Abstract
Background/Aim:
Clerodendrum myricoides is a Kenyan herbal plant used in the management of respiratory diseases. In the current study, we investigated in vitro antimicrobial activity, cytotoxicity, and phytochemical screening of C. myricoides
Materials and Methods:
Antimicrobial activities of C. myricoides organic fractions against array of microorganisms including: (i) Mycobacterium tuberculosis (MTB) H37Rv, (ii) Staphylococcus aureus, (iii) Klebsiella pneumoniae, (iv) Escherichia coli, (v) Candida albicans, (vi) Pseudomonas aeruginosa, (vii) Cryptococcus neoformans, (viii) Salmonella typhi, (ix) Shigella sonnei, and (x) Methicillin-resistant S. aureus (MRSA) were investigated by disc diffusion and microdilution techniques. Antituberculous activity was investigated using BACTEC MGIT 960 system while cytotoxicity was analyzed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay on HEp-2 cells. Finally, phytochemicals were screened using standard procedures.
Results:
Methanolic fractions exhibited a broad spectrum activity inhibiting 75% of test pathogens. It had the highest activity with minimal inhibition concentration (MIC) values of ≤62.5 µg/ml recorded against 62.5% tested microbes. It yielded the highest zone of inhibition of 20.3 mm (S. aureus), lowest MIC of <12.5 µg/ml (MTB), and the lowest minimal bactericidal concentration of 62.5 µg/ml (C. albicans), within the acceptable toxicity limit (CC50 >90 µg/ml). The phytochemicals largely believed to be responsible for the observed activity included: Alkaloid, phenols, anthraquinones, terpenoids, and flavonoids.
Conclusion:
Methanolic fraction had remarkable activity against MRSA, S. aureus, E. coli, S. sonnei, C. albicans, and MTB, which are of public health concerns due to drug resistance and as sources of community and nosocomial infections. To the best of our knowledge, this is the first report exploring the antituberculous activity of C. myricoides and thence a major output in search of novel, safe drug leads to mitigate the global tuberculosis threat.
KEY WORDS: Clerodendrum myricoides, cytotoxicity, ethnopharmacology, herbal medicine, infectious diseases, phytochemicals, tuberculosis
INTRODUCTION
The emergence and spread of drug resistance has severely compromised the efficacy of antimicrobial therapy and increased the threat of therapeutic failure [1], which now has become a matter of urgent attention [2]. This is particularly important because of the growing numbers of susceptible people such as: those with compromised immunity and the elderly [1]. In addition, the spread of resistance among infectious pathogens has revealed intricate mechanisms by which resistance coding genes are passed from one microbe to another, species difference notwithstanding. This has led to spread of resistance among Gram-positive, Gram-negative, acid-fast bacterial, and fungal pathogens. These pathogens are a cause for hospital- and community- acquired infections including and not limited to fluoroquinolone-resistant Pseudomonas aeruginosa, methicillin-resistant Staphylococcus aureus (MRSA), Klebsiella pneumoniae, Escherichia coli, and drug-resistant Mycobacterium tuberculosis (MTB) [1]. Furthermore, therapeutic options for emerging pathogenic strains are limited, forcing the use of a second and third line of antimicrobials agents. These second and third line classes of drugs are associated with serious adverse effects on the users [1-4]. Consequently, there is a need for concerted effort in prospecting for novel formulations which are lethal to resistant infectious microorganisms yet safe for humans to combat this on-going health scourge.
Plants provide unlimited prospects for novel potent antimicrobial agents because of their unmatched chemical diversity. However, despite their wide use and the fact that they form an integral part of the primary health care in many countries, there is paucity of information regarding their efficacy and more importantly, their safety levels. For this reason, the current study primarily aimed to evaluate the in vitro antimicrobial potency of Clerodendrum myricoides organic fractions against a panel of pathogenic microorganisms including S. aureus, MRSA, E. coli, K. pneumoniae, P. aeruginosa, Salmonella typhi, Shigella sonnei, Candida albican, Cryptococcus neoformans, and acid-fast MTB strain H37RV and also determine their safety by assaying for their in vitro acute toxicity on HEp-2 cells (human laryngeal carcinoma cell line ATCC CCL-23).
C. myricoides (Hochst.) R. Br. ex Vatke, locally known as “Munjuga-iria” among Mbeere community of Embu County in Kenya, is a member of Lamiaceae family. It is a shrub or small multi-stemmed tree characterized with ovate velvety alternate or 3-4-whorled leaves that often crowd near the ends of branches. The shrub is occasionally punctuated with few- purple-blue and white flowers with long exerted stamens terminal and axillary heads.
