Abstract
Aim:
Coconut water is a natural beverage that is a part of daily diet of many people. This study was designed to explore the anti-inflammatory activity of coconut water of different maturation stages (young and mature) with rat paw edema model of inflammation using plethysmometer.
Methodology:
For this study, albino rats were selected and divided into four equal groups (10 rats in each group). Group 1 was set as control and administered distilled water 1 ml orally; Groups 2 and 3 were treated with young and mature coconut water, respectively, at 4 ml/100 g dose orally. Group 4 was treated with the standard drug (ibuprofen) at 400 mg/70 kg. 0.1 ml of 1% w/v acetic acid was administered in the subplantar tissue of rat paw 30 min after oral treatments of groups. Plethysmometer was used to measure rat paw edema.
Results:
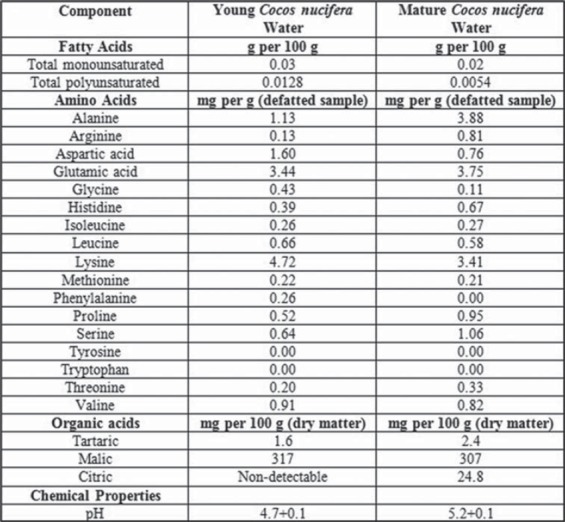
Results revealed that both coconut water possess significant anti-inflammatory activity (P < 0.001). In comparison to control, percent inhibition by young coconut water was 20.22%, 35.13%, 42.52%, and 36% at 1, 2, 3, and 4 h of acetic acid administration, respectively. However, maximum percent inhibition (42.52%) was observed in the second phase of the inflammatory process. On the other hand, percent inhibition by mature coconut water was 18.80%, 25.94%, 24.13%, and 18.66% at 1, 2, 3, and 4 h of acetic acid administration, respectively. However, maximum percent inhibition (25.94%) was observed in the first phase of the inflammatory process.
Conclusions:
This study strongly suggests the use of young coconut water for potent anti-inflammatory effect and mature coconut water for moderate anti-inflammatory effect.
KEY WORDS: Anti-inflammatory, coconut water, paw edema, salicylic acid
INTRODUCTION
Inflammation is a normal response that takes place in the presence of tissue injury. It is a frequent clinical observation [1]. Pain, swelling, local redness, edema, and loss of function are considered as classic signs of inflammatory process [2]. Nonsteroidal anti-inflammatory drugs (NSAIDs) are considered as most commonly used anti-inflammatory drugs worldwide. However, in addition to their high costs such drugs also present a high profile list of adverse effects and toxicity. Furthermore, one of the most common disadvantages of currently available drugs is that the symptoms of inflammation reappear after their discontinuation. Therefore, development and screening of new anti-inflammatory drugs are the need of time. For new drug discovery, plants are considered a rich source [3].
In the prevention of chronic ailments, nutrition can play a significant role. It is because most of the chronic ailments may be related to diet. The concept of “functional foods” explains that food should be considered not only for living purposes but also be considered as a great source of physical and mental health. It is because this consideration will aid in the reduction and prevention of risk factors for different diseases. It may also help in enhancing different physiological functions [4]. Food may be termed as “functional” if it satisfactorily demonstrates to affect different functions of the human body in a beneficial way. Moreover, food is termed as functional if it is beyond adequate nutritional effects, in such a way that is relevant to health or reduction in the risk of diseases [5]. Simplest examples of functional food include tomatoes, carrots, turmeric, and mustard. These items are considered as functional food items because these contain a high content of physiologically active composition [6]. Phytochemicals found in vegetables and fruits provide a synergistic and additive effects as a result of their significant antioxidant potential [7,8].
