Abstract
Cases of foodborne disease caused by Salmonella are frequently associated with the consumption of minimally processed produce. Bacterial cell surface components are known to be important for the attachment of bacterial pathogens to fresh produce. The role of these extracellular structures in Salmonella attachment to plant cell walls has not been investigated in detail. We investigated the role of flagella, fimbriae and cellulose on the attachment of Salmonella Typhimurium ATCC 14028 and a range of isogenic deletion mutants (ΔfliC fljB, ΔbcsA, ΔcsgA, ΔcsgA bcsA and ΔcsgD) to bacterial cellulose (BC)-based plant cell wall models [BC-Pectin (BCP), BC-Xyloglucan (BCX) and BC-Pectin-Xyloglucan (BCPX)] after growth at different temperatures (28°C and 37°C). We found that all three cell surface components were produced at 28°C but only the flagella was produced at 37°C. Flagella appeared to be most important for attachment (reduction of up to 1.5 log CFU/cm2) although both cellulose and fimbriae also aided in attachment. The csgD deletion mutant, which lacks both cellulose and fimbriae, showed significantly higher attachment as compared to wild type cells at 37°C. This may be due to the increased expression of flagella-related genes which are also indirectly regulated by the csgD gene. Our study suggests that bacterial attachment to plant cell walls is a complex process involving many factors. Although flagella, cellulose and fimbriae all aid in attachment, these structures are not the only mechanism as no strain was completely defective in its attachment.
Introduction
Over the past few decades there has been a fast growing and world-wide trend of greater consumption of fresh produce, such as fruits and vegetables, mainly due to a heightened consumer awareness of the benefits of a healthy diet [1,2]. Governments around the world have also encouraged the consumption of fresh produce in an attempt to proactively prevent various diseases such as heart disease, strokes, eye diseases and stomach cancers [3]. The prevalence of foodborne illness associated with consumption of minimally processed produce has, however, also been increasing rapidly [4,5]. Between 1996 and 2005, the consumption of leafy greens in the United States increased by 9% but the incidence of foodborne outbreaks associated with it increased by 39% [6]. Fresh produce is now recognized as the main cause of foodborne outbreaks around the world [7].
It was initially thought that enteric pathogens, which are usually found in the intestinal tracts of animals, would survive poorly on plant surfaces where microorganisms encounter harsh environmental conditions such as drastic temperature fluctuations, desiccation, sunlight and nutrient limitation but recent research [8–11] has shown otherwise. Salmonella in particular was previously largely only reported to be associated with foods of animal origin but is now the most commonly identified human bacterial pathogen associated with fresh produce [12,13].
Human foodborne pathogens need to establish themselves on surfaces, including plants, as a precursor to causing foodborne disease and therefore bacterial attachment is a crucial step in their transmission [14,15]. Cut surfaces of plant cell walls (PCWs) are especially vulnerable to the attachment of human foodborne bacterial pathogens as these surfaces lack the waxy cuticle which repels water that could carry pathogens [16,17]. These cut surfaces also exude nutrients and water which are favourable for the growth and survival of the pathogens. Some human pathogens are able to penetrate the internal tissues after attaching to cut plant surfaces which could protect them from chemical sanitizers [18]. Saggers et al. [19] suggested that PCW components at the PCW junction, particularly pectin, may provide receptor sites for Salmonella attachment. Our previous findings [20] also showed that pectin alone and pectin in combination with xyloglucan both increased the attachment of Salmonella strains.
Studies have found that bacterial cell surface components such as cellulose, flagella and fimbriae are important for the attachment of pathogens to fresh produce [17,21–23]. Flagella are long, thin surface appendages that extend up to 20μm and which are important for motility and chemotaxis [24]. Bacteria use flagella to move along the plant surface before finding a favourable attachment site [25]. Fimbriae are fine, hair-like protein appendages which contain adhesins on their tips with affinity to different sugar molecules and can be up to several micrometers long [26]. Cellulose, which consists of β(1–4)-linked glucose units secreted by bacterial cells, can hinder flagellar rotation and limit bacterial motility [27]. Production of both fimbriae and cellulose are regulated by the csgD gene in Salmonella. Expression of these structures are associated with biofilm formation and are important for the environmental persistence of Salmonella including enhancing its ability to avoid desiccation stress [28,29]. Temperature regulation of the expression of cellulose and fimbriae also takes place at the transcription level of csgD [30]. The csgD gene was the first enteric bacterial regulator identified to play a more influential role on enteric bacterial interaction with plants than in their animal hosts [10].
The specific mechanisms involved in the association between bacterial cell structures and PCW components exposed on cut PCWs have yet to be elucidated and up to now, very few genetic elements have been identified to be important for the attachment of human foodborne pathogens to plants. However, the ability to form biofilms has been correlated to better survival and stronger attachment to fresh produce [26], for example, Salmonella isolates sampled during tomato outbreaks produced biofilms and attached better to tomato leaflets compared to non-biofilm producing strains [31].
No studies reporting the interactions between bacterial surface components and specific PCW components have been reported. Most studies have focused only on the interactions between bacterial components and whole plant tissues. In this study we aimed to investigate how Salmonella cell surface structures (flagella, fimbriae and cellulose) influence attachment to major structural components of the PCW (cellulose, pectin and xyloglucan) after growth at 28°C (average environmental temperature) and 37°C (animal and human body temperature). Cases of salmonellosis usually peak during summer in most countries [32]. Most fresh produce have optimal growth temperatures within the range of 21°C to 32°C. For this reason, a growth temperature of 28°C was chosen as it coincides with the average summer temperatures in many countries, including China which is the top global producer of fresh vegetables [33].
