Abstract
Background:
Effective long-term management is the key to treatment of diabetes mellitus (DM) and its complications.
Aim:
To ascertain the ability of cryptolepine (CRP) in managing DM and some associated complications.
Materials and Methods:
Changes in fasting blood sugar (FBS), body weight, response to thermally-induced pain, and semen quality were assessed in normal and alloxan-induced diabetic rats treated with CRP (10, 30, or 100 mg/kg), glibenclamide (10 mg/kg), or normal saline (2 ml/kg) per os. Hematological profile, liver and kidney function tests, lipid profile, as well as liver, kidney, and pancreas histopathological examinations were also conducted to establish possible effects of CRP treatment.
Results:
CRP treatment reduced (P ≤ 0.001) FBS and body weight, inhibited (P ≤ 0.05 - 0.001) the latency to tail flick or withdrawal from pain stimulus. It did not alter (P > 0.05): Hematological parameters, elevated (P ≤ 0.05 - 0.001) plasma aspartate transaminase, alanine transaminase, and gamma-glutamyl transferase, reduced (P ≤ 0.01) plasma urea, and elevated (P ≤ 0.001) plasma creatinine associated with DM. CRP, however, reversed (P ≤ 0.05 - 0.001) DM-associated elevation (P ≤ 0.05 - 0.001) of plasma cholesterol, triglycerides, and low-density lipoproteins, and the reduction in high-density lipoproteins. CRP (10-30 mg/kg) showed dose-dependent regeneration of β-islet cells but could not repair degenerated liver and kidney tissue. CRP worsens dose-dependently (P ≤ 0.001) reduced sperm quality associated with DM.
Conclusion:
CRP abolishes hyperglycemia, weight loss, cold allodynia, neuropathic pain, and hyperlipidemia as well as pancreatic β-islet cell damage associated with DM. It, however, does not improve liver and kidney damage and lowered semen quality.
KEY WORDS: Cold allodynia, cryptolepine, diabetes mellitus complications, glibenclamide, paw withdrawal, tail flick test
INTRODUCTION
Diabetes mellitus (DM) is a pathologic condition characterized by abnormally high blood glucose resulting from insufficient levels of insulin and/or insulin resistance [1]. This metabolic disorder results in the generation of reactive oxygen species (ROS), which cause oxidative damage, specifically in the visceral organs such as the heart, kidney, and liver [2]. DM is really a global health issue with about 285 million people having diabetes globally as of 2010; Type 2 making up about 90% of the cases [3]. According to International Diabetes Federation, in 2013, an estimated 381 million people had DM; considering this prevalence, the number of DM individuals is estimated to almost double by 2030 with the greatest increase in prevalence expected in Asia and Africa [4]. DM is associated with several complications which result in damage to the eyes, kidneys, and nerves. Ocular complications, usually in the form of cataract and retinopathy (diabetic retinopathy), are the leading cause of visual impairment and blindness. Damage to the kidneys (diabetic nephropathy) could eventually lead to chronic kidney disease, whereas damage to the nerves of the body (diabetic neuropathy, DN), the most common complication of diabetes, manifests with symptoms such as numbness, tingling, pain, and altered pain sensation. In addition, proximal DN causes painful muscle wasting and weakness [5]. Other long-term complications of DM are related to damage of blood vessels, which is very much related to the impairment of fatty acid synthase and nitric oxide synthase that interact in the cells that line blood vessel walls [6]. Most of this excess risk is associated with an augmented prevalence of well-known risk factors such as hypertension, hyperlipidemia, obesity, and cardiovascular disease [7] as the majority (about 75%) of deaths in diabetics is due to coronary artery disease [8]. Other macrovascular diseases are stroke and peripheral vascular disease. The immunological and gastrointestinal systems are also not spared. Recent studies indicate that ROS plays a key intermediate role in the pathophysiology of DN [9]. Hyperglycemia, the main determinant of the initiation and progression of DN, not only generates more reactive oxygen metabolites but also attenuates antioxidative mechanisms through non-enzymatic glycosylation of antioxidant enzymes [1].
Cryptolepine (CRP), a naturally occurring indoloquinoline alkaloid (isolated from the roots of Cryptolepis sanguinolenta Family: Periplocaceae), is an antimalarial drug in Central and Western Africa [10]. It has, however, been perceived to be useful in the traditional management of DM. The aim of this study, therefore, was to ascertain the therapeutic usefulness of CRP in managing DM and some of its associated complication which includes weight loss, neuropathy, liver, kidney, and pancreatic tissue damage as well as decreased spermatocyte motility and viability.
MATERIALS AND METHODS
Experimental Animals
Laboratory-bred Sprague-Dawley rats (250-300 g; 8-10 weeks old) obtained from animal house of the Department of Biomedical and Forensic Sciences, School of Biological Sciences, University of Cape Coast, Ghana, were used in this study. The rats were kept under ambient conditions of temperature (23-26°C), relative humidity (60-70%), and 12 h light/dark cycle. Preceding the experimental session, water and rat chow were available ad libitum. All animals used were handled in accordance with the National Institute of Health Guidelines for Care and Use of Laboratory Animals and were approved by the Departmental Ethics Committee.
Drugs and Chemicals Used
Alloxan monohydrate (BDH Chemicals, England) was used to induce diabetes. Glibenclamide (Denk Pharma GmbH & Co. KG., Germany) was the selected reference hypoglycemic agent.
Induction of DM
Healthy Sprague-Dawley rats weighing 170-200 g (fed for 14 h with 5% glucose solution to prevent hypoglycemic shock) were injected intraperitoneally with 140 mg/kg alloxan monohydrate in distilled water [11] after their fasting blood sugar (FBS) were ascertained by the enzymatic glucose oxidase method using a commercial OneTouch Select™ blood glucose meter (LifeScan Inc, Milpitas, CA, China). Drops of blood for the test were obtained from the tail vein of the rats. 5 days after, rats with FBS above 15.0 mmol/L were considered diabetic and were selected for this study.
Antihyperglycemic Activity of CRP
Diabetic rats were grouped into five (n = 5). Group 1, diabetic (negative) control, rats were treated with 2 ml/kg physiological saline. Group 2 rats were treated with 10 mg/kg glibenclamide. Group 3-5 received 10, 30, and 100 mg/kg, respectively, CRP per os. Group 6, the sham control had rats, in which diabetes was not induced but was treated with vehicle (i.e., 2 ml/kg normal saline) and kept under the same experimental conditions as all the other groups. Treatments started from day 5 post-induction. FBS was measured before induction of diabetes (day 0), and on day 5, 7, 10, and 13 post-induction, 1 h after drug treatment.
Effect of CRP Treatment on Body Weight
Rats were weighed initially before induction of diabetes and grouped after induction of diabetes as described above. The weights of rats in all groups were monitored individually on days 5, 7, 9, 11, 13, and 15 post-induction using a Sartorius top loading balance (CPA3202S, Goettingen, Germany)
CRP in Managing Neuropathic Pain in DM
Cold allodynia [12] and Tail flick [13] tests were thermally-induced pain models used to assess neuropathic pain in the diabetic rats.
