Abstract
Objective:
According to our previous studies, propolis of Nigerian origin showed some evidence of hypoglycemic and hypolipidemic activities in addition to its ability to ameliorate oxidative-stress-induced organ dysfunction. This study was carried out to determine whether an ethanolic extract of Nigerian propolis (EENP) improves glycated hemoglobin A1c (HbA1c), fasting plasma glucose, very low-density lipoprotein (VLDL), and high-density lipoprotein (HDL) concentrations in rats that have alloxan diabetes.
Materials and Methods:
Diabetes was induced with alloxan (110 mg/kg). Animals were divided into 5 groups (n = 5); Group 1 was non-diabetic receiving normal saline and Group 2 was diabetic but also received only normal saline. Groups 3, 4, and 5 were diabetic receiving 200 mg/kg propolis, 300 mg/kg propolis, and 150 mg/kg metformin, respectively, for 42 days.
Results:
Hyperglycemia, elevated serum level of VLDL, elevated plasma level of HbA1c, and decreased levels of HDL were observed in the diabetic untreated animals. Nigerian propolis decreased blood glucose level and serum level of VLDL but elevated HDL level. These changes were significant (P < 0.05). The levels of plasma HbA1c were also reduced in the propolis-treated groups, and the reduction was significant (P < 0.05).
Conclusion:
Nigerian propolis contains compounds exhibiting hypoglycemic, antihyperlipidemic, and HbA1c reducing activities.
KEY WORDS: Glycated hemoglobin, high-density lipoprotein, Nigerian propolis, plasma glucose, very low-density lipoprotein
INTRODUCTION
Diabetes mellitus is a disease that can be a severe threat to public health as the number of adults that will be suffering from the disease by the year 2030 is estimated to be 439 million [1]. It was estimated that about 387 million people have diabetes worldwide, as of 2014, representing 3.8% of the adult population [2]. Alternative therapies with antihyperglycemic properties are more and more sought by diabetic patients for blood sugar control [3].
Propolis is a resinous substance that bees produce using materials collected from the buds and other parts of certain trees. It is used as a traditional herbal medicine in many countries. Over 300 components have been isolated from propolis. Moreover, they are mainly compounds of phenol (e.g., flavonoids and aromatic compounds), terpenes, and essential oil [4]. Propolis has been proven to have many bioactivities such as antipathogenic, antioxidative, immunoregulatory, antitumor, hepatoprotective, and anti-inflammatory and is now the focus of many biomedical research projects [5]. In general, propolis contains phenolic aldehydes, sesquiterpene, polyphenols, quinines, steroids, coumarins, amino acids, and inorganic compounds [6]. Plant origin and the region where it is collected bear a great significance to what the composition of propolis will be [7], hence, necessity of the prefix of source country (e.g., Nigerian propolis). The Nigerian propolis used for this study was obtained from south-western part of Nigeria – Abeokuta, Ogun State, Nigeria. Location coordinates of the site of collection goes thus: 7.15° North and 3.35° East. The propolis was collected during the rainy season. Despite the chemical differences, it is well known that samples of different geographical origins and chemical compositions usually demonstrates similar pharmacological properties [8]. Baccharis dracunculifolia shrub is the main plant source of Brazilian propolis, and prenylated p-coumaric acids are the prevalent biologically active substances in this propolis [9]. Chinese propolis is a propolis derived from poplar tree; and cinnamic acids, flavonoids, and their esters are the predominant active components in this propolis [10]. Earlier studies show that Chinese propolis helped in reducing fasting blood glucose and ameliorated oxidative stress and lipid metabolism in alloxan-induced diabetic rats [11]. Clinical application of propolis was useful in the control of blood glucose [12].
Chemical analysis of Nigerian propolis used for this study revealed that it is composed of alkaloids in appreciable amounts and also steroids, glycosides, saponins, and tannins in moderate amounts while other flavonoids and phlobatannins are in minute amounts. It also contains phenol compounds. The main plant source of this propolis is, however, yet to be determined.
