Abstract
Aim/Background:
The emergence of drug-resistant pathogens has drawn attention on medicinal plants for potential antimicrobial properties. The objective of the present study was the investigation of the antimicrobial activity of five plant essential oils on multidrug resistant Gram-negative bacteria.
Materials and Methods:
Basil, chamomile blue, origanum, thyme, and tea tree oil were tested against clinical isolates of Acinetobacter baumannii (n = 6), Escherichia coli (n = 4), Klebsiella pneumoniae (n = 7), and Pseudomonas aeruginosa (n = 5) using the broth macrodilution method.
Results:
The tested essential oils produced variable antibacterial effect, while Chamomile blue oil demonstrated no antibacterial activity. Origanum, Thyme, and Basil oils were ineffective on P. aeruginosa isolates. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration values ranged from 0.12% to 1.50% (v/v) for tea tree oil, 0.25-4% (v/v) for origanum and thyme oil, 0.50% to >4% for basil oil and >4% for chamomile blue oil. Compared to literature data on reference strains, the reported MIC values were different by 2SD, denoting less successful antimicrobial activity against multidrug resistant isolates.
Conclusions:
The antimicrobial activities of the essential oils are influenced by the strain origin (wild, reference, drug sensitive, or resistant) and it should be taken into consideration whenever investigating the plants’ potential for developing new antimicrobials.
KEY WORDS: Acinetobacter, antimicrobial activity, Escherichia coli, essential oil, multidrug resistant, Klebsiella, Pseudomonas
INTRODUCTION
Medicinal plants have been used for centuries by traditional medicine, first in India and China. In the ancient Western world, the Greeks contributed significantly to the rational development of herbal drugs with Hippocrates (460-377 BC), Aristotle (384-322), and Theophratus (circa 300 BC) to have dealt with the medicinal properties of herbs. According to the World Health Organization, still about 80% of the world population depends on traditional medicine for primary healthcare, especially in the developing countries [1,2]. Plant essential oils have formed the basis of pharmaceuticals and natural therapies being used for a wide variety of purposes, from treating infectious, systematic and inflammatory diseases to food preservation, and perfume and cosmetics production [3,4].
Over the past years, the emergence of drug-resistant pathogens has drawn attention on medicinal plants and their metabolites for potential antimicrobial properties. Multidrug-resistant Pseudomonas aeruginosa, Acinetobacter baumannii, extended-spectrum-beta-lactamase (ESBL) producing Escherichia coli, and carbapenemase-producing Klebsiella pneumoniae have become a worldwide major problem in the hospital environment and are the main causes of hospital-acquired infections or healthcare-associated infections, not excluding their potential of transmission in the community [5,6]. Such bacterial isolates can be resistant to all currently available antibiotics or may remain susceptible only to past agents such as the polymyxins [7].
One of the actions to mitigate the drug-resistance problem includes the development of new antimicrobials and in this sense essential oils are being investigated for potential antibacterial activities. Many plant oils or extracts have been reported to have antimicrobial properties and this is attributed to their ability to synthesize aromatic substances, most of which are phenols or oxygen-substituted derivatives [8,9]. However, most of the published studies deal with either non-pathogenic or reference bacterial strains and there is a scarcity of data about wild multidrug resistant isolates. The objective of this study was to determine the antimicrobial activity against multidrug resistant Gram-negative bacteria isolated from clinical samples, of plant essential oils, which are widely used in studies with non-pathogenic or reference strains but their actual effect against resistant pathogens is hardly addressed in the available literature.
