Abstract
Background:
Currently, there is an increasing interest in developing more efficient techniques for the extraction of phytochemicals. Microwaves and ultrasonic extraction methods are promising techniques that can be used for this purpose.
Objectives:
The purpose of this study was to investigate the impact of different extraction methods on yield, antioxidant and antimicrobial activities of volatile oil extracted from Trichodesma africanum.
Materials and Methods:
Volatile oil was extracted using microwave, ultrasonic, microwave-ultrasonic, and conventional hydrodistillation methods. The extracted oil was evaluated for antioxidant and antimicrobial activities. The antioxidant activity was assessed by 2,2-Diphenyl-1-picrylhydrazyl scavenging assay, whereas the antimicrobial activity was assessed by broth microdilution method. The antimicrobial activity of the volatile oils was examined against Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa American type culture collection reference strains, as well as against methicillin-resistant S. aureus (MRSA) and Candida albicans clinical isolates.
Results:
The volatile oil obtained by the four extraction methods in this study exhibited both antioxidant and antimicrobial activities. Among the four extraction methods used, the microwave-ultrasonic method yielded the largest amount (1.8% v/w) and the yield exhibited the highest antioxidant activity in terms of inhibition (91.83% ± 1.1). The minimum inhibitory concentrations for E. coli, P. aeruginosa, S. aureus, MRSA, and C. albicans were 3, 5, 6, 3, and 9, respectively.
Conclusion:
Among the extraction techniques used in this study, the microwave-ultrasonic method showed the best results. Moreover, this study suggests that T. africanum volatile oils contain active substances that could potentially be used both as natural preservatives in food and pharmaceutical industries as well as in developing new antimicrobial and antioxidant agents.
KEY WORDS: Antimicrobial, antioxidant, microwave-ultrasonic, Trichodesma africanum
INTRODUCTION
Medicinal plants are among the main sources of phytochemicals. These phytochemicals are secondary metabolites that plants can use to defend themselves against microbial infections, insect infestations, and herbivorous animals. Fortunately, many of these phytochemicals have been found to be pharmaceutically and medicinally important such as eugenol, ascaridole, cineole and many others [1]. Unfortunately, a lack of safety is a major problem that limits the use of various herbal products [2]. Many regulatory agencies including the United States Food and Drug Administration and the European medicines agency require that herbal extracts are standardized to ensure safety and efficacy of their medicinal use [3]. Accordingly, the method used for extraction is crucial because it may impact the final quality and the yield of the phytochemical constituents, especially when these constituents are sensitive and may undergo degradation [2]. Therefore, optimized extraction methods are important for reducing processing costs, time, energy, and improving yield. Conventional extraction methods, such as heating, boiling, or refluxing, have a negative impact on the quality and yield. Phytochemicals might be lost due to hydrolysis, oxidation and/or ionization during these extraction processes [4]. Recently, new extraction methods including ultrasound-assisted extraction and microwave-assisted extraction have emerged as promising techniques for the extraction of phytochemicals from plants and herbals [5].
Trichodesma africanum (L.) Sm., also known as African barbell in English and Lozeka in Arabic, belongs to the Boraginaceae family. This erect, harshly scabrid, annual, short-lived perennial herbaceous plant is of about 1 m in height. It forms branches mainly from the base and has a fistular stem which is densely covered with prickly stiff hairs of about 2 mm in length. The leaves of this plant are simple with entire margins that are covered on both surfaces with erect-patent, prickles [6-8]. T. africanum has been reportedly used in the folk medicine of several countries for the treatment of many clinical conditions including a cough and common cold [9]. In Iran, roots and leaves of T. africanum have been used for the treatment of several clinical conditions including common cold, chest congestions, chickenpox, scarlet fever, measles, bone fractures, headache, abdominal pain, mouth ulcer, and constipation [10]. In Nigeria, it has been used as used to induce diuresis [11]. In Pakistan, it has been described for the treatment of severe respiratory tract diseases [12]. Many studies have investigated the medicinal importance of the phytochemicals of T. africanum. It was shown that the methanolic extract of this plant has a remarkable inhibitory effect against the growth of many clinically important bacterial and fungal pathogens such as Staphylococcus aureus, Bacillus cereus, Escherichia coli, Klebsiella pneumonia, Pseudomonas aeruginosa, Salmonella typhi, and Candida albicans [13]. In addition, it has been shown that T. africanum methanolic extract exhibits antitumor activity in cell lines derived from several human cancers such as breast, colon, liver, and lung cancers [14].
