Abstract
Background
This meta-analysis enabled us to obtain a precise estimation of the association between gene polymorphisms on chromosome 1 (MTHFR, AGT, F5, IL-10, LEPR) and the susceptibility to pre-eclampsia (PE) in order to reach a uniform conclusion.
Material/Methods
Web of Science, PubMed, EMBASE, Cochran Library (CENTRAL), and Chinese databases (Chinese National Knowledge Infrastructure-CNKI and Wan Fang) were electronically searched to select relevant studies for this meta-analysis. We selected 95 case-control studies investigating 5 genes (MTHFR, AGT, F5, IL-10, and LEPR) with 8 SNPs. Odds ratios (OR) with their 95% confidence intervals (CI) were used for estimating the association.
Results
A total of 16 646 PE patients and 28 901 normal-pregnancy patients were included in this meta-analysis. The overall results suggested that rs1801133 of MTHFR (OR=1.17, 95% CI: 1.05–1.13) and rs6025 of F5 (OR=1.53, 95%CI: 1.07–2.20) are significantly associated with PE, whereas rs1801131 of MTHFR, rs699 and rs4762 of AGT, rs1800896 and rs1800871 of IL-10, and rs1137101 of LEPR have no significant association with PE. Subgroup analysis by ethnicity revealed that, except for MTHFR rs1801133 and F5 rs6025 in Caucasians, which were significantly associated with an increased risk of PE, none of these SNPs were significantly associated with PE. As suggested by a symmetric funnel plot in conjunction with the Egger’s test, there was no significant publication bias in MTHFR rs1801133 (P=0.318) and rs1801131 (P=0.204), F5 rs6025 (P=0.511), LEPR rs1137101 (P=0.511), AGT rs4762 (P=0.215) and rs699 (P=0.482), IL-10 rs1800871 (P=0.955), and rs1800896 (P=0.144).
Conclusions
This meta-analysis provides evidence that MTHFR rs1801133 and F5 rs6025 are associated with an increased risk of PE, especially in Caucasians. However, we do not have sufficient evidence to conclude there is a significant association between other gene polymorphisms and PE.
MeSH Keywords: Meta-Analysis as Topic; Polymorphism, Genetic; Pre-Eclampsia
Background
Pre-eclampsia (PE) has a cluster of symptoms, including hypertension and albuminuria, which usually appear together after 20 weeks of pregnancy. PE has an incidence rate of 2–8% in pregnant females and it is associated with lesions of the vascular (high blood pressure) and renal (albuminuria) systems [1,2]. Furthermore, PE is thought to be associated with eclampsia, HELLP syndrome (characterized by hemolysis, up-regulated liver enzymes, and low levels of platelets), kidney failure, lung edema, and hemorrhagic stroke; therefore, it poses enormous threats to the health of mothers and infants [3]. Although the etiology of PE is still unclear, it is suspected that various genetic and environmental factors are associated with the susceptibility to PE [2,4]. It has been observed that females who were born from a mother with PE have an increased risk of PE during their own gestation period. In addition, females with fathers who were born of mothers with PE have an increased risk of PE. Therefore, numerous investigations have been undertaken to clarify the role of genetic factors with significant effects on the prevalence of PE [5]. As suggested by previous reports, genetic polymorphisms of interleukin (IL)-10, methylenetetrahydrofolate reductase (MTHFR), angiotensinogen (AGT), leptin receptor (LEPR), and factor V (FV) might explain the potential role of genetic factors that affect the development of PE [6–10].
There are 2 procedures which are critical to the maintenance of pregnancy: one is the inhibition of T-helper 1 (Th1) lymphocytes and the other is the stimulation of Th2 lymphocytes [11–13]. IL-10 is believed to be involved in the etiology of PE due to its role in the reduction of inflammation-mediated vascular dysfunction and the regulation of trophoblastic infiltration [6].
Hyperhomocysteinemia has been confirmed to be involved in the pathogenesis of PE [14,15]. Furthermore, a high level of serum homocysteine is likely to be followed by endothelial disorders such as coronary artery disease [16] and atherosclerosis [17]. As a key enzyme for homocysteine and folate metabolism, MTHFR transforms 5, 10-methylenetetrahydrofolate to 5-methyltetrahydrofolate, which is a methyl donor in the transformation from homocysteine to methionine, and this implies a relatively negative correlation between homocysteine and folate [7,18].
AGT is converted into angiotensin II by angiotensin-converting enzyme and is a major component of the renin-angiotensin system (RAS) [19]. The RAS is also a strong regulating system that greatly affects blood pressure and water-salt balance, indicating its critical role in the development of PE [20]. Additionally, leptin, which is well known to regulate body weight, tends to be an important cytokine for the regulation of arterial blood pressure [21]. Elevated circulating leptin levels, along with the reduced soluble LEPR concentrations, are reported to be associated with susceptibility to PE [22]. The activated FV (FVa) accompanied by activated factor X (FXa) converts prothrombin into thrombin, and a connection between hypercoagulability and PE has been proposed [4].
The IL-10 gene, MTHFR gene, AGT gene, LEPR gene, and FV gene map to 1q31-q32, 1p36.3, 1q42.2, 1p31, and 1q23, respectively. We have noticed that inconsistent conclusions still exist among meta-analyses investigating the IL-10 gene [6,23–25]. Few studies have examined the relationship between the LEPR gene and PE risk, and there is no published meta-analysis focusing on the association between the LEPR gene and susceptibility to PE. Therefore, this study was designed to assess the association between multiple genetic polymorphisms and PE susceptibility in order to address the issue of contradictory findings resulting from heterogeneity.
