proliferation and differentiation. Furthermore, alginate gel-spheres enable high diffusion rates of macromolecules, which can be controlled to diffuse from the gel-microspheres at a high speed(18). Thus, alginate is biocompatible and has a slow degradation rate and good crosslinking property. This makes it a suitable agent to deliver PRP to the affected region (19).
Growth factors play crucial roles in bone regeneration. One that has been extensively employed for periodontal regeneration is BMP2 (20). BMP2 can efficiently promote osteogenic differentiation of stem cells (21, 22). However, BMP’s short biological half-lives, localized actions and rapid local clearance interfere during the process of bone regeneration clinically and its consistent release is still a challenge (23, 24). Hence, BMP2 genetically modified MSCs may prove to be of immense use due to its stabilized effect in the process of bone formation.
The aim of this paper was to investigate the effect of sustained release of PRP on BMP2 modified MSCs osteogenic differentiation in vitro and ex vivo on the wound healing of a fracture defect model as well as its effect on the calvarial suture closure.
MATERIALS AND METHODS
The animal experiments were approved by the IACUC ethics committee of the University at Buffalo.
PRP Preparation
PRP was prepared by a modification of the method of Landesberg et al (25). See supplementary Appendix 1 in the online Journal of Periodontology for the detail.
Fabrication and Degradability of Prp and Prp-Alginate Microspheres
A protocol developed by Lu et al was used for the PRP-alginate microspheres fabrication (26). Briefly, PRP was added to 1% alginate solution made from Sodium alginate (Sigma)**. The mixture was then dispensed via a syringe needle (26½-gauge) into 6% CaCl2 ††. The PRP alginate mixture was set by the diffusion of Ca2+ ions into the polymer mixture. After setting, the beads were incubated in CaCl2 solution for 5 min to complete the setting process. In order to maintain the same concentration of PRP in the beads, the beads were dissolved in 10% Sodium citrate solution by incubating the beads in it for one hour. The released platelets were then counted using a hemocytometer/neubauers chamber. Three different types of microspheres were fabricated; 1) Alginate microspheres only; 2) Alginate microspheres incorporating PRP; 3) PPP incorporating PRP (N=3) and the weight of the spheres were assessed using a weighing instrument at various time series of day 0, 7 and 14 respectively.
Quantification of Growth Factor Release (Pdgf) by Elisa
The supernatant concentrations of PDGF-AA, AB, and BB released from the alginate and Platelet poor plasma (PPP) beads were determined by Enzyme-Linked Immunosorbent Assay‡‡ following the manufacturer's instructions. See supplementary Appendix 1 in the online Journal of Periodontology for the detail.
Preparation and Culture of Mscs
Preparation and culturing MSCs was performed as previously described (27). See supplementary Appendix 1 in the online Journal of Periodontology for the detail.
BMP2 Gene Transfer
BMP2 adenovirus was generated and titered as previously described (27). For the transfection of MSCs, Ad-BMP2 adenovirus with serum-free media was added to MSCs. After 4 hours (h), serum was added to a final concentration of 2%, and cells were cultured for an additional 24 h. Cells were then cultured in osteogenic media (OS media is alpha-MEM medium§§, supplemented with 10% fetal bovine serum§§, L-glutamine (2 mmol/L), and penicillin/Streptomycin (100 U/ml), 50 μg/ml ascorbic acid, 10−8 M dexamethasone and 10 mM sodium β-gylcerolphosphate). See s supplementary Appendix 1 in the online Journal of Periodontology for the detail.
Cell Viability Assay
The cell viability was assayed using MTS cell viability assay kit‖‖ for optimization of the alginate microspheres concentration. There were four groups: 0, 0.5, 1 and 1.5% alginate. Experiments were conducted at a cell density of 4,000cells/cm2. Groups of MSC, MSC/BMP2, MSC + PRP, MSC/BMP2 + PRP and MSC, MSC + PRP (1%), MSC + PRP (2.5%), MSC + PRP (5%), MSC + PRP (10%) were evaluated for cell proliferation. After being induced with OS media for 2 days, the cells were incubated with 100 μl OS media and 20 μl MTS assay reagent for an additional 3 h. Finally, the supernatants were transferred to a new 96 well plate for recording the absorbance at 490 nm using a microplate reader.
Alkaline phosphatase activity (alp) assay and alizarin red assay
ALP activity was determined by using ALP assay kit (Sigma, Cat no: 245-325-0) †† following the manufacturer's instructions as described previously (22). The cells in the four groups of MSCs, MSCs/BMP2, MSCs + PRP (immediate) and MSCs/BMP2+PRP (immediate), and in the six groups of MSCs, MSCs/BMP2, MSCs + PRP (immediate), MSCs + PRP (sustained), MSCs/BMP2+PRP (immediate), MSCs/BMP2+PRP (sustained) were respectively cultured in OS media for 2 days and 7 days for ALP activity analysis. For the immediate release PRP experiments, 2.5% PRP was delivered to the cells one time only via the media on the first day. The sustained release of PRP experiments involved delivering 2.5% PRP every other day for 7 days.
To investigate whether the combination of BMP2 modified MSC with PRP in an alginate carrier affects the osteogenic differentiation and mineralization, the cells in groups of MSC(M), MSC + ALGINATE BEADS (M +AL), MSC + PRP (M + PRP), MSC + PRP (ALGINATE BEADS) (M+ PRP(AL)), MSC + BMP2(M/B2), MSC + BMP2(ALGINATE BEADS) (M/B2+AL), MSC/BMP2 + PRP (M/B2 + PRP),MSC/BMP2 + PRP(ALGINATE BEADS) (M/B2 + PRP(AL)) were plated in a 24-well plate and were supplemented with OS media for 7 days for ALP assay and 21 days for alizarin red assay. Alizarin Red S staining and calcium mineral content quantitation were performed as described previously (28) See supplementary Appendix 1 in the online Journal of Periodontology for the detail.
Hemocompatibility Test
The hemocompatibility experiments were conducted as previously described (29). Before conducting the test, anti-coagulated blood with 3.2% sodium citrate was used for this test,. The equilibrated alginate hydrogel was first equilibrated with saline solution (NaCl, 0.9 w/v at 37 °C for 24 h), and then transferred into a microcentrifuge tube with1 ml of PBS (pH 7.4) and incubated for 72 h. PBS was then, removed and 1 ml of 3.2% sodium citrate was added to each sample and incubated at 37 °C for 3 h. Positive and negative controls were prepared by adding the same amount of 3.2% sodium citrate to 1 ml of distilled water and PBS, respectively. Each tube was incubated and gently inverted twice every 30 min to guarantee the continuous contacting between alginate hydrogel and 3.2% sodium citrate blood. The fluids were transferred to a proper tube then centrifuged at 3000 rpm for 20 min. The hemoglobin released by hemolysis calculation by determination the optical densities (OD) of the supernatant. The supernatant was taken and its absorbance was monitored at 540 nm using a spectrophotometer.