Cold allodynia
Diabetic rats were put into five groups, A-E (n = 5). Group A, the control, was treated orally with normal saline (2 ml/kg). Groups B, C, and D were treated orally with CRP at doses of 10, 30, and 100 mg/kg, respectively, whereas Group E was treated orally with 10 mg/kg Morphine. Group F, the sham control, were normal rats, which were treated with 2 ml/kg normal saline but were subjected to the same experimental conditions as the diabetic rats. The temperature of cold water was set at 4°C and allowed to stabilize for 5 min (temperature of testing room 21 ± 1°C). Each hind paw of the animals was then placed into the cold water and the time (in seconds) taken for the first brisk lift paw to occur was recorded; there was alternation between right and left hind paws to prevent adaptation. The time to the brisk response is interpreted as the latency for cold pain withdrawal. Given that temperatures of 15°C and above are considered innocuous (based on human psychophysical data) and those rats explore their environment, 20 s was chosen as the upper time limit for a cold “pain” response. Any response with a latency >20 s was considered nonpainful [14]. A maximum cut-off time of 20 s was used to prevent tissue damage at the lower temperatures. Each rat was tested only once on any given test day to avoid any possible anesthetic or tissue damage effects that could be produced by repeated exposure to a cold environment. Test was done on day 0 (before induction of diabetes) as well as on day 5, 7, 9, 11, and 13 post-induction, 1 h after drug treatment.
Tail flick method
The tail flick test is a test of acute nociception, in which a moderately high-intensity thermal stimulus is directed to the tail of the rat. The time from onset of stimulation to a rapid flick/withdrawal of the tail from heat source is recorded. This experiment was done using hot water in a bath where temperature of the water was raised and maintained at 55°C. Diabetic rats were put into five groups, A-E (n = 5). Group A, the control, was treated orally with normal saline (2 ml/kg). Groups B, C, and D were treated orally with CRP at doses of 10, 30, and 100 mg/kg, respectively, whereas Group E was treated orally with 10 mg/kg Morphine. Group 6 was an sham control. The tail was immersed into the hot water 5 cm from the tip with the rats held vertically above the water bath. The cutoff time for the test was set to 10 s after which the subjects were withdrawn to prevent tissue damage. The time (in seconds) from the dipping of tail into the hot water and rapid flick/withdrawal from hot water (tail flicking latency) was recorded. Each animal was tested once at each day of pain assessment, to avoid voluntary adaptation to hot temperatures and also tissue damage. The test was conducted on day 0 (before induction of diabetes) and repeated on days 5, 7, 9, 11, 13, and 15 post-diabetic.
Collection of Blood Samples
By cardiac puncture [12] technique, samples of blood from animals from CRP treatment groups as well as the positive, negative, and sham control groups were collected at the end of treatment period and transferred into separate MediPlus vacutainer K3 EDTA tubes (Sunphoria Co. Ltd., Taiwan) for hematological studies or into serum separator tubes (SST) which containing silica particles and a serum separating gel (BD Vacutainer® Blood Collection Tube Product, USA) for chemical pathology studies.
Hematological Profile
Full blood count on samples was done using a hemato-analyzer (Sysmex XP - 300™ Automated Hematology Analyzer, USA).
Liver and Kidney Function Tests and Lipid Profile
Blood samples transferred into SSTs were allowed to clot and centrifuged at 3000 rpm for 10 min. The serum separated thereafter was collected into 5 ml sterile glass tubes for liver and kidney function tests as well as lipid profile using a clinical chemistry analyzer (Vital Scientific N. V, Netherlands) at Kasoa Government Hospital, in the Central Region of Ghana.
Histopathological Assessment
The pancreas, liver, and kidneys from treated and control animals were collected after sacrificing and dissection, re-sectioned and fixed in 10% phosphate-buffered formalin. The fixed organs were then processed using standard techniques [15]. Each of the organs was treated at all levels of tissue processing at Komfo Anokye Teaching Hospital in Kumasi, Ghana, and obtained slides observed under an Olympus trinocular light microscope (Jenoptik, Germany) at ×100 magnification power. Images were captured by a microscope camera with LC Micro software (Olympus Soft Imaging Solutions GmbH, Germany) connected to the third eyepiece of the microscope.
Effect of CRP Treatment on Semen Quality
Spermatocyte motility was determined according to the method described by Zemjanis [16]. Briefly, a drop of semen was collected from the caudal epididymis for the CRP -treated and the controls onto a glass slide. Sodium citrate buffer (2.9%) was then added to the semen and mixed until the desired dilution was obtained. Percentage motility was then evaluated microscopically within 2-4 min of semen collection. The total spermatozoa in the caudal epididymis sperm sample were counted from five large squares (volume: 0.5 mm3) using the improved Neubauer hemocytometer (depth 0.1 mm, area: 1/400 mm2; Yancheng Cordial Lab Glassware Co. Ltd., Jiangsu, China (Mainland). The sperm morphological abnormalities and percentage live/dead ratio was assessed using the method described by Wells and Awa [17]. These were obtained from a total count of 400 spermatozoa in smears obtained with Wells and Awa stains (0.2 g of Eosin and 0.6 g of fast green dissolved water and ethanol in ratio 2:1). Live/dead ratio was determined using 1% eosin and 5% nigrosin in 3% sodium citrate dehydration solution.
Statistical Analysis
GraphPad Prism Version 5 was used for data analyses. Data are presented as mean ± standard error of mean. Differences in data for sham, treatments, and the negative control (NC) were analyzed using one-way ANOVA. Further, comparisons were performed using Dunnett’s post-hoc test. P ≤ 0.05 was considered significant.
RESULTS
Effects of CRP on DM Hyperglycemia
Hyperglycemia was observed by day 3 post-induction with mean FBS of 4.56 ± 0.22 mmol/L elevating to 20.34 ± 1.29 mmol/L in diabetic rats. Treatment with CRP (10, 30, and 100 mg/kg) and glibenclamide (reference hypoglycemic drug) induced a significant reduction (P ≤ 0.001) in FBS to between 3.0 and 4.2 mmol/L. There were no significant changes in blood glucose levels in the sham control group [Figure 1].
Figure 1.

Cryptolepine (C) (10, 30, and 100 mg/kg) and glibenclamide (G) (10 mg/kg) treatments on, (a) The time course of fasting blood sugar and, (b) The area under the time-course curves (AUC) in alloxan-induced DM. values plotted are means±standard error of mean. †††P≤0.01 represents the significant difference between the normal and the diabetic state, ***P≤0.001; comparing drug treatments to the negative control (NC) (One-way ANOVA followed by Dunnett’s post-hoc test)
Effects of DM and CRP Treatment on Body Weight
With the induction of DM, there was a significant reduction (P ≤ 0.001) in body weight, i.e., 188.8 ± 2.7 g in normal rats to 152.9 ± 3.4 g in diabetic rats. Treatment with CRP (10 and 30 mg/kg) and glibenclamide caused significant increments (P ≤ 0.05) in the weight of the diabetic animals. There was, however, a consistent decrease in the weights of the diabetic but untreated (NC) group and the 100 mg/kg CRP treatment group [Figure 2].