Phytochemicals are explained as bioactive components found in grains, vegetables, and fruits. Synergistic and additive effects of phytochemicals in vegetables, fruits, and whole grains are held responsible for its potent anticancer and antioxidant activities. Use of a wide range of vegetables, fruits, and whole grains on a daily basis is considered as a practical strategy to optimize health [9].
Coconut (Cocos nucifera) is among significant plants that possess nutritional and medicinal properties. It is frequently cultivated for medicinal and nutritional purposes especially in tropical areas [10]. It belongs to the family Arecaceae. Total cultivation area of the coconut tree is 11 million hectares worldwide [11]. However, it may grow where ever there is adequate rainfall and warmth. Coconut water is a refreshing and nutritious beverage in its natural form. It is traditionally used all around the world due to its beneficial effects on human health. Coconut water [Figure 1] has a variety of health-related effects including antihyperlipidemic [12], antiulcerogenic, and cardioprotective effects [13]. The composition and quantity of coconut water changes as the fruit mature [Figures 4 and 5]. There is no research study conducted to compare the anti-inflammatory effects of young and mature coconut water. Rat is a commonly used animal species for preclinical model studies [14]. History shows that different people have been using plants to treat their ailments. For instance, there are more than 13,000 reports of plant extracts that possess anti-inflammatory effect [15]. The spectrum of biochemical effects from various phytochemicals might be able to influence different processes in the organism such as anti-inflammatory activity. Therefore, this study was designed to explore and compare anti-inflammatory effects of both young and mature coconut water in an animal model of inflammation.
Figure 1.
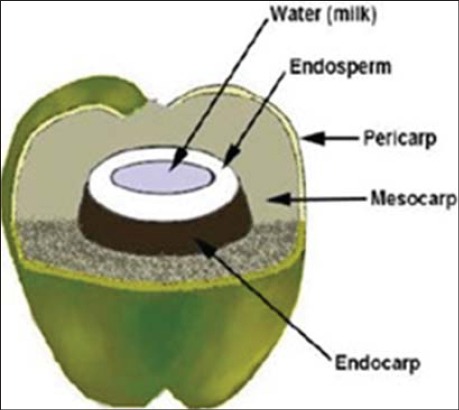
Coconut fruit (cross section)
Figure 2.

Young coconut fruit
Figure 3.

Mature coconut fruit
Figure 4.
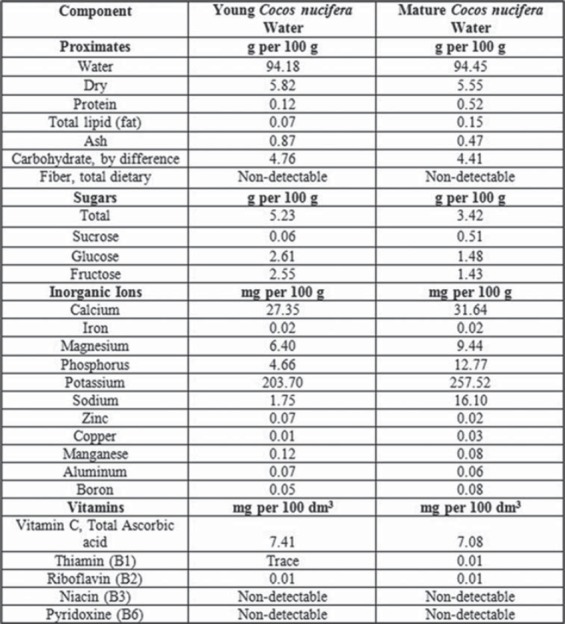
Figure 5.
METHODOLOGY
Collection of Coconut Water
Coconut fruits, both young and mature (Figures 2 and 3), were harvested from the coconut trees grown in Karachi city. Water from young and mature coconut fruits was collected after carefully cutting the fruits from the top, and the water was stored in the refrigerator for further use [18]. University Board of Advanced Studies and Research approved this study with no. 02181/Pharm.
Selection of Animals
To conduct this study, 40 rats of either sex were selected and divided into 4 equal groups. Their weights ranged from 200 to 250 g. Rats were housed with 12/12 h dark and light cycle and 21°C ± 1°C temperature. Animals were kept on standard animal diet and water ad libitum. Rats were handled according to Helsinki Resolution 1964 and divided into 4 groups of 10 rats each.