Material and Methods
Search strategy
Initially, a computer-based search of the online databases Web of Science, PubMed, EMBASE, Cochran Library (CENTRAL), and Chinese databases (Chinese National Knowledge Infrastructure-CNKI and Wan Fang) was conducted with no language constraint (up to September 2015) and the following searching terms were used: (“preeclampsia” OR “pre-eclampsia”) AND (“polymorphism” OR “single nucleotide polymorphism” OR “SNP” OR “variant”) AND (“factor V” OR “thrombophilia” OR “MTHFR” OR “methylenetetrahydrofolate reductase” OR “homocysteine” OR “interleukin-10” OR “IL-10” OR “angiotensinogen” OR “AGT” OR “leptin receptor” OR “LEPR”). References in the included articles containing meta-analyses were manually searched to identify additional related papers.
Inclusion criteria
Case-control studies investigating the association between genetic polymorphisms on chromosome 1 and PE susceptibility were considered. Females with new-onset high blood pressure (>140/90 mm Hg) and albuminuria (≥300 mg/24 h) at or after 20 weeks of pregnancy were diagnosed as having PE [26]. Females with a previous twin pregnancy and history of hormone therapy were excluded. Studies with genotype frequencies or adequate original data in the case and control groups were included, and genotyping was carried out using recognized methods. All included studies were done in humans. Publications with duplicate data were selected based on the sample size. Meta-analyses, editorials, or other articles irrelevant to the research subjects were excluded.
Quality assessment
The methodological quality of the eligible studies was assessed by 2 independent researchers using the Newcastle-Ottawa Quality Assessment Scale (NOS) and any discrepancies between them were solved by discussion. The score system of NOS was based on 3 perspectives: selection, comparability, and exposure. A score of 6 or more out of 8 stars represents good quality [27].
Data extraction
Relevant information was independently obtained from all included studies by 2 researchers and any inconsistencies between them were reviewed by a third researcher. The following information was selected: name of first author, publication date, country and ethnicity, method for genotyping, and the number of cases and controls for each genotype.
Statistical analysis
The chi-squared goodness-of-fit test was used to assess whether the observed genotype frequency in the control group complied with Hardy-Weinberg equilibrium (HWE), and a P value of less than 0.05 suggests significant deviation from HWE. If the genotype distribution did not comply with HWE, then the corresponding study was eliminated. The strength of association between genetic polymorphisms on chromosome 1 and PE susceptibility was measured by odds ratios (ORs) along with their corresponding 95% confidence intervals (CIs). Moreover, the pooled ORs were calculated under the allele model, and the Z-test with a significance level of 0.05 was used to determine whether a significant association existed between each SNP and PE susceptibility. Study heterogeneity was measured by using the I2 statistic and Cochran’s Q [28,29]. The random-effects model was used to summarize the ORs if I2 >50% and P value <0.1 [30]; whereas the fixed-effects model was used if there was no significant heterogeneity [28]. Potential publication bias was evaluated by a funnel plot, with a significance level of 0.05 [31]. The above analyses were all performed using R (version 3.2.1) statistical software.
Results
Literature selection
A total of 386 articles were discovered after the initial search strategy performed in Web of Science, PubMed, EMBASE, Cochran Library (CENTRAL), and Chinese databases (Chinese National Knowledge Infrastructure-CNKI and Wan Fang). By reviewing the abstracts and titles, 218 articles were excluded as not relevant to the research subject. After the exclusion of meta-analyses, reviews, studies without sufficient data, and studies with duplicate data, 95 articles with 150 eligible studies were selected (Figure 1). The methodological quality of these studies was systematically evaluated by 2 independent reviewers in accordance with the Newcastle-Ottawa Quality Assessment Scale (NOS). The NOS involves 3 main areas: study selection, comparability, and exposure. Each study was scored with answers to 8 questions, with a maximum score of 8. A total of 16 646 PE patients and 28 901 healthy mothers were included in the meta-analysis. The characteristics of the 150 included studies are summarized in Supplementary Figures 1, 2: 28 case-control studies involving F5 polymorphisms (rs6025); 23 case-control studies involving AGT polymorphisms (rs699, rs4762); 58 case-control studies involving MTHFR polymorphisms (rs1801131, rs1801133); 13 studies involving IL-10 polymorphisms (rs1800896, rs1800971); and 4 studies involving LEPR polymorphism (rs1137101).
Figure 1.
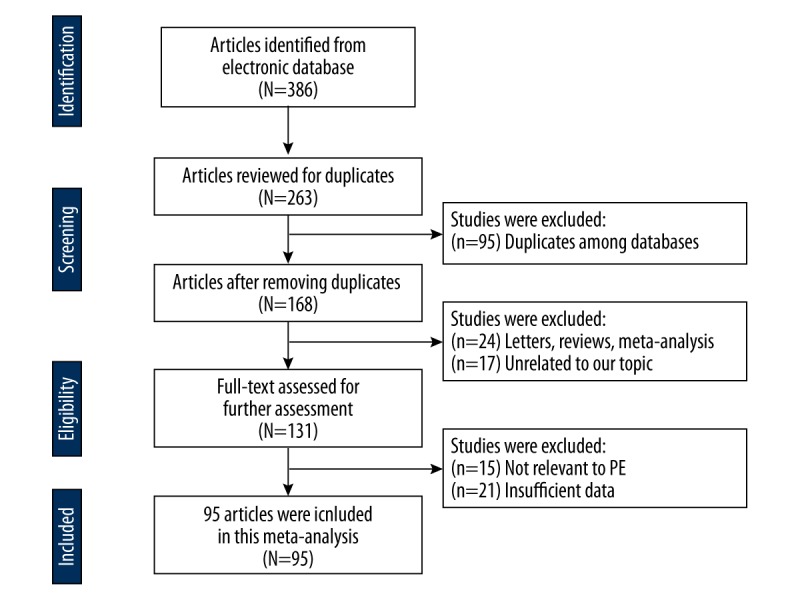
Literature selection flow chart.
