Abstract
Chinese herbal medicine (CHM) is an integral component of complementary/alternative medicine and it is increasingly becoming the preferred therapeutic modality for the treatment of liver fibrosis and hepatocellular carcinoma (HCC) worldwide. Accordingly, the World Health Organization (WHO) has attested to the popularity and efficacy of indigenous herbal therapies including CHM as a first line of treatment for some diseases including liver disorders. However, the WHO and drug discovery experts have always recommended that use of indigenous herbal remedies must go hand-in-hand with the requisite mechanistic elucidation so as to constitute a system of verification of efficacy within the ethnobotanical context of use. Although many CHM experts have advanced knowledge on CHM, nonetheless, more enlightenment is needed, particularly mechanisms of action of CHMs on fibro-hepato-carcinogenesis. We, herein, provide in-depth mechanisms of the action of CHMs which have demonstrated anti-fibro-hepatocarcinogenic effects, in pre-clinical and clinical studies as published in PubMed and other major scientific databases. Specifically, the review brings out the important signaling pathways, and their downstream targets which are modulated at multi-level by various anti-fibro-hepatocarcinogenic CHMs.
KEY WORDS: Chinese herbal medicine, fibro-hepato-carcinogenesis, immunomodulation, inflammation, mechanistic elucidation
INTRODUCTION
Chinese herbal medicine (CHM) that forms an integral component of traditional Chinese medicine (TCM) keeps growing popular worldwide [1,2]. The past few decades have seen increased scientific investigations on commonly used CHMs often reflective of the ethnobotanical context, in which they are used. As a result, it is common to find scientific studies on whole Chinese herbal formulas, whereby the pharmacological and therapeutic effects more often are attributed to the entire components of the herbal formula. This has always been the source of criticism of CHM therapy just like other indigenous herbal remedies, especially from strict adherents of western medicine. There has been a paradigm shift in recent years with regards to research on CHMs which have seen an incredible focus on mechanistic elucidation as well as structural and functional characterization of individual components of CHMs. Many scientific efforts have been made to highlight the mechanisms of the action of CHMs [3], but there is still more work to be done. Hepatitis B virus (HBV) endemicity correlates with the incidence of liver fibrosis and its attendant complications including cirrhosis and hepatocellular carcinoma (HCC). China is noted for a high incidence of HBV infections and alcohol abuse [4]. Coincidentally, these two factors are crucial risk factors for HCC [4]. Almost 80-90% of HBV-related HCC in Asia and Africa occur in China [5,6]. Although, many scientific efforts have been made to highlight the mechanism of action of CHMs [3] used in the treatment of hepatocarcinogenic disorders, nonetheless the mechanistic elucidation of CHMs remains incomplete. This review provides a mechanistic overview of CHMs which have demonstrated in vitro and in vivo anti-liver fibrosis, anti-cirrhosis, and anti-HCC effects featured in published scientific articles from PubMed and other major scientific databases. Specifically, it highlights the mechanisms of the action of CHMs in the light of specific therapeutic targets that can be explored in future studies.
CHM
CHM is an integral component of TCM. CHM has multi-compound composition, multi-modulatory, and multi-target action [1] [Figure 1]. It produces less adverse effects in the treatment of liver diseases [7-9]. In CHM practice, liver disease is assumed to be caused by a number of factors including poor blood circulation and dysregulated metabolism [10]. Thus, CHM therapy against liver disease is sorely to reduce blood stagnation, eliminate toxins and improve the immune system. CHM practice involves the use of either one herb/plant extract or a mixture of two or more herbal extracts based on a time-tested system of herbology. According to the principles and theories governing CHM practice, one pharmacologically active compound from one of the constituent herbs is normally regarded as “King herb” [11]. The “King herb” is the main medicine which exerts the expected therapeutic action. To enhance the therapeutic action of the “King herb,” the other component herbs play auxiliary functions, such as enhancing delivery of the “King herb” to target site, reduce toxicity/side effects of the “King herb,” and most importantly, provide synergistic effect to the “King herb.”
Figure 1.
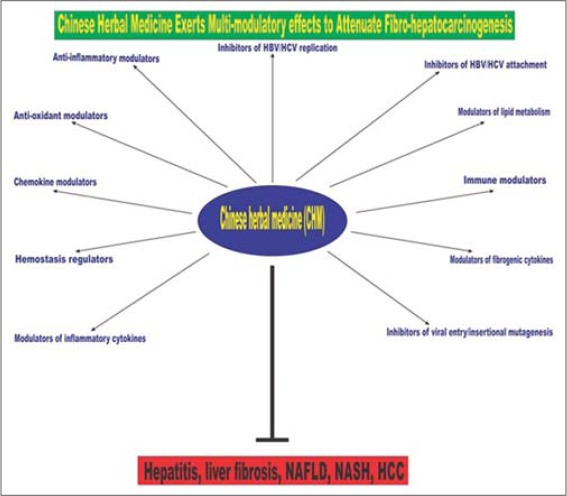
A diagrammatic depiction of the multi-modulatory and multi-target pharmacological effects of Chinese herbal medicine (CHM) which underpin the promising efficacy of CHM against liver disease in general
PATHOGENESIS OF FIBRO-HEPATOCARCINOGENESIS
Fibro-hepato-carcinogenesis epitomizes a spectrum of pathological events in the liver manifesting as liver fibrosis, cirrhosis and HCC if not treated at the initial stages. The whole pathological process begins as a result of dysregulated wound healing process secondary to chronic hepatic inflammation. Hepatic stellate cell (HSC) is the key hepatic cell implicated in liver fibrosis. Under normal physiological conditions, quiescent ito cells store retinoids (vitamin A) and play crucial homeostatic roles in the liver. However, in response to chronic inflammatory and fibrogenic stimuli, quiescent ito cells do not only transform into a fibrogenic phenotype (myofibroblasts) but also proliferate and increase the synthesis and the accumulation of extracellular matrix (ECM) in liver sinusoidal space. HSC morphological transformation represents the crucial pathological event for the initiation of fibrogenesis and its progression to fibrotic liver disease. As a result, increased output of fibrogenic and inflammatory genes mainly precede secretion of fibrogenic (transforming growth factor beta 1 [TGF-β1]), and inflammatory (tumor necrosis factor-alpha [TNF-α], interleukin 1 beta [IL-1b], IL-6) cytokines to sustain fibrogenesis. Furthermore, there is ECM accumulation, the proliferation of myofibroblasts, and recruitment and activation of other hepatic and non-hepatic cells in an autocrine and paracrine manner. If left untreated, liver fibrosis progresses to cirrhosis, but this transition can be hastened by comorbidity factors including HBV and hepatitis C viral (HCV) infections and alcohol abuse [Figure 2]. Cirrhosis is a manifestation of advanced liver fibrosis and it is characterized by hepatic nodules that progressively distort normal hepatic architecture and function resulting in increased resistance to portal blood flow. These pathological events elevate sinusoidal pressure leading to portal hypertension and the risk of HCC and death [12]. Underpinning fibro-hepatocarcinogenesis is a constellation of dysregulated cell signaling pathways mainly mediated by growth factors, cytokines, chemokines, transcriptional factors, and their resultant target genes. Thus, molecular underpinnings of fibro-hepatocarcinogenesis are not only diverse but also play crucial roles in homeostasis.
Figure 2.
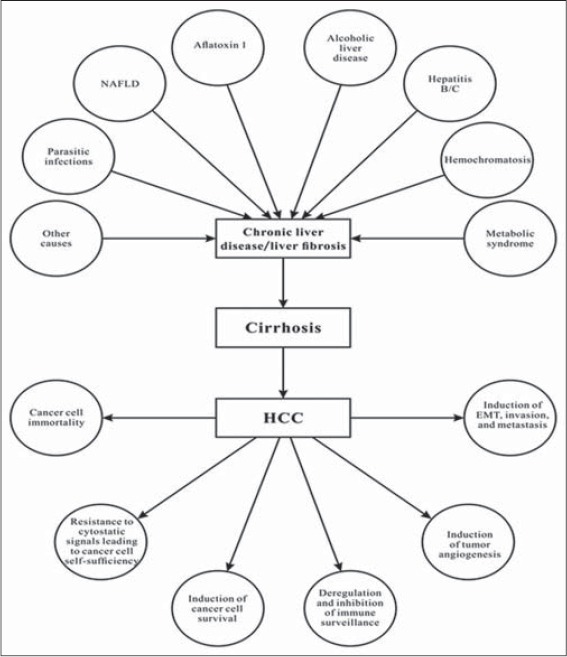
An illustration of the multi-etiology of fibro-hepato-carcinogenesis. Many etiological factors may act synergistically to promote progression of chronic liver injury to liver fibrosis and cirrhosis if left untreated, and this ultimately increases the risk of HCC and the manifestation of the six phenotypic hallmarks of HCC
The progression of liver fibrosis to cirrhosis through to HCC usually takes many years or decades. In view of this, most infected persons are asymptomatic [13]. However, this progression can be hastened within few months by factors such as neonatal liver disease, HCV infections, human immunodeficiency virus/HBV/HCV co-infections, severe delta hepatitis, and drug-induced liver disease [13]. HCC accounts for most liver-related mortality [14] and tumor progression is implicated as the main cause of death in HCC patients [15]. Furthermore, a significant percentage of patients may die from other complications arising from liver fibrosis and cirrhosis such as ascites, spontaneous peritonitis, hepatic encephalopathy, hepato-pulmonary syndrome, porto-pulmonary hypertension, and pain. The early detection and treatment of liver fibrosis and cirrhosis are crucial for overall management of HCC risk. However, the treatment of HCC is a major problem partly because of its complex nature such as high degree of cancer clonal heterogeneity, intra-tumor genetic heterogeneity, and emerging compensatory pathways in response to therapy-related inhibition of some pathways in cancer [5,16]. Among primary liver cancers, HCC represents the major histological subtype, accounting for 70-85% of the total liver cancer burden worldwide [4].
EPIDEMIOLOGY OF LIVER DISEASE IN CHINA
China has the largest population (1.3 billion people) in the world comprising 56 different ethnic groupings [17]. With the establishment of Central Cancer Registries by the Health Ministry of China in 2002 to take records of cancer cases and deaths, there has been a consistent increase in both the incidence and mortality of cancer [18-20]. For example, in 2006 the 3rd National Death Survey report showed that cancer is the second leading cause of death in China, before then a report from 2004 to 2005 had placed the national mortality rate of cancer at 135.88/100,000, with 170.17/100,000 in males and 99.97/100,000 in females (Ministry of Health, National Death Survey Report 2004-2005, Beijing). Liver cancer is the third reported cancer case in both rural and urban China and among the top 10 cancers in recent years [21]. HBV and alcohol abuse which account for the most reported cases of liver fibrosis and cirrhosis were reported to have changed, with the former decreasing while the latter increases [21]. Hospitalization due to the alcohol-related and non-viral-related cirrhosis was shown to have increased [21]. Viral related liver disease burden has increased quiet significantly. For example, in Guangdong province (the most populous province in China), the predicted annual cost of HBV-related liver disease was purged at RMB 10.8 billion [22], speculatively, more than twice the annual budget of a developing country. Meanwhile, novel therapeutic agents locked up in CHMs remain untapped or poorly explored. If this rich readily available ethnobotanical heritage is properly harnessed through cutting edge scientific approaches, it can save the increasing disease burden.