Phytochemical analysis of aerial parts of T. africanum has shown the presence of several phytochemicals of clinical importance such as alkaloids, sterols, triterpenes, tannins, anthraquinones, and pyrrolizidine [15]. T. africanum volatile oils have been found to consist mainly of caryophyllene oxide, γ-eiudesmol, α-muurolene, elemol, carvone, and β-caryophyllene and to a lesser extent of α-pinene [16]. Based on the above, the purpose of this study was to investigate the impact of extraction methods, on extraction yield, antioxidant, and antimicrobial activities of T. africanum volatile oils.
MATERIALS AND METHODS
Chemical Reagents
The following reagents were used to evaluate the antioxidant activity of T. africanum extracts: Methanol (Lobachemie, India), n-hexane (Frutarom LTD, Haifa), trolox ((s)-(-)-6 hydroxy-2,5,7,8-tetramethychroman-2-carboxylic acid) (Sigma-Aldrich, Denmark), and 2,2-Diphenyl-1-picrylhydrazyl (DPPH) (Sigma-Aldrich, Germany).
Reagents used for the screening of the antimicrobial activity of T. africanum volatile oil included nutrient broth that was ordered from Himedia, India, and dimethyl sulfoxide (DMSO), which was purchased from Riedeldehan, Germany.
Instrumentations
During this study, the following instruments were used: Ultrasonic-microwave cooperative extractor/reactor (CW-2000, China), rotary evaporator (Heidolph OB2000, VV2000, Germany), UV-visible spectrophotometer (Jenway 7315, England), grinder (Moulinex model, Uno. China), balance (Radw ag, AS 220/c/2, Poland), filter paper (Machrery-Nagel, MN 617 and Whatman no.1, USA), micropipettes (Finnpipette, Finland), incubator (Nuve, Turkey), syringe filter 0.45 µm pore size (Microlab, China), and 96-well plates (Greiner bio-one, North America).
Collection and Preparing Plant Materials
The leaves of T. africanum were collected during its flowering time from Bethlehem region (Palestine) during May 2015. Botanical identification was carried out by Pharmacognosist Dr. Nidal Jaradat from the Pharmacognosy and Herbal Products Laboratory, Faculty of Medicine and Health Sciences, An-Najah National University, Nablus. The identification process was conducted using live herbal specimens and photographs from books [17]. Voucher specimens were deposited in the pharmacognosy and herbal products laboratory under the code number: Pharm-PCT-2457.
To extract volatile oil, the leaves of T. africanum were separated carefully and then washed twice with distilled water. The washed leaves were dried for 10-14 days in the shade at room temperature to avoid damage and to minimize cross contamination of the separated leaves. Finally, the dried leaves were grounded well and the powder obtained was stored in cloth bags for future use.
Volatile Oils Extraction
Volatile oils from T. africanum leaves were extracted using several methods (shown below). The main aim behind using different extraction methods was to evaluate the efficiency of these methods in terms of the obtained yield as well and the extraction time. T. africanum volatile oils obtained by the different extraction methods used in this study were then examined for both of their antimicrobial and antioxidant activities.
Volatile Oil Extraction Methods
Hydro distillation
Simple hydrodistillation was carried out using clevenger apparatus with some modifications [18]. About 100 g of the T. africanum dried leaves were placed in a round-bottom flask. The leaves were mixed with about 500 ml distilled water and then boiled for 120 min at 100°C. Volatile oils were collected into a clean beaker (this procedure was repeated three times). The obtained volatile oil was chemically dried using CaCl2. The purified volatile oil was weighed and stored in tightly-closed amber-colored bottles at 4°C.
Microwave method
T. africanum volatile oil was extracted using microwave oven as described by Bousbia et al. with some modifications [19]. The power of the microwave oven was set at 1000 W. Clevenger apparatus with a 1 L round-bottom flask containing about 100 g of T. africanum dried leaves powder was placed inside the microwave oven. About 500 ml distilled water was then added into the flask containing the powder. The flask was then connected to clevenger apparatus. Microwave distillation was carried out three times for 15 min each at 100°C. The obtained volatile oil was collected into a clean beaker, chemically dried, weighed, and stored as mentioned above.