In order to investigate this, a bacterial cellulose (BC)-based PCW model was used. The PCW model was produced by culturing Gluconacetobacter xylinus, a BC-producing bacterium, in Hestrin and Schramm (HS) growth medium with the addition of pectin and/or xyloglucan. Formation of the PCW model mimics the natural process of PCW deposition in native PCWs [34]. The PCW model was also found to possess similar structural and chemical properties to native PCWs [34,35] and has been previously optimized for studying bacterial attachment to PCWs [36]. Another study [37] has shown that the trend and numbers of Salmonella cells attaching to the natural PCWs (potato tuber, apple fruit and lettuce leaves) and to BC composites were similar to each other. In comparison to the heterogeneous composition of native PCWs, the PCW model is more versatile as its chemical composition can easily be manipulated. This allows direct investigation on how bacterial cell surface components interact with individual components in the PCW without the interference of other factors which may complicate the study.
Materials and Methods
Bacterial strains and culture conditions
Gluconacetobacter xylinus ATCC 53524 and Salmonella enterica subspecies enterica serovar Typhimurium ATCC 14028 were obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA). Gene knockout mutants of S. Typhimurium ATCC 14028 used in attachment experiments and their sources are listed in Table 1. Gene expression in these mutants has been previously well confirmed and characterised [38–40].
Table 1. Genotype and characteristics of S. Typhimurium ATCC 14028 mutant strains used in this study.
| Genotype | Characteristics | Source or reference |
|---|---|---|
| ΔfliC fljB | Lacks phase 1 and 2 flagellin | Miao et al. [38] |
| ΔbcsA | Lacks cellulose | White et al. [29] |
| ΔcsgA | Lacks fimbriae | White et al. [29] |
| ΔcsgA bcsA | Lacks cellulose and fimbriae | As described in methods |
| ΔcsgD | Missing the major biofilm transcriptional regulator coding sequence, lacks cellulose and fimbriae | MacKenzie et al. [39] |
The G. xylinus strain which produces BC was cultured statically at 30°C for 72h in Hestrin and Schramm (HS) broth containing 2% (w/v) glucose, 0.5% (w/v) peptone, 0.5% (w/v) yeast extract, 0.27% (w/v) Na2HPO4 and 0.115% (w/v) citric acid [41]. G. xylinus was maintained on HS agar at 4°C which was prepared by adding 1.5% agar to the HS medium.
For the attachment experiments, wild type and mutant strains of S. Typhimurium ATCC 14028 were grown at either 28°C or 37°C in tryptic soy broth (TSB; Merck, Darmstadt, Germany) under shaking incubation (150rpm) (Lab Companion SK-600 benchtop shaker; Medline, UK). Production of cellulose and fimbriae by these strains at 28°C or 37°C were confirmed by monitoring their colony morphology on Luria-Bertani (LB; Merck, Darmstadt, Germany) medium without salt supplemented with 40μg/mL of Congo Red (CR; Sigma-Aldrich, Missouri, USA) and 20μg/mL Coomassie brilliant blue (CBB; Sigma-Aldrich, Missouri, USA) when grown at these two temperatures. Cellulose production was further confirmed using LB medium without salt supplemented with 50μg/mL of Calcofluor White (CW; Sigma-Aldrich, Missouri, USA), colonies which produce cellulose will fluoresce under UV light. Flagella production at both temperatures was determined using Ryu’s flagella stain as described by Kodaka et al. [42]. Salmonella strains were maintained on tryptic soy agar (TSA; Merck, Darmstadt, Germany) at 4°C.
Generation of S. Typhimurium 14028 ΔcsgA bcsA double mutant strain
The S. Typhimurium ATCC 14028 ΔbcsA strain was generated as previously described [29]. S. Typhimurium ΔbcsA cells were transformed with the pHSG415/ΔcsgA (formerly ΔagfA) construct prepared from S. Enteritidis 27655-3b genomic DNA [43] and selected on LB agar supplemented with 100μg/mL ampicillin. The ΔcsgA mutation was successfully performed through allelic exchange following established procedures [44]. Final ampicillin-sensitive S. Typhimurium ΔcsgA bcsA colonies were differentiated from ΔbcsA colonies by growth at 28°C on agar media (1% tryptone, 1.5% agar) supplemented with 100μg/mL Congo red; ΔcsgA bcsA colonies appeared light pink, whereas ΔbcsA colonies appeared orange or red. PCR was used to confirm the ΔcsgA mutation, using primers TAFPF (TACGCCAGGAAGGATCAAAACTAT) and TAFPR (GCCGTCGCGCACAGAGA); PCR products were purified and confirmed by DNA sequencing (Eurofins MWG Operon, Kentucky, USA).
Production of bacterial cellulose-based plant cell wall models
BC-based PCW models were produced as described in our previous paper [20]. Briefly, a primary inoculum of G. xylinus ATCC 53524 was prepared by transferring a colony grown on HS agar into HS broth which was incubated statically at 30°C for 72h. The primary inoculum was used for the scale-up production of all BC composites and was added to fresh HS medium with or without combinations of pectin and/or xyloglucan as shown below:
BC was produced with only HS medium without additional additives.
BC-Pectin (BCP) was produced by adding 0.1%, 0.3% and 0.5% w/v apple pectin (kindly donated by Herbstreith & Fox, Neuenbϋrg, Germany) to the HS medium and an optimal concentration of CaCl2 was added to form a low degree of esterification (DE) pectin gel, i.e. 3mM CaCl2 for 0.1% w/v pectin, 6mM CaCl2 for 0.3% w/v pectin and 12.5mM CaCl2 for 0.5% w/v pectin (R&M Chemicals, Malaysia).
BC-Xyloglucan (BCX) was produced by adding 0.1%, 0.3% and 0.5% w/v xyloglucan (Megazyme, County Wicklow, Ireland) to the HS medium.
BC-Pectin-Xyloglucan (BCPX) was produced by adding different combinations of pectin and xyloglucan (0.1%, 0.3% and 0.5% w/v), varying concentrations of calcium chloride was added according to the amount of pectin present as shown earlier.