CHMS EXERT ANTI-INFLAMMATORY EFFECTS
Chronic hepatic inflammation has been widely implicated in the initiation of liver fibrosis [23-25]. Many CHMs produce their effects by modulating pro-inflammatory factors including IL-1b, IL-2, IL-6, IL-8, IL-12, TNF-α, nuclear factor kappa B (NF-κB), prostaglandin E2 (PGE2), interferon gamma (IFN-γ), nitric oxide (NO), cyclooxygenase-2 (COX-2), intercellular adhesion molecule 1 (ICAM-1), and activator protein 1 (AP-1). The modulation of inflammatory mediators and their downstream protein scaffolds have become crucial targets for the treatment of liver fibrosis, cirrhosis, and HCC. Below are some specific inflammatory mediators modulated by some CHMs to cause attenuation of fibro-hepato-carcinogenesis.
NF-κB
NF-κB is an important target for therapy against liver fibrosis, in view of its role in inflammation. Lu et al. had demonstrated that myeloid differentiation protein 88 (MyD88) inactivated phosphorylation of IκBα in an NF-κB/IκBα trimmer complex leading to activation of IκBα and toll-like receptors (TLRs) [26], and this cascade led to the release of pro-inflammatory cytokines [27]. The role of NF-κB activation in inflammation, particularly how it induces the expression of pro-inflammatory cytokines, cell cycle regulatory molecules, and angiogenic factors have been elaborated [28]. By using a direct kinase assay and immunoblot analyses, it was shown that a seed extract of Phaseolus angularis markedly inhibited NF-κB expression and effectively ameliorated hepatic inflammation [29]. An extract of Cinnamomum cassia inhibited mRNA expression of induced nitric oxide synthase (iNOS), COX-2, and TNF-α through suppression of NF-κB activation [30]. Furthermore, an extract from the roots of Polygala tenuifolia inhibited the translocation of NF-κB by blocking TLR4 and MyD88 expression in lipopolysaccharide (LPS)-stimulated BV2 cell lines [31] indicating that TLRs and adaptor proteins such as MyD88 could be important targets of some CHMs.
TNF-α
A number of CHMs inhibited TNF-α expression to modulate TNF-α in experimentally induced liver fibrosis. CHMs with specific negative modulatory effects on TNF-α include extracts from Zanthoxylum schinifolium [32], P. tenuifolia [31], Clematis chinensis [33], and Angelica sinensis, and Sophora flavescens [34].
IL
Interleukins play crucial roles in the inflammatory reactions including cell adhesion, neutrophil aggregation, inflammatory gene expression, and release of neurotoxic substances to exacerbate inflammatory response. Inhibition of interleukins by CHMs may significantly account for the anti-inflammatory effects of most CHMs. Extracts from Glossogyne tenuifolia [35], Vitex trifolia [36], Glycyrrhiza uralensis [37], Scutellaria baicalensis and Andrographis paniculata [38], Caesalpinia sappan [39,40], and Phellodendron chinense [41] were shown to have down-regulated expression of IL-1β, IL-2, IL-6, and IL-12. IL-10 has severally been reported as a negative regulator of inflammation [42]. The mechanism of IL-10-dependent anti-inflammatory effect is linked to suppression of inflammatory cytokines [43]. Some CHMs were reported to have up-regulated IL-10 expression. Example, the root extracts of Astragalus membranaceus reversed down-regulation of IL-10 expression under colitis-inducing conditions [44].
IFN-γ
The role of IFN-γ in inflammation has been well-elaborated [45]. IFN-γ activates macrophages to release IL-1, TNF-α, IL-6, IL-8 and several other pro-inflammatory mediators, playing major roles in inflammation. A root extract of A. membranaceus markedly reduced the expression of IFN-γ [44].
PGE2
PGE2 causes vasodilation of peripheral blood capillaries at inflammatory site, thereby increasing vascular permeability, plasma exudation, edema, and inflammation and these effects can potentiate inflammatory reaction. Therefore, cessation of PGE2 activity may enhance anti-inflammation. An extract of Houttuynia cordata successfully inhibited PGE2 release in LPS-induced activation of mouse peritoneal macrophages [46]. Similarly, extracts of the flowers of Carthamus tinctorius markedly reduced the release of PGE2 [47].
iNOS and NO Production
Vascular dilatation, vascular permeability, cell infiltration, and release of pain mediators are all orchestrated by NO production under the regulation of iNOS in smooth muscle cells. Inhibition of this pathway may significantly halt inflammation and its associated complications. Lim et al. had reported inhibition of iNOS-dependent NO synthesis from RAW 264.7 cells by the action of phylligenin, a compound isolated from Forsythia koreana and it led to abrogation of the inflammatory response in the studied cells [48].
COX-2
COX-2 is a member of the cyclooxygenase family. It is constitutively expressed on inflammatory cells [49]. It is only expressed on tissues secondary to stimulation by inflammatory stimuli or tissue injury. A number of CHMs have been shown to markedly decrease iNOS and COX-2 expressions in experimental models of liver fibrosis. For example, extracts of the Ramulus of Taxillus liquidambaricola [50], and the aerial parts of Pogostemon cablin [51]. Further, some CHMs were shown to down-regulate a panel of pro-inflammatory mediators including IL-1β, TNF-α, iNOS, ICAM-1, and COX-2. A typical example is the extracts of A. sinensis and S. flavescens which significantly inhibited IL-1b, TNF-α, iNOS, ICAM-1, and COX-2 [34]. Extracts of P. angularis inhibited NF-κB and AP-1 [3].
Mitogen-activated Protein Kinases (MAPKs)
MAPK pathway is crucial in inflammatory responses, particularly its downstream mediators such as p38. Essentially, p38 enhances assembly and activation of leukocytes, regulates transcription factors and cytokine biosynthesis [3]. MCP-1 regulates many cells in the inflammatory process such as mononuclear cells, B cells, and T cells causing cell migration and aggregation at the site of inflammation. p38 and MCP-1 represent major targets for anti-inflammatory agents. Many CHMs exert their effects by inhibiting p38, and MCP-1 mRNA expression. For instance, extract of Z. schinifolium suppressed p38 and TNF-α-induced MCP-1 expression [32]. It was practically impossible to include in this review all CHMs with anti-inflammatory effects, and we seldom tried it, nonetheless those captured in this review comprehensively reflect the general picture. We wish to state that there are many other CHMs with anti-inflammatory effects, which readers can source elsewhere.
CHMS DEMONSTRATE INHIBITION OF HSC ROLE IN FIBROGENESIS
Many reports have conclusively implicated HSCs as the main hepatic cell responsible for liver fibrosis [52,53]. CHMs may interrupt one or more stages of HSC transformation, to attenuate liver fibrosis. For example, genipin, an isolate from one of the herbal components of Yinchenhao Tang, suppressed wound-induced HSC migration and proliferation to ameliorate liver fibrosis [54].
HSC Activation
HSC activation was also inhibited by Fuzheng Huayu, Chinese herbal formula, through blockade of fibronectin/integrin-5β1 signaling pathway [55,56]. Xiao Chaihu Tang inhibited HSC proliferation by suppressing cell secretion [57]. Root extracts of two Chinese herbs (A. membranaceus and Salvia miltiorrhizae) inhibited HSC activation and proliferation in keloid fibroblasts [58].
HSC Proliferation
Gypenosides inhibited platelet-derived growth factor (PDGF)-induced HSC proliferation via suppression of PDGF-Akt-p70S6k and inhibition of cyclin D1 and D2 expression [59]. Ganoderic and ganodenic acids derived from Ganoderma lucidum (“Lingzhi”) significantly inhibited HSC proliferation via suppression of platelet-derived growth factor β receptor (PDGFbR) phosphorylation [60].
HSC Apoptosis
Some CHMs selectively inhibited hepatocyte apoptosis but enhanced apoptosis of HSCs. Genipin, the pharmacologically active agent isolated from one of the herbal components of Yinchenhao Tang inhibited in vitro TGF-β1-induced hepatocyte apoptosis [61]. Subsequently, it was confirmed that genipin suppressed hepatocyte apoptosis in primary cultured murine hepatocytes via Fas-mediation [62]. Further genipin-treated mice resisted Ca2+-induced mitochondrial permeability transition (MPT) compared to control and model [63]. Tetrandrine, an isolate from the roots of Stephaniae tetrandrae potently induced apoptosis of T-HSC/Cl-6 cells by activating caspase-3 protease and cleavage of poly (ADP-ribose) polymerase [64].