MATERIALS AND METHODS
Microorganisms
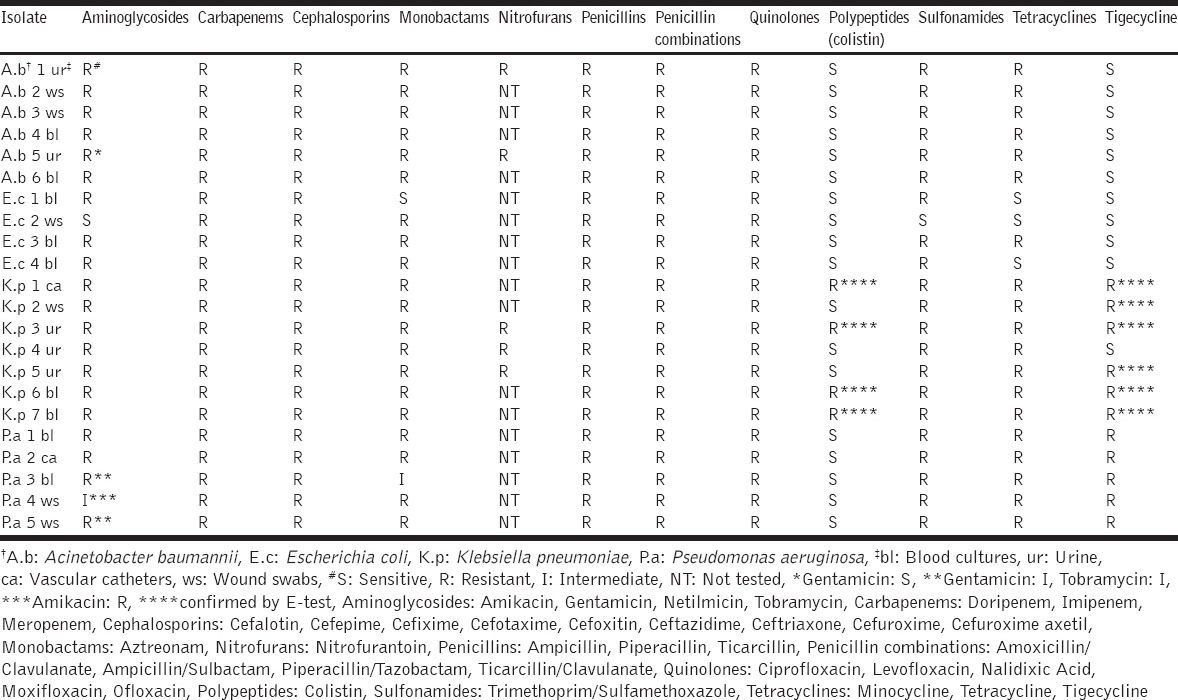
The bacterial strains used in this study were A. baumannii (n = 6), E. coli (n = 4), K. pneumoniae (n = 7) and P. aeruginosa (n = 5), isolated from blood cultures (n = 9), urine (n = 5), vascular catheters (n = 2), and wound swabs (n = 6) collected from equal number of hospitalized patients entering the University Hospital of Ioannina, Greece. Based on the susceptibility tests [Table 1] all the K. pneumoniae isolates were carbapenemase-producing and the E. coli isolates were producing ESBLs. Among the K. pneumoniae strains producing carbapenemases, four were resistant to all tested antibiotics, while the rest were sensitive only to colistin. Resistant bacteria to colistin and tigecycline were further confirmed by the E-test (BioMerieux SA, France).
Table 1.
Antimicrobial susceptibility testing
The isolation of the bacterial strains used in the present study was performed according to the following routine procedures employed by the Clinical Microbiology Laboratory of the University Hospital of Ioannina:
Blood specimens were inoculated directly into Bact/Alert® (BioMerieux SA, France) disposable culture bottles containing 30 ml of liquid substrate consisted of 22 ml of complex media and 8 ml of a charcoal suspension. The media component consists of soybean-casein digest (2.0% w/v), brain heart infusion solids (0.1% w/v), sodium polyanethol sulfonate (SPS) (0.05% w/v), pyridoxine HCl (0.001% w/v), menadione (0.0000725% w/v), hemin (0.000725% w/v), L-cysteine (0.03% w/v), and other complex amino acid and carbohydrate substrates in purified water. Moreover, the bottles contain an atmosphere of carbon dioxide (CO2) in oxygen under vacuum. The Bact/Alert® disposable culture bottles are commercially available, ready-to-be-used with the Bact/Alert® Microbial Detection System (BioMerieux SA, France), which is a fully automated blood culture system for detecting bacteremia, utilizing a colorimetric sensor and reflected light to monitor the presence and production of CO2 dissolved in the culture medium. When the growth of the microorganism produces CO2, the color of the gas-permeable sensor installed in the bottom of each culture bottle changes from blue-green to yellow. The lighter color results in an increase of reflectance units monitored by the system. Bottle reflectance is monitored and recorded by the instrument every 10 min.
Urine specimens were inoculated on Uricult Plus media (Orion Diagnostica, Finland), which are intended for diagnosing urinary tract infections by demonstration and presumptive detection of total bacteria count, Gram-negative bacteria, Enterococci, and E. coli in urine samples. Uricult Plus is a dip-slide system based on three agar media: The Cystine-lactose electrolyte-deficient agar intended for the determination of the total bacterial count in urine samples, the selective Mac Conkey agar supporting the growth of Gram-negative bacteria and the selective Enterococcus medium intended specifically for the detection of enterococci. The Uricult Plus slide-plates were incubated at 37°C for 24-48 h.