Composites were produced in enclosed plastic containers (1.5cm x 1.5cm x 1.5cm) incubated statically for 72h depending on the HS medium composition. During harvest, BC composites occur as a gelatinous layer floating above the growth medium. Harvested composites were rinsed in 6mM CaCl2 at 100rpm for 1h to remove media components. The range of pectin and xyloglucan concentrations were selected based on work carried out previously [20,36,37,45,46] which produced composites with characteristics that fall within average native PCW component concentrations. Chemical composition analysis of the BC composites were consistent when compared to each other [20].
Attachment to BC composites
Attachment experiments were carried out as described previously [20]. Early stationary phase cultures of S. Typhimurium ATCC 14028 and its mutants grown for 18h at either 28°C or 37°C were centrifuged at 5500 x g (Hettich D-78532, Tuttlingen, Germany) for 10 min at 4°C. The pellet was washed twice with phosphate buffer saline (PBS) (pH 7.4) (1st BASE, Singapore) and resuspended in PBS to an optical density at 600nm (UV/Vis spectrophotometer, Shimadzu UV mini-1240, USA) which corresponds to 108 CFU/mL for each strain.
After rinsing, BC composites (BC, BCP, BCX, BCPX) (1.5cm x 1.5cm, ~ 2mm thickness) were incubated in 10mL of each pathogenic bacteria suspension (108 CFU/mL) for 20 mins with gentle shaking (100rpm) at 25°C. After incubation, gentle rinsing (100rpm) was carried out in 6mM CaCl2 solution for a minute to remove loosely attached cells. Each composite was then placed in a stomacher bag filled with 50mL PBS and pummelled for a minute at 8 strokes/sec in BagMixer 400 (Interscience, France). The resulting stomached fluid was then serial diluted and appropriate dilutions were plated on xylose lysine deoxycholate agar (XLDA; Oxoid, UK) to enumerate the number of pathogenic bacteria attached to the BC composite (CFU/cm2 composite). There may be some variability in the surface chemistry of the surface exposed to the air compared to the other surfaces exposed to the HS medium. We have reasonably assumed that this will not significantly affect the attachment results as all surfaces of the composites were exposed to bacteria when the composites were fully immersed in the bacterial suspension.
Data analysis
All experiments were performed in triplicates with three independently grown bacterial cultures. Statistical analysis of results was performed using Statistical Package for the Social Sciences (SPSS) (PASW Statistics 18, SPSS Inc., USA). One-way analysis of variance (ANOVA) was used to compare significant differences between wild type and mutant strains of S. Typhimurium ATCC 14028 grown at the same temperature (either 28°C or 37°C) for their overall attachment to the BC composites. Another one-way ANOVA was carried out individually for each strain grown at a specific temperature (either 28°C or 37°C) to compare significant differences in numbers attaching to different BC composites. Independent sample t-tests were also conducted to determine significant differences for the same strain for its attachment to the BC composites when grown at two different temperatures (comparing 28°C and 37°C). Differences among the means were determined using Tukey’s method at 95% confidence level.
Results and Discussion
Production of cell surface components by S. Typhimurium ATCC 14028 wild type strains
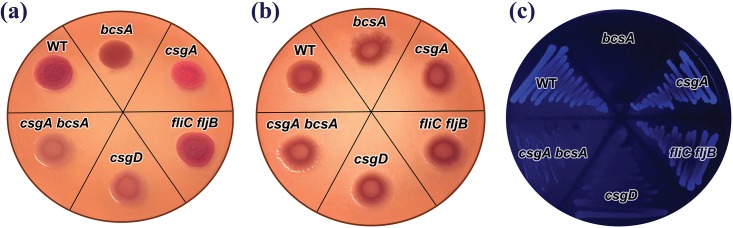
S. Typhimurium ATCC 14028 produced flagella when grown at 28°C and 37°C (shown in S1 Fig). However, the S. Typhimurium strain displayed temperature-dependent expression of cellulose and fimbriae as can be seen on CR and CW plates (Fig 1). As described by Romling et al. [47], the wild type strain of S. Typhimurium was able to produce cellulose and fimbriae at 28°C and showed rough, dry and red (rdar) phenotypes on the CR plate. Wild type colonies grown on the CW plate fluoresced under UV light. When grown at 37°C however, the strain lost the ability to produce these structures and appeared as smooth and white colonies (saw) on the CR plate and colonies formed on the CW plate did not fluoresce. White et al. [29] also noted that Salmonella only produce these extracellular structures at incubation temperatures of below 30°C and under nutrient-limited conditions at low osmolarity. According to Kader et al. [48], temperature regulation of the rdar morphotype is mediated by the temperature gradient in cyclic-di(3’→5’)-guanylic acid (c-di-GMP) concentrations. The c-di-GMP secondary messenger regulates cellulose and fimbriae production by affecting both CsgA and CsgD expression on the transcriptional and post-transcriptional levels respectively. Therefore, wild type Salmonella strains are expected to have lower levels of c-di-GMP at 37°C than at 28°C, this could be caused by either increased phosphodiesterase activity or reduced diguanylate cylase activity [48].
Fig 1. Colony morphology of Salmonella Typhimurium ATCC 14028 wild type and mutant strains.
Colonies formed on (a) Congo Red (CR) agar plate grown at 28°C, (b) CR agar plate grown at 37°C and (c) Calcofluor White (CW) agar plate grown at 28°C.