CHMS EXERT ANTI-OXIDANT AND ANTI-LIPID PER-OXIDATIVE EFFECTS
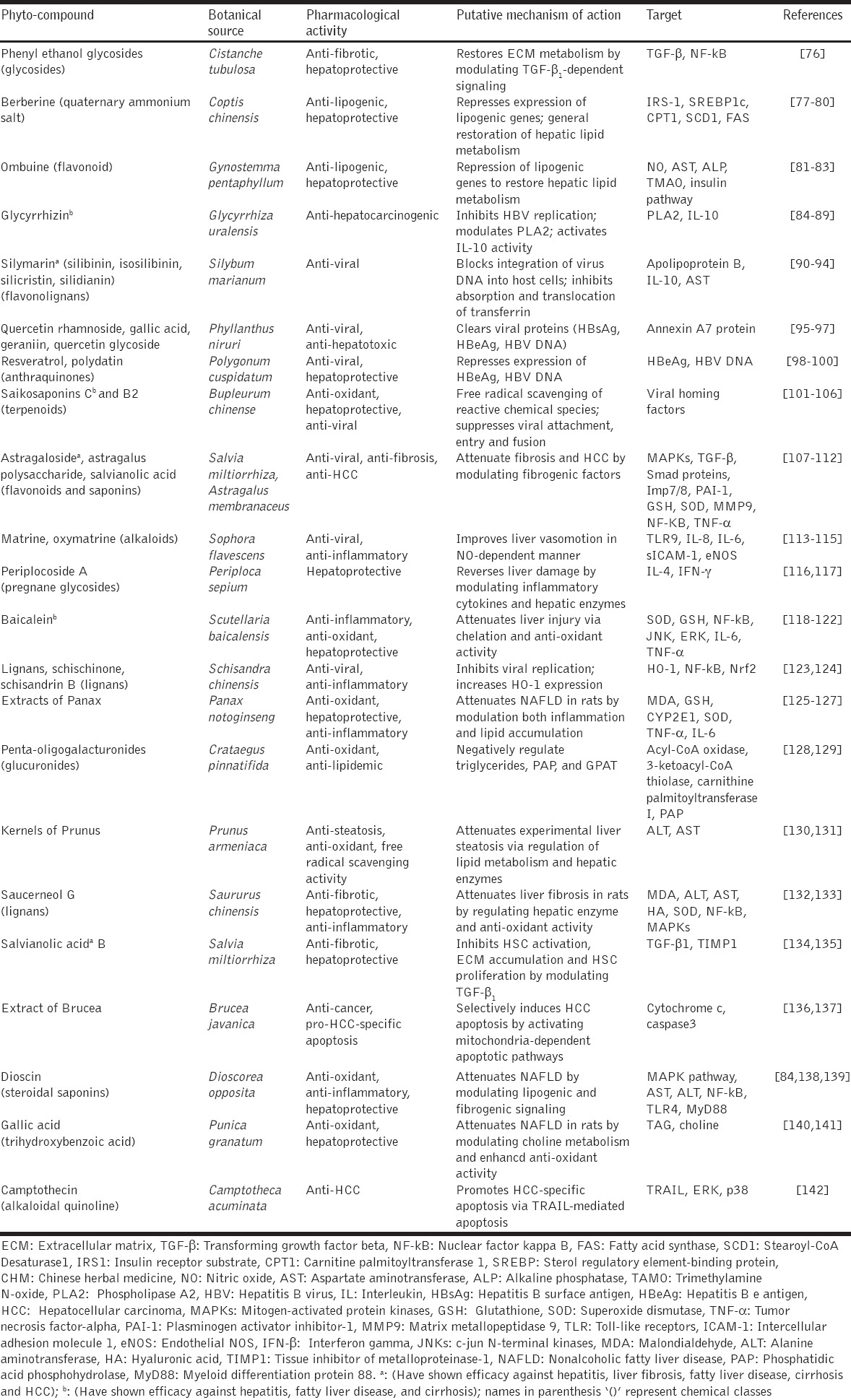
Many CHMs inhibit oxidant and lipid peroxidation whiles at the same time enhance in-built hepatic antioxidant machinery to attenuate reactive oxygen species (ROS)-mediated inflammation and fibrogenesis. The production of ROS in hepatocytes as well as perisinusoidal cells has been attributed to many factors including oxidant activity, lipid peroxidation, mitochondrion electron transport chain, damaged mitochondria, cytochrome P450 isoforms, e.g., P450 2E1 (CYP2E1), xanthine oxidases, nicotinamide adenine dinucleotide phosphate oxidases, and altered metabolism [65]. ROS-dependent oxidative stress causes increase in MPT leading to hepatocyte necrosis and apoptosis [24]. Moreover, ROS (e.g. hydrogen peroxide, superoxide radical, and nitrosative species) increases the expression of specific genes linked to fibrogenesis, among which are pro-collagen type 1, monocyte chemoattractant protein 1 (MCP-1), and tissue inhibitor of metalloproteinase-1 (TIMP-1) through activation of many signal transduction pathways and transcription factors such as c-jun N-terminal kinases, AP-1, and NF-κB [66]. ROS generated by activated Kupffer cells and damaged hepatocytes activate HSCs by increasing their fibrogenic potential. It is therefore of enormous significance in the treatment of liver disease to arrest or suppress oxidative stress and lipid peroxidation. A typical CHM shown to produce suppression of oxidant and lipid peroxidation activities is extracts from S. miltiorrhizae. Extracts from S. miltiorrhizae enhanced superoxide dismutase (SOD) activity whiles reducing malondialdehyde (MDA) levels in experimentally induced liver fibrosis [67]. Furthermore, extracts from S. miltiorrhizae up-regulated glutathione levels whilst at the same time reduced lipid peroxidation in a dose-dependent manner [68]. Other CHMs have shown significant anti-oxidant and anti-lipid peroxidation effects both in vitro and in vivo by reducing oxidant biomarkers (MDA, alanine aminotransferase, aspartate aminotransferase, total bilirubin, and alkaline phosphatase), fibrogenic biomarkers (hyaluronic acid, laminin, type III procollagen, and type IV collagen) but increased anti-oxidant activity (increased glutathione S-transferase and SOD activities). CHMs in this group (anti-oxidant and anti-lipid peroxidation promoters) worth mentioning include Panax notoginseng (Tianqi) extract [69], Gingko biloba (Yinxing) extract [70], berberine [71,72], Yichenhao Tang extract [72], extract of Solanum nigrum [73], Xiao Chaihu Tang [74], Handan Ganle, taurine [75], and several other CHMs [Table 1].
Table 1.
A list of some extracts and isolated phyto-compounds from CHMs and their mechanisms of action against fibro-hepato-carcinogenesis
CHMS EXERT ANTI-VIRAL REPLICATIVE EFFECTS
Cessation and inhibition of virus-derived ROS are important in the treatment of hepatitis-related liver fibrosis. Worldwide HBV and HCV have been acclaimed as the most common causes of chronic liver disease [4]. Pathologically, HBV can integrate into host genome (insertional mutagenesis) to induce chromosomal instability leading to liver disease progression. Unlike HBV, various HCV proteins such as core protein, the envelope, and non-structural proteins have been shown to exert oncogenic potential [24]. Several CHMs were shown to exert antiviral effects in both pre-clinical and clinical studies. In vitro berberine, artemisinin and artesunate inhibited viral reproduction [143]. Other CHMs including aucubin [144], nobiletin an isolate from the peelings of Citrus unshiu [145], and oxymatrine [146] inhibited viral reproduction and replication. Handan Ganle inhibited viral replication in patients with decompensated cirrhosis [147]. Moreover, Xiao Chai Hu Tang enhanced IFN-γ and antibody production against hepatitis B core and antigens in chronic HBV patients [148].
CHMS PRODUCE IMMUNOMODULATORY EFFECTS
TGF-β and PDGF are the two most potent fibrogenic cytokines [24] and have classically been considered to provide fibrogenic and proliferative stimuli to HSC.
TGF-β
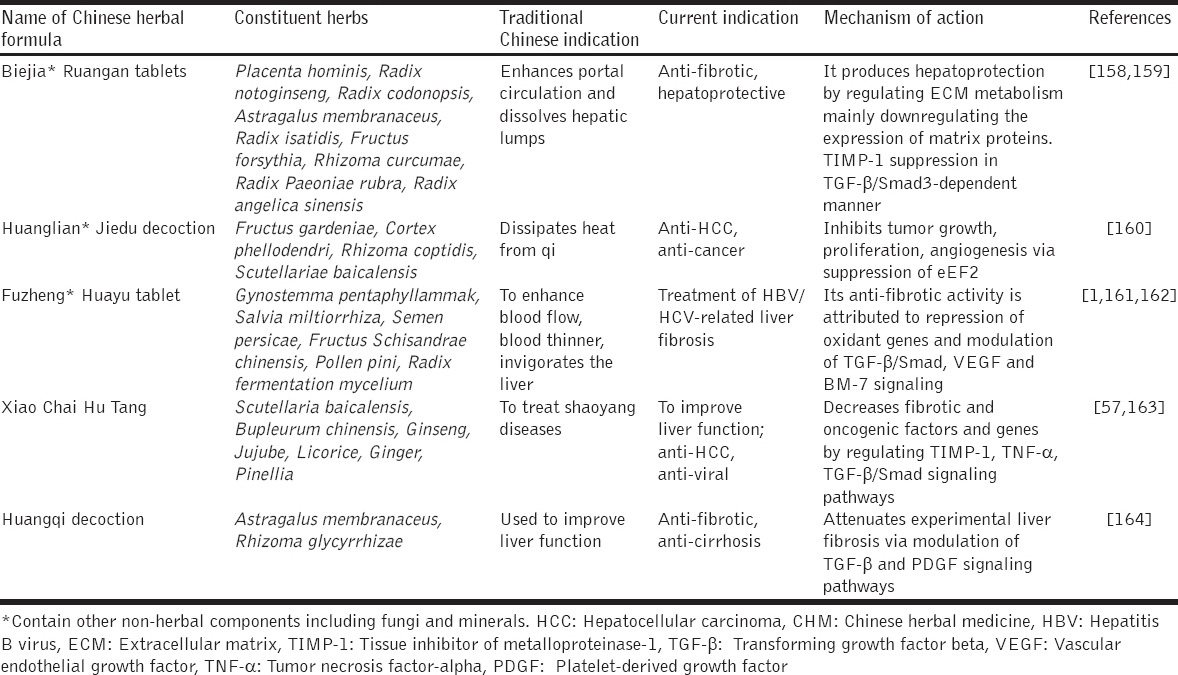
The specific role of TGF-β in liver fibrosis has severally been elucidated [149,150]. Yang et al. have shown effective modulation of TFG-β/Smad signaling by a synergized root extract derived from A. membranaceus and S. miltiorrhiza, which led to decreased fibrogenic biomarkers and liver fibrosis [9]. Subsequently, the synergized root extract inhibited TGF-β1-induced HepG2 cell proliferation and invasion by modulating TGF-β/Smad signaling [151]. The synergized root extract suppressed DEN-induced HCC, decreased pro-neoplastic markers (GGT and GST-P) and down-regulated PAI-1 mRNA expression in TGF-β1-stimulated HepG2 cells [152]. To further elaborate the mechanism of action of the synergized root extract, it was observed that it switched pSmad3L-dependent signaling (oncogenic) to that of pSmad3C (tumor suppression) [153]. S. miltiorrhiza extracts A&B down-regulated TGF-β1, and TIMP-1 gene expressions and blocked MAPK activity [154]. Rehin and emodin, isolated from Rheum palmatum inhibited TGF-β1 expression [155]. Buzhong Yiqi Tang and Renshen Yangrong Tang produced significant immunomodulatory effects to reduce liver fibrosis [156]. Put together, this observation with specific regard to the synergized root extract needs further investigations, in view of the fact that DEN-induced HCC model is highly sensitive and accurately mimic the pathological features of human liver fibrosis and HCC [157]. Many other Chinese herbal formulae modulate several signaling pathways at multi-level to produce anti-fibro-carcinogenic effects [Table 2].
Table 2.
Some CHM formulas, their composition and putative mechanisms of action against fibro-hepato-carcinogenesis
PDGF
Many other CHMs in like manner have modulated cytokines involved in fibrogenesis. Example, Ganoderma lucidum extract and Ganoderma polysaccharide inhibited HSC proliferation through blockade of PDGFbR phosphorylation [165]. Berberis anistata fruit extract down-regulated expression of NF-κB, α-SMA, and TGF-β1 [166,167]. Ginkgo biloba extract down-regulated expression of NF-κB, TGF-β1, and collagen genes [70]. Cordyceps polysaccharide inhibited PDGF expression [168].
CHM TARGETS SPECIFIC GENES
Some CHMs exert specific effects on some genes, especially genes implicated in fibro-hepato-carcinogenesis.
c-fos and c-jun
Tetrandrine down-regulated c-fos and c-jun gene expressions, while they up-regulated expression of Smad7 [169].