Association between chromosome 1 polymorphisms and PE
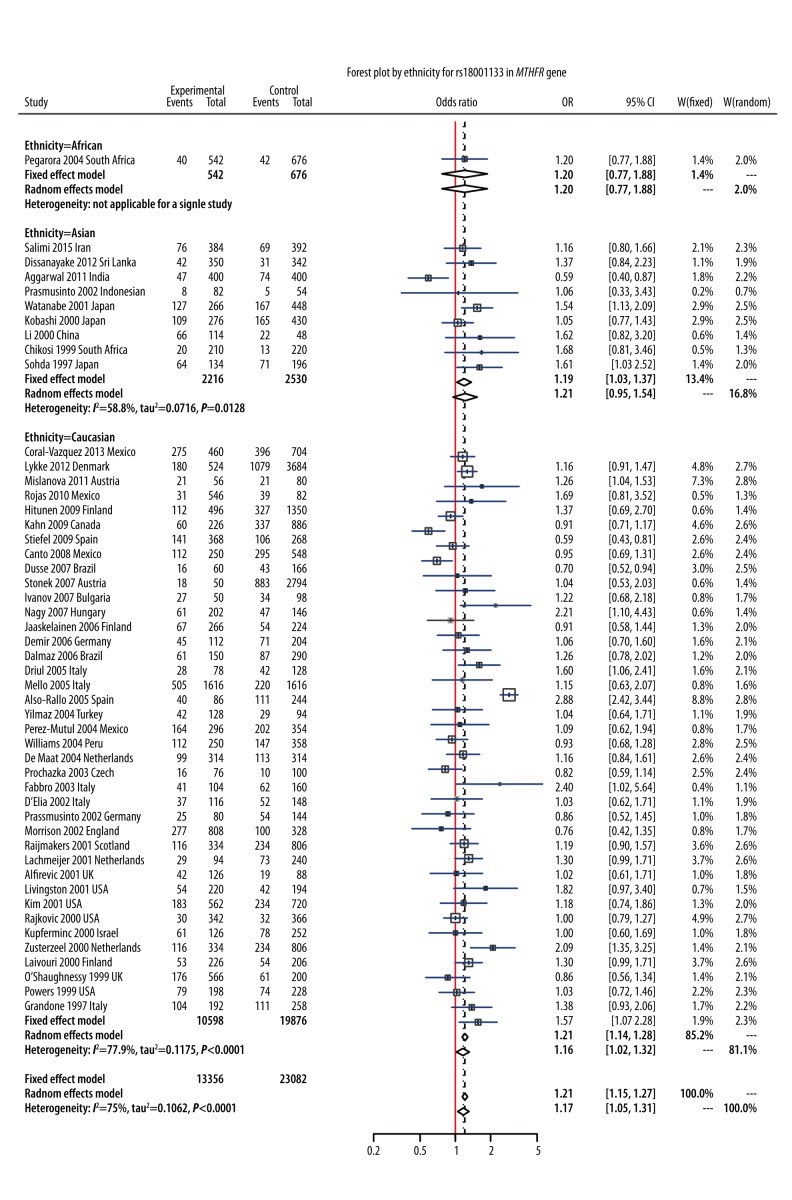
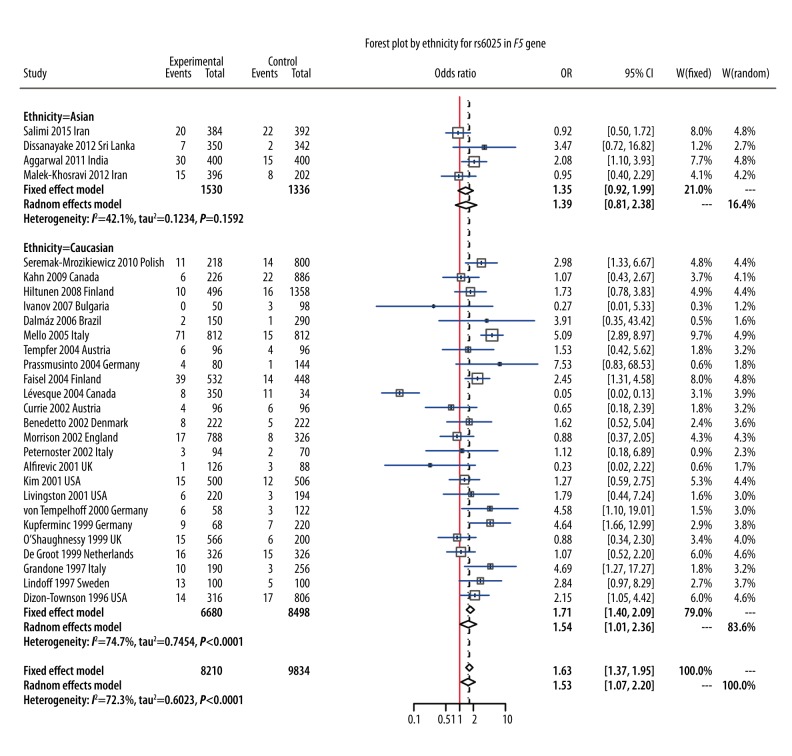
In this meta-analysis, we identified 8 polymorphisms on chromosome 1 that may be associated with PE susceptibility (Table 1). The random-effects model was used to synthesize evidence for studies involving rs6025, rs699, rs1801131, rs1801133, rs1800896, rs1800871, and rs1137101, whereas the fixed-effects model was used for rs4762 (I2=26.65%, P=0.266) (Table 1). Results of the meta-analysis showed that polymorphism of rs1801133 (6 678 cases/11 756 controls) was significantly associated with a 17% increase in risk of PE under the allelic model (OR=1.17, 95%CI: 1.05–1.13, P=0.002, Figure 2). Subgroup analysis by ethnicity revealed that this association was significant in Caucasians under the allelic model (OR=1.16, 95%CI: 1.02–1.32, Figure 2), but this association under the allelic model was not significant in Asians (OR=1.21, 95%CI: 0.95–1.54) or Africans (OR=1.20, 95%CI: 0.77–1.88, Figure 2). For rs6025 (4105 cases/4917 controls), the pooled result demonstrated that rs6025 was also significantly associated with a 53% increase in the risk of PE under the allelic model (OR=1.53, 95%CI: 1.07–2.20, P=0.024, Figure 3). Subgroup analysis by ethnicity suggested that this association was significant in Caucasians (OR = 1.54, 95% CI: 1.01–2.36, Figure 3) but not in Asians (OR=1.35, 95%CI: 0.92–1.99, Figure 3).