Effect of PCW components and temperature on Salmonella attachment to BC composites
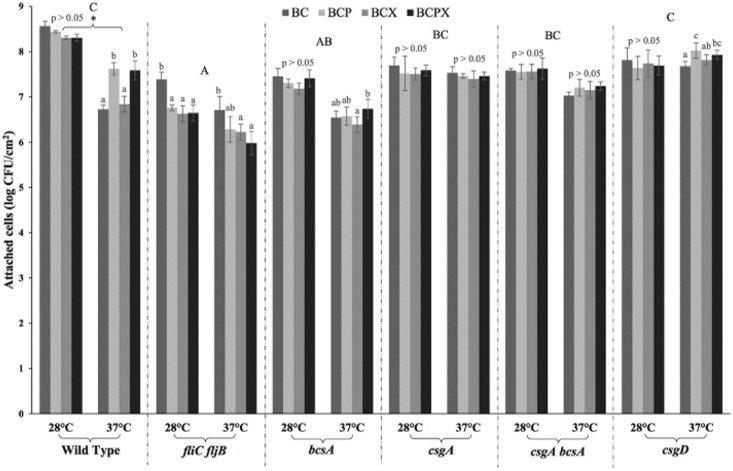
We found in this study that the varying levels of pectin and xyloglucan (0.1%, 0.3% and 0.5%) did not have significant effect on S. Typhimurium attachment to the BC composites (shown in S2 Fig), hence attachment numbers for each type of composite were collated and presented as an average value in Fig 2 for easy comparison. We initially expected the S. Typhimurium strains grown at 28°C (especially the wild type strain and the ΔfliC fljB mutant which can produce cellulose and fimbriae at 28°C) to have higher attachment levels to the BC composites than the same strains grown at 37°C. However, of the 6 strains only the wild type S. Typhimurium strain showed significant difference in the number of cells attached to the BC composites when grown at the two different temperatures (p<0.05) whereas others showed similar attachment for both temperatures (p>0.05). All strains grown at 28°C did not show significant differences in their attachment to the various BC composites (p>0.05) except for the ΔfliC fljB mutant which showed the differential attachment to the BC composites. The ΔfliC fljB mutant attached at lower levels compared to the other strains and its attachment to the BC composites containing pectin and/or xyloglucan was significantly higher than to the BC-only composite (p<0.05). This suggests that flagella may interact with pectin and xyloglucan, with the loss of flagella decreasing Salmonella attachment to the BC composites containing these PCW components. As Warriner and Namvar [49] have pointed out, human pathogen attachment to plants may involve specific recognition interactions between the bacterial cell surface and physical structures on the leaf. Cell surface components (such as cellulose, flagella and fimbriae) may harbour surface epitopes which enable pathogens to preferentially attach to cut surfaces and natural openings, such as stomata, which expose nutrients produced during photosynthesis [12,17]. A study by Saggers et al. [19] also indicated interactions between bacterial cells and PCW components as fewer S. Typhimurium cells were attached when less pectin was present in the potato tissue. It was also expected that the attachment for all S. Typhimurium strains grown at 37°C (except for the ΔfliC fljB mutant) would be similar to each other since cellulose and fimbriae are not produced at this temperature. This was not, however, the case as significant variations in the attachment of different strains were observed (p<0.05). Variations in the attachment of S. Typhimurium strains grown at different temperatures were most probably not due to the effect of cellulose and fimbriae structures but could be caused by other factors that have not yet been identified.
Fig 2. Attachment of S. Typhimurium ATCC 14028 (a) wild type, (b) ΔfliC fljB mutant, (c) ΔbcsA mutant, (d) ΔcsgA mutant, (e) ΔcsgA bcsA mutant and (f) ΔcsgD mutant grown at 28°C and 37°C to various BC composites (BC, BCP, BCX, BCPX).
Different lowercase letters indicate significant differences in attachment numbers between different BC composites within each strain grown at a specific temperature (One-way ANOVA & Tukey’s pairwise comparison at p<0.05). Different uppercase letters indicate significant differences in attachment numbers to BC composites between different strains (One-way ANOVA & Tukey’s pairwise comparison at p<0.05). Asterisk sign indicates significant differences in attachment numbers for the same strain grown at two different temperatures (28°C and 37°C) (independent samples t-test at p<0.05).
Role of cell surface structures in Salmonella attachment to BC composites
An overall comparison on the number of cells of the S. Typhimurium ATCC 14028 wild type and mutant strains attached to the BC composites presented in Fig 2 indicated that the different strains attached in significantly different numbers to the composites (p<0.05). It was shown that overall, the ΔfliC fljB and ΔbcsA mutants displayed significantly lower attachment levels compared to the wild type strain (p<0.05). Another comparison carried out on strains grown at 28°C showed that the wild type strain attached in significantly higher numbers as compared to all mutants (p<0.05). This indicates that cellulose, fimbriae and flagella were all involved in the attachment of S. Typhimurium to the PCW models. Lapidot and Yaron [23] suggested that bacterial surface appendages, such as flagella and curli fimbriae, may influence the initial reversible adhesion to plants, which is mediated by van der Waal interactions and hydrogen bonds. This initial adhesion is followed by stronger irreversible attachment which is mediated by electrostatic forces and dependent on extracellular components, such as bacterial cellulose.