Figure 2.

Cryptolepine (C) (10, 30, and 100 mg/kg) and glibenclamide (G) (10 mg/kg) treatments on, (a) The time course of cold allodynia and, (b) The area under the time-course curve (AUC), in alloxan-induced DM. Values plotted are means±standard error of mean, n=5. †††P≤0.01 represents the significant difference between the sham control (SH) and the diabetic state, *P≤0.05, ns P>0.05 comparing drug treatments to the negative control (NC) (One-way ANOVA followed by Dunnett’s post-hoc test)
Effect of CRP on DM Neuropathic Pain
Cold allodynia
Cold allodynia reduced significantly (P < 0.001) between normal and diabetes, i.e., from 11.41 ± 0.53 s to 6.68 ± 0.60 s. The 100 mg/kg CRP dose produced a significant (P ≤ 0.05) inhibition of cold allodynia in the diabetic rats; similar to morphine (P ≤ 0.01), the reference analgesic [Figure 3].
Figure 3.

Cryptolepine (C) (10, 30, and 100 mg/kg) and morphine(M) (10 mg/kg) treatments on, (a) the time course of cold allodynia and, (b) area under the time course curves (AUC) in alloxan-induced DM. Values plotted are means±standard error of mean. †††P≤0.01 represents the significant difference between the sham control (SH) and the diabetic state; ns P>0.05, *P≤0.05; comparing treatments to the negative control (NC) (One-way ANOVA followed by Dunnett’s post-hoc test)
Tail flick test
Latency period to pain reduced significantly (P ≤ 0.001) between normal and diabetic rats (i.e., from 3.52 ± 0.095 to 2.192 ± 0.093 s). Treatment with CRP (30 and 100 mg/kg) increased the latency to tail flicking (withdrawal) from hot water significantly (P ≤ 0.01); an effect similar to morphine (P ≤ 0.01), the reference analgesic [Figure 4].
Figure 4.
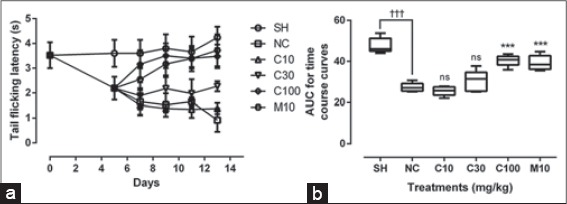
Cryptolepine (C) (10, 30, and 100 mg/kg) and morphine (M) (10 mg/kg) on, (a) the time course of neuropathic pain and, (b) Area under the treatment time course curves (AUC) in alloxan-induced DM. Values plotted are means±standard error of mean, (n=5). †††P≤0.01 represents the significant difference between sham control (SH) and the diabetic state; ns P>0.05, ***P≤0.05; comparing treatments to the negative control (NC) (One-way ANOVA followed by Dunnett’s post-hoc test)
Effect of CRP on Hematological Parameters in DM
Except platelet count which decreased significantly (P ≤ 0.001) with DM, there were no significant (P > 0.05) decrements in hematological parameters associated with DM in rats. CRP and glibenclamide treatments did to affect hematological parameters significantly [Table 1].
Table 1.
Hematological parameters in alloxan-induced diabetic rats and that following a 15 days treatment with 10, 30, and 100 mg/kg cryptolepine or 10 mg/kg glibenclamide

Liver Function Test
Although there were no significant (P > 0.05) changes in plasma levels of alkaline phosphatase, total protein (PROT), albumin (ALB), globulin (GLOB), and total bilirubin (TBIL) associated with DM, and treatment of DM with glibenclamide and CRP, the aspartate transaminase (AST), alanine transaminase (ALT), and gamma-glutamyl transferase (GGT) levels were significantly elevated (P ≤ 0.05 - 0.001) with DM. Treatment with glibenclamide resulted in a significant (P ≤ 0.001) reversal of these elevated parameters. CRP treatment, however, did not have any significant (P > 0.05) effect on the elevated parameters. In fact, AST was further elevated (P < 0.01) with CRP treatment [Table 2].
Table 2.
Blood biochemistry values of liver and kidney function tests and the lipid profile for normal and alloxan-induced diabetic rats and that following a 15 days treatment of diabetic rats with 10 mg/kg glibenclamide (G) or 10, 30, and 100 mg/kg cryptolepine (C)

Kidney Function Test
Associated with DM was a significant reduction (P ≤ 0.01) in plasma urea and a significant elevation (P ≤ 0.001) in creatinine. Glibenclamide treatment significantly reversed (P ≤ 0.001) these changes, however, CRP treatment could not alter significantly (P > 0.05) these changes [Table 2].
Lipid Profile
DM was also associated with a distorted lipid profile as there was significant elevation (P ≤ 0.05 - 0.001) of cholesterol (CHO), triglycerides (TAG), and low-density lipoproteins (LDL) and a significant reduction in high-density lipoproteins (HDL). Treatment with glibenclamide and CRP significantly reversed (P ≤ 0.05 - 0.001) the DM-associated distortion in lipid profile. Very LDL (VLDL) levels were, however, not significantly affected [Table 2].
Effect of CRP on Histopathology in DM
Pancreas
The normal control group showed normal histology of the islets of Langerhans and the exocrine acinar cells [Figure 5a]. Induction of DM resulted in necrosis of the pancreatic islets of Langerhans cells. Both cells also display degenerative changes within their nuclei [Figure 5b]. Glibenclamide treatment appeared to have normal islet cells [Figure 5c]. CRP treatment (10 and 30 mg/kg) showed a dose-dependent regeneration of islet cells [Figure 5d and e]. CRP (100 mg/kg) shows reduction of cells in the middle portion of the islets of Langerhans, which shows a sign of no or little regeneration, with degenerated and disorganized acinar cells [Figure 5f].
Figure 5.