Table 1.
Meta analysis of eight polymorphisms and PE susceptibility.
| Gene | SNP | Genetic model | OR [95% CI] | P odds ratio | Tau2 | I2 | P heterogeneity | Ethnicity | P publication bias | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Caucasians | Asian | African | |||||||||
| F5 | rs6025 | A vs. G | 1.53 [1.07,2.20] | 0.024 | 0.659 | 74.02% | 0.000 | 1.54 [1.01,2.36] | 1.35 [0.92,1.99] | – | 0.511 |
| AGT | rs699 | G vs. A | 1.12 [0.92,1.35] | 0.266 | 0.129 | 71.97% | 0.000 | 1.19 [0.90,1.56] | 0.99 [0.73,1.33] | 1.14 [0.72,1.81] | 0.482 |
| rs4762 | T vs. C | 0.93 [0.68,1.28] | 0.673 | 0.029 | 26.65% | 0.266 | – | 0.93 [0.50,1.72] | 1.07 [0.62.1.84] | 0.215 | |
| MTHFR | rs1801131 | T vs. C | 1.14 [0.93,1.40] | 0.196 | 0.054 | 59.61% | 0.012 | 1.17 [0.86,1.59] | 1.25 [0.98,2.18] | 0.91 [0.64,1.29] | 0.204 |
| rs1801133 | C vs. A | 1.17 [1.05,1.31] | 0.002 | 0.073 | 67.26% | 0.000 | 1.16 [1.02,1.32] | 1.21 [0.95,1.54] | 1.20 [0.77,1.88] | 0.318 | |
| IL-10 | rs1800896 | G vs. A | 0.91 [0.75,1.11] | 0.366 | 0.063 | 72.77% | 0.000 | 0.99 [0.90,1.10] | 0.98 [0.45,2.14] | 0.20 [0.07,0.58] | 0.144 |
| rs1800871 | T vs. C | 0.79 [0.58,1.07] | 0.655 | 0.166 | 82.99% | 0.000 | 0.94 [0.69,1.27] | 0.65 [0.41,1.03] | – | 0.694 | |
| LEPR | rs1137101 | G vs. A | 1.41 [0.93,2.12] | 0.114 | 0.126 | 69.59% | 0.024 | 1.44 [1.05,1.98] | 1.28 [0.46,3.55] | – | 0.486 |
Pooled odds ratios and 95% confidence intervals.
Figure 2.
Forest plot by ethnicity for rs18001133 in MTHFR gene.
Figure 3.
Forest plot by ethnicity for rs6025 in F5 gene.
As shown in Supplementary Figures 1–6, none of the polymorphisms of rs699 (1852 cases/4431 controls), rs4762 (395 cases/1246 controls), rs1800896 (1510 cases/3393 controls), rs1800871 (489 cases/1087 controls), rs1137101 (149 cases/499 controls), or rs1801131 (1390 cases/1818 controls) were significantly associated with PE susceptibility under the allelic model (all P values >0.05). Results of subgroup analysis by ethnicity were consistent with those of the overall analysis.
Publication bias
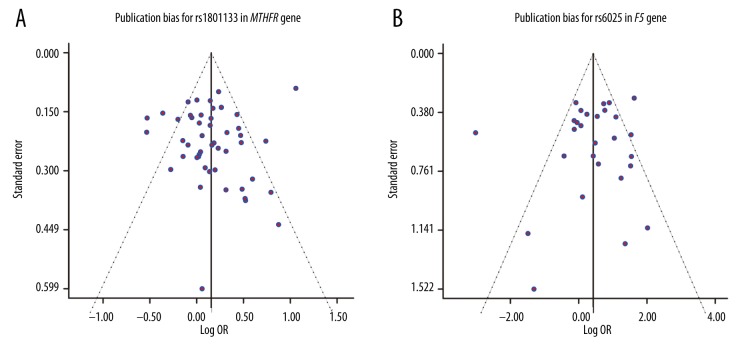
Potential publication bias of the included studies was assessed by the funnel plot, which revealed no significant publication bias in 8 SNPs under the allelic model (all P values >0.05): rs1801133, P=0.318 (Figure 4A); rs6025, P=0.511 (Figure 4B); rs1801131, P=0.204; rs1137101, P=0.511; rs4762, P=0.215; rs699, P=0.482; rs1800871, P=0.955; and rs1800896, P=0.144 (Supplementary Figure 7A–7F).
Figure 4.
Publication bias for rs1801133 and rs6025.
Discussion
Pre-eclampsia (PE) is a complex disease with great phenotypic diversity. It is a serious threat to the health of females during gestation. Although the pathogenesis of preeclampsia is extremely complex, previous studies showed that thrombophilia genes are associated with hypercoagulable state [32,33], which may partly explain the development of PE. In recent years, great attention has been paid to the role of SNPs in disease pathology; most of the published studies focused on the association between several SNPs and the susceptibility to PE, including AGT [34,35], ACE [36–39], F5 [4,40–44], MTHFR [7,18,45–49], and TNF-alpha [23,24,50,51]. The present study enabled us not only to explore the relationship between gene polymorphisms on chromosome 1 (MTHFR, AGT, F5, IL-10, LEPR) and susceptibility to PE, but also to assess potential heterogeneity among studies.