Role of flagella
Of the bacterial surface structures studied, flagella appeared to have the most important role in attachment as the ΔfliC fljB mutant attached in lowest numbers of all mutants to PCW models when grown at both temperatures. As compared to the wild type, attachment of the ΔfliC fljB mutant was reduced by ~1.5 and 0.9 log CFU/cm2 when grown at 28°C and 37°C, respectively. Flagella are known to be important for the motility of S. Typhimurium cells [50] and allow the bacteria to reach the attachment surface faster [51]. Flagella also mediate chemotaxis which can guide planktonic cells to swim towards sites with nutrients or towards cells attached to a surface [52]. Similarly, motility enables pathogens to enter and colonize stomata, wounds and openings in plants [13]. Flagella and motility mutants of S. Typhimurium failed to invade lateral root junctions of the Arabidopsis thaliana plants, which may be explained by the inability of the mutants to find entry points into the plant [25]. It was also found that mutations which impaired bacterial motility also reduced the ability of Salmonella to be internalized by plants [13]. We suggest that flagella enable the cells to move within the matrix of the BC composites where attachment can occur. There is also a possibility that the long flagella filament (which extends up to 20μm) may cause entanglement of Salmonella cells (~2μm) within the BC matrix. A study by Berger et al. [22] has shown that S. Senftenberg requires flagella to attach to salad leaves. In contrast to the results of our study, these authors found that the deletion of the fliC gene did not affect S. Typhimurium attachment to basil leaf epidermis. The different outcome of this previous study and ours may be due to the fact that the fljB gene was not deleted in the previous study. The normal expression of fljB (which encodes the phase-2 flagellin) could have substituted for the loss of phase-1 flagellin (encoded by fliC) and thus the ability of the S. Typhimurium to attach was not affected in their study. In addition to the use of flagella for motility and chemotaxis, S. Typhimurium also uses the flagella to sense external environments in order to regulate its own biogenesis and virulence [53]. It has been demonstrated that the flg22 peptide conserved in the Salmonella flagellin activates the plant immune system which then inhibits Salmonella colonization. Iniguez et al. [54] showed that the S. Typhimurium ΔfliC fljB mutant was more successful in colonizing roots in alfalfa, wheat and Arabidopsis plants. This could be due to the inability of the plant defence system in detecting colonization by the pathogens. Although the role of flagella in Salmonella attachment to PCWs can be studied using the PCW models and cut plant material, these models cannot be used to investigate the plant immune response to human pathogens. Interactions between plants and human pathogens may cause changes in the plant such as the production of free radicals, lignification, pH change and also phenolic and cellulose appositions in the PCW. This represents a limitation of our study and it is necessary to investigate this using native PCWs in future studies to obtain a complete picture of these interactions.
Roles of cellulose and fimbriae
In addition to the importance of flagella, our results also showed that cellulose and fimbriae were involved in the attachment of S. Typhimurium to PCWs. Single and double mutants of cellulose and fimbriae (ΔbcsA, ΔcsgA, ΔcsgA bcsA, ΔcsgD) which were grown at 28°C all displayed significantly lower attachment compared to the wild type strain (p<0.05). When these mutants were grown at 37°C, the ΔcsgA and ΔcsgA bcsA attached in similar numbers as compared to the wild type (p>0.05). The ΔfliC fljB and ΔbcsA mutants attached at significantly lower levels (p<0.05) whereas only the ΔcsgD mutant showed significantly higher attachment numbers compared to the wild type (p<0.05). The roles of cellulose and fimbriae in human pathogenic attachment to plants are not well understood yet but both attachment structures are known to contribute to aggregative multicellular behaviour, biofilm formation and protection against harsh environmental conditions [55,56].
Fimbriae, which are shorter in length, straighter and more numerous than flagella in numbers do not play a role in Salmonella motility. The function of adhesins on the fimbrial tip in determining its specific attachment properties to animal cells has been demonstrated [57]. Lectins, found on fimbriae and flagella structures, are able to recognize oligosaccharide units on the animal cells allowing specific attachment [58]. A similar process could be involved in bacterial attachment to plant surfaces. Cellulose play a role in cell-to-cell interactions and the formation of bacterial aggregates which may in turn favour bacterial attachment to a surface [58,59]. In addition to its role in attachment, cellulose also promotes bacterial persistence in the environment by conferring cells with resistance to adverse conditions, such as exposure to chlorine and bleach [60].
Some studies have shown that cellulose and fimbriae were involved in Salmonella attachment to parsley and alfalfa sprout seedlings [23,59]. A number of studies have found that S. enterica uses cellulose to adhere to plant roots [59,61]. Solomon et al. [62] found that high proportions of Salmonella strains isolated from fresh produce possess cellulose and fimbriae, which further supports this finding. Lapidot and Yaron [23] showed that both cellulose and fimbriae affect bacterial colonization although fimbriae played a more important role than cellulose in S. Typhimurium transfer from contaminated irrigation water to parsley. Another study by Barak et al. [59] also showed that the roles of cellulose and fimbriae in attachment are additive, with the initial attachment of a ΔcsgB bcsA double mutant further reduced compared with a ΔbcsA single mutant. Barak et al. [21] also observed that mutation in csgB, not csgA, reduced the ability of S. Newport to attach to alfalfa sprouts. They suggested that the curli fimbrial nucleator (encoded by csgB) may facilitate the initial attachment of Salmonella to plants even without the production of fimbriae.
In our study it appeared that the four cellulose and fimbriae mutants behaved similarly. This feature could be explained by the smaller roles these structures have in bacterial attachment such that the loss of either one or both of these structures did not greatly affect attachment of the strains. To the best of our knowledge, no study has a pleiotropic effect of these gene mutations on the general structure of the bacterial cell wall.
In addition to being involved in the regulation of cellulose and fimbriae expression, the csgD gene is also involved in the synthesis of the O antigen capsule and colanic acid which have been shown to modulate bacterial attachment to plants [59]. Colanic acid has been found to be associated with fimbriae in E. coli and is involved in the formation of Salmonella biofilms on animal cells. The O antigen capsule has been shown to protect bacterial cells from desiccation [28]. It was interesting to note that in our study the ΔcsgD mutant grown at both 28°C and 37°C had significantly higher ability to attach to the composites as compared to the ΔbcsA mutant (p<0.05). Studies [63,64] have shown that ΔcsgD mutants lacking cellulose and fimbriae have increased fliE (encoding the flagellum basal body) promoter activity and production of the FliC protein as compared to wild type cells. This can be explained as the synthesis of cellulose and fimbriae or flagella production are mutually exclusive from each other due to opposite regulation by the signalling molecule cyclic di-GMP [65]. Increased production of flagella-related genes could therefore have helped the ΔcsgD mutant to attach better to the BC composites. Further studies are required to confirm this.