Photomicrographs of the pancreas showing, (a) normal pancreatic islets of Langerhans (white arrows), (b) diabetic pancreas showing loss of cells mostly from the middle portion of the endocrine islets of Langerhans (arrow) with highly basophilic nuclei of surrounding secretory exocrine acinar cells, and the presence of connective tissue septum (arrow head), (c) glibenclamide treatment: Normal histology (white arrow) - sign of total regeneration, (d) 10 mg/kg cryptolepine (CRP) treatment: Reduction of cells in the middle portion of the Islets of Langerhans (arrows); a sign of little regeneration, (e) 30 mg/kg CRP treatment: Total regeneration of cells in the middle portion of the Islets of Langerhans (arrows) and the presence of an intralobular duct (asterisks) of the exocrine acinar, and (f) 100 mg/kg CRP treatment: Reduction of cells in the middle portion of the Islets of Langerhans (arrows) (H and E stain, ×100)
Liver
The normal histology of the liver has polygonal, tightly parked hepatocytes, containing basophilic central rounded nuclei separated by the hepatic sinusoids radiating from the central vein (CV) and with the presence of non-activated spindle-shaped Kupffer cells within the sinusoids [Figure 6a]. Induction of DM resulted in tissue appearing edematous, with the presence of activated Kupffer cells with some highly basophilic nuclei of some hepatocytes [Figure 6b]. Glibenclamide-treated diabetic animals appears to have normal liver histology with a very few highly basophilic hepatocytes [Figure 6c]. The 10 mg/kg CRP treatment showed disorganized hepatocytes with distorted margins of the CV. Some hepatocytes appeared degenerated with eosinophilic cytoplasm and little infiltration of mononuclear cells (Figure 6d). In the 30 mg/kg CRP-treated group, the histology showed mononuclear cells within sinusoids with normal nuclei in hepatocyte, and well-defined margins of the CV. The architecture of the hepatocyte appears normal (Figure 6e). There was, however, a loss of hepatocyte architecture in the 100 mg/kg CRP-treated group. Liver appears edematous with infiltration of lymphocytes within sinusoids but with well-defined CV. There is also demonstration of activation of Kupffer cells and the presence of few degenerative nuclei (necrosis) of the hepatocytes at the pyknotic stage (Figure 6f).
Figure 6.
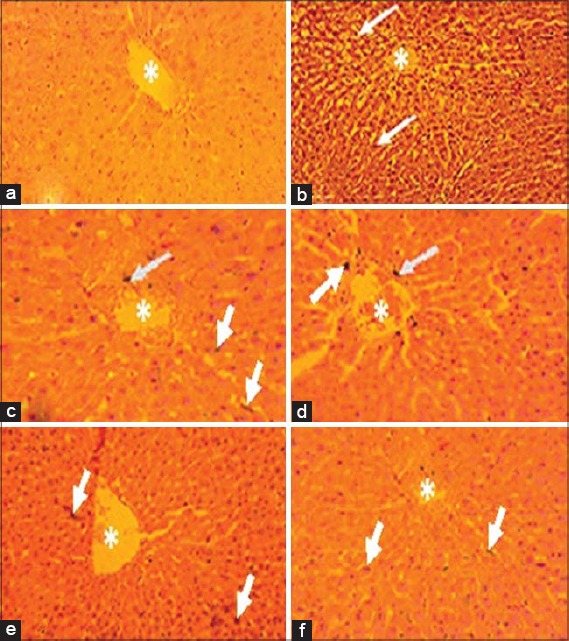
Photomicrographs of liver showing: (a) Normal histology with non-activate Kupffer cells and a normal hepatocytes and CV, (b) Diabetic liver showing edematous tissue with the presence of activated Kupffer cells (arrows), and highly basophilic nuclei of some hepatocytes, (c) glibenclamide treatment showing normal histology with very few highly basophilic hepatocytes, (d) 10 mg/kg cryptolepine (CRP) treatment showing slightly edematous tissue with few highly basophilic nuclei and the presence of activated Kupffer cells, (e) 30 mg/kg CRP treatment showing very mild edematous appearance with very few highly basophilic nuclei of hepatocytes and activated Kupffer cells (arrows), and (f) 100 mg/kg cryptolepine treatment showing the presence of numerous basophilic nuclei (H and E stain, ×100)
Kidney
The histology the kidney showed tubules and renal corpuscles, with well-defined capsular space (Figure 7a). The NC however had no capsular space around the glomerulus, distorted renal corpuscles, and localized coagulative necrosis of the tubules around the corpuscles [Figure 7b]. This was also seen in the 10 mg/kg CRP treated group [Figure 7d]. With glibenclamide treatment, there is capsular space (but not well-defined) and normal renal corpuscles, with little distortion in the renal tubules (Figure 7c). The 30 mg/kg CRP-treated animals have well-defined capsular space with normal renal corpuscles and normal renal tubules [Figures 7e]. With 100 mg/kg CRP treatment, however, there was edematous kidney with displaced glomeruli with empty Bowman’s capsule and also with distorted renal corpuscles with absence of capsular space [Figure 7f].
Figure 7.

Photomicrograph of kidney of cryptolepine (CRP)-treated alloxan-induced diabetic rats. (a) Sham: Normal histology of tubules, renal corpuscles, and a well-defined capsular space (white arrows), (b) negative control: Diminished outline of renal corpuscles and absence of capsular space (arrows), (c) glibenclamide treatment: Atrophy of tubules and corpuscles with intact capsular space (white arrow), (d) 10 mg/kg CRP treatment: Diminished outline of renal corpuscles (arrow) and absence of capsular space with localized degenerative areas (N) of the cortex of the kidney, (e) 30 mg/kg CRP treatment: Normal histology of tubules, renal corpuscles, and well-defined capsular space, (f) 100 mg/kg CRP treatment: Section reveals semi-capsular space (white arrows) around the glomerulus of the corpuscles and loss of glomerulus represented by an empty space (asterisk) in corpuscle (H and E stain, ×100)
Semen Examination
Following the induction of diabetes, there were significant decrements (P ≤ 0.001) in sperm count, sperm motility, and percentage live/dead ratio. CRP treatment did not improve these parameters but further reduced these dose-dependently (P ≤ 0.001) indicating no ameliorative effects of CRP on sperm abnormalities associated with DM [Table 3].
Table 3.
Effects of CRP treatment on sperm motility, viability, and sperm count

DISCUSSIONS
This study was conducted to ascertain the therapeutic usefulness of CRP in managing DM and some of its associated complications. Alloxan was used to induce experimental diabetes in Sprague-Dawley rats. This chemical selectively destroys insulin-producing pancreatic β-islet cells with a corresponding inverse changes in the plasma insulin concentration, and hence persistent hyperglycemia [18]. Persistently, high blood glucose directly increases hydrogen peroxide production by murine mesangial cells and lipid peroxidation of glomeruli and glomerular mesangial cells [19]. Hyperglycemia promotes glycosylation of circulating and cellular protein and may initiate a series of auto-oxidative reactions that culminate in the formation and accumulation of advanced glycosylation end-products (AGE) in tissue proteins [20]. AGE has oxidizing potential and can promote tissue damage by free radicals. In addition, increased lipid peroxidation impairs membrane functions by decreasing membrane fluidity and changing the activity of membrane-bound enzymes and receptors. Its products (lipid radicals and lipid peroxides) are harmful to the cells in the body and associated with atherosclerosis and damage to brain, kidney, liver, and other tissue [1,2]. In addition, diabetes and it associated hyperglycemia can be sources of DNA damage via the oxidation of DNA bases and sugar-phosphate binding sites [21,22]. The occurrence of these alterations can result in mutagenic effects and/or DNA replication arrest and could be associated with risks for developing DN in DM patients [23,24].