Ex-Vivo Bone Regeneration in Fracture Defect Model
A femur defect model was then generated as described previously with slight modification(30). Briefly, three wild type C57BL/6 four-week old mice were euthanized by CO2. The femur bone was isolated. Using the rongeur forceps, a 2 mm in width fracture defect was created in the middle of the femur shaft (n=6). 6% CaCl2 was aspirated and added drop by drop into a 1% alginic acid solution with or without 10% PRP (1 × 106 platelets/μl), and then incubated for 15 min in order to increase the microsphere strength. The CaCl2 – Alginate crosslinked microspheres were then incorporated into 5% alginic acid solution and were aspirated into a syringe. The microspheres were distributed equally and 3ml of 5% alginic acid contained 50 ± 10 microspheres. The solution was then pushed through the syringe resulting into the bursting of the beads and release of CaCl2 into the alginate solution, thus forming an alginate crosslinked hydrogel. Only alginate hydrogel (5%) was delivered to the control group, however, alginate gel (5%) incorporating PRP (50μl) was delivered to the fractured site area via a syringe, and then the femurs were placed in an OS media and cultured for 7 days. Pictures were obtained of the region of the fracture using a Nikon 85mm micro VR lens. A radiograph was obtained of the control and experimental samples in order to assess the repair of the fracture defect.
Effect of Prp on Cranial Sutures Obliteration (Coronal Suture and Sagittal Suture)
One wild type C57BL/6J eight-week old mouse was euthanized by CO2. The six embryos were then isolated from the uterus at embryonic day 15 (E15). To evaluate the effect of the Dura mater on suture closure, the Dura mater beneath the sagittal suture area was removed and cultured for 5 days in OS media supplemented with or without 150μl of PRP (1 × 106) platelets/μl (10%) in a humidified atmosphere of 5% CO2 at 37°C. After 5 days, calvaria were harvested, fixed and embedded in paraffin wax and sectioned to a thickness of 7 microns for hematoxylin and eosin staining (H & E).
Statistical Analysis
Statistical analysis was performed using 17.0 v. IBM SPSS. Where indicated, experimental data were reported as Mean ± SD of triplicate independent samples or indicated sample numbers. Data were analyzed using one-way analysis of variance (ANOVA), and Tukey's HSD test was applied as a post hoc test if statistical significance was determined. A value of p≤0.05 was considered statistically significant
RESULTS
Centrifugal Speed and Time Affects Platelet Efficiency and Alginate Controllably Releases Prp
The physics of the centrifugation process, time and acceleration are the fundamental parameters that define the composition of PRP sample (31). Studies have demonstrated that the concentrated WBCs within PRP can cause inevitable detrimental effects, which could disrupt the process of bone healing (32–34). To optimize the preparation of PRP with minimum white blood cells (WBCs), we evaluated the effect of centrifugation time at a low spin setting with respect to the concentration of WBC in the upper layer. The results showed that the highest platelet efficiency with decreased concentrations of WBC was established at 10 minutes as compared to the other time groups (Fig. 1A). The recovery efficiency of platelets increased as the centrifugal acceleration increased from 100 to 150 ×g and peaked at 150×g. It began to decrease at 200g (Fig. 1B). The highest recovery efficiency of PRP at 150g can be attributed to the effect of rouleaux formation, which increased the sedimentation rate, provided a more porous packing of red blood cells in the lower layer, and thus increasing the recovery volume of platelets.
Figure 1.
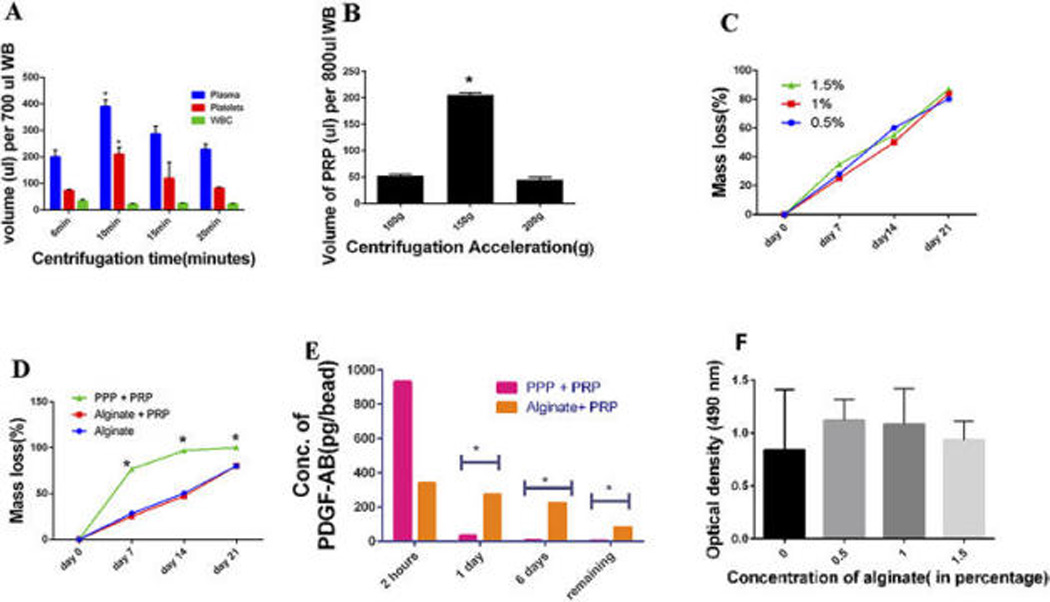
(A) Comparison between the volumes of PPP, PRP, and leucocytes in the upper layer after the first spin step of 150 ×g for 5, 10, 15 and 20 min. Volume of Whole Blood (WB):700 uL. (B) Recovery volume of PRP after the first spin step of WB (800uL) at centrifugal acceleration from 100 to 200 ×g for 10 min (N=3). (C) The formula for mass loss % was calculated as follows: Mass loss % = Original weight – weight after the estimated time period/ Original Weight. The mass loss (%) was assessed for the concentration of 0.5, 1 and 1.5% of alginate. There was no significant difference between the groups (p>0.05). Data represent the mean + SD of N= 3 samples. (D) The degradation rate for the group of alginate microsphere only, Alginate microsphere incorporating PRP and PPP incorporating PRP was assessed at 4 time points. The degradation rate of PPP as compared to Alginate with PRP and alginate alone was significantly higher (p<0.05).There was no difference in the degradation rates of Alginate containing PRP and Alginate alone. Data represent the mean + SD of N= 3 samples. (E) ELISA Assay: The concentration of PDGF was evaluated using an ELISA assay after samples were collected at various time points of 2 hours, 1 day and 6 days and the evaluation of the remaining growth factor where N=3 for the group of Alginate microsphere incorporating PRP and PPP incorporating PRP. PDGF release from the Alginate containing PRP was significantly higher than the PPP + PRP group and demonstrated a consistent release at various time points as compared to the PPP + PRP beads alone. (p<0.05). (F) MTS assay: Optimization of the concentration of the Alginate Microspheres; Effect of the concentration of Alginate Microspheres at 0, 0.5, 1 and 1.5% respectively at a cell seeding density of 4,000 cells/cm2. There was no significant difference in the effect of the various concentrations of alginate on cell viability at 4,000cells/cm2 (p>0.05)
After fabricating the alginate–PRP beads, we observed the bead shape and measured the diameter of the beads. The results showed that the fabricated alginate-PRP beads were uniformly shaped, with an average diameter of 1.9 ± 0.06 mm and an average volume of 2.5 ± 1.0 μL PRP was incorporated. PRP was uniformly distributed in the carriers, with the platelets found throughout the bead matrix, and enclosed within the beads. The intense red coloration of PRP within the carriers was prominent post fabrication and diminished thereafter. Matrix integrity was maintained throughout the 3-week study period. Platelet poor plasma (PPP) incorporating PRP displayed the fastest degradation rate at 7 days, while alginate incorporating PRP showed the slowest degradation rate (p<0.05). However, the various concentrations of alginate had relatively similar degradation rates (Fig. 1C)., with complete degradation occurring by 3 weeks (Fig. 1D). Hence, we decided to employ 1% alginate as a carrier for PRP for the rest of our studies. To study the growth factor release from the beads, the temporal release of PDGF from alginate and PPP were measured. As shown in (Fig. 1E) the release of PDGF from the alginate beads was consistent over a 6-day period when compared to the PPP carrier, which displayed negligible amount of PDGF by day 6 (p<0.05). There was 90 pg of PDGF remaining in the bead on day 6 ( Fig. 1E).