Glycated hemoglobin (hemoglobin A1c or HbA1c) is a type of hemoglobin primarily used to identify the average plasma glucose concentration over extended periods of time (at least 6-8 weeks) [13]. It is formed in a non-enzymatic pathway by hemoglobin’s normal exposure to high plasma glucose levels [14]. HbA1c is implicated in the genesis of diabetic complications such as retinopathy, nephropathy, and neuropathy [15]. It is estimated that the risks of these complications are decreased by approximately 3% for every 1 mmol/mol decrease in HbA1c [16]. Monitoring the HbA1c level in type-1 diabetic patients may help to improve treatment [16].
Diabetes mellitus is almost always associated with changes in plasma lipoproteins. To understand the mechanism of the changes in lipoproteins that occur in diabetes mellitus and how they may influence the complications that accompany this disorder, we must examine lipoprotein metabolism. There are multiple abnormalities of lipoprotein metabolism, primarily in very low-density lipoprotein (VLDL) and high-density lipoprotein (HDL) but also to some extent in low-density lipoprotein (LDL), which can potentially explain the increased atherogenesis in human diabetics.
Alloxan (2,4,5,6-pyrimidinetetrone) is a pyrimidine derivative which can bond with H2O molecules when in aqueous solution to form alloxan hydrate [17]. Alloxan is a toxic glucose analog that selectively destroys insulin-producing pancreatic β cells when administered to rodents and many other animal species [18,19]. This causes an insulin-dependent diabetes mellitus known as “Alloxan diabetes” in these animals, with features similar to Type 1 diabetes in humans [20]. Alloxan is selectively toxic to the β cells as it preferentially accumulates in these cells through uptake by the glucose transporter 2; hence, its usage in the induction of diabetes in laboratory animals [21].
This study showed the effect of ethanolic extract of Nigerian propolis (EENP) on plasma glucose, HbA1c, and some blood lipids (VLDL and HDL) in Type 1 diabetic rats.
MATERIALS AND METHODS
Drugs and Reagents
Nigerian propolis was obtained from south-western part of Nigeria, in Abeokuta town, Ogun State, during rainy season. The alloxan and metformin used were products of Sigma-Aldrich (Sigma, St. Louis, USA). The glucometer used in evaluating blood glucose was on-call-plus (ACON Biotech, Hang Zhou, China). A standard weighing scale was used for weight determination. The kits used for lipid profile was by Randox (UK). HbA1c was determined using the diabetes control and complications trial (DCCT) aligned clover A1c Analyzer (Infopia Co. Ltd., Korea) with a test range of 4-14%.
Extract Preparation
The fresh propolis was frozen (this makes it brittle) and broken into small pieces. 100 g of propolis was soaked in 500 ml of 95% ethanol. A total of 250 g of raw propolis was soaked. The mixture was allowed to stay for a week. This allowed for proper extraction of the propolis into the solvent (ethanol). After 1-week, the mixture was then filtered using Whatman No.1 filter paper. The filtrate was evaporated to dryness under low pressure and a maximum temperature of 60°C yielding a crude (EENP) weighing 29 g. The method used here, in the preparation of propolis extract is similar to that described in a publication by Musa et al. [22].
Laboratory Animals
The animals used for this study were bred in the animal house of the Department of Physiology, University of Ibadan, Nigeria, under standard laboratory conditions and were allowed free access to standard rat food and water. The study was performed in accordance with the ethical guidelines stipulated by the Ethical Committee of the College of the Institution. These guidelines are in accordance with the Helsinki Principles for laboratory animal use and care. Rats, weighing 160-200 g, were selected for the experiment.
Induction of Diabetes
Animals were fasted for 12 h. Diabetes was then induced by intraperitoneal injection of alloxan (110 mg/kg) dissolved in sterile normal saline (0.9%) after which they were allowed free access to food and water. The diabetic state was determined 72 h after alloxan administration. A pinch of blood collected from the tail was analyzed for glucose level (in each animal) with the aid of on-call-plus glucometer. Animals chosen for the diabetic model were identified and selected on the basis of blood glucose levels – higher than 230 mg/dl [21].