The selection of this plant was based on its ethnopharmacological use. C. myricoides roots are used in the management of respiratory infections, arthritis, malaria, tonsillitis, eye infections, and gonorrhea [5-7].
MATERIALS AND METHODS
Plant Organic Solvent Fractions Preparation
The plant for this study was identified through ethnobotanical approach whereby information regarding its use among the Mbeere community, Kenya, was gleaned from local herbalist and confirmed from literature [5,6]. This plant is not an endangered species, and it was collected in open community field, and therefore, no prior permission was required. The location for collection was around 0°46’27.0”S 37°40’54.9”E; −0.774156, 37.681908 of GPS co-ordinates. The identity was also confirmed by Botanists at Egerton University (Prof. Kariuki S. T. and Mr. Peter Amwogah) where voucher specimen number NSN13 was deposited, and the name checked as acceptable [8].
The plant roots were harvested, cleansed with tap water, shade dried at room temperature (23°C ± 2°C), and finely powdered using a mechanical grinder. Successive fractionation was done using different solvents of increasing polarity. 50 g of powdered sample was soaked in 200 ml of petro ether (PET) with intermittent shaking for 48 h then filtered and the resulting residue further re-extracted using the same fresh solvent for 24 h and the filtrates pooled together for further use. The resulting residue was air dried and further extracted with dichloromethane (DCM) followed by ethyl acetate (EA) and lastly methanol (MOH) following the same procedure. The solvent was vaporized from each filtrate using a rotary evaporator under conditions of reduced temperature and pressure, and the resulting dry extract weighed and stored in airtight sample bottles at −20°C. The extracts were reconstituted in dimethyl sulfoxide (DMSO) to form stock solution of different concentrations.
Antimicrobial Activity Assessment
One Gram-positive; S. aureus (ATTC 25923) strain and MRSA strain (clinical isolate); five Gram-negative; E. coli (ATTC 25922), K. pneumoniae (clinical isolate), P. aeruginosa (ATCC 27853), S. typhi (clinical isolate), and S. sonnei (clinical isolate); two Fungi; Candida albicans (ATTC 90028), C. neoformans (ATTC 66031), and acid-fast MTB strain H37Rv (ATCC 27294) were investigated for antimicrobial activity. These organisms were sourced from Kenya Medical Research Institute (KEMRI) – Nairobi-Kenya.
Disc Diffusion Assay
The antimicrobial activity was screened using agar disc diffusion method according to Clinical and Laboratory Standard Institute [9] and Mbaveng et al. [10] with slight modification. Briefly, fresh bacteria and fungi inoculums were prepared by suspending activated colonies in physiological saline water (0.85% NaCl). Using 0.5 McFarland turbidity standard, the bacteria and fungi suspensions were adjusted to 1.5 × 106 CFU/ml after which they were inoculated aseptically by swapping the surfaces of the Mueller-Hinton agar plates and Sabouraud dextrose agar (SDA) plates. Whatman filter paper (No. 1) discs of 6 mm diameter were made by punching the paper, and the blank discs sterilized in the hot air oven at 160°C for 1 h. They were then impregnated with the different organic solvent extracts (5 µg/disc for PET, DCM, and MOH fractions and 2.5 µg/disc for EA), carefully placed on the surface of the test plate at equidistant points using a sterile forceps and then culture incubated at 37°C (18 h) for bacteria and 27°C for fungi (48 h). Three standard drugs were used as positive controls: Oxacillin 10 µg/disc (Oxoid Ltd., Tokyo-Japan) and Gentamycin 10 µg/disc (Oxoid Ltd., Tokyo-Japan) for Gram-positive and Gram-negative bacteria, respectively. Nystatin 100 µg/disc (Oxoid Ltd, Tokyo-Japan) was used as the standard drug for all fungi while discs loaded with 10 µl of DMSO was used as negative controls. Antimicrobial activity was evaluated by determining the size of the zone of inhibition to the nearest (mm) using a ruler [11]. Extracts that gave a zone of more than 10 mm were considered to be active [9], and therefore, their minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) determined [11]. All tests were done in triplicate.