The methylenetetrahydrofolate reductase (MTHFR) gene encodes the enzyme 50, 100-methylenetetrahydrofolate reductase, located on chromosomal region 1p36.3. Many studies have indicated an association between MTHFR SNPs and risk of PE. However, the results were inconclusive due to significant heterogeneity resulting from differences in study population, ethnicity, and genotypes. Our meta-analysis provides evidence that MTHFR rs1801133 is significantly associated with increased risk of PE in Caucasians under the allelic model, but this association was not significant in Asians or Africans. In contrast, a study by Wu et al. concluded that there was a significant association between SNP of rs1801133 and PE susceptibility in Asians [7]. This inconsistency may be explained by 2 factors. Firstly, the current meta-analysis had a smaller sample size for Asians, which may have restricted its ability to detect any significant association. Secondly, only the allelic genetic model was incorporated in the meta-analysis, which may lead to a biased estimation of the association. Therefore, it is necessary to design studies with large sample sizes, particularly for Asians and Africans. For rs1801131, the overall analysis and the subgroup analysis did not provide sufficient evidence to suggest a significant association. As of September 2015, 3 other meta-analyses had suggested that there is no significant association between rs1801131 and PE [7,18,49]. Compared to the large number of cases and controls for rs1801133, we only have 1390 cases/1818 controls for rs1801131, which may significantly reduce the statistical power of a meta-analysis to detect a significant association. Therefore, further studies with large sample sizes should be carried out to increase the credibility of this conclusion. Overall, we concluded that MTHFR rs1801133 might be an effective marker for use in PE diagnosis.
F5 is considered to be a potential genetic factor for PE because it encodes an essential cofactor of the blood coagulation cascade. F5 has also been widely studied due to its common thrombophilic mutation [52,53]. Although the overall results suggested a significant association between polymorphism of F5 (rs6025) and PE, other studies have not provided sufficient evidence to conclude there is a significant association [53]. Our meta-analysis indicates that F5 rs6025 is associated with an increased risk of PE, which is contrary to the results of some other studies. This may be explained by the typical publication bias and time-lag bias inherent in smaller studies [54,55]. In addition, these smaller studies may also have failed to comply with strict standards. For example, some terms must be carefully defined, including paternity [56] and gravidity [57], because they may interact with genetic factors to affect PE susceptibility. Moreover, results from subgroup analysis may not be applicable to other ethnicities such as Africans because the included study did not involve African subjects. Thus, studies that incorporate different ethnicities, particularly Africans, should be designed to confirm the association between F5 (rs6025) and the susceptibility to PE.
AGT is a key effector in regulating the blood pressure, and the level of AGT among hypertensive patients is related to the SNPs of the AGT gene [58]. The expression of AGT rs699 was elevated in decidual spiral arteries, which play a vital role in developing several events that may trigger PE [59,60]. Lin et al. indicated that AGT rs699 was significantly associated with PE, whereas there was no significant association between AGT rs4762 and PE [8]. Similar results were also found in studies conducted by Ni et al. and Zhu et al. [35,39]. On the other hand, the present meta-analysis discovered that there was no significant association between AGT polymorphism (rs699, rs4762) and PE. This inconsistency may be attributable to the limited number of studies investigating AGT polymorphisms. Although ethnicity has been taken into consideration to explain the potential source of heterogeneity, the lack of information on gene-environment interaction or the interaction between several genes could have a substantial effect on the overall conclusion.
Interleukin 10 (IL-10) is located on the chromosome of 1q21-32 and it is an immune-regulatory cytokine associated with different biological functions [61]. Furthermore, IL-10 exerts regulatory effects on the balance of Th1/ Th2 and it is a crucial cytokine for females during gestation [13,62]. This study enabled us to investigate the association between IL-10 polymorphisms (rs1800896, rs1800871) and PE. The results suggest that rs1800896 or rs1800871 is not significantly associated with PE susceptibility. Results from subgroup analysis by ethnicity were consistent with those from the overall analysis. However, we should interpret these results with great caution since all of the included studies came from different regions, which may be considered as a confounding factor that influences the conclusion. Apart from that, other confounding factors, including gestational age at the sample collection time, body mass index, and assay sensitivity, should be taken into account in order to further investigate the association between IL-10 polymorphisms and PE susceptibility.
It has been suggested that the expression of leptin receptor (LEPR) significantly increases when inflammation occurs and that it plays an important role in immune responses [63]. Immune response is a factor for PE [63,64] and mutations in this gene have been shown to be associated with PE. For instance, a study by Fong et al. revealed that LEPR rs1137101 was significantly associated with PE, which is contrary to the results obtained from our meta-analysis. This discrepancy may also come from our small sample size, which significantly reduces the statistical power of the present meta-analysis to detect any significant association.
Conclusions
We conclude that rs1801133 of MTHFR and rs6025 of F5 are significantly associated with PE, whereas rs1801131 of MTHFR, rs699 and rs4762 of AGT, rs1800896 and rs1800871 of IL-10, and rs1137101 of LEPR have no significant association with PE. Studies with large sample sizes adjusting for various confounding factors should be designed to confirm the above conclusion. Nevertheless, our meta-analysis provides some evidence to help explain the mechanism of susceptibility to PE, which can be critical to the health of females during gestation.
Supplementary materials
Forest plot by ethnicity for rs699 in AGT gene.
Forest plot by ethnicity for rs4762 in AGT gene.
Forest plot by ethnicity for rs1800896 in IL-10 gene.
Forest plot by ethnicity for rs1800871 in IL-10 gene.
Forest plot by ethnicity for rs1137101 in LEPR gene.