Experimental Design
There were five groups of 5 rats per group:
Group 1: Control (non-diabetic); received normal saline
Group 2: Diabetic untreated; received normal saline
Group 3: Diabetic experimental; received 200 mg/kg of EENP as used by Bhadauria et al. [23]
Group 4: Diabetic experimental; received 300 mg/kg of EENP as used by Zamami et al. [24]
Group 5: Diabetic treated; received 150 mg/kg of metformin.
Drug Administration
The EENP was mixed with Tween 80 (polysorbate 80) for solubility [23], at the ratio of 200 mg propolis to 1 ml of Tween 80 before oral administration. The metformin and control groups were also given Tween 80 (proportionately mixed in) before oral administration. The administration continued for 42 days (a period sufficient for the formation of HbA1c). The fasting blood glucose and weight of all rats were measured weekly for 6 weeks.
Animal Sacrifice
After 42 days of treatment, animals were anesthetized and sacrificed by cervical dislocation and then dissected. Blood was obtained directly from the heart into heparinized and plain centrifuge bottles for plasma and serum samples, respectively.
Blood Sampling and Biochemical Analysis
Whole blood was used in the analysis of HbA1c with the aid of DCCT aligned clover A1c analyzer (Infopia Co. Ltd., Korea) that has a test range of 4-14%.
For serum preparation, blood samples (in plain centrifuge bottles) were allowed to stand for 30 min and then centrifuged at 3000 r/min for 15 min at 19°C to obtain the serum.
Obtained serum was analyzed for HDL and VLDL by an enzymatic method using commercial diagnostic kits (Randox, UK).
To determine the weekly levels of glucose, pinches of blood were obtained from the tip of the tails of the rats and dropped on the glucometer-strip which was prior inserted into the glucometer. The readings were then noted. The weekly amounts of weight of the rats were taken using a standard scale.
Statistical Analysis
Data were analyzed by one-way analysis of variance followed by Tukey-Kramer post-hoc test. The software used was GraphPad Prism (version 5, XML Project, November 2010) by GraphPad Software, Inc. California, USA. Results were presented as mean ± standard error of mean with statistical significance accepted at P ≤ 0.05.
RESULTS
To measure the effect of EENP on alloxan-induced diabetic rats, various biochemical assays were done in all experimental animals to approximate the levels of plasma glucose, serum VLDL, serum HDL, and plasma HbAlc. The following are the pharmacological effects discovered:
Glycemic Control
From the results, it can be seen that the 200 mg/kg propolis and the 300 mg/kg propolis caused a 67.98% and 72.76% (significant) drop in blood glucose, respectively (P < 0.001), compared to the 6.36% fluctuation of blood glucose level in the untreated group. The blood glucose level of the untreated group hovered on the high side while the blood glucose levels in the treated groups (including metformin-treated group which caused a 60.30% significant reduction (P < 0.001) in blood glucose level) significantly dropped over the period of administration with the highest percentage drop recorded in the 300 mg/kg propolis-treated group [Table 1]. Figure 1 is showing and comparing the weekly changes in blood glucose levels in the different groups from the 1st to 5th week. Alloxan administration had increased blood glucose level by the 1st week in both treated and untreated groups. Blood glucose level was significantly reduced (P < 0.001) in the metformin and propolis (both doses) treated groups from the 2nd week onward when compared to the diabetic control group.
Table 1.
Effects of propolis extracts on blood glucose level of alloxan diabetic rats
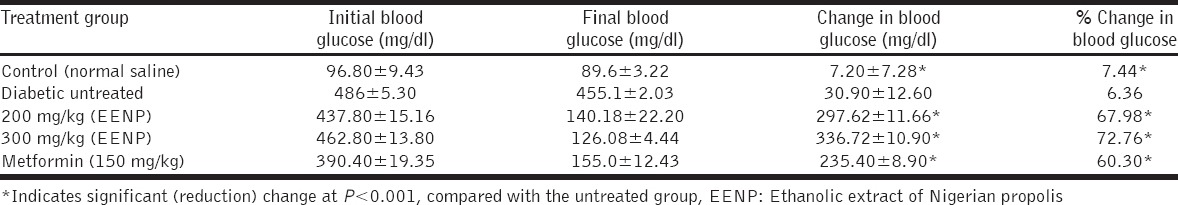
Figure 1.