To investigate the safety and biocompatibility of the alginate microspheres, MTS cell viability assay was performed using various concentrations of alginate at a cell density of 4,000 cells/cm2. The results showed no significant difference in cell viability among the three concentrations of alginate, indicating that alginate does not affect cell viability (Figs. 1F).
Prp can Promote MSC Cell Proliferation
In order to optimize and characterize the effect of PRP on the MSCs, the cells were cultured with different concentrations of PRP (2–10%). Cell proliferative activity result showed a significant PRP-dose dependent increase (Fig. 2A). Additionally, groups of MSC, MSC + PRP, MSC/BMP2 and MSC/BMP2+PRP were tested for their cell proliferative activity. The result showed a significantly higher proliferative activity in MSC + PRP compared to the other three groups (p<0.05) (Fig. 2B).
Figure 2.
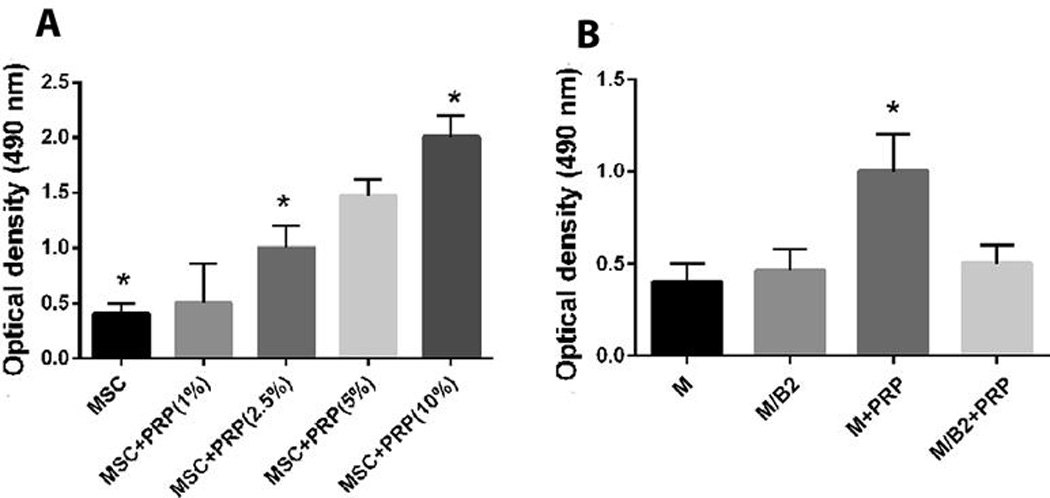
(A) Cell Proliferation activity of the various groups of MSC, MSC + PRP (1 %), MSC + PRP (2.5%), MSC + PRP (5%), MSC + PRP (10%) at a cell seeding density of 1×104 per well in a 96- well plate. Significant difference from the group of MSC vs MSC + PRP (2.5), MSC + PRP(2.5) vs MSC + PRP (10%), MSC vs MSC + PRP(10%) is indicated by * (p <0.01). (B) Cell Proliferation activity of the various groups of MSC, MSC/BMP2, MSC + PRP, MSC/BMP2 + PRP. The cellular proliferation activity of MSC + PRP was significantly higher than the rest of the groups as indicated by * (p<0.01). There is no difference among other three groups.
Sustained Release of Prp Increases Alp Activity
As shown in Fig. 3A, after 2 days following OS media stimulation, the combination of BMP2 modified MSCs with or without PRP dramatically promoted ALP activity compared to the groups without BMP2. ALP activity in MSCs/BMP2+PRP (immediate) is significantly higher than MSCs/BMP2 on day 2. In contrast, after 7 days following OS media (with 2.5%PRP in several doses) stimulation, the group of MSCs/BMP2+PRP (sustained) demonstrated a 0.35-fold higher ALP activity than that of MSCs/BMP2+PRP (immediate) group (N=3) *p<0.001. Moreover, there is significant difference between MSCs/BMP2+PRP (sustained) and MSCs/BMP2 (Fig. 3B) (p<0.05), suggesting that sustained release of PRP can more effectively promote osteoblast differentiation than fast release of PRP.
Figure 3.
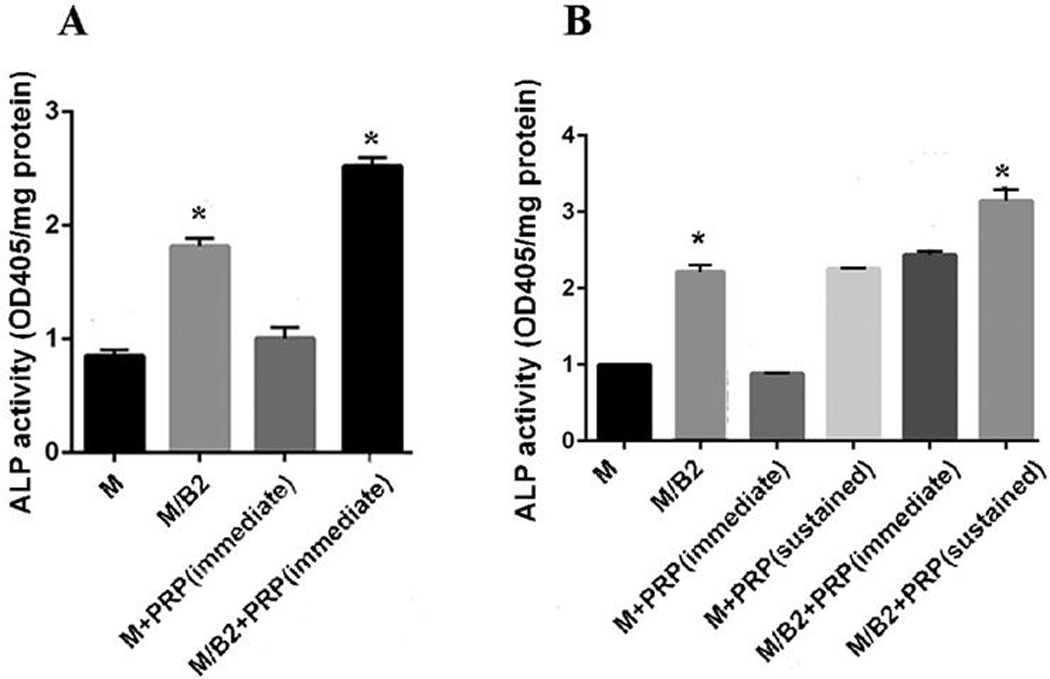
(A) Groups of MSC, MSC/BMP2, MSC + PRP (immediate) and MSC/BMP2 + PRP (immediate) were evaluated for their ALP Activity on day 2. MSC/BMP2 + PRP (immediate) demonstrated significantly higher ALP activity as compared to the other groups on day 2 (p<0.05). (B) Groups of MSC, MSC/BMP2, MSC + PRP (immediate), MSC+ PRP (sustained), MSC/BMP2 + PRP (immediate) and MSC/BMP2 + PRP (sustained) were evaluated for their ALP Activity on day 7. The group of MSC/BMP2 + PRP (sustained) demonstrated significantly higher ALP activity as compared to other groups* (p<0.05).