Ultrasonic-assisted extraction method
During this method, a suspension of the dried leaves powder was prepared in a screw-caped class bottle using about 100 g of the dried leaves powder, which was mixed with about 500 ml hexane. The screw-capped bottle containing the suspension was placed in the ultrasonic extractor apparatus. Volatile oils were extracted for 120 min at 100°C. During the extraction process, the ultrasonic extractor apparatus was adjusted at its maximum power (50 W and frequency of 40 kHz). Finally, the hexane was removed in a rotary evaporator at 30°C and the obtained volatile oil was stored as mentioned above [20].
Microwave-ultrasonic method
Microwave-ultrasonic is a recently developed method that is used for the extraction of volatile oil from medicinal plants. In this method, volatile oil was extracted using a microwave oven with ultrasonication. However, during the extraction process, the powder suspension being extracted was exposed to ultrasonic waves to improve the extraction process. In this study, the apparatus consisting of a microwave oven combined with an ultrasonic extractor was used (ultrasonic-microwave cooperative extractor/reactor (CW-2000, China). A 1 L round-bottom flask containing about 100 g of the dried leaves powder was placed in this apparatus. In this flask, the powder was suspended in about 500 ml deionized water. Then, the flask was connected with Clevenger apparatus, which was placed in the same apparatus. While carrying out the extraction process, the power of the microwave-ultrasonic extractor apparatus was adjusted at 1000 W. The ultrasonic power of the apparatus was adjusted at its maximum power as well (50 W and frequency of 40 kHz). The extraction process using this apparatus was conducted for 10 min at 100°C. This process was repeated for three times. The obtained volatile oil was collected into a clean beaker, chemically dried and stored as motioned above.
DPPH Radical-scavenging Activity
Trolox standard and plant working solutions
A stock solution of T. africanum volatile oil that was extracted by the four extraction methods used in this study was used to prepare a stock solution, in methanol and Trolox, at a concentration of 1 mg/ml. Each of these stock solutions was diluted in methanol to prepare 12 working solutions with the following concentrations: 1, 2, 3, 5, 7, 10, 20, 30, 40, 50, 80, 100 µg/ml.
Spectrophotometric Measurements
A freshly prepared DPPH solution (0.002% w/v) was mixed with both methanol and with each of the above-mentioned working solutions at 1:1:1 ratio. In addition, a negative control solution was prepared by mixing the above-mentioned DPPH solution with methanol in 1:1 ratio. Then, all of these solutions were incubated at room temperature in a dark cabinet for 30 min. By the end of the incubation period, the optical density of these solutions was determined spectrophotometrically at a wavelength of 517 nm using methanol as the blank solution.
Antioxidant Activity of T. africanum Volatile Oils
The antioxidant activity of T. africanum volatile oil and Trolox standard was determined in terms of inhibition percentage of DPPH activity using the following formula as follows:
Percentage of inhibition of DPPH activity (%) = (A-B)/A × 100%, where: A = Optical density of the blank and B = Optical density of the sample.
The antioxidant half-maximal inhibitory concentration (IC50) for each of the T. africanum volatile oil and Trolox standard solution as well as their standard deviations, were calculated by using BioDataFit edition 1.02 (data fit for biologist).
Data Analysis
The antioxidant activities of T. africanum volatile oil at different concentrations mentioned above were expressed in terms of the antioxidant activity of the Trolox standard. This was determined using the BioDataFit fitting program as follows:
Inhibition percentage according to Trolox= (Trolox IC50/volatile oil IC50) × 100%
Antimicrobial Tests
T. africanum volatile oils obtained by the four different extraction methods used in this study were investigated for both of their antibacterial and antifungal activities. The antibacterial activities of T. africanum volatile oils were examined against the growth of four reference bacterial strains obtained from the American type culture collection (ATCC) (S. aureus [ATCC 25923], E. coli [ATCC 25922], and P. aeruginosa [ATCC 27853]) as well as against the growth of a diagnostically-confirmed methicillin-resistant S. aureus (MRSA) clinical isolates. The antifungal activity of T. africanum volatile oils was examined against the growth of a diagnostically-confirmed C. albicans clinical isolate.