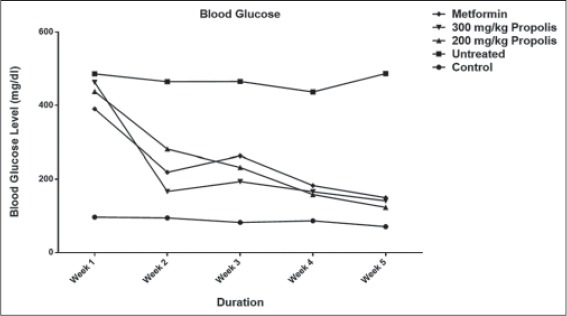
Effect of ethanolic extract of Nigerian propolis on blood glucose
Weight Amelioration
Table 2 compares change in body weight of the propolis-treated groups with the untreated group. The 200 mg/kg propolis group caused just a 0.5% increase in body weight, whereas the 300 mg/kg propolis caused an 11% increase in body weight. Both increments were significant (P < 0.01) when compared to the 21.91% weight loss in the untreated group as indicated by the negative sign. Metformin also significantly prevented weight loss when compared to untreated group. The untreated animals lost weight throughout the duration of the experiment while the treated ones gained weight with the most weight gain registered in the metformin and 300 mg/kg propolis-treated groups. Figure 2 is depicting and comparing the weekly change-in-weight of animals in the different groups. Note that the change-in-weight (∆ weight) is plotted against duration (in weeks) and not the absolute weight values. This is because the weight of the animals was not uniform at the start of the experiment, so it is reasonable working with and plotting the ∆ weight versus duration, instead of absolute weight values. A plummet is seen in the untreated group indicating loss of weight over the period while a spike is seen in the normal control group indicating continual weight gain over the period. All treated groups ameliorated weight loss significantly, compared to the untreated group.
Table 2.
Effects of propolis extracts on body weight of alloxan diabetic rats
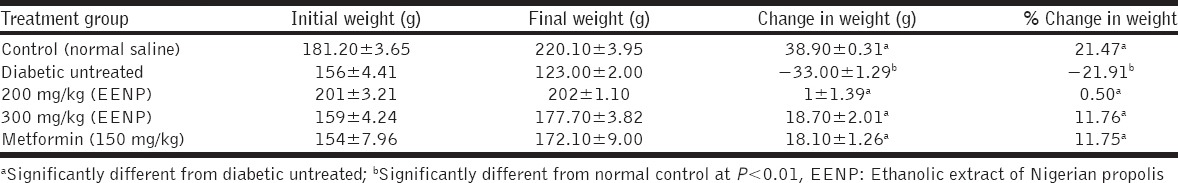
Figure 2.
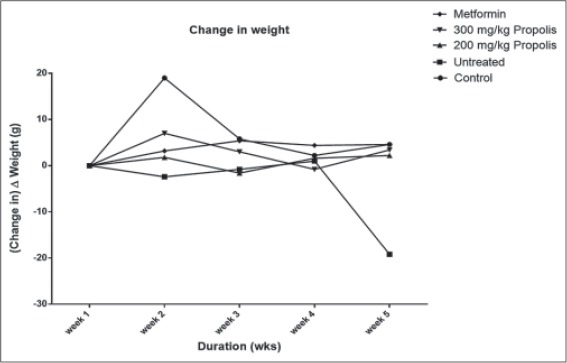
Effect of ethanolic extract of Nigerian propolis on body weight
HbA1c
Table 3 shows comparison between HbA1c levels in the treated groups, untreated group, and control group and at varying levels of significance. It is seen that high levels of HbA1C in all the treated groups (150 mg/kg, metformin; 200 mg/kg, propolis; 300 mg/kg propolis) are significantly prevented (P < 0.05) when compared to the untreated group. This indicates regulation of blood glucose level of the treated animals over the course of the experiment (6 weeks).
Table 3.