Smurf2
Glycyrrhizin an isolate from G. uralensis decreased NF-κB binding activity and also down-regulated smurf2 gene expression [63]. Buzhong Yiqi Tang and Renshen Yangrong Tang produced significant immunomodulatory effects to reduce liver fibrosis [156].
CONCLUSION
Admittedly, pathogenesis of liver disease is complex, normally enjoying the participation of many cell types, cytokines, chemokines, adhesion molecules and genes, notwithstanding, efforts should be made to tailor scientific investigations to specific targets which are crucial for treatment of liver diseases. It is heartwarming the array of isolated phyto-compounds from CHMs which have demonstrated efficacy against various pathological manifestations of fibro-hepato-carcinogenesis such as liver fibrosis, steatohepatitis, cirrhosis, and HCC. Indeed, there has been an increased effort to characterize these phyto-compounds in the light of their reported indigenous uses but more still needed to be done. For instance, efforts should be focused on structure activity relations of these compounds to help advance understanding on their specific effects at the molecular level. It is long held that Chinese herbal formulas are the historical antecedents of modern-day combination therapy, valid as it may be, it is important that future studies thoroughly investigate individual compounds as single chemical entities, then their combined effects can be predicted with certainty. As it is now, it is difficult to tell which compound or extract from which component herb is producing which effect and to what extent. Although the “one fits all” leaning of westernized medicine is without challenges, it is also important that “many fits all” characteristic of CHMs is subjected to thorough component analysis. We agree with others who are in support of combinatorial approach since it taps into the enhanced synergistic actions of many compounds with varying pharmacological activities. However, the question of herb-herb and herb-drug interactions remains outstanding just as toxicity details. It is worth notice that future studies should address these concerns. CHMs exert multi-modulatory and multi-target effects against pathological manifestations (liver fibrosis, cirrhosis, and HCC) of fibro-hepato-carcinogenesis but future research efforts must focus on structural and functional elucidation of single compounds isolated from herbal components of Chinese herbal formulae.
ACKNOWLEDGMENT
This study was supported by the National Natural Science Foundation of China (No. 81330081, No. 81302845).
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Song YN, Sun JJ, Lu YY, Xu LM, Gao YQ, Zhang W, et al. Therapeutic efficacy of fuzheng-huayu tablet based traditional chinese medicine syndrome differentiation on hepatitis-B-caused cirrhosis: A multicenter double-blind randomized controlled trail. Evid Based Complement Alternat Med. 2013;2013:709305. doi: 10.1155/2013/709305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu HX, Wang SR, Lei Y, Shang JJ. Characteristics and advantages of traditional Chinese medicine in the treatment of acute myocardial infarction. J Tradit Chin Med. 2011;31:269–72. doi: 10.1016/s0254-6272(12)60002-8. [DOI] [PubMed] [Google Scholar]
- 3.Wang Q, Kuang H, Su Y, Sun Y, Feng J, Guo R, et al. Naturally derived anti-inflammatory compounds from Chinese medicinal plants. J Ethnopharmacol. 2013;146:9–39. doi: 10.1016/j.jep.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 4.Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45:529–38. doi: 10.1016/j.jhep.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 5.Wu Q, Qin SK. Features and treatment options of Chinese hepatocellular carcinoma. Chin Clin Oncol. 2013;2:38. doi: 10.3978/j.issn.2304-3865.2013.09.07. [DOI] [PubMed] [Google Scholar]
- 6.Ismail BE, Cabrera R. Management of liver cirrhosis in patients with hepatocellular carcinoma. Chin Clin Oncol. 2013;2:34. doi: 10.3978/j.issn.2304-3865.2013.09.03. [DOI] [PubMed] [Google Scholar]
- 7.Tan K, Brayshaw N, Tomaszewski K, Troke P, Wood N. Investigation of the potential relationships between plasma voriconazole concentrations and visual adverse events or liver function test abnormalities. J Clin Pharmacol. 2006;46:235–43. doi: 10.1177/0091270005283837. [DOI] [PubMed] [Google Scholar]
- 8.Sun WY, Wei W, Wu L, Gui SY, Wang H. Effects and mechanisms of extract from Paeonia lactiflora and Astragalus membranaceus on liver fibrosis induced by carbon tetrachloride in rats. J Ethnopharmacol. 2007;112:514–23. doi: 10.1016/j.jep.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Yang Y, Yang S, Chen M, Zhang X, Zou Y, Zhang X. Compound Astragalus and Salvia miltiorrhiza extract exerts anti-fibrosis by mediating TGF-βeta/smad signaling in myofibroblasts. J Ethnopharmacol. 2008;118:264–70. doi: 10.1016/j.jep.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 10.Feng ZQ, Chu X, Huang NP, Wang T, Wang Y, Shi X, et al. The effect of nanofibrous galactosylated chitosan scaffolds on the formation of rat primary hepatocyte aggregates and the maintenance of liver function. Biomaterials. 2009;30:2753–63. doi: 10.1016/j.biomaterials.2009.01.053. [DOI] [PubMed] [Google Scholar]
- 11.Seeff LB, Lindsay KL, Bacon BR, Kresina TF, Hoofnagle JH. Complementary and alternative medicine in chronic liver disease. Hepatology. 2001;34:595–603. doi: 10.1053/jhep.2001.27445. [DOI] [PubMed] [Google Scholar]
- 12.Kuiper JJ, de Man RA, van Buuren HR. Review article: Management of ascites and associated complications in patients with cirrhosis. Aliment Pharmacol Ther. 2007;26:183–93. doi: 10.1111/j.1365-2036.2007.03482.x. [DOI] [PubMed] [Google Scholar]
- 13.Friedman SL. Hepatic Fibrosis: Pathogenesis, Diagnosis, and Emerging Therapies. Philadelphia, PA: Saunders; 2008. [DOI] [PubMed] [Google Scholar]
- 14.Fattovich G, Pantalena M, Zagni I, Realdi G, Schalm SW, Christensen E. European Concerted Action on Viral Hepatitis (EUROHEP). Effect of hepatitis B and C virus infections on the natural history of compensated cirrhosis: A cohort study of 297 patients. Am J Gastroenterol. 2002;97:2886–95. doi: 10.1111/j.1572-0241.2002.07057.x. [DOI] [PubMed] [Google Scholar]
- 15.Mukerji AN, Patel V, Jain A. Improving survival in decompensated cirrhosis. Int J Hepatol. 2012;2012:318627. doi: 10.1155/2012/318627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li F, Zhao C, Wang L. Molecular-targeted agents combination therapy for cancer: Developments and potentials. Int J Cancer. 2014;134:1257–69. doi: 10.1002/ijc.28261. [DOI] [PubMed] [Google Scholar]
- 17.You H, Ding W, Dang H, Jiang Y, Rountree CB. c-Met represents a potential therapeutic target for personalized treatment in hepatocellular carcinoma. Hepatology. 2011;54:879–89. doi: 10.1002/hep.24450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen J, Talwalkar JA, Yin M, Glaser KJ, Sanderson SO, Ehman RL. Early detection of nonalcoholic steatohepatitis in patients with nonalcoholic fatty liver disease by using MR elastography. Radiology. 2011;259:749–56. doi: 10.1148/radiol.11101942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen JY, Chen HL, Cheng JC, Lin HJ, Tung YT, Lin CF, et al. A Chinese herbal medicine, Gexia-Zhuyu Tang (GZT), prevents dimethylnitrosamine-induced liver fibrosis through inhibition of hepatic stellate cells proliferation. J Ethnopharmacol. 2012;142:811–8. doi: 10.1016/j.jep.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Chen W, Chen JY, Tung YT, Chen HL, Kuo CW, Chuang CH, et al. High-frequency ultrasound imaging to evaluate liver fibrosis progression in rats and yi guan jian herbal therapeutic effects. Evid Based Complement Alternat Med. 2013;2013:302325. doi: 10.1155/2013/302325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bao XY, Xu BB, Fang K, Li Y, Hu YH, Yu GP. Changing trends of hospitalisation of liver cirrhosis in Beijing, China. BMJ Open Gastroenterol. 2015;2:e000051. doi: 10.1136/bmjgast-2015-000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao J, Lin H, Liu T, Zeng W, Li X, Shao X, et al. Disease burden from hepatitis B Virus infection in guangdong province, China. Int J Environ Res Public Health. 2015;12:14055–67. doi: 10.3390/ijerph121114055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chor SY, Hui AY, To KF, Chan KK, Go YY, Chan HL, et al. Anti-proliferative and pro-apoptotic effects of herbal medicine on hepatic stellate cell. J Ethnopharmacol. 2005;100:180–6. doi: 10.1016/j.jep.2005.02.036. [DOI] [PubMed] [Google Scholar]
- 24.Mormone E, George J, Nieto N. Molecular pathogenesis of hepatic fibrosis and current therapeutic approaches. Chem Biol Interact. 2011;193:225–31. doi: 10.1016/j.cbi.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin X, Zhang S, Huang Q, Wei L, Zheng L, Chen Z, et al. Protective effect of Fufang-Liu-Yue-Qing, a traditional Chinese herbal formula, on CCl4 induced liver fibrosis in rats. J Ethnopharmacol. 2012;142:548–56. doi: 10.1016/j.jep.2012.05.040. [DOI] [PubMed] [Google Scholar]
- 26.Lu Z, Liu M, Stribinskis V, Klinge CM, Ramos KS, Colburn NH, et al. MicroRNA-21 promotes cell transformation by targeting the programmed cell death 4 gene. Oncogene. 2008;27:4373–9. doi: 10.1038/onc.2008.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila toll protein signals activation of adaptive immunity. Nature. 1997;388:394–7. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 28.Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–79. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 29.Yu T, Ahn HM, Shen T, Yoon K, Jang HJ, Lee YJ, et al. Anti-inflammatory activity of ethanol extract derived from Phaseolus angularis beans. J Ethnopharmacol. 2011;137:1197–206. doi: 10.1016/j.jep.2011.07.048. [DOI] [PubMed] [Google Scholar]