Experimental Protocol
All four groups received 0.1 ml of 1% acetic acid in the subplantar tissue of the rat’s paw 30 minutes after oral treatment of distilled water, young coconut water, mature coconut water, and ibuprofen. Group 1 received distilled water (1 ml orally) and served as control. Groups 2 and 3 served as treated groups for young coconut water and mature coconut water, respectively, and received respective coconut water orally at the dose of 4 ml/100 g [19]. Group 4 served as a standard group and received ibuprofen orally at the dose of 400 mg/70 kg.
Animal Model of Inflammation
1% w/v acetic acid induced paw edema model was used to study anti-inflammatory activity of young and mature coconut water. Prepared acetic acid (0.1 ml) was administered parenterally in the subplantar tissue of rat’s paw 30 min after oral treatments. Edema (sign of inflammation) in the paw of the animal was observed with the use of plethysmometer (Ugo Basile, Italy). The observations were made at different time intervals, i.e. before administration of acetic acid (baseline observation), immediately after administration of acetic acid, and after 1, 2, 3, and 4 h of acetic acid administration.
Plethysmometer
Paw of each rat was immersed in measuring tube of plethysmometer. The moment the paw is immersed in water, it is displaced, and this displacement is sensed by electrodes of platinum. Plethysmometer detects changes in conductance and an output signal is transmitted on the digital display that indicates measured volume displacement (resolution: 0.01 ml) [20]. Following formula was used to calculate percent inhibition in edema [21,22]:
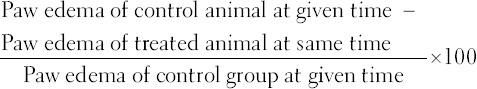
Statistical Analysis
Collected data are presented as mean ± standard deviation and analyzed by one-way ANOVA followed by post-hoc Bonferroni multiple comparisons. P < 0.001 is considered highly significant.
RESULTS
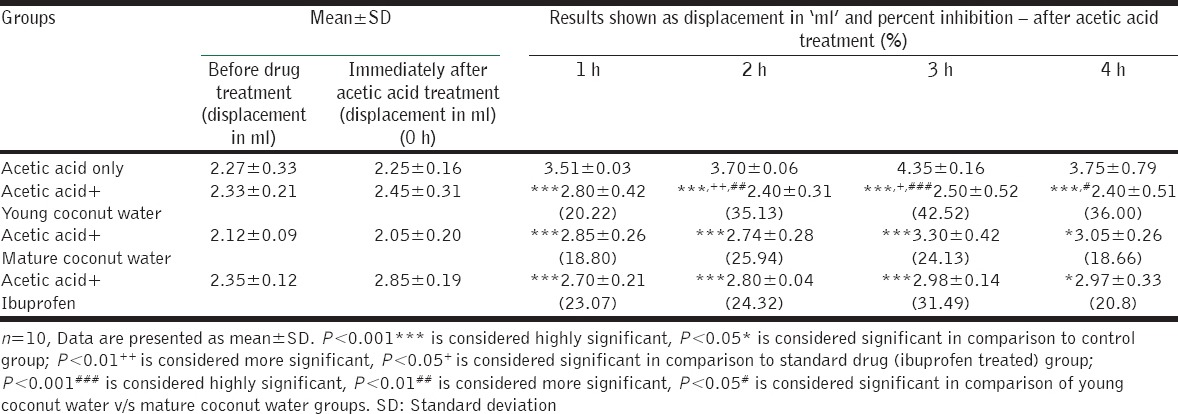
The results of this study are presented in Table 1. Young and mature coconut water showed remarkable anti-inflammatory effect. However, the intensity of the effect is varied.
Table 1.
Anti-inflammatory effect of young and mature coconut water
Effects after 1 h of Acetic Acid Administration
Ibuprofen (standard drug group), young coconut water and mature coconut water showed a highly significant decrease (P < 0.001) in rat paw edema after 1 h of acetic acid administration in comparison to control group. Moreover, the effects of both young and mature coconut water were similar to ibuprofen showing insignificant differences in rat paw edema between ibuprofen and young and mature water at 1 h.