Effect of treatment of alloxan-induced diabetic rats with ethanolic extract of Nigerian propolis (EENP) on glycated hemoglobin, VLDL, and HDL
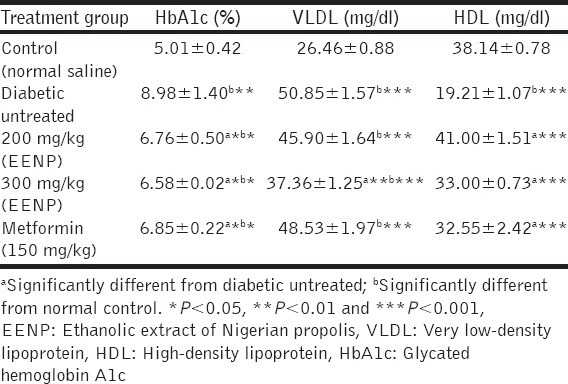
VLDL and HDL
Only the 300 mg/kg propolis caused a significant drop (P < 0.01) in VLDL level when compared with the untreated group, while 200 mg/kg propolis, 300 mg/kg propolis, and metformin caused significant increases (P < 0.001) in HDL levels when compared with the untreated group [Table 3].
DISCUSSION
Hyperglycemia is considered one of the primary causes of diabetes complications, and the presence of hyperglycemia is associated with increased morbidity and mortality [25]. HbA1c is highly correlated with long-term hyperglycemia, and hence, the presence of diabetic microvascular complications [26]. HbA1c is as effective a predictor of microvascular complications as fasting plasma glucose [27,28]. Measurement of HbA1c has been recommended for the diagnosis of diabetes and prediabetes [29]. Significant weight loss accompanies high level of blood glucose that occurs in diabetes. This is due to excessive gluconeogenesis that includes usage of body fats (lipolysis) for the synthesis of new glucose. The result of this study showed that Nigerian propolis was able to ameliorate the rise in blood glucose and the drop in weight of diabetic rats. This is in accordance with our earlier studies [30]. It also shows amelioration of dyslipidemia by propolis to a significant level. The level of HbA1c was also reduced significantly in the propolis-treated groups suggesting amelioration of hyperglycemia over the period of 6-week (period of diabetes and treatment) as against the untreated group. The period of 6 weeks was chosen to allow for proper glycation of hemoglobin to form HbA1c.
Earlier studies carried out on Croatian propolis [31], Chinese propolis [32], Brazilian propolis [32], and Egyptian propolis [33] among many others, revealed their diabetes ameliorating effects.
In this study, 200 mg/kg and 300 mg/kg EENP caused a significant decrease in blood glucose level when compared to the untreated diabetic group with the higher dose causing a more rapid decrease in blood glucose level. The significant hypoglycemic activity of this propolis suggests that propolis exacts its activity either by a direct or indirect mechanism in rats [34]. If the propolis had acted indirectly as a hypoglycemic agent, the effect observed when administered to alloxan-treated rats would have been minimal or absent due to the severe destructive effect of alloxan on the β cells of the pancreas [18]. Furthermore, propolis may have acted indirectly by rejuvenating the surviving β cells to secrete more of insulin instead of aiding the regeneration of necrotic pancreatic β cells. Furthermore, both doses of propolis ameliorated weight loss and caused a significant increase in rat weight when compared to the untreated diabetic group. Both the 200 mg/kg EENP and 300 mg/kg EENP significantly ameliorated HbA1c in diabetic rats; in correlation with the blood glucose lowering effect observed.
Diabetes mellitus is almost always associated with changes in plasma lipoproteins. To understand the mechanism of the changes in lipoproteins that occur in diabetes mellitus and how they may influence the complications that accompany this disorder, we must examine lipoprotein metabolism. There are multiple abnormalities of lipoprotein metabolism, primarily in VLDL and HDL but also to some extent in LDL, which can potentially explain the increased atherogenesis in human diabetics. In this study, increase in serum levels of VLDL and decreased level of HDL that were observed in the diabetic untreated groups agrees with the findings of Douillet et al. [35] and Naziroglu et al. [36]. A number of observations indicate that plasma HDL are low in untreated Type 1 diabetics [37] and HDL increases with degree of glycemic control. On the other hand, VLDL in individuals with Type 1 diabetes receiving adequate therapy need not be elevated [38], and it is now well-established that VLDL elevations in Type 1 diabetes are often well-correlated with degree of diabetic control [39]. The levels of VLDL in the 200 mg/kg EENP group rats did not change significantly when compared with the untreated group rats, whereas rats in the 300 mg/kg EENP group had their VLDL levels decreased significantly. HDL levels, however, increased in both 200 and 300 mg/kg EENP group rats significantly when compared with untreated group.