Determination of MIC and MBC
The MIC and MBC of the organic solvent fractions that exhibited antimicrobial activity against the test microorganisms were determined using broth microdilution technique. Stock solutions of PET, DCM, and MOH fractions were reconstituted using DMSO to form final stock concentration of 500 µg/ml while that of EA due to merger quantity available at 250 µg/ml. To 100 µl of nutrient broth in a sterile 96 well plate, 50 µl of varying concentrations of the organic solvent fractions (PET, DCM, and MOH fractions) obtained by serially diluting the active plant fractions resulting in concentrations ranging from 500 to 3.91 µg/ml while EA at 250-1.95 µg/m was added followed by 50 µl of test organisms previously diluted to equivalent of 0.5 McFarland standard. The choice of concentrations profiles was guided by the limiting material available for the study and care to have possible MIC within the acceptable limit of 100 µg/ml. Addition of the test organisms was done in all the wells with the exception of column 11 wells which contained neat DMSO and broth; this served as control to check for purity. The adequacy of the media to support the growth of the test organism was interrogated by putting the broth and the test organism in wells of column 12. The plates were then covered with a sterile “cling-on” sealer and incubated for 24 h at 37°C. Bacterial growth was evaluated by addition of 40 µl of 0.2 mg/ml p-iodonitrotetrazolium chloride (INT, Sigma) to each well and incubated for 30 min. The growth of bacteria was detected by formation of a pink-red coloration while inhibition of growth was signaled by persistence of a clear coloration. The lowest concentration that exhibited color change was considered as the MIC. MBC was determined by streaking a loopful of broth from wells that exhibited no color change onto sterile nutrient agar and SDA for bacteria and fungi, respectively, and thereafter incubated at 37°C for 24 h. The lowest concentration that exhibited no growth was considered as the MBC [12].
Antituberculous Activity
The H37Rv strain of MTB ATCC 27294 was sourced from the KEMRI, Nairobi. Before its use, it was rejuvenated on Lowenstein-Jensen slants for 14 days at 37°C following standard procedures [13]. The efficacy of the plant fractions against MTB was carried out using the BACTEC MGIT 960 system (BD, New York, USA). This is a fully automated, high volume, non-radiometric instrument that offers continuous monitoring of culture growth. Growth supplement (0.8 ml) containing a mixture of OADC - oleic acid, bovine albumin, dextrose, and catalase was added to five 7 ml BBL™ MGIT™ tube and labeled appropriately to provide essential substrates for rapid growth of mycobacteria. 100 µl of BBL™ MGIT™ streptomycin, isoniazid, rifampicin, ethambutol (SIRE) prepared aseptically according to the manufacturer’s instructions was added to corresponding labeled BBL™ MGIT™ tube followed by addition of 0.5 ml of 1% Mycobacterium suspension. Mycobacterium suspension was prepared by pipetting 0.1 ml Middlebrook 7H9 Broth containing Mycobacterium adjusted to 0.5 McFarland standard into 10 ml sterile saline aseptically. The BACTEC MGIT™ 960 system (BD, New York, USA) was then loaded following the manufacturer’s instructions and incubated at 37°C [14]. These served as the positive SIRE control (streptomycin at 1.0 µg/ml, isoniazid at 0.1 µg/ml, rifampicin at 1.0 µg/ml, and ethambutol at 5.0 µg/ml), whereas DMSO was used as a negative control. The procedure was repeated using PET, DCM, EA, and MOH organic solvent fractions. The organic solvent fractions were tested at varying concentrations ranging from 50 to 6.25 µg/ml (PET, DCM, and MOH) or 25-3.125 µg/ml (EA, due to limited amounts) to determine the MIC.
Phytochemical Analysis
Phytochemical analysis was done to establish the class(es) of ingredient(s) present in the active organic solvent fractions that could be responsible for activity and/or cytotoxicity using thin layer chromatography (TLC). TLC plates were developed with EA:PET (2:8) with few drops of glacial acetic acid before spraying with TLC visualizing reagent to give characteristic color changes [7,15-18]. The results were reported as (+) for presence and (−) for absence based on color intensity.
Dragendorff’s reagent for tests for alkaloids
The reagent was prepared by mixing two portions: Reagent A - 0.85 g of bismuth substrate prepared in a solution of 10 ml acetic acid and 40 ml water. Reagent B - 8 g of potassium iodide dissolved in 20 ml of water (stock solution was prepared by mixing volumes of solution A and B). The spray reagent was constituted by mixing 1 ml of the stock solution with 2 ml of fresh acetic acid and 10 ml of water. Formation of orange-brown spots on yellow background indicated the presence of alkaloids and other nitrogen compounds.
Ferric ferrocyanide reagent for phenolic test
About 10% iron chloride (FeCL3) was mixed with iron cyanide (FECN6) (0.1 g/10 ml). Ferric chloride (0.1 g) and potassium ferricyanide (K3F3CN3) (0.1 g) were freshly prepared by dissolving in 10 ml of distilled water. Equal portions of FeCL3 and FECN6 were mixed then sprayed to the plates and heated at 110°C. The instant blue change indicates the presence of phenols.
Vanillin reagent for terpenoids test
About 10% vanillin was dissolved in acidified ethanoic acid in ratio of 2:1, and thereafter sprayed onto the plates. The plates were then dried in the oven for 15 min. The presence of terpenoids was indicated by formation of different colors: Brown or dark green or purple.