Alginate Mediated Controlled Release of Prp Promotes Osteoblast Differentiation and Mineralization of Mscs in Vitro
M/B2 + PRP (AL) demonstrated significantly higher ALP activity (Fig. 4A) as compared to the other groups (p<0.05). This effect was also observed in alizarin red assay, where M/B2 + PRP(AL) demonstrated the highest mineralization activity, confirming that sustained release of PRP can promotes osteogenic differentiation and mineralization of MSCs in the presence of BMP2 (Figs. 4B and 4C).
Figure 4.
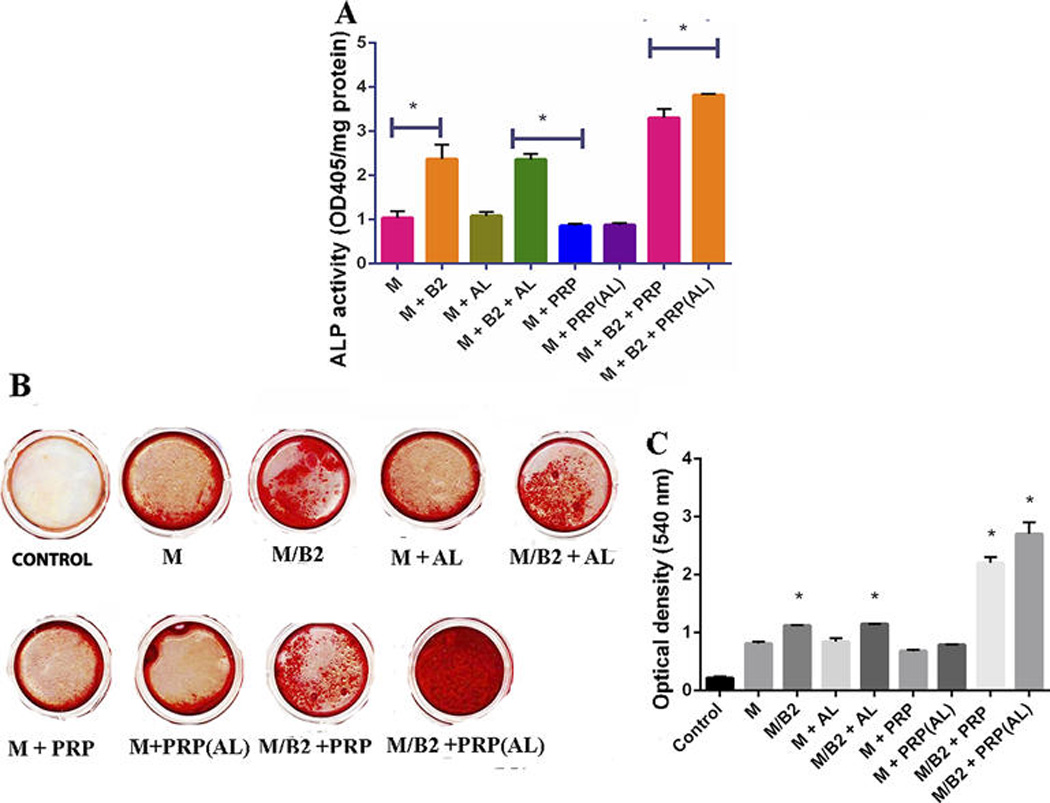
ALP Assay and Alizarin red assay: (A) The groups of MSC (M), MSC/BMP2( M/B2), MSC + ALGINATE BEADS(M + AL), MSC/BMP2 + ALGINATE BEADS(M/B2 + AL), MSC + PRP (M + PRP), MSC + PRP(ALGINATE BEADS) (M + PRP(AL)), MSC + BMP2 + PRP (M/B2 + PRP) and MSC/BMP2 + PRP(ALGINATE BEADS) ( M/B2 + PRP(AL)) were assessed for their ALP activity. The cells were induced with OS media for 7 days for ALP assay. Data represent the mean + SD of N= 3 samples. The ALP activity of the group of M/B2 + PRP (AL) was significantly higher than the other groups as marked as * (p<0.05). (B and C) Alizarin red: qualitative and quantitative: Cells were cultured in OS medium with 9 different groups (N=3). Control group: the empty wells were treated with OS medium. The groups of MSC vs MSC/BMP2, MSC vs MSC/BMP2 +ALGINATE, MSC vs MSC/BMP2 + PRP AND MSC vs MSC/BMP2 + PRP(ALGINATE) demonstrated a significant difference (p<0.05) as indicated by * on the graph.
Alginate Controlled Release of Prp Promotes Bone Regeneration for Bony Defects Ex-Vivo
To test the effect of alginate hydrogel with PRP on bone formation, we created femur bone fracture defect model(30). In order to increase the stiffness of the hydrogel, the percentage of alginate had to be increase (35). Hence, we first tested if the various concentrations of alginate were compatible with blood. The in vitro (murine) blood compatibility of the microspheres and gel scaffold was determined. As results of hemolysis test, the membrane materials were classified into three types according to their hemolytic index as follows: (a) hemolytic materials have hemolysis (%) >5%, (b) slightly hemolytic materials have hemolysis (%) between 2% and 5%, and (c) non-hemolytic materials have hemolysis (%) <2%. According to the classification of hemolytic tendency of polymeric materials, (American Society for Testing and Materials, 2000) F 756-00 (36), it is evident that the hemolysis values range from 1.16–1.24% with increasing alginate contents, which indicate a good compatibility of the microspheres and hydrogel scaffold. These results support the fact that alginate is highly hydrophilic and biocompatible polymeric material (see supplementary Table 1 in the online Journal of Periodontology).
To test the effect of increasing concentrations of PRP (2–10%) on osteogenic activity of MSCs. MSCs were cultured along with the various concentrations of PRP and supplemented with OS media for 7 days. The results depicted an increase in ALP activity with increasing concentrations of PRP with 10% PRP generating the highest ALP activity (Fig. 5A). Therefore, 10% PRP was chosen for the ex vivo femur fracture model. The pictures (N=3) of the specimens were then obtained and the defect width was measured and an independent t test statistical analysis was performed (Fig. 5B). Radiographic images were also obtained of the specimens. The control group results demonstrated a 15% gap fill (Figs. 5C and 5D) as compared to the experimental group, which demonstrated 75% gap fill. N=3, (p<0.05) (Figs. 5E and 5F).
Figure 5.