Forest plot by ethnicity for rs18001131 in MTHFR gene.
Publication bias of non-significant SNPs.
Acknowledgements
We acknowledge all authors whose publications were included in our meta-analysis.
Footnotes
Disclosure of conflict of interest
None.
Source of support: Departmental sources
References
- 1.Myatt L, Roberts JM. Preeclampsia: Syndrome or disease? Curr Hypertens Rep. 2015;17:83. doi: 10.1007/s11906-015-0595-4. [DOI] [PubMed] [Google Scholar]
- 2.Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010;376:631–44. doi: 10.1016/S0140-6736(10)60279-6. [DOI] [PubMed] [Google Scholar]
- 3.Amaral LM, Cunningham MW, Jr, Cornelius DC, LaMarca B. Preeclampsia: Long-term consequences for vascular health. Vasc Health Risk Manag. 2015;11:403–15. doi: 10.2147/VHRM.S64798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang X, Bai T, Liu S, Pan H, Wang B. Association between thrombophilia gene polymorphisms and preeclampsia: A meta-analysis. PLoS One. 2014;9:e100789. doi: 10.1371/journal.pone.0100789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Esplin MS, Fausett MB, Fraser A, et al. Paternal and maternal components of the predisposition to preeclampsia. N Engl J Med. 2001;344:867–72. doi: 10.1056/NEJM200103223441201. [DOI] [PubMed] [Google Scholar]
- 6.Lee YH, Kim JH, Song GG. Meta-analysis of associations between interleukin-10 polymorphisms and susceptibility to pre-eclampsia. Eur J Obstet Gynecol Reprod Biol. 2014;182:202–7. doi: 10.1016/j.ejogrb.2014.09.030. [DOI] [PubMed] [Google Scholar]
- 7.Wu X, Yang K, Tang X, et al. Folate metabolism gene polymorphisms MTHFR C677T and A1298C and risk for preeclampsia: a meta-analysis. J Assist Reprod Genet. 2015;32:797–805. doi: 10.1007/s10815-014-0408-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin R, Lei Y, Yuan Z, Ju H, Li D. Angiotensinogen gene M235T and T174M polymorphisms and susceptibility of pre-eclampsia: A meta-analysis. Ann Hum Genet. 2012;76:377–86. doi: 10.1111/j.1469-1809.2012.00722.x. [DOI] [PubMed] [Google Scholar]
- 9.Fong FM, Sahemey MK, Hamedi G, et al. Maternal genotype and severe preeclampsia: A HuGE review. Am J Epidemiol. 2014;180:335–45. doi: 10.1093/aje/kwu151. [DOI] [PubMed] [Google Scholar]
- 10.Rodger MA, Betancourt MT, Clark P, et al. The association of factor V leiden and prothrombin gene mutation and placenta-mediated pregnancy complications: A systematic review and meta-analysis of prospective cohort studies. PLoS Med. 2010;7:e1000292. doi: 10.1371/journal.pmed.1000292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xue H, Lin B, An J, Zhu Y, Huang G. Interleukin-10-819 promoter polymorphism in association with gastric cancer risk. BMC Cancer. 2012;12:102. doi: 10.1186/1471-2407-12-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denney JM, Nelson EL, Wadhwa PD, et al. Longitudinal modulation of immune system cytokine profile during pregnancy. Cytokine. 2011;53:170–77. doi: 10.1016/j.cyto.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thaxton JE, Sharma S. Interleukin-10: a multi-faceted agent of pregnancy. Am J Reprod Immunol. 2010;63:482–91. doi: 10.1111/j.1600-0897.2010.00810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fayed MR, Youssef M, Odah MM. Hyperhomocysteinemia is A risk marker for development of maternal pre-eclampsia. Boll Chim Farm. 2004;143:281–87. [PubMed] [Google Scholar]
- 15.Kim MW, Hong SC, Choi JS, et al. Homocysteine, folate and pregnancy outcomes. J Obstet Gynaecol. 2012;32:520–24. doi: 10.3109/01443615.2012.693984. [DOI] [PubMed] [Google Scholar]
- 16.Schaffer A, Verdoia M, Cassetti E, et al. Relationship between homocysteine and coronary artery disease. Results from a large prospective cohort study. Thromb Res. 2014;134:288–93. doi: 10.1016/j.thromres.2014.05.025. [DOI] [PubMed] [Google Scholar]
- 17.McCully KS. Homocysteine and the pathogenesis of atherosclerosis. Expert Rev Clin Pharmacol. 2015;8:211–19. doi: 10.1586/17512433.2015.1010516. [DOI] [PubMed] [Google Scholar]
- 18.Li X, Luo YL, Zhang QH, et al. Methylenetetrahydrofolate reductase gene C677T, A1298C polymorphisms and pre-eclampsia risk: A meta-analysis. Mol Biol Rep. 2014;41:5435–48. doi: 10.1007/s11033-014-3415-z. [DOI] [PubMed] [Google Scholar]
- 19.Shah DM. The role of RAS in the pathogenesis of preeclampsia. Curr Hypertens Rep. 2006;8:144–52. doi: 10.1007/s11906-006-0011-1. [DOI] [PubMed] [Google Scholar]
- 20.Afshariani R, Roozbeh J, Sharifian M, et al. Association between angiotensinogen M235T polymorphism and preeclampsia in Iranian pregnant women. J Family Reprod Health. 2014;8:169–73. [PMC free article] [PubMed] [Google Scholar]
- 21.Tsuda K. Leptin and nitric oxide in blood pressure regulation in humans. Am J Hypertens. 2014;27:1428. doi: 10.1093/ajh/hpu156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muy-Rivera M, Ning Y, Frederic IO, et al. Leptin, soluble leptin receptor and leptin gene polymorphism in relation to preeclampsia risk. Physiol Res. 2005;54:167–74. [PubMed] [Google Scholar]