The prevalent biologically active substances in Brazilian propolis are prenylated p-coumaric acids [9] while in Chinese propolis it is cinnamic acids, flavonoids, and their esters that are the predominant active components [10]. On carrying out chemical screening assay, Nigerian propolis though also contains glycosides, steroids, flavonoids, saponins, and tannins among others (see introduction above); alkaloids appear to be the predominant constituent compounds. Moreover, this group of compounds has been demonstrated to possess antihyperglycemic activities [40]. Research is, however, ongoing to determine which actual compounds are responsible for the antihyperglycemic and hypolipidemic effects.
CONCLUSION
Nigerian propolis significantly reduced the rise in blood glucose of alloxan-induced diabetic male rats and alleviated weight loss caused by this disease state. It also reduced the rate of HbA1c formation significantly over the period of its administration and ameliorated diabetic dyslipidemia by increasing the HDL levels. More research is, however, ongoing to determine which actual compound or compounds is responsible for the antihyperglycemic and hypolipidemic effects, thus creating chance for future components isolation and drug manufacture and to reveal a possible mechanism of action of this propolis.
ACKNOWLEDGMENT
Mr. B. Okon; Lab Technician, Experimental Laboratories, Department of Physiology, University of Ibadan, Ibadan, Nigeria.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Shi Y, Hu FB. The global implications of diabetes and cancer. Lancet. 2014;383:1947–8. doi: 10.1016/S0140-6736(14)60886-2. [DOI] [PubMed] [Google Scholar]
- 3.Pandey A, Tripathi P, Pandey R, Srivatava R, Goswami S. Alternative therapies useful in the management of diabetes: A systematic review. J Pharm Bioallied Sci. 2011;3:504–12. doi: 10.4103/0975-7406.90103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toreti VC, Sato HH, Pastore GM, Park YK. Recent progress of propolis for its biological and chemical compositions and its botanical origin. Evid Based Complement Alternat Med. 2013;2013:697390. doi: 10.1155/2013/697390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sforcin JM, Bankova V. Propolis: Is there a potential for the development of new drugs? J Ethnopharmacol. 2011;133:253–60. doi: 10.1016/j.jep.2010.10.032. [DOI] [PubMed] [Google Scholar]
- 6.Huang S, Zhang CP, Wang K, Li GQ, Hu FL. Recent advances in the chemical composition of propolis. Molecules. 2014;19:19610–32. doi: 10.3390/molecules191219610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christov R, Trusheva B, Popova M, Bankova V, Bertrand M. Chemical composition of propolis from Canada, its antiradical activity and plant origin. Nat Prod Res. 2006;20:531–6. doi: 10.1080/14786410500056918. [DOI] [PubMed] [Google Scholar]
- 8.Marquele FD, Di Mambro VM, Georgetti SR, Casagrande R, Valim YM, Fonseca MJ. Assessment of the antioxidant activities of Brazilian extracts of propolis alone and in topical pharmaceutical formulations. J Pharm Biomed Anal. 2005;39:455–62. doi: 10.1016/j.jpba.