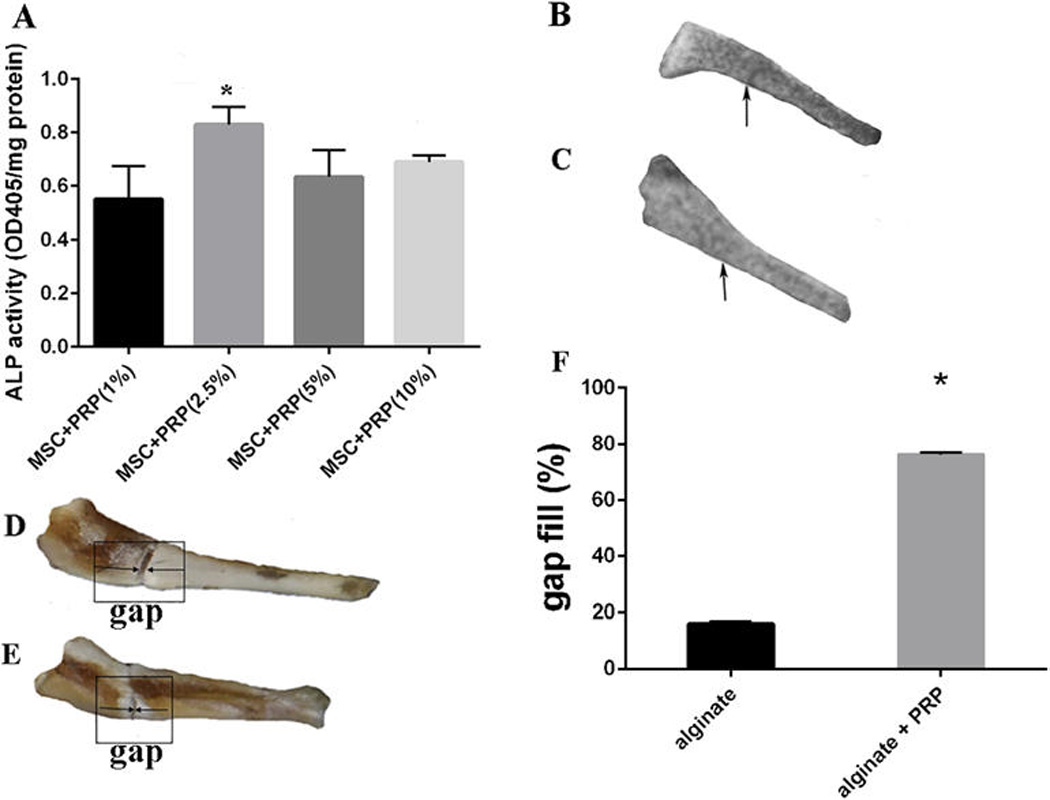
(A) Groups of MSC+PRP (1%), MSC+PRP (2.5%), MSC+ PRP (5%) and MSC + PRP (10%) were assessed for the optimization of PRP concentration. MSC + PRP (2.5%) demonstrated significantly higher ALP activity (N=3) as compared to the other three groups denoted by * (p<0.05). Effect of PRP on a 2 mm mouse femur defect: The gap fill % was calculated using the formula of Gap fill% = Original gap between the bone edges – gap between the bone edges post healing period × 100 Original gap between the bone edges. (B–C) Radiograph depicting complete closure of the defect in the alginate with PRP group (B), whereas the control group still displays the defect (C). (D–E): Images of femur defects with (D) or without PRP treatment (E). (F) Quantitative analysis of gap fill of the defect. 75% of the gap fill of the defect in the group containing alginate gel with PRP and 15% of gap fill was observed in the alginate only group. p<0.05.
Prp Promotes Calvarial Suture Closure
To study the effect of PRP generated from the dura mater on suture closure, we removed dura mater from calvarial explants of E15 mice and applied PRP to the OS media. After 5 days in culture, the widths of the sagittal sutures in PRP-treated explants closed more rapidly compared to the controls. The PRP cultured embryonic calvarias gross specimen depicted fusion of the sagittal suture (Fig. 6B) but not in the control group (Fig. 6A). The H&E stained histological sections of calvaria demonstrated overlapping of the coronal bone plates as well as the parietal bone plates, thus depicting a closure of the coronal and sagittal suture (Figs. 6D and 6F). However, control group has no apparent closure of the suture (Figs. 6C and 6E).
Figure 6.
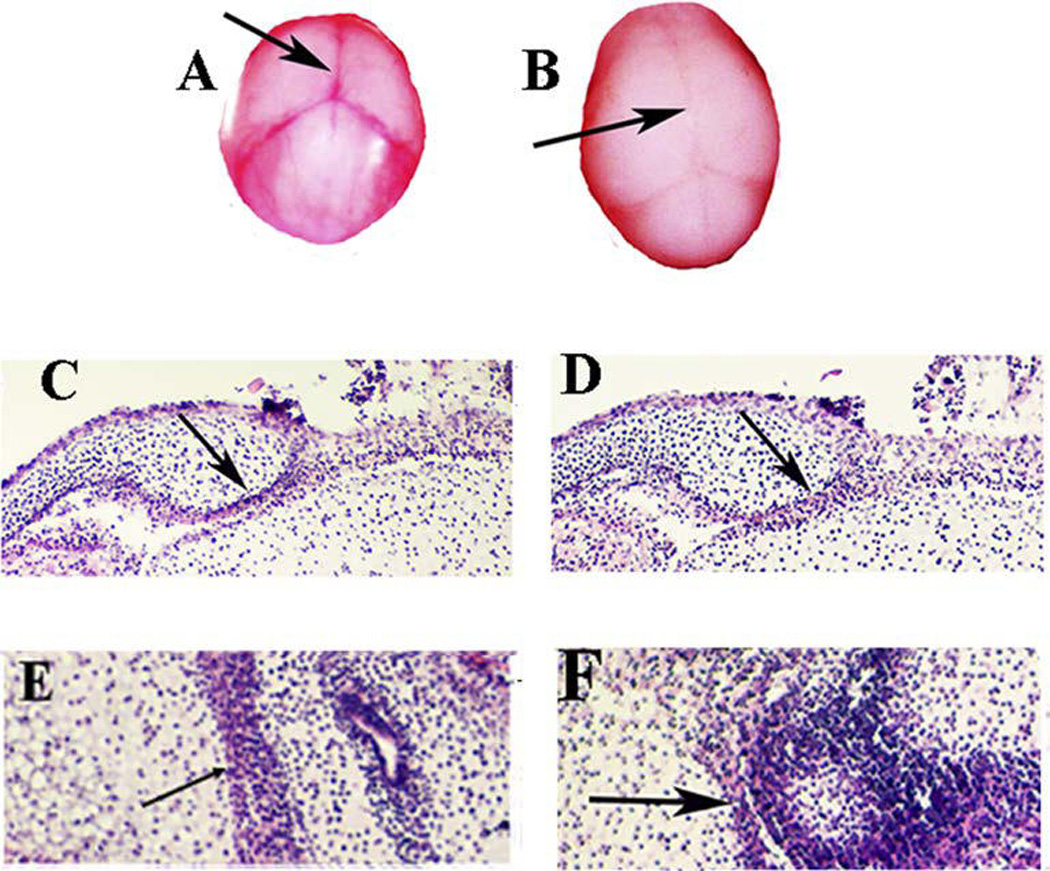
Effect of PRP on mouse calvarial suture closure (N=3): (A–B) Calvaria of the mouse were cultured for 5 days for the control group and the group with PRP (150ul) in the OS media (500uL). The group with the PRP shows an approximation of the sutures (Nikon micro VR 85mm) (B), whereas the group without the PRP does not demonstrate any suture closure (A). Histological section (coronal section (C and D) and transverse section (E and F) stained with H & E: The PRP groups showed an approximation of the coronal suture and the sagittal suture (D and F) and the control group failed to do so (C and E).