Conclusions
Taken together the results of our study demonstrate that S. Typhimurium cells grown in the animal host (at 37°C) do not produce cellulose and fimbriae and that these biofilm-forming structures will only form if the pathogens are released into the external environment which has a lower temperature (e.g.: 28°C). Flagella, fimbriae and cellulose all contribute to the interaction of Salmonella with intact plants in the environment, but we have shown that these structures are not the most important mechanisms for attachment of Salmonella to the BC composites which may be influenced by many other factors. Although the results from our study do not fully represent the real life phenomenon occurring on whole plants, a better understanding of the role of bacterial structures on the attachment of Salmonella to plants will aid in finding ways to remove pathogens from fresh produce more effectively.
Supporting Information
(TIF)
(PDF)
Acknowledgments
The authors would like to acknowledge Herbstreith and Fox, Neuenbürg, Germany for the kind donation of the apple pectin.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors have no support or funding to report.
References
- 1.Heaton JC, Jones K. Microbial contamination of fruit and vegetables and the behaviour of enteropathogens in the phyllosphere: a review. J Appl Microbiol. 2008; 104:613–626. [DOI] [PubMed] [Google Scholar]
- 2.Jacxsens L, Luning PA, van der Vorst JGAJ, Devlieghere F, Leemans R, Uyttendaele M. Simulation modelling and risk assessment as tools to identify the impact of climate change on microbiological food safety: the case study of fresh produce supply chain. Food Res Int. 2010; 43:1925–1935. [Google Scholar]
- 3.Berger CN, Sodha SV, Shaw RK, Griffin PM, Pink D, Hand P, et al. Fresh fruit and vegetables as vehicles for the transmission of human pathogens. Environ Microbiol. 2010; 12:2385–2397. 10.1111/j.1462-2920.2010.02297.x [DOI] [PubMed] [Google Scholar]
- 4.Tauxe RV. Emerging foodborne diseases: an evolving public health challenge. Emerg Infect Dis. 1997; 3:425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Viazis S, Akhtar M, Feirtag J, Diez-Gonzalez F. Reduction of Escherichia coli O157:H7 viability on leafy green vegetables by treatment with a bacteriophage mixture and trans-cinnamaldehyde. Food Microbiol. 2011; 28:149–157. 10.1016/j.fm.2010.09.009 [DOI] [PubMed] [Google Scholar]
- 6.Herman K, Ayers TL, Lynch M. Foodborne disease outbreaks associated with leafy greens, 1973–2006. International Conference on Emerging Infectious Diseases. Atlanta, GA; 2008. [Google Scholar]
- 7.Brandl MT. Fitness of human enteric pathogens on plants and implications for food safety. Annu Rev Phytopathol. 2006; 44:367–392. [DOI] [PubMed] [Google Scholar]
- 8.Holden N, Pritchard L, Toth I. Colonization outwith the colon: plants as an alternative environmental reservoir for human pathogenic enterobacteria. FEMS Microbiol Rev. 2008; 33:689–703. [DOI] [PubMed] [Google Scholar]
- 9.Krtinić G, Durić P, Ilić S. Salmonellae in food stuffs of plant origin and their implications on human health. Eur J Clin Microbiol Infect Dis. 2010; 29:1321–1325. 10.1007/s10096-010-1001-4 [DOI] [PubMed] [Google Scholar]
- 10.Barak JD, Schroeder BK. Interrelationships of food safety and plant pathology: the life cycle of human pathogens on plants. Annu Rev Phytopathol. 2012; 50:241–266. 10.1146/annurev-phyto-081211-172936 [DOI] [PubMed] [Google Scholar]
- 11.Tyler HL, Triplett EW. Plants as a habitat for beneficial and/or human pathogenic bacteria. Annu Rev Phytopathol. 2008; 46:53–73. 10.1146/annurev.phyto.011708.103102 [DOI] [PubMed] [Google Scholar]
- 12.Klerks MM, Franz E, van Gent-Pelzer M, Zijlstra C, van Bruggen AHC. Differential interaction of Salmonella enterica serovars with lettuce cultivars and plant-microbe factors influencing the colonization efficiency. ISME J. 2007; 1:620–631. [DOI] [PubMed] [Google Scholar]
- 13.Kroupitski Y, Pinto R, Brandl MT, Belausov E, Sela S. Interactions of Salmonella enterica with lettuce leaves. J Appl Microbiol. 2009; 106:1876–1885. 10.1111/j.1365-2672.2009.04152.x [DOI] [PubMed] [Google Scholar]
- 14.Bordas MA, Balebona MC, Zorrilla I, Borrgo JJ, Morinigo MA. Kinetics of adhesion of selected fish-pathogenic Vibrio strains to skin mucus of gilt-head sea bream (Sparus aurata L.). Appl Environ Microbiol. 1996; 62:3650–3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu L, Walker WA. Pathologic and physiologic interactions of bacteria with the gastrointestinal epithelium. Am J Clin Nutr. 2001; 73:1124–1130. [DOI] [PubMed] [Google Scholar]
- 16.Aruscavage D, Lee K, Miller S, LeJeune JT. Interactions affecting the proliferation and control of human pathogens on edible plants. J Food Sci. 2006; 71:R89–R99. [Google Scholar]
- 17.Kroupitski Y, Golberg D, Belausov E, Pinto R, Swartzberg D, Granot D, et al. Internalization of Salmonella enterica in leaves is induced by light and involves chemotaxis and penetration through open stomata. Appl Environ Microbiol. 2009; 75:6076–6086. 10.1128/AEM.01084-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takeuchi K, Matute CM, Hassan AN, Frank JF. Comparison of the attachment of Escherichia coli O157:H7, Listeria monocytogenes, Salmonella Typhimurium, and Pseudomonas fluorescens to lettuce leaves. J Food Prot. 2000; 63:1433–1437. [DOI] [PubMed] [Google Scholar]