- 30.Yu T, Lee S, Yang WS, Jang HJ, Lee YJ, Kim TW, et al. The ability of an ethanol extract of Cinnamomum cassia to inhibit Src and spleen tyrosine kinase activity contributes to its anti-inflammatory action. J Ethnopharmacol. 2012;139:566–73. doi: 10.1016/j.jep.2011.11.051. [DOI] [PubMed] [Google Scholar]
- 31.Cheong MH, Lee SR, Yoo HS, Jeong JW, Kim GY, Kim WJ, et al. Anti-inflammatory effects of Polygala tenuifolia root through inhibition of NF-?B activation in lipopolysaccharide-induced BV2 microglial cells. J Ethnopharmacol. 2011;137:1402–8. doi: 10.1016/j.jep.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 32.Cao LH, Lee YJ, Kang DG, Kim JS, Lee HS. Effect of Zanthoxylum schinifolium on TNF-αlpha-induced vascular inflammation in human umbilical vein endothelial cells. Vascul Pharmacol. 2009;50:200–7. doi: 10.1016/j.vph.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 33.Peng C, Perera PK, Li YM, Fang WR, Liu LF, Li FW. Anti-inflammatory effects of Clematis chinensis osbeck extract(AR-6) may be associated with NF-?B, TNF-α, and COX-2 in collagen-induced arthritis in rat. Rheumatol Int. 2012;32:3119–25. doi: 10.1007/s00296-011-2083-8. [DOI] [PubMed] [Google Scholar]
- 34.Han C, Guo J. Antibacterial and anti-inflammatory activity of traditional Chinese herb pairs, Angelica sinensis and Sophora flavescens. Inflammation. 2012;35:913–9. doi: 10.1007/s10753-011-9393-6. [DOI] [PubMed] [Google Scholar]
- 35.Wu MJ, Wang L, Ding HY, Weng CY, Yen JH. Glossogyne tenuifolia acts to inhibit inflammatory mediator production in a macrophage cell line by downregulating LPS-induced NF-kappa B. J Biomed Sci. 2004;11:186–99. doi: 10.1007/BF02256562. [DOI] [PubMed] [Google Scholar]
- 36.Matsui M, Kumar-Roine S, Darius HT, Chinain M, Laurent D, Pauillac S. Characterisation of the anti-inflammatory potential of Vitex trifolia L. (Labiatae), a multipurpose plant of the pacific traditional medicine. J Ethnopharmacol. 2009;126:427–33. doi: 10.1016/j.jep.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 37.Cho HJ, Lim SS, Lee YS, Kim JS, Lee CH, Kwon DY, et al. Hexane/ethanol extract of Glycyrrhiza uralensis licorice exerts potent anti-inflammatory effects in murine macrophages and in mouse skin. Food Chem. 2010;121:959–66. [Google Scholar]
- 38.Parichatikanond W, Suthisisang C, Dhepakson P, Herunsalee A. Study of anti-inflammatory activities of the pure compounds from Andrographis paniculata (burm. f.) nees and their effects on gene expression. Int Immunopharmacol. 2010;10:1361–73. doi: 10.1016/j.intimp.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 39.Wang YZ, Sun SQ, Zhou YB. Extract of the dried heartwood of Caesalpinia sappan L. attenuates collagen-induced arthritis. J Ethnopharmacol. 2011;136:271–8. doi: 10.1016/j.jep.2011.04.061. [DOI] [PubMed] [Google Scholar]
- 40.Wu SQ, Otero M, Unger FM, Goldring MB, Phrutivorapongkul A, Chiari C, et al. Anti-inflammatory activity of an ethanolic Caesalpinia sappan extract in human chondrocytes and macrophages. J Ethnopharmacol. 2011;138:364–72. doi: 10.1016/j.jep.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xian YF, Mao QQ, Ip SP, Lin ZX, Che CT. Comparison on the anti-inflammatory effect of cortex phellodendri Chinensis and cortex phellodendri amurensis in 12-O-tetradecanoyl-phorbol-13-acetate-induced ear edema in mice. J Ethnopharmacol. 2011;137:1425–30. doi: 10.1016/j.jep.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 42.Reutershan J, Basit A, Galkina EV, Ley K. Sequential recruitment of neutrophils into lung and bronchoalveolar lavage fluid in LPS-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2005;289:L807–15. doi: 10.1152/ajplung.00477.2004. [DOI] [PubMed] [Google Scholar]
- 43.Wanidworanun C, Strober W. Predominant role of tumor necrosis factor-alpha in human monocyte IL-10 synthesis. J Immunol. 1993;151:6853–61. [PubMed] [Google Scholar]
- 44.Ko JK, Chik CW. The protective action of radix Astragalus membranaceus against hapten-induced colitis through modulation of cytokines. Cytokine. 2009;47:85–90. doi: 10.1016/j.cyto.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 45.Kelchtermans H, Billiau A, Matthys P. How interferon-gamma keeps autoimmune diseases in check. Trends Immunol. 2008;29:479–86. doi: 10.1016/j.it.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 46.Li W, Zhou P, Zhang Y, He L. Houttuynia cordata, a novel and selective COX-2 inhibitor with anti-inflammatory activity. J Ethnopharmacol. 2011;133:922–7. doi: 10.1016/j.jep.2010.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jun MS, Ha YM, Kim HS, Jang HJ, Kim YM, Lee YS, et al. Anti-inflammatory action of methanol extract of Carthamus tinctorius involves in heme oxygenase-1 induction. J Ethnopharmacol. 2011;133:524–30. doi: 10.1016/j.jep.2010.10.029. [DOI] [PubMed] [Google Scholar]
- 48.Lim H, Lee JG, Lee SH, Kim YS, Kim HP. Anti-inflammatory activity of phylligenin, a lignan from the fruits of Forsythia koreana, and its cellular mechanism of action. J Ethnopharmacol. 2008;118:113–7. doi: 10.1016/j.jep.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 49.Dannenberg AJ, Altorki NK, Boyle JO, Dang C, Howe LR, Weksler BB, et al. Cyclo-oxygenase 2: A pharmacological target for the prevention of cancer. Lancet Oncol. 2001;2:544–51. doi: 10.1016/S1470-2045(01)00488-0. [DOI] [PubMed] [Google Scholar]
- 50.Deng JS, Chi CS, Huang SS, Shie PH, Lin TH, Huang GJ. Antioxidant, analgesic, and anti-inflammatory activities of the ethanolic extracts of Taxillus liquidambaricola. J Ethnopharmacol. 2011;137:1161–71. doi: 10.1016/j.jep.2011.07.041. [DOI] [PubMed] [Google Scholar]
- 51.Li YC, Xian YF, Ip SP, Su ZR, Su JY, He JJ, et al. Anti-inflammatory activity of patchouli alcohol isolated from pogostemonis herba in animal models. Fitoterapia. 2011;82:1295–301. doi: 10.1016/j.fitote.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 52.Friedman SL. Seminars in Liver Disease 1999: © 1999. New York: Thieme Medical Publishers, Inc; 1999. Cytokines and fibrogenesis; pp. 129–40. [DOI] [PubMed] [Google Scholar]
- 53.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–18. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kitano A, Saika S, Yamanaka O, Ikeda K, Reinach PS, Nakajima Y, et al. Genipin suppresses subconjunctival fibroblast migration, proliferation and myofibroblast transdifferentiation. Ophthalmic Res. 2006;38:355–60. doi: 10.1159/000096231. [DOI] [PubMed] [Google Scholar]
- 55.Liu JY, Hu JH, Zhu QG, Li FQ, Wang J, Sun HJ. Effect of matrine on the expression of substance P receptor and inflammatory cytokines production in human skin keratinocytes and fibroblasts. Int Immunopharmacol. 2007;7:816–23. doi: 10.1016/j.intimp.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 56.Liu C, Hu Y, Xu L, Liu C, Liu P. Effect of Fuzheng Huayu formula and its actions against liver fibrosis. Chin Med. 2009;4:12. doi: 10.1186/1749-8546-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen MH, Chen JC, Tsai CC, Wang WC, Chang DC, Tu DG, et al. The role of TGF-βeta 1 and cytokines in the modulation of liver fibrosis by Sho-saiko-to in rat’s bile duct ligated model. J Ethnopharmacol. 2005;97:7–13. doi: 10.1016/j.jep.2004.09.040. [DOI] [PubMed] [Google Scholar]
- 58.He S, Yang Y, Liu X, Huang W, Zhang X, Yang S, et al. Compound Astragalus and Salvia miltiorrhiza extract inhibits cell proliferation, invasion and collagen synthesis in keloid fibroblasts by mediating transforming growth factor-ß?/?Smad pathway. Br J Dermatol. 2012;166:564–74. doi: 10.1111/j.1365-2133.2011.10674.x. [DOI] [PubMed] [Google Scholar]
- 59.Chen MH, Chen SH, Wang QF, Chen JC, Chang DC, Hsu SL, et al. The molecular mechanism of gypenosides-induced G1 growth arrest of rat hepatic stellate cells. J Ethnopharmacol. 2008;8(117):309–17. doi: 10.1016/j.jep.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 60.Wang Z, Li Y, Banerjee S, Sarkar FH. Exploitation of the notch signaling pathway as a novel target for cancer therapy. Anticancer Res. 2008;28:3621–30. [PubMed] [Google Scholar]
- 61.Yamamoto M, Ogawa K, Morita M, Fukuda K, Komatsu Y. The herbal medicine Inchin-ko-to inhibits liver cell apoptosis induced by transforming growth factor beta 1. Hepatology. 1996;23:552–9. doi: 10.1053/jhep.1996.v23.pm0008617437. [DOI] [PubMed] [Google Scholar]
- 62.Yamamoto M, Miura N, Ohtake N, Amagaya S, Ishige A, Sasaki H, et al. Genipin, a metabolite derived from the herbal medicine Inchin-ko-to, and suppression of Fas-induced lethal liver apoptosis in mice. Gastroenterology. 2000;118:380–9. doi: 10.1016/s0016-5085(00)70220-4. [DOI] [PubMed] [Google Scholar]
- 63.Feng Y, Cheung KF, Wang N, Liu P, Nagamatsu T, Tong Y. Chinese medicines as a resource for liver fibrosis treatment. Chin Med. 2009;4:16. doi: 10.1186/1749-8546-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao X, Cui XY, Chen BQ, Chu QP, Yao HY, Ku BS, et al. Tetrandrine, a bisbenzylisoquinoline alkaloid from Chinese herb radix, augmented the hypnotic effect of pentobarbital through serotonergic system. Eur J Pharmacol. 2004;506:101–5. doi: 10.1016/j.ejphar.2004.10.046. [DOI] [PubMed] [Google Scholar]