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Teixeira EW, Message D, Negri G, Salatino A, Stringheta PC. Seasonal variation, chemical composition and antioxidant activity of Brazilian propolis samples. Evid Based Complement Alternat Med. 2010;7:307–15. doi: 10.1093/ecam/nem177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bankova V, Popova M, Trusheva B. Propolis volatile compounds: Chemical diversity and biological activity: A review. Chem Cent J. 2014;8:28. doi: 10.1186/1752-153X-8-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuliang HU, Hepburn HR, Xuan H, Chen M, Daya S, Radloff SE. Effects of propolis on blood glucose, blood lipid and free radicals in rats with diabetes mellitus. Pharm Res. 2005;51:147–52. doi: 10.1016/j.phrs.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 12.Hassan SH. Effect of propolis on blood glycemic control and lipid metabolism in diabetic rabbits. Int J Pharm Life Sci. 2014;5:4031. [Google Scholar]
- 13.Kilpatrick ES, Bloomgarden ZT, Zimmet PZ. Is haemoglobin A1c a step forward for diagnosing diabetes? BMJ. 2009;339:b4432. doi: 10.1136/bmj.b4432. [DOI] [PubMed] [Google Scholar]
- 14.Miedema K. Standardization of HbA1c and optimal range of monitoring. Scand J Clin Lab Invest Suppl. 2005;240:61–72. doi: 10.1080/00365510500236143. [DOI] [PubMed] [Google Scholar]
- 15.Lehman R, Krumholz HM. Tight control of blood glucose in long standing type 2 diabetes. BMJ. 2009;338:b800. doi: 10.1136/bmj.b800. [DOI] [PubMed] [Google Scholar]
- 16.Shubrook JH., Jr Risks and benefits of attaining HbA(1c) goals: Examining the evidence. J Am Osteopath Assoc. 2010;110:eS7–12. [PubMed] [Google Scholar]
- 17.Etuk EU. Animal models for studying diabetes mellitus. Agric Biol J N Am. 2010;1:130–4. [Google Scholar]
- 18.Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia. 2008;51:216–26. doi: 10.1007/s00125-007-0886-7. [DOI] [PubMed] [Google Scholar]
- 19.Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res. 2001;50:537–46. [PubMed] [Google Scholar]
- 20.Dunn JS, Sheehan HL, Mclethie NG. Necrosis of islets of langerhans produced experimentally. Lancet. 1943;1:484–7. [Google Scholar]
- 21.Danilova IG, Sarapultsev PA, Medvedeva SU, Gette IF, Bulavintceva TS, Sarapultsev AP. Anatomical Record. Hoboken, N.J 2007: 2014. Morphological restructuring of myocardium during the early phase of experimental diabetes mellitus. Available from: http://www.SciCurveOPEN.com . [DOI] [PubMed] [Google Scholar]
- 22.Musa TS, Salih NM, Ulaiwi WS. Detection of some active compounds in aqueous and ethanolic extracts of iraqi propolis and examine their antibacterial effects. Pak J Nutr. 2012;11:83–7. [Google Scholar]
- 23.Bhadauria M, Nirala SK, Shukla S. Propolis protects CYP 2E1 enzymatic activity and oxidative stress induced by carbon tetrachloride. Mol Cell Biochem. 2007;302:215–24. doi: 10.1007/s11010-007-9443-4. [DOI] [PubMed] [Google Scholar]
- 24.Zamami Y, Takatori S, Koyama T, Goda M, Iwatani Y, Doi S, et al. Effect of propolis on insulin resistance in fructose-drinking rats. Yakugaku Zasshi. 2007;127:2065–73. doi: 10.1248/yakushi.127.2065. [DOI] [PubMed] [Google Scholar]
- 25.Viana MV, Moraes RB, Fabbrin AR, Santos MF, Gerchman F. Assessment and treatment of hyperglycemia in critically ill patients. Rev Bras Ter Intensiva. 2014;26:71–6. doi: 10.5935/0103-507X.20140011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Curie CJ, Peters JR, Tynan A, Evans M, Heine RJ, Bracco OL. Survival as a function of HbA1c in people with type 2 diabetes: A retrospective cohort study. Lancet. 2010;375:481–9. doi: 10.1016/S0140-6736(09)61969-3. [DOI] [PubMed] [Google Scholar]