The antimicrobial activities of T. africanum volatile oils obtained by the four extraction methods used in this study were determined using broth microdilution method as described previously [21,22]. Briefly, T. africanum volatile oils were dissolved in 5% DMSO at a concentration of 132 mg/ml. The prepared T. africanum volatile oils solutions were filter-sterilized and then were serially micro-diluted (2 folds) 11 times in sterile nutrient broth. The dilution processes were carried out under aseptic conditions in 96 well plates. In the micro-wells that were assigned to evaluate the antibacterial activities of the extracted T. africanum volatile oils, the concentration of these oils ranged from 0.129 to 66 mg/ml. On the other hand, the concentrations of these volatile oils in the micro-wells assigned to evaluate their antifungal activities ranged from 55 to 0.065 mg/ml. In these plates, micro-well number 11 contained volatile oils-free nutrient broth, which was used as a positive control for microbial growth. On the other hand, micro-well number 12 contained volatile oils-free nutrient broth that was left uninoculated with any of the test microbes. This well was used as a negative control for microbial growth. Micro-wells numbers 1 to 11 were inoculated aseptically with the test microbes. At the time of inoculation, the final concentrations of microbial cells were about 5 × 105 and 0.5-2.5 × 103 colony-forming unit /ml for the tested bacterial pathogens and C. albicans, respectively. Each of the included microbes in this study was examined in duplicate for being inhibited by the obtained T. africanum volatile oils.
All the inoculated plates were incubated at 35°C. The incubation period lasted for about 18 h for those plates inoculated with the test bacterial strains and for about 48 h for those plates inoculated with C. albicans. The lowest concentration of T. africanum volatile oils, at which no visible microbial growth in that micro-well was observed, was considered as the minimal inhibitory concentration (MIC) of the examined T. africanum volatile oils.
Statistical Analysis
Volatile oil yield of different extraction methods and IC50 values was determined in triplicates. Results are expressed as means ± standard deviation (SD). Data were compared using ANOVA with multiple comparisons. The statistical significance was considered when the P < 0.05. Statistical significance is expressed in terms of *when the P < 0.05, **when the P ≤ 0.001, and ***when the P ≤ 0.0001.
RESULTS
Volatile Oil Yields
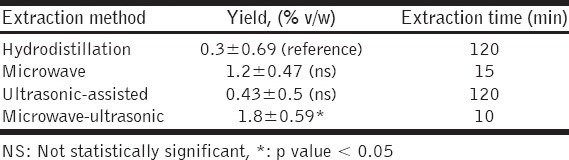
Comparing the amounts of volatile oil obtained from T. africanum by the four extraction methods used in this study, hydrodistillation method yielded the smallest amount with a dry weight yield of about 0.3% v/w after 2 h of extraction time, as shown in Table 1. The microwave method yielded a dry weight yield of about 1.2% v/w after about 15 min of extraction time. The amount of volatile oil obtained increased by ultrasonic-assisted method with a dry weight yield of about 0.43% v/w after 120 min of extraction time. The largest amount of volatile oils was obtained by the microwave-ultrasonic method, which gave a dry weight yield of about 1.8% v/w after only 10 min of extraction time.
Table 1.
Volatile oils yields obtained by four different extractions methods (at 100°C) versus extraction time
Antioxidant Activity using Trolox as Standard Equivalent
The free radical scavenging-activity of T. africanum volatile oils obtained by each of the extraction methods used in this study, have been tested by DPPH method using Trolox as a reference standard. The concentrations of the used volatile oils extracts as well as the Trolox standard ranged from 1 to 100 µg/ml. For measurement of the baseline scavenging activity, DPPH was diluted in the corresponding methanol solvent without any plant extract.
The calculated half-maximal IC50 for the Trolox standard was about 3.06 ± 0.54 µg/ml. The IC50 and percentage of inhibition for T. africanum volatile oils obtained by each of the used extraction methods are shown in Table 2 and clarified in [Figure 1].
Table 2.