- 65.Haorah J, Ramirez SH, Floreani N, Gorantla S, Morsey B, Persidsky Y. Mechanism of alcohol-induced oxidative stress and neuronal injury. Free Radic Biol Med. 2008;45:1542–50. doi: 10.1016/j.freeradbiomed.2008.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Higgins DF, Lappin DW, Kieran NE, Anders HJ, Watson RW, Strutz F, et al. DNA oligonucleotide microarray technology identifies fisp-12 among other potential fibrogenic genes following murine unilateral ureteral obstruction (UUO): Modulation during epithelial-mesenchymal transition. Kidney Int. 2003;64:2079–91. doi: 10.1046/j.1523-1755.2003.00306.x. [DOI] [PubMed] [Google Scholar]
- 67.Liu CL, Xie LX, Li M, Durairajan SS, Goto S, Huang JD. Salvianolic acid B inhibits hydrogen peroxide-induced endothelial cell apoptosis through regulating PI3K/Akt signaling. PLoS One. 2007;2:e1321. doi: 10.1371/journal.pone.0001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee TY, Chang HH, Wang GJ, Chiu JH, Yang YY, Lin HC. Water-soluble extract of Salvia miltiorrhiza ameliorates carbon tetrachloride-mediated hepatic apoptosis in rats. J Pharm Pharmacol. 2006;58:659–65. doi: 10.1211/jpp.58.5.0011. [DOI] [PubMed] [Google Scholar]
- 69.Lin CF, Wong KL, Wu RS, Huang TC, Liu CF. Protection by hot water extract of Panax notoginseng on chronic ethanol-induced hepatotoxicity. Phytother Res. 2003;17:1119–22. doi: 10.1002/ptr.1329. [DOI] [PubMed] [Google Scholar]
- 70.Ding J, Yu J, Wang C, Hu W, Li D, Luo Y, et al. Ginkgo biloba extract alleviates liver fibrosis induced by CCl in rats. Liver Int. 2005;25:1224–32. doi: 10.1111/j.1478-3231.2005.01169.x. [DOI] [PubMed] [Google Scholar]
- 71.Peng PL, Hsieh YS, Wang CJ, Hsu JL, Chou FP. Inhibitory effect of berberine on the invasion of human lung cancer cells via decreased productions of urokinase-plasminogen activator and matrix metalloproteinase-2. Toxicol Appl Pharmacol. 2006;214:8–15. doi: 10.1016/j.taap.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 72.Inao M, Mochida S, Matsui A, Eguchi Y, Yulutuz Y, Wang Y, et al. Japanese herbal medicine Inchin-ko-to as a therapeutic drug for liver fibrosis. J Hepatol. 2004;41:584–91. doi: 10.1016/j.jhep.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 73.Lin HM, Tseng HC, Wang CJ, Lin JJ, Lo CW, Chou FP. Hepatoprotective effects of Solanum nigrum Linn extract against CCl(4)-induced oxidative damage in rats. Chem Biol Interact. 2008;171:283–93. doi: 10.1016/j.cbi.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 74.Kusunose M, Qiu B, Cui T, Hamada A, Yoshioka S, Ono M, et al. Effect of Sho-saiko-to extract on hepatic inflammation and fibrosis in dimethylnitrosamine induced liver injury rats. Biol Pharm Bull. 2002;25:1417–21. doi: 10.1248/bpb.25.1417. [DOI] [PubMed] [Google Scholar]
- 75.Miyazaki T, Karube M, Matsuzaki Y, Ikegami T, Doy M, Tanaka N, et al. Taurine inhibits oxidative damage and prevents fibrosis in carbon tetrachloride-induced hepatic fibrosis. J Hepatol. 2005;43:117–25. doi: 10.1016/j.jhep.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 76.You SP, Zhao J, Ma L, Tudimat M, Zhang SL, et al. Preventive effects of phenylethanol glycosides from Cistanche tubulosa on bovine serum albumin-induced hepatic fibrosis in rats. Daru. 2015;23:52. doi: 10.1186/s40199-015-0135-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fan H, Chen YY, Bei WJ, Wang LY, Chen BT, Guo J. In vitro Screening for antihepatic steatosis active components within coptidis rhizoma alkaloids extract using liver cell extraction with HPLC analysis and a free fatty acid-induced hepatic steatosis HepG2 Cell assay. Evid Based Complement Alternat Med. 2013;2013:459390. doi: 10.1155/2013/459390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xing LJ, Zhang L, Liu T, Hua YQ, Zheng PY, Ji G. Berberine reducing insulin resistance by up-regulating IRS-2 mRNA expression in nonalcoholic fatty liver disease (NAFLD) rat liver. Eur J Pharmacol. 2011;668:467–71. doi: 10.1016/j.ejphar.2011.07.036. [DOI] [PubMed] [Google Scholar]
- 79.Xue M, Zhang L, Yang MX, Zhang W, Li XM, Ou ZM, et al. Berberine-loaded solid lipid nanoparticles are concentrated in the liver and ameliorate hepatosteatosis in db/db mice. Int J Nanomedicine. 2015;10:5049–57. doi: 10.2147/IJN.S84565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yeung CY, Lee FT, Wong HN. Effect of a popular Chinese herb on neonatal bilirubin protein binding. Biol Neonate. 1990;58:98–103. doi: 10.1159/000243239. [DOI] [PubMed] [Google Scholar]
- 81.Wang M, Wang F, Wang Y, Ma X, Zhao M, Zhao C. Metabonomics study of the therapeutic mechanism of Gynostemma pentaphyllum and atorvastatin for hyperlipidemia in rats. PLoS One. 2013;8:e78731. doi: 10.1371/journal.pone.0078731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Müller C, Gardemann A, Keilhoff G, Peter D, Wiswedel I, Schild L. Prevention of free fatty acid-induced lipid accumulation, oxidative stress, and cell death in primary hepatocyte cultures by a Gynostemma pentaphyllum extract. Phytomedicine. 2012;19:395–401. doi: 10.1016/j.phymed.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 83.Chou SC, Chen KW, Hwang JS, Lu WT, Chu YY, Lin JD, et al. The add-on effects of Gynostemma pentaphyllum on nonalcoholic fatty liver disease. Altern Ther Health Med. 2006;12:34–9. [PubMed] [Google Scholar]
- 84.Hong M, Li S, Tan HY, Wang N, Tsao SW, Feng Y. Current status of herbal medicines in chronic liver disease therapy: The biological effects, molecular targets and future prospects. Int J Mol Sci. 2015;16:28705–45. doi: 10.3390/ijms161226126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Abe M, Akbar F, Hasebe A, Horiike N, Onji M. Glycyrrhizin enhances interleukin-10 production by liver dendritic cells in mice with hepatitis. J Gastroenterol. 2003;38:962–7. doi: 10.1007/s00535-003-1179-7. [DOI] [PubMed] [Google Scholar]
- 86.Matsuo K, Takenaka K, Shimomura H, Fujii N, Shinagawa K, Kiura K, et al. Lamivudine and glycyrrhizin for treatment of chemotherapy-induced hepatitis B virus (HBV) hepatitis in a chronic HBV carrier with non-Hodgkin lymphoma. Leuk Lymphoma. 2001;41:191–5. doi: 10.3109/10428190109057970. [DOI] [PubMed] [Google Scholar]
- 87.van Rossum TG, de Jong FH, Hop WC, Boomsma F, Schalm SW. ‘Pseudo-aldosteronism’ induced by intravenous glycyrrhizin treatment of chronic hepatitis C patients. J Gastroenterol Hepatol. 2001;16:789–95. doi: 10.1046/j.1440-1746.2001.02382.x. [DOI] [PubMed] [Google Scholar]
- 88.van Rossum TG, Vulto AG, Hop WC, Schalm SW. Glycyrrhizin-induced reduction of ALT in European patients with chronic hepatitis C. Am J Gastroenterol. 2001;96:2432–7. doi: 10.1111/j.1572-0241.2001.04049.x. [DOI] [PubMed] [Google Scholar]
- 89.Fialla AD, Israelsen M, Hamberg O, Krag A, Gluud LL. Nutritional therapy in cirrhosis or alcoholic hepatitis: A systematic review and meta-analysis. Liver Int Off J Int Assoc Stud Liver. 2015;35:2072–8. doi: 10.1111/liv.12798. [DOI] [PubMed] [Google Scholar]
- 90.Mayer KE, Myers RP, Lee SS. Silymarin treatment of viral hepatitis: A systematic review. J Viral Hepat. 2005;12:559–67. doi: 10.1111/j.1365-2893.2005.00636.x. [DOI] [PubMed] [Google Scholar]
- 91.Adeyemo O, Doi H, Rajender Reddy K, Kaplan DE. Impact of oral silymarin on virus- and non-virus-specific T-cell responses in chronic hepatitis C infection. J Viral Hepat. 2013;20:453–62. doi: 10.1111/jvh.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Blaising J, Lévy PL, Gondeau C, Phelip C, Varbanov M, Teissier E, et al. Silibinin inhibits hepatitis C virus entry into hepatocytes by hindering clathrin-dependent trafficking. Cell Microbiol. 2013;15:1866–82. doi: 10.1111/cmi.12155. [DOI] [PubMed] [Google Scholar]
- 93.Flaig TW, Gustafson DL, Su LJ, Zirrolli JA, Crighton F, Harrison GS, et al. A phase I and pharmacokinetic study of silybin-phytosome in prostate cancer patients. Invest New Drugs. 2007;25:139–46. doi: 10.1007/s10637-006-9019-2. [DOI] [PubMed] [Google Scholar]
- 94.Flaig TW, Su LJ, Harrison G, Agarwal R, Glodé LM. Silibinin synergizes with mitoxantrone to inhibit cell growth and induce apoptosis in human prostate cancer cells. Int J Cancer. 2007;120:2028–33. doi: 10.1002/ijc.22465. [DOI] [PubMed] [Google Scholar]
- 95.Amin ZA, Bilgen M, Alshawsh MA, Ali HM, Hadi AH, Abdulla MA. Protective role of Phyllanthus niruri extract against thioacetamide-induced liver cirrhosis in rat model. Evid Based Complement Alternat Med. 2012;2012:241583. doi: 10.1155/2012/241583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu J, Lin H, McIntosh H. Genus Phyllanthus for chronic hepatitis B virus infection: A systematic review. J Viral Hepat. 2001;8:358–66. doi: 10.1046/j.1365-2893.2001.00307.x. [DOI] [PubMed] [Google Scholar]
- 97.Amin ZA, Abdulla MA, Ali HM, Alshawsh MA, Qadir SW. Assessment of in vitro antioxidant, antibacterial and immune activation potentials of aqueous and ethanol extracts of Phyllanthus niruri. J Sci Food Agric. 2012;92:1874–7. doi: 10.1002/jsfa.5554. [DOI] [PubMed] [Google Scholar]