Vascular catheters and wound swabs were cultured in blood agar, Mac Conkey agar (Oxoid, UK), Mannitol salt agar (Oxoid, UK), and Sabouraud Dextrose agar (Oxoid, UK) plates and were incubated at 37°C for 24-48 h.
Identification to species level was performed using the VITEK® 2 automated system (BioMerieux SA, France). This system uses advanced colorimetry, an identification technology enabling identification of routine clinical isolates (bacteria, yeast), and antibiotic susceptibility testing and resistance mechanism detection.
The selected Gram-negative isolates were stored at –70°C in Microbank® beads (Prolab diagnostics, Canada), a ready-to-use system for storage and retrieval of bacterial isolates, which is comprised of cryovials incorporating treated beads and a special cryopreservative solution enhancing longer survival of the fastidious microorganisms and higher quantitative recoveries. Prior to any experimentation, the so cryopreserved isolates were revived by subculturing in appropriate culture media.
The susceptibility testing was performed using the VITEK®2 automated system and the E-test (BioMerieux SA, France). Susceptibility to the following antibiotics was tested: Aminoglycosides (amikacin, gentamicin, netilmicin, and tobramycin), carbapenems (doripenem, imipenem, and meropenem), cephalosporins (cefalotin, cefepime, cefixime, cefotaxime, cefoxitin, ceftazidime, ceftriaxone, cefuroxime, and cefuroxime axetil), monobactams (aztreonam), nitrofurans (nitrofurantoin), penicillins (ampicillin, piperacillin, and ticarcillin), penicillin combinations (amoxicillin/clavulanate, ampicillin/sulbactam, piperacillin/tazobactam, and ticarcillin/clavulanate), quinolones (ciprofloxacin, levofloxacin, moxifloxacin, nalidixic acid, and ofloxacin), polypeptides (colistin), sulfonamides (trimethoprim/sulfamethoxazole) tetracyclines (minocycline and tetracycline), and tigecycline.
Essential Oils
The following five essential oils supplied by Sigma-Aldrich Co (Germany) were tested for antimicrobial properties:
Basil oil (FCC, comorictype, W211907), Ocimum basilicum L. (Lamiaceae).
Chamomile blue oil (W227307), Matricaria chamomilla, L. (Asteraceae).
Origanum oil (FCC, W282812), Thymus capitatus, L. (Labiatae).
Tea tree oil (W390208, Melaleuca alternifolia, (Myrtaceae).
Thyme oil-white (FCC, Kosher, W306509), Thymus vulgaris, L. (Lamiaceae).
For the used commercial oils, the supplier provided no data about their contents or chemical analysis, which is presumed to be the company’s copyright. However, a simple chemical analysis was performed in order to have a gross estimate of the components of the employed essential oils. For the identification of the components a QP 5000 Shimadzu instrument, equipped with a capillary column DB-5-MS, 30 × 0.32 mm, 0.25 µm, containing 5% phenyl-methylpolysiloxane (J&W Scientific, Folsom, CA, USA) was employed. The gas chromatography oven temperature was programmed as follows: initial temperature 55°C ramped at 5°C/min to 200°C, ramped 1°C/min to 210 (held for 2 min), and finally rampedto 270°C at 20°C/min and held for 3 min. The injector was set to 240°C in the splitless mode. The ion source and transfer were kept at 240°C and 290°C, respectively. In the full-scan mode, electronic ionization mass spectra at m/z of 50-450 were recorded at 70 eV. Helium was used as the carrier gas at 1.5 mL/min.
Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
Broth macrodilution assays were performed to determine the MIC and MBC for each essential oil, according to the Clinical and Laboratories Standards Institute (CLSI) protocol M7-A8 with some modifications [10].
Each essential oil was dispersed in a sterile tube containing Mueller-Hinton broth (MHB, Oxoid, UK) and was vortexed at room temperature, to obtain an initial stock solution of 8% (v/v). Subsequently, serial double-fold dilutions were prepared in sterile tubes containing MHB supplemented with 0.5% (v/v) Tween 20 (Serva, Germany). The final concentrations of each essential oil were 4, 2, 1, 0.5, 0.25, and 0.125 (v/v).