- 23.Daher S, Sass N, Oliveira LG, Mattar R. Cytokine genotyping in preeclampsia. Am J Reprod Immunol. 2006;55:130–35. doi: 10.1111/j.1600-0897.2005.00341.x. [DOI] [PubMed] [Google Scholar]
- 24.Xie C, Yao MZ, Liu JB, Xiong LK. A meta-analysis of tumor necrosis factor-alpha, interleukin-6, and interleukin-10 in preeclampsia. Cytokine. 2011;56:550–59. doi: 10.1016/j.cyto.2011.09.021. [DOI] [PubMed] [Google Scholar]
- 25.Yang W, Zhu Z, Wang J, et al. Evaluation of association of maternal IL-10 polymorphisms with risk of preeclampsia by A meta-analysis. J Cell Mol Med. 2014;18:2466–77. doi: 10.1111/jcmm.12434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.LeFevre ML Force USPST. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161:819–26. doi: 10.7326/M14-1884. [DOI] [PubMed] [Google Scholar]
- 27.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 28.Ioannidis JP, Patsopoulos NA, Evangelou E. Uncertainty in heterogeneity estimates in meta-analyses. BMJ. 2007;335:914–16. doi: 10.1136/bmj.39343.408449.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 30.DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28:105–14. doi: 10.1016/j.cct.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 31.Field AP, Gillett R. How to do a meta-analysis. Br J Math Stat Psychol. 2010;63:665–94. doi: 10.1348/000711010X502733. [DOI] [PubMed] [Google Scholar]
- 32.Dahlback B. Inherited resistance to activated protein C, a major cause of venous thrombosis, is due to a mutation in the factor V gene. Haemostasis. 1994;24:139–51. doi: 10.1159/000217094. [DOI] [PubMed] [Google Scholar]
- 33.Poort SR, Rosendaal FR, Reitsma PH, Bertina RM. A common genetic variation in the 3′-untranslated region of the prothrombin gene is associated with elevated plasma prothrombin levels and an increase in venous thrombosis. Blood. 1996;88:3698–703. [PubMed] [Google Scholar]
- 34.Zhao L, Dewan AT, Bracken MB. Association of maternal AGTR1 polymorphisms and preeclampsia: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2012;25:2676–80. doi: 10.3109/14767058.2012.708370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ni S, Zhang Y, Deng Y, et al. AGT M235T polymorphism contributes to risk of preeclampsia: evidence from a meta-analysis. J Renin Angiotensin Aldosterone Syst. 2012;13:379–86. doi: 10.1177/1470320312440903. [DOI] [PubMed] [Google Scholar]
- 36.Serrano NC, Diaz LA, Paez MC, et al. Angiotensin-converting enzyme I/D polymorphism and preeclampsia risk: evidence of small-study bias. PLoS Med. 2006;3:e520. doi: 10.1371/journal.pmed.0030520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buurma A, Turner R, Driessen A, et al. OS050. Genetic variants in pre-eclampsia: A meta-analysis. Pregnancy Hypertens. 2012;2:204. doi: 10.1016/j.preghy.2012.04.051. [DOI] [PubMed] [Google Scholar]
- 38.Buurma AJ, Turner RJ, Driessen JH, et al. Genetic variants in pre-eclampsia: A meta-analysis. Hum Reprod Update. 2013;19:289–303. doi: 10.1093/humupd/dms060. [DOI] [PubMed] [Google Scholar]
- 39.Zhu M, Zhang J, Nie S, Yan W. Associations of ACE I/D, AGT M235T gene polymorphisms with pregnancy induced hypertension in Chinese population: A meta-analysis. J Assist Reprod Genet. 2012;29:921–32. doi: 10.1007/s10815-012-9800-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodger MA, Walker MC, Smith GN, et al. Is thrombophilia associated with placenta-mediated pregnancy complications? A prospective cohort study. J Thromb Haemost. 2014;12:469–78. doi: 10.1111/jth.12509. [DOI] [PubMed] [Google Scholar]
- 41.Lin J, August P. Genetic thrombophilias and preeclampsia: A meta-analysis. Obstet Gynecol. 2005;105:182–92. doi: 10.1097/01.AOG.0000146250.85561.e9. [DOI] [PubMed] [Google Scholar]
- 42.Kosmas IP, Tatsioni A, Ioannidis JP. Association of Leiden mutation in factor V gene with hypertension in pregnancy and pre-eclampsia: A meta-analysis. J Hypertens. 2003;21:1221–28. doi: 10.1097/00004872-200307000-00002. [DOI] [PubMed] [Google Scholar]
- 43.Dudding TE, Attia J. The association between adverse pregnancy outcomes and maternal factor V Leiden genotype: A meta-analysis. Thromb Haemost. 2004;91:700–11. doi: 10.1160/TH03-10-0637. [DOI] [PubMed] [Google Scholar]
- 44.Staines-Urias E, Paez MC, Doyle P, et al. Genetic association studies in pre-eclampsia: Systematic meta-analyses and field synopsis. Int J Epidemiol. 2012;41:1764–75. doi: 10.1093/ije/dys162. [DOI] [PubMed] [Google Scholar]
- 45.Kosmas IP, Tatsioni A, Ioannidis JP. Association of C677T polymorphism in the methylenetetrahydrofolate reductase gene with hypertension in pregnancy and pre-eclampsia: A meta-analysis. J Hypertens. 2004;22:1655–62. doi: 10.1097/00004872-200409000-00004. [DOI] [PubMed] [Google Scholar]
- 46.Xia XP, Chang WW, Cao YX. Meta-analysis of the methylenetetrahydrofolate reductase C677T polymorphism and susceptibility to pre-eclampsia. Hypertens Res. 2012;35:1129–34. doi: 10.1038/hr.2012.117. [DOI] [PubMed] [Google Scholar]