Effects after 2 h and 3 h of Acetic Acid Administration
After 2 h and 3 h of acetic acid administration, ibuprofen, young coconut water, and mature coconut water showed a highly significant decrease (P < 0.001) in rat paw edema. The decrease of rat paw edema by mature coconut water is similar to ibuprofen as the difference in rat paw edema of these two groups is statistically insignificant. However, young coconut water showed better results than ibuprofen (standard drug group) at 2 h (P < 0.01) and 3 h (P < 0.05) of acetic acid administration.
Effects after 4 h of Acetic Acid Administration
After 4 h of acetic acid administration, young coconut water showed a highly significant decrease (P < 0.001) in rat paw edema. However, mature coconut water and ibuprofen showed an only significant decrease in paw edema (P < 0.05). Furthermore, at 4 h of acetic acid administration, the decrease in paw edema by young coconut water and mature coconut water is similar to ibuprofen as the difference between them is statistically insignificant.
Percent Inhibition of Rat Paw Edema
In comparison to control, percent inhibition of rat paw edema by young coconut water was 20.22%, 35.13%, 42.52%, and 36% at 1, 2, 3, and 4 h of acetic acid administration, respectively. However, maximum percent inhibition (42.52%) was observed in the second phase of the inflammatory process. On the other hand, percent inhibition by mature coconut water was 18.80%, 25.94%, 24.13%, and 18.66% at 1, 2, 3, and 4 h of acetic acid administration, respectively. However, maximum percent inhibition (25.94%) was observed in the first phase of the inflammatory process.
Comparison of Anti-inflammatory Effect of Young and Mature Coconut Water
At 1 h, the difference between rat paw edema of young and mature coconut water groups was found insignificant.
At 2, 3, and 4 h of acetic acid administration, the difference of rat paw edema between young and mature coconut water was more significant (P < 0.01), highly significant (P < 0.001), and significant (P < 0.05), respectively [presented by # in Table 1]. This difference in the results at 2, 3, and 4 h of inflammation shows the better anti-inflammatory effect of young coconut water than mature coconut water.
DISCUSSION
This study explored the anti-inflammatory effect of young and mature coconut water using acetic acid induced rat paw edema model. The effects were also compared with the standard drug, ibuprofen. Inflammation is induced by acetic acid as a result of inflammatory mediators. These inflammatory mediators are released in two phases. Platelet activation factor, serotonin, and histamine are released in the first phase. The time duration of this phase is first 90 min. However, prostaglandins (PGs), lysosomes, proteases, and kinins are released in the later phase (after 90 min) [23,24]. Synthesis of PGs, i.e. prostaglandin F2 alpha, prostaglandin E2 and synthesis of free radicals along with interleukin-1 (IL-1), IL-2 and tumor necrosis factor-alpha induces nociception and inflammation via stimulation of nociceptors [25]. Moreover, in addition to this induction, it may also stimulate cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase [26]. This eventually increases oxidative stress in inflamed tissue of rats paw. Edema at the site of inflammation is due to the release of bradykinin, histamine, and serotonin [27]. According to Panthong et al., NSAIDs produce its effect in the later phase of inflammation [28]. In this phase, they suppress the expression of COX-2 and hence reduce the synthesis of PGs [29].
Young and mature coconut water showed excellent anti-inflammatory effects [Table 1]. Coconut water contains flavonoids. These components are responsible for potent anti-inflammatory effect as they inhibit the synthesis of PGs [1,30,31]. Moreover, it is reported that if plants possess antioxidant activity, they show significant anti-inflammatory response [32,33]. Coconut water is reported to have antioxidant potential due to its unique composition including kinetin and micronutrients [16,34]. Coconut water is also reported to have the antihistaminic effect that further contributes to anti-inflammatory activity [35]. Furthermore, coconut water contains abscisic acid (ABA). ABA may contribute to the anti-inflammatory activity of water by acting on peroxisome proliferator-activated receptor-gamma (PPAR-γ). According to Kelly et al., the activation of PPAR-γ agonist produces direct inhibition of inflammation via action on nuclear factor-kappa B [36]. In addition to this, it may also inhibit monocyte chemoattractant protein-1 induced migration of monocytes [37,38].