CRP demonstrated an antihyperglycemic effect similar to glibenclamide, a sulfonylurea hypoglycemic agent. This may suggest that CRP could work via a similar mechanism as glibenclamide, which binds to and inhibits the ATP-sensitive potassium channels inhibitory regulatory subunit sulfonylurea receptor 1 [25] in pancreatic beta cells. This inhibition causes cell membrane depolarization, opening voltage-dependent calcium channels, resulting in an increase in intracellular calcium in the beta cell. This subsequently stimulates insulin release which enhances the utilization of glucose, and hence a fall in plasma glucose concentration [26].
DM, in this study, was associated with weight loss. In DM, because there is a lack of insulin, the processes of glucose transfer from the blood into cells, where it can be used the generate energy, is impaired. The body therefore, begins to utilize lipids and protein from muscles as an alternate source of fuel; causing a reduction in overall body weight. Induction of the release of insulin by glibenclamide and possibly by CRP would, therefore, reverse this weight loss as portrayed in this study.
As DM progresses, complications set in. DN is one such complication which results in allodynia and hyperalgesia (neuropathic pain) [27]. Allodynia refers largely to pain evoked by Aβ-fibers or low-threshold Ad- and C- nociceptive fibers in response to a non-nociceptive stimulus while hyperalgesia is increased pain sensitivity [28], which could be mediated by bradykinin B(1) receptors [29]. CRP, just like morphine, inhibited cold allodynia and thermal hyperalgesia. Morphine interacts with κ opioid receptorsand produces analgesia by causing hyperpolarization of interneurons within the dorsal spinal cord and depressing the release of transmitters such as enkephalin, serotonin, or norepinephrine, and specific receptors located on the nociceptive fibers that transmit pain sensation to the higher centers. In addition, morphine can interact with µ opioid receptors located in the supraspinal structures and activate the supraspinal system with the release of the endorphins inhibit the transmission of pain signals by these fibers [30,31]. The thermal antihyperalgesic properties of CRP may also be due to downregulation of bradykinin B(1) receptors, which have been found to be upregulated alongside the development of Type 1 diabetes [29], or possibly could be involved in raising the nociceptive threshold, or desensitization of nerve endings of A-delta-fiber and A-beta-fiber that are lowered with DM.
Although there were reductions of hematological parameters associated with DM in this study, these were statistically not significant, except for platelet count, which decreased significantly. DM has been reported to be associated with significant reduction in red blood cells (RBCs), Hemoglobin (Hb), mean corpuscular volume, mean corpuscular Hb concentration, mean corpuscular Hb, hematocrit, and red cell distribution width [32-34]. The occurrence of anemia in DM has been reported due to the increased non-enzymatic glycosylation of RBC membrane proteins by ROS [35]. Oxidation of these proteins and hyperglycemia in DM causes an increase in the production of lipid peroxides that lead to hemolysis of RBC [36]. A significant reduction in PLT has been reported in liver cirrhosis and other advanced liver diseases which may be associated with DM [37,38]. Thrombopoietin (TPO) is predominantly produced by the liver and constitutively expressed by hepatocytes. With the damage of hepatocytes, TPO production is reduced. This leads to reduced thrombopoiesis in the bone marrow and consequently to thrombocytopenia [39].
Among the parameters measured in the liver function test, AST, ALT, and GGT were elevated with DM. AST and ALT are enzymes present in hepatocytes and are associated with liver parenchymal cells. These enzymes are, therefore, significantly elevated in acute hepatocyte damage. The mutual rise in serum AST and ALT is a sure indication of hepatocellular damage since ALT is a more sensitive marker of hepatocellular damage [40]. An elevation of AST only would not have been an indication of hepatocyte damage since is not a specific indicator [41]. Cirrhosis results in significant elevation of AST and ALT [42,43] and is common in diabetic patients as more than 80% of patients with cirrhosis have been found to suffer glucose intolerance [44]. This clearly indicates that animals developed cirrhosis, and this assertion is further supported by the thrombocytopenia recorded in this study. GGT levels are elevated in biliary disease, cholelithiasis, and cholecystitis associated with DM [45]. It is conclusive, therefore, that DM is associated with hepatocellular damage. Unfortunately, neither glibenclamide nor CRP treatment could reverse this liver injury. The further elevation of AST in the CRP-treated groups, however, may not be further damage to hepatocytes as AST is not only from hepatocytes but could also be from RBC as well as cardiac and skeletal muscles [41]. Serum total proteins (ALB and GLOB) and TBIL (conjugated and unconjugated) were within normal ranges in DM indicating that diabetes may not cause chronic active hepatitis, liver cirrhosis, immune system over activity, and chronic inflammatory disorders [46] and does not have any deleterious effects on the liver’s synthetic function, hepatic metabolism, or biliary excretion.
Associated with induced DM was a significant reduction in plasma urea and a significant elevation in creatinine. Although a low level of urea is not usually a cause for concern in kidney function, it may be seen in severe liver disease [43]. Because urea is synthesized by the liver, severe liver failure causes a reduction of urea in the blood, with liver insufficiency the ammonia is not detoxified to urea. Persistent hyperglycemia is known to be a significant risk factor for diabetic nephropathy. Hyperglycemia may directly result in mesangial expansion and injury by an increase in the mesangial cell glucose concentration. The glomerular mesangium expands initially by cell proliferation and then by cell hypertrophy. Transforming growth factor β is particularly important in the mediation of expansion and later fibrosis via the stimulation of collagen and fibronectin [47]. This affects urea and creatinine clearance from blood. Creatinine is a more specific indicator of kidney function. It indicates, therefore, that the kidney was impaired in function. Clinical findings indicate that uncontrolled DM is the most common cause of kidney failure, accounting for nearly 44% of new cases [48], in the complication known as diabetic nephropathy [49]. Renal disease in diabetes is found to be caused by abnormalities of vasodilatation and generates ROS-mediated by endothelial-derived nitric oxide [50,51]. Glibenclamide treatment significantly reversed the changes in kidney function associated with induced DM; however, CRP treatment could not alter significantly these changes. A study aimed at elucidating the role of glibenclamide in the prevention of diabetic nephropathy indicated that glibenclamide attenuates some biochemical and histological changes produced by diabetic nephropathy [52]. This could be due to the tight regulation of DM offered by glibenclamide.