DISCUSSION
Studies have suggested that PRP can significantly increase MSC proliferative activity but inhibit osteogenic differentiation activity (37). In order to overcome this, BMP2 which acts by driving MSCs towards an osteogenic lineage was employed in this study (38). We have previously reported the significantly high rates of BMP2 on MSCs osteogenic differentiation and proliferation (22). Hence, for the first time in this study, our results demonstrated that controlled release of PRP in alginate beads along with BMP2 modified MSCs could significantly promote osteogenic differentiation, cellular proliferation and mineralization activity in vitro and that slow released PRP in alginate beads could accelerate bone healing ex vivo.
PRP is generally known to increase the cellular proliferative activity (39). Consistent to this finding, our result showed that PRP in M+PRP did increase cell proliferation in dose dependent manner compared to the groups of M and M/B2. Interestingly, we found that BMP2 increases osteogenic differentiation of MSCs and appears to have a role to play that is contrary to that of PRP in a 2-day culture, since in vitro studies have not shown an increased proliferative activity in M/B2+PRP compared to the groups of M and M/B2 (Fig. 2B). We also observed that immediate release of PRP could inhibit osteogenic potential of the MSCs on day 2 following OS media stimulation, suggesting that at the early stage of osteoblast differentiation, PRP alone promotes cell proliferation, but inhibits cell differentiation. This is supported by the findings from Lohmann et al(40). They found less osteogenic differentiation capacity in MSCs cultured with PRP. However, when BMP2 was used in conjunction with PRP, we found that PRP could promote osteogenic differentiation, suggesting that the complete release of the growth cytokines from PRP combined with BMP2 could promote both cell proliferation and differentiation. This result was confirmed by our further study of ALP activity assay on day 7 after OS media induction. We found that immediate release of PRP can still decrease ALP activity. However, ALP activity was significantly increased in the groups of sustained release of PRP compared to those in immediately released groups. Combination of PRP and BMP2 can significantly promote osteoblast differentiation of MSCs. The mechanism can probably be attributed to the fact that BMP2 could enable MSCs to differentiate while sustained release of cytokines from PRP could promote the cellular proliferation and differentiation of MSCs.
Since the quantity of cytokine released depended on the type of carrier, we had anticipated that factor release would be more rapid from the PPP carrier, given the greater degradation rate as compared to the alginate beads. This was valid for the release of PDGF, as a significantly higher concentration was consistently measured for the PPP beads as compared to the alginate beads. To form beads, the PRP is first mixed with alginate and then dropped into CaCl2 solution, hence due to this prolonged exposure, there appears to be a rapid crosslinking of the polymer chains by Ca2+ during bead formation, which results in the slow release of PDGF which was consistent with the finding of Lu et al (26). This can be explained by a small amount of PDGF remaining inside the bead on day 7. Since the alginate beads were formed by the inward diffusion of Ca2+ ions into the alginate gel, it took longer to degrade (26). Another advantage of the alginate carrier is its ability for flexibility in its design and its potential to control the distribution of the released factor(41).
Some studies demonstrated that the femur defect culture system is a high-throughput test model for scaffold/growth factor therapies for regenerative medicine(30, 42). The alginate appears to be a promising agent in the immobilization of fracture defect, thus enhancing the healing process(43, 44). When delivered with PPP, cytokines in PRP are released within few days and is often exhausted even before bone formation can begin in vivo or ex-vivo. Hence, alginate hydrogel bead appears to be a good carrier system to delay the release of the cytokines in a consistent manner (42). In addition to the extensively reported angiogenic effects of PRP cytokines, they are known to directly modulate osteoblast function, proliferation and differentiation, or acting indirectly through endothelial cells, including induction of chemotactic cell migration(30, 45). From our results it is evident that bone can be regenerated in a fracture defect ex vivo with the help of an alginate carrier system/PRP. The fracture defect healing may result from the differentiation of chondrogenic diaphyseal cells or cell migration and/or osteogenic differentiation from the periosteum along the femur edge. Studies that employed the combination of platelet releasates and MSCs from injectable composites have promoted bone regeneration in fracture models(46), which is in conjunction to our results of demonstrating excellent bone regeneration, the only exception to the use of PRP in our studies as compared to the use of platelet releastes in the other study. Besides this study, the histological section of the calvaria treated with PRP demonstrated faster obliteration of the sagittal and coronal cranial sutures. These findings could be attributed to the cell proliferative and differentiation activity of PRP on MSCs.
CONCLUSION
In summary, within the limitations of this study, delayed release PRP can be of significance in promoting osteogenic and cell proliferative activity of bmp-2 genetically modified MSCs in vitro as well in a bone fracture defect in vivo.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by the National Institute of Dental and Craniofacial Research and the National Institute of Aging, part of the National Institutes of Health, under Award Numbers DE023105 and AG048388 to S.Y. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Sigma Aldrich, MO, USA
Sigma Aldrich, MO, USA
Boster Immunoleader,CA, USA
Gibco, Life technologies, NY, USA
Promega, WI, USA
Life technologies, NY, USA
PerkinElmer, MA, USA
REFERENCES
- 1.Gallini G, Vecchi V, Canzi D. [Bone regeneration and new formation of connective attachment: theory, technic and critical review of the literature] Riv Ital Stomatol. 1984;53(1):5–17. PubMed PMID: 6382563. [PubMed] [Google Scholar]
- 2.Mellonig J. Periodontal regeneration with bone grafts. Dent Econ. 1993;83(5):100–101. PubMed PMID: 8243760. [PubMed] [Google Scholar]