Young coconut water decreased paw edema right from the first hour of inflammation (early phase). However, the percent inhibition of edema from young coconut water was found to be maximum in the second phase of inflammation. This result hence reveals that young coconut water showed the effect on histamine and serotonin (agents released in the first phase). However, since the maximum effect was observed in the third hour (second phase) of inflammation, it may also act on COX-2 and inhibits synthesis of PGs. This effect may be attributed to the presence of salicylic acid in young coconut water [39].
Mature coconut water also showed a decrease in rat paw edema right from the first hour of inflammation and maximum percent inhibition of edema was observed at the second hour (first phase). This proposes that mature coconut mainly acts via action on histamine and serotonin [23,24].
Difference in Effect of Young and Mature Coconut Water
The concentration of salicylic acid in coconut water starts decreasing as the fruit matures [39] suggesting that young coconut water contains more salicylic acid content than mature coconut water. It is probably because of this concentration difference; only young coconut water has shown a maximum effect in the second phase of inflammation as generally observed by NSAIDs also. Salicylic acid is one of the active components of aspirin (an example of NSAIDs).
CONCLUSION
Young and mature coconut water both possess significant anti-inflammatory activity. As the coconut water is widely used worldwide and is a part of daily diet of many people, this study could serve as a positive finding in using coconut water for their anti-inflammatory potential. Since young coconut water showed better results than mature coconut water and standard drug ibuprofen, this study strongly suggests the use of young coconut water for potent anti-inflammatory effect and mature coconut water for moderate anti-inflammatory effect. This proposed mechanism for anti-inflammatory effect is based on the composition of coconut water. However, further molecular level research may be conducted to uncover the more precise anti-inflammatory mechanism of action of young and mature coconut water.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Kumar S, Bajwa BS, Singh K, Kalia AN. Anti-inflammatory activity of herbal plants: A review. Int J Adv Pharm Biol Chem. 2013;2:272–81. [Google Scholar]
- 2.Percival M. Understanding the natural management of pain and inflammation. Clin Nutr Insights. 1999;4:1–5. [Google Scholar]
- 3.Srinivasan K, Muruganandan S, Lal J, Chandra S, Tandan SK, Prakash VR. Evaluation of anti-inflammatory activity of Pongamia pinnata leaves in rats. J Ethnopharmacol. 2001;78:151–7. doi: 10.1016/s0378-8741(01)00333-6. [DOI] [PubMed] [Google Scholar]
- 4.López-Varela S, González-Gross M, Marcos A. Functional foods and the immune system: A review. Eur J Clin Nutr. 2002;56(Suppl 3):S29–33. doi: 10.1038/sj.ejcn.1601481. [DOI] [PubMed] [Google Scholar]
- 5.Roberfroid MB. What is beneficial for health? The concept of functional food. Food Chem Toxicol. 1999;37:1039–41. doi: 10.1016/s0278-6915(99)00080-0. [DOI] [PubMed] [Google Scholar]
- 6.Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn Rev. 2010;4:118–26. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu RH. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am J Clin Nutr. 2003;78(3 Suppl):517S–20. doi: 10.1093/ajcn/78.3.517S. [DOI] [PubMed] [Google Scholar]
- 8.Liu RH. Potential synergy of phytochemicals in cancer prevention: Mechanism of action. J Nutr. 2004;134(12 Suppl):3479S–85. doi: 10.1093/jn/134.12.3479S. [DOI] [PubMed] [Google Scholar]
- 9.Liu RH. Nutritional Health. New Jeresy: Humana Press; 2012. Health benefits of phytochemicals in whole foods; pp. 293–10. [Google Scholar]
- 10.DebMandal M, Mandal S. Coconut (Cocos nucifera L: Arecaceae): In health promotion and disease prevention. Asian Pac J Trop Med. 2011;4:241–7. doi: 10.1016/S1995-7645(11)60078-3. [DOI] [PubMed] [Google Scholar]
- 11.Punchihewa PG, Arancon RN Coconut: Post-Harvest Operations. Inpho (Information Network on Post-Harvest Operations), FAO, Food and Agriculture Organization. 2005. Available from: http://www.fao.org/3/a-au999e.pdf .