IC50 for Trolox standard and T. africanum volatile oils obtained by four different extraction methods with their inhibition percentages according to Trolox standard
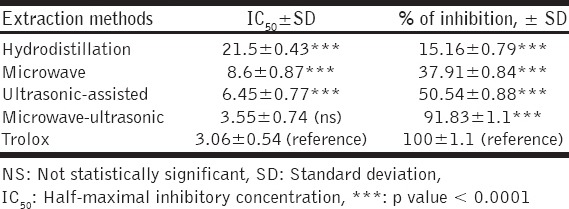
Figure 1.

Half-maximal inhibitory concentration percentages of Trichodesma africanum volatile oils yields obtained by four different extraction methods and their antioxidant-inhibition activities according to Trolox standard
Antimicrobial Activity
Volatile oils extracted by the four methods used in this study exhibit bioactivity against the growth of all microbes examined in this study.
On comparing the MICs of the volatile oils extracted by the four different methods, with the exception of C. albicans [Table 3 and Figure 2], clearly showed that there was a gradual decrease in the values of the MICs of volatile oils extracted by hydrodistillation, microwave, ultrasonic and microwave-ultrasonic, respectively. This indicates that the concentration of substances that have potential inhibiting-activity against microbial growth gradually increases in the volatile oil extracts obtained by extraction methods mentioned above. In all cases, the highest antimicrobial activity (lowest MIC) against microbes examined in this study, was seen in the extract obtained by microwave-ultrasonic method, indicating that among the four methods used in this study for extraction of volatile oil, this method was the most efficient one.
Table 3.
MICs of T. africanum volatile oils obtained by different extraction methods
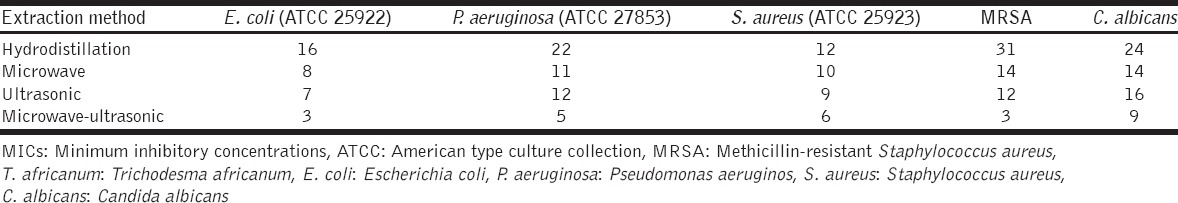
Figure 2.
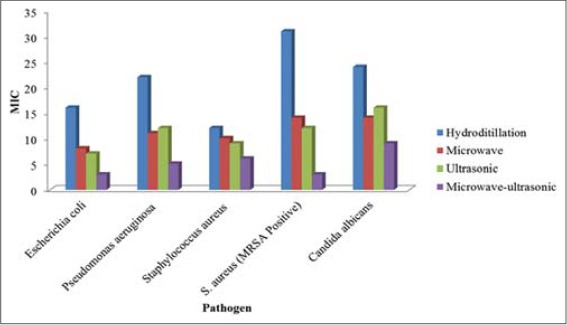
Minimum inhibitory concentrations of volatile oils extracted by hydrodistillation, ultrasonic, microwave and microwave-ultrasonic methods
DISCUSSION
According to World Health Organization, about 80% of developing countries populations utilize herbal medicines for the treatment of many diseases. In addition, about 25% of modern pharmacopoeia includes medications that are either phytochemicals of medicinal plants or their semi-synthetic derivatives [23]. Extraction techniques play an essential role in food, cosmetic and pharmaceutical industries. It is estimated that these techniques require up to 50% of the financial investments of these industries. Moreover, about 70% of the used energy used in food industries, is consumed by extraction techniques [24]. Recently, there has been an increasing-interest in developing more efficient and environment-friendly extraction techniques. These new techniques that are being developed, must guarantee decreasing environmental pollution and technical hazards, be more efficient in terms of quantity and the quality of the extracts as well as minimizing the financial cost [25]. The several new extraction techniques have been described recently, among which are microwave, ultrasonic, and microwave-ultrasonic assisted methods [26]. These methods showed a positive effect on quality and yield of the obtained medicinal agents due to the mechanism involved during the extraction process. In fact, through shear forces created by ultrasonic cavitations and cell walls are broken mechanically which results in an easier transfer of the phytochemical materials especially volatile oils. In addition, there is no chemical relationship in the ultrasound-assisted extraction, which means no chemical degradation in the product of interest [27]. Moreover, the used ultrasonic waves allow better penetration of the solvent into the herbal matrix which increases the contact surface between solid and liquid phases [28]. Therefore, the selection of the appropriate extraction method will depend on the kind of herb species and also the nature of extracts [27].