Tests for flavonoids
TLC plate loaded with samples was exposed to ammonia. The presence of flavonoids was confirmed by colored spots, e.g., yellow, pink, gray, and brown spots.
Tests for anthraquinones
Sample loaded TLC plate was sprayed with a solution of 10 ml MOH and 10 g potassium hydroxide. Change of the original yellow-brown to purple confirms presences of anthraquinones.
Cytotoxicity Testing
In vitro toxicity of active plant organic solvent fractions were tested using HEp-2 cells (human laryngeal carcinoma cell line ATCC CCL-23) by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT). HEp-2 cells were cultured in growth media comprising of 100 ml DMEM, 10 ml fetal bovine serum (FBS), 1 ml penstrep, 1 ml amphotericin B, and 1 ml L-glutamine. They were incubated at 37°C in 5% CO2 until they attained confluency. Thereafter, they were harvested by trypsinization and then 2 ml transferred into a 50 ml vial and adjusted to 1 × 105 cell/ml by topping up to 50 ml mark using growth media. 100 µl of the cell suspension was plated into wells of a microtiter plate in duplicates from A-H and then incubated for 48 h at 37°C and 5% CO2 in 100 µl growth media to form a confluent monolayer. Rows of cells containing medium without plant extracts were included to act as a negative control. The growth medium was replaced with 100 µl of maintenance medium (100 ml DMEM, 2 ml FBS, 1 ml penstrep, 1 ml amphotericin B, 1 ml L-glutamate, and 0.1 ml gentamycin). HEp-2 cells were then treated with varying concentrations of plant extracts (from 2.0 µg/ml to 500 µg/ml) for 48 h at 37°C after which 10 µl of 5 mg/ml MTT solution was added to all wells after aspirating off the plant extracts and further incubated for 4 h. Then, 100 µl of acidified isopropanol (0.04 N HCl in isopropanol) was added to dissolve formazan. The absorbance was read at 562 nm and 690 nm used as a reference using ELISA Scanning Multiwell Spectrophotometer (Multiskan Ex labs systems) and the percentage cell viability determined.
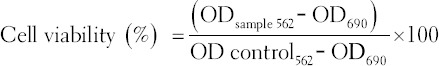
Statistical Analyses
Mean zones of inhibition of the plant extracts on the microbial samples were determined. One-way ANOVA was used to determine the effect of plant extracts on microbial samples. Mean zones of inhibitions were separated by Tukey’s test (P < 0.05). Cytotoxicity data were calculated as a percentage of untreated controls. Regression analysis was used to determine CC50 (concentration that kills 50% of the Vero cells). A sample was considered toxic if it had CC50 value of <90 µg/ml. All data were analyzed using SPSS version 20.
RESULTS
Antimicrobial Activity of C. myricoides
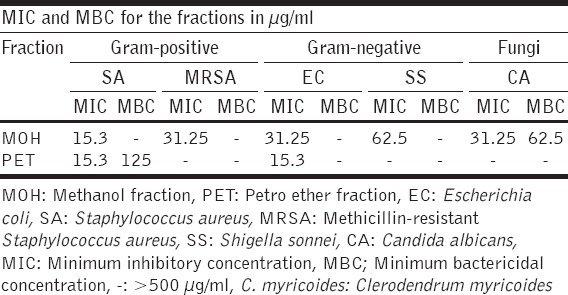
The methanolic solvent fraction exhibited broad spectrum activity inhibiting 75% (six out of eight of tested pathogens) while EA registered the least activity; 12.5% (inhibiting one out of eight test pathogens). The inhibitory effect differed significantly among the fractions (P < 0.05). The diameter of the zone of inhibition ranged from (20.3 ± 0.3) to (6.3 ± 0.3) mm. The activity was a broad spectrum with Gram-positives bacteria being more susceptible to the organic fractions than Gram-negative bacteria. S. aureus was most sensitive Gram-positive bacteria with the highest zone of inhibition of 20.3 ± 0.3 and the lowest MIC of 15.3 µg/ml. E. coli was the Gram-negative bacteria that gave the highest zone of inhibition of 14.7 ± 0.3 mm and MIC of 31.25 µg/ml. Among the fungi, C. albicans had the highest zone of inhibition of 12.3 ± 0.3 mm, MIC of 31.25 µg/ml, and MBC of 62.5 µg/ml [Tables 1 and 2]. The methanolic solvent fraction exhibited the best activity with MIC values of ≤62.5 µg/ml recorded against 62.5% (five of the eight) tested microbes which is less than 100 µg/ml; the set threshold for plant extract fractions [19]. DCM and EA solvent fractions gave inhibition zones of less than 10 mm. Therefore, these 2 fractions were not considered for MIC and MBC determination [Table 2].