- 12.Sandhya VG, Rajamohan T. Comparative evaluation of the hypolipidemic effects of coconut water and lovastatin in rats fed fat-cholesterol enriched diet. Food Chem Toxicol. 2008;46:3586–92. doi: 10.1016/j.fct.2008.08.030. [DOI] [PubMed] [Google Scholar]
- 13.Anurag P, Rajamohan T. Cardioprotective effect of tender coconut water in experimental myocardial infarction. Plant Foods Hum Nutr. 2003;58:1–12. [Google Scholar]
- 14.Kubatka P, Zihlavniková K, Kajo K, Péc M, Stollárová N, Bojková B, et al. Antineoplastic effects of simvastatin in experimental breast cancer. Klin Onkol. 2011;24:41–5. [PubMed] [Google Scholar]
- 15.Ramírez-Cisneros MÁ, Rios MY, Déciga-Campos M, Aguilar-Guadarrama AB. Phytochemical study and anti-inflammatory, antidiabetic and free radical scavenger evaluations of Krameria pauciflora methanol extract. Molecules. 2012;17:861–72. doi: 10.3390/molecules17010861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yong JW, Ge L, Ng YF, Tan SN. The chemical composition and biological properties of coconut (Cocos nucifera L.). water. Molecules. 2009;14:5144–64. doi: 10.3390/molecules14125144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santoso U, Kubo K, Ota T, Tadokoro T, Maekawa A. Nutrient composition of kopyor coconuts (Cocos nucifera L) Food Chem. 1996;57:299–304. [Google Scholar]
- 18.Khan MN, Rehman MU, Khan KW. A study of chemical composition of Cocos nucifera L. (Coconut) water and its usefulness as rehydration fluid. Pak J Bot. 2003;35:925–30. [Google Scholar]
- 19.Prathapan A, Rajamohan T. Antioxidant and antithrombotic activity of tender coconut water in experimental myocardial infarction. J Food Biochem. 2011;35:1501–7. [Google Scholar]
- 20.Tanko Y, Mohammed A, Okasha MA, Umah A, Magaji R. Anti-nociceptive and anti-inflammatory activities of ethanol extract of Syzygium aromaticum flower bud in Wistar rats and mice. Afr J Tradit Complement Altern Meds. 2008;5:209–12. doi: 10.4314/ajtcam.v5i2.31275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta M, Mazumder UK, Kumar RS, Gomathi P, Rajeshwar Y, Kakoti BB, et al. Anti-inflammatory, analgesic and antipyretic effects of methanol extract from Bauhinia racemosa stem bark in animal models. J Ethnopharmacol. 2005;98:267–73. doi: 10.1016/j.jep.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 22.Lamien CE, Guissou IP, Nacoulma OG. Anti-inflammatory, analgesie and antipyretic activities of Dicliptera verticillata. Int J Pharmacol. 2006;2:435–8. [Google Scholar]
- 23.Olajide OA, Makinde JM, Awe SO. Effects of the aqueous extract of Bridelia ferruginea stem bark on carrageenan-induced oedema and granuloma tissue formation in rats and mice. J Ethnopharmacol. 1999;66:113–7. doi: 10.1016/s0378-8741(99)00006-9. [DOI] [PubMed] [Google Scholar]
- 24.Salvemini D, Wang ZQ, Bourdon DM, Stern MK, Currie MG, Manning PT. Evidence of peroxynitrite involvement in the carrageenan-induced rat paw edema. Eur J Pharmacol. 1996;303:217–20. doi: 10.1016/0014-2999(96)00140-9. [DOI] [PubMed] [Google Scholar]
- 25.Choi EM. Antinociceptive and anti-inflammatory activities of pine (Pinus densiflora) pollen extract. Phytother Res. 2007;21:471–5. doi: 10.1002/ptr.2103. [DOI] [PubMed] [Google Scholar]
- 26.Pavlick KP, Laroux FS, Fuseler J, Wolf RE, Gray L, Hoffman J, et al. Role of reactive metabolites of oxygen and nitrogen in inflammatory bowel disease. Free Radic Biol Med. 2002;33:311–22. doi: 10.1016/s0891-5849(02)00853-5. [DOI] [PubMed] [Google Scholar]