- 3.Elsalanty ME, Genecov DG. Bone grafts in craniofacial surgery. Craniomaxillofac Trauma Reconstr. 2009;2(3):125–134. doi: 10.1055/s-0029-1215875. PubMed PMID: 22110806; PubMed Central PMCID: PMC3052656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng L, Wang Q. [The current situation and future of extracellular matrix materials for bone tissue engineering] Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2001;18(3):470–474. PubMed PMID: 11605519. [PubMed] [Google Scholar]
- 5.Khan Y, Yaszemski MJ, Mikos AG, Laurencin CT. Tissue engineering of bone: material and matrix considerations. J Bone Joint Surg Am. 2008;90(Suppl 1):36–42. doi: 10.2106/JBJS.G.01260. PubMed PMID: 18292355. [DOI] [PubMed] [Google Scholar]
- 6.Foschi F, Conserva E, Pera P, Canciani B, Cancedda R, Mastrogiacomo M. Graft materials and bone marrow stromal cells in bone tissue engineering. J Biomater Appl. 2012;26(8):1035–1049. doi: 10.1177/0885328210393046. PubMed PMID: 21363873. [DOI] [PubMed] [Google Scholar]
- 7.Heard RH, Mellonig JT. Regenerative materials: an overview. Alpha Omegan. 2000;93(4):51–58. PubMed PMID: 11212411. [PubMed] [Google Scholar]
- 8.McAllister BS, Haghighat K. Bone augmentation techniques. J Periodontol. 2007;78(3):377–396. doi: 10.1902/jop.2007.060048. PubMed PMID: 17335361. [DOI] [PubMed] [Google Scholar]
- 9.Pinipe J, Mandalapu NB, Manchala SR, Mannem S, Gottumukkala NV, Koneru S. Comparative evaluation of clinical efficacy of beta-tri calcium phosphate (Septodont-RTR) alone and in combination with platelet rich plasma for treatment of intrabony defects in chronic periodontitis. J Indian Soc Periodontol. 2014;18(3):346–351. doi: 10.4103/0972-124X.134573. PubMed PMID: 25024549; PubMed Central PMCID: PMC4095628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pradeep AR, Rao NS, Agarwal E, Bajaj P, Kumari M, Naik SB. Comparative evaluation of autologous platelet-rich fibrin and platelet-rich plasma in the treatment of 3-wall intrabony defects in chronic periodontitis: a randomized controlled clinical trial. J Periodontol. 2012;83(12):1499–1507. doi: 10.1902/jop.2012.110705. PubMed PMID: 22348695. [DOI] [PubMed] [Google Scholar]
- 11.Pacifici L, Casella F, Maggiore C. [Platelet rich plasma (PRP): potentialities and techniques of extraction] Minerva Stomatol. 2002;51(7–8):341–350. PubMed PMID: 12434129. [PubMed] [Google Scholar]
- 12.Menezes LM, Rao J. Long-term clinical evaluation of platelet-rich plasma in the treatment of human periodontal intraosseous defects: A comparative clinical trial. Quintessence Int. 2012;43(7):571–582. PubMed PMID: 22670252. [PubMed] [Google Scholar]
- 13.Alsousou J, Thompson M, Hulley P, Noble A, Willett K. The biology of platelet-rich plasma and its application in trauma and orthopaedic surgery: a review of the literature. J Bone Joint Surg Br. 2009;91(8):987–996. doi: 10.1302/0301-620X.91B8.22546. PubMed PMID: 19651823. [DOI] [PubMed] [Google Scholar]
- 14.Plachokova AS, Nikolidakis D, Mulder J, Jansen JA, Creugers NH. Effect of platelet-rich plasma on bone regeneration in dentistry: a systematic review. Clin Oral Implants Res. 2008;19(6):539–545. doi: 10.1111/j.1600-0501.2008.01525.x. PubMed PMID: 18422984. [DOI] [PubMed] [Google Scholar]
- 15.Everts PA, Knape JT, Weibrich G, Schonberger JP, Hoffmann J, Overdevest EP, et al. Platelet-rich plasma and platelet gel: a review. J Extra Corpor Technol. 2006;38(2):174–187. PubMed PMID: 16921694. [PMC free article] [PubMed] [Google Scholar]
- 16.Griffin XL, Smith CM, Costa ML. The clinical use of platelet-rich plasma in the promotion of bone healing: a systematic review. Injury. 2009;40(2):158–162. doi: 10.1016/j.injury.2008.06.025. PubMed PMID: 19084836. [DOI] [PubMed] [Google Scholar]
- 17.Ribeiro AJ, Neufeld RJ, Arnaud P, Chaumeil JC. Microencapsulation of lipophilic drugs in chitosan-coated alginate microspheres. Int J Pharm. 1999;187(1):115–123. doi: 10.1016/s0378-5173(99)00173-8. PubMed PMID: 10502618. [DOI] [PubMed] [Google Scholar]
- 18.Wan LS, Heng PW, Chan LW. Drug encapsulation in alginate microspheres by emulsification. J Microencapsul. 1992;9(3):309–316. doi: 10.3109/02652049209021245. PubMed PMID: 1403481. [DOI] [PubMed] [Google Scholar]
- 19.Hunt NC, Grover LM. Cell encapsulation using biopolymer gels for regenerative medicine. Biotechnol Lett. 2010;32(6):733–742. doi: 10.1007/s10529-010-0221-0. PubMed PMID: 20155383. [DOI] [PubMed] [Google Scholar]
- 20.Kato A, Miyaji H, Ishizuka R, Tokunaga K, Inoue K, Kosen Y, et al. Combination of Root Surface Modification with BMP-2 and Collagen Hydrogel Scaffold Implantation for Periodontal Healing in Beagle Dogs. Open Dent J. 2015;9:52–59. doi: 10.2174/1874210601509010052. PubMed PMID: 25674172; PubMed Central PMCID: PMC4319209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Betz OB, Betz VM, Nazarian A, Egermann M, Gerstenfeld LC, Einhorn TA, et al. Delayed administration of adenoviral BMP-2 vector improves the formation of bone in osseous defects. Gene Ther. 2007;14(13):1039–1044. doi: 10.1038/sj.gt.3302956. PubMed PMID: 17460719. [DOI] [PubMed] [Google Scholar]
- 22.He X, Dziak R, Yuan X, Mao K, Genco R, Swihart M, et al. BMP2 genetically engineered MSCs and EPCs promote vascularized bone regeneration in rat critical-sized calvarial bone defects. PLoS One. 2013;8(4):e60473. doi: 10.1371/journal.pone.0060473. PubMed PMID: 23565253; PubMed Central PMCID: PMC3614944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones AL, Bucholz RW, Bosse MJ, Mirza SK, Lyon TR, Webb LX, et al. Recombinant human BMP-2 and allograft compared with autogenous bone graft for reconstruction of diaphyseal tibial fractures with cortical defects. A randomized, controlled trial. J Bone Joint Surg Am. 2006;88(7):1431–1441. doi: 10.2106/JBJS.E.00381. PubMed PMID: 16818967. [DOI] [PubMed] [Google Scholar]
- 24.White AP, Vaccaro AR, Hall JA, Whang PG, Friel BC, McKee MD. Clinical applications of BMP-7/OP-1 in fractures, nonunions and spinal fusion. Int Orthop. 2007;31(6):735–741. doi: 10.1007/s00264-007-0422-x. PubMed PMID: 17962946; PubMed Central PMCID: PMC2266670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Landesberg R, Burke A, Pinsky D, Katz R, Vo J, Eisig SB, et al. Activation of platelet-rich plasma using thrombin receptor agonist peptide. J Oral Maxillofac Surg. 2005;63(4):529–535. doi: 10.1016/j.joms.2004.12.007. PubMed PMID: 15789326. [DOI] [PubMed] [Google Scholar]