- 19.Saggers EJ, Waspe CR, Parker ML, Waldron KW, Brocklehurst TF. Salmonella must be viable in order to attach to the surface of prepared vegetable tissues. J Appl Microbiol. 2008; 105:1239–1245. 10.1111/j.1365-2672.2008.03795.x [DOI] [PubMed] [Google Scholar]
- 20.Tan MSF, Rahman S, Dykes GA. Pectin and xyloglucan influence the attachment of Salmonella enterica and Listeria monocytogenes to bacterial cellulose-derived plant cell wall models. Appl Environ Microbiol. 2016; 82:680–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barak JD, Gorski L, Naraghi-Arani P, Charkowski AO. Salmonella enterica virulence genes are required for bacterial attachment to plant tissue. Appl Environ Microbiol. 2005; 71:5685–5691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berger CN, Shaw RK, Brown DJ, Mather H, Clare S, Dougan G, et al. Interaction of Salmonella enterica with basil and other salad leaves. ISME J. 2009; 3:261–265. 10.1038/ismej.2008.95 [DOI] [PubMed] [Google Scholar]
- 23.Lapidot A, Yaron S. Transfer of Salmonella enterica serovar Typhimurium from contaminated irrigation water to parsley is dependent on curli and cellulose, the biofilm matrix components. J Food Prot. 2009; 72:618–623. [DOI] [PubMed] [Google Scholar]
- 24.Wiedemann A, Virlogeux-Payant I, Chaussé A, Schikora A, Velge P. Interactions of Salmonella with animals and plants. Front Microbiol. 2015; 5:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooley MB, Miller WG, Mandrell RE. Colonization of Arabidopsis thaliana with Salmonella enterica and enterohemorrhagic Escherichia coli O157:H7 and competition by Enterobacter asburiae. Appl Environ Microbiol. 2003; 69:4915–4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yaron S, Römling U. Biofilm formation by enteric pathogens and its role in plant colonization and persistence. Microb Biotechnol. 2014; 7:496–516. 10.1111/1751-7915.12186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zorraquino V, García B, Latasa C, Echeverz M, Toledo-Arana A, Valle J, et al. Coordinated cyclic-di-GMP repression of Salmonella motility through YcgR and cellulose. J Bacteriol. 2013; 195:417–428. 10.1128/JB.01789-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gibson DL, White AP, Snyder SD, Martin S, Heiss C, Azadi P, et al. Salmonella produces an O-antigen capsule regulated by AgfD and important for environmental persistence. J Bacteriol. 2006; 188:7722–7730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.White AP, Gibson DL, Kim W, Kay WW, Surette MG, Acteriol JB. Thin aggregative fimbriae and cellulose enhance long-term survival and persistence of Salmonella. J Bacteriol. 2006; 188:3219–3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Römling U, Sierralta WD, Eriksson K, Normark S. Multicellular and aggregative behaviour of Salmonella Typhimurium strains is controlled by mutations in the agfD promoter. Mol Microbiol. 1998; 28:249–264. [DOI] [PubMed] [Google Scholar]
- 31.Cevallos-Cevallos JM, Gu G, Danyluk MD, van Bruggen AH. Adhesion and splash dispersal of Salmonella enterica Typhimurium on tomato leaflets: effects of rdar morphotype and trichome density. Int J Food Microbiol. 2012; 160:58–64. 10.1016/j.ijfoodmicro.2012.09.021 [DOI] [PubMed] [Google Scholar]
- 32.Naumova EN, Jagai JS, Matyas B, DeMaria A, MacNeill IB, Griffiths JK, et al. Seasonality in six enterically transmitted diseases and ambient temperature. Epidemiol Infect. 2007; 135:281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang SW. Global Trade Patterns in Fruits and Vegetables (U.S. Department of Agriculture, Economic Research Service, Washington DC) Agriculture and Trade Report No. WRS-04-06 Washington, DC; 2004. [Google Scholar]
- 34.Chanliaud E, Gidley M. In vitro synthesis and properties of pectin/Acetobacter xylinus cellulose composites. Plant J. 1999; 20:25–35. [DOI] [PubMed] [Google Scholar]
- 35.Whitney S, Gothard M, Mitchell J, Gidley M. Roles of cellulose and xyloglucan in determining the mechanical properties of primary plant cell walls. Plant Physiol. 1999; 121:657–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tan MSF, Wang Y, Dykes GA. Attachment of bacterial pathogens to a bacterial cellulose-derived plant cell wall model: a proof of concept. Foodborne Pathog Dis. 2013; 10:992–994. 10.1089/fpd.2013.1536 [DOI] [PubMed] [Google Scholar]
- 37.Tan MSF, Rahman S, Dykes GA. Relationship between cell concentration and Salmonella attachment to plant cell walls. Food Cont. 2016; 67:119–126. [Google Scholar]
- 38.Miao EA, Alpuche-Aranda CM, Dors M, Clark AE, Bader MW, Miller SI, et al. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat Immunol. 2006; 7:569–575. [DOI] [PubMed] [Google Scholar]
- 39.MacKenzie KD, Wang Y, Shivak DJ, Wong CS, Hoffman LJ, Lam S, et al. Bistable expression of CsgD in Salmonella enterica serovar Typhimurium connects virulence to persistence. Infect Immun. 2015; 83:2312–2326. 10.1128/IAI.00137-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zogaj X, Nimtz M, Rohde M, Bokranz W, Römling U. The multicellular morphotypes of Salmonella typhimurium and Escherichia coli produce cellulose as the second component of the extracellular matrix. Mol Microbiol. 2001; 39:1452–1463. [DOI] [PubMed] [Google Scholar]