Table 1.
Antimicrobial effects of C. myricoides extract fractions
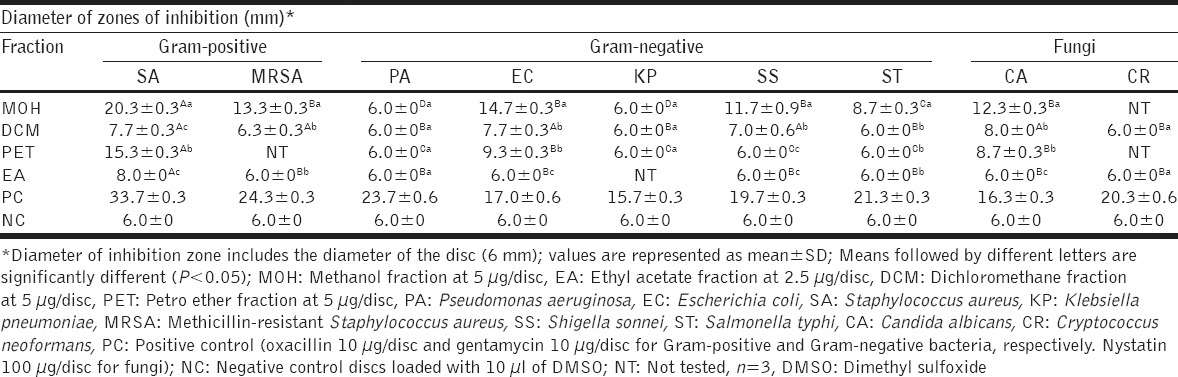
Table 2.
MIC and MBC for C. myricoides extract fractions
Antimycobacterial Activity of the Organic Solvent Fractions
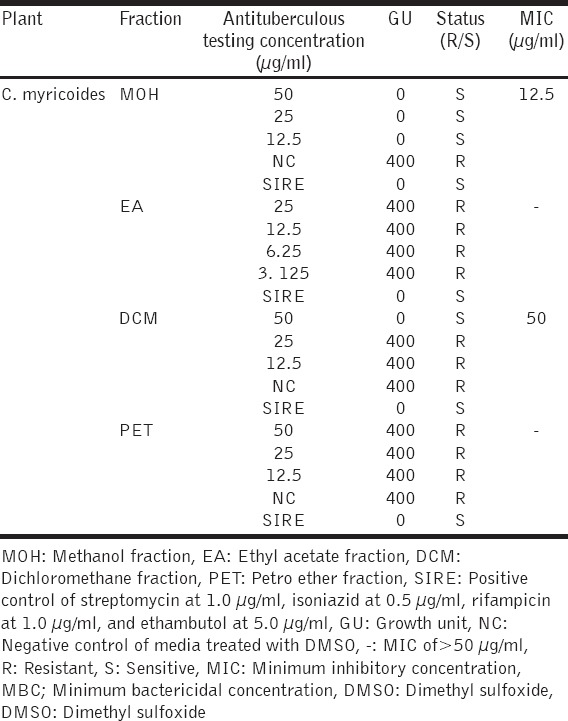
The antitubercular activity assay was done using varying concentrations of organic solvent extracts of C. myricoides by BACTEC MGIT™ 960 system (BD, New York-U.S.A) to determine MIC values. The results revealed that methanolic solvent extract was the most lethal to the tubercle with an MIC of <12.5 µg/ml. This was comparable to SIRE (positive control) which gave Zero GU, and it is indicative of a strong antituberculous activity. DCM solvent extract exhibited antituberculous activity with MIC of 50 µg/ml while EA and PET fractions had MIC of >50 µg/ml [Table 3].
Table 3.
Antituberculous activity for C. myricoides extract fractions
Preliminary Phytochemical Analysis
The qualitative phytochemical analysis results revealed that EA and DCM solvent extracts contained all the major classes of phytochemicals assayed for whereas PET and methanolic solvent extracts contained two and three classes, respectively [Table 4].
Table 4.
Phytochemical composition of C. myricoides extract fractions

Cytotoxicity Results of C. myricoides
The cytotoxicity results indicated that the PET, DCM, and EA solvent extracts were all not within the acceptable toxicity limits (CC50 >90 µg/ml) against HEp-2 cells. Interestingly, toxicity of the methanolic extract (which yielded the highest antibacterial and antitubercular activity) was within the acceptable toxicity limit with CC50 of >500 µg/ml) [Table 5].
Table 5.