- 27.Juarez DT, Demaris KM, Goo R, Mnatzaganian CL, Wong Smith H. Significance of HbA1c and its measurement in the diagnosis of diabetes mellitus: US experience. Diabetes Metab Syndr Obes. 2014;7:487–94. doi: 10.2147/DMSO.S39092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.d’Emden MC, Shaw JE, Colman PG, Colagiuri S, Twigg SM, Jones GR, et al. The role of HbA1c in the diagnosis of diabetes mellitus in Australia. Med J Aust. 2012;197:220–1. doi: 10.5694/mja12.10988. [DOI] [PubMed] [Google Scholar]
- 29.Incani M, Sentinelli F, Perra L, Pani MG, Porcu M, Lenzi A, et al. Glycated hemoglobin for the diagnosis of diabetes and prediabetes: Diagnostic impact on obese and lean subjects, and phenotypic characterization. J Diabetes Investig. 2015;6:44–50. doi: 10.1111/jdi.12241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amin A, Mustafa IO, Folarin RO, Onanuga IO, Ibrahim RB, Balogun WG. Effect of Nigerian propolis on glycemia, lipid profile, and oxidative stress markers in alloxan-induced diabetic rats. Pharmacol Online. 2013;2:149–58. [Google Scholar]
- 31.Oršolic N, Sirovina D, Koncic MZ, Lackovic G, Gregorovic G. Effect of Croatian propolis on diabetic nephropathy and liver toxicity in mice. BMC Complement Altern Med. 2012;12:117. doi: 10.1186/1472-6882-12-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu W, Li YH, Chen ML, Hu FL. Protective effects of Chinese and Brazilian propolis treatment against hepatorenal lesion in diabetic rats. Hum Exp Toxicol. 2011;30:1246–55. doi: 10.1177/0960327110387456. [DOI] [PubMed] [Google Scholar]
- 33.El-Sayed el-SM, Abo-Salem OM, Aly HA, Mansour AM. Potential antidiabetic and hypolipidemic effects of propolis extract in streptozotocin-induced diabetic rats. Pak J Pharm Sci. 2009;22:168–74. [PubMed] [Google Scholar]
- 34.Iwu MM, Igboko OA, Okunji CO, Tempesta MS. Antidiabetic and aldose reductase activities of biflavanones of Garcinia kola. J Pharm Pharmacol. 1990;42:290–2. doi: 10.1111/j.2042-7158.1990.tb05412.x. [DOI] [PubMed] [Google Scholar]
- 35.Douillet C, Bost M, Accominotti M, Borson-Chazot F, Ciavatti M. Effect of selenium and vitamin E supplements on tissue lipids, peroxides, and fatty acid distribution in experimental diabetes. Lipids. 1998;33:393–9. doi: 10.1007/s11745-998-0220-z. [DOI] [PubMed] [Google Scholar]
- 36.Naziroglu M, Dilsiz N, Cay M. Protective role of intraperitoneally administered vitamins C and E and selenium on the levels of lipid peroxidation in the lens of rats made diabetic with streptozotocin. Biol Trace Elem Res. 1999;70:223–32. doi: 10.1007/BF02783831. [DOI] [PubMed] [Google Scholar]
- 37.Sosenko JM, Breslow JL, Miettinen OS, Gabbay KH. Hyperglycemia and plasma lipid levels: A prospective study of young insulin-dependent diabetic patients. N Engl J Med. 1980;302:650–4. doi: 10.1056/NEJM198003203021202. [DOI] [PubMed] [Google Scholar]
- 38.Brunzell JD, Chait A, Bierman EL. The Diabetes Annual. Amsterdam: Elsevier; 1985. Plasma lipoproteins in human diabetes mellitus; pp. 463–79. [Google Scholar]
- 39.Gonen B, White N, Schonfeld G, Skor D, Miller P, Santiago J. Plasma levels of apoprotein B in patients with diabetes mellitus: The effect of glycemic control. Metabolism. 1985;34:675–9. doi: 10.1016/0026-0495(85)90097-6. [DOI] [PubMed] [Google Scholar]
- 40.Qiu S, Sun H, Zhang AH, Xu HY, Yan GL, Han Y, et al. Natural alkaloids: Basic aspects, biological roles, and future perspectives. Chin J Nat Med. 2014;12:401–6. doi: 10.1016/S1875-5364(14)60063-7. [DOI] [PubMed] [Google Scholar]