- 98.Peng W, Qin R, Li X, Zhou H. Botany, phytochemistry, pharmacology, and potential application of Polygonum cuspidatum Sieb. et Zucc: A review. J Ethnopharmacol. 2013;148:729–45. doi: 10.1016/j.jep.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 99.Zhang L, Wang G, Hou W, Li P, Dulin A, Bonkovsky HL. Contemporary clinical research of traditional Chinese medicines for chronic hepatitis B in China: An analytical review. Hepatology. 2010;51:690–8. doi: 10.1002/hep.23384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chang JS, Liu HW, Wang KC, Chen MC, Chiang LC, Hua YC, et al. Ethanol extract of Polygonum cuspidatum inhibits hepatitis B virus in a stable HBV-producing cell line. Antiviral Res. 2005;66:29–34. doi: 10.1016/j.antiviral.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 101.Lin L, Cai WM, Qin CJ, Miao LC, Yun LT, Hua Y, et al. Intervention of TLR4 signal pathway cytokines in severe liver injury with obstructive jaundice in rats. Int J Sports Med. 2012;33:572–9. doi: 10.1055/s-0031-1301318. [DOI] [PubMed] [Google Scholar]
- 102.Liu CT, Chuang PT, Wu CY, Weng YM, Chen W, Tseng CY. Antioxidative and in vitro hepatoprotective activity of Bupleurum kaoi leaf infusion. Phytother Res. 2006;20:1003–8. doi: 10.1002/ptr.1946. [DOI] [PubMed] [Google Scholar]
- 103.Chiang LC, Ng LT, Liu LT, Shieh DE, Lin CC. Cytotoxicity and anti-hepatitis B virus activities of saikosaponins from Bupleurum species. Planta Med. 2003;69:705–9. doi: 10.1055/s-2003-42797. [DOI] [PubMed] [Google Scholar]
- 104.Lin LT, Chung CY, Hsu WC, Chang SP, Hung TC, Shields J, et al. Saikosaponin b2 is a naturally occurring terpenoid that efficiently inhibits hepatitis C virus entry. J Hepatol. 2015;62:541–8. doi: 10.1016/j.jhep.2014.10.040. [DOI] [PubMed] [Google Scholar]
- 105.Huang W, Sun R. Study on hepatotoxicity on rats caused by crude extracts of total saikosaponins and correlation with oxidative damage mechanism. Zhongguo Zhong Yao Za Zhi. 2010;35:1745–9. doi: 10.4268/cjcmm20101320. [DOI] [PubMed] [Google Scholar]
- 106.Huang W, Sun R, Zhang Z. “Dose-time-toxicity” relationship study on hepatotoxicity caused by multiple dose of total Bupleurum saponin crude extracts to rats. Zhongguo Zhong Yao Za Zhi. 2010;35:3344–7. [PubMed] [Google Scholar]
- 107.Boye A, Wu C, Jiang Y, Wang J, Wu J, Yang X, et al. Compound Astragalus and Salvia miltiorrhiza extracts modulate MAPK-regulated TGF-ß/Smad signaling in hepatocellular carcinoma by multi-target mechanism. J Ethnopharmacol. 2015;169:219–28. doi: 10.1016/j.jep.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 108.Yue S, Hu B, Wang Z, Yue Z, Wang F, Zhao Y, et al. Salvia miltiorrhiza compounds protect the liver from acute injury by regulation of p38 and NF?B signaling in Kupffer cells. Pharm Biol. 2014;52:1278–85. doi: 10.3109/13880209.2014.889720. [DOI] [PubMed] [Google Scholar]
- 109.Zhang Y, Zhang Y, Xie Y, Gao Y, Ma J, Yuan J, et al. Multitargeted inhibition of hepatic fibrosis in chronic iron-overloaded mice by Salvia miltiorrhiza. J Ethnopharmacol. 2013;148:671–81. doi: 10.1016/j.jep.2013.05.028. [DOI] [PubMed] [Google Scholar]
- 110.Gao Y, Wang N, Zhang Y, Ma Z, Guan P, Ma J, et al. Mechanism of protective effects of Danshen against iron overload-induced injury in mice. J Ethnopharmacol. 2013;145:254–60. doi: 10.1016/j.jep.2012.10.060. [DOI] [PubMed] [Google Scholar]
- 111.Gao J, Liu ZJ, Chen T, Zhao D. Pharmaceutical properties of calycosin, the major bioactive isoflavonoid in the dry root extract of Radix astragali. Pharm Biol. 2014;52:1217–22. doi: 10.3109/13880209.2013.879188. [DOI] [PubMed] [Google Scholar]
- 112.Liu ZY. Progress in pharmacological research on radix Astragali. Zhong Xi Yi Jie He Za Zhi. 1991;11:312–4. [PubMed] [Google Scholar]
- 113.Ma ZJ, Li Q, Wang JB, Zhao YL, Zhong YW, Bai YF, et al. Combining oxymatrine or matrine with lamivudine increased its antireplication effect against the hepatitis B virus in vitro. Evid Based Complement Alternat Med. 2013;2013:186573. doi: 10.1155/2013/186573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Suo Z, Liu Y, Ferreri M, Zhang T, Liu Z, Mu X, et al. Impact of matrine on inflammation related factors in rat intestinal microvascular endothelial cells. J Ethnopharmacol. 2009;125:404–9. doi: 10.1016/j.jep.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 115.Yao N, Wang X. In vitro immunomodulatory activity of oxymatrine on Toll-like receptor 9 signal pathway in chronic hepatitis B. Am J Chin Med. 2014;42:1399–410. doi: 10.1142/S0192415X14500888. [DOI] [PubMed] [Google Scholar]
- 116.Wan J, Zhu YN, Feng JQ, Chen HJ, Zhang RJ, Ni J, et al. Periplocoside A, a pregnane glycoside from Periploca sepium Bge, prevents concanavalin A-induced mice hepatitis through inhibiting NKT-derived inflammatory cytokine productions. Int Immunopharmacol. 2008;8:1248–56. doi: 10.1016/j.intimp.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 117.Zhang J, Ni J, Chen ZH, Li X, Zhang RJ, Tang W, et al. Periplocoside A prevents experimental autoimmune encephalomyelitis by suppressing IL-17 production and inhibits differentiation of Th17 cells. Acta Pharmacol Sin. 2009;30:1144–52. doi: 10.1038/aps.2009.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kim SJ, Moon YJ, Lee SM. Protective effects of baicalin against ischemia/reperfusion injury in rat liver. J Nat Prod. 2010;73:2003–8. doi: 10.1021/np100389z. [DOI] [PubMed] [Google Scholar]
- 119.Zhao Y, Li H, Gao Z, Xu H. Effects of dietary baicalin supplementation on iron overload-induced mouse liver oxidative injury. Eur J Pharmacol. 2005;509:195–200. doi: 10.1016/j.ejphar.2004.11.060. [DOI] [PubMed] [Google Scholar]
- 120.Huang HL, Wang YJ, Zhang QY, Liu B, Wang FY, Li JJ, et al. Hepatoprotective effects of baicalein against CCl4-induced acute liver injury in mice. World J Gastroenterol. 2012;18:6605–13. doi: 10.3748/wjg.v18.i45.6605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wu YL, Lian LH, Wan Y, Nan JX. Baicalein inhibits nuclear factor-?B and apoptosis via c-FLIP and MAPK in D-GalN/LPS induced acute liver failure in murine models. Chem Biol Interact. 2010;188:526–34. doi: 10.1016/j.cbi.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 122.Wang JY, Lee CY, Pan PJ, Chang WC, Chiu JH, Chen WS, et al. Herb-induced autoimmune-like hepatitis in C57BL/6J mice. Liver Int. 2014;34:583–93. doi: 10.1111/liv.12266. [DOI] [PubMed] [Google Scholar]
- 123.Xue Y, Li X, Du X, Li X, Wang W, Yang J, et al. Isolation and anti-hepatitis B virus activity of dibenzocyclooctadiene lignans from the fruits of Schisandra chinensis. Phytochemistry. 2015;116:253–61. doi: 10.1016/j.phytochem.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 124.Checker R, Patwardhan RS, Sharma D, Menon J, Thoh M, Bhilwade HN, et al. Schisandrin B exhibits anti-inflammatory activity through modulation of the redox-sensitive transcription factors Nrf2 and NF-?B. Free Radic Biol Med. 2012;53:1421–30. doi: 10.1016/j.freeradbiomed.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 125.Liu F, Bai X, Ding RB, Hu YJ, Su H, Wan JB. UPLC/Q-TOFMS-based metabolomics studies on the protective effect of Panax notoginseng saponins on alcoholic liver injury. Am J Chin Med. 2015;43:695–714. doi: 10.1142/S0192415X15500433. [DOI] [PubMed] [Google Scholar]
- 126.Ding RB, Tian K, Cao YW, Bao JL, Wang M, He C, et al. Protective effect of Panax notoginseng saponins on acute ethanol-induced liver injury is associated with ameliorating hepatic lipid accumulation and reducing ethanol-mediated oxidative stress. J Agric Food Chem. 2015;63:2413–22. doi: 10.1021/jf502990n. [DOI] [PubMed] [Google Scholar]
- 127.Yang X, Liao M, Yang Z, Guo J, Gao Q. Effect of Panax notoginseng on genes expression of CYP and GST in liver tissues of rats. Zhongguo Zhong Yao Za Zhi. 2009;34:2390–3. [PubMed] [Google Scholar]
- 128.Li T, Li S, Dong Y, Zhu R, Liu Y. Antioxidant activity of penta-oligogalacturonide, isolated from haw pectin, suppresses triglyceride synthesis in mice fed with a high-fat diet. Food Chem. 2014;145:335–41. doi: 10.1016/j.foodchem.2013.08.036. [DOI] [PubMed] [Google Scholar]
- 129.Li TP, Zhu RG, Dong YP, Liu YH, Li SH, Chen G. Effects of pectin pentaoligosaccharide from Hawthorn (Crataegus pinnatifida Bunge. var. Major) on the activity and mRNA levels of enzymes involved in fatty acid oxidation in the liver of mice fed a high-fat diet. J Agric Food Chem. 2013;61:7599–605. doi: 10.1021/jf400283w. [DOI] [PubMed] [Google Scholar]
- 130.Ozturk F, Gul M, Ates B, Ozturk IC, Cetin A, Vardi N, et al. Protective effect of apricot (Prunus armeniaca L.). on hepatic steatosis and damage induced by carbon tetrachloride in wistar rats. Br J Nutr. 2009;102:1767–75. doi: 10.1017/S0007114509991322. [DOI] [PubMed] [Google Scholar]
- 131.Hokari A, Ishikawa T, Tajiri H, Matsuda T, Ishii O, Matsumoto N, et al. Efficacy of MK615 for the treatment of patients with liver disorders. World J Gastroenterol. 2012;18:4118–26. doi: 10.3748/wjg.v18.i31.4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lu Y, Hong TG, Jin M, Yang JH, Suh SJ, Piao DG, et al. Saucerneol G, a new lignan, from Saururus chinensis inhibits matrix metalloproteinase-9 induction via a nuclear factor kappa B and mitogen activated protein kinases in lipopolysaccharide-stimulated RAW264.7 cells. Biol Pharm Bull. 2010;33:1944–8. doi: 10.1248/bpb.33.1944. [DOI] [PubMed] [Google Scholar]
- 133.Wang L, Cheng D, Wang H, Di L, Zhou X, Xu T, et al. The hepatoprotective and antifibrotic effects of Saururus chinensis against carbon tetrachloride induced hepatic fibrosis in rats. J Ethnopharmacol. 2009;126:487–91. doi: 10.1016/j.jep.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 134.Parajuli DR, Zhao YZ, Jin H, Chi JH, Li SY, Kim YC, et al. Anti-fibrotic effect of PF2401-SF, a standardized fraction of Salvia miltiorrhiza, in thioacetamide-induced experimental rats liver fibrosis. Arch Pharm Res. 2015;38:549–55. doi: 10.1007/s12272-014-0425-2. [DOI] [PubMed] [Google Scholar]