Cytotoxicity of C. myricoides extract fractions

DISCUSSION
The search for potent antimicrobial ingredients from natural sources has recently received much attention. There are concerted efforts from scientific community to identify safe innovative therapeutic agents for various conditions including infectious diseases that can act as suitable pharmaceuticals agent to replace synthetic ones. Plant-derived phytocompounds can serve as molecular prototype to synthesize less toxic, yet highly effective drugs to combat the ever-evolving plethora of bugs. These phytochemicals have been demonstrated to have remarkable therapeutic potential against human microbes including fungi, bacteria, parasites, and viruses. Many studies have been conducted using various plants’ extracts to screen for antimicrobial activity as well as to unearth novel antimicrobial compounds [15].
In the present investigation, four different organic solvent fractions of C. myricoides were sequentially prepared using organic solvents of increasing polarity (PET, DCM, EA, and MOH fraction). Each of the organic solvent extracts was evaluated for their antimicrobial activity against selected Gram-positive, Gram-negative, acid-fast bacteria, and fungi, which are considered to be of public health concern. The efficacy of each fraction against Gram-positive, Gram-negative bacteria, and fungi was determined by disc diffusion and broth microdilution assays to evaluate zones of inhibition, MIC, and MBC. Efficacy against acid-fast bacteria was evaluated using BACTEC MGIT™ 960 system (BD, New York, USA) to determine MIC.
The antimicrobial activity results varied remarkably with respect to the organic solvent extract used. The observed variation in terms of efficacy among the fractions most probably may be due to differences in polarity of the organic solvents used during the extraction process that resulted in the differential distribution of bioactive ingredient among the extracts. This suggests that root part of C. myricoides contains several antibacterial and antifungal compounds of different polarities as supported by phytochemical studies [Table 4]. These phytochemicals are sequestered at different levels of polarity explaining the varied activity and cytotoxicity demonstrated by extracts. In general, the methanolic solvent extract was the most potent of all fractions with the highest zone of inhibition of 20.3 mm (S. aureus), lowest MIC of <12.5 µg/ml (MTB), and the lowest MBC (C. albicans). These results demonstrated that the most polar (MOH) solvent was capable of extracting active antimicrobial component(s) from the roots of C. myricoides. This strongly suggests that solvents play a key role in the extraction of active phytochemicals. In this case, MOH solvent seems to be a superior solvent for plant antimicrobial molecules extraction, and this is in agreement with previous studies [20-23].
The antimicrobial activity testing revealed that the organic solvent extract of C. myricoides has broad spectrum activity inhibiting the growth of Gram-positive, Gram-negative, acid-fast bacteria, and fungi. The potency against Gram-positive was significant (P < 0.05). For instance, methanolic fraction gave inhibition zone diameter of 20.3 mm and MIC of 31.25 µg/ml. The sensitivity of the Gram-positive microbes could be attributed to their cytoplasmic membrane which is simple, composed of a lipid bilayer, hence not an effective permeability barrier for most amphipathic compounds, and therefore, can be easily traversed by antibacterial. In contrast, Gram-negative bacteria have evolved a sophisticated, strong permeability barrier with an additional outer membrane comprising a highly hydrophilic lipopolysaccharide layer. This layer restricts penetration of hydrophobic and amphipathic compounds, which encompasses many drug compounds making them less sensitive [24,25]. The difference in sensitivity among tested strains also could be due to genetic differences between different strains, and this proofs the necessity of antibiogram before prescription as a precautionary measure in mitigating drug resistance development [26].
The qualitative screening of C. myricoides root extract’s revealed presence of alkaloids, phenols, anthraquinones, terpenoids, and flavonoids. Although there are several sub-classes of these phytochemicals, research has shown that members of a particular sub-class inflict damage on microbes by employing almost the same mechanism [22]. For instance, alkaloids are known to interfere with microbial cell wall and DNA besides enhancing the role of immune cells resulting in microbicidal activity. On the other hand, flavonoids, which are an effective antimicrobial agent, inactivate microbial enzymes and interfere with the bacterial cell wall by forming complexes with soluble proteins and the bacterial cell wall, respectively. Other studies have shown flavonoids to have antituberculous activity, and here, they function mechanistically by inhibiting de novo biosynthesis of fatty acid in Mycobacteria, inhibiting mycolic acid biosynthesis, proteasome inhibition, topoisomerase inhibition, inhibition of phosphatidyl-inositol 3-kinase, induction of cell cycle arrests, accumulation of p53 or enhanced expression of c-fos and c-myc genes [13,27]. The observed antitubercular activity of the MOH fraction could be due to presences of terpenoids, which have been shown to have capacity to traverse the highly hydrophobic tubercle envelope and interfere with the integrity of microbial membranes [28,29]. Similarly, anthraquinones and phenols, present in some of the fractions, are useful antimicrobial phytochemicals. Phenols demonstrate antimicrobial activity through enzyme inhibition by oxidized molecules (probably through reaction with sulfhydryl group(s) or through non-specific interaction with proteins), whereas anthraquinones exhibit their antimicrobial activity by inhibiting nucleic acid synthesis [22].