Overnight bacterial cultures on Mueller-Hinton agar (MHA, Oxoid, UK) were used to prepare the bacterial inocula. Each inoculum was adjusted with sterile saline to obtain the final suspension with turbidity analogous to that of 0.5 McFarland Standards, which equals to a concentration of 1-1.5 × 108 cfu/ml [11,12]. About 10 µl of the prepared bacterial inoculum were transferred to each tube containing the serial double-fold dilutions of the essential oil, giving a final bacterial concentration of 5 × 105 cfu/ml. The tubes were incubated aerobically at 37°C for 48 h. After the end of incubation, 10 µl of each dilution was inoculated onto MHA plates and incubated at 37°C for 24 and 48 h in order to determine the MIC and MBC, respectively. The MIC and MBC values were determined by viable counts in MHA, and the MIC was defined as the lowest concentration at which the inoculum viability was reduced up to 90% and MBC was defined as the lowest concentration at which the inoculum viability was reduced up to 99.9% or no apparent growth occurred [13].
Statistical Analysis
Statistical analysis was performed in SPSS (version 22.0. Armonk, NY: IBM Corp). The exhibited MICs and MBCs were grouped according to oil type and checked for normality by the Shapiro-Wilk test. Comparison between oil types was performed by one-way ANOVA, whereas differences between oil types were estimated by the Turkey’s test.
RESULTS
The antimicrobial susceptibility of the tested clinical isolates is presented in Table 1, and the MIC and MBC values of the selected essential oils against the tested drug-resistant isolates are presented in Tables 2 and 3. Basil, origanum, tea tree, and thyme essential oils presented antibacterial activity, but chamomile blue oil demonstrated no antibacterial action at all. The tea tree oil demonstrated consistent antimicrobial activity against all the tested clinical isolates and all four oils inhibited growth of A. baumanii isolates. However, origanum, thyme, and basil oils antigrowth effect on P. aeruginosa was poor [Tables 2 and 3].
Table 2.
Range of MIC and MBC values (% v/v) of selected essential oils against the tested bacteria
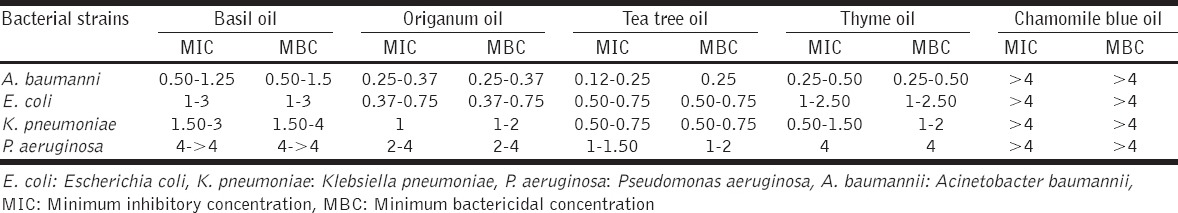
Table 3.
MIC and MBC values (% v/v) of selected essential oils against A. baumannii (1-6), E. coli (7-10), K. pneumoniae (11-17) and P. aeruginosa (18-22)
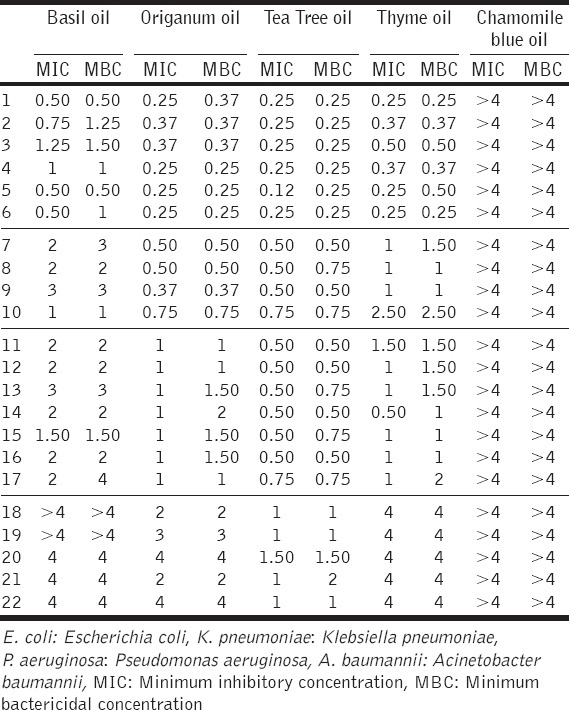
Statistically significant differences between the tested essential oils were determined by one-way ANOVA (F [3,133] = 7.403, P = 0.002). The Basil oil’s MIC and MBC values were significantly higher than the origanum and tea tree oil respective values (P < 0.05), but not statistically different than the thyme oil’s values. The thyme oil MICs and MBCs were significantly higher than the tea tree oil relevant values (P < 0.05), but not statistically different than the origanum oil corresponding values. For the tea tree oil, the recorded MIC and MBC values were not statistically different (P < 0.05), than the origanum oil respective values.