DM was also associated with a negative lipid profile as CHO, TAG, and LDL were significant elevated, and HDL was significant reduced [34,53]. Established DM results in an increased efflux of free fatty acids from adipose tissue and impaired insulin-mediated skeletal muscle uptake of free fatty acids increase fatty acid flux to the liver [54], with the subsequent increase in VLDL production and/or retarded clearance from the plasma of large VLDL. TAG enrichment of the lipolytic products through the action of cholesteryl ester transfer protein, together with hydrolysis of TAG and phospholipids by hepatic lipase, leads to results in increased production of IDLs and LDLs [55]. Plasma residence time of LDLs may be prolonged because of their relatively reduced affinity for LDL receptors [56]. Reduction in HDL associated with DM appears to be due to increased transfer of CHO from HDL to TAG-rich lipoproteins, with reciprocal transfer of TAG to HDL. TAG-rich HDL particles are hydrolyzed by hepatic lipase and, as a result, are rapidly catabolized and cleared from plasma [53]. Treatment with glibenclamide and CRP significantly reversed the DM-associated alteration in lipid profile due to controlled hyperglycemia [57].
Histological studies reveal destruction of islet of Langerhans cells of the pancreas by Alloxan used in inducing diabetes. Alloxan is rapidly taken into the β-islet of Langerhans cells where it is reduced, by SH-containing cellular compounds, reduced glutathione, cysteine and protein-bound sulfhydryl groups (including SH containing enzymes), to dialuric acid [58]. Dialuric acid is then re-oxidized back to alloxan establishing a redox cycle for the generation of superoxide radicals. The superoxide radicals undergo dismutation to hydrogen peroxide (reaction is catalyzed by superoxide dismutase). The ROS produced now cause damage to the DNA and a subsequent damage of pancreatic islets [58]. Hyperglycemic control (as seen with glibenclamide and CRP) results in significant reduction in the production of ROS, and hence, tissue susceptible to damage by ROS is protected. This could account for the regeneration/restoration of pancreatic β-islet cells accompanying treatment. Oxygen radicals generated in DM has tremendous damaging effect on the liver and kidney [59-61], as revealed by the blood biochemical and histopathological studies, and hence hyperglycemic control could be very helpful in restoring these organs. Glibenclamide and 30 mg/kg CRP treatment were moderately able to ameliorate the hepatocellular and kidney damage associated with DM due to their significant diabetes control. Drugs and chemicals rendering antioxidative properties can attenuate alloxan toxicity.
Similar to previous findings, there was reduction in the sperm motility and count as well as the percentage live/dead ratio while the number of abnormal spermatozoa also increased [62,63]. Observations from this study showed no ameliorative but further deterioration effect in semen quality by CRP. Decreased viability and spermatocyte cell count in DM could be attributed to damage of the secretory epithelial cells of the seminiferous tubules probably due to oxidative damage from glucose auto-oxidation and excessive production of superoxide radicals and formation of advanced glycation end products associated with DM [64]. Spermatozoa are highly susceptible to damage by excess concentration of ROS due to the high content of poly unsaturated fatty acid within their plasma membrane [65]. CRP could not reverse the oxidative stress on the spermatozoa caused by alloxan-induced diabetes because in vitro antioxidant activities of the compound are very minimal. CRP has been found to produce a variety of pharmacological effects including antimuscarinic activity [66], α-adrenoceptor antagonism [67], and cytotoxicity [68] which could adversely affect male fertility.
CONCLUSION
CRP treatment abolishes hyperglycemia, weight loss, cold allodynia, neuropathic pain, and undesirable lipid metabolism associated with DM as it has exhibited interesting antihyperglycemic, analgesic, and cytoprotective effect on the pancreas. However, it does not have a significant ameliorative effect on DM-induced disorders of the liver, the kidney histopathology, and quality of semen production.
ACKNOWLEDGMENTS
The authors are grateful to the workers of the animal house of the Biomedical Science for their assistance.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
AUTHORS CONTRIBUTION
EOA, GAK, SK, and RNAOM designed the experiment, analyzed the data, and prepared the final manuscript. DA, KKA, DK, and ADF contributed to the study design, performed the experiments, collected all data, and drafted the initial manuscript. All authors approved of the final manuscript.
REFERENCES
- 1.Orsolic N. Honey bee products and their polyphenolic compounds in treatment of diabetes. In: Govil JN, Singh VK, editors. Phytopharmacology and Therapeutic Values IV. USA: Studium Press; 2008. pp. 455–71. [Google Scholar]
- 2.Yue KK, Chung WS, Leung AW, Cheng CH. Redox changes precede the occurrence of oxidative stress in eyes and aorta, but not in kidneys of diabetic rats. Life Sci. 2003;73:2557–70. doi: 10.1016/s0024-3205(03)00662-3. [DOI] [PubMed] [Google Scholar]
- 3.Melmed S, Polonsky KS, Larsen PR, Kronenberg HM. Williams Textbook of Endocrinology. 12th ed. Philadelphia, PA: Elsevier/Saunders; 2011. pp. 1371–435. [Google Scholar]
- 4.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 5.US. Department of Health and Human Services. Diabetic Neuropathy: The Nerve Damage of Diabetes. Bethesda, MD: NIH Publication; 2009. pp. 9–3185. [Google Scholar]
- 6.Dryden J. Researchers Discover Root Cause of Blood Vessel Damage in Diabetes. St. Louis: Washington University; 2011. [Last accessed on 2016 May 01]. Available from: https://www.source.wustl.edu/2011/01/researchers-discover-root-cause-of-blood-vessel-damage-in-diabetes/ [Google Scholar]