On the other hand, it was of significant to assess the safety of C. myricoides because locally its root extracts are chewed by the human subject. Cytotoxicity assay results indicated that methanolic fraction of C. myricoides is within the acceptable toxicity limit (CC50> 500 µg/ml). However, EA (4.22 µg/ml), PET (14.05 µg/ml), and DCM (20.53 µg/ml) fractions were found to be highly toxic. Thus, MOH fraction is a good candidate for the antibacterial, antifungal, and antituberculous agent because of its observed remarkable, selective activity within the acceptable toxicity limits.
CONCLUSION
A major output of the current study is the identification of the methanolic solvent fraction which exhibited a broad spectrum activity inhibiting 75% (six out of eight of test pathogens). It had the best bioactivity, with MIC values of ≤62.5 µg/ml recorded against 62.5% (five of the eight) tested microbes which was less than 100 µg/ml; the set threshold for plant extract fractions. It yielded the highest zone of inhibition of 20.3 mm (S. aureus), lowest MIC of <12.5 µg/ml (MTB), and the lowest MBC (C. albicans). Of particular excitement is its high activity against MRSA, S. aureus, E. coli, S. sonnei, C. albicans, and MTB, which are currently posing great public health challenge due to drug resistance development and as major sources for community and hospital based infections. To the best of our knowledge, this is the first report exploring the antituberculous activity of C. myricoides and thence a major output in search of new safe drug leads to mitigate the global tuberculosis threat. However, more work is still required with a view of isolating pure compounds, determining the efficacy of the pure compound as well as elucidate the probable mechanism of action.
ACKNOWLEDGMENT
This research was supported by the International Foundation for Science, Stockholm, Sweden, through IFS grant No. F/5372-1 to Sospeter Ngoci Njeru. The authors would wish to acknowledge, Kenya Medical Research Institute (KEMRI) especially Dr. Bii C. among others, Prof. Anakalo Shitandi (Kisii University) and Prof. Matasyoh J.C. (Egerton University) for their support on this work.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, et al. Bad bugs, no drugs: No ESKAPE!An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Global Report for Research on Infectious Diseases of Poverty. 2012. [Last retrieved on 2014 Feb 05]. Available from: http://www.whqlibdoc.who.int/publications/2012/9789241564489_eng.pdf .
- 3.Bibi Y, Nisa S, Chaudhary FM, Zia M. Antibacterial activity of some selected medicinal plants of Pakistan. BMC Complement Altern Med. 2011;11:52. doi: 10.1186/1472-6882-11-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adnan M, Bibi R, Mussarat S, Tariq A, Shinwari ZK. Ethnomedicinal and phytochemical review of Pakistani medicinal plants used as antibacterial agents against Escherichia coli. Ann Clin Microbiol Antimicrob. 2014;13:40. doi: 10.1186/s12941-014-0040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riley BW, Brokensha D. The Mbeere in Kenya: Botanical Identity and Use. II. USA: University Press of America; 1988. pp. 76–7. [Google Scholar]
- 6.Kareru PG, Kenji GM, Gachanja AN, Keriko JM, Mungai G. Traditional medicines among the Embu and Mbeere peoples of Kenya. Afr J Tradit Complement Altern Med. 2006;4:75–86. doi: 10.4314/ajtcam.v4i1.31193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pascaline J, Charles M, Lukhoba C, George O. Phytochemical constituents of some medicinal plants used by the Nandis of South Nandi district, Kenya. J Anim Plant Sci. 2011;9:1201–10. [Google Scholar]
- 8. [Last accessed on 2015 Aug 25]. www.the plantlist.org. Available from: http://www.the plantlist.org .