- 27.Harriot M, Marion E, Martha A, Wellford S, William A. Inflammation induced by histamine, serotonine, bradykinin and compound 48/480 in the rat. Antagonists and mechanisms of action. J Pharmacol Exp Ther. 2004;191:300–2. [PubMed] [Google Scholar]
- 28.Panthong A, Kanjanapothi D, Taesotikul T, Phankummoon A, Panthong K, Reutrakul V. Anti-inflammatory activity of methanolic extracts from Ventilago harmandiana Pierre. J Ethnopharmacol. 2004;91:237–42. doi: 10.1016/j.jep.2003.12.025. [DOI] [PubMed] [Google Scholar]
- 29.Calixto JB, Medeiros R, Fernandes ES, Ferreira J, Cabrini DA, Campos MM. Kinin B1 receptors: Key G-protein-coupled receptors and their role in inflammatory and painful processes. Br J Pharmacol. 2004;143:803–18. doi: 10.1038/sj.bjp.0706012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ilavarasan R, Mallika M, Venkataraman S. Anti-inflammatory and antioxidant activities of Cassia fistula Linn bark extracts. Afr J Tradit Complement Altern Med. 2005;2:70–85. [Google Scholar]
- 31.Mascolo N, Capasso F, Menghini A, Fasulo MP. Biological screening of Italian medicinal plants for anti-inflammatory activity. Phytother Res. 1987;1:28–31. [Google Scholar]
- 32.Nguemfo EL, Dimo T, Dongmo AB, Azebaze AG, Alaoui K, Asongalem AE, et al. Anti-oxidative and anti-inflammatory activities of some isolated constituents from the stem bark of Allanblackia monticola Staner L. C (Guttiferae) Inflammopharmacology. 2009;17:37–41. doi: 10.1007/s10787-008-8039-2. [DOI] [PubMed] [Google Scholar]
- 33.Rathee P, Chaudhary H, Rathee S, Rathee D, Kumar V, Kohli K. Mechanism of action of flavonoids as anti-inflammatory agents: A review. Inflamm Allergy Drug Targets. 2009;8:229–35. doi: 10.2174/187152809788681029. [DOI] [PubMed] [Google Scholar]
- 34.Evans P, Halliwell B. Micronutrients: Oxidant/antioxidant status. Br J Nutr. 2001;85:S67–74. [PubMed] [Google Scholar]
- 35.Iwu MM. Handbook of African Medicinal Plants. Boca Raton: CRC Press; 1993. Pharmacognostical profile of selected medicinal plants. [Google Scholar]
- 36.Kelly D, Campbell JI, King TP, Grant G, Jansson EA, Coutts AG, et al. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-gamma and RelA. Nat Immunol. 2004;5:104–12. doi: 10.1038/ni1018. [DOI] [PubMed] [Google Scholar]
- 37.Tanaka T, Fukunaga Y, Itoh H, Doi K, Yamashita J, Chun TH, et al. Therapeutic potential of thiazolidinediones in activation of peroxisome proliferator-activated receptor gamma for monocyte recruitment and endothelial regeneration. Eur J Pharmacol. 2005;508:255–65. doi: 10.1016/j.ejphar.2004.10.056. [DOI] [PubMed] [Google Scholar]
- 38.Babaev VR, Yancey PG, Ryzhov SV, Kon V, Breyer MD, Magnuson MA, et al. Conditional knockout of macrophage PPARgamma increases atherosclerosis in C57BL/6 and low-density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2005;25:1647–53. doi: 10.1161/01.ATV.0000173413.31789.1a. [DOI] [PubMed] [Google Scholar]
- 39.Mahayothee B, Koomyart I, Khuwijitjaru P, Siriwongwilaichat P, Nagle M, Müller J. Phenolic compounds, antioxidant activity, and medium chain fatty acids profiles of coconut water and meat at different maturity stages. Int J Food Prop 2015. 2015 DOI: 10.1080/10942912.2015.1099042. [Google Scholar]