- 41.Hestrin S, Schramm M. Synthesis of cellulose by Acetobacter xylinum: preparation of freeze-dried cells capable of polymerizing glucose to cellulose. Biochem J. 1954; 58:345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kodaka H, Armfield AY, Lombard GL, Dowell VR. Practical procedure for demonstrating bacterial flagella. J Clin Microbiol. 1982; 16:948–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.White AP, Collinson SK, Banser PA, Gibson DL, Paetzel M, Strynadka NC, et al. Structure and characterization of AgfB from Salmonella Enteritidis thin aggregative fimbriae. J Mol Biol. 2001; 311:735–749. [DOI] [PubMed] [Google Scholar]
- 44.White AP, Allen-Vercoe E, Jones BW, DeVinney R, Kay WW, Surette MG. An efficient system for markerless gene replacement applicable in a wide variety of enterobacterial species. Can J Microbiol. 2007; 53:56–62. [DOI] [PubMed] [Google Scholar]
- 45.Cybulska J, Konstankiewicz K, Zdunek A, Skrzypiec K. Nanostructure of natural and model cell wall materials. Int Agrophysics. 2010; 24:107–114. [Google Scholar]
- 46.Cybulska J, Vanstreels E, Ho Q, Courtin C, Craeyveld V, Nicolaï B, et al. Mechanical characteristics of artificial cell walls. J Food Eng. Elsevier Ltd; 2010; 96:287–294. [Google Scholar]
- 47.Römling U, Bian Z, Hammar M, Sierralta WD, Normark S. Curli fibers are highly conserved between Salmonella Typhimurium and Escherichia coli with respect to operon structure and regulation. J Bacteriol. 1998; 180:722–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kader A, Simm R, Gerstel U, Morr M, Römling U. Hierarchical involvement of various GGDEF domain proteins in rdar morphotype development of Salmonella enterica serovar Typhimurium. Mol Microbiol. 2006; 60:602–616. [DOI] [PubMed] [Google Scholar]
- 49.Warriner K, Namvar A. The tricks learnt by human enteric pathogens from phytopathogens to persist within the plant environment. Curr Opin Biotechnol. 2010; 21:131–136. 10.1016/j.copbio.2010.01.004 [DOI] [PubMed] [Google Scholar]
- 50.Moens S, Vanderleyden J. Functions of bacterial flagella. Crit Rev Microbiol. 1996; 22:67–100. [DOI] [PubMed] [Google Scholar]
- 51.Reina LD, Fleming HP, Breidt F. Bacterial contamination of cucumber fruit through adhesion. J Food Prot. 2002; 65:1881–1887. [DOI] [PubMed] [Google Scholar]
- 52.Pratt LA, Kolter R. Genetic analysis of Escherichia coli biofilm formation: roles of flagella, motility, chemotaxis and type I pili. Mol Microbiol. 1998; 30:285–293. [DOI] [PubMed] [Google Scholar]
- 53.Wang Q, Suzuki A, Mariconda S, Porwollik S, Harshey RM. Sensing wetness: a new role for the bacterial flagellum. Embo J. 2005; 24:2034–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Iniguez AL, Dong Y, Carter HD, Ahmer BM, Stone JM, Triplett EW. Regulation of enteric endophytic bacterial colonization by plant defenses. Mol Plant-Microbe Interact. 2005; 18:169–178. [DOI] [PubMed] [Google Scholar]
- 55.Gu G, Hu J, Cevallos-Cevallos JM, Richardson SM, Bartz JA, van Bruggen AHC. Internal colonization of Salmonella enterica serovar Typhimurium in tomato plants. PLoS ONE. 2011; 6:e27340 10.1371/journal.pone.0027340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shaw RK, Lasa I, García BM, Pallen MJ, Hinton JCD, Berger CN, et al. Cellulose mediates attachment of Salmonella enterica serovar Typhimurium to tomatoes. Environ Microbiol Rep. 2011; 3:569–573. 10.1111/j.1758-2229.2011.00263.x [DOI] [PubMed] [Google Scholar]
- 57.Weening E, Barker J, Laarakker M, Humphries A, Tsolis R, Bäumler A. The Salmonella enterica serotype Typhimurium lpf, bcf, stb, stc, std,and sth fimbrial operons are required for intestinal persistence in mice. Infect Immun. 2005; 73:3358–3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rodríguez-Navarro DN, Dardanelli MS, Ruíz-Saínz JE. Attachment of bacteria to the roots of higher plants. FEMS Microbiol Lett. 2007; 272:127–136. [DOI] [PubMed] [Google Scholar]
- 59.Barak JD, Jahn CE, Gibson DL, Charkowski AO. The role of cellulose and O-antigen capsule in the colonization of plants by Salmonella enterica. Mol Plant Microbe Interact. 2007; 20:1083–1091. [DOI] [PubMed] [Google Scholar]
- 60.Solano C, Garcia B, Valle J, Berasain C, Ghigo J, Gamazo C, et al. Genetic analysis of Salmonella Enteritidis biofilm formation: critical role of cellulose. Mol Microbiol. 2002; 43:793–808. [DOI] [PubMed] [Google Scholar]
- 61.Charkowski A, Barak J, Sarreal C, Mandrell R. Differences in growth of Salmonella enterica and Escherichia coli O157:H7 on alfalfa sprouts. Appl Environ Microbiol. 2002; 68:3114–3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Solomon EB, Niemira BA, Sapers GM, Annous BA. Biofilm formation, cellulose production, and curli biosynthesis by Salmonella originating from produce, animal, and clinical sources. J Food Prot. 2005; 68:906–912. [DOI] [PubMed] [Google Scholar]
- 63.Marshall JM, Gunn JS. The O-antigen capsule of Salmonella enterica serovar Typhimurium facilitates serum resistance and surface expression of FliC. Infect Immun. 2015; 83:3946–3959. 10.1128/IAI.00634-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ogasawara H, Yamamoto K, Ishihama A. Role of the biofilm master regulator CsgD in cross-regulation between biofilm formation and flagellar synthesis. J Bacteriol. 2011; 193:2587–2597. 10.1128/JB.01468-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mika F, Hengge R. Small regulatory RNAs in the control of motility and biofilm formation in E. coli and Salmonella. Int J Mol Sci. 2013; 14:4560–4579. 10.3390/ijms14034560 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.