Regarding the chemical composition of the essential oils used in this study, the most abundant component in the case of basil oil was estragole. Carvacrol and thymol were identified as main constituents of origanum oil. The composition of tea tree oil presented high contents of terpinen-4-ol and p-cymene. The prevailing molecules of thyme oil were thymol, p-cymene, and linalool. Chamomile blue oil was rich in bisabolol and trans-b-farnesene. Typical chromatograms of the essential oils examined are shown in Figure 1.
Figure 1.
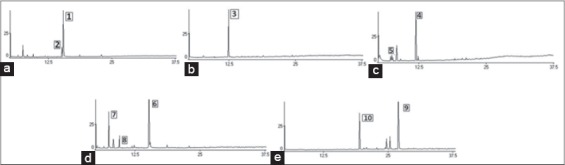
(a-e) Chromatograms of origanum oil (peak 1: Carvacrol, peak 2: Thymol), basil oil (peak 3: Estragole), tea tree oil (peak 4: Terpinen-4-ol, peak 5: p-cymene), thyme oil (peak 6: Thymol, peak 7: p-cymene, peak 8: Linalool), and chamomile blue oil (peak 9: Bisabolol, peak 10: Trans-b-farnesene)
DISCUSSION
The rapid evolution and spread of resistance among clinically important bacterial species constitutes a significant issue of outmost importance for public health. The emergence of antimicrobial resistance is the consequence of selective pressure imposed to microorganisms by the excessive use of antimicrobials mostly in the medical and veterinary practices. The major issue of this important health problem is that the appearance of resistance to antibiotics reduces the currently available therapeutic options for the treatment of infectious diseases signifying the need for the development of new antibiotic compounds. Plants produce a vast variety of phytochemicals that demonstrate a diversity of medicinal properties including antimicrobial effects. The principal phytochemicals present in plants are essential oils, phenolic compounds, alkaloids, polypeptides, and polyacetylenes [9].
Essential oils have shown antimicrobial properties against a number of Gram-negative and Gram-positive bacteria and in overall, their activity against the microbial cells of the same genera and species determined under the same conditions appears to be similar. However, some bacterial isolates may show a different response in comparison to the type strains [14,15]. Hence, to reach a decision on the antimicrobial activities of essential oils, it is important to use strains from different origins in order to simulate a more realistic situation instead of just using reference strains that may not reflect the actual behavior of the strains that can be found in nature, particularly in the clinical practice. The majority of the available published studies make use of reference strains, not clinical multidrug resistant isolates, and variable findings are recorded due to the diversity of the used methodologies.
Pertaining to the specific essential oils and isolates used in the present study, there are only two publications concerning the antimicrobial effect against clinical isolates. According to da Costa et al. [16] origanum oil inhibited A. baumannii, E. coli, and K. pneumoniae clinical isolates at MIC 0.12% (v/v) and P. aeruoginosa at 0.5% (v/v), while in our study it was significantly less effective (by 2SD). Hammer et al. [17] testing the tea tree oil toward clinical isolates, reported for A. baumannii and P. aeruoginosa MIC values 1% and 3% (v/v), while in our study the antibacterial performance of this oil was significantly better (by 2SD). However, for the K. pneumoniae the MIC values described by Hammer et al. [17] were significantly better (by 1SD) than those reported in the present study [Tables 2 and 3].
Diverse antimicrobial activities against A. baumanii, E. coli, K. pneumonia, and P. aeruginosa have been reported by researchers experimenting with reference strains. For E. coli, the MIC values reported for reference strains are ranging from 0.12% (v/v) for origanum oil to 0.5% (v/v) for basil [3,18-21]. In our study, the reported MIC values [Table 3] were much different (by 2SD) denoting much less successful antimicrobial activity of the origanum and Basil oils against multidrug resistant clinical isolates.