The main aim of this study was to evaluate these new extraction techniques in terms of both the quantity and the quality T. africanum volatile oils and to compare them with same volatile oils obtained by conventional hydrodistillation method. The obtained data showed that ultrasonic-assisted extraction method was comparable to hydrodistillation extraction method in terms of the extraction time (about 120 min) needed for T. africanum volatile oil extraction. However, both microwave and microwave-ultrasonic extraction methods were remarkably more efficient in terms of the extraction times needed for T. africanum volatile oil extraction, which were 15 and 10 minutes, respectively. This indicates that volatile oil extraction by both microwave and microwave-assisted extractions methods require about 8-12% of the time needed for the extraction of the same oil by both ultrasonic-assisted and the hydrodistillation methods. The microwave and the microwave-ultrasonic methods gave about 4-6 times more than the amount of T. africanum volatile oils (% v/w), obtained by the hydrodistillation method and about 3-4 times more than the amount of volatile oils obtained by the ultrasonic-assisted methods.
Our data clearly indicated that both the microwave and the microwave-ultrasonic extractions methods were much more efficient than ultrasonic method as well as conventional hydrodistillation extraction methods in terms of both of the extraction time and obtained yields of T. africanum volatile oils. Accordingly, it can be concluded that the microwave and the microwave-ultrasonic extractions methods are more efficient as well in terms of the consumed energy needed for the extraction process as well. In fact, our finding is in accordance with another similar study that has been conducted on the same plant where the extracted oils have been identified and analyzed using GC-MS techniques. According to this research, the major components of T. africanum volatile oils were caryophyllene oxide (15.6%), γ-eudesmol (13.7%), α-muurolene (10.5%), elemol (7.0%), carvone (6.8%), and β-caryophyllene (6.6%) while the minor component was α-pinene (0.1%) [16]. However, in a future study, this constituent will be extracted using different techniques and its quality and activity such as antioxidant and antimicrobial will be assessed. To evaluate the quality of the obtained T. africanum volatile oils by the four used extraction methods, their antioxidant activity as well as their antimicrobial activities were evaluated. Our results indicated that T. africanum volatile oils obtained by the microwave, the ultrasonic-assisted and the microwave-ultrasonic extraction methods, exhibited significant higher antioxidant activities in comparison to the antioxidant activity of same volatile oil obtained by the hydrodistillation extraction method. The antioxidant activities of the volatile oils obtained by the former mentioned three extraction methods were about 2.5, 3.3 and 6 times, respectively, higher than the antioxidant activity of same volatile oil obtained by the conventional hydrodistillation method. Antibacterial activity tests of the T. africanum volatile oils obtained by conventional hydrodistillation, microwave, ultrasonic-assisted and microwave ultrasonic, showed a gradual decrease, respectively, in their MICs against the growth of the tested bacterial pathogens. This gradual decrease in the MICs indicates that there is a gradual increase in the concentration of ingredients with potential antibacterial activity in these volatile oils. The lowest MICs against the growth of the tested bacterial pathogens were those of the volatile oil obtained the microwave-ultrasonic extraction methods, indicating that this extraction method was the best one in terms of the concentration of ingredients with potential antibacterial activity. Similar antimicrobial studies on this plant has been conducted by El-Ghazali et al. who tested the whole methanolic extract against Proteus vulgaris, E. coli, K. pneumonia, P. aeruginosa, S. aureus bacteria and the results were 9.5 ± 0.5, 10.5 ± 0.5, 12.5 ± 0.5, 10.5 ± 0.5, and14.0 ± 0.0 respectively. However, these results cannot be compared with our methods since we worked on essential oils instead of the crude extract.