- 7.Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–22. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Gara PT, Kushner FG, Ascheim DD, Casey DE, Chung MK, de Lemos JA, et al. ACCF/AHA guideline for the management of ST-elevation myocardial infarction. J Am Coll Cardiol. 2013;61:e78–140. doi: 10.1016/j.jacc.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 9.Matough FA, Budin SB, Hamid ZA, Alwahaibi N, Mohamed J. The role of oxidative stress and antioxidants in diabetic complications. Sultan Qaboos Univ Med J. 2012;12:5–18. doi: 10.12816/0003082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lisgarten JN, Coll M, Portugal J, Wright CW, Aymami J. The antimalarial and cytotoxic drug cryptolepine intercalates into DNA at cytosine-cytosine sites. Nat Struct Biol. 2002;9:57–60. doi: 10.1038/nsb729. [DOI] [PubMed] [Google Scholar]
- 11.Kanthlal SK, Kumar BA, Joseph J, Aravind R, Frank PR. Amelioration of oxidative stress by Tabernamontana divaricata on alloxan-induced diabetic rats. Anc Sci Life. 2014;33:222–8. doi: 10.4103/0257-7941.147429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parasuraman S, Raveendran R, Kesavan R. Blood sample collection in small laboratory animals. J Pharmacol Pharmacother. 2010;1:87–93. doi: 10.4103/0976-500X.72350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D’Amour FE, Smith DL. Method for determining loss of pain sensation. JPET. 1941;72:74–9. [Google Scholar]
- 14.Allchorne AJ, Broom DC, Woolf CJ. Detection of cold pain, cold allodynia and cold hyperalgesia in freely behaving rats. Mol Pain. 2005;1:36. doi: 10.1186/1744-8069-1-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.JoVE. Science Education Database. General Laboratory Techniques. Histological Sample Preparation for Light Microscopy. Cambridge, MA: JoVE; 2016. [Google Scholar]
- 16.Zemjanis R. Diagnostic and Therapeutic Techniques in Animal Reproduction. 2nd ed. Baltimore, MD, USA: Williams and Wilkins Company; 1970. Collection and evaluation of semen; pp. 139–56. [Google Scholar]
- 17.Wells ME, Awa OA. New technique for assessing acrosomal characteristics of spermatozoa. J Dairy Sci. 1970;53:227–32. doi: 10.3168/jds.S0022-0302(70)86184-7. [DOI] [PubMed] [Google Scholar]
- 18.Vouk VB, Sheehan PJ. Methods for Assessing the Effects of Chemicals on Reproductive Functions. New York: Wiley; 1983. pp. 263–75. [Google Scholar]
- 19.Rajbir B, Rawal S, Jatinder S, Ishari M. Effect of Aegle marmelos leaf extract treatment on diabetic neuropathy in rats: A possible involvement of A2 adrenoceptors. Int J Pharm Pharm Sci. 2012;4:632. [Google Scholar]
- 20.Ruiz-Muñoz LM, Vidal-Vanaclocha F, Lampreabe I. Enalaprilat inhibits hydrogen peroxide production by murine mesangial cells exposed to high glucose concentrations. Nephrol Dial Transplant. 1997;12:456–64. doi: 10.1093/ndt/12.3.456. [DOI] [PubMed] [Google Scholar]
- 21.Hunt JV, Bottoms MA, Mitchinson MJ. Oxidative alterations in the experimental glycation model of diabetes mellitus are due to protein-glucose adduct oxidation. Some fundamental differences in proposed mechanisms of glucose oxidation and oxidant production. Biochem J. 1993;291:529–35. doi: 10.1042/bj2910529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anjaneyulu M, Chopra K. Effect of irbesartan on the antioxidant defence system and nitric oxide release in diabetic rat kidney. Am J Nephrol. 2004;24:488–96. doi: 10.1159/000080722. [DOI] [PubMed] [Google Scholar]
- 23.Oršolic N, Gajski G, Garaj-Vrhovac V, Dikic D, ZŠ Prskalo, Sirovina D. DNA-protective effects of quercetin or naringenin in alloxan-induced diabetic mice. Eur J Pharmacol. 2011;656:110–8. doi: 10.1016/j.ejphar.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 24.Will JC, Vinicor F, Calle EE. Is diabetes mellitus associated with prostate cancer incidence and survival? Epidemiology. 1999;10:313–8. [PubMed] [Google Scholar]
- 25.Serrano-Martín X, Payares G, Mendoza-León A. Glibenclamide, a blocker of K (ATP) channels, shows antileishmanial activity in experimental murine cutaneous leishmaniasis. Antimicrob Agents Chemother. 2006;50:4214–6. doi: 10.1128/AAC.00617-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luzi L, Pozza G. Glibenclamide: An old drug with a novel mechanism of action? Acta Diabetol. 1997;34:239–44. doi: 10.1007/s005920050081. [DOI] [PubMed] [Google Scholar]
- 27.Sandkühler J. Models and mechanisms of hyperalgesia and allodynia. Physiol Rev. 2009;89:707–58. doi: 10.1152/physrev.00025.2008. [DOI] [PubMed] [Google Scholar]
- 28.Gabra BH, Benrezzak O, Pheng LH, Duta D, Daull P, Sirois P, et al. Inhibition of Type 1 diabetic hyperalgesia in streptozotocin-induced sprague-dawley versus spontaneous gene-prone BB/Worchester rats: Efficacy of a selective bradykinin B1 receptor antagonist. J Neuropathol Exp Neurol. 2005;64:782–9. doi: 10.1097/01.jnen.0000178448.79713.5f. [DOI] [PubMed] [Google Scholar]
- 29.Gabra BH, Sirois P. Kinin B1 receptor antagonists inhibit diabetes-induced hyperalgesia in mice. Neuropeptides. 2003;37:36–44. doi: 10.1016/s0143-4179(02)00148-8. [DOI] [PubMed] [Google Scholar]
- 30.Lipp J. Possible mechanisms of morphine analgesia. Clin Neuropharmacol. 1991;14:131–47. doi: 10.1097/00002826-199104000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Sprouse-Blum AS, Smith G, Sugai D, Parsa FD. Understanding endorphins and their importance in pain management. Hawaii Med J. 2010;69:70–1. [PMC free article] [PubMed] [Google Scholar]
- 32.Adeyi AO, Idowu BA, Mafiana CF, Oluwalana SA, Ajayi OL, Akinloye OA. Rat model of food-induced non-obese-type 2 diabetes mellitus: Comparative pathophysiology and histopathology. Int J Physiol Pathophysiol Pharmacol. 2012;4:51–8. [PMC free article] [PubMed] [Google Scholar]
- 33.Colak S, Geyikoglu F, Aslan A, Deniz GY. Effects of lichen extracts on haematological parameters of rats with experimental insulin-dependent diabetes mellitus. Toxicol Ind Health. 2014;30:878–87. doi: 10.1177/0748233712466130. [DOI] [PubMed] [Google Scholar]
- 34.Akomas SC, Okafor AI, Ijioma SN. Glucose level, haematological parameters and lipid profile in ficus sur treated diabetic rats. Compr J Agric Biol Sci. 2014;2:5–11. [Google Scholar]
- 35.Oyedemi SO, Yakubu MT, Afolayan AJ. Antidiabetic activities of aqueous leaves extract of Leonotis leonurus in streptozotocin induced diabetic rats. J Med Plant Res. 2011;5:119–25. [Google Scholar]
- 36.Arun GS, Ramesh KG. Improvement of insulin sensitivity by perindopril in spontaneously hypertensive and streptozotocin-diabetic rats. Indian J Pharmacol. 2002;34:156–64. [Google Scholar]