The MIC values reported in the present study and the reciprocal values reported for K. pneumoniae reference strains [3] are different by 2SD and 1SD for origanum and thyme oils, respectively, indicating less successful antimicrobial effect against resistant clinical isolates. Concerning the basil and tea tree oil’s activity [Table 3] against K. pneumoniae no difference was observed between the tested resistant clinical isolates and the reference strains tested by Hammer et al. [3].
Regarding the P. aeruginosa strains and the activity of tea tree oil, a significant difference (by 2SD) was observed with this oil performing much better against the tested resistant clinical isolates than the reference strains tested by other researchers [3,22-24]. The antimicrobial activity of the origanum and basil oil against P. aeruginosa was poor and our findings coincide with those reported on reference strains [3,25].
Based on the afore-mentioned literature data and the results of the present study, much different MIC values are recorded between the reference and clinical resistant isolates. Studies employing reference strains are showing more efficient performance of the tested essential oils; however, in the case of clinical isolates and particularly in the case of the multidrug resistant isolates used in the present study, essential oils are less efficacious. This finding can be attributed to the strain origin rather than to the methodological differences reported by other researchers [4,13,18,26-29].
In the present study, we used multidrug resistant strains of Gram-negative bacteria isolated from hospitalized subjects. The Gram-negative bacteria are considered to be more resistant to essential oils than the Gram-positives [30]. This is largely attributed to the different structure of their cell wall which is more complex in Gram-negatives, not allowing the easy penetration of antibiotics and drugs, including the phenolic compounds (e.g., thymol, carvacrol, and eugenol) which are present in the essential oils [31,32]. Thus, the possible mechanism of action of the essential oils and their compounds is based on their ability to disrupt the bacterial cell wall and the cytoplasmic membrane; this mode of action consequently leads to cell lysis and leakage of intracellural compounds [33]. Considering that an intact external cell envelope is a prerequisite for the bacterium survival protecting the cell cytoplasm from the external environment, any changes in the permeability of the cell wall and cytoplasmic membrane can influence the bacterial growth. Whenever antibacterial compounds are present in the environment surrounding microorganisms, the bacteria are forced to react by altering the synthesis of fatty acids and membrane proteins to modify the permeability of the membrane [34,35]. The essential oils have the potential to alter both the permeability and the function of the membrane proteins, particularly the essential oils, which are rich in phenolics, can penetrate into the phospholipids layer of the bacterial cell wall, bind to proteins and block their normal functions. Because of their lipophilic nature, essential oils and their compounds can influence the percentage of unsaturated fatty acids and their structure [30,36]. However, because of the variety of molecules present in plant extracts, the antimicrobial activity of the essential oils cannot be attributed to a single mechanism but to a number of diverse biochemical and structural mechanisms at various sites of the bacterial cell outer and inner components affecting the functions of cell membrane, cytoplasm, enzymes, proteins, fatty acids, ions, and metabolites.
CONCLUSIONS
A detailed examination of all the factors potentially influencing the antimicrobial activity of the essential oils should be ideal, but it is rather difficult to implement as evidenced by the existing relevant literature. However, any additional data do contribute to the increase of knowledge in the field. Concerning our study significant differences were observed between our results and the results of other researchers who experimented with non-clinical/non-resistant isolates. Our findings indicate that the essential oils’ antimicrobial activities are influenced by the strain origin (wild, reference, drug sensitive, or resistant) and this observation should be taken into consideration whenever investigating the plants’ potential for developing new antimicrobials. Nevertheless, the identification of the exact compounds encompassing a true antimicrobial effect is a prerequisite in order to optimize their potential therapeutic use. Yet, microbes are very good survivors having a remarkable ability to adapt to hostile environments, such as being surrounded by antimicrobials, thus meticulous investigation of their resistance mechanisms is necessary in order to encounter successfully the emergence of antibiotic resistance.