The antifungal activities of the obtained volatile oils indicated that the volatile oils obtained by microwave, ultrasonic-assisted, and microwave ultrasonic extraction methods showed a significantly lower MICs-thus higher antifungal activity than the same oil obtained by the conventional hydrodistillation method. The highest antifungal activity was exhibited by the volatile oil obtained by the microwave ultrasonic extraction method. Since both the highest antibacterial and antifungal activities (lowest MICs) were exhibited by the volatile oil obtained microwave-ultrasonic extraction method indicates that this extraction method is the best one among the used ones in this study and further investigations required to identify the changes in the constituents of these volatile oils which extracted by these four different methods. Although only one medicinal plant was included in this study (T. africanum), the obtained results are encouraging in terms of applying these recent extraction methods for volatile oil extraction from other medicinal plants.
CONCLUSION
Environment-friendly and recently-developed methods for the extraction of volatile oils from T. africanum were evaluated in this study in terms of their quantitative and qualitative efficiency in comparison to the conventional hydrodistillation method. Our results indicated that these recent extraction methods were significantly more efficient than the conventional hydrodistillation method in terms of the extraction time, the obtained yields as well as in terms of their antioxidant and antimicrobial activities. In addition, this implies that these recent extraction methods are more-likely to be more economical in terms of the consumed energy, water, and the financial cost needed for the extraction process. Among the four extraction methods that were evaluated in this study, the microwave-ultrasonic extraction method was the best one since volatile oil obtained by this method always gave the best one in terms of yield (1.8% v/w) and antioxidant activities (91.83% ± 1.1%) of inhibition, while the MICs for E. coli, P. aeruginosa, S. aureus, MRSA and C. albicans were 3, 5, 6, 3, and 9, respectively.
ACKNOWLEDGMENTS
The authors acknowledge the assistance of the technicians Mohamad Arar and Linda Esa.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Cespedes CL, Alarcon J, Aqueveque PM, Lobo T, Becerra J, Balbontin C, et al. New environmentally-friendly antimicrobials and biocides from Andean and Mexican biodiversity. Environ Res. 2015;142:549–62. doi: 10.1016/j.envres.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Hinneburg I, Neubert RH. Influence of extraction parameters on the phytochemical characteristics of extracts from buckwheat (Fagopyrum esculentum) herb. J Agric Food Chem. 2005;53:3–7. doi: 10.1021/jf049118f. [DOI] [PubMed] [Google Scholar]
- 3.Rousseaux CG, Schachter H. Regulatory issues concerning the safety, efficacy and quality of herbal remedies. Birth Defects Res B Dev Reprod Toxicol. 2003;68:505–10. doi: 10.1002/bdrb.10053. [DOI] [PubMed] [Google Scholar]
- 4.Han F, Guo Y, Gu H, Li F, Hu B, Yang L. Application of alkyl polyglycoside surfactant in ultrasonic-assisted extraction followed by macroporous resin enrichment for the separation of vitexin-2?-O-rhamnoside and vitexin from Crataegus pinnatifida leaves. J Chromatogr B Analyt Technol Biomed Life Sci. 2016;1012-1013:69–78. doi: 10.1016/j.jchromb.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 5.Hayat K, Hussain S, Abbas S, Farooq U, Ding B, Xia S, et al. Optimized microwave-assisted extraction of phenolic acids from citrus mandarin peels and evaluation of antioxidant activity in vitro. Sep Purf Technol. 2009;70:63–70. [Google Scholar]
- 6.Al-Yahya MA, Suud Ga-M. Saudi Plants: A Phytochemical and Biological Approach. Saudi Arabia: King Abdulaziz City for Science and Technology; 1990. [Google Scholar]