- 9.Clinical and Laboratory Standard Institute. CLSI Methods for Determining Bactericidal Activity of Antimicrobial Agents. Tentative Standards M26-T. Wayne: National Committee for Clinical Laboratory Standards; 2007. [Last accessed on 2015 Aug 25]. Available from: http://www.shop.clsi.org/site/Sample_pdf/M26A_sample.pdf . [Google Scholar]
- 10.Mbaveng AT, Ngameni B, Kuete V, Simo IK, Ambassa P, Roy R, et al. Antimicrobial activity of the crude extracts and five flavonoids from the twigs of Dorstenia barteri (Moraceae) J Ethnopharmacol. 2008;116:483–9. doi: 10.1016/j.jep.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 11.Mothana RA, Abdo SA, Hasson S, Althawab FM, Alaghbari SA, Lindequist U. Antimicrobial, antioxidant and cytotoxic activities and phytochemical screening of some yemeni medicinal plants. Evid Based Complement Alternat Med. 2010;7:323–30. doi: 10.1093/ecam/nen004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lai HY, Lim YY, Kim KH. Blechnum orientale Linn - A fern with potential as antioxidant, anticancer and antibacterial agent. BMC Complement Altern Med. 2010;10:15. doi: 10.1186/1472-6882-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mariita R, Ogol C, Oguge N, Okemo P. Antitubercular and phytochemical investigation of methanol extracts of medicinal plants used by the Samburu community in Kenya. Trop J Pharm Res. 2010;9:379–85. [Google Scholar]
- 14.Becton Dickson and Company. Becton Dickinson Product and Procedure Manual MA-0029; BACTEC 12B Mycobacteria Culture Vials Middlebrook 7H12”. Sparks, MD, USA: Becton Dickson and Company; 2007. [Google Scholar]
- 15.Houghton PJ, Raman A. Laboratory Hand Book for the Fractionation of Natural Extracts. 1st ed. London: Chapman and Hall; 1998. ; pp. 1–153. [Google Scholar]
- 16.Edeoga HO, Okwu DE, Mbaebie BO. Phytochemical constituents of some Nigerian medicinal plants. Afr J Biotechnol. 2005;4:685–8. [Google Scholar]
- 17.Ngoci SN, Matasyoh JC, Mwaniki CG, Mwendia CM. Phytochemical and cytotoxicity testing of Indigofera lupatana Baker F. J Anim Plant Sci. 2011;11:1364–73. [Google Scholar]
- 18.Somboro AA, Patel K, Diallo D, Sidibe L, Chalchat JC, Figueredo G, et al. An ethnobotanical and phytochemical study of the African medicinal plant Guiera senegalensis J. F. Gmel. J Med Plants Res. 2011;5:1639–51. [Google Scholar]
- 19.Kuete V, Kamga J, Sandjo LP, Ngameni B, Poumale H, Ambassa P, et al. Antimicrobial activities of the methanol extract, fractions and compounds from Ficus polita Vahl. (Moraceae) BMC Complement Altern Med. 2011;11:6. doi: 10.1186/1472-6882-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eloff JN. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med. 1998;64:711–3. doi: 10.1055/s-2006-957563. [DOI] [PubMed] [Google Scholar]
- 21.Ngoci SN, Matasyoh JC, Mwaniki CG, Mwendia CM. Antibacterial activity of methanol root extract of Indigofera lupatana Baker F. Eastern J Med. 2012;17:11–6. [Google Scholar]
- 22.Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12:564–82. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fankam AG, Kuete V, Voukeng IK, Kuiate JR, Pages JM. Antibacterial activities of selected Cameroonian spices and their synergistic effects with antibiotics against multidrug-resistant phenotypes. BMC Complement Altern Med. 2011;11:104. doi: 10.1186/1472-6882-11-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohamed LT, El-Nur BS, Abdelrahman MN. The Antibacterial, antiviral activities and phytochemical screening of some Sudanese medicinal plants. Eurasia J Biosci. 2010;4:8–16. [Google Scholar]
- 25.Lewis K, Ausubel FM. Prospects for plant-derived antibacterials. Nat Biotechnol. 2006;24:1504–7. doi: 10.1038/nbt1206-1504. [DOI] [PubMed] [Google Scholar]
- 26.Yimta F, Simplice MR, Francos N, Sidor NG, de Dieu TJ, Roger KJ. Antibacterial activity of methanol extract and fractions from Kalanchoe crenata, Terminalia avicennioides and Sarcocephalus latifolius. Pharmacologia. 2014;5:199–204. [Google Scholar]
- 27.Yuan E, Liu B, Ning Z, Chen C. Preparative separation of flavonoids in Adinandra nitida leaves by high-speed counter - Current chromatography and their effects on human epidermal carcinoma cancer cells. Food Chem. 2009;115:1158–63. [Google Scholar]
- 28.Edwards PA, Ericsson J. Sterols and isoprenoids: Signaling molecules derived from the cholesterol biosynthetic pathway. Annu Rev Biochem. 1999;68:157–85. doi: 10.1146/annurev.biochem.68.1.157. [DOI] [PubMed] [Google Scholar]
- 29.Gobalakrishnana R, Kulandaivelu M, Bhuvanesweri R, Kandavel D, Kannan L. Screening of wild plant species for antibacterial activity and phytochemical analysis of Tragia involucrata L. J Pharm Anal. 2013;3:460–5. doi: 10.1016/j.jpha.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