ACKNOWLEDGMENTS
This research was financially supported by the HERAKLEITOS Project 61/1733/7, funded by the EPEAEK Administration Office of the Greek Ministry of Education.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Gurib-Fakim A. Medicinal plants: Traditions of yesterday and drugs of tomorrow. Mol Aspects Med. 2006;27:1–93. doi: 10.1016/j.mam.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Prabuseenivasan S, Jayakumar M, Ignacimuthu S. In vitro antibacterial activity of some plant essential oils. BMC Complement Altern Med. 2006;6:39. doi: 10.1186/1472-6882-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hammer KA, Carson CF, Riley TV. Antimicrobial activity of essential oils and other plant extracts. J Appl Microbiol. 1999;86:985–90. doi: 10.1046/j.1365-2672.1999.00780.x. [DOI] [PubMed] [Google Scholar]
- 4.Moreno PR, da Costa-Issa FI, Rajca-Ferreira AK, Pereira MA, Kaneko TM. Native Brazilian plants against nosocomial infections: A critical review on their potential and the antimicrobial methodology. Curr Top Med Chem. 2013;13:3040–78. doi: 10.2174/15680266113136660219. [DOI] [PubMed] [Google Scholar]
- 5.Lee NY, Lee CC, Huang WH, Tsui KC, Hsueh PR, Ko WC. Carbapenem therapy for bacteremia due to extended-spectrum-ß-lactamase-producing Escherichia coli or Klebsiella pneumoniae: Implications of ertapenem susceptibility. Antimicrob Agents Chemother. 2012;56:2888–93. doi: 10.1128/AAC.06301-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lucena A, Dalla Costa LM, Nogueira KS, Matos AP, Gales AC, Paganini MC, et al. Nosocomial infections with metallo-beta-lactamase-producing Pseudomonas aeruginosa: Molecular epidemiology, risk factors, clinical features and outcomes. J Hosp Infect. 2014;87:234–40. doi: 10.1016/j.jhin.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Magiorakos AP, Srinivasan A, Carey RB, Carmeli ME, Falagas CG, Giske S, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–81. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 8.Perumal Samy R, Gopalakrishnakone P. Therapeutic potential of plants as anti-microbials for drug discovery. Evid Based Complement Alternat Med. 2010;7:283–94. doi: 10.1093/ecam/nen036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12:564–82. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: Approved Standard. 7th ed. Wayne, PA: National Committee for Clinical Laboratory Standards; 2009. pp. M7–A8. [Google Scholar]
- 11.Marsik FJ. Antimicrobial susceptibility testing procedures. In: Mahon CR, Lehman DC, Manuselis G, editors. Textbook of Diagnostic Microbiology. 4th ed. Missouri: Saunders, Elsevier; 2011. pp. 280–5. [Google Scholar]
- 12.CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically. Approved Standards. 7th ed. Wayne, PA: National Committee for Clinical Laboratory Standards; 2006. pp. M7–A7. [Google Scholar]
- 13.Moreira MR, Ponce AG, Del Valle CE, Roura SI. Inhibitory parameters of essential oils to reduce a foodborne pathogen. LWT-Food Sci Technol. 2005;38:565–70. [Google Scholar]
- 14.Chorianopoulos NG, Evergetis ET, Aligiannis N, Mitakou S, Nychas GJ, Haroutian SA. Correlation between chemical composition of Greek essential oils and their antibacterial activity against food-borne pathogens. Nat Prod Commun. 2007;2:419–26. [Google Scholar]
- 15.Canillac N, Mourey A. Effects of several environmental factors on the anti-Listeria monocytogenes activity of an essential oil of Picea excelsa. Int J Food Microbiol. 2004;92:95–103. doi: 10.1016/j.ijfoodmicro.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 16.da Costa AC, Calvacanti dos Santo BM, Filho LS, Lima EO. Antibacterial activity of the essential oil of Origanum vulgare L. (Lamiaceae) against bacteria multiresistant isolates from nosocomial patients. Rev Bras Farmacogn. 2009;19:236–41. [Google Scholar]
- 17.Hammer KA, Carson CF, Riley TV. Susceptibility of transient and commensal skin flora to the essential oil of Melaleuca alternifolia (tea tree oil) Am J Infect Control. 1996;24:186–9. doi: 10.1016/s0196-6553(96)90011-5. [DOI] [PubMed] [Google Scholar]
- 18.Peñalver P, Huerta B, Borge C, Astorga R, Romero R, Perea A. Antimicrobial activity of five essential oils against origin strains of the Enterobacteriaceae family. APMIS. 2005;113:1–6. doi: 10.1111/j.1600-0463.2005.apm1130101.x. [DOI] [PubMed] [Google Scholar]