- 37.Afdhal N, McHutchison J, Brown R, Jacobson I, Manns M, Poordad F, et al. Thrombocytopenia associated with chronic liver disease. J Hepatol. 2008;48:1000–7. doi: 10.1016/j.jhep.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 38.Hayashi H, Beppu T, Shirabe K, Maehara Y, Baba H. Management of thrombocytopenia due to liver cirrhosis: A review. World J Gastroenterol. 2014;20:2595–605. doi: 10.3748/wjg.v20.i10.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peck-Radosavljevic M. Thrombocytopenia in liver disease. Can J Gastroenterol. 2000;14:60D–6. doi: 10.1155/2000/617428. [DOI] [PubMed] [Google Scholar]
- 40.Koffuor GA, Boye A, Ofori-Amoah J, Kyei S, Nouoma CK, Debrah AP. Evaluating muco-suppressant, anti-tussive and safety profile of Polyscias fruticosa (L.). Harms (Araliaceae) in asthma management. Br J Med Med Res. 2015;10:1–11. [Google Scholar]
- 41.Obici S, Otobone FJ, da Silva Sela VR, Ishida K, da Silva JC, Nakamura CV, et al. Preliminary toxicity study of dichloromethane extract of Kielmeyera coriacea stems in mice and rats. J Ethnopharmacol. 2008;115:131–9. doi: 10.1016/j.jep.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 42.Murali AR, Carey WD. Liver Test Interpretation - Approach to the Patient with Liver Disease: A Guide to Commonly Used Liver Tests. The Cleveland Clinic Foundation. 2014. [Last accessed on 2016 Feb 02]. Available from: http://www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/hepatology/guide-to-common-liver-tests/
- 43.American Association for Clinical Chemistry, (AACC) Lab Tests AST© 2001-2016. [Last accessed on 2016 Feb 02]. Available from: https://www.labtestsonline.org/understanding/analytes/ast/tab/test/
- 44.Tolman KG, Fonseca V, Dalpiaz A, Tan MH. Spectrum of liver disease in Type 2 diabetes and management of patients with diabetes and liver disease. Diabetes Care. 2007;30:734–43. doi: 10.2337/dc06-1539. [DOI] [PubMed] [Google Scholar]
- 45.Levinthal GN, Tavill AS. Liver disease and diabetes mellitus. Clin Diabetes. 1999;17:2. [Google Scholar]
- 46.Rochling FA. Evaluation of abnormal liver tests. Clin Cornerstone. 2001;3:1–12. doi: 10.1016/s1098-3597(01)90074-2. [DOI] [PubMed] [Google Scholar]
- 47.Butt S, Hall P, Nurko S. Diabetic Nephropathy Cleveland Clinic. Center for Continuing Education. The Cleveland Clinic Foundation. 2010. [Last accessed on 2016 May 03]. Available from: http://www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/nephrology/diabetic-nephropathy/
- 48.National Institute of Diabetes and Digestive and Kidney Diseases. Kidney Disease of Diabetes. NIH Publication No. 14-3925. 2014 [Google Scholar]
- 49.Dabla PK. Renal function in diabetic nephropathy. World J Diabetes. 2010;1:48–56. doi: 10.4239/wjd.v1.i2.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Creager MA, Lüscher TF, Cosentino F, Beckman JA. Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part I. Circulation. 2003;108:1527–32. doi: 10.1161/01.CIR.0000091257.27563.32. [DOI] [PubMed] [Google Scholar]
- 51.Akbar DH, Hagras MM, Amin HA, Khorshid OA. Comparison between the effect of glibenclamide and captopril on experimentally induced diabetic nephropathy in rats. J Renin Angiotensin Aldosterone Syst. 2013;14:103–15. doi: 10.1177/1470320312460881. [DOI] [PubMed] [Google Scholar]
- 52.Hall PM. Prevention of progression in diabetic nephropathy. Diabetes Spectr. 2006;19:18–24. [Google Scholar]
- 53.Krauss RM. Lipids and lipoproteins in patients with Type 2 diabetes. Diabetes Care. 2004;27:1496–504. doi: 10.2337/diacare.27.6.1496. [DOI] [PubMed] [Google Scholar]
- 54.Boden G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes. 1997;46:3–10. [PubMed] [Google Scholar]
- 55.Krauss RM. Atherogenicity of triglyceride-rich lipoproteins. Am J Cardiol. 1998;81:13B–7. doi: 10.1016/s0002-9149(98)00032-0. [DOI] [PubMed] [Google Scholar]
- 56.Berneis KK, Krauss RM. Metabolic origins and clinical significance of LDL heterogeneity. J Lipid Res. 2002;43:1363–79. doi: 10.1194/jlr.r200004-jlr200. [DOI] [PubMed] [Google Scholar]
- 57.Khan HA, Sobki SH, Khan SA. Association between glycaemic control and serum lipids profile in Type 2 diabetic patients: HbA1c predicts dyslipidaemia. Clin Exp Med. 2007;7:24–9. doi: 10.1007/s10238-007-0121-3. [DOI] [PubMed] [Google Scholar]
- 58.Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res. 2001;50:537–46. [PubMed] [Google Scholar]
- 59.Jaeschke H, Gores GJ, Cederbaum AI, Hinson JA, Pessayre D, Lemasters JJ. Mechanisms of hepatotoxicity. Toxicol Sci. 2002;65:166–76. doi: 10.1093/toxsci/65.2.166. [DOI] [PubMed] [Google Scholar]
- 60.Forbes JM, Coughlan MT, Cooper ME. Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes. 2008;57:1446–54. doi: 10.2337/db08-0057. [DOI] [PubMed] [Google Scholar]
- 61.Noeman SA, Hamooda HE, Baalash AA. Biochemical study of oxidative stress markers in the liver, kidney and heart of high fat diet induced obesity in rats. Diabetol Metab Syndr. 2011;3:17. doi: 10.1186/1758-5996-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.La Vignera S, Condorelli R, Vicari E, D’Agata R, Calogero AE. Diabetes mellitus and sperm parameters. J Androl. 2012;33:145–53. doi: 10.2164/jandrol.111.013193. [DOI] [PubMed] [Google Scholar]
- 63.Omu AE, Al-Bader MD, Al-Jassar WF, Al-Azemi MK, Omu FE, Mathew TC, et al. Antioxidants attenuates the effects of insulin dependent diabetes mellitus on sperm quality. Bioenergetics. 2014;3:113. [Google Scholar]
- 64.Rabbani SI, Devi K, Khanam S. Inhibitory effect of glimepiride on nicotinamide-streptozotocin induced nuclear damages and sperm abnormality in diabetic Wistar rats. Indian J Exp Biol. 2009;47:804–10. [PubMed] [Google Scholar]
- 65.Zieglgänsberger W, Berthele A, Tölle TR. Understanding neuropathic pain. CNS Spectr. 2005;10:298–308. doi: 10.1017/s1092852900022628. [DOI] [PubMed] [Google Scholar]
- 66.Petrenko AB, Yamakura T, Baba H, Shimoji K. The role of N-methyl-D-aspartate (NMDA) receptors in pain: A review. Anesth Analg. 2003;97:1108–16. doi: 10.1213/01.ANE.0000081061.12235.55. [DOI] [PubMed] [Google Scholar]
- 67.Petrenko O, Zaika A, Moll UM. Deltanp73 facilitates cell immortalization and cooperates with oncogenic Ras in cellular transformation in vivo. Mol Cell Biol. 2003;23:5540–55. doi: 10.1128/MCB.23.16.5540-5555.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ameyaw EO, Owusu Agyei PE, Boampong JN, Koranteng KA, Kyei S. Ethanolic root extract of Jatropha curcas L. ameliorates paclitaxel-induced neuropathic pain in rats. Res Rev A J Pharmacol. 2013;3:10–4. [Google Scholar]