- 47.Wang XM, Wu HY, Qiu XJ. Methylenetetrahydrofolate reductase (MTHFR) gene C677T polymorphism and risk of preeclampsia: an updated meta-analysis based on 51 studies. Arch Med Res. 2013;44:159–68. doi: 10.1016/j.arcmed.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 48.Li X, Luo Y, Chen Q. The association between methylenetetrahydrofolate reductase C677T polymorphism and pre-eclampsia risk: Appraisal of a recent meta-analysis. Hypertens Res. 2013;36:467–68. doi: 10.1038/hr.2012.201. [DOI] [PubMed] [Google Scholar]
- 49.Zusterzeel PL, Visser W, Blom HJ, et al. Methylenetetrahydrofolate reductase polymorphisms in preeclampsia and the HELLP syndrome. Hypertens Pregnancy. 2000;19:299–307. doi: 10.1081/prg-100101991. [DOI] [PubMed] [Google Scholar]
- 50.Molvarec A, Jermendy A, Nagy B, et al. Association between tumor necrosis factor (TNF)-alpha G-308A gene polymorphism and preeclampsia complicated by severe fetal growth restriction. Clin Chim Acta. 2008;392:52–57. doi: 10.1016/j.cca.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 51.Bombell S, McGuire W. Tumour necrosis factor (-308A) polymorphism in pre-eclampsia: meta-analysis of 16 case-control studies. Aust N Z J Obstet Gynaecol. 2008;48:547–51. doi: 10.1111/j.1479-828X.2008.00924.x. [DOI] [PubMed] [Google Scholar]
- 52.Rajkovic A, Mahomed K, Rozen R, et al. Methylenetetrahydrofolate reductase 677 C --> T polymorphism, plasma folate, vitamin B(12) concentrations, and risk of preeclampsia among black African women from Zimbabwe. Mol Genet Metab. 2000;69:33–39. doi: 10.1006/mgme.1999.2952. [DOI] [PubMed] [Google Scholar]
- 53.Alfirevic Z, Mousa HA, Martlew V, et al. Postnatal screening for thrombophilia in women with severe pregnancy complications. Obstet Gynecol. 2001;97:753–59. doi: 10.1016/s0029-7844(01)01190-5. [DOI] [PubMed] [Google Scholar]
- 54.Kaiser T, Brennecke SP, Moses EK. Methylenetetrahydrofolate reductase polymorphisms are not a risk factor for pre-eclampsia/eclampsia in Australian women. Gynecol Obstet Invest. 2000;50:100–2. doi: 10.1159/000010291. [DOI] [PubMed] [Google Scholar]
- 55.Kobashi G, Yamada H, Asano T, et al. Absence of association between a common mutation in the methylenetetrahydrofolate reductase gene and preeclampsia in Japanese women. Am J Med Genet. 2000;93:122–25. doi: 10.1002/1096-8628(20000717)93:2<122::aid-ajmg8>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 56.Martinelli P, Grandone E, Colaizzo D, et al. Familial thrombophilia and the occurrence of fetal growth restriction. Haematologica. 2001;86:428–31. [PubMed] [Google Scholar]
- 57.Prochazka M, Krcova V, Kudela M, Slavik L. [Occurrence of gene mutations in factor V Leiden, prothrombin and methylenetetrahydrofolate reductase in patients with pre-eclampsia]. Ceska Gynekol. 2003;68:162–66. [in Czech] [PubMed] [Google Scholar]
- 58.Jeunemaitre X, Soubrier F, Kotelevtsev YV, et al. Molecular basis of human hypertension: Role of angiotensinogen. Cell. 1992;71:169–80. doi: 10.1016/0092-8674(92)90275-h. [DOI] [PubMed] [Google Scholar]
- 59.Morgan T, Craven C, Lalouel JM, Ward K. Angiotensinogen Thr235 variant is associated with abnormal physiologic change of the uterine spiral arteries in first-trimester decidua. Am J Obstet Gynecol. 1999;180:95–102. doi: 10.1016/s0002-9378(99)70156-0. [DOI] [PubMed] [Google Scholar]
- 60.Morgan T, Craven C, Nelson L, et al. Angiotensinogen T235 expression is elevated in decidual spiral arteries. J Clin Invest. 1997;100:1406–15. doi: 10.1172/JCI119661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hsu HJ, Yang YH, Shieh TY, et al. TGF-beta1 and IL-10 single nucleotide polymorphisms as risk factors for oral cancer in Taiwanese. Kaohsiung J Med Sci. 2015;31:123–29. doi: 10.1016/j.kjms.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 62.Brogin Moreli J, Cirino Ruocco AM, Vernini JM, et al. Interleukin 10 and tumor necrosis factor-alpha in pregnancy: aspects of interest in clinical obstetrics. ISRN Obstet Gynecol. 2012;2012:230742. doi: 10.5402/2012/230742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Poston L. Leptin and preeclampsia. Semin Reprod Med. 2002;20:131–38. doi: 10.1055/s-2002-32504. [DOI] [PubMed] [Google Scholar]
- 64.Molvarec A, Szarka A, Walentin S, et al. Serum leptin levels in relation to circulating cytokines, chemokines, adhesion molecules and angiogenic factors in normal pregnancy and preeclampsia. Reprod Biol Endocrinol. 2011;9:124. doi: 10.1186/1477-7827-9-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Forest plot by ethnicity for rs699 in AGT gene.
Forest plot by ethnicity for rs4762 in AGT gene.
Forest plot by ethnicity for rs1800896 in IL-10 gene.
Forest plot by ethnicity for rs1800871 in IL-10 gene.
Forest plot by ethnicity for rs1137101 in LEPR gene.
Forest plot by ethnicity for rs18001131 in MTHFR gene.
Publication bias of non-significant SNPs.