- 135.Fugh-Berman A. Herb-drug interactions. Lancet. 2000;355:134–8. doi: 10.1016/S0140-6736(99)06457-0. [DOI] [PubMed] [Google Scholar]
- 136.Lau FY, Chui CH, Gambari R, Kok SH, Kan KL, Cheng GY, et al. Antiproliferative and apoptosis-inducing activity of Brucea javanica extract on human carcinoma cells. Int J Mol Med. 2005;16:1157–62. [PubMed] [Google Scholar]
- 137.Yue Y, Yang Y, Shi L, Wang Z. Suppression of human hepatocellular cancer cell proliferation by Brucea javanica oil-loaded liposomes via induction of apoptosis. Arch Med Sci. 2015;11:856–62. doi: 10.5114/aoms.2015.53306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Liu M, Xu Y, Han X, Yin L, Xu L, Qi Y, et al. Dioscin alleviates alcoholic liver fibrosis by attenuating hepatic stellate cell activation via the TLR4/MyD88/NF-?B signaling pathway. Sci Rep. 2015;5:18038. doi: 10.1038/srep18038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liu M, Xu L, Yin L, Qi Y, Xu Y, Han X, et al. Corrigendum: Potent effects of dioscin against obesity in mice. Sci Rep. 2015;5:12183. doi: 10.1038/srep12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chao J, Huo TI, Cheng HY, Tsai JC, Liao JW, Lee MS, et al. Gallic acid ameliorated impaired glucose and lipid homeostasis in high fat diet-induced NAFLD mice. PLoS One. 2014;9:e96969. doi: 10.1371/journal.pone.0096969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hsu CL, Yen GC. Effect of gallic acid on high fat diet-induced dyslipidaemia, hepatosteatosis and oxidative stress in rats. Br J Nutr. 2007;98:727–35. doi: 10.1017/S000711450774686X. [DOI] [PubMed] [Google Scholar]
- 142.Jayasooriya RG, Choi YH, Hyun JW, Kim GY. Camptothecin sensitizes human hepatoma Hep3B cells to TRAIL-mediated apoptosis via ROS-dependent death receptor 5 upregulation with the involvement of MAPKs. Environ Toxicol Pharmacol. 2014;38:959–67. doi: 10.1016/j.etap.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 143.Romero MR, Efferth T, Serrano MA, Castaño B, Macias RI, Briz O, et al. Effect of artemisinin/artesunate as inhibitors of hepatitis B virus production in an “in vitro” replicative system. Antiviral Res. 2005;68:75–83. doi: 10.1016/j.antiviral.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 144.Chang IM. Antiviral activity of aucubin against hepatitis B virus replication. Phytother Res. 1997;11:189–92. [Google Scholar]
- 145.Suzuki M, Sasaki K, Yoshizaki F, Oguchi K, Fujisawa M, Cyong JC. Anti-hepatitis C virus effect of citrus unshiu peel and its active ingredient nobiletin. Am J Chin Med. 2005;33:87–94. doi: 10.1142/S0192415X05002680. [DOI] [PubMed] [Google Scholar]
- 146.Chen Y, Mao B, Jiang J. [Relationship between serum load of HBV-DNA and therapeutic effect of oxymatrine in patients with chronic hepatitis B]. Zhongguo Zhong xi yi jie he za zhi Zhongguo Zhongxiyi jiehe zazhi. Chin J Integr Tradit West Med/Zhongguo Zhong Xi Yi Jie He Xue Hui, Zhongguo Zhong Yi Yan Jiu Yuan Zhu Ban. 2002;22:335–6. [PubMed] [Google Scholar]
- 147.Yang Q, Xie RJ, Geng XX, Luo XH, Han B, Cheng ML. Effect of Dan Shao Huaxian capsule on expression of matrix metalloproteinase-1 and tissue inhibitor of metalloproteinase-1 in fibrotic liver of rats. World J Gastroenterol. 2005;11:4953–6. doi: 10.3748/wjg.v11.i32.4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cheng PW, Ng LT, Lin CC. Xiao chai hu tang inhibits CVB1 virus infection of CCFS-1 cells through the induction of Type I interferon expression. Int Immunopharmacol. 2006;6:1003–12. doi: 10.1016/j.intimp.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 149.Furukawa F, Matsuzaki K, Mori S, Tahashi Y, Yoshida K, Sugano Y, et al. p38 MAPK mediates fibrogenic signal through Smad3 phosphorylation in rat myofibroblasts. Hepatology. 2003;38:879–89. doi: 10.1053/jhep.2003.50384. [DOI] [PubMed] [Google Scholar]
- 150.Gressner AM, Weiskirchen R. Modern pathogenetic concepts of liver fibrosis suggest stellate cells and TGF-βeta as major players and therapeutic targets. J Cell Mol Med. 2006;10:76–99. doi: 10.1111/j.1582-4934.2006.tb00292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Liu X, Yang Y, Zhang X, Xu S, He S, Huang W, et al. Compound Astragalus and Salvia miltiorrhiza extract inhibits cell invasion by modulating transforming growth factor-beta/Smad in HepG2 cell. J Gastroenterol Hepatol. 2010;25:420–6. doi: 10.1111/j.1440-1746.2009.05981.x. [DOI] [PubMed] [Google Scholar]
- 152.Rui W, Xie L, Liu X, He S, Wu C, Zhang X, et al. Compound Astragalus and Salvia miltiorrhiza extract suppresses hepatocellular carcinoma progression by inhibiting fibrosis and PAI-1 mRNA transcription. J Ethnopharmacol. 2014;151:198–209. doi: 10.1016/j.jep.2013.10.022. [DOI] [PubMed] [Google Scholar]
- 153.Hu X, Rui W, Wu C, He S, Jiang J, Zhang X, et al. Compound Astragalus and Salvia miltiorrhiza extracts suppress hepatocarcinogenesis by modulating TGF-βompound astraga. J Gastroenterol Hepatol. 2013;29:1284–91. doi: 10.1111/jgh.12490. [DOI] [PubMed] [Google Scholar]
- 154.Zhao JF, Liu CH, Hu YY, Xu LM, Liu P, Liu C. Effect of salvianolic acid B on Smad3 expression in hepatic stellate cells. Hepatobiliary Pancreat Dis Int. 2004;3:102–5. [PubMed] [Google Scholar]
- 155.Zhan Y, Li D, Wei H, Wang Z, Huang X, Xu Q, et al. Emodin on hepatic fibrosis in rats. Chin Med J (Engl) 2000;113:599–601. [PubMed] [Google Scholar]
- 156.Abe S, Ishibashi H, Tansho S, Hanazawa R, Komatsu Y, Yamaguchi H. Protective effect of oral administration of several traditional Kampo-medicines on lethal Candida infection in immunosuppressed mice. Nihon Ishinkin Gakkai Zasshi. 2000;41:115–9. doi: 10.3314/jjmm.41.115. [DOI] [PubMed] [Google Scholar]
- 157.Chen G, Dai ZK, Liang RG, Xiao SJ, He SQ, Zhao HL, et al. Characterization of diethylnitrosamine-induced liver carcinogenesis in Syrian golden hamsters. Exp Ther Med. 2012;3:285–92. doi: 10.3892/etm.2011.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhou J, Chen XM, Liu SW, Fu B, Hong Q, Wang SJ. Effects of Biejia Ruangan Tablet-containing serum on matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 expression in cultured renal interstitial fibroblasts. Chin J Integr Med. 2015;21:152–6. doi: 10.1007/s11655-014-1784-0. [DOI] [PubMed] [Google Scholar]
- 159.Yang FR, Fang BW, Lou JS. Effects of Fufang Biejia Ruangan pills on hepatic fibrosis in vivo and in vitro. World J Gastroenterol. 2013;19:5326–33. doi: 10.3748/wjg.v19.i32.5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang N, Feng Y, Tan HY, Cheung F, Hong M, Lao L, et al. Inhibition of eukaryotic elongation factor-2 confers to tumor suppression by a herbal formulation Huanglian-Jiedu decoction in human hepatocellular carcinoma. J Ethnopharmacol. 2015;164:309–18. doi: 10.1016/j.jep.2015.02.025. [DOI] [PubMed] [Google Scholar]
- 161.Wang RQ, Mi HM, Li H, Zhao SX, Jia YH, Nan YM. Modulation of IKKß/NF-?B and TGF-ß1/Smad via Fuzheng Huayu recipe involves in prevention of nutritional steatohepatitis and fibrosis in mice. Iran J Basic Med Sci. 2015;18:404–11. [PMC free article] [PubMed] [Google Scholar]
- 162.Liu C, Jiang CM, Liu CH, Liu P, Hu YY. Effect of Fuzhenghuayu decoction on vascular endothelial growth factor secretion in hepatic stellate cells. Hepatobiliary Pancreat Dis Int. 2002;1:207–10. [PubMed] [Google Scholar]
- 163.Deng G, Kurtz RC, Vickers A, Lau N, Yeung KS, Shia J, et al. A single arm phase II study of a Far-Eastern traditional herbal formulation (sho-sai-ko-to or xiao-chai-hu-tang) in chronic hepatitis C patients. J Ethnopharmacol. 2011;136:83–7. doi: 10.1016/j.jep.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 164.Zhang GB, Song YN, Chen QL, Dong S, Lu YY, Su MY, et al. Actions of Huangqi decoction against rat liver fibrosis: A gene expression profiling analysis. Chin Med. 2015;10:39. doi: 10.1186/s13020-015-0066-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Park EJ, Ko G, Kim J, Sohn DH. Antifibrotic effects of a polysaccharide extracted from Ganoderma lucidum, glycyrrhizin, and pentoxifylline in rats with cirrhosis induced by biliary obstruction. Biol Pharm Bull. 1997;20:417–20. doi: 10.1248/bpb.20.417. [DOI] [PubMed] [Google Scholar]
- 166.Watanabe A, Obata T, Nagashima H. Berberine therapy of hypertyraminemia in patients with liver cirrhosis. Acta Med Okayama. 1982;36:277–81. doi: 10.18926/AMO/30659. [DOI] [PubMed] [Google Scholar]
- 167.Zhang BJ, Xu D, Guo Y, Ping J, Chen LB, Wang H. Protection by and anti-oxidant mechanism of berberine against rat liver fibrosis induced by multiple hepatotoxic factors. Clin Exp Pharmacol Physiol. 2008;35:303–9. doi: 10.1111/j.1440-1681.2007.04819.x. [DOI] [PubMed] [Google Scholar]
- 168.Gong HY, Wang KQ, Tang SG. Effects of cordyceps sinensis on T lymphocyte subsets and hepatofibrosis in patients with chronic hepatitis B. Hunan Yi Ke Da Xue Xue Bao. 2000;25:248–50. [PubMed] [Google Scholar]
- 169.Hsu YC, Chiu YT, Cheng CC, Wu CF, Lin YL, Huang YT. Antifibrotic effects of tetrandrine on hepatic stellate cells and rats with liver fibrosis. J Gastroenterol Hepatol. 2007;22:99–111. doi: 10.1111/j.1440-1746.2006.04361.x. [DOI] [PubMed] [Google Scholar]