- 19.Smith-Palmer A, Stewart J, Fyfe L. Antimicrobial properties of plant essential oils and essences against five important food-borne pathogens. Lett Appl Microbiol. 1998;26:118–22. doi: 10.1046/j.1472-765x.1998.00303.x. [DOI] [PubMed] [Google Scholar]
- 20.Mann CM, Markham JL. A new method for determining the minimum inhibitory concentration of essential oils. J Appl Microbiol. 1998;84:538–44. doi: 10.1046/j.1365-2672.1998.00379.x. [DOI] [PubMed] [Google Scholar]
- 21.Preuss HG, Echard B, Enig M, Brook I, Elliott TB. Minimum inhibitory concentrations of herbal essential oils and monolaurin for Gram-positive and Gram-negative bacteria. Mol Cell Biochem. 2005;272:29–34. doi: 10.1007/s11010-005-6604-1. [DOI] [PubMed] [Google Scholar]
- 22.Longbottom CJ, Carson CF, Hammer KA, Mee BJ, Riley TV. Tolerance of Pseudomonas aeruginosa to Melaleuca alternifolia (tea tree) oil is associated with the outer membrane and energy-dependent cellular processes. J Antimicrob Chemother. 2004;54:386–92. doi: 10.1093/jac/dkh359. [DOI] [PubMed] [Google Scholar]
- 23.Papadopoulos CJ, Carson CF, Hammer KA, Riley TV. Susceptibility of pseudomonads to Melaleuca alternifolia (tea tree) oil and components. J Antimicrob Chemother. 2006;58:449–51. doi: 10.1093/jac/dkl200. [DOI] [PubMed] [Google Scholar]
- 24.Mickiene R, Bakutis B, Baliukoniene V. Antimicrobial activity of two essential oils. Ann Agric Environ Med. 2011;18:139–44. [PubMed] [Google Scholar]
- 25.Sokovic M, Glamoclija J, Marin PD, Brkic D, van Griensven LJ. Antibacterial effects of the essential oils of commonly consumed medicinal herbs using an in vitro model. Molecules. 2010;15:7532–46. doi: 10.3390/molecules15117532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Radulovic NS, Blagojevic PD, Stojanovic-Radic ZZ, Stojanovic NM. Antimicrobial plant metabolites: Structural diversity and mechanism of action. Curr Med Chem. 2013;20:932–52. [PubMed] [Google Scholar]
- 27.Suppakul P, Miltz J, Sonneveld K, Bigger SW. Antimicrobial properties of basil and its possible application in food packaging. J Agric Food Chem. 2003;51:3197–207. doi: 10.1021/jf021038t. [DOI] [PubMed] [Google Scholar]
- 28.Sienkiewicz M, Lysakowska M, Ciecwierz J, Denys P, Kowalczyk E. Antibacterial activity of thyme and lavender essential oils. Med Chem. 2011;7:674–89. doi: 10.2174/157340611797928488. [DOI] [PubMed] [Google Scholar]
- 29.Holley R, Patel D. Improvement in shelf-life and safety of perishable foods by plant essential oils and smoke antimicrobials. Food Microbiol. 2005;22:273–92. [Google Scholar]
- 30.Nazzaro F, Fratianni F, De Martino L, Coppola R, De Feo V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals (Basel) 2013;6:1451–74. doi: 10.3390/ph6121451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trombetta D, Castelli F, Sarpietro MG, Venuti V, Cristani M, Daniele C, et al. Mechanisms of antibacterial action of three monoterpenes. Antimicrob Agents Chemother. 2005;49:2474–8. doi: 10.1128/AAC.49.6.2474-2478.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tiwari BK, Valdramidis VP, O’Donnell CP, Muthukumarappan K, Bourke P, Cullen PJ. Application of natural antimicrobials for food preservation. J Agric Food Chem. 2009;57:5987–6000. doi: 10.1021/jf900668n. [DOI] [PubMed] [Google Scholar]
- 33.Lopez-Romero JC, González-Ríos H, Borges A, Simões M. antibacterial effects and mode of action of selected essential oils components against Escherichia coli and Staphylococcus aureus. Evid Based Complement Alternat Med. 2015;2015:795435. doi: 10.1155/2015/795435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burt SA, Reinders RD. Antibacterial activity of selected plant essential oils against Escherichia coli O157: H7. Lett Appl Microbiol. 2003;36:162–7. doi: 10.1046/j.1472-765x.2003.01285.x. [DOI] [PubMed] [Google Scholar]
- 35.Mrozik A, Piotrowska-Seget Z, Labuzek S. Changes in whole cell-derived fatty acids induced by naphthalene in bacteria from genus Pseudomonas. Microbiol Res. 2004;159:87–95. doi: 10.1016/j.micres.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 36.Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oils - A review. Food Chem Toxicol. 2008;46:446–75. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]