- 7.Retief E. The genus Trichodesma (Boraginaceae: Boraginoideae) in southern Africa. Bothalia. 2002;32:151–66. [Google Scholar]
- 8.Boulos L. Flora of Egypt Checklist. Cairo: Al Hadara Publishing; 1995. [Google Scholar]
- 9.El-Ghazali GE, Al-Khalifa KS, Saleem GA, Abdallah EM. Traditional medicinal plants indigenous to Al-Rass province, Saudi Arabia. J Med Plants Res. 2010;4:2680–3. [Google Scholar]
- 10.Safa O, Soltanipoor MA, Rastegar S, Kazemi M, Nourbakhsh Dehkordi K, Ghannadi A. An ethnobotanical survey on hormozgan province, Iran. Avicenna J Phytomed. 2013;3:64–81. [PMC free article] [PubMed] [Google Scholar]
- 11.Soladoye M, Oyesiku O. A Textbook of Medicinal Plants from Nigeria Lagos. Nigeria: UNILAG Press; 2008. Taxonomy of Nigerian Medicinal Plants. [Google Scholar]
- 12.Tareen RB, Bibi T, Khan MA, Ahmad M, Zafar M, Hina S. Indigenous knowledge of folk medicine by the women of Kalat and Khuzdar regions of Balochistan, Pakistan. Pak J Bot. 2010;42:1465–85. [Google Scholar]
- 13.Abdallah Emad M, El-Ghazali Gamal E. Screening for antimicrobial activity of some plants from Saudi folk medicine. Glob J Res Med Plants Indig Med. 2013;2:189–97. [Google Scholar]
- 14.Moustafa SM, Menshawi BM, Wassel GM, Mahmoud K, Mounier M. Screening of some plants in Egypt for their cytotoxicity against four human cancer cell lines. Screening. 2014;6:1074–84. [Google Scholar]
- 15.Sharawy SM, Alshammari AM. Checklist of poisonous plants and animals in Aja Mountain, Hail region, Saudi Arabia. Aust J Basic Appl Sci. 2008;3:2217–25. [Google Scholar]
- 16.Ahmed S, Ibrahim M, Khalid K. Investigation of essential oil constituents isolated from Trichodesma africanum (L.). grow wild in Egypt. Res J Med Plant. 2015;9:248–51. [Google Scholar]
- 17.Tristram HB. The Survey of Western Palestine: The Fauna and Flora of Palestine. Vol. 5. London: Committee of the Palestine Exploration Fund; 1884. [Google Scholar]
- 18.Clevenger J. Apparatus for the determination of volatile oil. J Am Pharm Assoc. 1928;17:345–9. [Google Scholar]
- 19.Bousbia N, Vian MA, Ferhat MA, Petitcolas E, Meklati BY, Chemat F. Comparison of two isolation methods for essential oil from rosemary leaves: Hydrodistillation and microwave hydrodiffusion and gravity. Food Chem. 2009;114:355–62. [Google Scholar]
- 20.Da Porto C, Decorti D, Kikic I. Flavour compounds of Lavandula angustifolia L. to use in food manufacturing: Comparison of three different extraction methods. Food Chem. 2009;112:1072–8. [Google Scholar]
- 21.Wikler MA. Performance Standards for Antimicrobial Susceptibility Testing: Seventeenth Informational Supplement. USA: Clinical and Laboratory Standards Institute; 2007. [Google Scholar]
- 22.Forbes BA, Sahm DF, Weissfeld AS. Study Guide for Bailey and Scott’s Diagnostic Microbiology. USA: Mosby; 2007. [Google Scholar]
- 23.Bodeker G, Bhat K, Burley J, Vantomme P. Medicinal Plants for Forest Conservation and Health Care. Rome: FAO; 1997. [Google Scholar]
- 24.Clark JH, Macquarrie DJ. Handbook of Green Chemistry and Technology. New York: John Wiley & Sons; 2008. [Google Scholar]
- 25.William M. Green Solvents for Chemistry-Perspectives and Practice. USA: Oxford University Press; 2003. [Google Scholar]
- 26.Ali M, Watson IA. Comparison of oil extraction methods, energy analysis and biodiesel production from flax seeds. Int J Energ Res. 2014;38:614–25. [Google Scholar]
- 27.Wang L, Weller CL. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci Technol. 2006;17:300–12. [Google Scholar]
- 28.Lianfu Z, Zelong L. Optimization and comparison of ultrasound/microwave assisted extraction (UMAE) and ultrasonic assisted extraction (UAE) of lycopene from tomatoes. Ultrason Sonochem. 2008;15:731–7. doi: 10.1016/j.ultsonch.2007.12.001. [DOI] [PubMed] [Google Scholar]