- 26.Lu HH, Vo JM, Chin HS, Lin J, Cozin M, Tsay R, et al. Controlled delivery of platelet-rich plasma-derived growth factors for bone formation. J Biomed Mater Res A. 2008;86(4):1128–1136. doi: 10.1002/jbm.a.31740. PubMed PMID: 18181109. [DOI] [PubMed] [Google Scholar]
- 27.He X, Dziak R, Mao K, Genco R, Swihart M, Li C, et al. Integration of a novel injectable nano calcium sulfate/alginate scaffold and BMP2 gene-modified mesenchymal stem cells for bone regeneration. Tissue Eng Part A. 2013;19(3–4):508–518. doi: 10.1089/ten.tea.2012.0244. PubMed PMID: 22994418; PubMed Central PMCID: PMC3542881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang S, Wang C. The intraflagellar transport protein IFT80 is required for cilia formation and osteogenesis. Bone. 2012;51(3):407–417. doi: 10.1016/j.bone.2012.06.021. PubMed PMID: 22771375; PubMed Central PMCID: PMC3412883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamouna E-RSK Elbadawy A, Tamerc Tamer M, El-Meligyb Mahmoud A, Mohy Eldina Mohamed S. Poly (vinyl alcohol)-alginate physically crosslinked hydrogel membranes for wound dressing applications: Characterization and bio-evaluation. Arabian Journal of Chemistry. 2015;8(1):38–47. [Google Scholar]
- 30.Smith EL, Kanczler JM, Gothard D, Roberts CA, Wells JA, White LJ, et al. Evaluation of skeletal tissue repair, part 1: assessment of novel growth-factor-releasing hydrogels in an ex vivo chick femur defect model. Acta Biomater. 2014;10(10):4186–4196. doi: 10.1016/j.actbio.2014.06.011. PubMed PMID: 24937137. [DOI] [PubMed] [Google Scholar]
- 31.Perez AG, Lana JF, Rodrigues AA, Luzo AC, Belangero WD, Santana MH. Relevant aspects of centrifugation step in the preparation of platelet-rich plasma. ISRN Hematol. 2014;2014:176060. doi: 10.1155/2014/176060. PubMed PMID: 25006472; PubMed Central PMCID: PMC4005024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sweeney JD, Holme S, Heaton WA, Nelson E. White cell-reduced platelet concentrates prepared by in-line filtration of platelet-rich plasma. Transfusion. 1995;35(2):131–136. doi: 10.1046/j.1537-2995.1995.35295125735.x. PubMed PMID: 7825208. [DOI] [PubMed] [Google Scholar]
- 33.McCarrel TM, Minas T, Fortier LA. Optimization of leukocyte concentration in platelet-rich plasma for the treatment of tendinopathy. The Journal of bone and joint surgery American volume. 2012;94(19) doi: 10.2106/JBJS.L.00019. e143(1-8) PubMed PMID: 23032594. [DOI] [PubMed] [Google Scholar]
- 34.El-Sharkawy H, Kantarci A, Deady J, Hasturk H, Liu H, Alshahat M, et al. Platelet-rich plasma: growth factors and pro- and anti-inflammatory properties. Journal of periodontology. 2007;78(4):661–669. doi: 10.1902/jop.2007.060302. PubMed PMID: 17397313. [DOI] [PubMed] [Google Scholar]
- 35.Ahearne M, Yang Y, El Haj AJ, Then KY, Liu KK. Characterizing the viscoelastic properties of thin hydrogel-based constructs for tissue engineering applications. J R Soc Interface. 2005;2(5):455–463. doi: 10.1098/rsif.2005.0065. PubMed PMID: 16849205; PubMed Central PMCID: PMC1618501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.American Society for Testing and Materials A. Standard Practices for Assessment of Haemolytic Properties of Materials. ASTM. 2000 F 756-00. [Google Scholar]
- 37.Drengk A, Zapf A, Sturmer E, Sturmer K, Frosch K. Influence of platelet rich plasma (PRP) on chondrogenic differentiation and proliferation of chondrocytes (CC) and mesenchymal stem cells (MSC) J Stem Cells Regen Med. 2007;2(1):180–181. PubMed PMID: 24692986. [PubMed] [Google Scholar]
- 38.Graham S, Leonidou A, Lester M, Heliotis M, Mantalaris A, Tsiridis E. Investigating the role of PDGF as a potential drug therapy in bone formation and fracture healing. Expert Opin Investig Drugs. 2009;18(11):1633–1654. doi: 10.1517/13543780903241607. PubMed PMID: 19747084. [DOI] [PubMed] [Google Scholar]
- 39.Mishra A, Tummala P, King A, Lee B, Kraus M, Tse V, et al. Buffered platelet-rich plasma enhances mesenchymal stem cell proliferation and chondrogenic differentiation. Tissue Eng Part C Methods. 2009;15(3):431–435. doi: 10.1089/ten.tec.2008.0534. PubMed PMID: 19216642; PubMed Central PMCID: PMC2819709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Copland IB, Garcia MA, Waller EK, Roback JD, Galipeau J. The effect of platelet lysate fibrinogen on the functionality of MSCs in immunotherapy. Biomaterials. 2013;34(32):7840–7850. doi: 10.1016/j.biomaterials.2013.06.050. PubMed PMID: 23891515. [DOI] [PubMed] [Google Scholar]
- 41.Lin SS, Landesberg R, Chin HS, Lin J, Eisig SB, Lu HH. Controlled release of PRP-derived growth factors promotes osteogenic differentiation of human mesenchymal stem cells. Conf Proc IEEE Eng Med Biol Soc. 2006;1:4358–4361. doi: 10.1109/IEMBS.2006.260847. PubMed PMID: 17947081. [DOI] [PubMed] [Google Scholar]
- 42.Smith EL, Kanczler JM, Gothard D, Roberts CA, Wells JA, White LJ, et al. Evaluation of skeletal tissue repair, part 2: enhancement of skeletal tissue repair through dual-growth-factor-releasing hydrogels within an ex vivo chick femur defect model. Acta Biomater. 2014;10(10):4197–4205. doi: 10.1016/j.actbio.2014.05.025. PubMed PMID: 24907660. [DOI] [PubMed] [Google Scholar]
- 43.Bhoj M, Zhang C, Green DW. A First Step in De Novo Synthesis of a Living Pulp Tissue Replacement Using Dental Pulp MSCs and Tissue Growth Factors, Encapsulated within a Bioinspired Alginate Hydrogel. J Endod. 2015;41(7):1100–1107. doi: 10.1016/j.joen.2015.03.006. PubMed PMID: 25958179. [DOI] [PubMed] [Google Scholar]
- 44.Keshaw H, Forbes A, Day RM. Release of angiogenic growth factors from cells encapsulated in alginate beads with bioactive glass. Biomaterials. 2005;26(19):4171–4179. doi: 10.1016/j.biomaterials.2004.10.021. PubMed PMID: 15664644. [DOI] [PubMed] [Google Scholar]
- 45.Man Y, Wang P, Guo Y, Xiang L, Yang Y, Qu Y, et al. Angiogenic and osteogenic potential of platelet-rich plasma and adipose-derived stem cell laden alginate microspheres. Biomaterials. 2012;33(34):8802–8811. doi: 10.1016/j.biomaterials.2012.08.054. PubMed PMID: 22981779. [DOI] [PubMed] [Google Scholar]
- 46.McCanless JD, Jennings LK, Bumgardner JD, Cole JA, Haggard WO. Hematoma-inspired alginate/platelet releasate/CaPO4 composite: initiation of the inflammatory-mediated response associated with fracture repair in vitro and ex vivo injection delivery. J Mater Sci Mater Med. 2012;23(8):1971–1981. doi: 10.1007/s10856-012-4672-9. PubMed PMID: 22588505. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


